Keywords: aging, animal models, artery stiffening, pulse wave velocity, sex differences
Abstract
The aorta stiffens with aging in both men and women, which predicts cardiovascular mortality. Aortic wall structural and extracellular matrix (ECM) remodeling, induced in part by chronic low-grade inflammation, contribute to aortic stiffening. Male mice are an established model of aortic aging. However, there is little information regarding whether female mice are an appropriate model of aortic aging in women, which we aimed to elucidate in the present study. We assessed two strains of mice and found that in C57BL/6N mice, in vivo aortic stiffness (pulse wave velocity, PWV) was higher with aging in both sexes, whereas in B6D2F1 mice, PWV was higher in old versus young male mice, but not in old versus young female mice. Because the age-related stiffening that occurs in men and women was reflected in male and female C57BL/6N mice, we examined the mechanisms of stiffening in this strain. In both sexes, aortic modulus of elasticity (pin myography) was lower in old mice, occurred in conjunction with and was related to higher plasma levels of the elastin-degrading enzyme matrix metalloproteinase-9 (MMP-9), and was accompanied by higher numbers of aortic elastin breaks and higher abundance of adventitial collagen-1. Plasma levels of the inflammatory cytokines interferon-γ, interleukin 6, and monocyte chemoattractant protein-1 were higher in both sexes of old mice. In conclusion, female C57BL/6N mice exhibit aortic stiffening, reduced modulus of elasticity and structural/ECM remodeling, and associated increases in MMP-9 and systemic inflammation with aging, and thus are an appropriate model of aortic aging in women.
NEW & NOTEWORTHY Our study demonstrates that with aging, female C57BL/6N mice exhibit higher in vivo aortic stiffness, reduced modulus of elasticity, aortic wall structural and extracellular matrix remodeling, and elevations in systemic inflammation. These changes are largely reflective of those that occur with aging in women. Thus, female C57BL/6N mice are a viable model of human aortic aging and the utility of these animals should be considered in future biomedical investigations.
INTRODUCTION
The aorta is the primary blood vessel responsible for delivering blood from the heart to the systemic circulation (1). The aorta is a large artery that is, under normal physiological conditions, highly elastic and dampens the pulsatile blood flow ejected from the heart (1, 2). This dampening effect is protective of the microvasculature and target organs, particularly in organs that receive large volumes of blood flow and have delicate, low-resistance vascular beds, such as the brain and kidneys (1). With advancing age, increases in aortic stiffness, as assessed in vivo by the reference standard measure carotid-femoral pulse wave velocity (c-fPWV), occur in both men and women (3–6). This increase in stiffness directly contributes to the development of cardiovascular diseases (CVD) (3), and increases in c-fPWV can predict (4–7) future incidence of CVD. Furthermore, c-fPWV is an independent predictor of numerous age-related conditions in men and women, including cognitive decline (8, 9), chronic kidney disease (10, 11), and impaired glucose tolerance and type 2 diabetes mellitus (11–13).
Structural changes to the arterial wall largely contribute to the increase in aortic stiffness in vivo. Fragmentation and degradation of the arterial structural protein elastin and consequently decreased elastic energy lead to aortic dilation/enlargement and a compensatory increase in the deposition of the structural protein collagen that provides rigidity to the arterial wall (1–3, 14). Other age-related changes in the arterial structure include an increase in the thickness of the intimal and medial layers (1–3). These adverse, yet adaptive, arterial structural changes are in part due to an increase in inflammation that occurs with aging (1, 15).
As the demographics in the United States and other developed nations shift toward a more aged population (16, 17), the prevalence of CVD is projected to increase by 30–35% (18). Despite extensive research in this field, there is still a need for effective interventions that prevent or treat age-related aortic stiffening and aortic wall structural and extracellular matrix remodeling. Use of appropriate animal models in biomedical research is essential for various reasons including 1) screening interventions for safety and efficacy (19, 20) and 2) providing mechanistic insight that is difficult, or impossible, to obtain in humans (19–22).
A key feature necessary for the use of animal models in biomedical research is that the model must completely, or mostly, reflect the human condition (19). Despite data demonstrating that age-related aortic stiffening and CVD occur in both men and women (e.g., 2, 21, 22), the majority of research to date examining mechanisms of and treatments for age-related aortic stiffening have been conducted in male rodent models (23–29). Some recent studies have begun to examine mechanisms of arterial function and aging in female mice (30–33), but there is still a paucity of information regarding the utility of female mice as an appropriate model of age-related aortic stiffening and structural remodeling and their relevance to aortic aging in women, especially late in life.
The goals of this study were to first determine whether female mice are an appropriate model (i.e., reflective of the human condition) of age-related aortic stiffening in vivo (aortic PWV, the rodent corollary to c-fPWV in humans) using two mouse models that are commonly studied in the context of conditions and diseases of aging, the B6D2F1 and C57BL/6N strains (25, 34, 35). In the strain(s) that were reflective of the human condition, we next sought to determine whether in vivo aortic stiffening with aging is accompanied by and related to aortic wall structural and extracellular matrix remodeling and systemic inflammation. Finally, we aimed to determine whether there were any sex differences in these outcomes between young and old mice.
MATERIALS AND METHODS
Ethical Approval
All protocols and procedures for this study were submitted to and approved by the University of Colorado Boulder Institutional Animal Care and Use Committee (Protocol No. 08-03-LES-01 and 2618) and adhered to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (36).
Study Design and Experimental Animals
We conducted a cross-sectional study in young and old, male and female, B6D2F1 and C57BL/6N mice to determine whether female mice were a viable model of age-related changes in aortic stiffness, aortic wall structural and extracellular matrix remodeling, and systemic (plasma) inflammation that occur in humans. Mice were studied at the following ages based on the age of physical maturity in young mice and the age of median lifespan for each sex in old mice (34). Young (studied at 6 mo of age) male and female B6D2F1 mice (n = 6 or 7/sex) were obtained from Charles River (Wilmington, MA). Old male (studied at 31 mo of age; n = 9) and old female (studied at 28 mo of age; n = 9) B6D2F1 mice were obtained from the National Institute of Aging colony (maintained by Charles River). Young male and female (studied at 6–7 mo of age; n = 10/sex) C57BL/6N mice were obtained from Charles River, and old male (studied at 27 mo of age, n = 20) and female (studied at 29 mo of age, n = 20) C57BL/6N mice were obtained from the National Institute of Aging colony. C57BL/6N mice (7 old males and 7 old females) died before euthanasia because of natural aging processes, resulting in final group sizes of n = 13/sex. All female mice were virgin, and we did not control for estrous cycle to increase generalizability of our findings. Mice were given ≥4 wk to adjust to our facilities at the University of Colorado Boulder before any testing. Mice were group-housed with a 12-h:12-h light/dark cycle and given ad libitum access to pelleted and irradiated NIH-31 Open formula mouse/rat sterilizable diet (stored at room temperature, Teklad 7917; Envigo, Indianapolis, IN) and drinking water.
Experimental Procedures
Investigators were blinded to the age and sex of the mice for biochemical assessments and data analyses.
In vivo aortic stiffness.
Aortic stiffness was assessed in vivo as aortic PWV via Doppler ultrasonography (Doppler Signal Processing Workstation, Indus Instruments, Webster, TX) as previously described by our laboratory (26, 27, 37–39). Mice were placed supine on a heating pad under light inhaled isoflurane anesthesia (1.5–2.0%) in O2. Front and hind limbs were secured to electrodes to monitor heart rate, which was maintained between ∼400 and 500 beats/min. Doppler probes were placed on the transverse aortic arch and abdominal aorta. Three consecutive 2-s ultrasound tracings were recorded, and the time between the ECG R wave to the beginning of the Doppler signal (i.e., average pre-ejection time) was determined for each location. PWV (in cm/s) was calculated by dividing the distance between the two probes by the difference in the pre-ejection times of the aortic arch and abdominal aorta (timeabdominal – timearch).
Euthanasia and tissue collection.
Mice were euthanized using a method approved under the American Veterinary Medical Association guidelines (40). Mice were anesthetized under inhaled anesthesia (open-drop method) and euthanized via cardiac exsanguination. Whole blood was heparinized and centrifuged at 10,000 rpm (9,391 rcf) for 10 min at room temperature to obtain plasma, which was then stored at −80°C for analyses described in Plasma MMP-9 and Plasma proinflammatory cytokines/chemokines. The heart was removed, cleaned, and weighed. The aorta was excised and rinsed in physiological saline solution (PSS), cleared of perivascular adipose tissue, and sectioned and stored as described in Ex vivo aortic modulus of elasticity—elastin-dominant region, Aortic diameter and IMT, and Elastin breaks, media cell count, and adventitila collagen-1 abundance.
Ex vivo aortic modulus of elasticity—elastin-dominant region.
Two 1 mm segments of thoracic aorta were collected, stored in PSS, and frozen at −80°C. Aortic modulus of elasticity (resistance to deformation within the elastic region of the elastic modulus) in the elastin-dominant region was assessed via pin (force) myography (DMT, Arhaus, Denmark) as previously described (37, 41, 42). Briefly, thawed aorta rings were mounted onto two pins in a phosphate-buffered saline (PBS) bath heated to 37°C. After three rounds of prestretching (pins displaced to 1 mm), the aortic diameter was increased until a force of 1 mN was reached and then was incrementally increased by 50 μm every 3 min until the vessel reached mechanical failure. The force corresponding to each stretching interval was recorded and used to calculate stress and strain and to construct a stress-strain curve:
where d is diameter and di is the initial diameter, and
where L is one-dimensional load, H is intima-media thickness, and D is vessel length.
The borders of the low-force, elastin-dominant region of the stress-strain curve were established by fitting a seventh polynomial equation to the data (r2 > 0.99; RStudio, Boston, MA) and then computing the roots of the equation. The first root was considered the boundary between the very low-force region and the point at which elastin fibers are initially engaged, and the second root was considered the upper boundary of the elastin-dominant region (43, 44). The modulus of elasticity was defined as the slope of a linear equation fitted to the stress-strain data between the first and second roots where the curvature is ∼0.
Plasma MMP-9.
Abundance of plasma matrix metalloproteinase-9 (MMP-9) in plasma was assessed using a quantikine ELISA kit per the manufacturer’s instructions (R&D Systems, Minneapolis, MN; Cat. No. MMPT90).
Aortic diameter and IMT.
The aortic diameter and intima-media thickness (IMT) were assessed in 1-mm aortic rings that were frozen in optimal cutting temperature (OCT) compound (Tissue-Tek) in liquid nitrogen-cooled methylbutane and stored at −80°C until the time of cryostat sectioning (Leica CM1520, Leica biosystems, Weltzar, Germany). Sections (7 μm) were plated on microscope slides and imaged under a bright-field microscope (Nikon Eclipse TS100; 4× magnification), and ImageJ software (National Institutes of Health, Bethesda, MA; RRID:SCR_003070) was used to quantify the aortic diameter and IMT (45). The differentiation between the medial and adventitial layers was determined as the point between the regular banding patterns of the external elastic lamellae and the diffuse pattern of the adventitia.
Elastin breaks, media cell count, and adventitial collagen-1 abundance.
Sections (∼1 mm) of thoracic aorta were excised and frozen in OCT compound, as described in the Aortic diameter and IMT. Sections (7 μm; Leica CM1520) were plated on poly-l-lysine-coated microscope slides, fixed in 2% paraformaldehyde for 10 min, washed with PBS, and permeabilized for 15 min (0.1% Triton X-100). Slides were blocked with 5% donkey serum for 1 h in a humidified chamber, incubated with an anti-collagen-1 primary antibody, (1:200; Cat. No. 1310-01; RRID: AB_2753206; Southern Biotech, Birmingham, AL) for 1 h, washed with PBS, and incubated with a species-specific fluorescent secondary antibody (1:200; AlexaFluor 647; Invitrogen, Waltham, MA) for 30 min. Slides were washed, stained with DAPI (1:1,000; Cat. No. 62248; ThermoFisher, Waltham, MA) for 5 min, and cured overnight with Invitrogen ProLong Gold mounting media (Cat. No. P36934; Invitrogen). The slides were then imaged using an EVOS m7000 (Cat. No. AMF7000; ThermoFisher) fluorescence microscope at 20× magnification under identical conditions and analyzed using Invitrogen Celleste 5.0 Image Analysis Software (RRID:SCR_022692). Images for assessing elastin breaks were obtained between 470 and 525 nm, where the elastic lamellae autofluorescence, as previously described (46, 47). Elastin breaks were determined as points in which there were large discontinuous regions in the elastic lamellae. These breaks were confirmed across two to three images per animal to reduce the likelihood of determining an elastin break when the lamellae were merely out of the focal plane of the microscope. The number of elastin breaks was averaged across n = 2 or 3 samples/animal. Media cell count was obtained by automatically counting the number of cells (identified with DAPI) within the borders of the internal and external elastic lamellae. The adventitial abundance of collagen-1 was determined as the average intensity (in arbitrary units, AU) of the collagen-positive area across n = 4–6 samples/animal. Specificity of the collagen-1 antibody was determined using negative controls (secondary antibody-only conditions) in a subset of the samples.
Plasma proinflammatory cytokines/chemokines.
Levels of interleukin (IL)-6, interferon (IFN)-γ, monocyte chemoattractant protein (MCP)-1, tumor necrosis factor (TNF)-α, and IL-1β were determined in plasma samples using a custom mouse G-Series semiquantitative cytokine antibody array (Cat. No. AAM-CUST-GS; RayBiotech, Peachtree Corners, GA). Plasma was diluted 2× and loaded into each microplate well. The assay was performed per the manufacturer’s instructions. The array slides were shipped to the manufacturer for scanning and data extraction, and data were sorted using the Excel-based GSM5 S4 Software, which was provided by the manufacturer.
Arterial blood pressure.
Systolic blood pressure (SBP) and diastolic BP (DBP) were measured using the CODA noninvasive tail-cuff system (Kent Scientific, Torrington, CT) as we have previously described (27, 38, 39). The average pressure readings were calculated from 20 collection cycles (after 5 acclimatization cycles) obtained each day for 3 consecutive days.
Statistics
Power calculations were performed using G*power 3.1 (RRID: SCR_013726) for our primary outcome variable, aortic PWV. Previously, our laboratory obtained effect sizes of 1.35 when comparing aortic PWV between young and old male mice. With this effect size, n = 6 mice per condition were required to achieve 99% statistical power. Additional mice were studied in each group to ensure sufficient PWV traces were obtained and to account for age-related attrition.
Statistical analyses were conducted using GraphPad Prism version 9.4.0 (GraphPad Software, San Diego, CA; RRID:SCR_002798). Data were assessed for statistical outliers (ROUT test; Q = 1%), and outliers were excluded from final analyses. All variables were assessed using two-way (age × sex) analysis of variance (ANOVA) with Šidák’s post hoc test when a main effect was observed. Relations between specific variables were assessed using simple linear regression. Data incorporated into these regressions were first assessed for normal distribution (Shapiro–Wilk normality test) and were log-transformed to account for skewness when P < 0.05. Statistical significance was set to α = 0.05. Data are presented as means ± SD.
RESULTS
In Vivo Aortic Stiffness
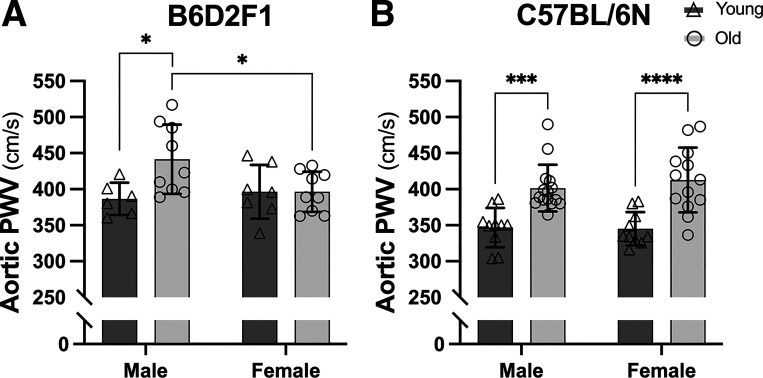
In B6D2F1 mice, we observed that aortic PWV was higher in males (P = 0.016 vs. young) but not in females (P > 0.999 vs. young) with aging (Fig. 1A), whereas both male and female C57BL/6N mice exhibited elevations in aortic PWV with aging (both P < 0.001 vs. young within sex; Fig. 1B). There were sex differences in aortic PWV between old B6D2F1 mice (P = 0.027), but no sex differences between young B6D2F1 (P = 0.866) or young (P = 0.992) and old (P = 0.629) C57BL/6N mice. Given that the age-related changes that occur in men and women were accurately reflected in male and female C57BL/6N mice, we further examined mechanisms of aortic stiffening in this strain.
Figure 1.
In vivo aortic stiffness is higher with aging in both male and female C57BL/6N mice, but only in male B6D2F1 mice. Aortic pulse wave velocity (PWV) in male and female, young (6–7 mo) and old (28–31 mo) B6D2F1 (A) and young (6–7 mo) and old (27–29 mo) C57BL/6N (B) mice. Data are represented as means ± SD; n = 6–15/group. Statistics are two-way mixed (age × sex) ANOVA with Šidák’s post hoc test. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Aortic Wall Extracellular Matrix and Structural Remodeling
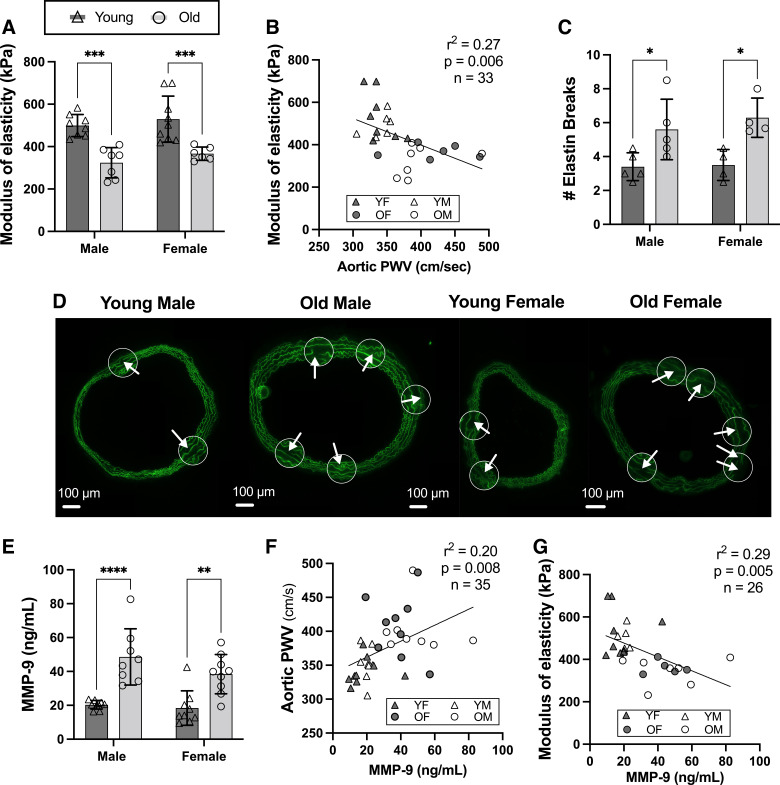
In both male and female C57BL/6N mice, we observed lower aortic modulus of elasticity with aging (Fig. 2A; both P < 0.001 vs. young within sex), which was inversely related to in vivo aortic stiffness (Fig. 2B; r2 = 0.27; P = 0.006) and accompanied by higher numbers of aortic elastin breaks (Fig. 2, C and D; both P < 0.03 vs. young within sex). In addition, plasma levels of a key elastin degrading enzyme, matrix metalloproteinase-9 (MMP-9) (48, 49), were elevated with aging (Fig. 2E; both P < 0.01 vs. young within sex), and MMP-9 levels were positively related to aortic stiffness (Fig. 2F; r2 = 0.20; P = 0.008) and inversely related to aortic modulus of elasticity (Fig. 2G; r2 = 0.29; P = 0.005). There were no sex differences in any of these outcomes (all P > 0.288).
Figure 2.
Aortic modulus of elasticity is lower and elastin breaks and matrix metalloproteinase-9 (MMP-9) are higher with aging in both male and female mice. In male and female, young (6–7 mo) and old (27–29 mo) C57BL/6N mice, aortic modulus of elasticity (elastin dominant, low-force region of the stress-strain curve) (A), aortic modulus of elasticity is inversely related to aortic stiffness (aortic pulse wave velocity, PWV) (B), number of aortic elastin breaks (C) with representative images below (D) [within each aortic ring, there are zoomed in visuals (white circles) and arrows denoting the elastin breaks], and plasma levels of MMP-9 (E), and plasma MMP-9 levels are positively related to aortic stiffness (PWV; F) and inversely related to aortic modulus of elasticity (G). Data are represented as means ± SD; n = 4–9/group unless otherwise noted. Statistics are two-way mixed (age × sex) ANOVA with Šidák’s post hoc test and simple linear regression. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
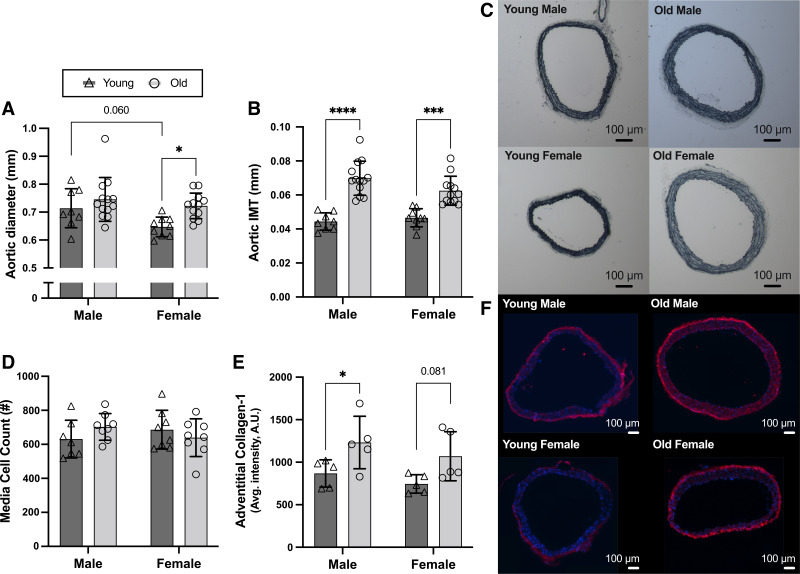
Aortic diameter tended to be higher in young males than young females (P = 0.060) and was higher in old versus young female mice (P = 0.016) but was not different between old versus young males (P = 0.444; Fig. 3, A and C). Aortic IMT was higher in both old versus young male and female mice (Fig. 3, B and C; both P < 0.001), likely due to medial cell hypertrophy (vs. hyperplasia), as there were no differences in medial cell count between male or female old versus young mice (Fig. 3D; both P > 0.352 vs. young), similar to previous reports (23). These structural changes were accompanied by a higher adventitial abundance of collagen-1 [primary isoform of collagen in arteries (50)] in both old male (Fig. 3, E and F; P = 0.049) and female mice (P = 0.081), with a similar average magnitude of age-related difference in both sexes. There were no sex differences in aortic IMT, medial cell count, nor adventitial abundance of collagen-1 (all P > 0.190).
Figure 3.
Aortic wall structural and extracellular matrix remodeling in male and female mice with aging. In male and female, young (6–7 mo) and old (27–29 mo) C57BL/6N mice, aortic diameter (A) and aortic intima-media thickness (IMT; B), measured in 7 μm segments of thoracic aorta; representative images of aortic diameter and IMT (C); media cell count using DAPI staining (D); and adventitial abundance of collagen-1 (E), measured via immunofluorescence in 7 μm segments of thoracic aorta; representative images of DAPI (blue) and collagen-1 (red) (F). Data are represented as means ± SD; n = 5–13/group. Statistics are two-way mixed (age × sex) ANOVA with Šidák’s post hoc test. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Circulating Markers of Inflammation
Circulating (plasma) levels of the proinflammatory cytokines/chemokines interferon-γ (IFN-γ), interleukin 6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) were elevated with aging in both male and female mice, whereas there were no effects of age on levels of interleukin-1β (IL-1β) or tumor necrosis factor-α (TNF-α) (Table 1). There was a significant interaction between age and sex for IL-1β, but there was no significant main effect of either age or sex (Table 1). There were no differences across sex for any of the other circulating inflammatory markers (Table 1).
Table 1.
Plasma cytokine/chemokine levels
| Male |
Female |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Cytokine | Young | Old | Young | Old | Age | Sex | Interaction |
| IFN-γ | 1,006 ± 607 | 2,128 ± 1,301 | 1,626 ± 1,033 | 2,432 ± 2,276 | 0.0373 | 0.3081 | 0.7264 |
| IL-1β | 354 ± 43 | 463 ± 114 | 516 ± 194 | 415 ± 135 | 0.9166 | 0.1953 | 0.0203 |
| IL-6 | 2,970 ± 724 | 6,320 ± 3,132 | 2,794 ± 572 | 8,261 ± 4,197 | <0.0001 | 0.3162 | 0.2309 |
| MCP-1 | 727 ± 134 | 1,088 ± 526 | 891 ± 131 | 1,427 ± 855 | 0.0121 | 0.1489 | 0.6118 |
| TNF-α | 583 ± 171 | 603 ± 165 | 648 ± 114 | 610 ± 176 | 0.4700 | 0.8624 | 0.5581 |
Values are means ± SD (n = 9–13/group) and are expressed as arbitrary units of intensity relative to an internal positive control. Plasma was analyzed by cytokine array. IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin 6; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-α. Statistics are two-way mixed (age × sex) ANOVA with Sidák’s post hoc test. Boldface indicates significant value.
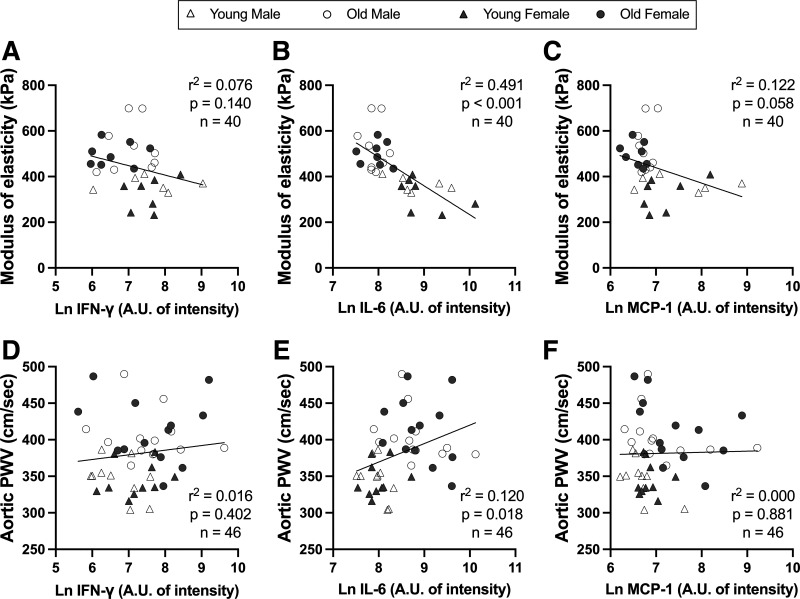
Relations were assessed between the aortic modulus of elasticity or in vivo stiffness and the circulating (plasma) markers of inflammation that were higher with aging (Table 1). Inflammatory markers were log-transformed to account for skewness (Shapiro–Wilk normality test: all P < 0.05). Aortic modulus of elasticity was inversely related to IL-6 (Fig. 4B; r2 = 0.491; P < 0.001) and MCP-1 (Fig. 4C; r2 = 0.122; P = 0.058). Aortic stiffness was positively associated with IL-6 (Fig. 4E; r2 = 0.120; P = 0.018). There were no relations between aortic modulus of elasticity and IFN-γ (Fig. 4A; r2 = 0.076; P = 0.140) or between aortic stiffness and IFN-γ (Fig. 4D; r2 = 0.016; P = 0.402) or MCP-1 (Fig. 4F; r2 = 0.000; P = 0.881).
Figure 4.
Relations between aortic modulus of elasticity and in vivo stiffness [pulse wave velocity (PWV)] and select inflammatory markers that were higher with aging. In male and female, young (6–7 mo) and old (27–29 mo) C57BL/6N mice, relations between aortic modulus of elasticity and plasma levels of interferon-γ (IFN-γ; A), interleukin-6 (IL-6; B), and monocyte chemoattractant protein-1 (MCP-1; C) and between aortic stiffness and plasma levels of IFN-γ (D), IL-6 (E), and MCP-1 (F); n = 10–14/group. Plasma was analyzed by cytokine array and reported as arbitrary units (AU) of intensity. All cytokines/chemokines were log-transformed to account for skewness (Shapiro–Wilk normality test; all P < 0.05). Statistics are simple linear regression.
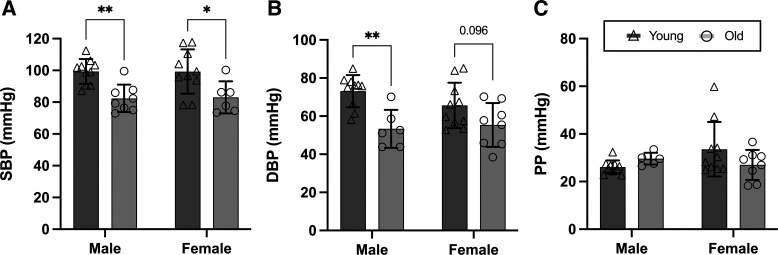
Blood Pressure
DBP was lower in old versus young male mice (Fig. 5B; P = 0.002) and tended to be lower in old versus young female mice (Fig. 5B; P = 0.096). SBP was lower in old versus young male and female mice (Fig. 5A; P < 0.001), and there was no effect of age on PP (Fig. 4C; P = 0.554) in either male or female mice. There were no sex differences in these outcomes (all P > 0.347).
Figure 5.
Systolic blood pressure (SBP) and diastolic BP (DBP) are lower in old vs. young male and female mice, and pulse pressure (PP) is not different. Tail-cuff BP in young (6–7 mo) and old (27–29 mo), male and female C57BL/6N mice: SBP (A); DBP (B); and PP (C). Data are represented as means ± SD; n = 6–10/group. Statistics are two-way mixed (age × sex) ANOVA with Šidák’s post hoc test. *P < 0.05; **P < 0.01.
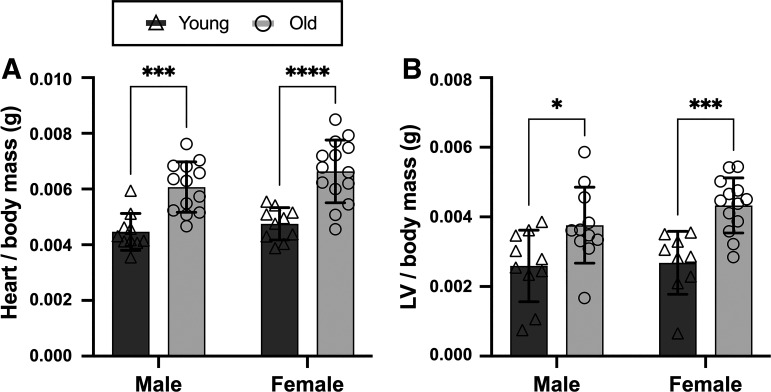
Heart and Left Ventricular Masses
These age-related aortic functional and structural changes were accompanied by elevations in the mass of the heart (Fig. 6A) and left ventricle (LV) (Fig. 6B) (normalized to body mass) in old versus young male and female mice (all P < 0.015). There were no sex differences in heart or LV mass (both P > 0.106).
Figure 6.
Heart and left ventricular (LV) masses are higher in old vs. young male and female mice. Ratio of heart (A) and left ventricular (B) masses to body mass in young (6–7 mo) and old (27–29 mo), male and female C57BL/6N mice is shown. Data are represented as means ± SD; n = 9–14/group. Statistics are two-way mixed (age × sex) ANOVA with Šidák’s post hoc test. *P < 0.05; ***P < 0.001; ****P < 0.0001.
DISCUSSION
Our results demonstrate that in vivo aortic stiffness, as assessed by aortic PWV, is higher in old versus young both male and female C57BL/6N mice, and in male but not female B6D2F1 mice. As such, female C57BL/6N mice appear to be an appropriate model of in vivo aortic stiffening with aging in women. Subsequently, in C57BL/6N mice, we explored whether mechanisms that contribute to aortic stiffening with aging were apparent in these mice, and observed reduced aortic modulus of elasticity, aortic wall structural and extracellular matrix remodeling, and increases in systemic inflammation in both sexes.
Female Mice in Vascular Aging Research
Aspects of CVD risk, trajectories, presentation, and responsiveness to/effectiveness of various cardiovascular-targeted interventions differ between men and women. Accordingly, organizations such as the National Institutes of Health, the American Heart Association, and the American Physiological Society have emphasized the need to consider sex as a biological variable to improve the quality and generalizability of biomedical research (51–56). Male mice have been established as a model of aortic aging, and the majority of vascular aging research (e.g., 23–27, 57, 58), and cardiovascular research in general (54, 55, 59), to date has been conducted solely using male mouse models. Recently, investigators have begun to examine sex differences in 1) mechanisms of vascular function using transgenic mouse models and 2) impairments in vascular function in middle to early late life (30–33). These previous studies have demonstrated that there are sex differences in normal arterial function and the time course of arterial aging in midlife, which can be attributed to sexually dimorphic arterial abundance of elastin and mineralocorticoid and estrogen receptors. We sought to contribute to this emerging body of literature by assessing changes in aortic function and structure in female mice in late life, their relevance to human aortic aging, and thus viability as a model for screening interventions for improving vascular aging in late life, which to our knowledge have not been thoroughly characterized. This study provides the necessary validation of female C57BL/6N mice as a model of aortic aging in women, which will aid the vascular aging research community in addressing important biomedical research questions that pertain to sex as a biological variable. Furthermore, our study demonstrates that female mice of different strains exhibit variations in aortic aging, suggesting strain-specific utility of mice as models for aortic aging in humans.
Trajectories of Aortic Aging in Humans and Mouse Models
In humans, c-fPWV is higher in older compared with young men and women, with a similar magnitude of increase with aging in both sexes (3, 60), although increases in c-fPWV may be delayed temporarily during midlife in women (57). We observed that the in vivo aortic stiffening that occurs with aging in men and women was reflected in male and female C57BL/6N mice, and in male but not in female B6D2F1 mice, suggesting that C57BL/6N mice are a more appropriate model of age-related aortic stiffening in humans. As such, we sought to determine if the mechanistic phenotypes, including aortic wall structural changes and systemic inflammation, that accompany and likely contribute to in vivo aortic stiffening were similar between old male and female C57BL/6N mice, and similar to those that occur in humans.
In humans, aortic compliance, which reflects the elastic properties of the artery, can be estimated in vivo (61). These data show reductions in aortic compliance with aging in both men and women, which occurs to a greater extent in men (2, 62). Mechanical and tensile properties of human arteries can be assessed ex vivo using numerous techniques (63) and collectively demonstrate altered mechanical properties (64–66) and reduced aortic elastic energy with aging (67). These studies did not report data separately by sex. We assessed the elastin dominant component of the aortic elastic modulus by determining the slope of the linear portion of the low-force region of the stress-strain curve (i.e., modulus of elasticity). Consistent with data in humans and previous findings in mice (37, 41, 44), modulus of elasticity in this region was lower with aging in the present study, and we did not observe any sex differences.
In line with the findings discussed earlier, we observed an inverse relation between aortic stiffness (PWV) and the low-force, elastin-dominant modulus of elasticity. The Moens–Korteweg equation predicts a positive relation between PWV and elastic modulus. However, elastic modulus in this equation is typically obtained from the high-force region of the stress-strain curve and may encompass artery stiffness that would not typically be observed within the normal physiological pressure range. Thus, we believe assessing the relation between PWV and the low-force region of the elastic modulus curve is relevant and does not contradict this equation and previous findings, but rather is an alternate way to compare these other relevant aspects of arterial mechanics and physiology.
Increased aortic stiffness and reduced modulus of elasticity are largely due to aortic wall structural remodeling (1, 3, 68, 69). Data in humans collected during biopsies and in postmortem tissues demonstrate that aortic elastin fragmentation/disorganization and collagen content increase with age (64, 70–73), consistent with what we observed in the present study. These data in humans are not reported separately by sex, and we did not observe any sex differences in these outcomes.
MMP-9 is a key enzyme that degrades elastin and is indicated in aortic stiffening (1, 74–78). Circulating levels of MMP-9 are higher with aging in male mice (79), whereas in humans, conflicting data have been reported concerning changes with aging and differences across sex (80–82). We observed an increase in circulating MMP-9 with aging in both male and female mice, which was positively related to aortic stiffness and inversely related to aortic modulus of elasticity, suggesting a potential role of MMP-9-mediated elastin degradation in increased aortic stiffness and reduced modulus of elasticity in the C57BL/6N mouse model.
In humans, aortic root diameter and IMT are higher with advanced age in both men and women, but aortic diameter is larger in men at any given age (2, 3, 83). Aortic wall thickness has been reported to be higher in men with aging, although this has not always been observed (2, 83, 84). We similarly observed an age-related increase in aortic diameter and IMT in male and female mice but did not observe any sex differences.
Serum concentrations of inflammatory cytokines are elevated with aging and associated with and/or implicated in the development of aortic stiffening in humans and male C57BL/6N mice (1, 27, 85–87). As such, we measured an array of circulating (plasma) inflammatory cytokines and observed elevated levels of IFN-γ, IL-6, and MCP-1 with aging in mice, reflecting the increase in systemic inflammation observed in humans with aging. Furthermore, aortic stiffness was positively related to IL-6, and aortic modulus of elasticity was inversely related to IL-6 and MCP-1, suggesting a potential role for systemic inflammation in mediating increases in stiffness and decreases in modulus of elasticity in the aging aorta of these mice, but future studies should investigate whether this relation is causal in both sexes.
Blood Pressure and Cardiac Hypertrophy
In humans, noninvasive assessments of brachial artery BP show that SBP increases and DBP is unchanged or decreases with aging, resulting in a widening of PP (3), and this increase in SBP is linked to arterial stiffening (88). In contrast, when using a noninvasive corollary of this measure (tail-cuff plethysmography) in C57BL/6N mice, we have not observed similar increases in SBP and PP with aging (27, 44), consistent with the present study. Direct, invasive intra-arterial assessments of BP are more precise and indicate age-related increases in SBP and PP in male C57BL/6N and transgenic mice (89, 90) and in male and female C57BL/6J mice (33). Therefore, we note that tail-cuff plethysmography may not accurately capture age-related changes in central blood pressure, which is a limitation of the study.
Increases in aortic stiffness and consequent increases in aortic impedance contribute to adverse cardiac remodeling, in particular left ventricular hypertrophy (91–93). We measured heart and left ventricular masses normalized to body mass and observed increases in both measures in male and female mice, consistent with what is observed with aging in men and women (94).
Experimental Considerations Regarding Sex Hormones
Sex hormones influence aspects of vascular function and aging. For example, PWV varies across the estrous cycle in young female C57BL/6N mice (95). We did not control for estrous cycle in the present study to increase the generalizability of our findings. However, it is possible that in our study, fluctuations in hormone levels in young female mice and consequent changes in PWV could have contributed to the lack of an age-related difference in PWV in female B6D2F1 mice.
In addition, our study examined mice during early adulthood and late life but did not include assessments in midlife. The estrous cycle of female mice becomes irregular and typically ceases in midlife (96), which may influence the timing of age-related changes in vascular function in a sexually dimorphic way. For example, female relative to male C57BL/6J mice have delayed aortic stiffening in midlife (12–18 mo of age) (31). However, intracarotid PWV is not different between male and female C57BL/6 mice at 13 mo of age (32), and aortic PWV is equivalent in male and female wild-type mice (unknown strain) at 24 mo of age (30). The age of female mice relative to follicular failure and potential sex differences in the timing of age-related changes in vascular function should be considered in future studies and when translating findings from mice to humans. These considerations are particularly relevant in the context of identifying mechanisms of or treatments for aortic/arterial aging in pre- versus postmenopausal women.
Conclusions
Our findings demonstrate that female C57BL/6N mice generally reflect the major aortic structural and functional changes that occur with aging in women, and therefore are an appropriate model for use in biomedical investigations. Future studies should consider the use of both male and female C57BL/6N mice in the context of identifying sex differences in mechanisms of aortic aging and identifying or developing treatment strategies for preventing or improving aortic aging.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health Grants T32 AG000279 (to A.G.L.), F31 HL160173 (to A.G.L.), F32 HL151022 (to Z.S.C), and K99 HL159241 (to Z.S.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G.L., M.C.Z., and Z.S.C. conceived and designed research; A.G.L., R.V., M.C.Z., A.J.L., S.A.M., N.T.G., N.S.V., and Z.S.C. performed experiments; A.G.L., A.J.L., K.R.L., and Z.S.C. analyzed data; A.G.L. and Z.S.C. interpreted results of experiments; A.G.L. prepared figures; A.G.L. drafted manuscript; A.G.L., R.V., M.C.Z., A.J.L., S.A.M., N.T.G., N.S.V., K.R.L., K.L.M., D.R.S., and Z.S.C.edited and revised manuscript; A.G.L., R.V., M.C.Z., A.J.L., S.A.M., N.T.G., N.S.V., K.R.L., K.L.M., D.R.S., and Z.S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jill Miyamoto-Ditmon and Monvi Kudumula for technical assistance. Graphical abstract was created using a licensed version of BioRender.
REFERENCES
- 1. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 74: 1237–1263, 2019. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols WW, O’Rourke MF, Vlachopoulo C. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles (6th ed.). London: CRC Press, 2011. [Google Scholar]
- 3. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res 128: 864–886, 2021. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 5. Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, Yamane K, Kohno N. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J 69: 259–264, 2005. doi: 10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- 6. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 7. Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 8. Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51: 99–104, 2008. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 9. Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 45: 494–501, 2005. doi: 10.1053/j.ajkd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11. Vasan RS, Pan S, Xanthakis V, Beiser A, Larson MG, Seshadri S, Mitchell GF. Arterial stiffness and long-term risk of health outcomes: the Framingham Heart Study. Hypertension 79: 1045–1056, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, Wu S. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res 127: 1491–1498, 2020. doi: 10.1161/CIRCRESAHA.120.317950. [DOI] [PubMed] [Google Scholar]
- 13. Tian X, Zuo Y, Chen S, Zhang Y, Zhang X, Xu Q, Wu S, Wang A. Hypertension, arterial stiffness, and diabetes: a prospective cohort study. Hypertension 79: 1487–1496, 2022. doi: 10.1161/HYPERTENSIONAHA.122.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res 110: 298–308, 2016. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 15. Zanoli L, Briet M, Empana JP, Cunha PG, Mäki-Petäjä KM, Protogerou AD, Tedgui A, Touyz RM, Schiffrin EL, Spronck B, Bouchard P, Vlachopoulos C, Bruno RM, Boutouyrie P; Association for Research into Arterial Structure, Physiology (ARTERY) Society, the European Society of Hypertension (ESH) Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries. Vascular consequences of inflammation: a position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J Hypertens 38: 1682–1698, 2020. doi: 10.1097/HJH.0000000000002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q 87: 842–862, 2009. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 18. Mohebi R, Chen C, Ibrahim NE, McCarthy CP, Gaggin HK, Singer DE, Hyle EP, Wasfy JH, Januzzi JL Jr.. Cardiovascular disease projections in the United States based on the 2020 census estimates. J Am Coll Cardiol 80: 565–578, 2022. doi: 10.1016/j.jacc.2022.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maurer KJ, Quimby FW. Animal models in biomedical research. In: Laboratory Animal Medicine (3rd ed.). American College Laboratory Animal Medicine (Academic Press), 2015, p. 1497–1534. [Google Scholar]
- 20. Barré-Sinoussi F, Montagutelli X. Animal models are essential to biological research: issues and perspectives. Future Sci OA 1: FSO63, 2015. doi: 10.4155/fso.15.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seals DR. Translational physiology: from molecules to public health. J Physiol 591: 3457–3469, 2013. doi: 10.1113/jphysiol.2013.253195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Human Genome Research Institute. Animal Models. https://www.genome.gov/genetics-glossary/Animal-Model [2021 Jun 18].
- 23. De Moudt S, Hendrickx JO, Neutel C, De Munck D, Leloup A, De Meyer GRY, Martinet W, Fransen P. Progressive aortic stiffness in aging C57Bl/6 mice displays altered contractile behaviour and extracellular matrix changes. Commun Biol 5: 605, 2022. doi: 10.1038/s42003-022-03563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie W, Ke Y, You Q, Li J, Chen L, Li D, Fang J, Chen X, Zhou Y, Chen L, Hong H. Single-cell RNA sequencing and assay for transposase-accessible chromatin using sequencing reveals cellular and molecular dynamics of aortic aging in mice. Arterioscler Thromb Vasc Biol 42: 156–171, 2022. doi: 10.1161/ATVBAHA.121.316883. [DOI] [PubMed] [Google Scholar]
- 25. Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol 285: H1464–H1470, 2003. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- 26. Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol 599: 911–925, 2021. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clayton ZS, Hutton DA, Brunt VE, VanDongen NS, Ziemba BP, Casso AG, Greenberg NT, Mercer AN, Rossman MJ, Campisi J, Melov S, Seals DR. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am J Physiol Heart Circ Physiol 321: H185–H196, 2021. doi: 10.1152/ajpheart.00118.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindesay G, Ragonnet C, Chimenti S, Villeneuve N, Vayssettes-Courchay C. Age and hypertension strongly induce aortic stiffening in rats at basal and matched blood pressure levels. Physiol Rep 4: e12805, 2016. doi: 10.14814/phy2.12805. doi: 10.14814/phy2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol 51: 263–271, 2011. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang TY, Zhao BJ, Wang T, Wang J. Effect of aging and sex on cardiovascular structure and function in wildtype mice assessed with echocardiography. Sci Rep 11: 22800, 2021. doi: 10.1038/s41598-021-02196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 320: H169–H180, 2021. doi: 10.1152/ajpheart.00262.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogola BO, Abshire CM, Visniauskas B, Kiley JX, Horton AC, Clark-Patterson GL, Kilanowski-Doroh I, Diaz Z, Bicego AN, McNally AB, Zimmerman MA, Groban L, Trask AJ, Miller KS, Lindsey SH. Sex differences in vascular aging and impact of GPER deletion. Am J Physiol Heart Circ Physiol 323: H336–H349, 2022. doi: 10.1152/ajpheart.00238.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hawes JZ, Cocciolone AJ, Cui AH, Griffin DB, Staiculescu MC, Mecham RP, Wagenseil JE. Elastin haploinsufficiency in mice has divergent effects on arterial remodeling with aging depending on sex. Am J Physiol Heart Circ Physiol 319: H1398–H1408, 2020. doi: 10.1152/ajpheart.00517.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. In: The Mouse in Biomedical Research (2nd ed.), edited by Fox JG. Burlington, MA: American College Laboratory Animal Medicine (Elsevier), 2007. [Google Scholar]
- 35. Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Research Council of the National Academics. The Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academic Press, 2011. [Google Scholar]
- 37. Casso AG, VanDongen NS, Gioscia-Ryan RA, Clayton ZS, Greenberg NT, Ziemba BP, Hutton DA, Neilson AP, Davy KP, Seals DR, Brunt VE. Initiation of 3,3-dimethyl-1-butanol at midlife prevents endothelial dysfunction and attenuates in vivo aortic stiffening with ageing in mice. J Physiol 600: 4633–4651, 2022. doi: 10.1113/JP283581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brunt VE, Casso AG, Gioscia-Ryan RA, Sapinsley ZJ, Ziemba BP, Clayton ZS, Bazzoni AE, VanDongen NS, Richey JJ, Hutton DA, Zigler MC, Neilson AP, Davy KP, Seals DR. Gut microbiome-derived metabolite trimethylamine N-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension 78: 499–511, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brunt VE, Greenberg NT, Sapinsley ZJ, Casso AG, Richey JJ, VanDongen NS, Gioscia-Ryan RA, Ziemba BP, Neilson AP, Davy KP, Seals DR. Suppression of trimethylamine N-oxide with DMB mitigates vascular dysfunction, exercise intolerance, and frailty associated with a Western-style diet in mice. J Appl Physiol (1985) 133: 798–813, 2022. doi: 10.1152/japplphysiol.00350.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Schaumburg, IL: American Veterinary Medical Association, 2020. [Google Scholar]
- 41. Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, Vázquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R, Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol 597: 2361–2378, 2019. doi: 10.1113/JP277336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol 295: H1451–H1459, 2008. doi: 10.1152/ajpheart.00127.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2018. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu X, Turcotte R, Seta F, Zhang Y. Micromechanics of elastic lamellae: unravelling the role of structural inhomogeneity in multi-scale arterial mechanics. J R Soc Interface 15: 20180492, 2018. doi: 10.1098/rsif.2018.0492. doi: 10.1098/rsif.2018.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen JZ, Sawada H, Moorleghen JJ, Weiland M, Daugherty A, Sheppard MB. Aortic strain correlates with elastin fragmentation in fibrillin-1 hypomorphic mice. Circ Rep 1: 199–205, 2019. doi: 10.1253/circrep.CR-18-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol 44-46: 224–231, 2015. doi: 10.1016/j.matbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lau AC, Duong TT, Ito S, Yeung RS. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arthritis Rheum 58: 854–863, 2008. doi: 10.1002/art.23225. [DOI] [PubMed] [Google Scholar]
- 50. Xu J, Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta 1842: 2106–2119, 2014. doi: 10.1016/j.bbadis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clayton JA, Gaugh MD. Sex as a biological variable in cardiovascular diseases: JACC Focus Seminar 1/7. J Am Coll Cardiol 79: 1388–1397, 2022. doi: 10.1016/j.jacc.2021.10.050. [DOI] [PubMed] [Google Scholar]
- 52. Shansky RM, Murphy AZ. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci 24: 457–464, 2021. doi: 10.1038/s41593-021-00806-8. [DOI] [PubMed] [Google Scholar]
- 53.National Institutes of Health. Consideration of Sex as a Biological Variable in NIH-funded Research (Online). https://orwh.od.nih.gov/sites/orwh/files/docs/NOT-OD-15-102_Guidance.pdf. [Last accessed, 17 Feb. 2023].
- 54. Jung RG, Stotts C, Makwana D, Motazedian P, Di Santo P, Goh CY, Verreault-Julien L, Simard T, Ramirez FD, Hibbert B. Methodological rigor in preclinical cardiovascular research: contemporary performance of AHA scientific publications. Circ Res 129: 887–889, 2021. doi: 10.1161/CIRCRESAHA.121.319921. [DOI] [PubMed] [Google Scholar]
- 55. Ramirez FD, Motazedian P, Jung RG, Di Santo P, MacDonald Z, Simard T, Clancy AA, Russo JJ, Welch V, Wells GA, Hibbert B. Sex bias is increasingly prevalent in preclinical cardiovascular research: implications for translational medicine and health equity for women: a systematic assessment of leading cardiovascular journals over a 10-year period. Circulation 135: 625–626, 2017. doi: 10.1161/CIRCULATIONAHA.116.026668. [DOI] [PubMed] [Google Scholar]
- 56. Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 57. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 58. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 59. Chang DH, Dumanski SM, Ahmed SB. Female sex-specific considerations to improve rigor and reproducibility in cardiovascular research. Am J Physiol Heart Circ Physiol 324: H279–H287, 2023. doi: 10.1152/ajpheart.00462.2022. [DOI] [PubMed] [Google Scholar]
- 60.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 31: 2338–2350, 2010. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pannier BM, Avolio AP, Hoeks A, Mancia G, Takazawa K. Methods and devices for measuring arterial compliance in humans. Am J Hypertens 15: 743–753, 2002. doi: 10.1016/s0895-7061(02)02962-x. [DOI] [PubMed] [Google Scholar]
- 62. Sonesson B, Hansen F, Stale H, Länne T. Compliance and diameter in the human abdominal aorta–the influence of age and sex. Eur J Vasc Surg 7: 690–697, 1993. doi: 10.1016/s0950-821x(05)80718-2. [DOI] [PubMed] [Google Scholar]
- 63. Camasão DB, Mantovani D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater Today Bio 10: 100106, 2021. doi: 10.1016/j.mtbio.2021.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giudici A, Li Y, Yasmin, Cleary S, Connolly K, McEniery C, Wilkinson IB, Khir AW. Time-course of the human thoracic aorta ageing process assessed using uniaxial mechanical testing and constitutive modelling. J Mech Behav Biomed Mater 134: 105339, 2022. doi: 10.1016/j.jmbbm.2022.105339. [DOI] [PubMed] [Google Scholar]
- 65. Taghizadeh H, Tafazzoli-Shadpour M. Characterization of mechanical properties of lamellar structure of the aortic wall: effect of aging. J Mech Behav Biomed Mater 65: 20–28, 2017. doi: 10.1016/j.jmbbm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 66. Amabili M, Asgari M, Breslavsky ID, Franchini G, Giovanniello F, Holzapfel GA. Microstructural and mechanical characterization of the layers of human descending thoracic aortas. Acta Biomater 134: 401–421, 2021. doi: 10.1016/j.actbio.2021.07.036. [DOI] [PubMed] [Google Scholar]
- 67. Jadidi M, Habibnezhad M, Anttila E, Maleckis K, Desyatova A, MacTaggart J, Kamenskiy A. Mechanical and structural changes in human thoracic aortas with age. Acta Biomater 103: 172–188, 2020. doi: 10.1016/j.actbio.2019.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pierce GL, Coutinho TA, DuBose LE, Donato AJ. Is it good to have a stiff aorta with aging? Causes and consequences. Physiology (Bethesda) 37: 154–173, 2022. doi: 10.1152/physiol.00035.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience 39: 7–18, 2017. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fritze O, Romero B, Schleicher M, Jacob MP, Oh DY, Starcher B, Schenke-Layland K, Bujan J, Stock UA. Age-related changes in the elastic tissue of the human aorta. J Vasc Res 49: 77–86, 2012. doi: 10.1159/000331278. [DOI] [PubMed] [Google Scholar]
- 71. Cattell MA, Anderson JC, Hasleton PS. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta 245: 73–84, 1996. doi: 10.1016/0009-8981(95)06174-6. [DOI] [PubMed] [Google Scholar]
- 72. Hosoda Y, Minoshima I. Elastin content of the aorta and the pulmonary artery in the Japanese. Angiology 16: 325–332, 1965. doi: 10.1177/000331976501600604. [DOI] [PubMed] [Google Scholar]
- 73. Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 39: 13–20, 1977. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 74. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology (Bethesda) 28: 391–403, 2013. doi: 10.1152/physiol.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yasmin, McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25: 372, 2005. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 76. Medley TL, Cole TJ, Dart AM, Gatzka CD, Kingwell BA. Matrix metalloproteinase-9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vasc Biol 24: 1479–1484, 2004. doi: 10.1161/01.ATV.0000135656.49158.95. [DOI] [PubMed] [Google Scholar]
- 77. Liu PY, Tsai WC, Lin CC, Hsu CH, Haung YY, Chen JH. Invasive measurements of pulse wave velocity correlate with the degree of aortic valve calcification and severity associated with matrix metalloproteinases in elderly patients with aortic valve stenosis. Clin Sci (Lond) 107: 415–422, 2004. doi: 10.1042/CS20040098. [DOI] [PubMed] [Google Scholar]
- 78. Wang M, Kim SH, Monticone RE, Lakatta EG. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 65: 698–703, 2015. doi: 10.1161/HYPERTENSIONAHA.114.03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12: 772–783, 2013. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, McClure CD, Finklea L, Spinale FG, Zile MR. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail 13: 530–540, 2007. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Trentini A, Manfrinato MC, Castellazzi M, Bellini T. Sex-related differences of matrix metalloproteinases (MMPs): new perspectives for these biomarkers in cardiovascular and neurological diseases. J Pers Med 12: 1196, 2022. doi: 10.3390/jpm12081196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paczek L, Michalska W, Bartlomiejczyk I. Trypsin, elastase, plasmin and MMP-9 activity in the serum during the human ageing process. Age Ageing 37: 318–323, 2008. doi: 10.1093/ageing/afn039. [DOI] [PubMed] [Google Scholar]
- 83. Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo SY, Liu TH, Ou DY, O'Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol 139: 1119–1129, 1991. [PMC free article] [PubMed] [Google Scholar]
- 84. Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH. Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population-based sample. J Vasc Surg 53: 950–957, 2011. doi: 10.1016/j.jvs.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 85. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J 53: 258–261, 2012. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 87. Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κ B signalling. J Hypertens 33: 2477–2482, 2015. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 64: 210–214, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H365–H375, 2016. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18: 1429–1433, 2012. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 92. Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation 94: 3362–3368, 1996. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 93. Milnor WR. Arterial impedance as ventricular afterload. Circ Res 36: 565–570, 1975. doi: 10.1161/01.res.36.5.565. [DOI] [PubMed] [Google Scholar]
- 94. Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: changes in structure and function. J Am Coll Cardiol 10: 42A–47A, 1987. doi: 10.1016/s0735-1097(87)80447-3. [DOI] [PubMed] [Google Scholar]
- 95. Kehmeier MN, Bedell BR, Cullen AE, Khurana A, D'Amico HJ, Henson GD, Walker AE. In vivo arterial stiffness, but not isolated artery endothelial function, varies with the mouse estrous cycle. Am J Physiol Heart Circ Physiol 323: H1057–H1067, 2022. doi: 10.1152/ajpheart.00369.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology 153: 3571–3578, 2012. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.