Abstract
In critically ill patients infected with SARS-CoV-2, early leukocyte recruitment to the respiratory system was found to be orchestrated by leukocyte trafficking molecules accompanied by massive secretion of proinflammatory cytokines and hypercoagulability. Our study aimed to explore the interplay between leukocyte activation and pulmonary endothelium in different disease stages of fatal COVID-19. Our study comprised 10 COVID-19 postmortem lung specimens and 20 control lung samples (5 acute respiratory distress syndrome, 2 viral pneumonia, 3 bacterial pneumonia, and 10 normal), which were stained for antigens representing the different steps of leukocyte migration: E-selectin, P-selectin, PSGL-1, ICAM1, VCAM1, and CD11b. Image analysis software QuPath was used for quantification of positive leukocytes (PSGL-1 and CD11b) and endothelium (E-selectin, P-selectin, ICAM1, VCAM1). Expression of IL-6 and IL-1β was quantified by RT-qPCR. Expression of P-selectin and PSGL-1 was strongly increased in the COVID-19 cohort compared with all control groups (COVID-19:Controls, 17:23, P < .0001; COVID-19:Controls, 2:75, P < .0001, respectively). Importantly, P-selectin was found in endothelial cells and associated with aggregates of activated platelets adherent to the endothelial surface in COVID-19 cases. In addition, PSGL-1 staining disclosed positive perivascular leukocyte cuffs, reflecting capillaritis. Moreover, CD11b showed a strongly increased positivity in COVID-19 compared with all controls (COVID-19:Controls, 2:89; P = .0002), indicating a proinflammatory immune microenvironment. Of note, CD11b exhibited distinct staining patterns at different stages of COVID-19 disease. Only in cases with very short disease course, high levels of IL-1β and IL-6 mRNA were observed in lung tissue. The striking upregulation of PSGL-1 and P-selectin reflects the activation of this receptor–ligand pair in COVID-19, increasing the efficiency of initial leukocyte recruitment, thus promoting tissue damage and immunothrombosis. Our results show that endothelial activation and unbalanced leukocyte migration play a central role in COVID-19 involving the P-selectin–PSGL-1 axis.
Keywords: CD11b, CD62P, COVID-19, leucocyte migration, P-selectin, PSGL-1, CD162, SARS-CoV-2
Introduction
The emergence of SARS-CoV-2 virus and the outbreak of a global pandemic presented unprecedented medical and economic challenges.1 Key pathological features observed in fatal COVID-19 were pulmonary complications, a prominent prothrombotic state, and a failure of the immune response.2, 3, 4 Accumulating evidence suggests that SARS-CoV-2 can directly affect endothelial cells with the consequent immune response accompanied by massive secretion of proinflammatory cytokines resulting in dysregulation of the endothelium, leukocyte activation, neutrophil extracellular trap (NET) generation, and platelet consumption.5, 6, 7 These pathways can induce a prothrombotic state, namely immunothrombosis, which can lead to severe thrombotic complications.8 , 9
Therefore, there is an urgent clinical need to further elucidate the interplay between pulmonary endothelia and the inflammatory compartment in fatal COVID-19. Understanding these pathogenic mechanisms offers important opportunities to adapt therapeutic approaches attenuating inflammation, endothelial damage, thrombosis, and systemic microangiopathy. The interaction between the leukocytes and the endothelium comprises a well-defined and regulated multistep cascade involving consecutive steps of adhesive interactions and migratory events for leukocytes.10
This process comprises 4 consecutive steps: slow rolling, adhesion strengthening, intraluminal crawling, and finally transcellular migration through the endothelium.10 , 11 The initial rolling is mediated by L-selectin (CD62L) expressed on leukocytes and E-selectin and P-selectin (CD62P) stored in the α-granules of platelets and in the endothelial cells. Upon activation, P-selectin is translocated to the cell membrane, and overexpression indicates activation of endothelium and platelets. The main ligand for these selectins is P-selectin glycoprotein ligand (PSGL)-1 (CD162) expressed both on neutrophils and certain endothelial cells. Subsequently, firm adhesion is mediated through macrophage antigen-1 (Mac-1/CD11b) expressed on neutrophils and macrophages and its ligand intercellular adhesion molecule (ICAM)-1 expressed on the vascular endothelium.12 The third step, intravascular crawling, involves CD11b and other β2-integrins.13 Before the final step, the transendothelial migration, vascular cell adhesion molecules (VCAM)-1 and ICAM-1 form the so-called docking structures on endothelial cells.14 Severe cases of COVID-19 are characterized by elevated levels of P-selectin, a biomarker of endothelial degranulation and platelet activation.15 However, according to existing literature, only the soluble form of P-selectin (sP-selectin) in sera15, 16, 17, 18 or P-selectin on the mRNA level19 have been studied so far. Thus, in our study, we aimed to visualize the expression of this marker and other antigens relevant for leukocyte activation and extravasation in lung tissue by immunohistochemistry (IHC) in COVID-19 in comparison to other inflammatory lung disorders.
PSGL-1 was primarily regarded as an adhesion molecule that binds P-selectin on activated endothelium,20 and is now progressively recognized as an important regulator of many facets of immune responses by myeloid cells and T cells in settings of homeostasis and inflammation.21 PSGL-1 is broadly expressed on most immune cells, including neutrophils, monocytes, dendritic cells (DCs), and lymphocytes, and mediates interactions with P-selectin and E-selectin present in activated endothelium.
CD11b is a β-integrin involved in neutrophil adhesion, diapedesis, and phagocytosis and is stored in primary and secondary intracellular granules within unstimulated neutrophils and macrophages.22 In addition to its role as an adhesion molecule, CD11b is used as a marker of neutrophil activation. Its activation is linked to several neutrophil functions, such as the oxidative burst, phagocytosis, and release of proteolytic enzymes.23 CD11b is normally expressed at very low levels on neutrophils and becomes elevated when neutrophils encounter pathogens or their products.24, 25, 26
In this study, we examined the expression of adhesion molecules by IHC on endothelium and leukocytes in COVID-19 postmortem lung specimens in different stages of the disease (early-stage capillaritis, exudative, and proliferative phase). We compared the results with a control group comprising acute respiratory distress syndrome [ARDS] of other causes, pneumonia, and normal lung specimens.
Dysregulated IL-1β and IL-6 responses have been implicated in the pathogenesis of severe COVID-19.27 , 28 Endothelial cells are a target of these cytokines, and they regulate several aspects of vascular homeostasis and cellular inflammation. Based on our previous limited data, we also aimed to determine the expression of these cytokines by RT-qPCR in our COVID-19 cohort and controls.
Materials and Methods
Patients
The COVID-19 group comprises postmortem lung samples of patients whose primary cause of death was considered SARS-CoV-2 (n = 10) infection as determined in a complete autopsy. All cases occurred during the first wave of the pandemic in spring 2020, thus representing an uninitiated cohort homogeneously infected with the alpha variant of SARS-CoV-2. According to histologic patterns, the COVID-19 cohort was divided into 3 subgroups: (1) a vascular stage with edema, incipient epithelial damage, and endothelialitis, generally characterizing the early phase of infection; (2) an epithelial stage of fully developed exudative diffuse alveolar damage (DAD); and (3) the organizing/fibrotic stage of DAD (Table 1 ).2 , 29 , 30 Testing for COVID-19 had been performed on nasopharyngeal swabs taken during intensive care unit (ICU) hospitalization, and positivity was confirmed on FFPE tissue by qRT-PCR. Significant levels of SARS-CoV-2 RNA were detected in the lungs of all patients. The clinical data of 10 cases were obtained from medical records during hospitalization in the ICU at Tübingen University Hospital (Tübingen, Germany) (Table 1). The control group (n = 20) was composed of postmortem (5 ARDS, 2 viral pneumonia, and 3 bacterial pneumonia) and surgical lung samples (5 samples of normal lung from lung cancer resection specimens and 5 normal lung specimens from bullectomy due to spontaneous pneumothorax).
Table 1.
Clinical data of the patients
| Case | Age (y) | Sex | Days from admission until death | Days in ICU | Comorbidities | Clinical cause of death | Stage |
|---|---|---|---|---|---|---|---|
| COVID-19-1 | 78 | F | 1 (home care) | — | Cardiac pacemaker, obesity | Pneumonia | Vascular |
| COVID-19-2 | 50 | M | 9 | 8 | Bronchial asthma, peripheral pulmonary emphysema | Ventricular fibrillation | Vascular |
| COVID-19-3 | 59 | M | 12 | 8 | Arterial hypertension, intrinsic bronchial asthma | ARDS, multiorgan failure | Vascular |
| COVID-19-4 | 56 | M | 20 | 12 | Arterial hypertension | Multiorgan failure with progressive liver dystrophy | Epithelial |
| COVID-19-5 | 61 | M | 18 | 10 | Arterial hypertension | ARDS, multiorgan failure | Epithelial |
| COVID-19-6 | 58 | M | 21 | 8 | Diabetes type 2, obesity | ARDS | Epithelial |
| COVID-19-7 | 72 | M | 19 | 12 | NST EMI in 3-vessel disease | Multiorgan failure, acute liver dystrophy | Fibrotic |
| COVID-19-8 | 78 | M | 8 | 4 | Coronary artery disease, cardiomegaly, atherosclerosis | ARDS, multiorgan failure, liver hemophagocytosis | Fibrotic |
| COVID-19-9 | 74 | M | 32 | 15 | Coronary artery disease, pulmonary valve stenosis | Multiorgan failure with progressive liver dystrophy | Fibrotic |
| COVID-19-10 | 86 | F | 11 | 9 | Arterial hypertension | ARDS with progressive organization, pulmonary aspergillosis | Fibrotic |
ARDS, acute respiratory distress syndrome; F, female; M, male; NST EMI, non-ST-elevation myocardial infarction.
Tissue Macroarray
Representative lung blocks of the control group were selected after reviewing all original glass slides from the surgical resection specimens. Recut hematoxylin and eosin–stained sections were annotated for tissue macroarray (TMA) construction. Areas with the most pronounced changes were chosen. Two 6 mm cores from each block were targeted, providing double sampling of all 20 cases with a total of 40 cores.
Immunohistochemistry
Routine histologic stains were performed on formalin-fixed and paraffin-embedded samples following standard protocols. Immunohistochemistry was performed on an automated immunostainer (Ventana Benchmark Ultra, Roche Diagnostics). Antibody details are included in Table 2 .
Table 2.
Antibodies used for immunohistochemistry
| Antibody | Company | Catalog code | Dilution |
|---|---|---|---|
| E-selectin (CD62E) | Abcam | ab18981 | 1:400 |
| P-selectin (CD62P) | Invitrogen | MA5-16567 | 1:100 |
| PSGL-1 | Abcam | ab110096 | 1:400 |
| CD11b | Abcam | ab133357 | 1:2000 |
| VCAM-1 | Abcam | ab134047 | 1:250 |
| ICAM-1 | Novocastra | NCL-CD54-307 | 1:15 |
Image Data Acquisition
All immune-stained slides were scanned using a Ventana DP 200 digital scanner at 40× magnification. Expression of adhesion molecules was recorded and evaluated using QuPath software (version 0.3.0) open-source software for digital pathology image analysis.31 Detailed information is given in the Supplementary Material.
SARS-Cov-2 RNA and IL-1β and IL-6 mRNA Detection
For RNA isolation, native tissue samples obtained during autopsy were placed in 500 μL peqGOLD TriFast RNA/DNA/Protein Isolation Reagent (peqLab Biotechnologie Erlangen). Tissue samples were mechanically shredded. After adding chloroform and incubating the samples for 10 minutes at room temperature, the samples were centrifuged, and the aqueous supernatant containing the RNA was precipitated by adding 250 μL isopropanol. The RNA precipitate was washed with 75% ethanol and dried. The pellet was resuspended in 25 μL RNase-free water.
Real-time quantitative PCR was performed using the GeneFinder COVID-19 Plus RealAmp Kit assay (ELITech Group) according to the manufacturer's suggestions. RT-qPCR was carried out using a Rotor-Gene-Q (Qiagen) applying FAM fluorophore for the RdRp gene and JOE (ABI) fluorophore for the N gene. A positive test result was defined as a cycle threshold (Ct) ≤40 for individual targets.
Quantitative RT-PCR for 200 ng of RNA was used to perform one-step quantitative real-time reverse-transcription PCR (TaqMan RNA-to-CT 1-Step Kit, Applied Biosystems,) at the appropriate annealing temperature for 40 cycles on a 7300 Real-Time PCR System (Applied Biosystems). Data analysis was performed as relative quantification in relation to the expression of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase as internal standard. Specific primers and probes were purchased from MWG Biotech. Primers were as follows: hIL1b-fwd GCACGAT GCACCTGTACGAT, hIL1b-rev AGACATCACCAAG CTTTTTTGCT, hIL1b-probe ACTGAAC TGCACGCTCCGGGACTC, hIL6-fwd GGTACATCCTCGACGGCATCT, hIL6-rev GTGCCTC TTTGCTGCTTTCAC, hIL6-probe TGTTACTCTTGTTACATGTCTCCTTTCTCAGGGCT.
In Situ Hybridization
To detect SARS-CoV-2 RNA (plus strand RNA), 4-μm–thick lung tissue sections, including negative and positive controls, were hybridized using specific probes for SARS-CoV-2 (Advanced Cell Diagnostics, ACD) followed by the RNAscope 2.5 HD Detection Kit Red from ACD according to the manufacturer’s protocol.
Statistical Analysis
All data are expressed as mean ± SD. Data sets were normally distributed, with similar variances between compared groups. All statistical analysis for adhesion molecules was performed using JMP software (SAS Institute) and was conducted using 1-way analysis of variance analyses to compare 2 or more groups. The Dunnett post hoc multiple comparison test was used to determine statistical significance when analysis of variance results were significant. The nonparametric Mann–Whitney U-test was used to evaluate differences in cytokine levels and was conducted using GraphPad Prism (v8.4.3, GraphPad Software). The P values <.05 were considered significant. Significance was indicated as follows: ∗P < .05, ∗∗P < .005, and ∗∗∗P < .001. Error bars represent the SEM.
Results
Normal Lung
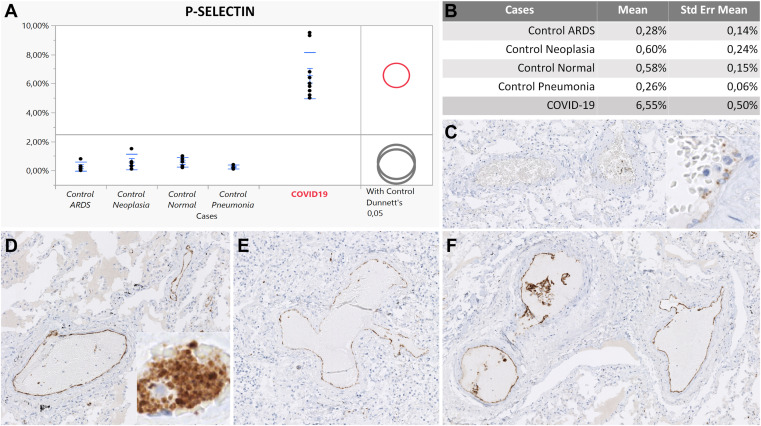
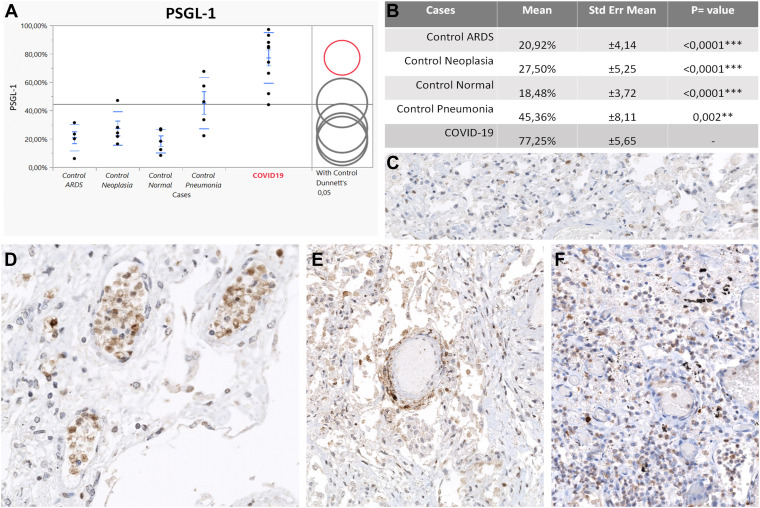
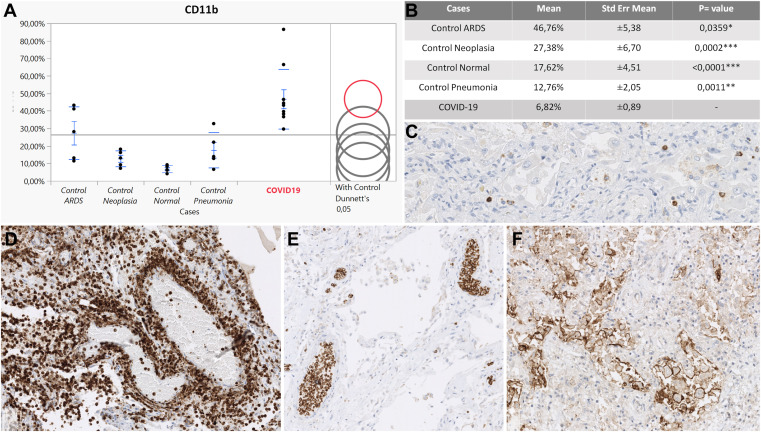
The 5 normal lung samples showed comparable results. P-selectin mediates the initial step of the adhesion cascade (rolling) and is constitutively expressed at low levels by pulmonary endothelium and platelets. P-selectin expression was almost undetectable in the small vessels of uninflamed lungs, indicating nonactivated endothelium (0, 60%; 0, 58%) (Table 3 ). PSGL-1 expression was detected on scattered leukocytes in normal lung tissue from bullectomy specimens showing an overall reduced positivity compared with non-COVID-19 pneumonia cases, ARDS, and most of all, COVID-19 cases (18, 48%) (Table 3). Of note, PSGL-1 expression was upregulated on leukocytes in normal lung tissue from lung resections for lung cancer (27, 50%) (Table 3) compared with normal lung tissue from bullectomy. PSGL-1 expressing epithelial cells (bronchopulmonary squamous metaplasia, pneumocyte type II hyperplasia, and bronchial glands) and endothelium were excluded from the analysis. CD11b staining revealed only scattered positive interstitial leukocytes in normal controls and was generally low (6, 82%; 12, 76%) (Table 3).
Table 3.
Statistical analysis of the difference between COVID-19 group and controls
| Cases | Mean (%) | SE | P value |
|---|---|---|---|
| E-selectin | |||
| COVID-19 | 1,07 | ±0.28 | |
| ARDS | 1,88 | ±0.22 | .2524 |
| Pneumonia | 2,46 | ±0.34 | .0159a |
| Neoplasia | 1,52 | ±0.34 | .7551 |
| Normal | 0,84 | ±0.41 | .9652 |
| P-selectin | |||
| COVID-19 | 6,55 | ±0.50 | |
| ARDS | 0,28 | ±0.14 | <.0001c |
| Pneumonia | 0,26 | ±0.06 | <.0001c |
| Neoplasia | 0,60 | ±0.24 | <.0001c |
| Normal | 0,58 | ±0.15 | <.0001c |
| PSGL-1 | |||
| COVID-19 | 77,25 | ±5.65 | |
| ARDS | 20,92 | ±4.14 | <.0001c |
| Pneumonia | 45,36 | ±8.11 | .002b |
| Neoplasia | 27,50 | ±5.25 | <.0001c |
| Normal | 18,48 | ±3.72 | <.0001c |
| ICAM-1 | |||
| COVID-19 | 1,18 | ±0.56 | |
| ARDS | 4,04 | ±1.15 | .0555 |
| Pneumonia | 3,44 | ±1.02 | .1687 |
| Neoplasia | 1,82 | ±1.03 | .9524 |
| Normal | 2,54 | ±0.47 | .5958 |
| VCAM-1 | |||
| COVID-19 | 2,17 | ±1.02 | |
| ARDS | 6,40 | ±2.14 | .3627 |
| Pneumonia | 17,10 | ±3.93 | <.0001c |
| Neoplasia | 5,30 | ±1.22 | .6281 |
| Normal | 5,68 | ±1.65 | .5307 |
| CD11b | |||
| Normal | 6,82 | ±0.89 | <.0001c |
| COVID-19 | 46,76 | ±5.38 | |
| ARDS | 27,38 | ±6.70 | .0359a |
| Pneumonia | 17,62 | ±4.51 | .0011b |
| Neoplasia | 12,76 | ±2.05 | .0002c |
ARDS, acute respiratory distress syndrome.
P < 0.05.
P < 0.005.
P < 0.001.
Acute Respiratory Distress Syndrome and Pneumonia
The overall positivity of P-selectin in our control group with non-COVID inflammatory conditions was significantly lower compared with the COVID-19 group, reflecting a relatively reduced endothelial and platelet activation. Immunostaining revealed focally P-selectin–positive platelets, which exhibited a finely punctuated cytoplasmic positivity, implying only basal expression in the alpha granules and thus representing quiescent thrombocytes (Fig. 1 C, inset). Moreover, in most of the vessels, P-selectin was almost undetectable (Fig. 1C), and none of the controls showed prominent P-selectin–positive intravascular platelet aggregates that characterized COVID-19 cases as described in COVID-19. PSGL-1 was also lower compared with COVID-19 cases, but among controls, PSGL-1 was highly expressed in the pneumonia group. However, none of the controls showed intravascular PSGL-1–positive leukocyte aggregates, as observed in the COVID-19 cohort. PSGL-1 expression was found mostly in the interstitial immune cell component (Fig. 2 C). CD11b showed a significantly lower expression in the controls overall compared with COVID-19, with the highest expression in non-COVID-19 pneumonia (Fig. 3 C).
Figure 1.
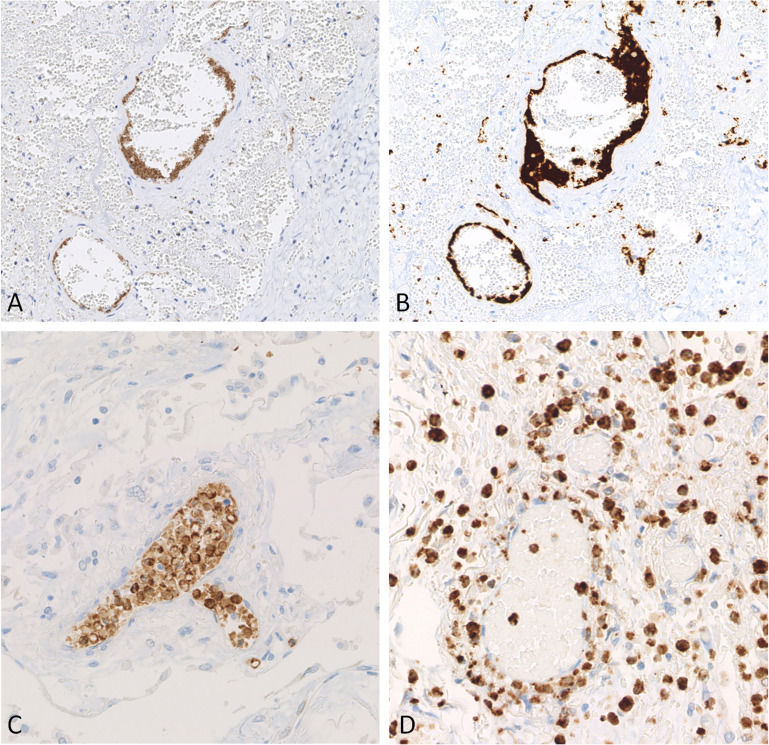
P-selectin expression in pulmonary vessels. (A) Upregulation of P-selectin in COVID-19 (red circle) compared with controls (gray circles). The difference between comparison group (COVID-19) and controls is represented by the distance between the circles. (B) Statistically significant difference between COVID-19 group and controls according to a Dunnett test. (C) Lower P-selectin expression in a pneumonia case. Inset shows a weaker, finely punctuated P-selectin intensity of platelets in the same case. (D, E) P-selectin positivity on the luminal surface of endothelial cells in COVID-19. (F) P-selectin positivity on endothelial cells and platelet aggregates in a COVID-19 case. Original magnification, 100× (C–F); 200× (insets, C, D).
Figure 2.
PSGL-1 expression on leukocytes. (A) Upregulation of PSGL-1 in COVID-19 (red circle) compared with controls (gray circles). The difference between comparison group (COVID-19) and controls is represented by the distance between the circles. (B) Statistically significant difference between COVID-19 group and controls according to Dunnett's test. (C) PSGL-1 expression in an acute respiratory distress syndrome control case. (D) PSGL-1 positive leukocytes coalescing to form intravascular white cell plugs in a COVID-19 patient in the vascular stage. (E) PSGL-1 intensely positive leukocytes arranged in a capillaritis picture in a COVID-19 case. (F) PSGL-1-positive granulocytes in the epithelial stage of a COVID-19 patient. Original magnification, 200× (C–F).
Figure 3.
CD11b expression on leukocytes. (A) Upregulation of CD11b in COVID-19 (red circle) compared with controls (gray circles). The difference between comparison group (COVID-19) and controls is represented by distance between circles. (B) Statistically significant difference between COVID-19 group and controls according to a Dunnett test. (C) CD11b expression in a pneumonia control case. (D) Neutrophilic capillaritis in a COVID-19 vascular stage. (E) CD11b-positive intravascular white cell plugs in the epithelial stage of a COVID-19 patient. (F) CD11b-positive macrophages and giant cells in the fibrotic stage of a COVID-19 case. Original magnification, 200× (C, F); 100× (D, E).
COVID-19
Immunohistochemical analysis of adhesion molecules revealed a highly significant upregulation of P-selectin, its ligand (PSGL-1), and CD11b in the COVID-19 cohort compared with the controls. P-selectin expression was found to be extremely upregulated in the COVID-19 collective compared with all the control groups (COVID-19:ARDS, 23:39, P < .0001; COVID-19:pneumonia, 25:19, P < 0001; COVID-19:neoplasia, 10:91, P < .0001; COVID-19:normal, 11:29, P < .0001) reflecting altered platelet–endothelium–leukocyte interactions (Table 3). P-selectin showed a peculiar pattern of expression in the COVID-19 group when compared with the controls. The immunoreaction showed a membranous positivity of endothelial cells, indicating the translocation of P-selectin from Weibel–Palade bodies to the luminal surface of pulmonary vessels (Fig. 1). Such findings are consistent with endothelial activation in COVID-19 cases. Moreover, P-selectin IHC revealed strong membranous/cytoplasmic staining of platelet aggregates intimately adhered to the luminal surface of lung blood vessels, likely representing activated platelets in the totality of the COVID-19 group (10/10) and thus contributing to the overall P-selectin positivity (Fig. 1D, inset). Notably, P-selectin upregulation in COVID-19 was documented in all stages of disease with the highest peak in vascular phase (MV: 7, 37%, SE ± 0, 9), indicating endothelial and platelet activation as the first pathophysiologic event in severe COVID-19 (Table 3; Supplementary Table S1). In comparison with control groups, COVID-19 cases showed a prominent upregulation of PSGL-1 in leukocytes (COVID-19:ARDS, 3:69; P < .0001; COVID-19:neoplasia, 2:81; P < .0001; COVID-19:normal, 4:18; P < .0001; COVID-19:pneumonia, 1:7; P = .0020) (Table 3; Supplementary Table S1). Early COVID-19 cases in the vascular stage were characterized by a strong PSGL-1 positivity both in endothelial cells and in the intravascular leukocyte accumulations of postcapillary venules comprising mainly monocytes and immature granulocytes (Fig. 2D). In the epithelial stage of COVID-19, PSGL-1 staining highlighted the prominent alveolar granulocyte accumulations (Fig. 2F), whereas the cases classified as late DAD in the fibrotic stage exhibited weaker positivity, mainly of the macrophage population. Focally, PSGL-1 disclosed cuffs of positive leukocytes infiltrating tunica intima and media of numerous small vessels in vascular, epithelial, and fibrotic stages, highlighting a role of this adhesion molecule in COVID-19 capillaritis (Fig. 2E). Patients with COVID-19 showed a strong upregulation of CD11b in leukocytes in comparison with controls (COVID-19:ARDS, 1:71; P = 0.0359; COVID-19:pneumonia, 3:66; P = .0011; COVID-19:neoplasia, 3:66; P = .0002; COVID-19:normal, 6:82; P < .0001) indicating a proinflammatory immune microenvironment (Table 3; Supplementary Table S1). Furthermore, CD11b showed a unique expression pattern in COVID-19. The vascular and epithelial phases were dominated by CD11b+ granulocytes and monocytes as interstitial infiltrates or at times, in intravascular cellular plugs (Fig. 3E). In some cases, CD11b-positive neutrophils and monocytes participated in thrombus formation alongside with fibrin deposits. Most macrophages were negative. On the other hand, the fibrotic phase of COVID-19 was characterized by strongly CD11b-positive macrophages and giant cells clustering in alveolar spaces (Fig. 3F). Irrespective of disease progression, CD11b-positive mononuclear and neutrophilic cell infiltrates were detected within the walls of many small vessels, in particular, of postcapillary venules characterizing the histologic picture of neutrophilic capillaritis (Fig. 3D). Although statistically not significant, E-selectin, ICAM-1, and VCAM-1 showed a downregulation in patients with COVID-19 compared to patients with other pneumonia types. Detailed information is included in the Supplementary Material.
Quantitative Reverse-Transcription Polymerase Chain Reaction
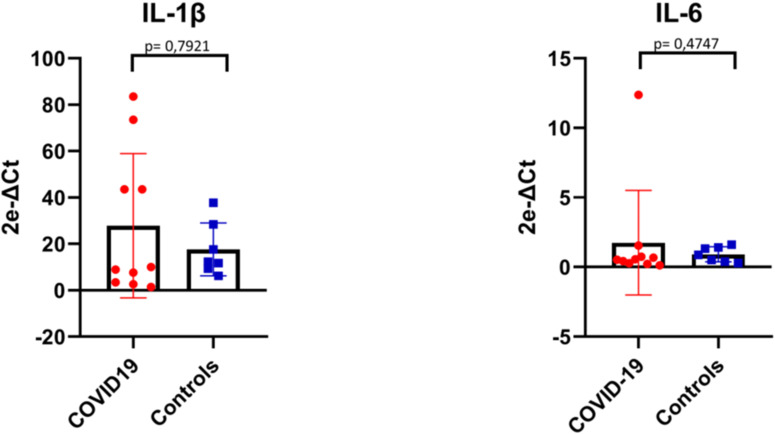
The overall comparison between cytokine mRNA levels in COVID-19 and controls did not show any statistically significant differences neither for IL-1β (P = .7921) nor IL-6 (P = .4747) (Supplementary Fig. S3; Supplementary Table S2). However, cytokine mRNA levels were high in the lung of cases with very short disease course (Supplementary Table S2), whereas no significant levels were detected in the other patients, congruent with a decrease in serum IL-6 levels before death.32 This finding suggests that the massive secretion of cytokines occurs early in disease, but it should not be considered a reliable marker of disease severity due to its transiency. In fact, the increase of cytokine is rapidly followed by high and durable expression of P-selectin at tissue level in all stages of disease, representing a hallmark of COVID-19 cases.
Discussion
Dysregulated leukocyte migration and a prothrombotic state have been identified as key factors in COVID-19 pathogenesis.33 , 34 The present study provides new insights into the contribution of adhesion molecules and, in particular, of the P-selectin–PSGL-1 axis to COVID-19 pathogenesis with the identification of a unique expression pattern of molecules involved leukocyte–endothelium interaction in patients with fatal outcome. We demonstrate for the first time on tissue level that endothelial and platelet activation by massively increased P-selectin expression both on vessels and platelets of patients with COVID-19 is a unique feature that characterizes fatal SARS-CoV-2 infection. In addition, we show that platelet and endothelial activation is a constant finding in all disease stages and shows its peak in the vascular stage, shortly after infection. Elevated P-selectin expression promotes leukocyte–endothelial and leukocyte–platelet adhesion35 and release of citrullinated histones and neutrophil extracellular trap (NET) formation,36 promoting the formation of platelet–neutrophil or platelet–monocyte aggregates by binding of P-selectin to its ligand PSGL-1 expressed on the surface of leukocytes.35 Accordingly, elevated sP-selectin has been recently described in patients with COVID-19,16, 17, 18 but the explanation of its origin and localization in tissues has been lacking so far. Our study supports these findings and indicates that elevated sP-selectin may originate both from endothelial and platelet shedding. Moreover, our data highlight the key role of platelet activation in SARS-CoV-2–infected patients. In addition to their role in primary hemostasis, platelets are an integral part of the immune response to pathogens.37 The functional interdependence and the coordinated activation of both processes, designated as thromboinflammation, may drive adverse effects, such as thrombosis, multiple organ failure, and death.38 Our findings further support the hypothesis that deregulated primary coagulation in association with inflammation may constitute a main pathogenetic mechanism underlying SARS-CoV-2–induced pulmonary damage. Several studies have demonstrated that statin use in patients hospitalized with COVID-19 is associated with lower inpatient mortality.39, 40, 41, 42, 43, 44, 45, 46. The explanation for this finding could be that statins are known as potent P-selectin inhibitors.47
Further evidence of the dysregulated leukocyte migration in COVID-19 was the upregulation of PSGL-1 in our COVID-19 cohort. The importance of PSGL-1–P-selectin interactions for acute inflammation is well established,20 , 48 , 49 but recently, a further role of PSGL-1–P-selectin for the preservation of immune homeostasis has been demonstrated.21 , 50 In monocyte-derived DCs, the interaction between PSGL-1 and P-selectin triggers a tolerogenic program characterized by increased TGF-β, IL-10, and indolamine 2,3-dioxygenase mRNA.51 Moreover, PSGL-1–P-selectin interaction reduces class II major histocompatibility complex expression on DCs, inducing a tolerogenic phenotype that promotes the generation of regulatory T cells.52 Furthermore, PSGL-1 signaling in T cells was found to control their activation by upregulating the inhibitory receptor PD-1, and thus representing a negative regulator of the T-cell response.53 PSGL-1 also offers a regulatory role in B cells.54 In particular, it has been demonstrated that PSGL-1+ B cells are part of the B reg population in humans expressing IL-10.55 , 56 In this context, the upregulation of PSGL-1 and its ligand in our COVID-19 group suggests an activation of regulatory pathways, which may be responsible for the failure and depletion of the T- and B-cell response as previously reported.57, 58, 59, 60, 61
Furthermore, our analysis revealed an overexpression of CD11b in COVID-19–infected patients compared with other inflammatory states. Although CD11b can be expressed by several leukocyte subsets, neutrophils are the most abundant expressors of this integrin, in particular activated ones. Although neutrophils are potent antimicrobial cells, they can contribute to host tissue damage when hyperactivated.62 Interestingly, we found cuffs of CD11b-positive neutrophils around small vessels in the lungs of patients with COVID-19, constituting the picture of neutrophilic capillaritis, especially in vascular and epithelial stages (Fig. 3D). Moreover, in most cases, we found white cell plugs in the microvasculature, in particular postcapillary venules, exhibiting a kind of inflammatory leukostasis (Fig. 3E). The finding of intravascular cell plugs, simulating a leukemic leukostasis, is not exclusive of the COVID-19 pneumonia cases according to our experience and published data.63 Other viral and, most of all, bacterial infections may induce this phenomenon in small vessels of the lungs. However, this is very rare in non–COVID-19 pneumonia, and none of our controls showed such prominent intravascular leukocyte accumulations as observed in the COVID-19 cases. This condition may contribute to SARS-CoV-2 pathogenesis resulting in increased oxidative stress, vascular damage, and hypoxia. These findings point to an aberrant neutrophil response in severe COVID-19 and imply a central role for a dysfunctional neutrophil recruitment and activation in disease pathophysiology, perhaps more than a direct viral cytopathic effect (Fig. 4 ). Our results concerning the role of neutrophils in COVID-19 pathophysiology are consistent with other publications.64, 65, 66, 67 Conversely, other groups using single-cell sequencing focused on other immune cell types for technical reasons, underestimating the granulocytic compartment.5 , 68 Although early cases show high IL-6 and IL-1β mRNA levels in lung tissue, critically ill patients with COVID-19 share the upregulation of this cytokine profile with sepsis and other ARDS causes. However, some features of adhesion molecule expression may uniquely characterize SARS-CoV-2 infection. Interestingly, fatal COVID-19 cases themselves are heterogeneous, characterized by the presence of different histologic patterns in different areas of the lung that do not always correlate with the length of the disease. In fact, in some patients, the progression of the disease was faster than others, most likely reflecting underlying immunologic differences.30
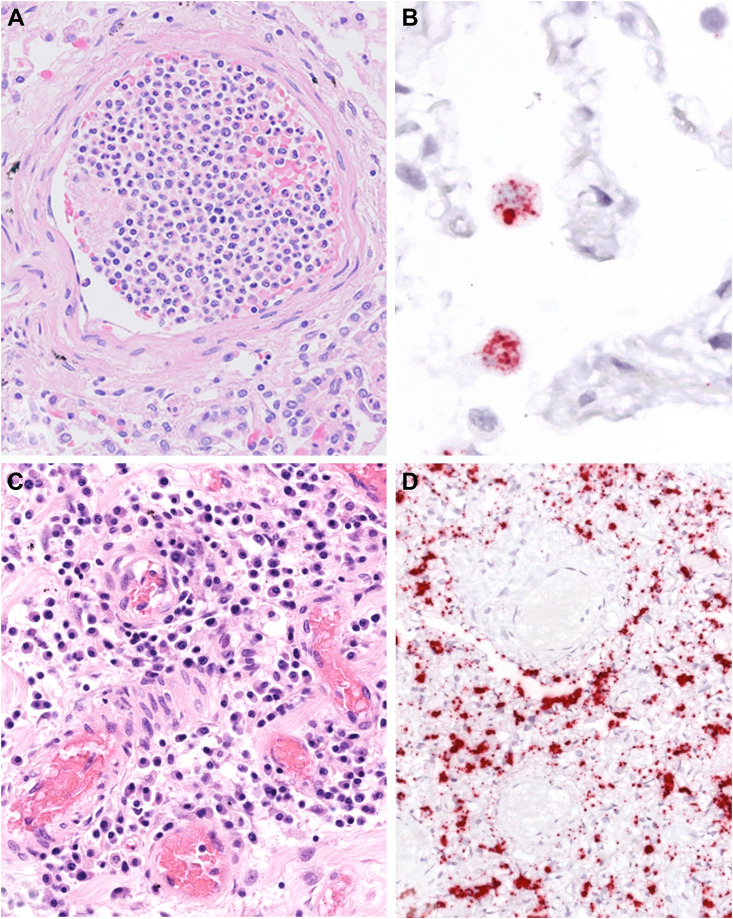
Figure 4.
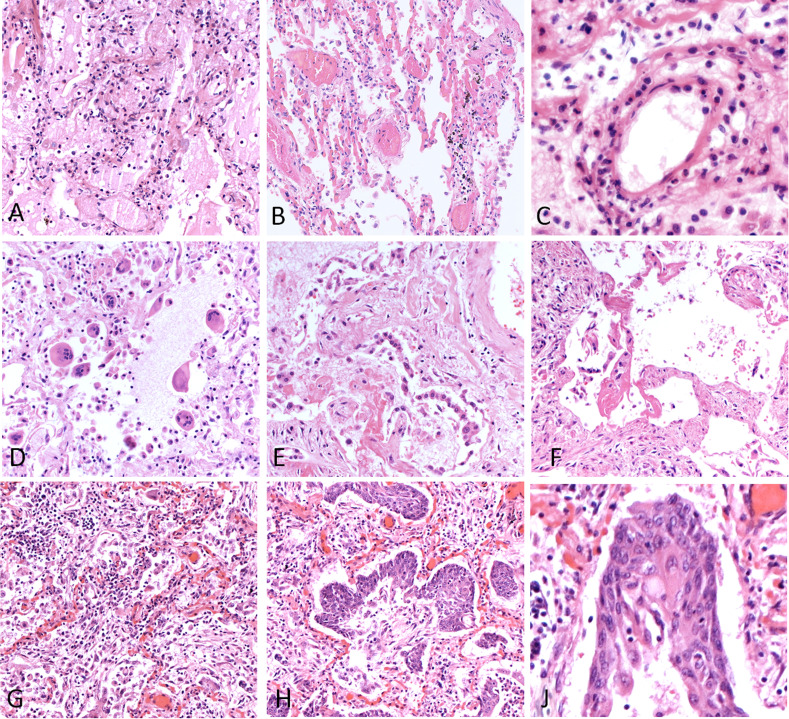
SARS-CoV-2 RNA distribution and inflammatory infiltrates. (A) White cell plugs constituted mainly by neutrophils were a constant finding in the lungs of COVID-19 patients. (B) In situ hybridization (ISH) for SARS-CoV-2 RNA highlights positive macrophages in most of the cases. (C) Vasculitis pattern in a COVID-19 lung sample. (D) ISH shows absence of SARS-CoV-2 RNA in vessel walls and endothelial cells. However, the immune infiltrate and pneumocytes show numerous virus RNA signals. Original magnification 200× (A, C, D); 400× (B).
Limitations of our study include the small sample size, the lack of cases infected by SARS-CoV-2 variants, and our focus on fatal COVID-19, which may not reflect the immunologic setting of the mild and chronic disease course. However, the fact that our COVID-19 samples belong to the first wave of the pandemic (February–April 2020) and are thus restricted to unvaccinated, immunologically uninitiated patients may represent a strength of our study.
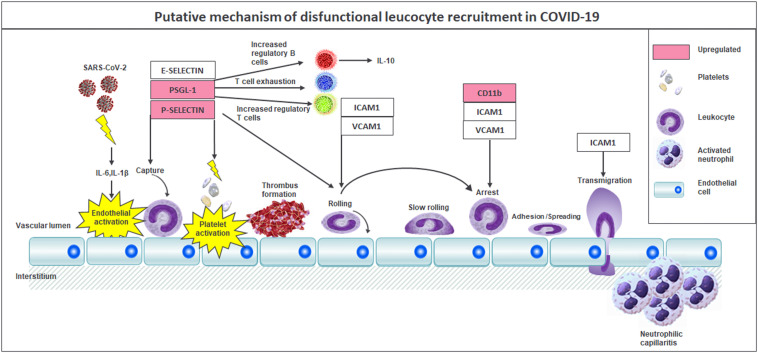
In conclusion, our data support a disease model whereby activation of a dysfunctional P-selectin–PSGL1 axis is followed by neutrophil activation and attraction into sites of injury and the formation of platelet–leukocyte aggregates, precipitating organ injury and failure (Fig. 5 ). The model that we propose may not only explain the pulmonary pathology of SARS-CoV-2 but may also apply to other organs.69 Finally, these findings suggest that patients with COVID-19 may benefit from drugs such as P-selectin or PSGL-1 inhibitors, which may prevent platelet-endothelium activation and immunothrombosis. Such therapies have also been proposed in other septic settings with promising results and are well tolerated.70
Figure 5.
Putative mechanism of pathogenesis and dysfunctional leukocyte recruitment in COVID-19. Cytokine release in the early phase of infection. P-selectin hyperexpression indicates endothelial and platelet activation, which leads to micro- and macrothrombosis. PSGL-1 upregulation determines the increase of the regulatory T and B cell population and T cell exhaustion. Neutrophilic capillariitis comprising CD11b-positive cells.
Acknowledgments
We thank Dr Martina Sauter for performing in situ hybridization experiments and Sandra Bundschuh for excellent technical assistance.
Author Contributions
F.F. and M.G. helped in conceptualization. H.B. provided data curation investigation, M.G., J.M., H.B., K.G., and V.W. helped in investigation. L.Q., H.B., K.K., U.V., and C.M.S. provided methodology. F.F. helped with project administration and supervision. K.K. and H.B. provided validation. M.G., F.F., and K.K. helped in writing – original draft. L.Q., H.B., F.F., K.K., T.B., and P.R. helped in writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding
This study was supported by special funds from the state of Baden-Württemberg for autopsy-based COVID-19 research and the DEFEAT PANDEMICs network funded by the BMBF to FF. TB received grant support from the German Research Foundation and the Herzstiftung (BA5158/4 and TSG-Study). K.K. received basic funding from the Ministry of Science, Research, and Arts of the State of Baden-Württemberg (COVID-19 Funding) and from Deutsche Herzstiftung.
Declaration of Competing Interests
This study was supported by special funds from the state of Baden-Württemberg for autopsy-based COVID-19 research and the DEFEAT PANDEMICs network funded by the BMBF to FF. TB received grant support from the German Research Foundation and the Herzstiftung (BA5158/4 and TSG-Study). K.K. received basic funding from the Ministry of Science, Research, and Arts of the State of Baden-Württemberg (COVID-19 Funding) and from Deutsche Herzstiftung.
Footnotes
The online version contains supplementary material available at https://doi.org/10.1016/j.labinv.2023.100179.
Supplementary Material
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
Supplementary Figure S4.
Supplementary Figure S5.
Supplementary Figure S6.
References
- 1.Holmes E.C., Goldstein S.A., Rasmussen A.L., et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184(19):4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bösmüller H., Traxler S., Bitzer M., et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477(3):349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bösmüller H., Matter M., Fend F., Tzankov A. The pulmonary pathology of COVID-19. Virchows Arch. 2021;478(1):137–150. doi: 10.1007/s00428-021-03053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto-Pérez L., Fortes J., Soto C., et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33(11):2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delorey T.M., Ziegler C.G.K., Heimberg G., et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595(7865):107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karki R., Sharma B.R., Tuladhar S., et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilosi M., Poletti V., Ravaglia C., et al. The pathogenic role of epithelial and endothelial cells in early-phase COVID-19 pneumonia: victims and partners in crime. Mod Pathol. 2021;34(8):1444–1455. doi: 10.1038/s41379-021-00808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventura A., Vecchié A., Dagna L., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa M., Kanno H., Zhou Y., et al. Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19. Nat Commun. 2021;12(1):7135. doi: 10.1038/s41467-021-27378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nourshargh S., Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Friedl P., Weigelin B. Interstitial leukocyte migration and immune function. Nature Immunology. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 12.Hyun Y.-M., Choe Y.H., Park S.A., Kim M. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) distinctly regulate neutrophil extravasation through hotspots I and II. Exp Mol Med. 2019;51(4):1–13. doi: 10.1038/s12276-019-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippi M.-D. Neutrophil transendothelial migration: updates and new perspectives. Blood. 2019;133(20):2149–2158. doi: 10.1182/blood-2018-12-844605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim I., Moon S.-O., Kim S.H., Kim H.J., Koh Y.S., Koh G.Y. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-κB activation in endothelial cells. J Biol Chem. 2001;276(10):7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 15.Mosarla R., Myndzar K., Xia Y., Barrett T., Berger J. Soluble P-selectin, platelet activity and risk of morbidity and mortality in COVID-19. J Am Coll Cardiol. 2022;79(9):1845. doi: 10.1016/S0735-1097(22)02836-4. [DOI] [Google Scholar]
- 16.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/s2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manne B.K., Denorme F., Middleton E.A., et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yatim N., Boussier J., Chocron R., et al. Platelet activation in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):113. doi: 10.1186/s13613-021-00899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore K.L., Stults N.L., Diaz S., et al. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlow D.A., Gossens K., Naus S., Veerman K.M., Seo W., Ziltener H.J. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230(1):75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 22.Fan S.-T., Edgington T.S. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J Immunol. 1993;150(7):2972–2980. doi: 10.4049/jimmunol.150.7.2972. [DOI] [PubMed] [Google Scholar]
- 23.Lau D., Mollnau H., Eiserich J.P., et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci. 2005;102(2):431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid M.C., Khan S.Q., Kaneda M.M., et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat Commun. 2018;9(1):5379. doi: 10.1038/s41467-018-07387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polanowska-Grabowska R., Wallace K., Field J.J., et al. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol. 2010;30(12):2392–2399. doi: 10.1161/atvbaha.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton E.A., He X.Y., Denorme F., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J.S., Lee J.Y., Yang J.W., et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamura A.P.D., Tavares da Silva M.R., Hümmelgen A.L., et al. Immunity and inflammatory biomarkers in COVID-19: a systematic review. Rev Med Virol. 2021;31(4) doi: 10.1002/rmv.2199. [DOI] [PubMed] [Google Scholar]
- 29.Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannone G., Caponio V.C.A., De Stefano I.S., et al. Lung histopathological findings in COVID-19 disease - a systematic review. Infect Agent Cancer. 2021;16(1):34. doi: 10.1186/s13027-021-00369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bankhead P., Loughrey M.B., Fernández J.A., et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talwar D., Kumar S., Acharya S., et al. Interleukin 6 and its correlation with COVID-19 in terms of outcomes in an intensive care unit of a rural hospital:a cross-sectional study. Indian J Crit Care Med. 2022;26(1):39–42. doi: 10.5005/jp-journals-10071-24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Meijenfeldt F.A., Havervall S., Adelmeijer J., et al. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality. Res Pract Thromb Haemost. 2021;5(1):132–141. doi: 10.1002/rth2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cambien B., Wagner D.D. A new role in hemostasis for the adhesion receptor P-selectin. Trends Mol Med. 2004;10(4):179–186. doi: 10.1016/j.molmed.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126(2):242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semple J.W., Italiano J.E., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 38.Jenne C., Urrutia R., Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35(3):254–261. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- 39.Fung K.W., Baik S.H., Baye F., Zheng Z., Huser V., McDonald C.J. Effect of common maintenance drugs on the risk and severity of COVID-19 in elderly patients. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0266922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow R., Lee J., Noh H., et al. The association between statin and COVID-19 adverse outcomes: national COVID-19 cohort in South Korea. Ann Palliat Med. 2022;11(4):1297–1307. doi: 10.21037/apm-21-3464. [DOI] [PubMed] [Google Scholar]
- 41.Chow R., Simone C.B., Ii, Prsic E.H., Shin H.J. Cost-effectiveness analysis of statins for the treatment of hospitalized COVID-19 patients. Ann Palliat Med. 2022;11(7):2285–2290. doi: 10.21037/apm-21-2797. [DOI] [PubMed] [Google Scholar]
- 42.Kashour T., Halwani R., Arabi Y.M., et al. Statins as an adjunctive therapy for COVID-19: the biological and clinical plausibility. Immunopharmacol Immunotoxicol. 2021;43(1):37–50. doi: 10.1080/08923973.2020.1863984. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A., Madhavan M.V., Poterucha T.J., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels L.B., Ren J., Kumar K., et al. Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masana L., Correig E., Rodríguez-Borjabad C., et al. Effect of statin therapy on SARS-CoV-2 infection-related mortality in hospitalized patients. Eur Heart J Cardiovasc Pharmacother. 2022;8(2):157–164. doi: 10.1093/ehjcvp/pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniels L.B., Sitapati A.M., Zhang J., et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruni F., Pasqui A.L., Pastorelli M., et al. Different effect of statins on platelet oxidized-LDL receptor (CD36 and LOX-1) expression in hypercholesterolemic subjects. Clin Appl Thromb Hemost. 2005;11(4):417–428. doi: 10.1177/107602960501100408. [DOI] [PubMed] [Google Scholar]
- 48.Moore K.L. Structure and function of P-selectin glycoprotein ligand-1. Leuk Lymphoma. 1998;29(1–2):1–15. doi: 10.3109/10428199809058377. [DOI] [PubMed] [Google Scholar]
- 49.McEver R.P., Cummings R.D. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(11 Suppl):S97–S103. PMID 9413410. [PubMed] [Google Scholar]
- 50.Hidalgo A., Peired A.J., Wild M.K., Vestweber D., Frenette P.S. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26(4):477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urzainqui A., Martínez del Hoyo G., Lamana A., et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179(11):7457–7465. doi: 10.4049/jimmunol.179.11.7457. [DOI] [PubMed] [Google Scholar]
- 52.Abadier M., Ley K. P-selectin glycoprotein ligand-1 in T cells. Curr Opin Hematol. 2017;24(3):265–273. doi: 10.1097/moh.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 53.Tinoco R., Carrette F., Barraza M.L., et al. PSGL-1 Is an immune checkpoint regulator that promotes T cell exhaustion. Immunity. 2016;44(5):1190–1203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosser E.C., Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Sattler S., Ling G.-S., Xu D., et al. IL-10-producing regulatory B cells induced by IL-33 (BregIL-33) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun. 2014;50:107–122. doi: 10.1016/j.jaut.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui D., Zhang L., Chen J., et al. Changes in regulatory B cells and their relationship with rheumatoid arthritis disease activity. Clin Exp Med. 2015;15(3):285–292. doi: 10.1007/s10238-014-0310-9. [DOI] [PubMed] [Google Scholar]
- 57.Barnova M., Bobcakova A., Urdova V., et al. Inhibitory immune checkpoint molecules and exhaustion of T cells in COVID-19. Physiol Res. 2021;70(S2):S227–S247. doi: 10.33549/physiolres.934757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roe K. A role for T-cell exhaustion in long COVID-19 and severe outcomes for several categories of COVID-19 patients. J Neurosci Res. 2021;99(10):2367–2376. doi: 10.1002/jnr.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra K.P., Singh M., Saraswat D., Ganju L., Varshney R. Dysfunctional state of T cells or exhaustion during chronic viral infections and COVID-19: a review. Viral Immunol. 2022;35(4):284–290. doi: 10.1089/vim.2022.0002. [DOI] [PubMed] [Google Scholar]
- 60.Zheng H.Y., Zhang M., Yang C.X., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mortaz E., Alipoor S.D., Adcock I.M., Mumby S., Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018;9:2171. doi: 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reed W.P., Chick T.W., Jutila K., Butler C., Goldblum S. Pulmonary leukostasis in fatal human pneumococcal bacteremia without pneumonia. Am Rev Respir Dis. 1984;130(6):1184–1187. doi: 10.1164/arrd.1984.130.6.1184. [DOI] [PubMed] [Google Scholar]
- 64.Borges L., Pithon-Curi T.C., Curi R., Hatanaka E. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reusch N., De Domenico E., Bonaguro L., et al. Neutrophils in COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aschenbrenner A.C., Mouktaroudi M., Krämer B., et al. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Med. 2021;13(1):7. doi: 10.1186/s13073-020-00823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meizlish M.L., Pine A.B., Bishai J.D., et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5(5):1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rendeiro A.F., Ravichandran H., Bram Y., et al. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021;593(7860):564–569. doi: 10.1038/s41586-021-03475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fox S.E., Heide R.S.V. COVID-19: The heart of the matter-pathological changes and a proposed mechanism. J Cardiovasc Pharmacol Ther. 2021;26(3):217–224. doi: 10.1177/1074248421995356. [DOI] [PubMed] [Google Scholar]
- 70.Wong D.J., Park D.D., Park S.S., et al. A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood. 2021;138(13):1182–1193. doi: 10.1182/blood.2020009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.