Keywords: melanin, microvascular function, nitric oxide, skin blood flow, UV radiation
Abstract
Ultraviolet radiation (UVR) exposure acutely reduces nitric oxide (NO)-dependent cutaneous vasodilation. In addition, increased constitutive skin melanin is associated with attenuated NO-dependent cutaneous vasodilation. However, the impact of within-limb variation in skin melanization, associated with seasonal UVR exposure, on NO-dependent cutaneous vasodilation is unknown. We investigated the effect of within-limb variation in skin melanin on NO-dependent cutaneous vasodilation. Intradermal microdialysis fibers were placed in the inner-upper arm, ventral forearm, and dorsal forearm of seven adults (33 ± 14 yr; 4 M/3 F) with constitutively light skin pigmentation. Melanin-index (M-index; an index of skin pigmentation), measured via reflectance spectrophotometry, confirmed differences in sun exposure among sites. A standardized local heating (42°C) protocol induced cutaneous vasodilation. After attaining a stable elevated blood flow plateau, 15 mM NG-nitro-l-arginine methyl ester (l-NAME; NO synthase inhibitor) was infused to quantify the NO contribution. Laser-Doppler flowmetry (LDF) measured red cell flux and cutaneous vascular conductance (CVC = LDF/mean arterial pressure) and was normalized to maximal (%CVCmax; 28 mM sodium nitroprusside + 43°C local heating). Dorsal forearm M-index was higher [50.5 ± 11.8 au (arbitrary units)] compared with the ventral forearm (37.5 ± 7.4 au; P ≤ 0.03) and upper arm (30.0 ± 4.0 au; P ≤ 0.001) M-index. Cutaneous vasodilation responses to local heating were not different among sites (P ≥ 0.12). Importantly, neither the magnitude of the local heating plateau (dorsal: 85 ± 21%; ventral: 70 ± 21%; upper: 87 ± 15%; P ≥ 0.16) nor the NO-mediated component of that response (dorsal: 59 ± 15%; ventral: 54 ± 13%; upper: 55 ± 11%; P ≥ 0.79) was different among sites. These data suggest that within-limb differences in skin pigmentation secondary to seasonal UVR exposure do not alter NO-dependent cutaneous vasodilation.
NEW & NOTEWORTHY Locally derived endothelial nitric oxide (NO) contributes to the full expression of cutaneous vasodilation responses. Acute ultraviolet radiation (UVR) exposure attenuates NO-mediated vasodilation of the cutaneous microvasculature. Our findings suggest that in constitutively lightly pigmented skin, variation in skin melanin due to seasonal exposure to UVR does not alter the NO contribution to cutaneous vasodilation. Seasonal UVR exposure does not impact the NO-mediated cutaneous microvascular function.
INTRODUCTION
Locally derived endothelial nitric oxide (NO) is a critical contributor to the full expression of cutaneous vasodilation responses (1,2). Consequently, greater NO-mediated vasodilation is associated with a healthy vascular phenotype (3). NO production and bioavailability are influenced by numerous mechanisms including the expression and coupling of the endothelial NO synthase (eNOS) dimer, oxidative stress, and inflammation (4,5). As such, any physiological stressor that reduces eNOS activity and/or increases oxidative stress or inflammation may negatively impact microvascular endothelial function.
The folate metabolite 5-methyltetrahydrofolate (5-MTHF) promotes the stabilization of endothelial NO synthase (NOS) and bioavailability of NO by 1) increasing the production of tetrahydrobiopterin (BH4), an essential cofactor in the coupling of eNOS, and 2) acting as a potent antioxidant, directly scavenging reactive oxygen species (ROS; 5,6). Ultraviolet radiation (UVR) may degrade 5-MTHF directly and/or indirectly through increased production of reactive ROS (7–9). Our laboratory has previously demonstrated reduced NO-mediated vasodilation in the cutaneous microvasculature after an acute exposure to UV-B or broad-spectrum UVR (10,11). However, local perfusion of ascorbate (a nonspecific antioxidant) or 5-MTHF improved NO-mediated vasodilation after UV-B exposure (10), such that there was no difference between nonexposed control skin and UV-B-exposed skin treated with ascorbate or 5-MTHF. Together, our data suggest that UVR exposure acutely reduces NO-mediated cutaneous vasodilation through direct and/or indirect photodegradation of 5-MTHF.
In contrast to the impact of UVR exposure on 5-MTHF, UVB radiation exposure catalyzes the production of vitamin D from epidermal and dermal stores of 7-dehydrocholesterol (12,13). UV-B-induced cutaneous vitamin D synthesis is decreased, however, when skin melanin concentrations are greater (i.e., a darker skin pigmentation) due to increased absorption of UV-B by skin melanocytes (14,15). Importantly, vitamin D may promote endothelial health by 1) increasing eNOS expression, 2) suppressing ROS production and/or increasing ROS scavenging, and/or 3) reducing inflammation (16, 17). In this context, our laboratory has demonstrated that constitutive skin pigmentation is negatively associated with serum vitamin D concentrations ([25(OH)D]) and the NO contribution to cutaneous vasodilation during local heating in healthy young adults (14, 18). Furthermore, serum [25(OH)D] was directly related to the NO contribution to cutaneous vasodilation, suggesting that microvascular endothelial function was greatest in those who were vitamin D sufficient (14). Thus, vitamin D concentrations and the NO contribution to cutaneous vasodilation responses were typically highest in those with lightly pigmented skin. Together, those findings suggest that lower vitamin D concentrations contribute to lesser NO bioavailability in otherwise healthy darkly pigmented young adults.
In sum, our laboratory has demonstrated 1) acute UVR-induced reductions in NO-mediated cutaneous vasodilation, secondary to direct and/or indirect degradation of 5-MTHF, and 2) persistently reduced NO-mediated cutaneous vasodilation in those with constitutively moderate to dark skin pigmentation, secondary to vitamin D insufficiency/deficiency. However, it remains unclear whether within-individual variation in skin melanin concentrations, secondary to seasonal UVR exposure, influences NO-dependent cutaneous vasodilation in individuals with constitutively light skin pigmentation. Therefore, this study aimed to compare NO-dependent cutaneous vasodilation in areas of tanned skin that was regularly exposed to UVR to that of relatively unexposed/untanned skin on the same limb in individuals with constitutively light skin pigmentation. We hypothesized that 1) NO-dependent cutaneous vasodilation would be reduced in tanned skin relative to untanned skin and 2) NO-dependent cutaneous vasodilation would be negatively associated with increases in tanning-induced skin pigmentation.
METHODS
Subjects
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University. Written and verbal consents were obtained voluntarily from all subjects before participation, according to the Declaration of Helsinki. Seven healthy subjects (4 men and 3 women) underwent an initial screening that included physical examination (i.e., height, weight, blood pressure, and heart rate). Subjects with Fitzpatrick skin type I and II were enrolled (19). As this was a strictly within-subject study design, participants were included without regard to age, blood pressure, BMI, or blood biochemistry. Subjects with rash, skin disease, disorders of pigmentation, known skin allergies, allergies to folic acid, or kidney disease were excluded. Subjects were regularly active outdoors (cycling, running, golfing, etc.) according to self-report. Female subjects were premenopausal and menstrual cycle phase was not considered or recorded.
Based on previously published data (18) demonstrating the difference in NO-mediated vasodilation in subjects with lightly pigmented skin compared with darkly pigmented skin, we determined a priori, using an effect size of 1.21 (α = 0.05; power = 0.8), that six subjects would be sufficient to detect within- and between-group differences in the %NO contribution to the local heating response.
Assessment of Skin Pigmentation
Skin pigmentation was measured by skin reflectance spectrophotometry (DermaSpectrometer; Cortex Technology, Hadsund, Denmark) to determine the melanin index (M-index) of the skin on the subject’s dorsal and ventral aspects of the forearm and inner aspect of the upper arm. These sites were chosen as they provide a range of high (dorsal forearm), moderate (ventral forearm), and low (inner-upper arm) sun exposure. Lower and higher M-indices are related to lighter and darker skin pigmentation, respectively.
Experimental Protocol
Data were collected during late summer and early fall (August–October) to maximize the potential effect of seasonal exposure to UVR. All protocols were performed on a single day in a thermoneutral laboratory with the subject in a semisupine position with the experimental arm supported at heart level. Intradermal microdialysis fibers (10 mm, 55-kDa cutoff membrane; CMA, Holliston, MA) were simultaneously placed into the dermal layer of the ventral and dorsal aspects of the forearm and the inner aspect of the upper arm for a total of three fibers. Pharmacological agents were dissolved in lactated Ringer solution just before use, sterilized using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), and wrapped in foil to prevent degradation because of light exposure. Solutions were perfused through the microdialysis fibers at a rate of 2 µL/min (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems, West Lafayette, IN; 1). Local red blood cell flux was measured directly over each microdialysis site throughout the protocol using an integrated laser-Doppler flowmetry (LDF) probe placed in a local heating unit (moorLAB, temperature monitor, SHO2; Moor Instruments, Axminster, UK). Mean arterial pressure (MAP) was calculated for each phase of the protocol using blood pressure taken from an automated blood pressure monitor (CardioCap; GE Healthcare, Milwaukee, WI).
Each fiber was initially perfused with lactated Ringer solution during the first ∼60 min to allow for resolution of hyperemia associated with fiber placement. After this period, baseline data were collected (∼20 min) before beginning a local heating (42°C) protocol, as described previously (1,2). This heating protocol elicits an initial peak mediated by the axon reflex, after which there is a gradual rise and eventual plateau in blood flow (4, 20). After observing a stable local heating plateau (∼40 min), 15 mM NG-nitro-l-arginine methyl ester (l-NAME; NOS inhibitor) was perfused through all sites, allowing for quantification of NO-dependent vasodilation (%NO; 4,5). After a stable l-NAME plateau, 28 mM sodium nitroprusside (SNP; USP, Rockville, MD) was perfused through all sites, and local temperature was increased to 43°C to induce maximal vasodilation (2, 20).
Data Acquisition and Analysis
LDF data were recorded at 40 Hz and stored for offline analysis (PowerLab/LabChart, ADInstruments, Colorado Springs, CO). CVC was calculated as red blood cell flux divided by MAP and expressed as a percentage of CVCmax for each phase of the local heating protocol (2, 21). The percentage of cutaneous vasodilation mediated by NO (%NO) was calculated from the difference between the local heating and l-NAME plateaus.
Two-way repeated-measures ANOVA was used to detect differences among the three experimental sites at each phase of the local heating protocol and the NO contribution to vasodilation (GraphPad Prism, v. 9.2, GraphPad Software, San Diego, CA). One-way repeated-measures ANOVA was used to detect differences in M-index. Data were checked for normality using Shapiro–Wilk test for all statistical analyses. A nonnormal distribution was found for the local heating plateau; therefore, a nonparametric test was conducted for that phase only. To determine whether the magnitude of increase in skin pigmentation associated with seasonal tanning influences the potential change in NO-mediated vasodilation, simple linear regression analysis was used to assess the relation between M-index and NO-dependent vasodilation, with all three sites from each subject being entered into the analysis. Significance was set a priori and accepted at α = 0.05. All values are presented as means ± standard deviation.
RESULTS
Subject characteristics are presented in Table 1. Subjects who were >40 yr of age, had blood pressure >130/80 mmHg, or BMI ≥30 did not quantitatively influence the results. M-index was higher (i.e., pigmentation was darkest) on the dorsal aspect of the forearm compared with the ventral and inner aspects of the upper arm (both P < 0.05); however, M-index was not significantly different between the ventral aspect of the forearm and the inner-upper arm (P = 0.27).
Table 1.
Subject characteristics
| Means ± SD | Range | |
|---|---|---|
| Age, yr | 33 ± 14 | 20–65 |
| Systolic BP, mmHg | 126 ± 12 | 101–141 |
| Diastolic BP, mmHg | 75 ± 10 | 65–90 |
| BMI, kg/m2 | 23 ± 3 | 21–30 |
| M-index, au | ||
| Dorsal forearm | 50.5 ± 11.8 | 39.1–72.7 |
| Ventral forearm | 37.5 ± 7.4* | 27.7–47.6 |
| Upper arm | 30.0 ± 4.0* | 23.8–35.0 |
Data are presented as means ± SD and ranges; n = 7 (4 men and 3 women). au, Arbitrary units; BP, blood pressure; M-index, a skin reflectance measure of melanization. *P < 0.05 compared with dorsal forearm.
Figure 1 illustrates a representative tracing of red cell flux in response to the standardized local heating protocol for one subject. The decrease in red cell flux by NOS inhibition via local administration of l-NAME is indicated.
Figure 1.
A representative tracing of red cell flux in response to the standardized local heating protocol for one subject. The percent decrease in red cell flux with NOS inhibition by local administration of NG-nitroarginine-l-methyl ester (l-NAME) is indicated by the arrow. NOS, NO synthase; SNP, sodium nitroprusside.
Presented in Fig. 2 is %CVCmax for each phase of the local heating protocol at each of the three sites. There were no differences in %CVCmax among the three sites at any phase of the local heating protocol. Importantly, CVCmax was similar among the sites (dorsal: 1.73 ± 0.75 flux/mmHg; ventral: 1.80 ± 0.53 flux/mmHg; upper: 2.04 ± 0.86 mmHg; P ≥ 0.23).
Figure 2.
Comparisons in %CVCmax among the three sites at each phase of the local heating protocol (n = 7). There were no differences in %CVCmax due to tanning-induced increases in skin pigmentation during baseline, at the initial peak, or during the local heating or post-l-NAME plateaus. Data were analyzed using two-way repeated-measures ANOVA and are presented as means ± SD with individual data points. %CVCmax, percent maximal cutaneous vascular conductance.
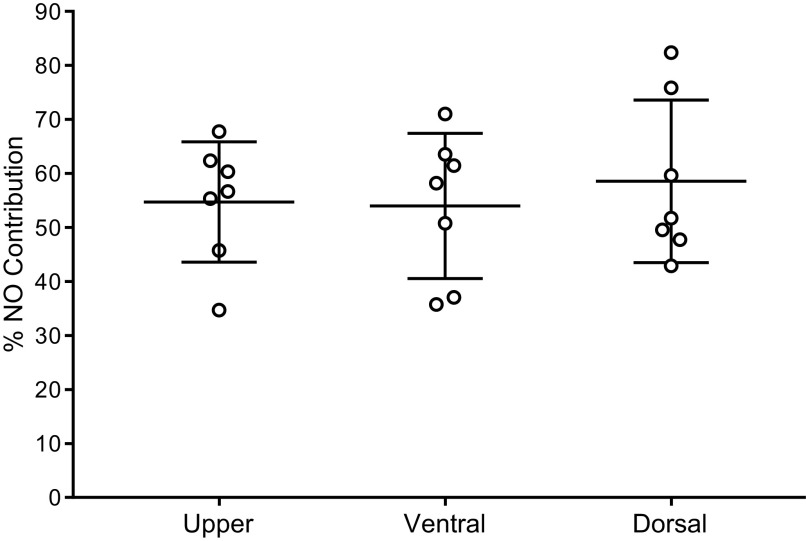
Figure 3 depicts the %NO contribution to cutaneous vasodilation in response to local heating at each site. There were no differences in %NO-mediated vasodilation among the sites. Furthermore, there was no association between %NO-mediated vasodilation and M-index (r = 0.24, P = 0.30).
Figure 3.
Comparisons in the percent contribution of nitric oxide (%NO Contribution) to the local heating plateau among the three sites (n = 7). Data were analyzed using two-way repeated-measures ANOVA and are presented as means ± SD with individual data points.
DISCUSSION
The primary finding of this study was that within-limb variation in M-index due to seasonal UVR exposure, unlike interindividual differences in constitutive skin pigmentation, did not alter the magnitude of the cutaneous vasodilation response to local heating or the NO-mediated component of that response. In addition, there was no association between seasonal tanning-induced increases in skin pigmentation and NO-mediated vasodilation of the cutaneous microvasculature. These data suggest that seasonal tanning-induced increases in skin melanin concentrations do not impact NO-dependent cutaneous vasodilation.
Nitric oxide is a critical component of the vasodilatory response to local heating in healthy adults (1, 3). Adequate bioavailability of 5-MTHF contributes to the full expression of NO-mediated cutaneous vasodilation responses through its role as an eNOS cofactor and by acting as a potent antioxidant (5). In this context, we previously demonstrated (10,11) that acute UVR exposure attenuated NO-mediated cutaneous vasodilation via photodegradation of 5-MTHF, directly and/or indirectly by increasing local production of ROS (10). Recovery time between UVR exposure and return to normal NO-mediated cutaneous vasodilation remains unclear.
In contrast to the potential deleterious effects of acute UVR exposure on NO-mediated cutaneous vasodilation observed by our laboratory, chronic UVR exposure, particularly in the UV-B spectrum, promotes adequate vitamin D bioavailability (22). In turn, vitamin D may promote healthy endothelial function by increasing eNOS expression and/or by reducing oxidative stress and inflammation (16,17). Indeed, our laboratory has shown that vitamin D status is directly related to NO-mediated cutaneous vasodilation, such that those who are vitamin D sufficient have a greater NO contribution to cutaneous vasodilation responses relative to those who are vitamin D insufficient or deficient (14). Melanin within the skin absorbs UVB such that darker skin pigmentation impairs vitamin D synthesis (23). As such, individuals with constitutively dark skin pigmentation who are living in areas with relatively low UVR exposure and/or high seasonality are at greatest risk of vitamin D deficiency (15, 24).
The findings of the present study are in contrast with previous studies from our laboratory demonstrating 1) acute UVR-induced reductions in NO-mediated cutaneous vasodilation and 2) persistently reduced NO-mediated cutaneous vasodilation in those with constitutively darkly pigmented skin (10,11, 14, 18). One possible explanation for this difference may be the duration/frequency of UVR exposure. UVR exposure that would typically degrade folate results in rapid generation of protective immediate pigment darkening and, subsequently, delayed tanning (7). Therefore, acute and local impacts of UVR exposure on folate and NO bioavailability may be transient (resolve after a few hours) and there may be a protective effect of tanning-induced increases in skin melanin concentrations on subsequent exposures.
However, the contrast between the present findings and our previous data demonstrating differences in NO-mediated cutaneous vasodilation between darkly and lightly pigmented young adults (14, 18) is more likely explained by the differences between constitutive and tanning-induced skin pigmentation in terms of vitamin D status. Vitamin D status is a mediator in the relation between constitutive skin pigmentation and NO-dependent microvascular function; that is, vitamin D insufficiency or deficiency may contribute to persistent microvascular dysfunction in otherwise healthy darkly pigmented adults (14). The cohort in this study consisted of individuals with constitutively light skin pigmentation, with seasonal changes in UVR resulting in within-limb variation in M-index. Therefore, the potential mechanisms of function/dysfunction (i.e., vitamin D deficiency vs. UV-induced 5-MTHF degradation) differ from those of a cohort with constitutively dark skin pigmentation. Although we did not measure vitamin D status in this study, our laboratory has demonstrated that those with constitutively light skin pigmentation are typically vitamin D sufficient and that vitamin D supplementation does not augment microvascular function in those individuals (18). Furthermore, tanning-induced increases in M-index in lightly pigmented individuals suggest likely increases in vitamin D bioavailability via regular sun exposure.
An important consideration in this study is that although participants were regularly active outdoors (cycling, running, golfing, etc.), no data were collected regarding frequency/duration of outdoor activities in each subject, or the temporality of the most recent exposure in relation to the experimental visit. Thus, there is likely variation in the daily amount of UVR that subjects were exposed to and the proximity of the most recent exposure that was unaccounted for. For these reasons, we looked at the relation between M-index and %NO-mediated vasodilation and found no association between tanning-induced increases in skin pigmentation and %NO-mediated vasodilation. However, we cannot rule out the possibility that a greater magnitude of exposure in a region with more intense and less seasonal UVR may result in cutaneous microvascular dysfunction in lightly pigmented individuals. Another potential limitation is the small sample size; however, given 1) the within-subject nature of this study, 2) our a priori power analysis suggesting six subjects would be sufficient to identify significant differences if they existed, and 3) the small effect sizes observed in our data, we are confident that testing additional subjects would not meaningfully alter our findings.
In summary, this study demonstrated that in constitutively lightly pigmented skin, seasonal UVR exposure-induced variation in M-index does not alter the NO contribution to cutaneous vasodilation responses to local heating. These data suggest that repeated seasonal UVR exposure does not impact NO-mediated cutaneous microvascular function.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health Grant R0 AG067471 (to W.L.K.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.L.K. and S.T.W. conceived and designed research; K.G.F. and S.T.W. performed experiments; K.G.F. analyzed data; K.G.F., W.L.K. and S.T.W. interpreted results of experiments; K.G.F. and S.T.W. prepared figures; K.G.F. drafted manuscript; K.G.F., W.L.K., and S.T.W. edited and revised manuscript; K.G.F., W.L.K., and S.T.W. approved final version of manuscript.
REFERENCES
- 1. Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 3. Holowatz LA. Human cutaneous microvascular ageing: potential insights into underlying physiological mechanisms of endothelial function and dysfunction. J Physiol 586: 3301, 2008. doi: 10.1113/jphysiol.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol 304: R164–R169, 2013. doi: 10.1152/ajpregu.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev 75: 61–70, 2017. doi: 10.1093/nutrit/nuw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25: 81–88, 2011. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moan J, Nielsen KP, Juzeniene A. Immediate pigment darkening: its evolutionary roles may include protection against folate photosensitization. FASEB J 26: 971–975, 2012. doi: 10.1096/fj.11-195859. [DOI] [PubMed] [Google Scholar]
- 8. Off MK, Steindal AE, Porojnicu AC, Juzeniene A, Vorobey A, Johnsson A, Moan J. Ultraviolet photodegradation of folic acid. J Photochem Photobiol B 80: 47–55, 2005. doi: 10.1016/j.jphotobiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 9. Steindal AH, Juzeniene A, Johnsson A, Moan J. Photodegradation of 5-methyltetrahydrofolate: biophysical aspects. Photochem Photobiol 82: 1651–1655, 2006. doi: 10.1562/2006-06-09-RA-915. [DOI] [PubMed] [Google Scholar]
- 10. Wolf ST, Stanhewicz AE, Jablonski NG, Kenney WL. Acute ultraviolet radiation exposure attenuates nitric oxide-mediated vasodilation in the cutaneous microvasculature of healthy humans. J Appl Physiol (1985) 125: 1232–1237, 2018. doi: 10.1152/japplphysiol.00501.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolf ST, Berry CW, Stanhewicz AE, Kenney LE, Ferguson SB, Kenney WL. Sunscreen or simulated sweat minimizes the impact of acute ultraviolet radiation on cutaneous microvascular function in healthy humans. Exp Physiol 104: 1136–1146, 2019. doi: 10.1113/EP087688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet 36, Suppl 11: S54–S60, 2004. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- 13. Holick MF. Vitamin D deficiency. N Engl J Med 357: 266–281, 2007. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14. Wolf ST, Dillon GA, Alexander LM, Jablonski NG, Kenney WL. Skin pigmentation is negatively associated with circulating vitamin D concentration and cutaneous microvascular endothelial function. Am J Physiol Heart Circ Physiol 323: 490–498, 2022. doi: 10.1152/ajpheart.00309.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young AR, Morgan KA, Ho TW, Ojimba N, Harrison GI, Lawrence KP, Jakharia-Shah N, Wulf HC, Cruickshank JK, Philipsen PA. Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: a comparison of extreme phenotypes. J Invest Dermatol 140: 1418–1426 e1, 2020. doi: 10.1016/j.jid.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 16. Wolf ST, Kenney WL. The vitamin D-folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiol 317: R491–R501, 2019. doi: 10.1152/ajpregu.00136.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and endothelial function. Nutrients 12: 575, 2020. doi: 10.3390/nu12020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf ST, Jablonski NG, Ferguson SB, Alexander LM, Kenney WL. Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. Am J Physiol Heart Circ Physiol 319: H906–H914, 2020. doi: 10.1152/ajpheart.00631.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol 75: 93–96, 2009. doi: 10.4103/0378-6323.45238. [DOI] [PubMed] [Google Scholar]
- 20. Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 21. Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol (1985) 109: 1239–1246, 2010. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother 3: 118–126, 2012. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Libon F, Cavalier E, Nikkels AF. Skin color is relevant to vitamin D synthesis. Dermatology 227: 250–254, 2013. doi: 10.1159/000354750. [DOI] [PubMed] [Google Scholar]
- 24. Ames BN, Grant WB, Willett WC. Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities? Nutrients 13: 499, 2021. doi: 10.3390/nu13020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.