Keywords: descending pain modulation, electrophysiology, firing properties, inflammatory pain, vlPAG
Abstract
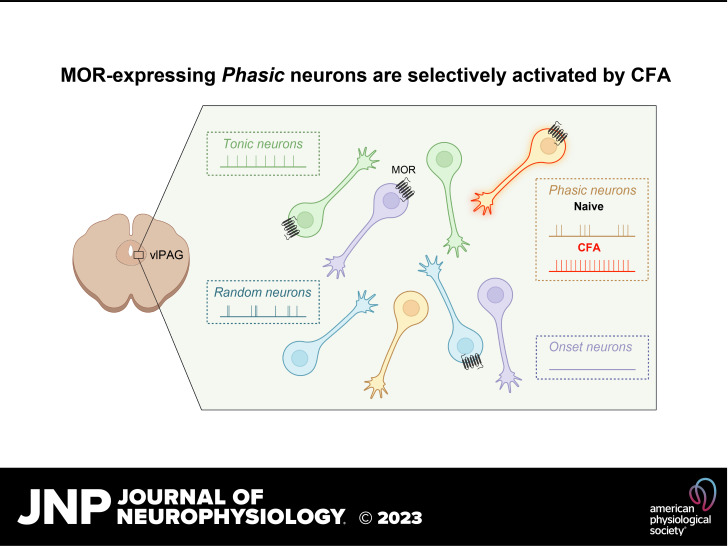
The ventrolateral periaqueductal gray (vlPAG) is a key brain area within the descending pain modulatory pathway and an important target for opioid-induced analgesia. The vlPAG contains heterogeneous neurons with respect to neurotransmitter content, receptor and channel expression, and in vivo response to noxious stimuli. This study characterizes intrinsic membrane properties of vlPAG neurons to identify neuron types that respond to inflammation and determine whether the pain-responsive neurons are inhibited by opioids. Surveying 382 neurons identified four neuron types with distinct intrinsic firing patterns: Phasic (48%), Tonic (33%), Onset (10%), and Random (9%). Mu-opioid receptor (MOR) expression was determined by the ability of a selective MOR agonist (DAMGO) to activate G protein-coupled inwardly rectifying potassium channel (GIRK) currents. Opioid-sensitive neurons were observed within each neuron type. Opioid sensitivity did not correlate with other intrinsic firing features, including low-threshold spiking that has been previously proposed to identify opioid-sensitive GABAergic neurons in the vlPAG of mice. Complete Freund’s adjuvant (CFA)-induced acute inflammation (2 h) had no effect on vlPAG neuron firing patterns. However, persistent inflammation (5–7 days) selectively activated Phasic neurons through a significant reduction in their firing threshold. Opioid-sensitive neurons were strongly activated compared with the opioid-insensitive Phasic neurons. Overall, this study provides a framework to further identify neurons activated by persistent inflammation so that they may be targeted for future pain therapies.
NEW & NOTEWORTHY Intrinsic firing properties define four distinct vlPAG neuron populations, and a subset of each population expresses MORs coupled to GIRK channels. Persistent, but not acute, inflammation selectively activates opioid-sensitive Phasic vlPAG neurons. Although the vlPAG is known to contribute to the descending inhibition of pain, the activation of a single physiologically defined neuron type in the presence of persistent inflammation represents a mechanism by which the vlPAG participates in descending facilitation of pain.
INTRODUCTION
The ventrolateral periaqueductal gray (vlPAG) is involved in many behavioral circuits associated with survival such as threat, fear, and pain, as well as vital autonomic functions like breathing, feeding, and respiration (1–4). vlPAG stimulation (electrical and chemical) induces strong antinociception in animals (5–7) and pain relief in humans (8–10), demonstrating that nonspecific activation of vlPAG neurons promotes descending inhibition of nociception. Early studies proposed that the analgesia produced by vlPAG stimulation occurs via activation of glutamatergic rostral ventromedial medulla (RVM)-projecting vlPAG neurons (11, 12). This hypothesis has been supported by more recent studies in which selectively activating glutamatergic vlPAG neurons with chemogenetics produced analgesia (13). Pharmacological studies blocking GABA receptors on vlPAG neurons determined that GABA promotes nociception (14, 15). GABAergic inputs from extrinsic afferents and intrinsic, locally projecting vlPAG GABAergic neurons target vlPAG neurons that project to the RVM (16). Chemogenetic activation of vlPAG GABAergic neurons confirms their ability to produce hyperalgesia (13) and suggests that a subset of vlPAG neurons can produce descending facilitation of nociception. These studies demonstrate that artificially manipulating separate populations of vlPAG neurons can promote descending inhibition or descending facilitation, prompting the important follow-up question of whether acute or chronic pain activates a specific population of vlPAG neurons.
Inflammation activates subsets of neurons within the vlPAG and downstream RVM as indicated by Fos expression (17–19); however, cellular heterogeneity within the vlPAG (20) prompts further investigation into the specificity of inflammation-mediated neuronal activation. RVM neurons have been characterized by whether they are activated (ON-cells) or inhibited (OFF-cells) by noxious stimuli in vivo, providing a useful framework for ongoing studies (21). Increased spontaneous firing of ON-cells and decreased firing of OFF-cells within the first 60 min after Complete Freund’s adjuvant (CFA) injection coincide with hyperalgesia (22). Importantly, RVM ON-cells selectively express postsynaptic mu-opioid receptors (MORs) (23), suggesting that opioids can directly inhibit RVM neurons that respond to noxious stimuli. In vivo electrophysiological studies in the vlPAG observe the same ON- and OFF-cells within the RVM (24, 25), with both neuron types showing sustained increases in spontaneous activity during the development of neuropathic pain (26). However, the in vivo approach is less fruitful in the vlPAG for several reasons, including lower spontaneous activity observed in the vlPAG because of inhibitory afferent drive limiting the feasibility of in vivo recordings (2, 24, 25), sparse direct vlPAG efferents to the dorsal horn limiting the functional implications of vlPAG ON- and OFF-cell firing (27), and heterogeneous MOR expression (28, 29).
Subpopulations of neurons can also be determined by characterizing intrinsic membrane and firing properties in vitro, providing a framework for identifying adaptations induced by specific stimuli, behaviors, or physiological states (i.e., pain) (30–36). Subpopulations of vlPAG neurons can be selectively activated by afferent inputs (35), opioids (37), cannabinoids (33), and inflammatory mediators (34), prompting us to ask whether inflammation activates the vlPAG broadly or whether a neuron subpopulation with specific intrinsic membrane properties is activated. Persistent inflammatory pain enhances GABA tone within the vlPAG of male rats (38) and neuropathic pain produces hypofunction of glutamatergic neurotransmission through pre- and postsynaptic mechanisms (39), but there is limited information regarding how pain influences the firing of vlPAG neurons. Furthermore, since the vlPAG is a key site for opioid-mediated analgesia with only a subpopulation of vlPAG neurons expressing MORs (28), it is valuable to determine the opioid sensitivity of any pain-activated population. Previous studies in mice determined that a subpopulation of tonic-firing GABAergic vlPAG neurons that exhibited T-type calcium channel-mediated low-threshold spiking (LTS) exclusively expressed postsynaptic MORs (40), providing additional intrinsic firing properties to use to investigate the selectivity of pain state-activated vlPAG neurons. To address these questions, the present study conducted a large-scale survey of the intrinsic membrane and firing properties of vlPAG neurons, determined distinct neuron types and their opioid sensitivity, and then used this baseline data to identify whether acute or persistent inflammation selectively activated subpopulations of vlPAG neurons.
METHODS
Animals
Female and male Long-Evans (LE) rats (from Harlan Laboratories and bred in house; 30–90 postnatal days for electrophysiology) were group-housed with environmental enrichment squares and toy bones. Lights were on a 12:12-h light-dark cycle, and food and water were provided ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Oregon Health & Science University. To determine whether in vitro neuron types observed in LE rats aligned with neuron types observed in Sprague-Dawley (SD) rats, which were used for previous studies in the laboratory, a subset of recordings (nSD = 70) used SD rats. The same neuron firing patterns existed in both LE and SD rats, with no strain differences in neuron type proportions (nLE = 331, nSD = 46; chi-square, P = 0.10). Subsequently, data for LE and SD rats were combined.
CFA Treatment
Rats were lightly anesthetized with isoflurane, and CFA (a heat-killed Mycobacterium tuberculosis in mineral oil, 1 mg/mL, 0.1-mL volume injected; Sigma-Aldrich) was injected subcutaneously into the plantar surface of the left hind paw. The CFA injection produced an intense tissue inflammation of the hind paw characterized by erythema, edema, and hyperalgesia (41). Rats were used 2 h or 5–7 days after injections (6 days for Fos immunohistochemistry).
Fos Immunohistochemistry
Male and female LE rats were deeply anesthetized with isoflurane and perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (PFA) either 2 h or 6 days after CFA injection in the hind paw. The brains were postfixed for an additional 24 h before being transferred and incubated in 30% sucrose in PBS at 4°C for 2–3 days. Brains were then frozen in dry ice and stored at 80°C until coronal brain slices (40 µm) were cut with a Leica CM2050 S cryostat. Sections were washed in PBS, blocked in 3% normal goat serum (NGS) in PBS with 0.25% Triton X-100 (PBS-Tx), and incubated for 24 h at 4°C with anti-Fos antibody (1:8,000 dilution; Cell Signaling Technology, catalog no. 2250) in blocking solution. Sections were then washed in PBS and incubated with biotinylated goat anti-rabbit secondary antibody (1:600 dilution; Vector Laboratories, catalog no. BA-1000) in PBS-Tx and 1% NGS for 2 h at room temperature. After another wash in PBS, sections were incubated in avidin-biotin-peroxidase complex (ABC Elite kit, PK-6100; Vector Laboratories) in PBS containing 0.5% Triton X-100. Finally, sections were developed in 3,3-diaminobenzidine (DAB) for 3–5 min (until uniform vlPAG staining occurred with minimal increase in background staining). Sections were then mounted onto chromalumgelatin-coated slides. After drying, the slides were dehydrated with a progression of alcohol (30%, 60%, 90%, 100%, 100% ethanol) followed by CitriSolv (Fisher Scientific) before coverslipping with Permount (Sigma-Aldrich). Bright-field images of immunoreactive neurons in the vlPAG were captured digitally with an Olympus BX51 fluorescence microscope at ×10 magnification. Regions of interest within the vlPAG were measured, and Fos-labeled neurons were manually quantified by an investigator blind to the treatment conditions, to give a Fos+ nuclei/mm2 value. The four sections per rat were averaged so that each rat represented an n = 1 for staining across the rostral-caudal extent of the ventrolateral column of the PAG.
Electrophysiology
Rats were anesthetized with isoflurane, and brains were quickly removed and immersed in ice-cold sucrose artificial cerebrospinal fluid (aCSF) containing the following (in mM): 75 NaCl, 2.5 KCl, 0.1 CaCl2, 6 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 2.5 dextrose, and 50 sucrose. Coronal slices containing the vlPAG were cut 220 µm thick with a vibratome (Leica Microsystems). Slices were placed in a holding chamber oxygenated with aCSF containing (in mM) 126 NaCl, 21.4 NaHCO3, 11.1 dextrose, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, and 1.2 NaH2PO4 and equilibrated with 95% O2-5% CO2 at 32°C until recording. Brain slices were placed into a recording chamber on an upright Zeiss Examiner Z1 and superfused with 32°C aCSF. Whole cell recordings were conducted with pipettes (3–4 MΩ) with KMeSO4 internal solution containing the following (in mM): 138 CH3KO4S, 10 HEPES, 10 KCl, 1 MgCl2, 1 EGTA, 0.3 CaCl2, 4 MgATP, and 3 NaGTP, pH 7.3–7.4. A junction potential of 15 mV was corrected for at the beginning of the experiments, and access was monitored throughout. During whole cell voltage-clamp recordings, neurons were held at −70 mV. During current-clamp recordings, no holding current was used. Data acquisition was completed with an Axopatch 200B Amplifier (Molecular Devices) at 5 kHz and low-pass filtered at 2 kHz. Currents were digitized with InstruTECH ITC-18 (HEKA), collected via AxoGraph. Data acquisition and analysis were completed with AxoGraph (AxoGraph Scientific). All analyses were blind to treatment.
Neuron Type Characterization
We recorded from 233 neurons in the vlPAG from naive and 148 neurons from CFA-treated LE and SD rats. The sex of the rats was confirmed for most of the neurons in the dataset (naive: nmale = 101 and nfemale = 121; CFA: nmale = 67 and nfemale = 81). The figures comparing features observed in naive and CFA conditions are shown with all naive data points included. However, since the original characterization done in naive rats was completed first, we compared data in CFA-treated rats to naive rats recorded within the same time frame. This ensured that 1) the effects when comparing all naive data points to CFA were not influenced by the larger n in the naive group and 2) we controlled for new breeder lines, housing, or handling stress, etc.
Firing patterns were evaluated with a series of depolarizing current steps in current clamp. Specifically, we used 2-s-long depolarizing steps from 0 pA to 120 pA in 20-pA increments, with a 1-s delay between steps. Only neurons with stable resting membrane potentials (RMPs) that exhibited action potentials that crossed 0 mV when depolarized by current step protocols were used for electrophysiological analysis. Spontaneous firing frequency was calculated as the total number of action potentials during the full 3-s recording with 0-pA current injection. The firing frequency during the 2-s-long depolarizing current steps was determined by dividing the total number of action potentials by the time spent firing; for some neurons this was the full 2-s current step, and for others this required manually measuring the time firing (from the firing threshold of the first action potential until the end of the afterhyperpolarization of the last action potential). LTS was assessed with a 500-ms-long hyperpolarizing current step (−50 pA), which either did or did not elicit a spike upon the offset of the current step. A short poststep latency was required to determine the presence of LTS and distinguish from cells that were spontaneously active.
Input resistance (Rin) was calculated with the steady-state current response from a −10-mV hyperpolarizing step in voltage clamp. Resting membrane potential (RMP) was determined by averaging the baseline traces before the series of depolarizing current steps. Capacitance values were calculated from a −10-mV hyperpolarizing voltage step with AxoGraph X. Firing threshold was determined with a series of 500-ms-long current steps starting with −50 pA and increasing in 10-pA increments up to 120 pA. The current injection where the first action potential occurred during the step was used for the firing threshold.
Opioid-Mediated G Protein-Coupled Inwardly Rectifying Potassium Channel Currents
G protein-coupled inwardly rectifying potassium channel (GIRK) current responses to MOR activation by DAMGO were evaluated in a subset of neurons that exhibited a stable baseline for 2–3 min before drug application, after completing the intrinsic membrane property protocols. Neurons were held at −70 mV in voltage clamp. After 2–3 min of a stable baseline, a maximal concentration of the selective MOR agonist DAMGO (5 µM) was superfused over the slice until the outward current reached a maintained, stable peak. The nonselective opioid receptor antagonist naloxone (10 µM) was then superfused until a steady return baseline was achieved. GIRK currents were measured as the difference between the peak of the current and an average of currents measured at baseline and reversal in the presence of naloxone. A neuron was considered opioid insensitive if there was no change in current induced by the agonist. In some experiments, GABAB-mediated GIRK currents were elicited with the GABAB agonist baclofen (20 µM) followed by antagonist CGP 35348 (10 µM). GIRK current traces were analyzed blind to the neuron type and treatment.
Loose-Patch Recordings
Pipettes with KMeSO4 internal solution (2–3 MΩ) were used to get a cell-attached loose-patch seal (42) to passively measure firing rates of intact, spontaneously active vlPAG neurons without altering the intracellular milieu. After 2–3 min of a stable baseline, the MOR agonist DAMGO (5 µM) was superfused over the slice for 4–5 min, followed by antagonist naloxone (10 µM). Traces were excluded from the data set if there was runup or rundown throughout the entire recording.
Experimental Design and Statistical Analysis
All data are expressed as means ± SE. Data were analyzed with Prism 9 (GraphPad Software). Each cell was considered an independent observation. It is important to note that we recorded cells from male and female animals in each data set, which are detailed in the figure legends. Differences between groups were assessed by ANOVA and t tests or Kruskal–Wallis test for nonparametric comparisons when appropriate (significance denoted with P values on graphs, in text, or in figure legends).
RESULTS
vlPAG Neurons Exhibit 4 Distinct Firing Patterns
To begin to define heterogeneity within the vlPAG, the firing pattern and associated intrinsic membrane properties were evaluated. Neurons within the vlPAG could be divided into four neuron types based on distinct firing patterns: Phasic, Tonic, Onset, and Random. Since the vlPAG is a sexually dimorphic region (19, 43–45), both female and male rats were used throughout the studies, but we observed no notable sex differences in the firing patterns, prevalence of identified neuron types, or the associated properties. Subsequently, the recordings from female and male rats were combined for analysis. The total number of cells from male and female rats for each data set are displayed in the figure legends.
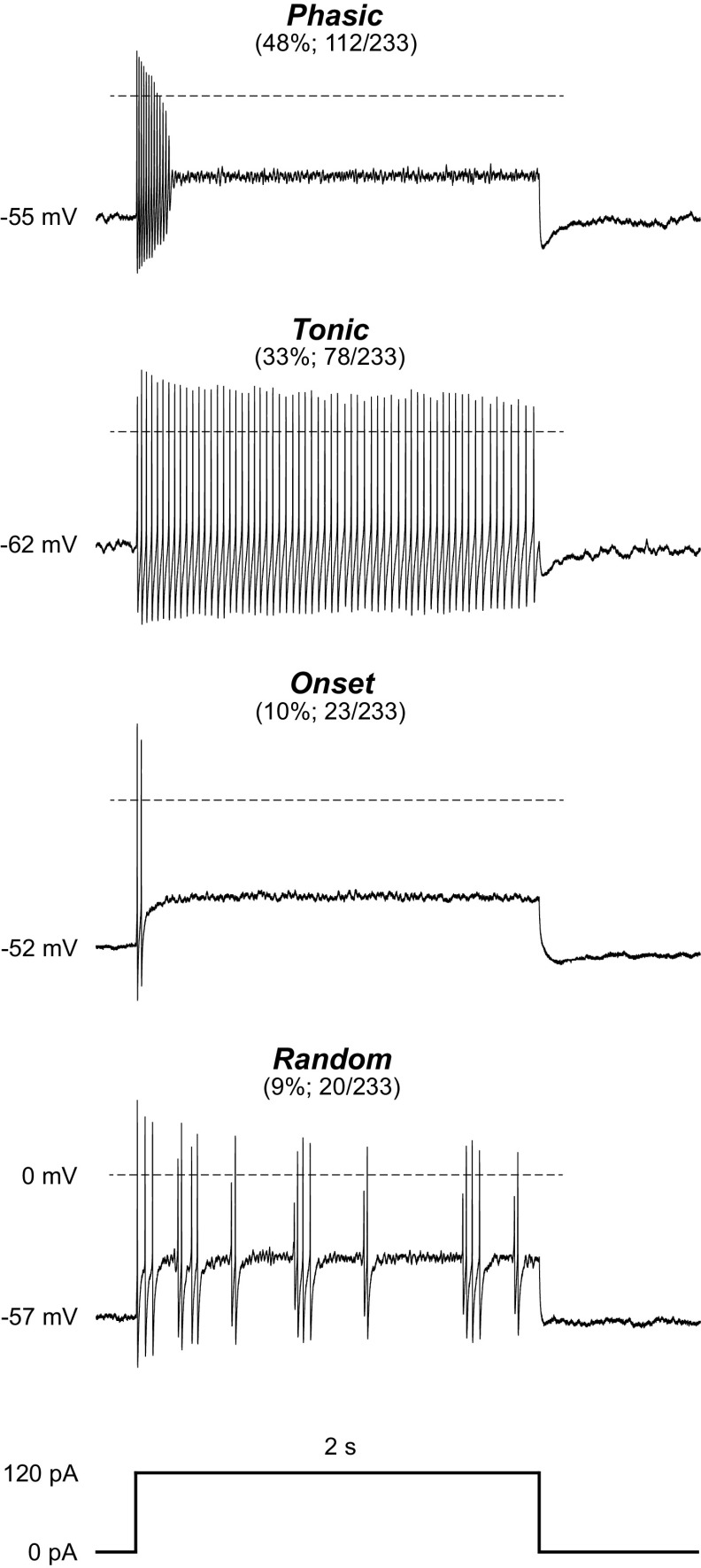
The firing patterns and prevalence of these four neuron types are shown in Fig. 1. Of the 233 neurons in naive rats, 112 (48%) displayed firing patterns where firing duration decreased as the stimulus intensity increased (Phasic). The second largest group (33%; 78/233) fired throughout each of the depolarizing steps above 20 pA (Tonic). Approximately 10% (23/233) of the neurons fired one or two action potentials at the beginning of each depolarizing step (Onset), and another 9% (20/233) displayed randomly timed action potentials throughout (Random). Notably, Random neurons do not appear to exhibit burst-firing spontaneously or in response to depolarizing current steps. Conversely, Phasic neurons can fire in distinct bursts either spontaneously or during depolarization. Because of the many sexually dimorphic features within the vlPAG it is also important to note that these neuron types were seen with comparable prevalence in naive males and females (nfemale = 121, nmale = 101; chi-square, P = 0.4237). Previous work in the laboratory has used SD rats, so we also examined intrinsic membrane properties from this strain and observed the same neuron types in similar proportions in naive rats: 57% Phasic (25/44), 18% Tonic (8/44), 11% Onset (5/44), and 14% Random (6/44).
Figure 1.
The ventrolateral periaqueductal gray (vlPAG) contains 4 neuron types defined by distinct firing patterns. Representative traces from vlPAG neurons from naive rats showing 4 distinct neuron types based on their response to a 2-s-long 120-pA depolarizing current step. The proportion of neurons exhibiting the firing pattern out of the total neurons recorded in naive rats and the resulting percentage are shown in parentheses. Resting membrane potential is noted at the beginning of each trace, and the dotted line represents 0 mV. One hundred twelve Phasic neurons were observed from 29 female (56 neurons) and 21 male (50 neurons) naive rats; 78 Tonic neurons were observed from 22 female (38 neurons) and 19 male (37 neurons) naive rats; 23 Onset neurons were observed from 9 female (15 neurons) and 6 male (7 neurons) naive rats; 20 Random neurons were observed from 8 female (12 neurons) and 5 male (7 neurons) naive rats. A subset of cells from Sprague-Dawley (SD) rats was grouped with the Long-Evans (LE) cells after showing no differences in firing patterns observed and prevalence of neuron types (nSD = 33, nLE = 200; chi-square = 6.34, df = 3, P = 0.096).
All neurons were evaluated for intrinsic membrane properties including resting membrane potential (RMP), membrane resistance (Rin), capacitance, and spontaneous firing activity (Table 1). All neurons have a high Rin averaging ∼1–2 GΩ. Comparisons between the two most prevalent firing patterns show that RMPs of Tonic neurons are more hyperpolarized compared with Phasic neurons and Tonic neurons have a significantly larger capacitance than Phasic neurons. These differences in intrinsic membrane properties further underscore the distinction between Phasic and Tonic neuron types based on firing patterns. The topographical distribution of each neuron type was evaluated for a large subset of neurons, showing no unique organization or clustering of the different types across the rostral-caudal axis of the vlPAG (n = 117; chi-square, P = 0.2284; most caudal vlPAG slice 1: 17/54 or 31% Phasic, 19/54 or 35% Tonic, 14/54 or 26% Onset, 4/54 or 7% Random; slice 2: 11/54 or 20% Phasic, 16/54 or 30% Tonic, 26/54 or 48% Onset, 1/54 or 2% Random; slice 3: 14/39 or 36% Phasic, 8/39 or 21% Tonic, 16/39 or 41% Onset, 1/39 or 3% Random; most rostral vlPAG slice 4: 6/18 or 33% Phasic, 5/18 or 28% Tonic, 5/18 or 28% Onset, 2/18 or 11% Random).
Table 1.
Electrophysiological properties of 4 neuron types in naive rats
| Phasic | Tonic | Onset | Random | |
|---|---|---|---|---|
| Resting membrane potential, mV | −55 ± 0.6 | −62 ± 0.9 | −52 ± 1.2 | −57 ± 1.7 |
| Membrane resistance, GΩ | 2.2 ± 0.3 | 1.6 ± 0.2 | 1.8 ± 0.4 | 0.8 ± 0.1 |
| Capacitance, pF | 42 ± 1.4 | 67 ± 3.5 | 49 ± 4.8 | 64 ± 6.8 |
| Spontaneous firing | 65% (73/112) | 94% (73/78) | 0% (0/23) | 37% (7/20) |
Values are means ± SE. Number of neurons recorded (ntotal = 233; nmale = 101, nfemale = 121): Phasic (ntotal = 112; nmale = 50, nfemale = 56), Tonic (ntotal = 78; nmale = 37, nfemale = 38), Onset (ntotal = 23; nmale = 7, nfemale = 15), and Random (ntotal = 20; nmale = 7, nfemale = 12). Significant differences were identified in resting membrane potential (1-way ANOVA, F3,227 = 19.45, P < 0.0001; Tukey’s multiple comparisons; Phasic vs. Tonic, P < 0.0001, Tonic vs. Onset, P < 0.0001, Onset vs. Random, P < 0.04), with no significant differences in resting membrane potential between males and females among these neuron types (2-way ANOVA, F1,212 = 2.12, P = 0.15). No significant differences were observed in the membrane resistance of these neuron types either when grouping males and females together (F3,219 = 2.54, P = 0.06) or when looking at sex as a factor (F1,205 = 0.02, P = 0.88). Differences in capacitance were identified (F3,218 = 18.49, P < 0.0001; Tukey’s multiple comparisons test; Phasic vs. Tonic, P < 0.0001, Phasic vs. Random, P = 0.001, and Tonic vs. Onset, P = 0.01), with no further differences between males and females (F1,202 = 0.03, P = 0.86). Spontaneous firing is given as an overall percentage, with the exact number of neurons firing at rest over the total number of that type of neuron shown in parentheses.
Intrinsic Firing Patterns and Membrane Properties of Phasic and Tonic Neurons
The two most prevalent neuron types represented in our data set, Phasic and Tonic neurons, have substantial differences in spontaneous activity, firing patterns, and frequency in response to depolarizing current steps. The majority of Phasic neurons were spontaneously active (71/103, 69%; Table 1) and either fired evenly spaced action potentials or shorter bursts of action potentials with pauses of variable duration (Fig. 2A, bottom trace).
Figure 2.
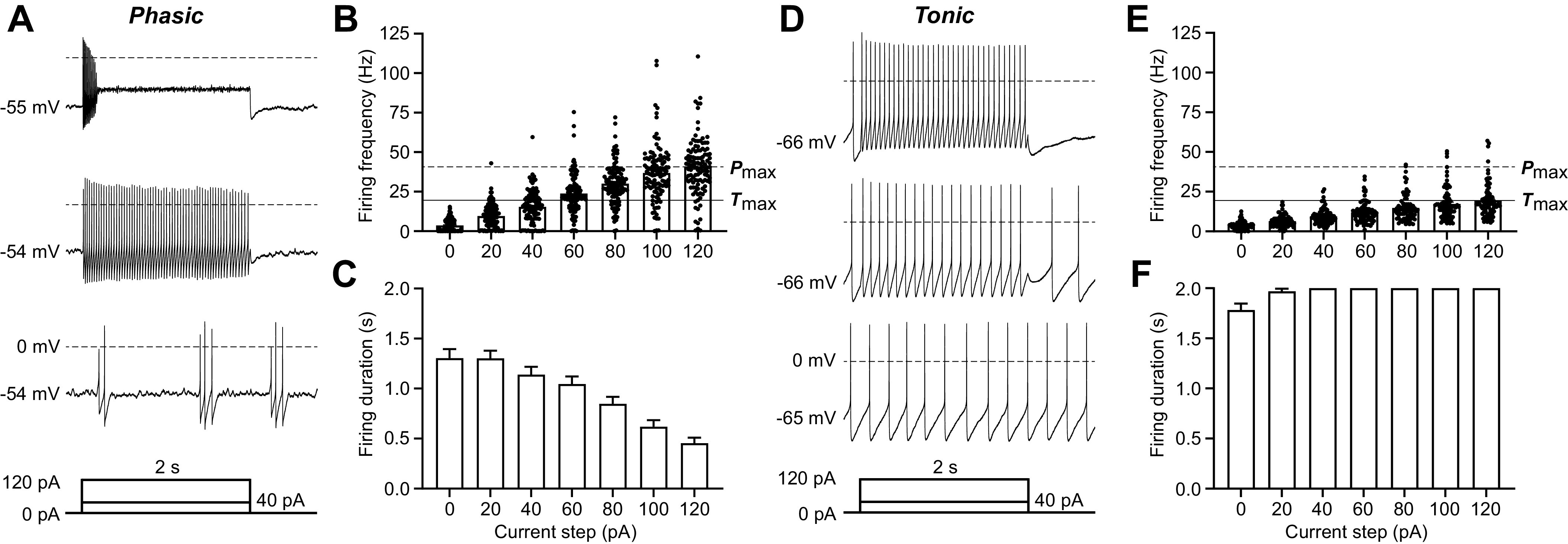
Defining features of the 2 most common ventrolateral periaqueductal gray (vlPAG) neuron types: Phasic and Tonic neurons. A: representative trace of a recording from a Phasic vlPAG neuron from a naive rat in response to 0-pA (bottom trace), 40-pA (middle trace), and 120-pA (top trace) current injections. Current injections were 2 s long after a 50-ms delay, followed by 250-ms return to baseline (as shown in the current protocol schematic below the traces). B: firing frequency of all Phasic neurons for current steps ranging from 0 pA to 120 pA in 20-pA increments (ntotal = 112; nfemale = 56, nmale = 50). The dashed line labeled Pmax indicates the average firing frequency at the maximally depolarizing current step (120 pA) for Phasic neurons. The solid line labeled Tmax indicates the average firing frequency at the maximally depolarizing current step (120 pA) for Tonic neurons. C: total firing duration of all Phasic neurons throughout each of the 2-s depolarizing current steps (ntotal = 112; nfemale = 56, nmale = 50). D: representative trace of a recording from a Tonic vlPAG neuron from a naive rat in response to 0-pA (bottom trace), 40-pA (middle trace), and 120-pA (top trace) current injections. E: firing frequency of all Tonic neurons for current steps ranging from 0 pA to 120 pA in 20-pA increments (ntotal = 78; nfemale = 38, nmale = 37). F: compiled data showing the total firing duration of all Tonic neurons throughout each of the 2-s depolarizing current steps (ntotal = 78; nfemale = 38, nmale = 37).
Phasic neurons increased in firing frequency from an average of 4 Hz with 0-pA current injection to an average of 41 Hz in response to the maximal current injection of 120 pA (indicated by Pmax; Fig. 2B); however, the firing duration decreased and action potential amplitudes became attenuated as the depolarizing steps increased in intensity (Fig. 2, A, top trace, and C). The decrease in firing duration and attenuation in action potential amplitude were also observed with repeated shorter (200 ms) current steps of 60 pA, 120 pA, and 180 pA (data not shown). In contrast, a higher percentage of Tonic neurons were spontaneously active with a comparable average firing frequency of 4 Hz (71/77, 92%; Table 1) and reached a lower average firing rate of 20 Hz (indicated by Tmax) in response to the maximally depolarizing current step of 120 pA (Fig. 2, D and E). Tonic neurons were defined by stable, evenly spaced firing for the full duration of each current step (Fig. 2F), which was also observed in response to shorter, repeated current steps (data not shown). Tonic neurons exhibited significantly slower firing frequency in response to depolarization compared with Phasic neurons (2-way ANOVA, effect of neuron type, F1,187 = 72.17, P < 0.0001).
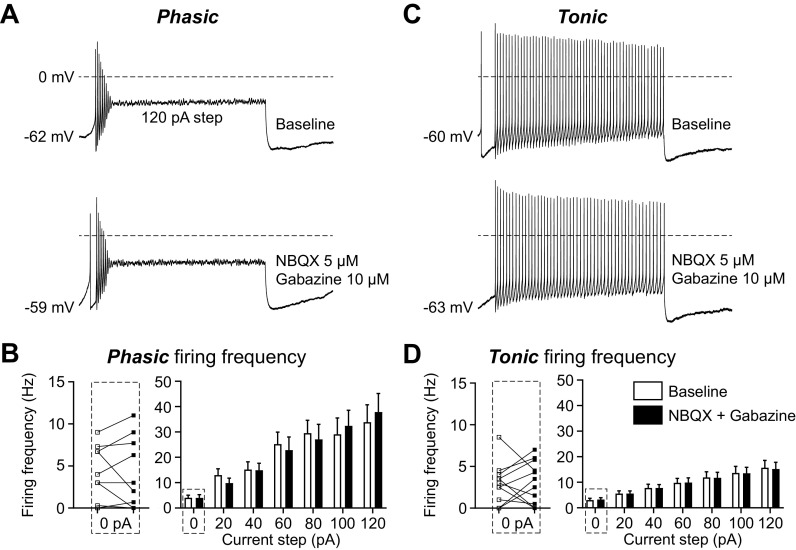
To determine whether the firing pattern and frequency were intrinsic and independent of inhibitory and excitatory synaptic inputs, we used AMPA- and GABAA-receptor blockers [5 µM 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) and 10 µM gabazine] to block the synaptic inputs onto vlPAG neurons. Both Phasic and Tonic neurons maintain the same firing patterns (Fig. 3, A and C) and firing frequency at rest and in response to a series of depolarizing current steps in the absence of synaptic inputs (Fig. 3, B and D). The effect of removing synaptic inputs on spontaneous firing (0 pA; Fig. 3, B and D, left) reveals subpopulations within both the Phasic and Tonic groups that have a different balance in excitatory and inhibitory inputs. Some neurons receive predominantly inhibitory inputs, resulting in increased firing by the addition of synaptic blockers. Other neurons receive predominantly excitatory inputs, producing decreased firing in the presence of blockers. Overall, these data indicate that the vlPAG has two main neuron types based on distinct intrinsic membrane properties that are maintained in the absence of synaptic inputs.
Figure 3.
The firing patterns of Phasic and Tonic neurons are maintained in the absence of synaptic inputs. A: representative traces of a recording from a Phasic ventrolateral periaqueductal gray (vlPAG) neuron from a naive rat in response to 120-pA current injections before (top) and after (bottom) synaptic blockers for glutamate [2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) 5 µM] and GABA (gabazine 10 µM). B: compiled data showing the firing frequency of all Phasic neurons for current steps ranging from 0 pA to 120 pA in 20-pA increments before (white bars) and after (black bars) synaptic blockers (2-way ANOVA, effect of synaptic blockers, F1,18 = 0.002, P = 0.96; n = 10). Individual experiments from the 0-pA step, showing the effect of synaptic blockers on spontaneous firing frequency (white squares are baseline and black squares are after blockers), are shown on left (Wilcoxon test, P = 0.8125). C: representative traces of a recording from a Tonic vlPAG neuron from a naive rat in response to 120-pA current injections before (top) and after (bottom) synaptic blockers. D: compiled data showing the firing frequency of all Tonic neurons for all current steps before and after synaptic blockers (2-way ANOVA, effect of synaptic blockers, F1,20 = 0.0007, P = 0.98; n = 11). Individual experiments from the 0-pA step, showing the effect of synaptic blockers on spontaneous firing frequency, are shown on left (Wilcoxon test, P = 0.85).
MOR-Mediated GIRK Currents Are Observed within All 4 vlPAG Neuron Types
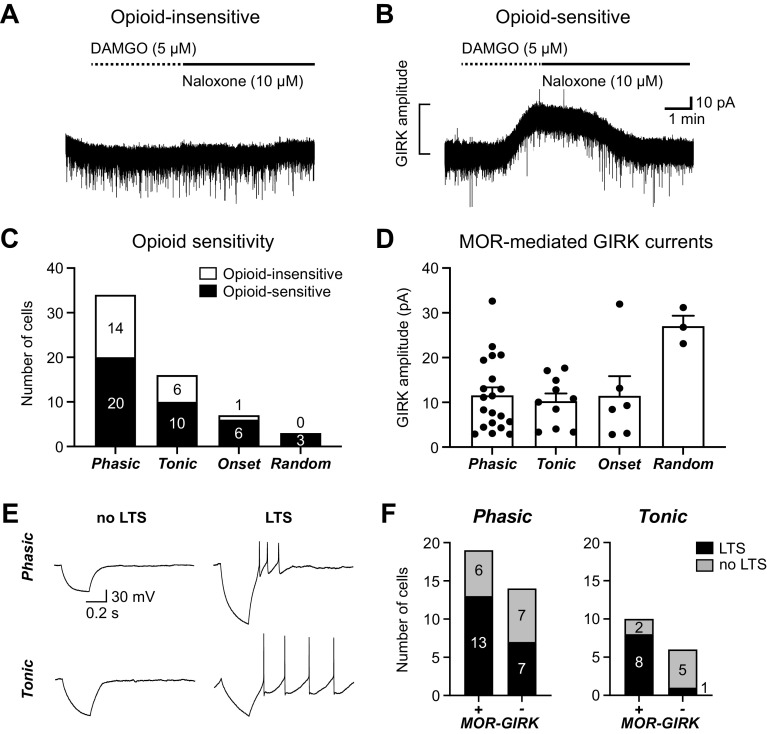
To determine whether postsynaptic MORs are selectively expressed on a single vlPAG neuron type defined by intrinsic firing properties, the MOR agonist DAMGO (5 µM) was used to elicit GIRK currents after evaluation of the firing patterns of each neuron. Opioid-insensitive neurons showed no current response to DAMGO application (Fig. 4A), whereas opioid-sensitive neurons, with GIRK-coupled MORs, showed an outward K+ current in response to DAMGO that was reversed by the MOR antagonist naloxone (Fig. 4B). Opioid-mediated GIRK currents were elicited in a subset of the Phasic, Tonic, and Onset neurons (Fig. 4C). All three Random neurons were opioid sensitive, but the small sample may have precluded observing opioid-insensitive neurons in this group. Opioid-insensitive neurons in each group exhibited GABAB-mediated GIRK currents, consistent with GIRK channel expression in most neurons, as we noted in our previous study (46). GIRK current amplitudes were an average of ∼10 pA in Phasic, Tonic, and Onset neurons (Fig. 4D). The most common neurons, Phasic and Tonic, showed no significant differences in the proportion of opioid-sensitive neurons observed (Fisher’s exact test, P > 0.9999) or the GIRK current amplitudes (unpaired t test, t28 = 0.4514, P = 0.6552).
Figure 4.
Opioid-mediated G protein-coupled inwardly rectifying potassium channel (GIRK) currents are observed in all 4 neuron types. A: a representative trace from an opioid-insensitive ventrolateral periaqueductal gray (vlPAG) neuron in which the selective mu-opioid receptor (MOR) agonist DAMGO (5 µM) did not elicit an outward GIRK current. B: a representative trace from an opioid-sensitive vlPAG neuron in which DAMGO (5 µM) elicited an outward GIRK current that was reversed by the MOR antagonist naloxone (10 µM). C: number of neurons exhibiting DAMGO-mediated GIRK current responses (opioid sensitive) compared with those that did not (opioid insensitive) in each neuron type (n = 60). Opioid insensitivity was observed in 21 neurons from 16 naive rats (nfemale = 7, nmale = 9), and opioid sensitivity was observed in 39 neurons from 31 naive rats (nfemale = 18, nmale = 13). D: DAMGO-mediated GIRK current amplitudes (pA) for opioid-sensitive neurons in each neuron type (1-way ANOVA, main effect of neuron type, F3,35 = 3.9, P = 0.02; Tukey’s post hoc test Phasic vs. Random P = 0.01, Tonic vs. Random P = 0.01, and Onset vs. Random P = 0.04; n = 39). E: sample whole cell traces in response to 500-ms-long −50-pA hyperpolarizing current steps, demonstrating neurons with and without low-threshold spikes (LTS) in both Phasic and Tonic neuron populations. F: the proportion of LTS and non-LTS neurons in opioid-sensitive (MOR-GIRK+) and opioid-insensitive (MOR-GIRK−) groups of Phasic (left; Fisher’s exact test, P = 0.47) and Tonic (right; Fisher’s exact test, P = 0.035) neurons.
Loose-patch recordings demonstrated clear, reversible DAMGO-mediated inhibition of firing in spontaneously active neurons (46/56 or 82% of neurons from naive animals and 33/37 or 89% of neurons from CFA-treated animals were inhibited ≥25%). However, the inability to reliably observe opioid effects on the firing of identified neuron types motivated our search for additional firing features that are correlated with opioid sensitivity.
Neurons exhibiting LTS have been previously described to be linked to opioid sensitivity in the vlPAG of mice, with 5/5 LTS neurons having MOR-mediated GIRK currents and 4/4 non-LTS neurons having no GIRK response (40). We found that both Phasic and Tonic groups had neurons with and without LTS (Fig. 4E). LTS did not completely predict opioid sensitivity in our data set, with neurons in opioid-sensitive and -insensitive groups exhibiting LTS in both neuron types. However, opioid-sensitive Tonic neurons had a significantly greater proportion of LTS neurons (8/10) than the opioid-insensitive Tonic population (1/6) (Fig. 4F, right). Conversely, both opioid-sensitive and -insensitive Phasic neurons had several LTS and non-LTS neurons (Fig. 4F, left).
Persistent Inflammation Selectively Activates a Subset of Opioid-Sensitive Phasic Neurons
Defining intrinsic firing properties in naive rats provided the framework to evaluate changes in intrinsic properties after acute and persistent inflammation. We started by confirming that we can replicate several previously published studies that showed vlPAG activation induced by CFA, using Fos expression as a marker for neuronal activity. Both acute (2 h) and persistent (6 days) inflammation enhance Fos expression in the vlPAG (Fig. 5, A and B), so we examined firing properties at these time points. The proportions of the four firing patterns were the same between the naive condition and after CFA (nnaive = 233 nCFA 5-7days = 149; chi-square = 3.823, P = 0.2812), suggesting that cells maintained firing patterns during inflammation. Interestingly, when we looked at the spontaneous firing frequency of vlPAG neurons, Tonic neurons remained unchanged across both post-CFA time points (Fig. 5C), whereas the Phasic neurons approximately doubled their firing frequency after persistent inflammation compared with naive and the acute inflammation time point (Fig. 5D). The proportion of spontaneously inactive Phasic neurons decreased in recordings from vlPAG slices after persistent inflammation (39/112 or 35% inactive in naive vs. 6/41 or 15% after CFA 5–7 days; Fisher’s exact test, P = 0.02).
Figure 5.
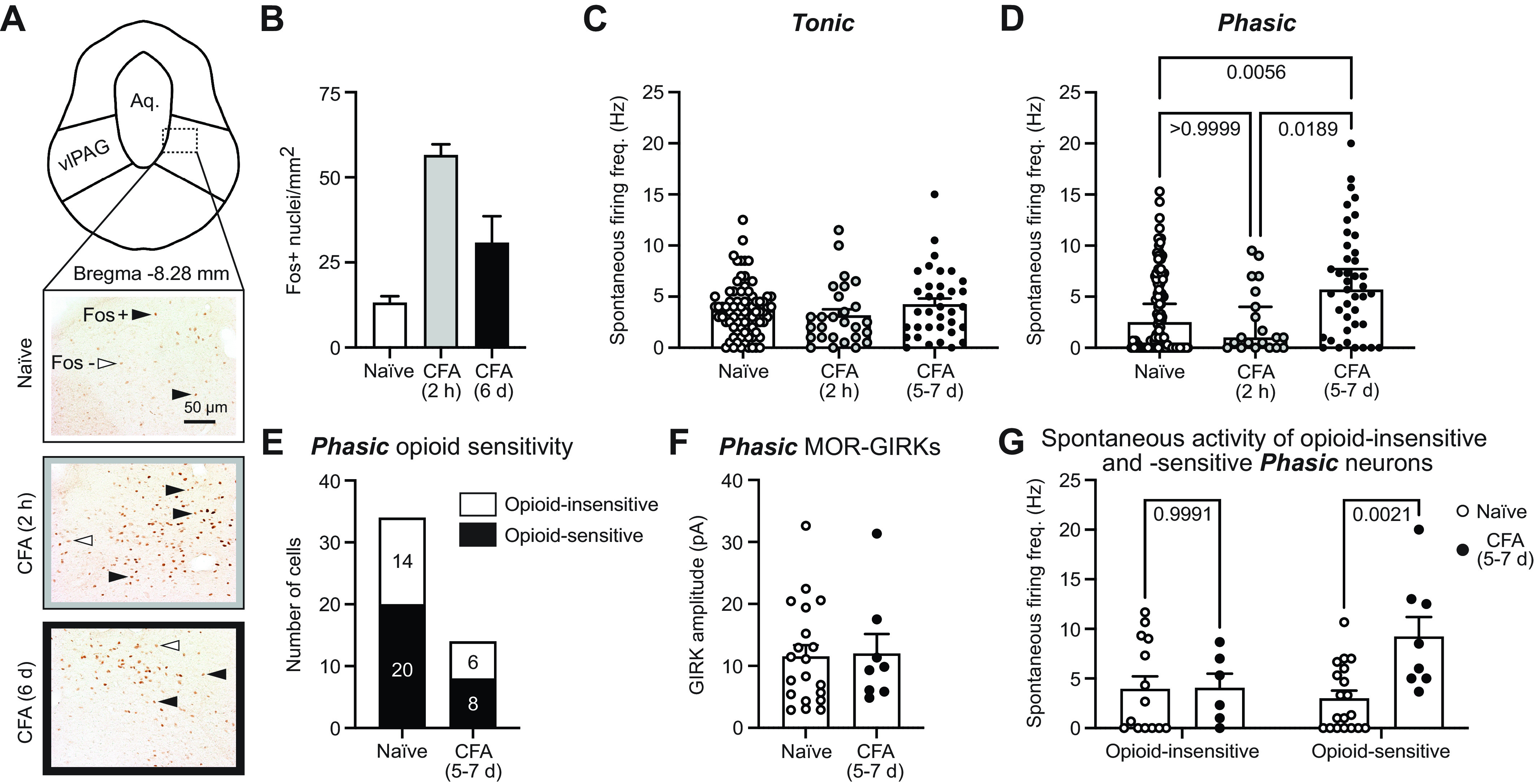
Persistent inflammation selectively activates opioid-sensitive Phasic neurons. A: region of interest [ventrolateral periaqueductal gray (vlPAG)] outlined and representative image location demonstrated with a dashed box on a diagram of bregma −8.28 mm (data are quantified from the full rostral-caudal axis of vlPAG); cerebral aqueduct is labeled “Aq.” Representative images from Fos immunohistochemistry of tissue from naive rats or rats euthanized 2 h or 6 days after Complete Freund’s adjuvant (CFA) injection to the hind paw. Black arrowheads indicate Fos+ nuclei that were above our quantification threshold. White arrowheads are Fos– neurons below our detection threshold. Scale bar, 50 µm for all images. B: average Fos+ nuclei/mm2 for naive rats and those 2 h or 6 days after CFA injections (3 males and 3 females in the naive group, 2 males and 2 females in each of the CFA-treated groups). C: spontaneous firing frequency (no current injection) of Tonic neurons is unaltered by acute or persistent inflammation whether the CFA groups are compared to all naive recordings (Kruskal–Wallis test, P = 0.29; nnaive = 78, nfemale = 38, nmale = 37; nCFA 2 h = 27, nfemale = 20, nmale = 7; nCFA 5-7days = 35, nfemale = 16, nmale = 19) or a subset of naive recordings from the same time frame as the CFA recordings (data not shown; Kruskal–Wallis test, P = 0.28; nnaive = 25, nfemale = 13, nmale = 12; nCFA 2 h = 27, nfemale = 20, nmale = 7; nCFA 5-7days = 35, nfemale = 16, nmale = 19). D: persistent inflammation significantly increases the spontaneous firing frequency (no current injection) of Phasic neurons compared with all naive recordings (Kruskal–Wallis test, P = 0.0031; multiple comparisons, naive vs. CFA 5–7 days, P = 0.0056 and CFA 2 h vs. CFA 5–7 days, P = 0.0189; nnaive = 112, nfemale = 56, nmale = 50; nCFA 2 h = 20, nfemale = 15, nmale = 5; nCFA 5-7days = 41, nfemale = 19, nmale = 23) or a subset of naive recordings from the same time frame as the CFA recordings (data not shown; Kruskal–Wallis test, P = 0.0011; multiple comparisons, naive vs. CFA 5–7 days, P = 0.0023 and CFA 2 h vs. CFA 5–7 days, P = 0.020; nnaive = 34, nfemale = 18, nmale = 15; nCFA 2 h = 20, nfemale = 15, nmale = 5; nCFA 5-7days = 41, nfemale = 19, nmale = 22). E: the same proportion of opioid-sensitive Phasic neurons is observed in the naive and persistent inflammation conditions (Fisher’s exact test, P > 0.99). F: DAMGO-mediated G protein-coupled inwardly rectifying potassium channel (GIRK) current amplitudes (pA) of opioid-sensitive Phasic neurons are unchanged by persistent inflammation (unpaired t test, t27 = 1.379, P = 0.89, nnaive = 20, nCFA 5-7days = 8). G: comparing the spontaneous firing frequency of opioid-sensitive and -insensitive Phasic neurons before and after persistent inflammation reveals a significant effect of CFA treatment (2-way ANOVA, P = 0.025) and an interaction between treatment and opioid sensitivity (2-way ANOVA, P = 0.028).
To determine whether the increase in spontaneous firing of Phasic neurons was a result of changes to intrinsic firing properties, we examined the impact of synaptic inputs (using NBQX and gabazine as described above) on spontaneous firing frequencies, RMP, and firing threshold. Removing synaptic inputs had no consistent effect on the spontaneous firing frequency, with some Phasic cells firing faster and others slower (meanbaseline = 7.6 Hz, meansynaptic blockers = 4.3 Hz; paired t test, t14 = 1.9, P = 0.08). The RMP was also similar across the two inflammation time points (meannaive = −55.31 mV, meanCFA 5-7days = −55.39 mV; unpaired t test, t152 = 0.06, P = 0.95). In naive rats, 14/33 Phasic neurons required a depolarizing current step to fire, whereas after persistent inflammation only 3/37 required a depolarizing current step and 9/37 fired during hyperpolarizing steps, resulting in a significant reduction in firing threshold after persistent inflammation (meannaive = −7.0 pA, meanCFA5-7days = −2.4 pA; unpaired t test, t68 = 3.036, P = 0.0034). These findings confirmed that the enhanced spontaneous firing of Phasic neurons was a result of an intrinsic alteration to the firing threshold.
To determine whether the most strongly activated Phasic neurons from CFA-treated rats were opioid sensitive, we evaluated DAMGO-mediated GIRK currents. The proportion of opioid-sensitive Phasic neurons was similar in naive and CFA-treated rats (Fig. 5E), with comparable GIRK current amplitudes (Fig. 5F). Importantly, the spontaneous firing frequencies of opioid-sensitive Phasic neurons are significantly increased after persistent inflammation, whereas CFA treatment had no effect on the opioid-insensitive Phasic neurons (Fig. 5G). Furthermore, persistent inflammation has no impact on the spontaneous firing frequency of opioid-sensitive or -insensitive Tonic neurons (mean naive opioid-insensitive = 4.8 Hz, CFA 5–7 days opioid-insensitive = 4.7 Hz, naive opioid-sensitive = 4.9 Hz, CFA 5–7 days opioid-insensitive = 4.6 Hz; no effect of CFA treatment, 2-way ANOVA, F1,25 = 0.0372, P = 0.8486; no interaction between treatment and opioid sensitivity, 1-way ANOVA, F1,25 = 0.0038, P = 0.95).
DISCUSSION
The membrane properties of vlPAG neurons were examined in naive rats to create a framework to identify neuron-specific alterations in firing after acute (2 h) or persistent (5–7 days) inflammation. In all conditions, we observe four types of neurons with distinct, intrinsic firing patterns that do not segregate with opioid sensitivity (determined by MOR-mediated GIRK currents). Despite robust Fos expression after acute inflammation, intrinsic firing properties remain unchanged until the persistent inflammation time point, when Phasic neurons display an increase in the average spontaneous firing frequency and lowered firing threshold. Further assessment of opioid sensitivity after persistent inflammation revealed that the strongly activated Phasic neurons are opioid-sensitive.
Neuronal Heterogeneity in Descending Pain Modulation
Neuronal firing properties and response to noxious stimuli in vivo have been used to define neuron types that have functionally distinct impacts on descending pain modulation in the downstream RVM (2, 21, 22, 47). Neurons with the same in vivo response to noxious stimuli have been identified in the vlPAG (24); however, the technical limitations of identifying pain-responsive neurons within the vlPAG with in vivo recordings make it more difficult to characterize the vlPAG neurons compared with those in the RVM. Other studies using chemogenetics have found that distinct populations within the vlPAG, defined by neurotransmitter content (GABA and glutamate), differentially alter nociception (13). Specifically, activating glutamatergic neurons or inhibiting GABA neurons produces analgesia, whereas activating GABA neurons produces hyperalgesia. These studies provide important confirmation of results from prior foundational studies where in vivo electrical stimulation and pharmacological inhibitors to globally activate or inhibit vlPAG neurons proposed circuit mechanisms where activation of vlPAG glutamatergic projection neurons promotes analgesia (descending inhibition) and GABA release or activation of GABAA receptors promotes nociception (descending facilitation) (5, 6, 11, 12, 14, 15, 48, 49). However, selective activation of an entire population of GABAergic or glutamatergic neurons does not provide information regarding the physiologically relevant activation of specific types of vlPAG neurons by acute or persistent noxious stimuli.
A growing number of studies have identified significant heterogeneity in neurotransmitter content of vlPAG neurons, which further complicates the interpretation of studies that use neurotransmitters as the sole markers for functionally distinct neuronal subtypes. For example, selective activation of dynorphin-expressing glutamate neurons within the vlPAG produces dynorphin-mediated pain facilitation at the level of the RVM (50), which directly opposes the analgesia produced by activating all vlPAG glutamate neurons. Similarly, another recent study shows that activating a population of RVM-projecting glutamate neurons that express somatostatin facilitates nociception (51). In addition, a subpopulation of Chx10-expressing glutamate neurons mediate freezing behaviors (35), indicating that vlPAG glutamatergic neurons are involved in multiple behaviors. Multiplexed error-robust fluorescence in situ hybridization (MERFISH) was recently used to identify >100 inhibitory and excitatory vlPAG populations that play unique roles in many different behaviors (52), some of which have been shown to coexpress GABA and glutamate markers (53) and corelease dopamine and glutamate (54). These studies demonstrate diverse functionality within these neuron populations and underscore the importance of defining cellular heterogeneity by many different features (i.e., firing pattern, molecular markers, afferent inputs, efferent targets, etc.) (20).
The vlPAG plays a key role in opioid-induced analgesia, and the disinhibition hypothesis suggests that postsynaptic MOR expression on GABAergic interneurons within the vlPAG promotes analgesia (11, 48, 55–58). Thus, we considered the possibility that opioid sensitivity may map onto our neuron types defined by firing properties. MORs are expressed on a subpopulation of postsynaptic cell bodies within the vlPAG and are coupled to GIRKs, such that MOR agonist binding produces an outward K+ current that hyperpolarizes the neurons (28, 46). Our survey of 60 vlPAG neurons revealed that postsynaptic MOR expression can be found in a subset of neurons within each of the four types, with both opioid-sensitive and opioid-insensitive neurons within the Phasic, Tonic, and Onset groups. Thus, MOR expression does not exclusively map onto the four neuron types defined by firing properties.
It is important to note that we define opioid sensitivity with MOR-mediated GIRK currents. Opioid receptors couple to signaling cascades other than GIRK channels, so opioid mRNA expression does not equate to the presence of MOR-GIRK currents. Additionally, we know that opioids inhibit release from GABAergic and glutamatergic presynaptic terminals that impinge on vlPAG neurons (16, 37, 59–61). The effect of opioids on vlPAG neuron activity can depend on the balance of the excitatory and inhibitory presynaptic inputs that are being inhibited as well as the presence of GIRK-coupled MORs. As a result, some neurons are inhibited and others are disinhibited by opioid administration (37).
LTS is another marker that has been proposed to correlate with opioid sensitivity in the vlPAG neurons from mice (40). Specifically, Park et al. (40) identified a small population of “fast-spiking” GABAergic vlPAG neurons, and half of these neurons had LTS mediated by low-voltage-activated T-type Ca2+ channels and all were opioid sensitive. Within our larger data set of Phasic and Tonic neurons in rats, LTS was observed in both opioid-sensitive and -insensitive neurons.
Persistent Inflammation Selectively Activates Phasic vlPAG Neurons
With a few exceptions, our current understanding of how vlPAG subpopulations participate in descending pain modulation is based on artificial circuit manipulations. Studies that selectively activate or inhibit neurons based on viral strategies targeting different genetic markers provide interesting insights into possible circuit mechanisms; however, they fail to illuminate how pain (or other stimuli) impacts innate physiology. Our extensive survey of firing properties was intended to create a useful framework to identify the neuron-specific impact of opioids and activation induced by acute and persistent inflammation.
Noxious stimuli induce Fos expression in the vlPAG (17, 18). Typically, reexposure to the stimulus or stimulus-associated cues is used to induce acute reactivation of neurons because Fos is a marker for strong neuronal activation. Alternatively, in this study we deliberately did not reexpose the rats to a pain stimulus, in order to determine whether the initial CFA injection produced ongoing activation of specific vlPAG neurons that could be measured with whole cell patch-clamp electrophysiology. Fos labeling was detected at the acute time point (2 h) after CFA injection and was maintained over baseline levels at the persistent (6 days) time point. Fos protein expression peaks 90–120 min after a stimulus (62), indicating that our 2 h time point most closely demonstrates Fos expression driven by the cellular activity within the first 30 min after CFA injection. Downstream RVM neuronal activity is known to be altered over the first 30 min after CFA (22). The robust Fos expression after acute and persistent inflammation confirmed that CFA-mediated inflammation produces ongoing activation of vlPAG neurons.
In contrast to the Fos data, spontaneous firing of vlPAG Phasic neurons is enhanced exclusively at the persistent inflammation time point, suggesting a time-dependent adaptation. The enhanced spontaneous activation is selective to the Phasic population, with Tonic neurons exhibiting no changes in firing activity after acute or persistent inflammation. Further evaluation showed that this activation of Phasic neurons was driven by a significant reduction in the intrinsic firing threshold. The more robust Fos expression observed 2 h after CFA injection, which is not reflected in any increase in spontaneous firing frequencies observed at 2 h, may indicate the importance of intact afferent inputs that can influence vlPAG cellular activity in vivo (25) but are not present in our slices.
Next, we looked to see whether opioid sensitivity was altered in the Phasic neurons that were strongly activated by persistent inflammation. We found a comparable proportion of opioid-sensitive Phasic neurons with similar-amplitude DAMGO-mediated GIRK currents in both naive and CFA-treated rats. Interestingly, we found that the subset of vlPAG Phasic neurons that are selectively activated by persistent inflammation is the opioid-sensitive subpopulation. Selective activation of the opioid-sensitive Phasic population with persistent inflammation is consistent with a population of vlPAG neurons that promote descending facilitation of pain. Enhanced activation of a subpopulation of vlPAG neurons that facilitate pain has been proposed in a neuropathic pain model in vivo (26). In addition, a recent study provides further support for descending facilitation of pain from the vlPAG. In this study, selectively activating MOR-expressing vlPAG neurons in vivo facilitates the response to noxious stimuli (63). However, it is important to note that the in vivo activation of PAG-MOR neurons activates all MOR-expressing neurons, which includes the opioid-sensitive subset of neurons from each of our four identified neuron groups. Thus, further studies will determine whether activation of MOR-expressing Phasic neurons is sufficient to produce descending facilitation.
The targeted activation of opioid-sensitive Phasic neurons by persistent inflammation prompts many interesting future lines of investigation. Exploring further Phasic neuron-specific receptor and channel expression could illuminate the mechanism by which inflammation selectively activates these neurons. Additionally, identifying unique expression profiles for these neurons can provide us with pharmacological methods to selectively manipulate Phasic neuron activation. These methods would 1) solidify the impact of the selective Phasic neuron activation by persistent inflammation on ongoing nociception and 2) provide a potential novel therapeutic that reverses Phasic neuron activation induced by inflammation.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
The studies were supported by National Institute of Drug Abuse (NIDA) R01 DA042565 (SLI) and F31 DA052114-01 (C.A.B.) and National Institute of Neurological Disease and Stroke (NINDS) R01 NS120486 (S.L.I.). K.B.M. was supported by the National Science Foundation Graduate Research Fellowship Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.B.M. and S.L.I. conceived and designed research; K.B.M. and C.A.B. performed experiments; K.B.M., C.A.B., B.C., and S.L.I. analyzed data; K.B.M. and S.L.I. interpreted results of experiments; K.B.M. and B.C. prepared figures; K.B.M. drafted manuscript; K.B.M. and S.L.I. edited and revised manuscript; K.B.M. and S.L.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Mary Heinricher for critical reading of the manuscript. Graphical abstract created with BioRender and published with permission.
REFERENCES
- 1. Bodnar RJ. Supraspinal circuitry mediating opioid antinociception: antagonist and synergy studies in multiple sites. J Biomed Sci 7: 181–194, 2000. doi: 10.1007/BF02255465. [DOI] [PubMed] [Google Scholar]
- 2. Heinricher MM, Ingram SL. Brainstem and nociceptive modulation. In: The Senses: A Comprehensive Reference (2nd ed.), edited by Fritzsch B. Oxford, UK: Elsevier, 2020, vol. 5, p. 249–271. [Google Scholar]
- 3. Silva C, McNaughton N. Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog Neurobiol 177: 33–72, 2019. doi: 10.1016/j.pneurobio.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 4. Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53: 95–104, 2000. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 5. Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164: 444–445, 1969. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 6. Mayer DJ, Liebeskind JC. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res 68: 73–93, 1974. doi: 10.1016/0006-8993(74)90534-4. [DOI] [PubMed] [Google Scholar]
- 7. Soper WY, Melzack R. Stimulation-produced analgesia: evidence for somatotopic organization in the midbrain. Brain Res 251: 301–311, 1982. doi: 10.1016/0006-8993(82)90747-8. [DOI] [PubMed] [Google Scholar]
- 8. Barbaro NM. Studies of PAG/PVG stimulation for pain relief in humans. Prog Brain Res 77: 165–173, 1988. doi: 10.1016/s0079-6123(08)62783-1. [DOI] [PubMed] [Google Scholar]
- 9. Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci 12: 515–519, 2005. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10. Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 197: 183–186, 1977. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 11. Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7: 309–338, 1984. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 12. Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res 170: 85–93, 1979. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- 13. Samineni VK, Grajales-Reyes JG, Copits BA, O’Brien DE, Trigg SL, Gomez AM, Bruchas MR, Gereau RW 4th.. Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. eNeuro 4: ENEURO.0129-16.2017, 2017. doi: 10.1523/ENEURO.0129-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 397: 37–46, 1986. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- 15. Behbehani MM, Jiang M, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain 40: 195–204, 1990. doi: 10.1016/0304-3959(90)90070-T. [DOI] [PubMed] [Google Scholar]
- 16. Winters BL, Lau BK, Vaughan CW. Cannabinoids and opioids differentially target extrinsic and intrinsic GABAergic inputs onto the periaqueductal grey descending pathway. J Neurosci 42: 7744–7756, 2022. doi: 10.1523/JNEUROSCI.0997-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keay KA, Bandler R. Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci Lett 154: 23–26, 1993. doi: 10.1016/0304-3940(93)90162-e. [DOI] [PubMed] [Google Scholar]
- 18. Keay KA, Li QF, Bandler R. Muscle pain activates a direct projection from ventrolateral periaqueductal gray to rostral ventrolateral medulla in rats. Neurosci Lett 290: 157–160, 2000. doi: 10.1016/s0304-3940(00)01329-x. [DOI] [PubMed] [Google Scholar]
- 19. Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray‐rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol 496: 723–738, 2006. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McPherson KB, Ingram SL. Cellular and circuit diversity determines the impact of endogenous opioids in the descending pain modulatory pathway. Front Syst Neurosci 16: 963812, 2022. doi: 10.3389/fnsys.2022.963812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fields H, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci 3: 2545–2552, 1983. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cleary DR, Heinricher MM. Adaptations in responsiveness of brainstem pain-modulating neurons in acute compared with chronic inflammation. Pain 154: 845–855, 2013. doi: 10.1016/j.pain.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinricher M, Morgan M, Fields H. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience 48: 533–543, 1992. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 24. Heinricher MM, Cheng ZF, Fields HL. Evidence for two classes of nociceptive modulating neurons in the periaqueductal gray. J Neurosci 7: 271–278, 1987. doi: 10.1523/JNEUROSCI.07-01-00271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tryon VL, Mizumori SJ, Morgan MM. Analysis of morphine-induced changes in the activity of periaqueductal gray neurons in the intact rat. Neuroscience 335: 1–8, 2016. doi: 10.1016/j.neuroscience.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samineni VK, Premkumar LS, Faingold CL. Neuropathic pain induced enhancement of spontaneous and pain evoked neuronal activity in the periaqueductal gray that is attenuated by gabapentin. Pain 158: 1241–1253, 2017. doi: 10.1097/j.pain.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 187: 513–531, 1979. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- 28. Chieng B, Christie M. Hyperpolarization by opioids acting on μ‐receptors of a sub‐population of rat periaqueductal gray neurones in vitro. Br J Pharmacol 113: 121–128, 1994. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of μ‐opioid receptors to γ‐aminobutyric acid‐immunoreactive and medullary‐projecting periaqueductal gray neurons. J Comp Neurol 419: 532–542, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 30. Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, Bossert JM, Shaham Y, Bonci A, Hope BT. Bidirectional modulation of intrinsic excitability in rat prelimbic cortex neuronal ensembles and non-ensembles after operant learning. J Neurosci 37: 8845–8856, 2017. doi: 10.1523/JNEUROSCI.3761-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol 539: 817–836, 2002. doi: 10.1113/jphysiol.2001.013437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li JN, Sheets PL. The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J Physiol 596: 6289–6305, 2018. doi: 10.1113/JP276935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelley LK, Middleton J, Gilpin NW, Lightfoot SH, Hill MN. Chronic THC vapor rescues inflammation-related thermal hyperalgesia and causes cell type-specific modifications in vlPAG neurons (Preprint). bioRxiv 2021.09.23.461562, 2021. doi: 10.1101/2021.09.23.461562. [DOI]
- 34. Pati D, Kash TL. Tumor necrosis factor-α modulates GABAergic and dopaminergic neurons in the ventrolateral periaqueductal gray of female mice. J Neurophysiol 126: 2119–2129, 2021. doi: 10.1152/jn.00251.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaaga CE, Brown ST, Raman IM. Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. eLife 9: e54302, 2020. doi: 10.7554/eLife.54302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adke AP, Khan A, Ahn HS, Becker JJ, Wilson TD, Valdivia S, Sugimura YK, Gonzalez SM, Carrasquillo Y. Cell-type specificity of neuronal excitability and morphology in the central amygdala. eNeuro 8: ENEURO.0402-20.2020, 2021. doi: 10.1523/ENEURO.0402-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lau BK, Winters BL, Vaughan CW. Opioid presynaptic disinhibition of the midbrain periaqueductal grey descending analgesic pathway. Br J Pharmacol 177: 2320–2332, 2020. doi: 10.1111/bph.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tonsfeldt KJ, Suchland KL, Beeson KA, Lowe JD, Li MH, Ingram SL. Sex differences in GABAA signaling in the periaqueductal gray induced by persistent inflammation. J Neurosci 36: 1669–1681, 2016. doi: 10.1523/JNEUROSCI.1928-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho YC, Cheng JK, Chiou LC. Hypofunction of glutamatergic neurotransmission in the periaqueductal gray contributes to nerve-injury-induced neuropathic pain. J Neurosci 33: 7825–7836, 2013. doi: 10.1523/JNEUROSCI.5583-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park C, Kim JH, Yoon BE, Choi EJ, Lee CJ, Shin HS. T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray. Proc Natl Acad Sci USA 107: 14857–14862, 2010. doi: 10.1073/pnas.1009532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain 35: 313–326, 1988. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 42. Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42: 939–946, 2004. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 43. Fullerton EF, Doyle HH, Murphy AZ. Impact of sex on pain and opioid analgesia: a review. Curr Opin Behav Sci 23: 183–190, 2018. doi: 10.1016/j.cobeha.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Loyd DR, Wang X, Murphy AZ. Sex differences in μ-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 28: 14007–14017, 2008. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav Brain Res 177: 126–133, 2007. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McPherson KB, Leff ER, Li MH, Meurice C, Tai S, Traynor JR, Ingram SL. Regulators of G-protein signaling (RGS) proteins promote receptor coupling to G-protein-coupled inwardly rectifying potassium (GIRK) channels. J Neurosci 38: 8737–8744, 2018. doi: 10.1523/JNEUROSCI.0516-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanegas H, Barbaro NM, Fields HL. Tail-flick related activity in medullospinal neurons. Brain Res 321: 135–141, 1984. doi: 10.1016/0006-8993(84)90689-9. [DOI] [PubMed] [Google Scholar]
- 48. Reichling DB, Basbaum AI. Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: I. GABA‐immunoreactive projection neurons in the periaqueductal gray and nucleus raphe magnus. J Comp Neurol 302: 370–377, 1990. doi: 10.1002/cne.903020213. [DOI] [PubMed] [Google Scholar]
- 49. Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science 174: 1351–1354, 1971. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- 50. Nguyen E, Smith KM, Cramer NP, Holland R, Bleimeister I, Flores-Felix K, Silberberg H, Keller A, Pichon CL, Ross SE. Medullary kappa-opioid receptor neurons inhibit pain and itch through a descending circuit. Brain 145: 2586–2601, 2022. doi: 10.1093/brain/awac189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Huang X, Xin WJ, He S, Deng J, Ruan X. Somatostatin neurons from periaqueductal gray to medulla facilitate neuropathic pain in male mice. J Pain 2023: S1526-5900(23)00010-X, 2023. doi: 10.1016/j.jpain.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 52. Vaughn E, Eichhorn S, Jung W, Zhuang X, Dulac C. Three-dimensional interrogation of cell types and instinctive behavior in the periaqueductal gray (Preprint). bioRxiv 2022.06.27.497769, 2022. doi: 10.1101/2022.06.27.497769. [DOI]
- 53. Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, Sabatini BL. Molecular and anatomical organization of the dorsal raphe nucleus. eLife 8: e46464, 2019. doi: 10.7554/eLife.46464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li C, Sugam JA, Lowery-Gionta EG, McElligott ZA, McCall NM, Lopez AJ, McKlveen JM, Pleil KE, Kash TL. Mu opioid receptor modulation of dopamine neurons in the periaqueductal gray/dorsal raphe: a role in regulation of pain. Neuropsychopharmacology 41: 2122–2132, 2016. doi: 10.1038/npp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jensen TS, Yaksh TL. Comparison of the antinociceptive effect of morphine and glutamate at coincidental sites in the periaqueductal gray and medial medulla in rats. Brain Res 476: 1–9, 1989. doi: 10.1016/0006-8993(89)91529-1. [DOI] [PubMed] [Google Scholar]
- 56. Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114: 83–103, 1976. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- 57. Barbaresi P, Manfrini E. Glutamate decarboxylase-immunoreactive neurons and terminals in the periaqueductal gray of the rat. Neuroscience 27: 183–191, 1988. doi: 10.1016/0306-4522(88)90229-1. [DOI] [PubMed] [Google Scholar]
- 58. Kalyuzhny AE, Wessendorf MW. Relationship of μ‐and δ‐opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol 392: 528–547, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 59. Vaughan C, Ingram S, Connor M, Christie M. How opioids inhibit GABA-mediated neurotransmission. Nature 390: 611–614, 1997. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- 60. Vaughan C, Christie M. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol 498: 463–472, 1997. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18: 10269–10276, 1998. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237: 192–197, 1987. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 63. Nguyen E, Chiang M, Nguyen CC, Ross SE. Brainstem modulation of nociception by periaqueductal gray neurons expressing the mu-opioid receptor (Preprint). bioRxiv 2022.08.12.503787, 2022. doi: 10.1101/2022.08.12.503787. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.