
Keywords: exercise, fluid regulation, heat stress, menstrual cycle
Abstract
We tested the hypothesis that women may be more at risk of becoming dehydrated during physical work in the heat in the early follicular phase (EF), compared with the late follicular (LF) and mid-luteal (ML) phases of the menstrual cycle when allowed free access to drink. Twelve healthy, eumenorrheic, unacclimated women (26 ± 5 yr) completed three trials (EF, LF, and ML phases) involving 4 h of exposure to 33.8 ± 0.8 °C, 54 ± 1% relative humidity. Each hour, participants walked on a treadmill for 30 min at a rate of metabolic heat production of 338 ± 9 W. Participants drank a cool, flavor-preferred non-caloric sport drink ad libitum. Nude body weight was measured pre- and post-exposure, and percent changes in body weight loss were interpreted as an index of changes in total body water. Total fluid intake and urine output were measured and sweat rate was estimated from changes in body mass corrected for fluid intake and urine output. Fluid intake was not different between phases (EF: 1,609 ± 919 mL; LF: 1,902 ± 799 mL; ML: 1,913 ± 671; P = 0.202). Total urine output (P = 0.543) nor sweat rate (P = 0.907) differed between phases. Percent changes in body mass were not different between phases (EF: −0.5 ± 0.9%; LF: −0.3 ± 0.9%; ML: −0.3 ± 0.7%; P = 0.417). This study demonstrates that the normal hormonal fluctuations that occur throughout the menstrual cycle do not alter fluid balance during physical work in the heat.
NEW & NOTEWORTHY The effect of the menstrual cycle on fluid balance during physical work in the heat when fluids are freely available is unknown. This study demonstrates that fluid balance is not modified in women across three distinct phases of the menstrual cycle during physical work in the heat These results indicate that when women have free access to cool fluid during physical work in the heat, they respond similarly across all three phases to maintain fluid homeostasis across the menstrual cycle.
INTRODUCTION
Approximately 15 million laborers regularly work in hot environments, while the incidence of occupational heat exposure is predicted to increase in the coming decades, occurring secondary to climate change (1). Roughly 1–2 million of these laborers are women, with more women expected to be employed in industries requiring physical work in hot environments in the coming years (2). Notably, biological sex is considered a risk factor for health and safety susceptibility during heat exposure (1).
Compared with temperate ambient conditions, there is a greater risk of dehydration during physical work in the heat due to increased fluid output caused by sweating (3, 4). Indeed, dehydration is prevalent in laborers working in the heat (5), with up to 69% of workers being dehydrated at the end of a work shift (6). Fluid regulation controls extracellular fluid volume and maintains serum osmolality and electrolyte concentrations (e.g., sodium) by modulating fluid intake (e.g., thirst) and fluid output (e.g., urine concentration and production). Proper fluid balance is important because dehydration (i.e., a hypovolemic, hyperosmotic state caused by reductions in body water) can reduce mental and physical capacity, which includes impairments in cognitive function and decision-making, and alterations in cardiovascular functioning and thermoregulatory control (7). Thus, standard best practices are for workers to have ready access to cool fluids in the workplace to prevent dehydration (8).
Research examining fluid balance and/or the physiological implications of dehydration during physical work in the heat has generally been conducted in men or examined in women in the low hormone phase of the menstrual cycle (i.e., early follicular phase) (9). This ignores fluctuations in estrogen and progesterone occurring across the menstrual cycle in women that can influence fluid regulation hormones (10–12), which may affect overall fluid balance. For example, increased progesterone concentrations are associated with elevations in aldosterone concentrations (13), whereas an increase in estrogen lowers the osmotic threshold for the release of arginine vasopressin (AVP) (14), both of which stimulate renal water retention and thirst (11, 12). Therefore, the physiological effects of these female sex hormones are likely heightened during the phases in which the concentrations of these hormones are elevated (i.e., the late follicular and mid-luteal phases) (11). Although there has been relatively extensive research investigating circulating sex hormones and their effects on fluid regulation (9, 10, 13–16), a gap still exists involving the role of menstrual cycle hormones on fluid balance when ad libitum drinking is permitted during exercise in the heat. This knowledge gap is concerning given that occupational and public health hydration recommendations encourage people to drink cool fluids, which should be readily available, when exposed to heat (8, 17, 18). Due to the hormonal influences of estrogen and progesterone and their potential to shift the osmotic thresholds that modulate fluid retention and intake, we hypothesized that when women are provided free access to fluid, they will drink less and retain less fluid in the early follicular phase (low estrogen and progesterone), thereby potentially, exacerbating the likelihood of becoming dehydrated during physical work in the heat compared with the late follicular (high estrogen) and mid-luteal (high estrogen, high progesterone) phases of the menstrual cycle.
METHODS
Participants
To our knowledge, there were no direct data (e.g., those including drinking behavior across the menstrual cycle) to inform a sample size calculation. That said, previous evidence indicated that the Cohen’s dz effect size associated with the impact of endogenous menstrual cycle hormones on circulating arginine vasopressin concentrations (16) is 0.707. Thus, an a priori power calculation using this effect size was carried out using G-Power v. 3.1.9.4 (19), which determined that 12 participants were required to achieve 80% power with α = 0.05. Therefore, 12 healthy, eumenorrheic women between the ages of 18 to 34 yr participated in this study. Participants were eligible if they self-reported no known cardiovascular, metabolic, renal, or neurological diseases. Additional eligibility criteria included being physically active, nonsmokers, not currently taking any type of hormonal contraceptive, and/or having abstained from contraceptives for >6 mo. Participating women were not pregnant, which was confirmed through a urine pregnancy test taken at each visit, self-reported to be normally menstruating, and reported no menstrual cycle-specific disorder as assessed by a menstrual cycle questionnaire. Participants were considered recreationally active as they all met the minimum physical activity requirements of participating in at least 150 min/wk of aerobic exercise (20). Participants provided verbal and written informed consent after being fully informed of the experimental procedures and possible risks. This study was approved by the Indiana University Institutional Review Board (IRB #: 2004319694) and was carried out according to the most recent revision of the Declaration of Helsinki, except for registration in a database.
To determine menstrual cycle status and confirm study eligibility, we adhered to the recommended screening criteria for the determination of physiologically natural, menstrual cycles through the confirmation of eumenorrheic menstrual cycle length (≥ 21 days and ≤ 35 days), presence of urinary luteinizing hormone (LH) surge, and basal body temperature exhibiting normal patterns, all of which were tracked for two whole menstrual cycles (21, 22). These three measures were our strict inclusion criteria to determine if women were eligible to complete the experimental protocol. Hormone levels were measured during the experimental visits as a tertiary measure to confirm the menstrual cycle phase.
Details of menstrual cycle tracking are as follows. During the screening visit (Visit 1), participants were asked to track their menstrual cycles through the FitrWoman App (Orreco Ltd., Galway, Ireland) as a method to log when they started menstrual bleeding throughout the duration of the study. The study team had access to FitrWoman app data via a remote desktop as part of the FitrCoach platform (Orreco Ltd., Galway, Ireland). This minimized participant burden throughout the tracking process. Prior to experimental testing, oral temperature was measured for at least two cycles, every morning upon waking, using a digital thermometer (Medical Thermometer, B.Weiss Personal Care, Shenzhen, China) to provide a measure of the increase in body temperature from the follicular to the mid-luteal phase that should occur if ovulation is present. The women also took a daily urinary ovulation test (Ovukit Self-Test, Cen-Med Enterprise, New Brunswick, NJ) between days 10–20 of their menstrual cycle to detect the surge in luteinizing hormone that occurs on average 14 ± 1 day post-menses, to confirm the occurrence of ovulation. Blood samples were collected at the beginning of each experimental visit and were retrospectively measured for serum estradiol (intra-assay CV: 10.6 ± 7.6%), progesterone (intra-assay coefficient of variation (CV): 5.9 ± 2.2%), and testosterone (intra-assay CV: 7.8 ± 3.0%) concentrations using commercially available assay kits (Eagle Biosciences, Amherst, NH). The ratio of estradiol to progesterone (E2/P4 ratio) was calculated as the quotient of estradiol (ng/mL) to progesterone (ng/mL). One woman in our study had contaminated baseline hormone samples, which resulted in post hoc measured nonphysiological hormone levels. However, we were able to confirm that she had a normal menstrual cycle length, we observed a surge in luteinizing hormone mid-cycle, and she exhibited the fluctuating temperature pattern indicative of a eumenorrheic menstrual cycle profile. Therefore, data from this participant were included in statistical analyses.
Experimental Protocol
In a randomized crossover design, participants completed three experimental trials marked by a distinct phase of the menstrual cycle predicted out based on historical tracking data from the two previous cycles: early follicular (EF, day 3 ± 2), late follicular (LF, day 13 ± 2), and mid-luteal (ML, day 21 ± 2). Notably, if we were unable to conduct the experimental trial in back-to-back menstrual cycle phases for a given subject (e.g., due to scheduling conflicts, randomization, etc.), we continued tracking the participant’s menstrual cycles using the aforementioned methods until the participant was able to be scheduled. Blinding of the experimental conditions was not possible in this study, but participants were blinded from the research hypotheses. The order of experimental trials (Visits 2–4) was assigned in blocks of three where one participant began Visit 2 in the early follicular phase, one participant in the late follicular phase, and one participant in the mid-luteal phase in a randomized order (Fig. 1).
Figure 1.
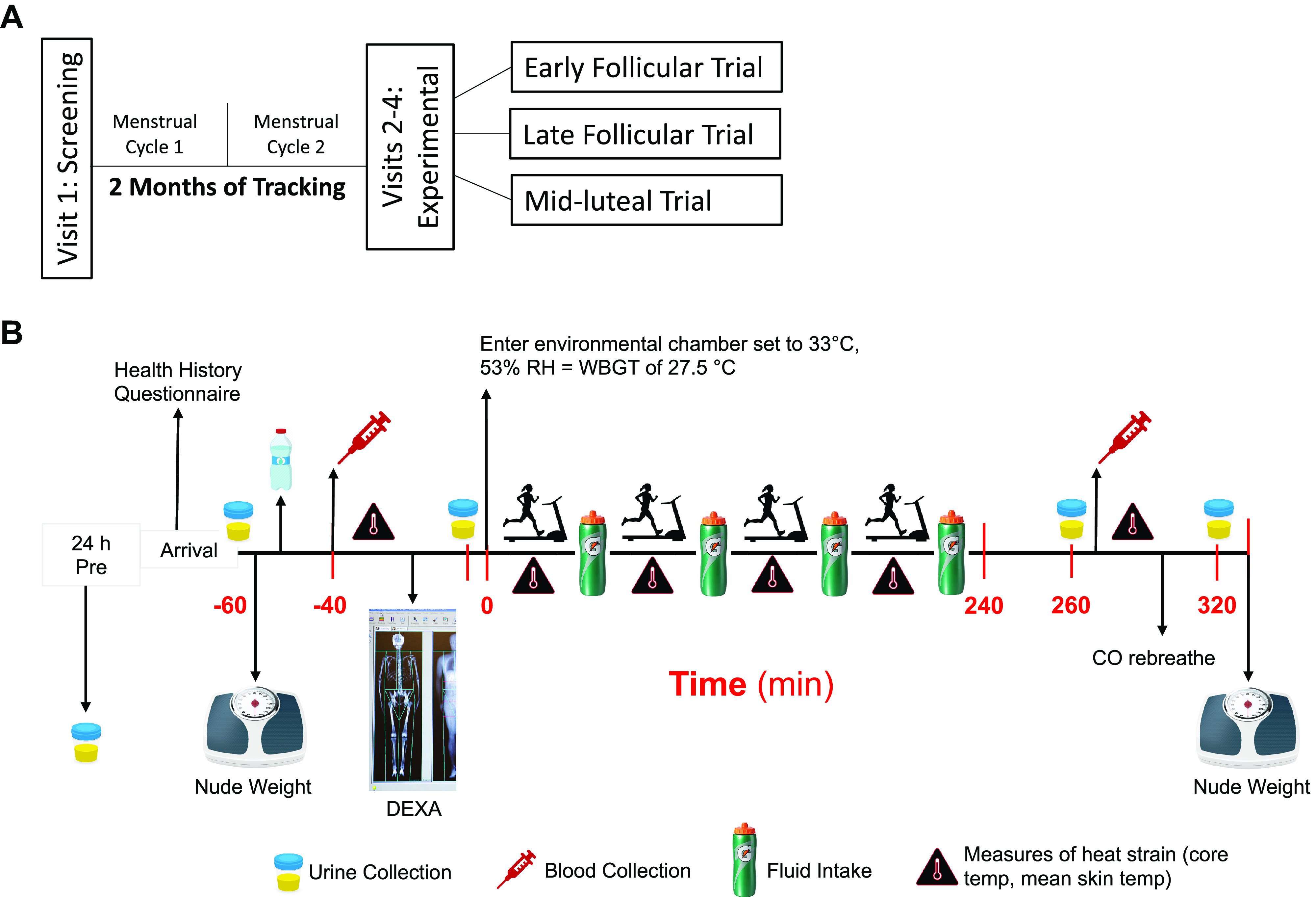
A: schematic of experimental design. Participants completed, in a randomized crossover design, a total of four visits including three experimental trials (Visits 2–4), each during the early follicular, late follicular, and mid-luteal phase. B: timeline of data collection procedures.
Participants reported to the temperature-controlled laboratory (21.1 ± 0.9°C) for three experimental trials after abstaining from exercise, caffeine, and alcohol for 12 h and food for 2 h. Participants were encouraged to arrive at the laboratory well-hydrated but were not given any specific fluid intake instructions. Participants were advised to maintain their normal exercise routines and diet throughout the duration of the experimental period. To ensure this was the case, a diet and fluid log was collected in the 24 h preceding the experimental trial and participants were instructed to complete a 24-h urine collection before the visit which was analyzed via MyFitnessPal.com. To account for diurnal changes, each experimental visit was completed at the same time of day (±1 h) within a participant.
Upon arrival at the laboratory for each experimental visit, participants completed a health history update questionnaire to ensure no relevant changes in their health history had occurred. Participants were then instructed to void their bladder and euhydration was confirmed using this urine sample (i.e., urine specific gravity <1.020) (23). Participants measured their nude body mass, consumed 250 mL of cool tap water to establish a baseline urine flow rate and better control for pre-exercise hydration, and were instrumented with iButtons and a Polar heart rate monitor and laid in the supine position. Following 20 min of rest in the temperate laboratory, pre-exercise measurements of heart rate, mean arterial pressure, core and skin temperatures were collected and culminated in the collection of a venous blood sample and voiding of the bladder. After this, body composition was measured via dual X-ray absorptiometry (Lunar iDXA, GE Healthcare, IL).
Following pre-exercise data collection, participants exercised in the heat for 4 h in an environmental chamber set to 33.8 ± 0.8°C, 54 ± 1% relative humidity. Participants wore a long sleeve button down shirt and long pants to mimic the clothing worn by physical laborers. Four hours was chosen to simulate half of a workday. The selected environmental conditions elicit a wet bulb globe temperature of 27.5°C, which is the average hot environmental conditions often encountered in outdoor occupations (8, 17, 18). During this 4-h period, participants walked on a treadmill for 30 min/h at a treadmill speed and grade to elicit a rate of metabolic heat production of 300–400 W. Based on the Compendium of Physical Activities (24), this rate of metabolic heat production is the average workload encountered in outdoor occupational settings that are regularly exposed to heat stress. Notably, we expected this clamped rate of heat production to result in the same volume of sweat loss within a woman across the menstrual cycle (25). Moreover, this work-to-rest ratio (30 min walking:30 min resting) is in accordance with the National Institute of Occupational Safety and Health (NIOSH) heat stress recommendations for this wet bulb globe temperature (WBGT) and rate of metabolic heat production (8, 17, 18). Participants remained seated for the entirety of the 30-min rest period. Gas exchange measures (e.g., oxygen uptake, ventilation) were collected for 5 min at the start of each hour of exercise and metabolic heat production was calculated from these data. Fluid was readily available in two 32-ounce Gatorade branded water bottles (Fig. 1B) placed in an open cooler with ice directly next to the treadmill or chair at arm’s reach for easy access. Fluid intake was monitored throughout the entire 4 h in the chamber by noting every time a participant took a drink and weighing the bottles at the beginning and end of each hour. Any time a bottle was finished, we immediately refilled the bottle with the chosen Gatorade zero flavor. Measures of heat strain were monitored every 15 min and urine was collected (if needed) during the rest periods. Participants were administered perceptual questionnaires every 15 min.
Upon completion of exercise in the heat, participants returned to the temperate laboratory environment for post-exercise measurements. Participants laid in the supine position for 20 min of rest. Following this period, post-exercise measurements of heart rate, mean arterial pressure, core and skin temperatures were collected, a final blood draw and urine sample were obtained, and nude body mass was measured at the end. This was followed by a carbon monoxide rebreathing procedure. The carbon monoxide rebreathing procedure was conducted after exercise in the heat to ensure that the administration of carbon monoxide did not interfere with measures during exercise in the heat and because there is no evidence that hemoglobin mass is altered by a single bout of low to moderate intensity exercise (26).
Instrumentation and Measurements
Standing height was measured during the first screening visit using a stadiometer (Holtain Limited, Seritex, Wales, UK) and recorded to the nearest cm and nude body mass was measured to the nearest g using a digital scale (Sauter, Balingen, Germany) at the beginning and end of each experimental visit. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Body fat percentage was measured via dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Chicago, IL) collected during each experimental visit. Core temperature was measured continuously with an ingestible telemetry capsule (n = 10, HQ, Palmetto, FL) or rectal temperature (n = 2, Covidien, Medtronic, Minneapolis, MN) the latter of which was measured when participants were contraindicated for ingesting the telemetry capsule. Weighted mean skin temperature was calculated from the measurement of skin temperature on the chest, triceps, thigh, and calf on the right side of the body (Thermochron iButtons, Maxim Integrated, San Jose, CA) (27). Whole body sweat loss and sweat rate were calculated from changes in body mass, corrected for fluid intake and urine loss. Heart rate was continuously measured using a Polar (Bethpage, NY) heart rate monitor. Expired gases were sampled for volume, oxygen, and carbon dioxide fractions, which were used to determine oxygen uptake and carbon dioxide production during exercise (Parvo Medics, Salt Lake City, UT). We estimated metabolic heat production from the ratio of oxygen uptake and carbon dioxide production (28). Blood pressure was measured manually and in duplicate measures by the same operator during both rest and exercise for all experimental trials within each subject.
Plasma aldosterone (intra-assay CV: 12.7 ± 6.9%, inter-assay CV: 10.3 ± 4.0%) and plasma renin activity (intra-assay CV: 11.1 ± 7.9%, inter-assay CV: 17.7 ± 5.5%), and plasma copeptin, an indirect marker of AVP (29) were measured (intra-assay CV: 2.9 ± 2.1%, inter-assay CV: 8.9 ± 8.6%) in duplicate using commercially available ELISA kits (Eagle Biosciences, Amherst, NH; My BioSource, San Diego, CA). Urine flow rate was calculated as urine volume divided by the time (min) between each bladder void. Urine-specific gravity was measured using refractometry (Atago, Tokyo, Japan). Urine and serum osmolality (Advanced Instruments, Norwood, MA) and sodium concentrations (Medica Corporation, Bedford, MA) were measured in duplicate using commercially available systems. Sodium clearance was calculated as the quotient of urine sodium and serum sodium multiplied by urine flow rate, and free water clearance was calculated as UFR × (1 − [Uosm ÷ Posm]), where UFR is urine flow rate and Uosm and Posm are the osmolalities of the urine and plasma.
Carbon monoxide (CO) rebreathing was used to measure total hemoglobin mass, and through this measure, we calculated red blood cell volume and absolute plasma volume from hemoglobin concentration and hematocrit using methods previously reported in our laboratory (30, 31). Following at least 15 min of supine rest, the CO rebreathing procedures consisted of a bolus of 99.5% chemically pure CO in the amount of 1.0 mL CO per kg body weight delivered via a 100 mL syringe and inhaled by the participant. The participant rebreathed for 2 min from a closed spirometer system connected to a 5 L anesthetic bag filled with 100% O2 while remaining in the supine position. Before and at 4 min and 6 min after the start of the rebreathe, finger prick blood samples were collected in duplicate. The obtained capillary blood was sampled by a blood gas analyzer (OSM3, Radiometer) for the determination of carboxy-hemoglobin concentration, which permitted the calculation of hemoglobin mass. Plasma volume was calculated from total blood volume minus erythrocyte volume (32).
A standardized script was used to verbally explain ratings-of-perceived exertion (RPE; 6–20 Borg scale), ratings of thirst (1–9), thermal comfort (1–4), and thermal sensation (1–7) using visual analog scales. In brief, participants rated their RPE between 6 and 20, where 6 implies “no effort” and 20 indicates “maximal effort” (33). Participants were also asked “how thirsty do you feel now?” on a scale from 1 to 9, where 1 is “not thirsty at all” and 9 is “very, very thirsty” (34). In addition, the thermal comfort scale was from 1 to 4 where 1 signifies “comfortable” and 4 signifies “very uncomfortable” and the thermal sensation scale is rated as 1 = cold, 4 = neutral, and 7 = hot (35).
Participants had free access to a cool (10–15°C) non-caloric sport drink [Gatorade Zero (160 mg sodium and 50 mg potassium per 360 mL) (PepsiCo, OK)] during exercise in the heat, and the volume consumed was measured every hour. Ad libitum fluid intake permitted the assessment of body fluid balance, which is a function of both fluid intake and output. This measure also enhanced the external validity of our protocol, given that the workplace hydration recommendations encourage cool fluids to be freely available throughout the workday, particularly in hot conditions (17). A noncaloric sport drink was used given the recommendations for electrolyte replacement when heat exposure exceeds 2 h in duration (8, 17, 18) and to reduce the risk of hyponatremia. Additional considerations for using a noncaloric beverage were to ensure calorie intake did not affect drinking behavior. During the screening visit (Visit 1), each participant had the opportunity to choose their preferred flavor of noncaloric sport drink and consumed the same flavor for each experimental visit. According to the Nutrition Facts label, the constituents of the beverages did not differ depending on the flavor of the sport drink.
Data and Statistical Analyses
Pre-exercise data were analyzed using a repeated measures one-way linear mixed model. Data during exercise and data obtained pre- and post-exercise were analyzed using two-way linear mixed models with repeated factors of menstrual cycle phase and time. When the assumption of sphericity was violated, the Geisser-Greenhouse correction was applied. Data normality and the residuals of the linear mixed model were determined to be normally distributed in all cases and thus, no corrections were necessary. If a linear mixed model revealed a significant main effect or interaction, pairwise comparisons were made with Sidak’s post hoc test. A priori statistical significance was set at P ≤ 0.05. For transparency, exact F (including degrees of freedom) and P values are reported where possible and data are reported as means ± SD and/or as mean and individual values. All data were analyzed with Prism software (v. 9; GraphPad Software, La Jolla, CA).
RESULTS
Screening and 24-h Pre-Visit
Participant characteristics obtained during the screening visit (Visit 1) and through menstrual cycle tracking are displayed in Table 1. There were no differences in the 24-h urine collection measures or in the dietary recall measures across the phases (Table 2).
Table 1.
Participant characteristics
| Age, yr | 26 ± 5 |
| Height, cm | 164 ± 4 |
| Body mass, kg | 64.6 ± 6.7 |
| Body mass index, kg/m2 | 24 ± 2 |
| Mean arterial pressure, mmHg | 87 ± 6 |
| Menstrual cycle length, days | 30 ± 3 |
| Day of LH surge | 15 ± 1 |
Means ± SD. All data are n = 12. LH, luteinizing hormone.
Table 2.
24-h pre-experimental visit
| Early Follicular | Late Follicular | Mid-Luteal | P Value | F Value | |
|---|---|---|---|---|---|
| Fluid intake, mL | 2,743 ± 1,293 | 2,184 ± 1,635 | 2,918 ± 1,717 | 0.431 | (F2, 22 = 0.87) |
| Urine volume, mL | 1,673 ± 985 | 3,122 ± 4101 | 1,820 ± 699 | 0.287 | (F1, 12 = 1.28) |
| USG, au | 1.012 ± 0.006 | 1.010 ± 0.004 | 1.010 ± 0.005 | 0.276 | (F1, 15 = 1.36) |
| Urine osmolality, mosmol/kg | 443 ± 220 | 402 ± 158 | 439 ± 149 | 0.646 | (F2, 16 = 0.34) |
| Urine sodium, mmol/L | 83 ± 45 | 91 ± 37 | 92 ± 40 | 0.637 | (F2, 22 = 0.46) |
| Dietary sodium, mg | 2,054 ± 980 | 2,166 ± 1134 | 1,953 ± 1637 | 0.786 | (F2, 19 = 0.24) |
| Dietary protein, g | 92 ± 27 | 77 ± 32 | 83 ± 40 | 0.475 | (F2, 18 = 0.75) |
| Dietary fat, g | 64 ± 25 | 61 ± 20 | 57 ± 34 | 0.715 | (F2, 16 = 0.29) |
| Dietary carbohydrate, g | 185 ± 61 | 182 ± 64 | 198 ± 82 | 0.757 | (F2, 17 = 0.25) |
Data were analyzed with one-way linear mixed model. USG, urine specific gravity. One-way repeated measures linear mixed model F- and P-values are reported, and data are presented as means ± SD. All data are n = 12.
Pre-Exercise Measures
Serum estradiol (F1, 14 = 13.48) and progesterone (F2, 17 = 19.67) differed across menstrual cycle phase (both one-way repeated measures linear mixed modes: P ≤ 0.001). Estradiol was lower during the EF phase compared with both the LF (P ≤ 0.012) and ML phases (P ≤ 0.001) but there were no differences between the LF and ML phases (P ≥ 0.891) (Table 3). As expected, progesterone was higher during the ML phase compared with the EF and LF phases (P ≤ 0.002) with no difference between EF and LF phases (P = 0.581). Testosterone did not differ between any of the phases (P = 0.209) (Table 3). There were no differences in pre-exercise cardiovascular and thermal measures (all one-way repeated measures linear mixed models: P ≥ 0.744), except for core temperature (F1, 13 = 4.33, P = 0.053), with pairwise comparisons revealing a lower core temp during the LF phase compared with the ML phase (P = 0.041) (Table 3). Pre-exercise water and electrolyte homeostasis measures did not differ across the menstrual cycle (all one-way repeated measures linear mixed models: P ≥ 0.072) between phases (Table 4). Likewise, nude body mass (one-way repeated measures linear mixed model: (F2, 19 = 0.62, P = 0.529) and hemoglobin mass (one-way repeated measures linear mixed model: (F2, 19 = 0.21, P = 0.780) did not differ between phases at pre-exercise measures. All data at screening and pre-exercise are n = 12 except for sex hormone measures where data are n = 11 due to nonphysiological hormone levels.
Table 3.
Pre-exercise sex hormone concentrations
| Early Follicular |
Late Follicular |
Mid-Luteal |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol, ng/mL | Progesterone, ng/mL | E2/P4 Ratio | Testosterone, ng/mL | Estradiol, ng/mL | Progesterone, ng/mL | E2/P4 Ratio | Testosterone, ng/mL | Estradiol, ng/mL | Progesterone, ng/mL | E2/P4 Ratio | Testosterone, ng/mL | |
| 1 | 36.1 | 0.4 | 90.4 | 1.3 | 104.7 | 0.4 | 259.3 | 1.3 | 86.1 | 4.4 | 19.7 | 1.2 |
| 2 | 72.7 | 0.4 | 190.8 | 0.9 | 161.6 | 0.4 | 405.1 | 0.8 | 148.6 | 9.0 | 16.6 | 1.1 |
| 3 | 75.7 | 0.3 | 280.3 | 1.7 | 65.8 | 0.2 | 307.4 | 1.8 | 91.5 | 0.5 | 183.4 | 1.8 |
| 4 | 67.2 | 0.6 | 111.0 | 1.5 | 107.7 | 0.5 | 215.8 | 1.5 | 137.0 | 6.4 | 21.3 | 1.6 |
| 5 | 58.5 | 0.5 | 107.7 | 1.3 | 100.9 | 0.5 | 220.3 | 1.4 | 146.5 | 7.7 | 19.0 | 1.3 |
| 6 | 84.4 | 0.2 | 449.0 | 1.4 | 224.8 | 0.4 | 642.2 | 2.1 | 137.0 | 9.0 | 15.2 | 2.2 |
| 7 | 74.9 | 0.4 | 184.1 | 1.9 | 79.0 | 0.2 | 389.3 | 1.6 | 128.3 | 0.3 | 403.6 | 2.0 |
| 8 | 49.9 | 0.3 | 154.3 | 1.2 | 100.3 | 0.5 | 200.9 | 1.2 | 95.7 | 4.4 | 21.8 | 1.0 |
| 9 | 87.5 | 0.5 | 174.2 | 1.7 | 95.1 | 0.5 | 196.4 | 1.8 | 143.5 | 12.7 | 11.3 | 1.8 |
| 10 | 58.9 | 1.9 | 31.1 | 1.2 | 105.4 | 10.7 | 9.9 | 1.5 | 106.4 | 11.9 | 8.9 | 1.3 |
| 11 | ||||||||||||
| 12 | 50.0 | 0.4 | 126.4 | 1.5 | 77.7 | 0.7 | 114.5 | 1.7 | 64.4 | 6.5 | 9.9 | 1.5 |
| Mean | 65.1 | 0.6# | 172.7 | 1.4 | 111.2* | 1.4# | 269.2 | 1.5 | 116.8* | 6.6 | 66.4 | 1.5 |
| SD | 15.9 | 0.5 | 112.1 | 0.3 | 45.0 | 3.1 | 167.3 | 0.4 | 29.1 | 4.1 | 122.7 | 0.4 |
| % BF | 31.6 ± 6.0 | 29.9 ± 5.1 | 31.6 ± 5.9 | |||||||||
All data are n = 12 except hormone levels due to nonphysiological hormone levels in one participant. E2/P4 Ratio = estradiol/progesterone ratio; % BF = percentage of body fat. Analyzed by one-way linear mixed model. *Significantly different from the EF phase (P ≤ 0.012). #Significantly different from the mid-luteal (ML) phase (P ≤ 0.002). Bold text (participant 10) highlights one woman where the peak increase in estradiol concentrations was likely missed in the late follicular (LF) phase. Italicized text (participants 3 and 7) highlights two women where the progesterone concentrations did not reach the expected absolute levels in the LF and ML phases.
Table 4.
Pre-exercise measures
| Early Follicular | Late Follicular | Mid-Luteal | P Value | F Value | |
|---|---|---|---|---|---|
| Cardiovascular and thermal measures |
|
||||
| Mean arterial pressure, mmHg | 87 ± 9 | 86 ± 7 | 86 ± 9 | 0.915 | (F1, 16 = 0.04) |
| Heart rate, beats/min | 62 ± 9 | 61 ± 11 | 65 ± 10 | 0.934 | (F2, 17 = 2.87) |
| Core temperature, °C | 37.1 ± 0.3 | 36.8 ± 0.6# | 37.2 ± 0.3 | 0.053 | (F1, 13 = 4.33) |
| Mean skin temperature, °C | 32.3 ± 0.8 | 32.5 ± 0.8 | 32.4 ± 0.9 | 0.744 | (F2, 19 = 0.28) |
| Water and electrolyte homeostasis measures | |||||
| Urine specific gravity, au | 1.004 ± 0.004 | 1.003 ± 0.005 | 1.005 ± 0.006 | 0.267 | (F2, 16 = 1.41) |
| Serum osmolality, mosmol/kg | 290 ± 6 | 288 ± 2 | 287 ± 5 | 0.382 | (F2, 30 = 0.99) |
| Urine osmolality, mosmol/kg | 174 ± 141 | 160 ± 154 | 234 ± 224 | 0.180 | (F1, 13 = 2.01) |
| Serum sodium, mmol/L | 141 ± 1 | 141 ± 1 | 140 ± 1 | 0.072 | (F2, 21 = 3.04) |
| Urine sodium, mmol/L | 57 ± 51 | 41 ± 29 | 47 ± 48 | 0.422 | (F1, 13 = 0.79) |
Data were analyzed with one-way linear mixed model. One-way repeated measures linear mixed model F- and P-values are reported, and data are presented as means ± SD #Significantly different from ML phase (P = 0.041). All data are n = 12.
Thermal Measures
WBGT (EF: 28 ± 0°C; LF: 28 ± 1°C; ML: 28 ± 1°C) inside the environmental chamber was not different between phases (one-way repeated measures linear mixed model: F2, 20 = 1.88, P = 0.181. Likewise, chamber temperature (F2, 17 = 2.07, P = 0.163) and relative humidity (F2, 22 = 2.29, P = 0.125) did not differ between phases. Core temperature was higher in ML versus EF phase during baseline rest (0 min) (P = 0.016) but not different at 30 min (P = 0.230) or 60 min (P = 0.191) into exercise. However, at 90 min, core temperature was higher again during the ML versus EF (P = 0.443), but this difference was not observed at 120 min into exercise (P = 0.834) and thereafter at 150 min (P = 0.519), 180 min (P = 0.507), 210 min (P = 0.164), and 240 min (P = 0.130). There were no differences in core temperature compared with the LF phase at baseline rest (P ≥ 0.180) (Fig. 2A). Although there was a significant main effect of time for mean skin temperature (Fig. 2B), there were no differences between phases at baseline rest (P ≥ 0.502) and the only difference was observed at 150 min when the ML was higher compared with both the EF and LF phases (P < 0.001) (Fig. 2B). There were no differences in the rate of metabolic heat production at hours 1, 2 and 4 (P ≥ 0.185) (Fig. 2C). However, metabolic heat production in the LF phase was slightly lower compared with the ML phase at hour 3 (P = 0.050) (Fig. 2C). Sweat rate did not differ between phases (EF: 0.3 ± 0.1 L/h; LF: 0.3 ± 0.1 L/h; ML: 0.3 ± 0.1 L/h) (one-way repeated measures linear mixed model: Fig. 2D).
Figure 2.

Core temperature (A), mean skin temperature (B), and metabolic heat production (C) throughout 4 h of exercise in the heat. Baseline resting data upon entry into the chamber is 0 min. Timepoints 60 min, 120 min, 180 min, and 240 min are resting measures while timepoints 30 min, 90 min, 150 min, and 210 min are exercise measures. Data are presented as means ± SD and were analyzed using a two-way linear mixed model with Sidak post hoc comparisons. P values from the two-way linear mixed model and post hoc comparisons are reported. D: total sweat rate throughout exercise in the heat are presented as individual values and analyzed using one-way linear mixed model with actual F and P values reported. All data are n = 12. #Significantly different from the ML phase (P ≤ 0.050). EF, early follicular; LF, late follicular; ML, mid-luteal.
Cardiovascular and Metabolic Measures
A significant main effect of time (F3, 35 = 63.56, P < 0.001) and phase (F2, 20 = 9.39, P = 0.002) was observed for heart rate (Fig. 3A) but pairwise comparisons indicated that heart rate was not different between phases throughout any timepoints spent in the environmental chamber (P ≥ 0.133) (Fig. 3A). There was also a significant main effect of time (F4, 38 = 18.28, P < 0.001) for mean arterial pressure and pairwise comparison identified a lower mean arterial pressure during the LF phase at 120 min compared to the EF phases (P = 0.038) but mean arterial pressure was not different at any other timepoints between the phases (P ≥ 0.199) (Fig. 3B).
Figure 3.

Heart rate (A) and mean arterial pressure (B) throughout 4 h of exercise in the heat. Baseline resting data upon entry into the chamber is 0 min. Timepoints 60 min, 120 min, 180 min, and 240 min are resting measures while timepoints 30 min, 90 min, 150 min, and 210 min are exercise measures. Data are presented as means ± SD and were analyzed using a two-way linear mixed model with Sidak post hoc comparisons. F and P values from the two-way linear mixed model and post hoc comparisons are reported. All data are n = 12. *Significantly different from the EF phase (P ≤ 0.038). EF, early follicular; LF, late follicular; ML, mid-luteal.
Perceptual Measures
A significant main effect of time (F4, 40 = 16.91, P < 0.001) and phase (F2, 20 = 5.61, P = 0.013) was observed for thermal sensation (Fig. 4A). Pairwise comparisons demonstrated that thermal sensation differed at 30 min between EF and ML phases (P = 0.005), at 90 min between the EF and LF phases (P = 0.042) and at 150 min between the LF and ML phases (P = 0.034) and the EF and ML phases (P = 0.051). There were otherwise no differences in pairwise comparisons between phases in thermal sensation (P ≥ 0.151). Although there was a significant main effect of time (F3, 31 = 8.17, P = 0.001) (Fig. 4B), pairwise comparisons of thermal comfort show no differences in ratings between phases at any chamber timepoints (P ≥ 0.278). A significant main effect of phase (F2, 21 = 3.90, P = 0.038) was observed for ratings of thirst (Fig. 4C) and pairwise comparisons showed lower thirst sensations in the LF phase compared with the ML phase at 210 min and 240 min (P ≤ 0.022) but no differences between phases were observed at any other timepoints (P ≥ 0.058). Ratings of perceived exertion were not different at any timepoints between any phase (P ≥ 0.237).
Figure 4.

Rating of thermal sensation (A), rating of thermal comfort (B), rating of thirst (C), and rating of perceived exertion (RPE) (D) throughout 4 h of exercise in the heat. Baseline resting data upon entry into the chamber is 0 min. Timepoints 60, 120, 180, and 240 are resting measures while timepoints 30, 90, 150, and 210 are exercise measures. Data are presented as means ± SD and were analyzed using a two-way linear mixed model with Sidak post-hoc comparisons. F and P values from the two-way linear mixed model and post-hoc comparisons are reported. All data are n = 12. *Significantly different from the EF phase (P ≤ 0.035). #Significantly different from the ML phase (P ≤ 0.030). EF, early follicular; LF, late follicular; ML, mid-luteal.
Hormone Measures
Pre-exercise plasma levels of copeptin during the EF phase (98.1 ± 29.9 pg/mL) and the LF phase (109.2 ± 38.7 pg/mL) were both lower compared with the ML phase (124.8 ± 41.8 pg/mL) (P ≤ 0.037). Similarly, post-exercise plasma copeptin during the EF phase (99.5 ± 31.5 pg/mL) and the LF phase (104.1 ± 36.5 pg/mL) remained lower compared with the ML phase (120.3 ± 42.0 pg/mL) (P ≤ 0.028). Pre-exercise plasma aldosterone during the EF phase (162.8 ± 92.8 pg/mL) and the LF phase (164.8 ± 68.1 pg/mL) were both lower compared with the ML phase (395.6 ± 294.0 pg/mL) (P ≤ 0.003). Similarly, post-exercise plasma aldosterone during the EF phase (182.1 ± 176.9 pg/mL) and the LF phase (148.3 ± 77.1 pg/mL) remained lower compared with the ML phase (349.3 ± 233.2 pg/mL) (P ≤ 0.038). Plasma renin activity showed a significant main effect of phase (F1, 13 = 5.2, P = 0.037) (Fig. 5C) but pairwise comparisons identified no statistical differences between any phases at pre-exercise (P ≥ 0.180) or post-exercise (P ≥ 0.109).
Figure 5.

Plasma copeptin (A), plasma aldosterone (B), and plasma renin (C) at pre-exercise and post-exercise during the EF, LF, and ML phases of the menstrual cycle. Data are presented as means ± SD and were analyzed using a two-way linear mixed model with Sidak post hoc comparisons. F and P values from the two-way linear mixed model and post hoc comparisons are reported. *Significantly different from the EF phase. $Significantly different from the LF phase (P ≤ 0.034). Data are n = 12. EF, early follicular; LF, late follicular; ML, mid-luteal.
Hydration Measures
Fluid temperature (EF:11.4 ± 1.1°C; LF: 11.1 ± 1.3°C; ML: 11.0 ± 1.5°C) was not different between any phase throughout the entire 4 h spent in the heat (one-way repeated measures linear mixed model: P = 0.174) nor was total fluid intake different between phases (EF: 1609 ± 919 mL; LF: 1902 ± 799 mL; ML: 1913 ± 671; F1, 15 = 1.80, P = 0.202) (Fig. 6, A and D). Total urine output also was not different between phases (EF: 833 ± 546 mL; LF: 905 ± 593 mL; ML: 940 ± 438; F2, 17 = 0.55, P = 0.543) (Fig. 6B). Percent changes in body mass from pre- to post-exercise were not different between phases (EF: −0.5 ± 0.9%; LF: −0.3 ± 0.9%; ML: −0.3 ± 0.7%; F2, 16 = 0.83, P = 0.417) (Fig. 6C). Although there was a significant main effect of time (F1, 11 = 13.54, P = 0.004) (Fig. 6E), pairwise comparisons revelated no differences in plasma volume at pre-exercise (P ≥ 0.291) or post-exercise (P ≥ 0.257) between any phase nor were there changes from pre- to post-exercise within each phase (P ≥ 0.102) (Fig. 6E).
Figure 6.

Total fluid intake (A), urine output (B), percent change in body mass (C), fluid intake (D) during each hour of exercise, plasma volume (E), and the percent change in plasma volume from pre- to post-exercise in the heat (F). Data are presented as means ± SD and data over time were analyzed using a two-way linear mixed model with Sidak post hoc comparisons. Other data was analyzed using a one-way linear mixed model. F and P values from the two-way linear mixed model and post hoc comparisons and one-way linear mixed model are reported. All data are n = 12. EF, early follicular; LF, late follicular; ML, mid-luteal.
Serum osmolality was lower at post-exercise during the ML phase compared to the LF phase (LF: 287 ± 3 mosmol/kg vs. ML: 283 ± 4 mosmol/kg; P = 0.014) but there was no difference between the ML and EF phases (EF: 286 ± 3 mosmol/kg, P = 0.225) (Fig. 7A). Moreover, serum osmolality decreased from pre-exercise to post-exercise in the ML phase (P = 0.046) but did not change in the EF (P = 0.076) or LF (P = 0.592) phases. Urine osmolality was not different between phases (main effect of phase: F2, 16 = 0.62, P = 0.502) and did not change over time (main effect of time: F1, 11 = 1.68, P = 0.221) (Fig. 7B). Free water clearance also did not differ between phases (main effect of phase: F2, 18 = 1.78, P = 0.199) or over time (main effect of time: F1, 11 = 4.28, P = 0.063) (Fig. 7C). There were no differences in serum sodium at pre-exercise (Table 4) but a higher serum sodium concentration was observed in the EF and LF phases compared with the ML phase post-exercise (EF: 141 ± 1 mmol/L; LF: 140 ± 1 mmol/L; ML: 139 ± 1 mmol/L; P ≤ 0.053). Urine sodium was lower at post-exercise in the LF and ML phases compared with the EF phase (EF: 66 ± 44 mmol/L; LF: 29 ± 21 mmol/L; ML: 33 ± 29 mmol/L; P ≤ 0.051) and there was no difference in pre- to post- in any phase (P ≥ 0.420). Finally, sodium clearance was greater in the EF phase compared to the ML phase at pre-exercise (EF: 1.6 ± 0.7 mmol/L vs. ML: 0.7 ± 0.6 mmol/L; P = 0.028) and post-exercise (EF: 1.0 ± 0.5 mmol/L vs. ML: 0.5 ± 0.2 mmol/L; P = 0.044). The LF phase was not different from the EF (P ≥ 0.098) or ML (P ≥ 0.272) phases at either timepoint. Sodium clearance decreased in the EF phase from pre- to post-exercise (P = 0.034) but there were no differences in the LF (P = 0.215) or ML (P = 0.337) phases from pre- to post-exercise.
Figure 7.

Serum osmolality (A), urine osmolality (B), free water clearance (C), serum sodium (D), urine sodium (E), and sodium clearance pre- to post-exercise in the heat (F). Data are presented as means ± SD and data over time were analyzed using a two-way linear mixed model with Sidak post hoc comparisons. F and P values from the two-way linear mixed model and post hoc comparisons are reported. All data are n = 12. *Significantly different from the EF phase (P = 0.033). #Significantly different from the ML phase (P ≤ 0.044). $Significantly different from the LF phase (P = 0.014). EF, early follicular; LF, late follicular; ML, mid-luteal.
DISCUSSION
In contrast with our hypothesis, ad libitum fluid and electrolyte intake and fluid output (sweat and urine loss) during physical work in the heat did not differ between the three distinct phases of the menstrual cycle. Thus, percent changes in body mass, an index of acute changes in total body water, did not differ between the EF, LF, and ML phases of the menstrual cycle (Fig. 6C). Although we observed the expected increases in aldosterone and copeptin, a surrogate for arginine vasopressin, during the ML phase pre-exercise (Fig. 5, A and B), this did not translate to differences in fluid intake or fluid conservation. To our knowledge, this is the first study to investigate the fluid balance responses (i.e., both fluid and electrolyte intake and output) to physical work in the heat within women across three phases of the menstrual cycle.
Fluctuations in sex hormones in women likely influence fluid and electrolyte balance in women (14, 36, 37). When estrogen and progesterone concentrations are elevated during the ML phase, studies have reported that plasma aldosterone and renin activity are both greater at rest (38) and during exercise (39) compared to the follicular phase. We observed elevations in plasma aldosterone pre- and post-exercise, and while we saw an effect of phase on plasma renin activity (PRA), pairwise comparisons did not reveal differences between phases (Fig. 5C), unlike previous studies (36, 40–42). That said, Szmuilowicz et al. (43) similarly observed a plasma renin activity-independent luteal phase increase in aldosterone and suggested that progesterone may directly influence adrenal aldosterone production, as progesterone receptor expression has been detected in adrenal capsular cells in female mice (44). In addition, progesterone is a precursor in the aldosterone biosynthetic pathway, and increased substrate taken up by the adrenal cells could contribute to elevated aldosterone production in the ML phase (43). However, the renin-angiotensin II-aldosterone system is one of the major pathways that regulate drinking (45–47) and this PRA-independent increase in aldosterone/absence of elevated PRA may be one reason why fluid intake was not influenced in this study.
The actions of augmented aldosterone to retain sodium were witnessed in our study. A lower sodium clearance was identified in the ML phase at both pre- and post-exercise (Fig. 7F) compared with the EF phase for the same total urine output, a phase in which aldosterone concentrations were increased. It should be noted, however, that these findings were likely impacted by the consumption of fluid with electrolytes, which promotes sodium and fluid retention, as opposed to fluid without electrolytes (e.g., water). Interestingly, we also observed a lower serum osmolality post-exercise in the ML phase compared to the LF phase (Fig. 7A). Vokes et al. (48) similarly demonstrated a downward resetting of the osmoreceptors during the luteal phase following a hypertonic saline infusion before water intake. A lowering of the osmotic threshold post-exercise for the same fluid intake could help explain why we saw a decrease in serum osmolality and not an increase in urine output or free water clearance during the ML phase. Furthermore, Stachenfeld et al. (12) found that the combined oral contraceptive pill (estrogen-progestin) elicited a lower plasma osmolality operating point represented by the osmotic regulation of arginine vasopressin and thirst, as they also did not observe effects on free water clearance in response to hypertonic saline infusion, dehydration, or rehydration. Consistent with this evidence, we observed that elevated copeptin levels were present before exercise in the ML phase, and thus, may have contributed to the lower serum osmolality post-exercise in the ML phase. On the other hand, we did not witness an influence of perceptual thirst and subsequently saw no changes in fluid intake throughout physical work in the heat during the ML phase.
Finally, it is well understood that thirst and AVP are also sensitive to volume stimuli detected via changes in baroreceptor activation and is another reason we suspected that exercise-induced fluid loss would lead to increases in thirst and ad libitum fluid intake. Given the modest change in body mass (<1%), it is reasonable that participants were not dehydrated enough to stimulate a response from AVP to increase thirst and recover the lost fluid. Furthermore, most of our participants consumed high amounts of fluid in the 24-h before arriving at the laboratory, which may have contributed to the findings that euhydration was well maintained. In fact, our participants never rated above “a little thirsty” according to their ratings of thirst (Fig. 3C), which stayed relatively consistent throughout the 4 h. Furthermore, roughly a 10% loss in plasma volume is required before any increase in either thirst or AVP is observed (12) and it is likely our participants prevented a loss in plasma volume by freely drinking (Fig. 6E), which therefore, prevented the increase in thirst.
Notably, we let the participants choose their preferred flavor of Gatorade Zero, which may have influenced the participants to drink more based on preferred palatability, as it has been shown to be a significant factor in rehydration (49–51). Furthermore, evidence suggests that the distinct hormonal profiles of the menstrual cycle may affect both olfactory and gustatory perception, which could alter palatability to drink more towards the end of the menstrual cycle (mid-late luteal phase) (52). Similarly, mood state has been shown to change based on menstrual phase status (53) and it’s possible that mood state may have altered the behavioral response to drink. Therefore, both palatability and mood could have influenced our results and the behavioral response on osmoregulation. However, the flavor of Gatorade Zero was the same in all phases of the menstrual cycle, and we ultimately observed no differences in fluid intake. Thus, it is likely that differences in mood and/or taste did not alter the behavior to drink in this case.
Potential Limitations
There are a few factors to consider when interpreting these findings. First, fluid was placed directly next to each participant when walking on the treadmill and at rest in the heat chamber. Convenient access to drink was used to mitigate confounding with other behavioral aspects (i.e., getting up or working to get a drink). In an external environment of physical labor, the degree of preoccupation for an individual is much greater than what our participants experienced in our study and may distract more from properly restoring fluid intake (54). This could help explain why participants continued to drink even when thirst ratings were low. The NIOSH guidelines recommend workers arrive at the worksite hydrated and that the worksite have cool fluids readily available (8). These recommendations guided our study design. However, we acknowledge that this may not always be what is encountered in the workplace where, for example, workers regularly arrive at the worksite dehydrated (6). Thus, our findings support that when following the NIOSH recommendations, the risk of dehydration is low in women across the menstrual cycle. However, they may not be readily translated to conditions in which adherence to the NIOSH recommendations is relatively poor.
Second, the environmental conditions and workload were chosen to mimic conditions experienced in a physical labor environment (8, 17, 18). Thus, our observations may not be representative of what might occur during higher-intensity exercise/physical labor or more severe environmental conditions.
Third, our a priori power analysis was conducted using AVP as there were no other studies, to our knowledge, that measured ad libitum fluid intake in a similar population across phases of the menstrual cycle. Thus, it is possible that we were underpowered with regard to one of our primary outcome variables (i.e., fluid intake). Nevertheless, the findings of the current study provide benchmark data from which future studies investigating the effects of the menstrual cycle on fluid balance can be appropriately designed.
Finally, while a strength of our study is including testing during three phases of the menstrual cycle, we could not prospectively identify the menstrual phase based on serum hormone concentrations, as this was only possible post hoc. Therefore, it is possible that we missed the primary peak of estradiol in the LF phase of one participant (Table 3, participant 10 highlighted in bold text). This may explain why we did not observe higher copeptin levels, as other studies would suggest (10, 14, 55, 56). Moreover, our reported estradiol values for the LF phase fall within the range closer to mid-follicular values (21). Notably, two of our subjects did not exhibit progesterone values within the range we would expect to observe during the ML phase (Table 3, participants 3 and 7 highlighted in italic text). Both participants did show an increase in progesterone during the ML phase but not to the anticipated magnitude based on population references (21, 22). We removed the data from participants 3, 7, and 10 from data analyses and the statistical inferences of our primary outcome variables (e.g., percent changes in body weight, fluid intake, etc.) did not change. That said, we acknowledge that these post hoc analyses may have been underpowered. Nevertheless, we elected to include these participants in the manuscript because they were eligible based on our a priori inclusion methodology (i.e., cycle length, luteinizing hormone surge, basal body temperature fluctuations) and because their inclusion did not modify the conclusions of this study. To this end, it is worth noting that multiple studies report that the observed intra-woman cycle variability can be classified as normal for a eumenorrheic-defined woman in good health based on our inclusion criteria (57–59). Most of the variation that occurs within a cycle is during the follicular phase (57, 58), which could explain why these three participants’ hormone levels were slightly off from the expected hormonal profiles of the late follicular and mid-luteal phases. Strikingly, studies have indicated that a within-women cycle length difference of greater than 7 days but less than 14 days is relatively common, with at least 40% of all participants regularly experiencing this variation (58, 60). Therefore, even with diligently tracked cycles and rigorous methods, it is very likely that significant between-cycle variations occur in up to 40% of eumenorrheic classified participants based on cycle length, LH surge, and basal body temperature. To circumvent this issue, future studies should either over-recruit (by upwards to 40%) to account for potential within-woman between-cycle differences in estradiol and progesterone concentrates or plan to test sex hormone levels the day of experimental testing (61). Unfortunately, given the time and cost-intensive nature of this study, over-recruitment was not feasible. Moreover, we do not have the capability for point-of-care assessment of estradiol or progesterone, nor are we aware whether such technology exists.
Perspectives and Significance
An increasing number of women are entering the manual labor workforce (2). Furthermore, with climate change, there is expected to be a greater incidence of occupational heat exposure (62). Thus, research is required to address whether biological sex is a risk factor for health and safety susceptibility during physical work in the heat (1). One potential factor that may modulate any potential differential impacts of sex on health and safety is dehydration. Importantly, despite acknowledgment that sex is a modifying factor for hydration during physical work in the heat, the current National Institute of Occupational Safety and Health (8), American College of Sports Medicine (ACSM) (23), and National Athletic Trainers Association (63) hydration recommendations do not directly address biological sex, while the female menstrual cycle is unmentioned as a potential modulator of fluid regulation. Our data indicate that the normal hormonal fluctuations that occur throughout the menstrual cycle do not alter the fluid balance, defined as a function of fluid in versus. fluid out, during physical labor in the heat among euhydrated eumenorrheic women. This indicates that in occupational heat stress recommendation-compliant scenarios, women can maintain fluid balance across the menstrual cycle when fluids are readily available, despite changes in sex hormone concentrations. As our participants simulated only half a workday and began work in a euhydrated state, a more comprehensive understanding of fluid balance and regulation in women during physical work in the heat is warranted. Additional studies are required to better understand the effect of biological sex on fluid balance during physical work in the heat and perhaps could repeat this protocol with ad lib intake only during rest periods during work shifts and/or post shift rehydration to address the lack of thirst in our protocol.
Conclusion
The present study demonstrates that ad libitum fluid intake during moderate-intensity physical work in the heat is not affected by natural fluctuations in estrogen and progesterone across the menstrual cycle in euhydrated eumenorrheic women. By extension, the loss of body water (sweat and urine loss) did not differ across the menstrual cycle. Although we observed elevated copeptin (AVP surrogate) and aldosterone during the ML phase of the menstrual cycle, this did not translate to differences in fluid balance or drinking behavior. Collectively, our results indicate that when women have free access to cool fluid during physical work in the heat, they respond similarly across all three phases to maintain fluid homeostasis across the menstrual cycle.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by the Indiana University Graduate School Grant, American College of Sports Medicine (ACSM) Foundation Carl V. Gisolfi Memorial Fund, and a grant from the National Institute of Occupational Safety and Health (R01OH011528).
DISCLOSURES
J.A.F. has received consultant fees from Orreco Ltd. and G.B. is employed by Orreco Ltd. Z.J.S. has received consultant fees from Otsuka Holdings Co., Ltd. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.A.F., T.B.B., B.J.M., S.J.C., R.F.C., and Z.J.S. conceived and designed research; J.A.F., C.S.G., R.A., T.B.B., and Z.J.S. performed experiments; J.A.F., T.B.B., G.B., and Z.J.S. analyzed data; J.A.F., T.B.B., G.B., T.M., B.J.M., S.J.C., R.F.C., and Z.J.S. interpreted results of experiments; J.A.F., T.B.B., and Z.J.S. prepared figures; J.A.F., T.B.B., and Z.J.S. drafted manuscript; J.A.F., C.S.G., R.A., T.B.B., G.B., T.M., B.J.M., S.J.C., R.F.C., and Z.J.S. edited and revised manuscript; J.A.F., C.S.G., R.A., T.B.B., G.B., T.M., B.J.M., S.J.C., R.F.C., and Z.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the subjects for participating in our study.
REFERENCES
- 1.World Health Organization. Why Gender and Health? (Online). Geneva: WHO, 2011. https://www.who.int/news-room/questions-and-answers/item/gender-and-health [Access date: Dec 2019]. [Google Scholar]
- 2.U.S. Bureau of Labor Statistics. Labor Force Statistics from the Current Population Survey (Online). United States Department of Labor, 2022. https://www.bls.gov/cps/ [Access April 2022]. [Google Scholar]
- 3. Cheuvront SN, Carter RI, Sawka MN. Fluid balance and endurance exercise performance. Curr Sports Med Rep 2: 202–208, 2003. doi: 10.1249/00149619-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 4. Rehrer NJ. Fluid and electrolyte balance in ultra-endurance sport. Sports Med 31: 701–715, 2001. doi: 10.2165/00007256-200131100-00001. [DOI] [PubMed] [Google Scholar]
- 5. Flouris AD, Dinas PC, Ioannou LG, Nybo L, Havenith G, Kenny GP, Kjellstrom T. Workers’ health and productivity under occupational heat strain: a systematic review and meta-analysis. Lancet Planet Health 2: e521–e531, 2018. doi: 10.1016/S2542-5196(18)30237-7. [DOI] [PubMed] [Google Scholar]
- 6. Piil JF, Lundbye-Jensen J, Christiansen L, Ioannou L, Tsoutsoubi L, Dallas CN, Mantzios K, Flouris AD, Nybo L. High prevalence of hypohydration in occupations with heat stress—Perspectives for performance in combined cognitive and motor tasks. PLoS One 13: e0205321, 2018. doi: 10.1371/journal.pone.0205321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheuvront SN, Kenefick RW. Dehydration: physiology, assessment, and performance effects. Compr Physiol 4: 257–285, 2014. doi: 10.1002/cphy.c130017. [DOI] [PubMed] [Google Scholar]
- 8. Jacklitsch BL, Williams WJ, Musolin K, Coca A, Kim J-H, Turner N. Occupational Exposure to Heat and Hot Environments. Atlanta, GA: Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 2016. [Google Scholar]
- 9. Stachenfeld NS, Gleim GW, Zabetakis PM, Nicholas JA. Fluid balance and renal response following dehydrating exercise in well-trained men and women. Eur J Appl Physiol Occup Physiol 72: 468–477, 1996. doi: 10.1007/bf00242277. [DOI] [PubMed] [Google Scholar]
- 10. Forsling ML, Akerlund M, Strömberg P. Variations in plasma concentrations of vasopressin during the menstrual cycle. J Endocrinol 89: 263–266, 1981. doi: 10.1677/joe.0.0890263. [DOI] [PubMed] [Google Scholar]
- 11. Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol (1985) 86: 1092–1096, 1999. doi: 10.1152/jappl.1999.86.3.1092. [DOI] [PubMed] [Google Scholar]
- 12. Stachenfeld NS. Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev 36: 152–159, 2008. doi: 10.1097/JES.0b013e31817be928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol (1985) 98: 1991–1997, 2005. doi: 10.1152/japplphysiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 14. Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Physiol 283: E711–E721, 2002. doi: 10.1152/ajpendo.00192.2002. [DOI] [PubMed] [Google Scholar]
- 15. Giersch GEW, Charkoudian N, Morrissey MC, Butler CR, Colburn AT, Caldwell AR, Kavouras SA, Casa DJ. Estrogen to progesterone ratio and fluid regulatory responses to varying degrees and methods of dehydration. Front Sports Act Living 3: 722305, 2021. [Erratum in Front Sports Act Living 4: 848595, 2022]. doi: 10.3389/fspor.2021.722305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spruce BA, Baylis PH, Burd J, Watson MJ. Variation in osmoregulation of arginine vasopressin during the human menstrual cycle. Clin Endocrinol (Oxf) 22: 37–42, 1985. doi: 10.1111/j.1365-2265.1985.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Occupational Safety and Health. Prevent Heat-Related Illness or Death of Outdoor Workers (Online). https://www.cdc.gov/niosh/docs/wp-solutions/2013-143/default.html.
- 18.OSHA-NIOSH. Protecting Workers from Heat Illness (Online). https://www.osha.gov/heat.
- 19. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 20.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 21. Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, McKee SA, Allen SS. Determining menstrual phase in human biobehavioral research: a review with recommendations. Exp Clin Psychopharmacol 24: 1–11, 2016. doi: 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elliott-Sale KJ, Minahan CL, de Jonge XAKJ, Ackerman KE, Sipilä S, Constantini NW, Lebrun CM, Hackney AC. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med 51: 843–861, 2021. doi: 10.1007/s40279-021-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS; American College of Sports Medicine. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 24. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43: 1575–1581, 2011. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 25. Giersch GEW, Morrissey MC, Katch RK, Colburn AT, Sims ST, Stachenfeld NS, Casa DJ. Menstrual cycle and thermoregulation during exercise in the heat: a systematic review and meta-analysis. J Sci Med Sport 23: 1134–1140, 2020. doi: 10.1016/j.jsams.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 26. Robach P, Boisson R-C, Vincent L, Lundby C, Moutereau S, Gergelé L, Michel N, Duthil E, Féasson L, Millet GY. Hemolysis induced by an extreme mountain ultra-marathon is not associated with a decrease in total red blood cell volume. Scand J Med Sci Sports 24: 18–27, 2014. doi: 10.1111/j.1600-0838.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- 27. Schlader ZJ, O'Leary MC, Sackett JR, Johnson BD. Face cooling reveals a relative inability to increase cardiac parasympathetic activation during passive heat stress. Exp Physiol 103: 701–713, 2018. doi: 10.1113/EP086865. [DOI] [PubMed] [Google Scholar]
- 28. Cramer MN, Jay O. Partitional calorimetry. J Appl Physiol (1985) 126: 267–277, 2019. doi: 10.1152/japplphysiol.00191.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab 96: 1046–1052, 2011. doi: 10.1210/jc.2010-2499. [DOI] [PubMed] [Google Scholar]
- 30. Foss JL, Constantini K, Mickleborough TD, Chapman RF. Short-term arrival strategies for endurance exercise performance at moderate altitude. J Appl Physiol (1985) 123: 1258–1265, 2017. doi: 10.1152/japplphysiol.00314.2017. [DOI] [PubMed] [Google Scholar]
- 31. Gore CJ, Bourdon PC, Woolford SM, Ostler LM, Eastwood A, Scroop GC. Time and sample site dependency of the optimized co-rebreathing method. Med Sci Sports Exerc 38: 1187–1193, 2006. doi: 10.1249/01.mss.0000222848.35004.41. [DOI] [PubMed] [Google Scholar]
- 32. Heinicke K, Wolfarth B, Winchenbach P, Biermann B, Schmid A, Huber G, Friedmann B, Schmidt W. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med 22: 504–512, 2001. doi: 10.1055/s-2001-17613. [DOI] [PubMed] [Google Scholar]
- 33. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 34. Riebe D, Maresh CM, Armstrong LE, Kenefick RW, Castellani JW, Echegaray ME, Clark BA, Camaione DN. Effects of oral and intravenous rehydration on ratings of perceived exertion and thirst. Med Sci Sports Exerc 29: 117–124, 1997. doi: 10.1097/00005768-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 35. Gagge AP, Stolwijk JAJ, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1: 1–20, 1967. doi: 10.1016/0013-9351(67)90002-3. [DOI] [PubMed] [Google Scholar]
- 36. Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL, Selected C. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol (1985) 91: 1893–1901, 2001. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 37. Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol (1985) 96: 1011–1018, 2004. doi: 10.1152/japplphysiol.01032.2003. [DOI] [PubMed] [Google Scholar]
- 38. De Souza MJ, Maresh CM, Maguire MS, Kraemer WJ, Flora-Ginter G, Goetz KL. Menstrual status and plasma vasopressin, renin activity, and aldosterone exercise responses. J Appl Physiol (1985) 67: 736–743, 1989. doi: 10.1152/jappl.1989.67.2.736. [DOI] [PubMed] [Google Scholar]
- 39. Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Regul Integr Comp Physiol 249: R186–R191, 1985. doi: 10.1152/ajpregu.1985.249.2.R186. [DOI] [PubMed] [Google Scholar]
- 40. Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol 13: 446–452, 2002. doi: 10.1681/ASN.V132446. [DOI] [PubMed] [Google Scholar]
- 41. Michelakis AM, Yoshida H, Dormois JC. Plasma renin activity and plasma aldosterone during the normal menstrual cycle. Am J Obstet Gynecol 123: 724–726, 1975. doi: 10.1016/0002-9378(75)90495-0. [DOI] [PubMed] [Google Scholar]
- 42. Sundsfjord JA, Aakvaag A. Plasma renin activity, plasma renin substrate and urinary aldosterone excretion in the menstrual cycle in relation to the concentration of progesterone and oestrogens in the plasma. Acta Endocrinol (Copenh) 71: 519–529, 1972. doi: 10.1530/acta.0.0710519. [DOI] [PubMed] [Google Scholar]
- 43. Szmuilowicz ED, Adler GK, Williams JS, Green DE, Yao TM, Hopkins PN, Seely EW. Relationship between aldosterone and progesterone in the human menstrual cycle. J Clin Endocrinol Metab 91: 3981–3987, 2006. doi: 10.1210/jc.2006-1154. [DOI] [PubMed] [Google Scholar]
- 44. Uotinen N, Puustinen R, Pasanen S, Manninen T, Kivineva M, Syvälä H, Tuohimaa P, Ylikomi T. Distribution of progesterone receptor in female mouse tissues. Gen Comp Endocrinol 115: 429–441, 1999. doi: 10.1006/gcen.1999.7333. [DOI] [PubMed] [Google Scholar]
- 45. Andersson B, Olsson K, Rundgren M. ADH in regulation of blood osmolality and extracellular fluid volume. JPEN J Parenter Enteral Nutr 4: 88–96, 1980. doi: 10.1177/014860718000400207. [DOI] [PubMed] [Google Scholar]
- 46. Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the rain of the rat. J Physiol 210: 457–474, 1970. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 48. Vokes TJ, Weiss NM, Schreiber J, Gaskill MB, Robertson GL. Osmoregulation of thirst and vasopressin during normal menstrual cycle. Am J Physiol Regul Integr Comp Physiol 254: R641–R647, 1988. doi: 10.1152/ajpregu.1988.254.4.R641. [DOI] [PubMed] [Google Scholar]
- 49. Burdon CA, Johnson NA, Chapman PG, O'Connor HT. Influence of beverage temperature on palatability and fluid ingestion during endurance exercise: a systematic review. Int J Sport Nutr Exerc Metab 22: 199–211, 2012. doi: 10.1123/ijsnem.22.3.199. [DOI] [PubMed] [Google Scholar]
- 50. Boulze D, Montastruc P, Cabanac M. Water intake, pleasure and water temperature in humans. Physiol Behav 30: 97–102, 1983. doi: 10.1016/0031-9384(83)90044-6. [DOI] [PubMed] [Google Scholar]
- 51. Armstrong LE, Hubbard RW, Szlyk PC, Matthew WT, Sils IV. Voluntary dehydration and electrolyte losses during prolonged exercise in the heat. Aviat Space Environ Med 56: 765–770, 1985. [PubMed] [Google Scholar]
- 52. Stanić Ž, Pribisalić A, Bošković M, Bućan Cvitanić J, Boban K, Bašković G, Bartulić A, Demo S, Polašek O, Kolčić I. Does each menstrual cycle elicit a distinct effect on olfactory and gustatory perception? Nutrients 13: 2509, 2021. doi: 10.3390/nu13082509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Freemas JA, Baranauskas MN, Constantini K, Constantini N, Greenshields JT, Mickleborough TD, Raglin JS, Schlader ZJ. Exercise performance is impaired during the mid-luteal phase of the menstrual cycle. Med Sci Sports Exerc 53: 442–452, 2021. doi: 10.1249/MSS.0000000000002464. [DOI] [PubMed] [Google Scholar]
- 54. Maughan R, Shirreffs S. Exercise in the heat: challenges and opportunities. J Sports Sci 22: 917–927, 2004. doi: 10.1080/02640410400005909. [DOI] [PubMed] [Google Scholar]
- 55. Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol (1985) 87: 1016–1025, 1999. doi: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 56. Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 274: R187–R195, 1998. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- 57. Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med 2: 83, 2019. doi: 10.1038/s41746-019-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs 35: 376–384, 2006. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 59. Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil 12: 77–126, 1967. [PubMed] [Google Scholar]
- 60. Creinin MD, Keverline S, Meyn LA. How regular is regular? An analysis of menstrual cycle regularity. Contraception 70: 289–292, 2004. doi: 10.1016/j.contraception.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 61. Janse DE Jonge X, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc 51: 2610–2617, 2019. doi: 10.1249/MSS.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 62.Intergovernmental Panel on Climate Change. IPCC, 2021: Summary for policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 2021, p. 3−32. [Google Scholar]
- 63. Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BSE, Roberts WO, Stone JA. National athletic trainers’ association position statement: fluid replacement for athletes. J Athl Train 35: 212–224, 2000. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.


