Keywords: aquaporin 2, insulin-regulated aminopeptidase, water loading, vasopressin
Abstract
The objective of this study was to understand the response of mice lacking insulin-regulated aminopeptidase (IRAP) to an acute water load. For mammals to respond appropriately to acute water loading, vasopressin activity needs to decrease. IRAP degrades vasopressin in vivo. Therefore, we hypothesized that mice lacking IRAP have an impaired ability to degrade vasopressin and, thus, have persistent urinary concentration. Age-matched 8- to 12-wk-old IRAP wild-type (WT) and knockout (KO) male mice were used for all experiments. Blood electrolytes and urine osmolality were measured before and 1 h after water load (∼2 mL sterile water via intraperitoneal injection). Urine was collected from IRAP WT and KO mice for urine osmolality measurements at baseline and after 1 h administration of the vasopressin type 2 receptor antagonist OPC-31260 (10 mg/kg ip). Immunofluorescence and immunoblot analysis were performed on kidneys at baseline and after 1 h acute water load. IRAP was expressed in the glomerulus, thick ascending loop of Henle, distal tubule, connecting duct, and collecting duct. IRAP KO mice had elevated urine osmolality compared with WT mice due to higher membrane expression of aquaporin 2 (AQP2), which was restored to that of controls after administration of OPC-31260. IRAP KO mice developed hyponatremia after an acute water load because they were unable to increase free water excretion due to increased surface expression of AQP2. In conclusion, IRAP is required to increase water excretion in response to an acute water load due to persistent vasopressin stimulation of AQP2.
NEW & NOTEWORTHY Insulin-regulated aminopeptidase (IRAP) degrades vasopressin, but its role in urinary concentration and dilution is unknown. Here, we show that IRAP-deficient mice have a high urinary osmolality at baseline and are unable to excrete free water in response to water loading. These results reveal a novel regulatory role for IRAP in urine concentration and dilution.
INTRODUCTION
Regulation of water balance is critical for living organisms. Urinary concentration and dilution by the kidney play a key role in regulating body water balance. In response to hypertonic stress, water is reabsorbed resulting in concentrated urine, whereas in response to hypotonic stress, excess water is excreted by preventing water reabsorption. Vasopressin is a peptide hormone required for the concentration of urine. When extracellular fluid tonicity rises, circulating vasopressin increases and binds to vasopressin type 1a, vasopressin type 1b, and vasopressin type 2 receptors (V2Rs). Stimulation of V2R in the distal nephron increases the phosphorylation and apical membrane abundance of the water channel aquaporin 2 (AQP2), which promotes water reabsorption and urinary concentration, which counteracts the initial rise in extracellular fluid tonicity. When extracellular fluid tonicity falls, circulating vasopressin levels decrease. Consequently, decreased activation of V2R leads to decreased AQP2 activity, which increases water excretion and dilutes urine production to restore extracellular fluid tonicity.
Although the response to vasopressin is well studied, less is known about the role of vasopressin degradation. In humans, circulating vasopressin is cleared rapidly with an estimated plasma half-life of 10–30 min (1). Vasopressin is cleaved by insulin-regulated aminopeptidase (IRAP), a zinc metalloprotease that is expressed in multiple tissues including the kidney (2–4). Vasopressin is the only in vivo established biological substrate for IRAP (2). In muscle cells and adipocytes, IRAP colocalizes with glucose transporter type 4 (GLUT4) and is involved in its membrane translocation (3, 5). Total body IRAP knockout (KO) mice have higher levels of circulating vasopressin, decreased GLUT4 expression, and an impaired response to insulin (6). IRAP KO mice also have decreased vasopressin mRNA and peptide levels in the brain relative to wild-type (WT) controls, suggesting a negative feedback loop between circulating vasopressin and vasopressin production by the brain (6). In the kidney, IRAP is found in tubular epithelial cells of the proximal tubule, the ascending limb of the loop of Henle, the distal tubule, connecting tubules, and collecting ducts, but IRAP and GLUT4 were localized in different subcellular domains (7). The widespread distribution of IRAP in the kidney suggests that IRAP plays a role in regulating vasopressin signaling in the kidney as well as other potential functions in nephron segments that do not express vasopressin receptors. Interestingly, IRAP KO mice are reported to have higher expression of AQP2 in membrane fractions (7). However, the specific role that IRAP plays in hypotonic and hypertonic stress has never been studied.
The elevated levels of circulating vasopressin coupled with higher expression of AQP2 in membrane fractions suggested the possibility that IRAP KO mice have altered water balance. Therefore, we studied the response of IRAP KO mice to acute water restriction and acute water loading. We found that IRAP KO mice have higher urine osmolality and more abundant membrane AQP2 at baseline relative to WT controls. IRAP KO mice were able to concentrate urine after water restriction but were unable to increase water excretion in response to acute water loading, which correlated with higher expression, membrane abundance, and phosphorylation of AQP2. Moreover, when treated with the V2R antagonist OPC-31260, IRAP KO mice were able to produce dilute urine. Therefore, we conclude that IRAP deficiency leads to a V2R-dependent increase in AQP2 activity, which impairs water excretion in response to an acute water load.
METHODS
Mice
All animal experiments were performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center. IRAP KO mice were obtained from Jackson Laboratories (No. 027187, JAX) on a C57BL6/J background. The generation of these mice was originally described by Keller et al. (6). Male age-matched C57BL/6J WT and IRAP KO mice aged 8–12 wk were used for all experiments. All mice had ad libitum access to standard 4.5% fat mouse chow (5L0D, LabDiets) throughout the experiment.
Water Balance Experiments
Water restriction.
Twenty-four hours before euthanasia, water was removed from the cages of the water-restricted group; food access was maintained throughout (8).
Acute water loading.
Before euthanasia, mice were weighed and then given 10% of body weight in grams in milliliters of intraperitoneal sterile water. Spot urine was collected before and after water restriction and before and 60 min after acute water loading.
V2R Antagonism
Following baseline measurements, IRAP mice were injected intraperitoneally with OPC-31260 (10 mg/kg, O1266, Sigma) (9, 10). After 60 min, spot urine was collected for osmolality measurement.
Glomerular Filtration Rate
Glomerular filtration rate (GFR) was measured in conscious, unrestrained IRAP WT and KO mice (n = 5/group) by injecting 90 µL FITC-sinistrin (0.15 mg/g body wt in sterile saline) via the retroorbital plexus as previously described (11). After 90 min, the FITC-sinistrin half-life was recorded using MPD Studio software (MediBeacon, Mannheim, Germany) and converted into GFR (in µL/min/100 g body wt). Results are expressed as microliters per minute.
Osmolality and Electrolytes
Urine was collected from 8- to 12-wk-old IRAP WT versus KO male mice at baseline and following water restriction and acute water load. Urine osmolality was measured using a freezing point osmometer from Precision Systems (No. 6002, Natick, MA). Blood electrolytes were measured at baseline and following an acute water load using commercially available iStat Chem8+ single-use test cartridges and an i-STAT1 blood analyzer (Abbot). Collected blood was allowed to clot at room temperature for 30 min. It was then spun at 4°C for 5 min at 5,000 RPM in a 5425 R tabletop centrifuge (Eppendorf). Serum was then collected, and osmolality was measured using a freezing point osmometer from Precision Systems (No. 6002).
Blood Pressure Measurement
Baseline systolic pressure was measured in conscious, restrained IRAP WT and KO mice (n = 5/group) via tail plethysmography for 4 days using a six-channel blood pressure transducer (BP-2000 Series II Blood Pressure Analysis System, Visitech Systems). Data were continuously recorded for 90 min, and all pressure measurements were made during midmorning hours.
Proteinuria
At 8−12 wk of age, IRAP WT and KO mice (n = 10/group) were placed in metabolic cages and urine was collected. Urinary protein excretion was measured by a Bradford assay using a commercially available reagent (Cat. No. 5000006, Bio-Rad), and data were normalized to urinary creatinine (Cat. No. 80350, Crystal Chem). Results are expressed as protein excretion (in mg/dL)/creatinine (in mg/dL).
Immunoblot Analysis
Whole kidneys were lysed in RIPA buffer (Thermo Fisher Scientific) with protease and phosphatase inhibitors (Roche) using Tissue Tearor (model 985370, Daigger Scientific). Samples were spun at 4°C for 30 min at 15,000 RPM in a 5425 R tabletop centrifuge (Eppendorf), the supernatant was collected, and total protein was quantified with a BCA Assay (Pierce). Equal amounts of protein were loaded in MiniProtean TGX precast gels (Bio-Rad) using the Precision Plus Single color protein weight standard (Bio-Rad). Proteins were transferred to nitrocellulose membranes using Transblot Turbo (Bio-Rad). Total protein was quantified with Ponceau-S (Thermo Fisher Scientific). Membranes were then blocked with 5% nonfat dry milk and incubated with primary and secondary antibodies, as shown in the appendix (Table A1). Chemiluminescent signals were captured with an iBright FL1500 (Thermo Fisher Scientific), and band quantification was performed with ImageJ. Statistical analysis was performed with Prism 9 software. For both total AQP2 and phosphorylated AQP2 (phospho-Ser269) quantification, bands from both glycosylated (∼37 kDa) and nonglycosylated (25 kDa) were added and used as representative of total AQP2 expression.
Immunofluorescence
After euthanasia, kidneys were removed and placed in 3.7% formaldehyde, 10 mM sodium m-periodate, 40 mM phosphate buffer, and 1% acetic acid at room temperature overnight. The fixed tissue was then dehydrated using graded ethanols, paraffin embedded, and sectioned (5 µm). Tissue sections were then mounted on glass slides. The antibodies used are shown in the appendix (Table A1). Tissue was imaged using a Nikon TiE fully motorized inverted fluorescent microscope. Colocalization analysis was performed with NIS-Elements software, and Pearson’s correlation was calculated in sections of WT and KO mice for ezrin (AF-647) and AQP2 (FITC) at baseline and after OPC-31260 (n = 4 WT, 4 KO, and 4 KO + OPC-31260). Two images were taken per mouse per experiment, five tubules were selected per image, and Pearson’s correlation analysis was run on each tubule. Pearson’s correlation graph for the average of the five tubules selected in the representative image is shown. Individual values for each tubule were graphed and analyzed with one-way ANOVA with the Tukey test for multiple comparisons. Images were analyzed by an investigator who was blinded to genotype and treatment conditions. For post water loading, two images were taken per mouse and stained for AQP2-FITC and phosphorylated AQP2 (phospho-Ser269, AF-647) (n = 4 WT and 4 KO). One representative image is shown.
Real-Time Quantitative PCR
RNA was isolated from whole kidneys with TRIzol reagent (Invitrogen) following the manufacturer’s instructions. cDNA was synthesized from equal amounts (1 μg) of total RNA from each sample using the SuperScript IV First-strand Synthesis Sytem kit (Invitrogen). Quantitative RT-PCR was carried out using TaqMan real-time PCR (7900HT, Applied Biosystems). All gene probes and master mix were purchased from Applied Biosystems. The probes used in the experiments were as follows: mouse Aqp2 (Mm00437575_m1), mouse arginine vasopressin receptor 2 (Avpr2; Mm01193534_g1), mouse aquaporin 3 (Mm01208559_m1), and mouse ribosomal protein S18 (RPS18; Mm02601777). Amplification of specific PCR products was confirmed by the 2(−ΔΔCT) method (where CT is threshold cycle) with dissociation curve analysis for each primer. Data were normalized to RPS18.
Statistics
Data are shown as means ± SD. Between-group comparisons were made with two-way ANOVA with repeated measures (for water restriction, water loading, and OPC-31260 experiments), with a Sidak post hoc test, one-Way ANOVA with a post hoc Tukey test, or two-tailed Student’s t test as indicated in the figures and tables. P < 0.05 was used as the significance threshold. All analyses were performed with Prism 9 software.
RESULTS
IRAP Was Expressed in Multiple Sites Along the Nephron
To understand the physiological role of IRAP in the kidney, we used immunohistochemistry to determine where IRAP is expressed along the nephron. Cells expressing IRAP included podocytes (demarcated by nephrin; Fig. 1, A–C), in the proximal convoluted tubules (demarcated by Lotus tetragonolobus lectin; Fig. 1, D and G), in the distal convoluted tubules (demarcated by Dolichos biflorus lectin; Fig. 1, D–G), in the thick ascending limb of Henle (demarcated by Na+-K+-2Cl− cotransporter), and in the collecting duct (demarcated by AQP2; Fig. 1, E–K). As expected, there was no expression of IRAP in KO mice (see Fig. A1, A–F, in the appendix).
Figure 1.

Expression of insulin-regulated aminopeptidase (IRAP) along the nephron. IRAP was expressed in the podocytes (labeled with nephrin; A–C), in the proximal tubule [labeled with Lotus tetragonolobus lectin (LTL); D, F, and G], in the distal convoluted tubule [labeled with Dolichos biflorus lectin (DBA); E–G], in the loop of Henle [labeled with Na+-K+-2Cl− cotransporter (NKCC2); I–K], in the cortical collecting ducts [labeled with aquaporin 2 (AQP2); H, J, and K], and in the medullary collecting ducts (L, M, and N). Scale bar = 20 μm. Blue indicates DAPI.
IRAP KO Could Concentrate Urine Further After Water Restriction but Could Not Decrease Urine Osmolality After an Acute Water Load
IRAP cleaves vasopressin in vivo and IRAP KO mice have high levels of vasopressin at baseline (6). We, therefore, assessed the baseline kidney phenotype of IRAP KO mice by measuring body weight, blood pressure, GFR with FITC-sinistrin, proteinuria, urine osmolality, serum osmolality, and blood electrolytes at baseline (Table 1). We found no differences between IRAP KO and WT control mice except for urine osmolality, where IRAP KO mice had higher urine osmolality at baseline (2,506 ± 309 vs. 1,007 ± 212 mosmol/kgH2O/L) than WT controls (P < 0.001; Fig. 2A). These data are consistent with the urinary concentrating effects of vasopressin.
Table 1.
Baseline characteristics of IRAP WT vs. KO mice
| IRAP WT | IRAP KO | |
|---|---|---|
| Body weight, g | 25 ± 1.5 | 25 ± 2.4 |
| Systolic blood pressure, mmHg | 122 ± 6.6 | 128 ± 1.9 |
| Serum Na+ | 142 ± 1.9 | 143 ± 2.6 |
| Serum K+ | 5.3 ± 0.83 | 5.6 ± 0.38 |
| Serum Cl− | 104 ± 1.8 | 108 ± 4.0 |
| Glomerular filtration rate, µL/min | 251 ± 20 | 254 ± 16 |
| Urinary protein/creatinine, (mg/dL)/(mg/dL) | 4.7 ± 1.8 | 3.5 ± 0.72 |
| Serum osmolality, mosmol/kgH2O/L | 325 ± 5.0 | 331 ± 3.8 |
Data are presented as means ± SD; n = 6 per group for all measures except serum osmolality, where n = 4 per group. There were no significant differences between insulin-regulated aminopeptidase (IRAP) wild-type (WT) and knockout (KO) mice at baseline in body weight, systolic blood pressure, blood Na+, K+, and Cl−, glomerular filtration rate, urinary protein, or serum osmolality. Between-group comparisons were performed using an unpaired Student’s t test.
Figure 2.
Insulin-regulated aminopeptidase (IRAP) knockout (KO) mice have persistently elevated urinary osmolality (UOsm). IRAP KO mice had higher UOsm at baseline vs. wild-type (WT) controls (P = 0.0019 for the interaction and P < 0.001 WT vs. KO at baseline; A). IRAP KO mice concentrated their urine further after 24-h fluid restriction (A) and lost less weight than WT mice (P = 0.0486 for the interaction; B). IRAP KO mice were unable to decrease UOsm after an acute water load (P = 0.0031 for the interaction and P = 0.010 for WT vs. KO at baseline; C). IRAP KO mice had lower blood Na+ levels after water loading; the interaction was not significant (P = 0.0538 and *P = 0.0173 for WT vs. KO after water load; D). ns, nonsignificant. Data in A−C are presented as means ± SD and were analyzed with two-way ANOVA with repeated measures and a Šídák's multiple comparisons test. n = 8 per group for water restriction experiments and n = 9 per group in water load experiments. Data in D are presented as means ± SD and were analyzed with two-way ANOVA (n = 4 per group) and a Šídák’s multiple comparisons test for between-group comparisons.
Given the high urine osmolality at baseline, we explored the response of IRAP KO mice to 24-h water restriction. After water restriction, both WT and IRAP KO mice increased urine osmolality to similar levels (3,626 ± 283 vs. 4,200 ± 232 mosmol/kgH2O/L, respectively; Fig. 2A), but WT mice lost more weight relative to KO mice (Fig. 2B). These results suggest that IRAP KO mice were more efficient at conserving water during periods of water restriction.
Given the high baseline urine osmolality in IRAP KO mice, we hypothesized that IRAP KO mice would be unable to decrease urine osmolality in response to an acute water load. We, therefore, performed acute water loading [10% of total body weight (in g) in mL of sterile water intraperitoneally] and collected urine 1 h after water loading for both WT and KO mice. WT mice could dilute their urine from a mean urine osmolality of 1,007 ± 212 to 577 ± 273 mosmol/kgH2O/L (P = 0.027), whereas IRAP KO mice could not (2,505 ± 309 vs. 2,740 ± 400 mosmol/kgH2O/L, not significant; Fig. 2C). The inability to decrease urine osmolality after water loading suggested water retention in IRAP KO mice. This was confirmed by the fact that although blood Na+ decreased in both WT and KO mice, levels were lower in IRAP KO mice compared with WT mice (127 ± 3.8 vs. 133 ± 3.1 mEq/L, P = 0.0173; Fig. 2D and Table A2 in the appendix). These results suggest that IRAP KO mice retained water due to persistent urinary concentration in IRAP KO mice following an acute water challenge.
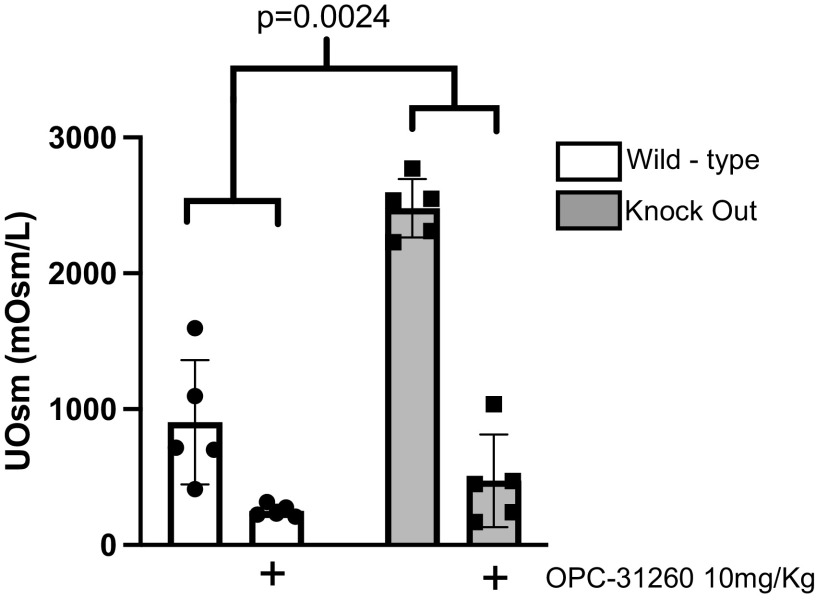
High Urine Osmolality in IRAP KO Mice Was Dependent on V2R Activation
Increased circulating vasopressin (6) and persistently high urine osmolality suggested increased V2R signaling in IRAP KO mice. To determine whether the increased urinary concentration was mediated by V2R, we treated WT and KO mice with a V2R antagonist [OPC-31260 (10 mg/kg ip)]. This treatment resulted in a drop in urine osmolality to similar levels (251 ± 44 vs. 472 ± 340 mosmol/kgH2O/L) in both WT and KO mice (Fig. 3). We then performed quantitative PCR for V2R to evaluate differences in expression between WT and KO mice. We found decreased V2R mRNA in IRAP KO mice at baseline (Fig. A2A in the appendix), suggesting negative feedback regulation. Together, these results suggest that increased V2R activation contributed to the persistent urine concentration in IRAP KO mice.
Figure 3.
High urinary osmolality in insulin-regulated aminopeptidase (IRAP) knockout (KO) mice requires vasopressin type 2 receptor activation. IRAP knockout (KO) mice have higher urinary osmolality (UOsm) at baseline vs. wild-type (WT) controls. After treatment with the vasopressin type 2 receptor antagonist OPC-31260, UOsm between WT and KO mice was not different. Data are presented as means ± SD and were analyzed with two-way ANOVA with repeated measures and a Šídák's multiple comparisons test; n = 5 per group. P = 0.0024 for the interaction and P = 0.0007 for WT vs. KO mice at baseline; there was no significant difference between WT vs. KO mice after OPC-31260.
IRAP KO Mice Have Higher AQP2 Levels at Baseline and After Acute Water Load
Based on the persistent urinary concentration and response to OPC-31260, we hypothesized that AQP2 expression and phosphorylation would be increased in IRAP KO mice. Surprisingly, we found no differences in the expression of total AQP or phosphorylated AQP2 (phospho-Ser269; Fig. 4, A–D); however, in IRAP KO mice, AQP2 was found predominantly in the apical membrane, as denoted by colocalization with the membrane protein ezrin (Fig. 5, E–H) compared with WT controls (Fig. 5, A–D). After treatment with OPC-31260, the apical distribution of AQP2 decreased in IRAP KO mice (Fig. 5, I–L). Taken together, these data suggest that increased apical abundance of AQP2 contributed to the elevated baseline urine osmolality and was dependent on V2R activity (Fig. 5M).
Figure 4.

Whole kidney expression of total aquaporin 2 (AQP2) and phosphorylated AQP2 is the same in insulin-regulated aminopeptidase (IRAP) wild-type (WT) vs. knockout (KO) mice. Immunoblots of whole kidney lysates of IRAP WT and KO mice had the same amount of total AQP2 (A and B) and phosphorylated AQP2 (Ser269; A and C) at baseline relative to total protein loading control. Data are presented as means ± SD; n = 6 WT mice and n = 5 KO mice. Between-group comparisons were performed using an unpaired Student’s t test. ns, no significant difference.
Figure 5.

Apical membrane expression of total aquaporin 2 (AQP2) is higher in insulin-regulated aminopeptidase (IRAP) knockout (KO) mice at baseline. Immunofluorescence of total AQP2 and the membrane protein ezrin in mouse collecting duct sections showed that colocalization of total AQP2 (B, F, and J) with apical the membrane protein marker ezrin (A, E, and I) was higher in KO mice (G, H, and M) vs. wild-type (WT) mice (C, D, and M) but decreased in KO mice after treatment with OPC-31260 (10 mg/kg; K, L, M). Colocalization analysis was performed with NIS-Elements software. Pearson’s correlation for ezrin (magenta) and AQP2 (green) at baseline and after OPC was calculated in 10 tubules per mouse (n = 4 WT, 4 KO, and 4 KO + OPC). Pearson’s correlation graphs for the average of the five tubules selected in each representative image are shown (D, H, and L). Individual tubule Pearsons’ correlation coefficient values are shown (M). Data in M are presented as means ± SD. Between-group comparisons were performed with a one-way ANOVA with a post hoc Tukey test for multiple comparisons. ****P < 0.001. Scale bar = 20 μm. Blue indicates DAPI.
Acute water loading decreases vasopressin signaling with a subsequent decrease in V2R activation and decreased apical AQP2 membrane abundance and phosphorylation (12–16). Therefore, we hypothesized that the inability of IRAP KO mice to decrease urine osmolality after an acute water load was due to increased AQP2 expression. This was indeed the case, as after water loading IRAP KO mice had higher expression of both total and phosphorylated AQP2 relative to WT controls in whole kidney lysates (Fig. 6, A–C). Phosphorylated AQP2 (phospho-Ser269) was predominantly localized in the apical membrane (Fig. 6, D–I).
Figure 6.

After an acute water load, insulin-regulated aminopeptidase (IRAP) knockout (KO) mice have higher expression of total and phosphorylated aquaporin 2 (AQP2). Immunoblots of whole kidney lysates of IRAP KO mice after water loading had higher expression of both total AQP2 (A and B) and phosphorylated AQP2 (Ser269; A and C) relative to wild-type (WT) water-loaded controls. Immunofluorescence of total AQP2 (green) and phosphorylated AQP2 (Ser269; red) in mouse collecting duct sections showed that IRAP KO mice had higher expression of total AQP2 (D and F vs. G and I) and phosphorylated AQP2 (Ser269; E and F vs. H and I) vs. WT controls. Data in B and C are presented as means ± SD; n = 4 per group. Between-group comparisons were performed using an unpaired Student’s t test. *P = 0.0286 vs. WT and #P = 0.0299 vs. WT. ns, no significant difference between groups. Scale bar = 20 μm. Blue indicates DAPI.
DISCUSSION
Appropriate vasopressin signaling is required for the maintenance of water homeostasis. Stimulation of V2Rs in the kidney by vasopressin leads to higher activity and apical membrane abundance of AQP2 in collecting ducts with consequent increases in water retention and urine osmolality. In the present study, we found that IRAP KO mice had higher urine osmolality at baseline compared with WT controls due to persistent activation of V2R. Furthermore, after an acute water load, they were unable to downregulate AQP2 and decrease urine osmolality. Therefore, we conclude that IRAP deficiency leads to a persistent V2R-dependent increase in AQP2 membrane abundance, which impairs water excretion in response to an acute water load.
One of the key observations from our study was that IRAP KO mice achieve water balance and maintain normal serum osmolality at baseline despite high levels of circulating vasopressin and elevated urine osmolality. This result is consistent with prior observations that mice infused with desmopressin (dDAVP) and kept on a normal diet can maintain water balance and keep normal blood Na+ levels (17, 18). Prior observations in IRAP KO mice (6) have suggested a negative feedback loop wherein the high circulating vasopressin leads to decreased brain vasopressin mRNA and circulating vasopressin protein in IRAP KO mice. Thus, we believe that the negative regulation of central vasopressin secretion is contributing to water balance in IRAP KO mice.
IRAP KO mice were able to further concentrate urine above baseline after water restriction and lost less weight than WT controls (Fig. 2). The postrestriction weight difference between WT and KO mice is likely related to the intrinsic water retention in the KO mice, which limits water losses immediately postrestriction, whereas WT mice lose water (and body weight) until maximal water retention sets in. In healthy individuals, acute water loading causes a drop in circulating vasopressin levels, decreased membrane abundance of AQP2, and decreased water permeability in the collecting ducts, which leads to free water excretion (12–15). We found that at baseline, IRAP KO mice have higher urinary osmolality relative to WT controls due to V2R-mediated higher abundance of apical membrane AQP2 (Fig. 5), and acute water loading failed to decrease apical membrane abundance and phosphorylation of AQP2 (Fig. 6).
The effects of persistently elevated vasopressin have been studied in the past. Many of these studies have used dDAVP infusion as a model (17–21). dDAVP is a synthetic analog of vasopressin that cannot be degraded by IRAP (22, 23). This is highly relevant because dDAVP infusion models have been used to study a phenomenon called vasopressin escape. In vasopressin escape, persistent activation of V2R via dDAVP in combination with water loading leads to decreased expression of Aqp2 mRNA, AQP2 protein, and a paradoxical drop in urine osmolality despite high dDAVP levels (18–21). Elegant studies have shown that vasopressin escape is mediated through vasopressin-independent mechanisms that decrease V2R signaling, Aqp2 mRNA, and AQP2 protein (18–21). Since IRAP does not degrade dDAVP, vasopressin escape cannot be explained by changes in IRAP expression or activity.
Our results suggest that IRAP is involved in decreasing vasopressin signaling in response to acute water loading. Whether this is a function of IRAP directly degrading vasopressin within the kidney or elsewhere in the body cannot be answered with our current model.
Perspectives and Significance
The evolutionary conservation of the vasopressin system across multiple species points to its physiological importance. Prior work has shown that there is a clear interplay between vasopressin and insulin (24–26). There are multiple studies that have shown an association between elevated vasopressin levels, insulin resistance, and worse cardiovascular outcomes (24–29). Moreover, prior research suggests that increased vasopressin signaling can lead to worsened kidney function, directly or indirectly (via activation of the renin-angiotensin-aldosterone system) (30–35). Our results suggest that a lack of IRAP leads to increased vasopressin signaling in the kidney. We speculate that IRAP in the kidney plays a role in regulating local vasopressin signaling, which, in turn, might alter kidney function. Therefore, the study of IRAP activity and regulation in the kidney remains a critical area that remains to be explored.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants K08DK135931-01 (to J.P.A.), DK51265, DK95785, DK62794, DK7569, and P30DK114809 (to R.C.H. and M.Z.Z.), DK116964 (to G.B.), DK069921 and DK127589 (to R.Z.), DP5OD033412 (to A.S.T.), and K08DK134879-01A1 (to F.B.); Veterans Affairs Merit Award 00507969 (to R.C.H.) and Veterans Affairs Merit Award I01-BX002196 (to R.Z.); an American Society of Nephrology-Kidney Cure career development award (to J.A.W.); Intramural Research Program of the NIH Grant ES103361-01 (to J.A.W.); and the Vanderbilt Center for Kidney Disease. J.P.A. is a Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program Scholar. F.B. is supported by a postdoctoral Ben J. Lipps fellowship from the American Society of Nephrology and a Vanderbilt Faculty Research Scholar Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Z., G.B., J.A.W., R.Z., and J.P.A. conceived and designed research; Y.Z., J.C., J.B.T., S.K., and J.P.A. performed experiments; Y.Z., F.B., J.B.T., and J.P.A. analyzed data; Y.Z., J.C., A.S.T., F.B. J.A.W., R.Z., R.C.H., and J.P.A. interpreted results of experiments; Y.Z. and J.P.A. prepared figures; Y.Z. and J.P.A. drafted manuscript; Y.Z., J.C., A.S.T., F.B., G.B., J.A.W., S.K., M.Z., R.Z., R.C.H., and J.P.A. edited and revised manuscript; R.C.H. and J.P.A. approved final version of manuscript.
APPENDIX
Table 1A shows the primary and secondary antibodies as well as the conditions used in this study.
Table A1.
List of antibodies and conditions used for immunohistochemistry and immunoblot analysis
| Antibody Target | Host Species | Catalog Number | Vendor | Dilution and Application | Time |
|---|---|---|---|---|---|
|
Primary antibodies | |||||
| Irap | Rabbit | 6918S | Cell Signaling | IF 1:1,000, WB 1:1,000 | 1 hr RT |
| Nephrin | Goat | AF3159 | Novus bio | IF 1:200 | 1 hr RT |
| AQP2 FITC | Mouse | sc-515770 | Santa Cruz Biotechnology | IF 1:100, WB 1:500 | 1 hr RT |
| AQP2 - phospho | Rabbit | p112-269t | Phosphosolutions | WB 1:1,000 | 1 hr RT |
| NKCC2 | Sheep | NKCC2 total | MRC PPU Reagents | WB 1:1,000 | 1 hr RT |
| Ezrin | Rabbit | 3145 | Cell Signaling | IF 1:500 | 1 hr RT |
| LTL | None | FL-1321-2 | Vector Laboratories | IF 1:200, IHC 1:200 | 30 min RT |
| DBA | None | RL-1032-2 | Vector Laboratories | IF 1:200, IHC 1:200 | 30 min RT |
| Secondary antibodies | |||||
| Anti-rabbit IgG | Goat | 111-035-003 | Jackson Immunoresearch | WB 1:5,000 | 1 hr RT |
| Anti-rabbit IgG AF 647 | Donkey | 711-605-152 | Jackson Immunoresearch | WB 1:5,000 | 1 hr RT |
| Anti-sheep IgG | mouse | 213-032-177 | Jackson Immunoresearch | WB 1:5,000 | 1 hr RT |
AQP2, aquaporin 2; AQP2 - phospho, phosphorylated aquaporin 2; DBA, Dolichos biflorus lectin; IF, immunofluorescence; IRAP, insulin-regulated aminopeptidase; LTL, Lotus tetragonolobus lecti; NKCC2, Na+-K+-2Cl− cotransporter; RT, room temperature; WB, Western blot.
Although blood Na+ decreased in both WT and KO mice, levels were lower in IRAP KO mice compared with WT mice (Table A2).
Table A2.
Blood electrolytes in IRAP WT vs. KO mice before and after an acute water load
| IRAP WT |
IRAP KO |
|||
|---|---|---|---|---|
| Baseline | After Water Load | Baseline | After Water Load | |
| Blood Na+ | 142 ± 1.9 | 133 ± 3.1 | 143 ± 2.6 | 127 ± 3.8* |
| Blood K+ | 5.3 ± 0.83 | 5.1 ± 0.84 | 5.6 ± 0.38 | 5.2 ± 0.58 |
| Blood Cl− | 104 ± 1.8 | 99 ± 3.5 | 108 ± 4.0 | 94 ± 2.6 |
Values are means ± SD; n = 4 animals per group. IRAP, insulin-regulated aminopeptidase; KO, knockout; WT, wild-type. *P = 0.0173 vs. WT mice after water load (by two-way ANOVA and a Šídák's multiple comparisons test).
As expected, there was no expression of IRAP in KO mice (Fig. A1, A–F).
Figure A1.
Insulin-regulated aminopeptidase (IRAP) knockout (KO) mice do not express IRAP in the kidney, whereas wild-type (WT) mice do. A−F: podocytes were labeled with nephrin, proximal tubules were labeled with Lotus tetragonolobus lectin (LTL), distal tubules were labeled with Dolichos biflorus lectin (DBA), loops of Henle were labeled with Na+-K+-2Cl− cotransporter (NKCC2), and collecting ducts were labeled with AQP2 Scale bars = 10 μm in A, B, E, and F and 50 μm in C and D. Blue indicates DAPI. G: immunoblot analysis showing IRAP in whole kidney lysates from WT mice but not from KO mice.
We found decreased V2R mRNA in IRAP KO mice at baseline (Fig. A2A), suggesting negative feedback regulation.
Figure A2.
Insulin-regulated aminopeptidase (IRAP) knockout (KO) mice had less vasopressin receptor type 2 mRNA [arginine vasopressin receptor 2 (Avpr2)] relative to wild-type (WT) controls (A). IRAP wild-type (WT) and KO mice expressed similar levels of aquaporin 2 (Aqp2) mRNA (B) and aquaporin 3 (Aqp3) mRNA (C) at baseline.
REFERENCES
- 1. Czaczkes JW, Kleeman CR, Koenig M. Physiologic studies of antidiuretic hormone by its direct measurement in human plasma. J Clin Invest 43: 1625–1640, 1964. doi: 10.1172/JCI105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallis MG, Lankford MF, Keller SR. Vasopressin is a physiological substrate for the insulin-regulated aminopeptidase IRAP. Am J Physiol Endocrinol Physiol 293: E1092–E1102, 2007. doi: 10.1152/ajpendo.00440.2007. [DOI] [PubMed] [Google Scholar]
- 3. Keller SR. The insulin-regulated aminopeptidase: a companion and regulator of GLUT4. Front Biosci 8: s410–s420, 2003. doi: 10.2741/1078. [DOI] [PubMed] [Google Scholar]
- 4. Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J Biol Chem 270: 23612–23618, 1995. [Erratum in J Biol Chem 270: 30236, 1995]. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- 5. Jordens I, Molle D, Xiong W, Keller SR, McGraw TE. Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles. Mol Biol Cell 21: 2034–2044, 2010. doi: 10.1091/mbc.e10-02-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keller SR, Davis AC, Clairmont KB. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J Biol Chem 277: 17677–17686, 2002. doi: 10.1074/jbc.M202037200. [DOI] [PubMed] [Google Scholar]
- 7. Albiston AL, Yeatman HR, Pham V, Fuller SJ, Diwakarla S, Fernando RN, Chai SY. Distinct distribution of GLUT4 and insulin regulated aminopeptidase in the mouse kidney. Regul Pept 166: 83–89, 2011. doi: 10.1016/j.regpep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8. Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE. Dehydration parameters and standards for laboratory mice. J Am Assoc Lab Anim Sci 52: 233–239, 2013. [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamura Y, Ogawa H, Yamashita H, Chihara T, Miyamoto H, Nakamura S, Onogawa T, Yamashita T, Hosokawa T, Mori T, Tominaga M, Yabuuchi Y. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br J Pharmacol 105: 787–791, 1992. doi: 10.1111/j.1476-5381.1992.tb09058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sinha S, Dwivedi N, Tao S, Jamadar A, Kakade VR, Neil MO, Weiss RH, Enders J, Calvet JP, Thomas SM, Rao R. Targeting the vasopressin type-2 receptor for renal cell carcinoma therapy. Oncogene 39: 1231–1245, 2020. doi: 10.1038/s41388-019-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scarfe L, Schock-Kusch D, Ressel L, Friedemann J, Shulhevich Y, Murray P, Wilm B, de Caestecker M. Transdermal measurement of glomerular filtration rate in mice. J Vis Exp 140: 58520, 2018. doi: 10.3791/58520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saito T, Ishikawa SE, Sasaki S, Fujita N, Fushimi K, Okada K, Takeuchi K, Sakamoto A, Ookawara S, Kaneko T, Marumo F, Saito T. Alteration in water channel AQP-2 by removal of AVP stimulation in collecting duct cells of dehydrated rats. Am J Physiol Renal Physiol 272: F183–F191, 1997. doi: 10.1152/ajprenal.1997.272.2.F183. [DOI] [PubMed] [Google Scholar]
- 13. Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frøkiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 10: 647–663, 1999. doi: 10.1681/ASN.V103647. [DOI] [PubMed] [Google Scholar]
- 14. Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 52: 2340–2352, 1973. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olesen ETB, Fenton RA. Aquaporin 2 regulation: implications for water balance and polycystic kidney diseases. Nat Rev Nephrol 17: 765–781, 2021. doi: 10.1038/s41581-021-00447-x. [DOI] [PubMed] [Google Scholar]
- 16. Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishikawa S, Saito T, Kasono K. Pathological role of aquaporin-2 in impaired water excretion and hyponatremia. J Neuroendocrinol 16: 293–296, 2004. doi: 10.1111/j.0953-8194.2004.01177.x. [DOI] [PubMed] [Google Scholar]
- 18. Saito T, Higashiyama M, Nagasaka S, Sasaki S, Saito T, Ishikawa SE. Role of aquaporin-2 gene expression in hyponatremic rats with chronic vasopressin-induced antidiuresis. Kidney Int 60: 1266–1276, 2001. doi: 10.1046/j.1523-1755.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 19. Lee JW, Alsady M, Chou CL, de Groot T, Deen PMT, Knepper MA, Ecelbarger CM. Single-tubule RNA-Seq uncovers signaling mechanisms that defend against hyponatremia in SIADH. Kidney Int 93: 128–146, 2018. doi: 10.1016/j.kint.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997. doi: 10.1172/JCI119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ecelbarger CA, Chou CL, Lee AJ, DiGiovanni SR, Verbalis JG, Knepper MA. Escape from vasopressin-induced antidiuresis: role of vasopressin resistance of the collecting duct. Am J Physiol Renal Physiol 274: F1161–F1166, 1998. doi: 10.1152/ajprenal.1998.274.6.F1161. [DOI] [PubMed] [Google Scholar]
- 22. Stegner H, Artman HG, Leake RD, Fisher DA. DDAVP (1-desamino-8-D-arginine vasopressin) clearance rate. Acta Endocrinol (Copenh) 107: 204–206, 1984. doi: 10.1530/acta.0.1070204. [DOI] [PubMed] [Google Scholar]
- 23. Gazis D, Sawyer WH. Elimination of infused arginine-vasopressin and its long-acting deaminated analogue in rats. J Endocrinol 78: 179–186, 1978. doi: 10.1677/joe.0.0780179. [DOI] [PubMed] [Google Scholar]
- 24. Vokes TP, Aycinena PR, Robertson GL. Effect of insulin on osmoregulation of vasopressin. Am J Physiol Endocrinol Physiol 252: E538–E548, 1987. doi: 10.1152/ajpendo.1987.252.4.E538. [DOI] [PubMed] [Google Scholar]
- 25. Baylis PH, Zerbe RL, Robertson GL. Arginine vasopressin response to insulin-induced hypoglycemia in man. J Clin Endocrinol Metab 53: 935–940, 1981. doi: 10.1210/jcem-53-5-935. [DOI] [PubMed] [Google Scholar]
- 26. Spruce BA, McCulloch AJ, Burd J, Orskov H, Heaton A, Baylis PH, Alberti KG. The effect of vasopressin infusion on glucose metabolism in man. Clin Endocrinol (Oxf) 22: 463–468, 1985. doi: 10.1111/j.1365-2265.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 27. Jansen LT, Suh H, Adams JD, Sprong CA, Seal AD, Scott DM, Butts CL, Melander O, Kirkland TW, Vanhaecke T, Dolci A, Lemetais G, Perrier ET, Kavouras SA. Osmotic stimulation of vasopressin acutely impairs glucose regulation: a counterbalanced, crossover trial. Am J Clin Nutr 110: 1344–1352, 2019. doi: 10.1093/ajcn/nqz236. [DOI] [PubMed] [Google Scholar]
- 28. Enhörning S, Melander O. The vasopressin system in the risk of diabetes and cardiorenal disease, and hydration as a potential lifestyle intervention. Ann Nutr Metab 72, Suppl 2: 21–27, 2018. doi: 10.1159/000488304. [DOI] [PubMed] [Google Scholar]
- 29. Enhörning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm E, Groop L, Lyssenko V, Orho-Melander M, Newton-Cheh C, Melander O. Plasma copeptin and the risk of diabetes mellitus. Circulation 121: 2102–2108, 2010. doi: 10.1161/CIRCULATIONAHA.109.909663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 18: 497–506, 2003. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 31. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 311: F1087–F1108, 2016. doi: 10.1152/ajprenal.00340.2016. [DOI] [PubMed] [Google Scholar]
- 32. Wannamethee SG, Welsh P, Papacosta O, Lennon L, Whincup PH, Sattar N. Copeptin, insulin resistance, and risk of incident diabetes in older men. J Clin Endocrinol Metab 100: 3332–3339, 2015. doi: 10.1210/JC.2015-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kosmas CE, Silverio D, Tsomidou C, Salcedo MD, Montan PD, Guzman E. The impact of insulin resistance and chronic kidney disease on inflammation and cardiovascular disease. Clin Med Insights Endocrinol Diabetes 11: 1179551418792257, 2018. doi: 10.1177/1179551418792257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whaley-Connell A, Sowers JR. Insulin resistance in kidney disease: is there a distinct role separate from that of diabetes or obesity? Cardiorenal Med 8: 41–49, 2017. doi: 10.1159/000479801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45: 275–280, 2005. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.