Abstract
Hypogonadism in males confers elevated cardiovascular disease (CVD) risk by unknown mechanisms. Recent radiological evidence suggests that low testosterone (T) is associated with mediobasal hypothalamic (MBH) gliosis, a central nervous system (CNS) cellular response linked to metabolic dysfunction. To address mechanisms linking CNS androgen action to CVD risk, we generated a hypogonadal, hyperlipidemic mouse model with orchiectomy (ORX) combined with hepatic PCSK9 overexpression. After 4 wk of high-fat, high-sucrose diet (HFHS) consumption, despite equal body weights and glucose tolerance, androgen-deficient ORX mice had a more atherogenic lipid profile and increased liver and leukocyte inflammatory signaling compared with sham-operated control mice. Along with these early CVD risk indicators, ORX markedly amplified HFHS-induced astrogliosis in the MBH. Transcriptomic analysis further revealed that ORX and high-fat diet feeding induced upregulation of inflammatory pathways and downregulation of metabolic pathways in hypothalamic astrocytes. To interrogate the role of sex steroid signaling in the CNS in cardiometabolic risk and MBH inflammation, central infusion of T and dihydrotestosterone (DHT) was performed on ORX mice. Central DHT prevented MBH astrogliosis and reduced the liver inflammatory signaling and monocytosis induced by HFHS and ORX; T had a partial protective effect. Finally, a cross-sectional study in 41 adult men demonstrated a positive correlation between radiological evidence of MBH gliosis and plasma lipids. These findings demonstrate that T deficiency in combination with a Western-style diet promotes hypothalamic gliosis concomitant with increased atherogenic risk factors and provide supportive evidence for regulation of lipid metabolism and cardiometabolic risk determinants by the CNS action of sex steroids.
NEW & NOTEWORTHY This study provides evidence that hypothalamic gliosis is a key early event through which androgen deficiency in combination with a Western-style diet might lead to cardiometabolic dysregulation in males. Furthermore, this work provides the first evidence in humans of a positive association between hypothalamic gliosis and LDL-cholesterol, advancing our knowledge of CNS influences on CVD risk progression.
Keywords: astrocyte, cardiovascular risk, hypogonadism, hypothalamic gliosis, testosterone
INTRODUCTION
A growing body of evidence suggests that hypogonadism is a major contributor to cardiovascular disease (CVD) incidence (1–4). Hypogonadism is common, estimated to be as high as 38.7% in men over the age of 45 yr (5), and those whose plasma testosterone (T) levels are within the lowest tertile have a 20–30% higher risk of developing CVD (3, 4, 6). At the present time, the safety and efficacy of testosterone replacement therapy (TRT) is hotly debated since some studies have shown increased CV events with TRT (7, 8). This uncertainty provides a rationale to develop a deeper understanding of the links between androgens and CVD for more targeted approaches to therapy.
Orchiectomy (ORX) in rodents serves as a useful model to interrogate mechanisms relating androgen signaling to cardiometabolic health and to identify key tissues involved in this relationship. As in humans, androgen deficiency promotes cardiometabolic dysfunction including fat mass accumulation, glucose intolerance, and hepatic steatosis, particularly when combined with long-term consumption of a high-fat diet (HFD) more similar to the typical Western diet than normal rodent chow (9, 10). Moreover, ORX in mouse models susceptible to CVD (e.g., LDL receptor knockout, apolipoprotein E knockout) increases total plasma concentrations of cholesterol, triglycerides (TG), and apolipoprotein B (APOB)-containing lipoproteins and accelerates atherosclerosis (11–13). Importantly, restoration of physiological testosterone levels both reestablishes lipid homeostasis and reduces inflammatory signaling, together acting to improve cardiometabolic health (13, 14); however, given the widespread expression of the androgen receptor (AR) (15), the tissue-level mechanisms underlying these protective effects of testosterone remain unclear. Since neuron-specific AR-deficient male mice develop obesity, insulin resistance, and hypothalamic inflammation (16), the brain is a potential site of androgen action that mediates cardiometabolic protection.
In response to central nervous system (CNS) insults, resident glial cells including microglia and astrocytes adopt an activated phenotype and accumulate at sites of injury, a process called gliosis (17). In the context of metabolism, gliosis arises in the mediobasal hypothalamus (MBH) of rodents fed obesogenic diets before increases in body weight (18–20), and both microglial and astrocytic inflammatory activation promote obesity pathogenesis and metabolic dysfunction (21–23). Importantly, magnetic resonance imaging (MRI) studies have confirmed that MBH gliosis also occurs in obese humans (18, 24) and is inversely correlated with serum testosterone levels independently of body mass index (BMI) (25). Conversely, in rodents, testosterone treatment reduces gliosis after brain injury (26–29). Together, these data suggest that androgen action in the CNS via MBH glia modulates metabolic function, a hypothesis we tested by determining whether testosterone deficiency amplifies gliosis and determinants of CVD risk and whether central androgen treatment can reverse these phenotypes (26–29).
MATERIALS AND METHODS
Animals
Adult male wild-type (WT) C57BL/6J, Aldh1l1Cre/ERT2, and ROSA26eGFP-L10a mice were obtained from Jackson Laboratory and housed in temperature-controlled rooms with a 14:10-h light-dark cycle under specific pathogen-free conditions. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care Committee at the University of Washington.
Mouse model of hypercholesterolemia/atherosclerosis.
Male C57BL/6J mice were given a single dose of 1 × 1011 vector genome copies of AAV8-D377Y-mPCSK9 (Vector Biolabs) or saline control through retroorbital injection. Mice were switched to high-fat, high-sucrose diet (HFHS; see description of the diet below) at least 14 days after virus administration. To confirm the efficiency of the method, plasma cholesterol, triglycerides, and lipoprotein profiles were determined (see below).
Mouse model for TRAP in astrocytes.
The generation of Aldh1l1eGFP-L10a mice for immunoprecipitation of ribosomes [translating ribosome affinity purification (TRAP)] was achieved by crossing Aldh1l1CreERT2 heterozygotes to homozygous Rosa26eGFP-L10a mice. The presence of the CreERT2 or wild-type allele was determined with the following protocol: primers for internal control: fwd 5′-CTG TCC CTG TAT GCC TCT GG-3′, rev 5′-AGA TGG AGA AAG GAC TAG GCT ACA-3′; transgene: fwd 5′-CTT CAA CAG GTG CCT TCC A-3′, rev 5′-GGC AAA CGG ACA GAA GCA-3′. PCR reaction was performed with Lucigen EconoTaq PLUS (Fisher Scientific, catalog no. NC0421795) and the thermocycle program as follows: 1) 94°C for 2 min, 2) 10 cycles with annealing temperature decreasing 0.5°C per cycle (denature: 94°C for 20 s, anneal: 65°C for 15 s, extension: 68°C for 10 s), 3) 28 cycles (denature: 94°C for 15 s, anneal: 60°C for 15 s, extension: 72°C for 10 s), 4) 72°C for 2 min. Amplicon sizes were 198 bp for the CreERT2 transgene and 415 bp for the internal control. Six-week-old Aldh1l1eGFP-L10a mice were administered four daily intraperitoneal injections of tamoxifen (TMX, 0.4 mg dissolved in 200 μL warm purified corn oil; Sigma, T5648) to induce Cre-mediated recombination. Specificity of L10a-enhanced green fluorescent protein (eGFP) expression exclusively in astrocytes was confirmed by immunochemistry (IHC) (Supplemental Fig. S4, available at https://doi.org/10.6084/m9.figshare.22064822). Twelve weeks after TMX administration, mice were subjected to ORX or sham surgery (see below).
Diets, Hormones, and Virus
Animals were fed regular chow, high-fat diet [HFD; D12492, Research Diets; 60% kcal from fat (lard), 20% kcal from carbohydrates (maltodextrin and sucrose in ∼2-to-1 ratio)], or high-fat, high-sucrose diet with added cholesterol [HFHS; F4997, BioServ; 58.9% kcal from fat (lard), 26.2% kcal from carbohydrates (maltodextrin and sucrose in ∼1-to-2 ratio) with 0.15% added cholesterol] as described previously (30). Testosterone and dihydrotestosterone (DHT) were obtained from Sigma (catalog nos. T1500 and A8380). AAV8-D377Y-mPCSK9 was purchased from Vector Biolabs.
Surgical Procedures
Bilateral orchiectomy.
Mice were anesthetized with continuous administration of 3% isoflurane. A 1-cm skin incision was made in the scrotum, and each testicle was removed one at a time after ligation of the testicular artery. The incision was closed with surgical suture (6-0; Ethicon). Sham-operated animals went through the same surgical procedure without removal of the testes. All mice were provided analgesia with buprenorphine (0.05 mg/kg sc) administered postoperatively.
Testosterone replacement procedure.
The Silastic capsule method was used to provide systemic testosterone based on diffusion (31, 32). A 10-mm section of Silastic tubing (Dow Corning; 1.57-mm inner diameter and 3.18-mm outer diameter) was filled with testosterone, and the end of the tubing was sealed with water-resistant silicon rubber (Multi-Purpose Sealant; Dow Corning). During ORX, testosterone-replaced mice also underwent placement of an intrascapular subcutaneous Silastic implant, whereas control mice received empty Silastic tubing.
Chronic intracerebroventricular infusion.
Four groups of 12- to 16-wk-old male C57BL/6J mice underwent implantation of a lateral ventricle (LV) cannula (Brain Infusion Kit; ALZET) using standard stereotaxic coordinates (0.7 mm posterior to bregma, 1.3 mm lateral, and 2.1 mm below the skull surface). A catheter tube filled with sterile saline was connected to the cannula and implanted subcutaneously in the intrascapular region of the animal. After 14 days of recovery time from LV cannulation, mice were subjected to ORX or sham surgery and the catheter tube connected to the LV cannula was attached to a subcutaneously implanted osmotic minipump (model 1004, 28-day infusion at 0.11 µL/h; Alzet) containing vehicle (2-hydroxypropyl-β-cyclodextrin 20% in sterile saline), testosterone (0.9 µg/µL), or DHT (0.2 µg/µL). The concentrations of testosterone and DHT were based on previous rodent studies showing that these doses induce CNS androgen signaling when infused centrally (33, 34). Mice were switched to HFHS 3 days after minipump implantation, and measurements of body weight and food intake were continued for the entirety of the 28-day infusion.
Animal Model of Chemical Castration
A model of secondary hypogonadism was achieved by repeated administration of acyline, a competitive GnRH antagonist, as described previously (35). Briefly, two groups of C57BL/6J male mice were subcutaneously injected three times a week (Monday, Wednesday, and Friday) with either vehicle (sterile saline) or acyline (100 µg/mouse) for 6 wk. At week 2, mice were switched to HFHS diet for the last 4 wk.
Body Composition
Determination of body fat and lean mass was performed by quantitative magnetic resonance spectroscopy (EchoMRI, Houston, TX) in conscious mice with the Energy Balance Core of the NIH-funded Nutrition Obesity Research Center at the University of Washington (36).
Glucose Tolerance Testing
Intraperitoneal glucose tolerance tests (GTTs) were conducted in 5-h-fasted mice by intraperitoneal injection of 30% d-glucose (2 g/kg). Blood glucose levels were measured periodically over 120 min in tail capillary blood applied to a handheld glucose meter (OneTouch Ultra).
Plasma Analyses
Plasma total cholesterol and triglyceride concentrations were measured with colorimetric assay kits from Wako (catalog no. 635-50981) and Sigma (catalog no. T2449), respectively. Apolipoprotein B (APOB) was measured by an ELISA kit obtained from Abcam (catalog no. ab230932). The cholesterol content of plasma lipoprotein fractions was measured after separation of lipoproteins by fast-phase liquid chromatography (FPLC) according to the method of Garber et al. (37). A 100-μL aliquot of plasma obtained retroorbitally at the end of the study was applied to a Superose 6 HR 10/30 column (10 mm × 300 mm; Amersham Biosciences Corp). Sixty 0.5-mL fractions were collected, and fractions 11–42 were analyzed for cholesterol content. Values for the amount of cholesterol within each of the lipoprotein classes were determined by quantifying the area under the curve from each lipoprotein profile with fractions 15–20 for very low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL), fractions 21–27 for low-density lipoprotein (LDL), and fractions 28–35 for high-density lipoprotein.
Flow Cytometry
Retroorbital blood was collected and incubated with RBC lysis buffer to eliminate erythrocytes. Samples were incubated in consecutive steps with viability dye (eBioscience, catalog no. 65-0863) for 20 min, Fc blocker (Serotec, catalog no. BUF041B) for 10 min, and a mixed antibody solution for 30 min. The antibodies used were allophycocyanin (APC)-labeled anti-CD115 (eBioscience, clone AFS98), phycoerythrin (PE)-Cy7-labeled anti-GR1 (eBioscience, clone RB6-8C5), PE-labeled anti-CD45 (eBioscience, clone 30-F11), and FITC-labeled anti-CCR2 (BioLegend, clone SA203G11). Flow cytometry was conducted on a FACSCanto RUO (BD), and single live cells were gated based on their forward versus side scatter profile and exclusion of the viability dye. Clusters were identified as Ly6Chi monocytes by CD45+CD115+GR1hi marker status and as Ly6Clo monocytes by CD45+CD115+GR1lo status. Total cell counts were analyzed with a Cellometer Auto 2000 Cell Viability Counter (Nexcelom Bioscience).
Tissue Processing
For immunohistochemical (IHC) studies, animals were anesthetized with a ketamine + xylazine cocktail (140 mg of ketamine and 12 mg of xylazine/kg body weight) and then perfused with ice-cold PBS followed by 4% paraformaldehyde (PFA) in PBS with a peristaltic pump (4 mL/min). Brains were removed, postfixed in 4% PFA overnight at 4°C, placed in 25% sucrose, cryosectioned at 30 µm in the coronal plane through the hypothalamus with a freezing microtome (Leica SM2010R), and stored in freezing solution (phosphate buffer, 30% sucrose, 30% ethylene glycol) at −20°C for IHC staining.
For RNA extraction and TRAP, animals were anesthetized with CO2 and rapidly decapitated. Trunk blood was collected, and leukocytes were isolated by centrifugation for further RNA extraction. Epididymal fat, liver, and hypothalamus were dissected and stored at −80°C until RNA extraction and TRAP.
Translating Ribosome Affinity Purification
Protein A/G magnetic beads (Pierce catalog no. 88803) were precoated with anti-GFP monoclonal antibodies (33.3 µg per 200 µL of beads of 19C8 and 19F7; Heintz Lab; Rockefeller University catalog no. Htz-GFP-19C8, RRID: AB_2716737) for 4 h. Antibody-coated beads were washed three times with low-salt buffer (1% NP-40, 100 mM KCl, 50 mM Tris·HCl pH 7.4, 12 mM MgCl2) and stored at 4°C until use the next day. One hypothalamus per sample (6 or 7 mice/group) was gently homogenized with a Dounce prefilled with 1 mL of buffer (low-salt buffer plus 100 µg/mL cycloheximide, EDTA-free protease inhibitor tablet, 1 mg/mL heparin, 5 µL/mL RNasin, and 1 mM DTT). Homogenized samples were spun at 10,000 g for 10 min, 50 µL of sample was saved as “input” fraction, and the remaining sample was incubated with 200 µL of antibody-coated beads overnight. The unbound fraction was stored as “supernatant,” and beads were washed four times with high-salt buffer (same as low-salt buffer but with 300 mM KCl, 100 µg/mL cycloheximide, and 0.5 mM DTT). The immunoprecipitated fraction was obtained by elution from the beads with RLT buffer (Qiagen). RNAs were purified with the Qiagen RNeasy micro kit (catalog no. 74004).
TRAP-RNA Sequencing
Extracted RNA from the supernatant and immunoprecipitated fractions was used to prepare Illumina-compatible sequencing libraries with QuantSeq 3′ mRNA-Seq Library Prep Kit-FWD (catalog no. 15, Lexogen) according to the manufacturer’s protocol. Cycle numbers for library amplification were determined with the PCR Add-on Kit for Illumina (catalog no. 020.96, Lexogen). After amplification and purification, sequencing library quality was assessed with the Agilent 4200 Tapestation system. The final sequencing libraries were then sequenced on the Illumina NextSeq 500 system. After adaptor trimming with Trim Galore! (v. 0.6.4_dev), the sequencing data were aligned to a decoy-aware index (Gencode GRCm38, release M23) with Salmon (v. 1.9.0) (with the –noLengthCorrection and –numBootstraps 100 arguments) (38, 39). FastQC (v. 0.11.9) and MultiQC (v. 1.11) were used for quality control and to obtain alignment statistics (40). An average of 11.83 million reads were aligned per sample (SD 2.03) (83.26% mapping rate, SD 5.01%).
Cell type proportion estimation.
To evaluate the immunoprecipitation efficiency and correct for possible differences in cell type composition between samples, the deconvolution software MuSiC (v. 0.2.0) was used to estimate cell type proportions (41). Single-cell RNA sequencing (RNA-seq) data from mouse hippocampi were used as a reference (from mousebrain.org) (42). The data were read and processed using SeuratDisk (v. 0.0.0.9019) and Seurat (v. 4.1.1) (43, 44). The gene-summarized raw counts from the TRAP-seq data were extracted with the R package Tximeta (v. 1.10) (with the countsFromAbundance=”no” argument) (45). Cell type proportions were calculated using the average of the outputs of two methods: MuSiC and nonnegative least squares (NNLS) and differences between groups assessed with a t test (rstatix v. 0.7.0).
RNA sequencing analysis.
The R packages Wasabi (v. 1.0.1) and Sleuth (v. 0.30.0) were used to prepare the data and perform gene-level analysis of the TRAP-seq data (sleuth_prep gene_mode = T) (46). Sample groups were compared either with a pairwise comparison (HFD Sham vs. HFD ORX) to test for the effect of ORX in the presence of a high-fat diet or with an ordinal regression to test for genes for which changes between the chow diet and HFD are amplified by the treatment (ORX) (ordered changes following the Chow Sham < HFD Sham < HFD ORX or Chow Sham > HFD Sham > HFD ORX pattern). All analyses were corrected for cell type composition to avoid the confounding effect of differences in immunoprecipitation efficiency and/or cell type proportion between samples. A likelihood ratio test was performed to obtain significance levels (P values, false discovery rate adjusted with the Benjamini–Hochberg procedure) and a Wald test to obtain the effect size (log2 fold change). Gene annotations were obtained with the annotable package (v. 0.1.91). The package fgsea (v. 1.16.0; minimum geneset size minSize = 20) was used for gene set enrichment analysis, using pathways from msigDB (47–50). The C2-CP, C5-GO.MF, C5-GO.BP, and Hallmark pathway sets of msigDB were extracted with the msigdf package (v. 7.4). Signed test statistics from the differential expression analysis were used as a ranking metric. Fgsea’s function collapsePathways was subsequently used to keep only independent pathways. The full list of noncollapsed results is available in Supplemental Data S1, available at http://doi.org/10.6084/m9.figshare.22065005, saved with the openxlsx package, v. 4.2.5). All analyses were performed in R (v. 4.0.5) using the tidyverse packages (v. 1.3.2) for data wrangling and a combination of the packages ggplot2 (v. 3.3.6), ggradar (v. 0.2), ggven (v. 0.1.9), lemon (v. 0.4.5), RColorBrewer (v. 1.1–3), and cowplot (v. 1.1.1) for plotting. The raw and processed data are available on the Gene Expression Omnibus website [Home–GEO–NCBI (nih.gov)] under the GEO accession number GSE214683. The code to reproduce the results is available on the GitHub repository https://github.com/novonordisk-research/ORX_HFD_Astrocytes_TRAPseq.
Real-Time PCR
Total RNA was extracted with the RNeasy mini kit according to the manufacturer’s instructions (Qiagen, catalog no. 74106) and reverse-transcribed with Multiscribe Reverse Transcriptase (Applied Biosystems). Levels of mRNA for Il1b, Il6, Il10, Tnf, Ikbkb, Nfkbia, Arg1, Ccl2, Icam1, Vcam1, Emr1, Cd68, and Rn18S (internal control) were measured by semiquantitative real-time PCR (40 cycles of 95°C for 15 s and 60°C for 1 min) on an ABI Prism 7900 HT (Applied Biosystems) with SYBR Green PCR Supermix (Bio-Rad). The primer sequences used were as follows: Il1b fwd: 5′- TACAAGGAGAGACAAGCAACGACA-3′, rev: 5′- GATCCACACTCTCCAGCTGCA-3′; Il6 fwd: 5′- GTGGCTAAGGACCAAGACCA-3′, rev: 5′- GGTTTGCCGAGTAGACCTCA-3′; Il10 fwd: 5′- GCTCTTACTGACTGGCATGAG-3′, rev: 5′- CGCAGCTCTAGGAGCATGTG-3′; Tnf fwd: 5′- CATCTTCTCAAAACTCGAGTGACAA-3′, rev: 5′- TGGGAGTAGATAAGGTACAGCCC-3′; Ikbkb fwd: 5′- CTGCGGGAAGGAGCTGTC-3′, rev: 5′- GGCTTCAGGTCTCGATGGAT-3′; Nfkbia fwd: 5′- TGCCTGGCCAGTGTAGCAGTCTT-3′, rev: 5′- CAAAGTCACCAAGTGCTCCACGAT-3′; Arg1 fwd: 5′- AACACGGCAGTGGCTTTAACC-3′, rev: 5′- GGTTTTCATCTGGCGCATTC-3′; Ccl2 fwd: 5′- CTTCTGGGCCTGCTGTTCA-3′, rev: 5′- CAAGCCTACTCATTGGGATCA-3′; Icam1 fwd: 5′- GGCATTGTTCTCTAATGTCTCC-3′, rev: 5′- GCTCCAGGTATATCCGAGCTTC-3′; Vcam1 fwd: 5′- TGCACAGTCCCTAATGTGTATCC-3′, rev: 5′- GACTTTATGCCCATTTCCTCCA-3′; Emr1 fwd: 5′- AATCGCTGCTGGTTGAATACAG-3′, rev: 5′- CCAGGCAAGGAGGACAGAGTT-3′; Cd68 fwd: 5′- GAGCCCGAGTACAGTCTACC-3′, rev: 5′- ATTCTGCGCCATGAATGTCC-3′; Rn18S fwd: 5′- CGGACAGGATTGACAGATTG-3′, rev: 5′- CAAATCGCTCCACCAACTAA-3′. The relative gene expression was calculated by the method, where CT is threshold cycle (51).
Immunohistochemical Staining
Free-floating 30-µm coronal brain sections were washed with PBS, blocked with 2.5% normal donkey serum (Jackson ImmunoResearch Laboratories, catalog no. 017-000-121), and incubated overnight with Cy3-conjugated mouse monoclonal anti-glial fibrillary acidic protein (GFAP) (1:1,000; Sigma, catalog no. C9205) and rabbit polyclonal Anti-Iba1 (1:1,000; Wako, catalog no. 019-19741). To visualize the GFP expression in Aldh1l1eGFP-L10a mice, chicken anti-GFP (1:5,000; Abcam, catalog no. ab13970) was used. After primary incubation overnight at 4°C, all wells were processed for 1 h with the corresponding fluorescent secondary antibodies (Alexa Fluor 488 donkey anti-rabbit for Anti-Iba1, 1:500, Life Technologies catalog no. R37118; Alexa Fluor 488 donkey anti-chicken for Anti-GFP, 1:500, Jackson ImmunoResearch catalog no. 703-545-155). Stained sections were imaged with a cooled CCD camera attached to a DS-Ri1 epifluorescence microscope (Nikon, Japan). Microglia were counted manually in a blinded fashion. Microgliosis and astrogliosis were quantified by calculating the percent area occupied by Iba1 and GFAP staining, respectively, in a defined region of interest on six anatomically matched coronal sections per animal with ImageJ (NIH). Replicate values from each animal were individually averaged before determination of group means (n = 4 or 5/group).
For Sholl analysis of microglia morphology, imaging was performed on a Keyence BZ-X800 using a ×63 oil-immersion objective. z stacks were generated at 1-µm z-step size. We used the SNT plugin of ImageJ to generate the traces of microglial processes. Then the Sholl analysis plugin was run for each microglia to determine intersections at each Sholl radius.
Human Study
To determine the relationship between plasma lipid concentrations and MBH gliosis in men, plasma samples and brain MRI images from a previously conducted cross-sectional study were utilized (25). For the present analyses, the study cohort comprised 41 men with and without obesity who were otherwise healthy and recruited from the University of Washington Twin Registry (52). Participants were included on the basis of availability of plasma samples and previously analyzed brain MRI images. The detailed study protocol and recruitment methods have been published previously (25, 53). Fasting plasma concentrations of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides were measured at the Northwest Lipid Metabolism and Diabetes Research Center (NWLMDRC; Seattle, WA) through validated methods that have been previously described (54). Methods for brain MRI image acquisition and analyses have been reported in detail elsewhere (25).
Statistical Analyses
Data are presented as means ± SE. Statistical analysis using Prism (GraphPad) involved unpaired two-tailed Student’s t tests and one- and two-way ANOVA with post hoc Bonferroni testing. D’Agostino–Pearson normality test was run for each of the data sets. Mann–Whitney test was performed when normality was not reached. Probability (P) values of <0.05 were considered statistically significant.
RESULTS
Androgen Deficiency in Combination with HFHS Consumption Increases Levels of Proatherogenic Lipoproteins and Peripheral Inflammation
For this study, we sought a mouse model with a “humanized” lipid profile to facilitate examination of early cardiometabolic risk markers at the 4 wk HFHS feeding time point. Hepatic overexpression of a gain-of-function variant of PCSK9 achieved through intravenous injection of AAV-D377Y-mPCSK9 elicits functional LDL receptor deficiency (55), resulting in susceptibility to HFHS-induced hyperlipidemia including substantial elevations in triglycerides (Supplemental Fig. S1A, available at http://doi.org/10.6084/m9.figshare.22064813) and total cholesterol (Supplemental Fig. S1B) from elevations in VLDL, IDL, and LDL species (Supplemental Fig. S1C).
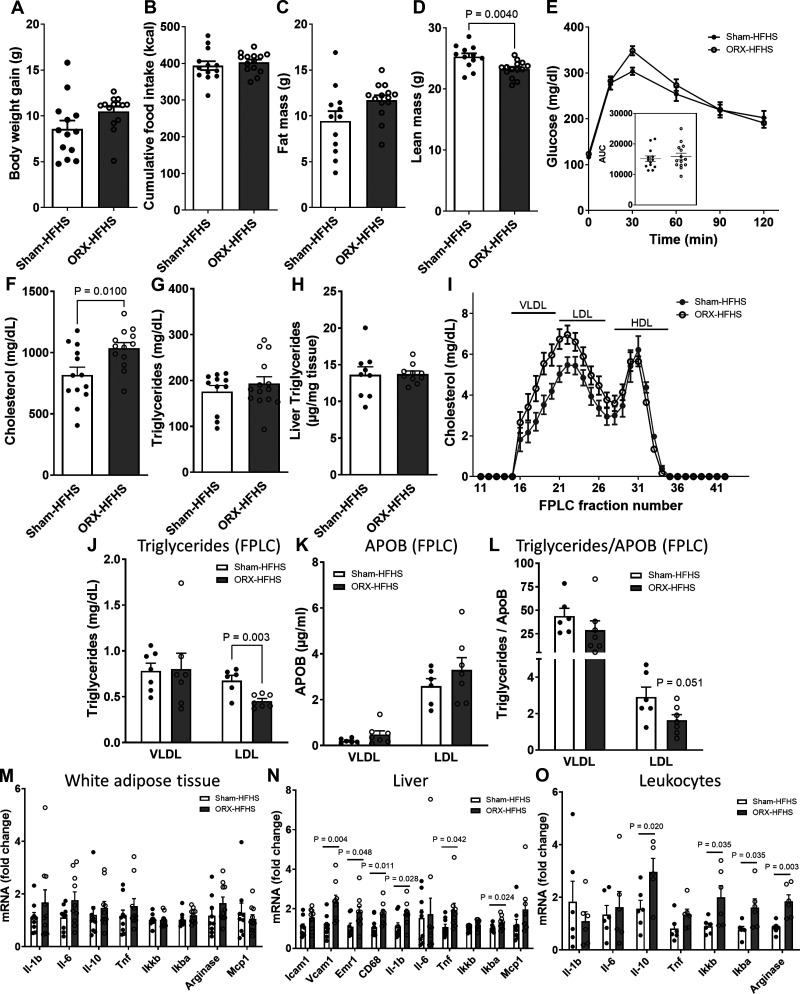
Using this mouse model, we first investigated whether the determinants of cardiometabolic risk induced by obesogenic diet consumption are modified by androgen deficiency in a body weight-independent manner. In rodents, ORX-induced hypogonadism exacerbates diet-induced weight gain, but only after at least 8 wk of obesogenic diet feeding (10). Accordingly, we observed no significant differences between ORX and sham-operated C57BL/6J mice in body weight gain, cumulative food intake, fat mass, or glucose tolerance after 4 wk of HFHS feeding (Fig. 1, A–C and E). As expected, ORX-HFHS mice had lower lean mass (Fig. 1D), a result of muscle loss from androgen deprivation.
Figure 1.
Orchiectomized (ORX) mice exposed to high-fat, high-sucrose diet (HFHS) for 4 wk have increased markers of cardiovascular disease (CVD) risk despite no metabolic changes. Metabolic and inflammatory parameters measured in sham-operated (Sham) and ORX mice injected with AAV- D377Y-mPCSK9 and fed 4 wk of HFHS. A–D: body weight gain (A), cumulative food intake (B), fat mass (C), and lean mass (D) (n = 13 or 14/group). E: intraperitoneal glucose tolerance test (2 g/kg). Inset, area under the curve (AUC). F–H: plasma cholesterol (F), plasma triglycerides (TG) (G), and liver TG content (H) (n = 9 or 10/group). I: lipoprotein distribution of cholesterol measured by fast-phase liquid chromatography (FPLC) (pooled plasma of 2 or 3 mice/n, n = 4 and n = 6 for Sham-HFHS and ORX-HFHS, respectively). Fractions 15–20 contain VLDL, fractions 21–27 contain LDL, and fractions 28–35 contain HDL. J–L: TG (J) and apolipoprotein B (APOB) (K) measured in the VLDL peak fraction (17) and the LDL peak fraction (22) and TG measured in VLDL and LDL fractions normalized to APOB (L), providing an estimate of TG molecules/lipoprotein particle. M–O: mRNA levels of inflammatory markers in white adipose tissue (M), liver (N), and circulating leukocytes (O). In A–L, data are presented as means ± SE. In M–O, data are presented as fold change relative to Sham-HFHS. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Next, we evaluated whether the addition of androgen deprivation with ORX to 4-wk HFHS feeding in AAV-D377Y-mPCSK9-treated mice would exacerbate their hyperlipidemia. We observed an elevation of total plasma cholesterol (ORX-HFHS vs. Sham-HFHS; Fig. 1F), but not plasma or liver triglycerides (TG) (Fig. 1, G and H), with a shift toward cholesterol carried by very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and low-density lipoprotein (LDL) species (Fig. 1I), a pattern highly predictive of atherosclerosis progression (56). Importantly, ORX in chow-fed mice did not increase plasma cholesterol levels (Sham: 84.5 ± 4.9, ORX: 90.5 ± 7.4 mg/dL; n = 5/group, P value = 0.52), indicating that androgen deficiency only in combination with obesogenic diet predisposes to hyperlipidemia.
We then evaluated the level of TG and APOB in the peak fractions of VLDL and LDL obtained by FPLC. Interestingly, the TG content in the LDL fractions was reduced in ORX-HFHS mice (Fig. 1J), with no marked changes in APOB (Fig. 1K). Since there is one APOB molecule per lipoprotein particle in APOB-containing lipoproteins, the lower TG-to-APOB ratio observed in the LDL fraction (Fig. 1L) indicates the presence of LDL particles enriched in cholesterol relative to triglycerides in ORX-HFHS mice compared with Sham-HFHS control mice.
Finally, we assessed markers of cardiometabolic risk by measuring inflammatory gene expression in white adipose tissue (WAT), liver, and circulating leukocytes in sham-operated and ORX C57BL/6J mice on 4-wk HFHS. As expected, based on the absence of body weight and fat mass differences between groups, no differences were observed in WAT (Fig. 1M). In contrast, inflammatory gene expression in the liver including NF-κB pathway intermediates (Ikbkb and Nfkbia) and the proinflammatory cytokine Il6 was markedly increased in ORX-HFHS mice along with elevation of myeloid cell markers Emr1 (also known as F4/80) and Cd68 (Fig. 1N). Similarly, circulating leukocytes manifested signs of increased NF-κB pathway activity (Fig. 1O).
Thus, these data indicate that even before ORX increases the fat mass gain and glucose intolerance elicited by HFHS feeding, androgen deprivation promotes lipid alterations and signs of systemic cardiometabolic dysregulation.
Hypogonadism in Male Mice Amplifies Hypothalamic Gliosis Induced by Western-Type Diets
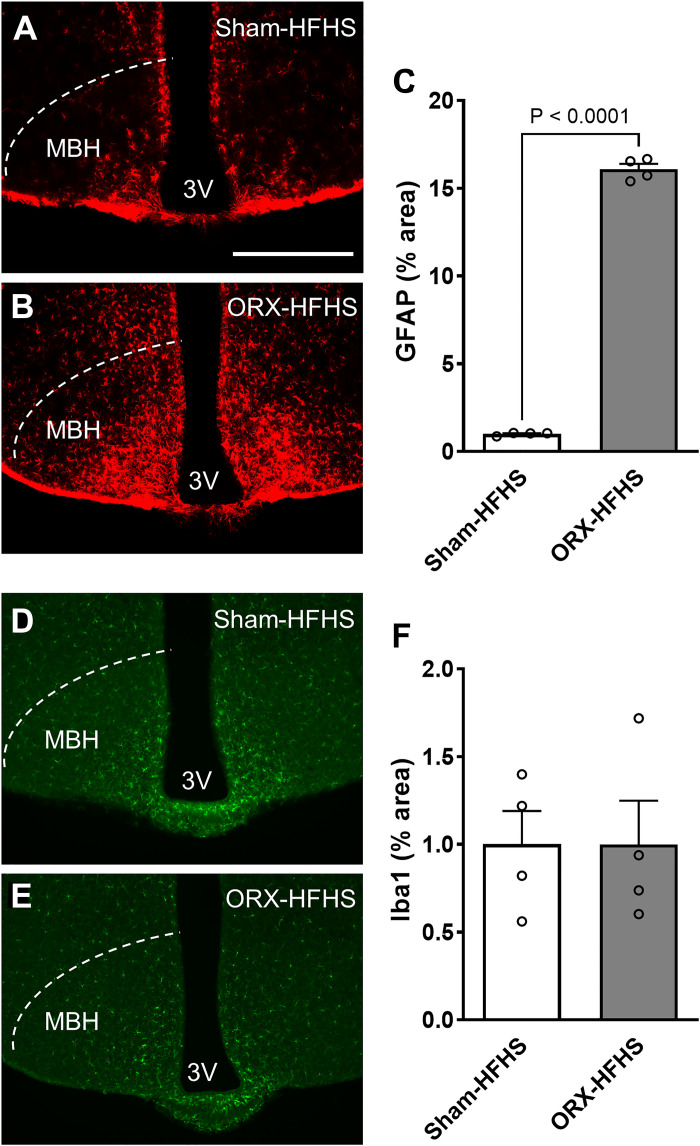
Given that diet-induced gliosis in the MBH promotes systemic metabolic dysfunction (21–23), we next determined the impact on MBH glia of combining androgen deprivation with HFHS. Strikingly, ORX mice fed HFHS showed a dramatic increase in GFAP immunostaining in the MBH compared with sham-operated control mice (Fig. 2, A–C), indicating substantial activation of hypothalamic astrocytes. In contrast, microglial activation measured by Iba1 density and Sholl analysis of processes revealed no differences between groups (Fig. 2, D–F, and Supplemental Fig. S2, available at http://doi.org/10.6084/m9.figshare.22064810).
Figure 2.
Orchiectomized (ORX) mice exposed to high-fat, high-sucrose diet (HFHS) have increased astrocytosis but not microgliosis in the mediobasal hypothalamus. A and B: representative images showing glial fibrillary acidic protein (GFAP) immunoreactivity in the mediobasal hypothalamus (MBH) of sham-operated (Sham)-HFHS (A) and ORX-HFHS (B) mice. 3V, third ventricle. Scale bar, 500 µm. C: quantification of GFAP-positive area percentage in the MBH from 6 sections per animal. D and E: representative images showing Iba1 immunoreactivity in the MBH of Sham-HFHS (D) and ORX-HFHS (E) mice. F: quantification of Iba1-positive area percentage in the MBH from 6 sections per animal. Data are presented as means ± SE of 4 mice per group.
To further characterize the relationship between astrogliosis and androgen deficiency, we analyzed hypothalamic sections from wild-type ORX mice fed either chow or a HFD (60% fat by calories) with no added cholesterol (Supplemental Fig. S3, available at http://doi.org/10.6084/m9.figshare.22064819). Although ORX did not alter glial profiles in chow-fed mice (Supplemental Fig. S3, A, C, and E), ORX combined with HFD feeding induced a profound astrogliosis (Supplemental Fig. S3, B, D, and E), indicating that the enhancement of astrogliosis by ORX is diet specific and requires neither severe hyperlipidemia nor ingested cholesterol. Importantly, peripheral testosterone replacement in ORX mice on HFD prevented the development of hypothalamic astrogliosis (Supplemental Fig. S3, F–I). Finally, we demonstrated that MBH astrogliosis occurs to a similar degree with secondary hypogonadism (chemical castration with a GnRH antagonist) as with primary (ORX-induced) androgen deficiency (Supplemental Fig. S3, J–N). Together, these data support a critical role for testosterone rather than other testicular products or gonadotrophins in the regulation of gliosis during obesogenic diet feeding.
Androgen Deficiency in Combination with HFD Alters Transcriptomic Profile of Hypothalamic Astrocytes to More Activated Phenotype
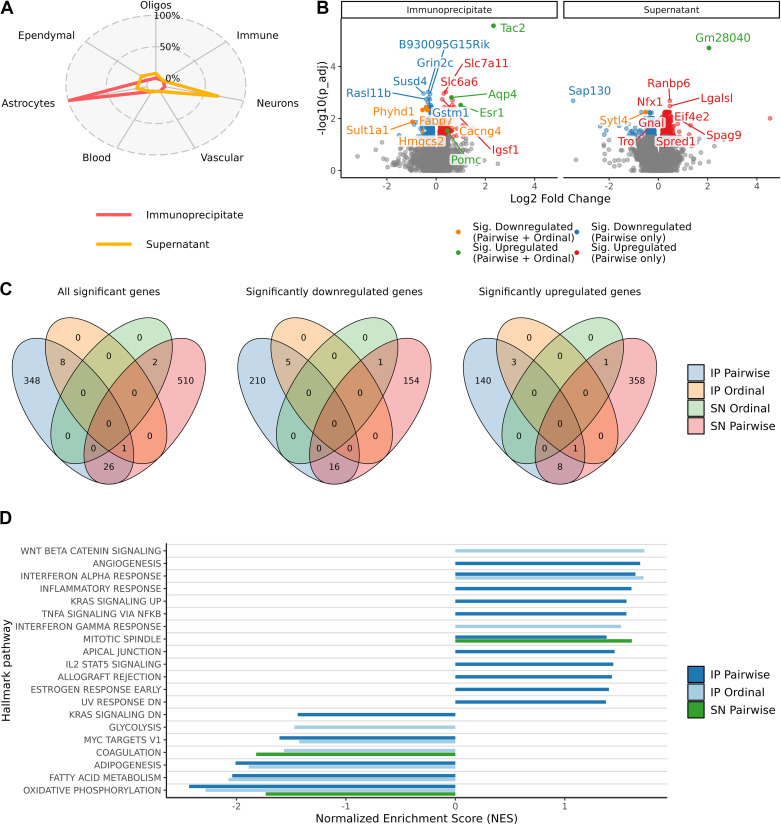
To further determine the impact of hypogonadism on diet-induced gliosis, we conducted transcriptomic profiling of hypothalamic astrocytes using ribosome capture (TRAP)-based mRNA purification and sequencing (57) in Aldh1l1eGFP-L10a mice subjected to ORX and 4-wk HFD feeding. In this mouse model, regulatory elements of the pan-astrocytic gene Aldh1l1 direct Cre-mediated recombination of the eGFP-L10a cassette, resulting in astrocyte-specific eGFP labeling of ribosomes. First, to confirm the predicted expression pattern, we used IHC with GFP and astrocytic marker GFAP antibodies to identify cells with eGFP-L10a ribosome fusion proteins in Aldh1l1eGFP-L10a mice (Supplemental Fig. S4). Although ALDH1L1 is expressed in nearly all astrocytes, GFAP is limited to certain astrocyte subsets (58) Thus, all GFAP-positive cells coexpressed GFP, but some GFP-labeled cells were GFAP negative (Supplemental Fig. S4). Consistent with previous reports, GFP-positive cells did not costain with NeuN (neuronal marker) or Iba1 (microglial marker) (Refs. 59, 60 and data not shown), a cellular specificity that was confirmed at the transcriptional level as well (see below). Next, we performed TRAP on microdissected hypothalami from Aldh1l1eGFP-L10a mice under three experimental conditions: Sham-Chow (n = 7), Sham-HFD (n = 6), and ORX-HFD (n = 7). Immunoprecipitation (IP) fractions of hypothalamic lysates were generated with GFP antibodies, yielding astrocyte ribosomes from which translating mRNAs were eluted. mRNAs from supernatant (SN) fractions were also collected to assess overall changes in hypothalamic gene expression. Using publicly available mouse hypothalamus single-cell RNA-seq data (42) and the cell type decomposition software MuSiC (41), we estimated that the IP fractions were composed of 97.7% astrocyte RNAs on average (SD 2.8%) (Fig. 3A and Supplemental Fig. S5A, available at http://doi.org/10.6084/m9.figshare.22064825), demonstrating the remarkable cellular specificity of the Aldh1l1-Cre driver. The most prevalent cell types predicted in the SN fractions were neurons (67.2%, SD 6.1%), astrocytes (12.9%, SD 7.1%), and ependymal cells (11.7%, SD 1.9%) (Fig. 3A and Supplemental Fig. S5A). No statistically significant differences in cellular composition were observed between sample groups (Supplemental Fig. S5B), but intersample variability was observed. For this reason, all subsequent differential expression analyses were corrected for cellular composition to account for differences in yields of specific cell types and efficiencies of immunoprecipitation.
Figure 3.
Androgen deficiency in combination with high-fat diet (HFD) feeding promotes a proinflammatory and hypometabolic transcriptomic profile in hypothalamic astrocytes. A: cell type proportion estimations with the deconvolution software MuSiC for each cell fraction [immunoprecipitate (IP) and supernatant (SN)] performed in hypothalamic samples from 3 groups of Aldh1l1eGFP-L10a mice [sham operated (Sham)-regular chow (Chow), Sham-HFD, and orchiectomized (ORX)-HFD]. B: volcano plot representing the significantly altered genes (blue = down; red = up) in the pairwise comparison (Sham-HFD vs. ORX-HFD) and with the additional ordinal regression analysis (Sham-Chow > Sham-HFD > ORX-HFD = orange or Sham-Chow < Sham-HFD < ORX-HFD = green) in both the IP (left) and SN (right) fractions. Genes with the most significant changes are labeled. C: Venn diagrams indicating overlap of genes with altered expression levels in the different analyses performed. Left: all genes. Center: downregulated genes. Right: upregulated genes. D: gene set enrichment analysis showing over- and underrepresentation of expression changes in hallmark pathways (all genes included). The top 10 over- and underenriched in each analysis (adjusted P value ≤ 0.05) are presented (no pathways were significantly enriched for the SN Ordinal test). Data obtained from individual hypothalamic lysates (Sham-Chow n = 7, Sham-HFD n = 6, ORX-HFD n = 7).
In a pairwise comparison between Sham-HFD and ORX-HFD groups, changes in gene expression were observed in both astrocyte-enriched IP fractions (152 upregulated genes and 221 downregulated genes) and astrocyte-depleted SN fractions (368 upregulated genes and 171 downregulated genes) (Fig. 3, B and C, and Supplemental Data S1 for the full list; Pairwise only). Thus, androgen deprivation has broad effects on hypothalamic gene expression in the context of HFD feeding.
To further clarify the interaction between ORX and HFD, we performed an ordinal regression analysis to identify genes that followed the pattern Sham-Chow > Sham-HFD > ORX-HFD or Sham-Chow < Sham-HFD < ORX-HFD. In the astrocyte-enriched IP fractions, four genes (Tac2, Esr1, Pomc, and Aqp4) showed increased levels of gene expression across groups whereas five (Phyhd1, Fabp7, Cacng4, Sult1a1, and Hmgcs2) decreased. For the mixed-cell SN fractions, the increasing and decreasing genes were Gm28040 and Sytl4, respectively (Fig. 3, B and C, and Supplemental Data S1 for the full list; Pairwise + Ordinal). Although these genes were not statistically significant in a pairwise Sham-Chow to Sham-HFD comparison (data not shown), the ordinal regression analysis suggests potential combinatorial effects of HFD and ORX.
We next evaluated changes in specific biological pathways using gene set enrichment analyses. As expected, based on the astrogliosis observed with the GFAP immunoreactivity (Fig. 2, B and C, and Supplemental Fig. S3), astrocyte mRNAs from ORX-HFD mice were positively enriched for inflammatory pathways including those designated “interferon alpha/gamma response,” “inflammatory response,” and “Tnfa signaling via Nfkb.” Interestingly, metabolic pathways were negatively enriched in ORX-HFD astrocytes including “fatty acid metabolism” and “oxidative phosphorylation” [Fig. 3D, Supplemental Fig. 6 (available at http://doi.org/10.6084/m9.figshare.22064816), and Supplemental Data S1 for the full list of results, including gene set enrichment analyses using MSigDB’s Canonical Pathways sets as well as Gene Ontology BP (Biological Process) and MF (Molecular Function) sets].
These findings overall suggest that hypogonadism induced by ORX in combination with HFD induces reactivity in astrocytes and a reduced metabolic capacity, which may alter their normal functions in the hypothalamus, such as modulating neuronal metabolism and synaptic activity.
Central Androgen Infusion in ORX Mice Fed with HFHS Diet Prevents Hypothalamic Gliosis and Atherogenic Risk Factors
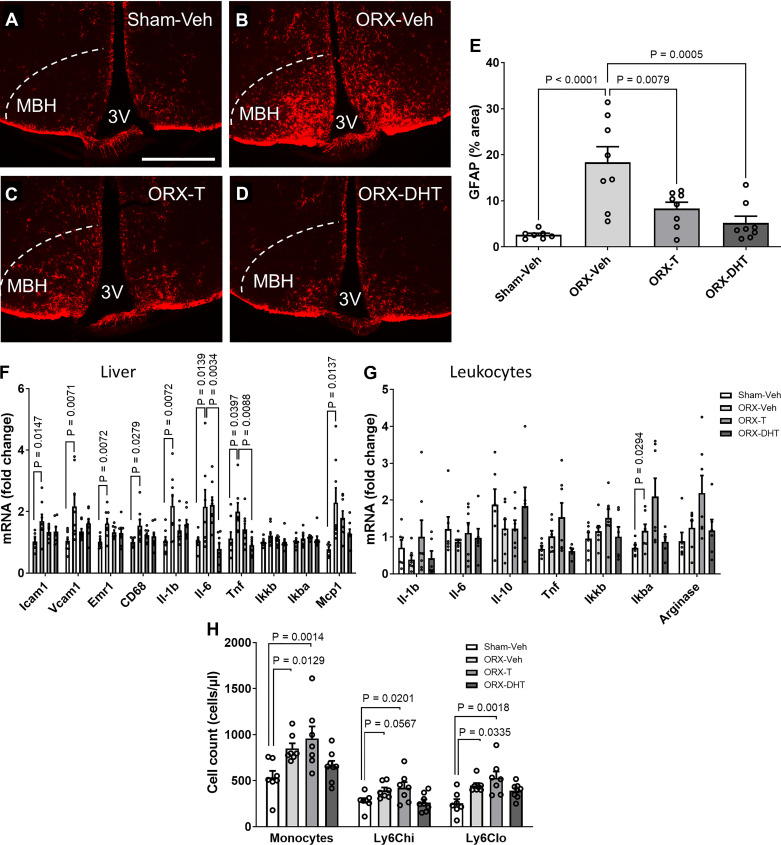
The physiological effects of testosterone (T) can be mediated by direct activation of the androgen receptor (AR) or via estrogen receptors after aromatization of T to estradiol. To test the capacity of CNS androgen signaling to prevent hypothalamic gliosis and the development of a proatherogenic risk profile as observed in ORX-HFHS mice (Figs. 1 and 2), we performed 28-day lateral ventricle intracerebroventricular infusion of vehicle (Veh), T, or DHT (a nonaromatizable androgen) in ORX mice that concurrently received HFHS.
As observed in the previous cohort (Fig. 2, A–C), ORX mice on HFHS for 4 wk had an ∼10-fold increase in GFAP immunostaining in the MBH compared with their sham-operated controls (Fig. 4, A, B, and E), an effect that was largely abolished by intracerebroventricular infusion of either T or DHT (Fig. 4, A–E). Importantly, the androgens infused intracerebroventricularly did not detectably alter circulating levels of T or DHT (Supplemental Fig. S7, A and B, available at http://doi.org/10.6084/m9.figshare.22064828), indicating that their action occurred directly within the CNS to reduce gliosis.
Figure 4.
Chronic central infusion of testosterone (T) and dihydrotestosterone (DHT) reduces hypothalamic astrogliosis and peripheral risk markers in orchiectomized (ORX) mice exposed to high-fat, high-sucrose diet (HFHS). A–D: representative images showing glial fibrillary acidic protein (GFAP) immunoreactivity in the mediobasal hypothalamus (MBH) of sham operated (Sham)-vehicle (Veh) (A), ORX-Veh (B), ORX-T (C), and ORX-DHT (D). 3V, third ventricle. Scale bar, 500 µm. All mice were injected with AAV-D377Y-mPCSK9 and fed with HFHS and received intracerebroventricular infusion for 28 days. E: quantification of GFAP-positive area percentage in the MBH from 6 sections per animal. F and G: mRNA levels of inflammatory markers in liver (F) and circulating leukocytes (G). H: quantification of total monocyte and monocyte subset cell numbers determined by flow cytometry. In E and H, data are presented as means ± SE of 8 mice per group. In F and G, data are presented as fold change relative to Sham-Veh.
We then determined whether intracerebroventricular treatment with T and DHT in ORX mice prevents peripheral inflammation. The elevated inflammatory gene expression profile observed in the livers of ORX-Veh mice compared with Sham-Veh was almost completely prevented by central infusion of T and DHT (Fig. 4F), whereas the inflammatory profile in leukocytes was also curtailed by DHT but not by T (Fig. 4G). Similarly, the elevated number of circulating monocytes in ORX mice was prevented by central DHT but not by T (Fig. 4H). Importantly, body weight gain and cumulative food intake were not different among groups (Supplemental Fig. 7, C and D). Together, these data suggest that androgen action in the CNS can have a positive impact on cardiometabolic risk by reducing gliosis and systemic inflammatory signaling.
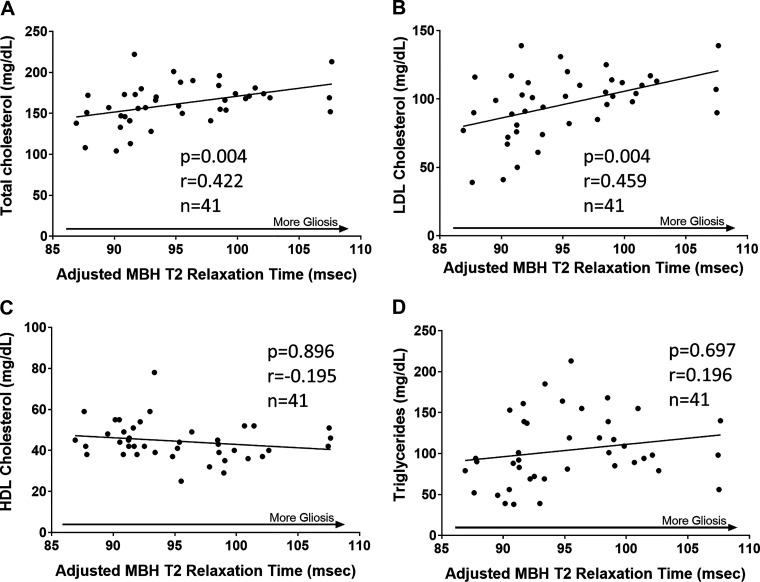
Positive Correlation between MBH Gliosis and Plasma Total Cholesterol and LDL-Cholesterol in Humans
Based on the mouse studies detailed above, we investigated the relationship between plasma lipid concentrations and MBH gliosis in a cohort of 41 healthy adult men who had previously undergone MRI scanning of the MBH region to measure gliosis [using T2 relaxation time; see Berkseth et al. (25)]. As described in Table 1 in Berkseth et al. (25), participants ranged from 19 to 48 yr of age (mean age 29.7 ± 10.3 yr) with BMIs from 20.0 to 39.8 kg/m2 (mean BMI 29.6 ± 5.8 kg/m2) and mean circulating concentrations of free testosterone, total testosterone, and estradiol within the reference ranges. MBH T2 relaxation time was positively associated with plasma concentrations of both total cholesterol and LDL-cholesterol (Fig. 5, A and B) even after adjusting for BMI. In contrast, no correlations were observed between MBH T2 relaxation time and plasma HDL or triglycerides (Fig. 5, C and D). Thus, independent of obesity status, MBH gliosis and atherogenic LDL-cholesterol are correlated in men, suggesting the possibility of mechanistic links among deficient androgen signaling, MBH glial cell activation, and the generation of atherogenic lipids.
Figure 5.
Radiological evidence that hypothalamic gliosis is associated with total cholesterol and LDL-cholesterol in men. Scatterplot and regression line showing correlations between mean bilateral mediobasal hypothalamus (MBH) T2 relaxation time and plasma concentrations of total cholesterol (A), LDL-cholesterol (B), HDL-cholesterol (C), and triglycerides (D). Correlation P values were determined through generalized estimating equations. Data are adjusted for T2 relaxation times in control regions in the putamen and amygdala. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
DISCUSSION
Although hypogonadism in men is associated with elevated cardiometabolic risk, the underlying mechanisms explaining this relationship are not completely understood. The present data implicate a central action of hypogonadism to promote atherogenic risk profiles in both rodent models and humans. Here we show that androgen deficiency amplifies HFHS feeding-induced hypothalamic astrogliosis concomitant with hyperlipidemia, peripheral inflammation, leukocytosis, and liver fat accumulation. Notably, these early markers of CVD risk appear before changes in weight gain, adiposity, and glucose intolerance. Furthermore, chronic androgen infusion into the CNS of ORX mice prevents hypothalamic astrogliosis and significantly reduces peripheral inflammatory signaling, suggesting that central androgen receptor signaling may reduce metabolic dysfunction in obese mice. Finally, we provide evidence of a positive association between radiological evidence of hypothalamic gliosis and plasma LDL-cholesterol levels in human subjects, supporting the concept that CNS gliosis is a potential contributor to CVD risk in susceptible individuals.
In rodents, androgen deficiency in combination with consumption of a Western-type diet increases susceptibility to obesity and glucose intolerance beyond the effect of diet alone (9, 10, 13). However, these metabolic alterations only occur after several additional months of HFD feeding after ORX. Here, we studied short-term exposure to HFHS or HFD to identify adiposity-independent effects of androgens on gliosis and CVD risk factors. As expected, after 4 wk of HFHS/HFD feeding there were no metabolic alterations or elevations in well-established markers of obesity and insulin resistance such as Tnf and Il1b in adipose tissue (61, 62). Nevertheless, mice with androgen deficiency fed a short-term obesogenic diet developed peripheral indicators of cardiometabolic risk including increased levels of total cholesterol, VLDL-cholesterol, and LDL-cholesterol, similar to the inverse correlation observed in humans between T levels and serum lipids/CVD risk (1–4). Interestingly, plasma and liver TG were unchanged despite elevated VLDL-cholesterol, raising the possibility that the combination of low T and HFHS diet consumption enhanced hepatic VLDL production or impeded hepatic clearance of these particles without hindering hydrolysis of their TG content. In addition to the lipid changes observed, liver and leukocyte inflammation and monocytosis were increased in ORX-HFHS mice. These data are consistent with clinical studies showing an inverse association between T levels and systemic inflammation (63–65), indicating that the mouse model recapitulates many aspects of human CVD pathogenesis.
Hypothalamic gliosis occurs in both early HFD feeding and established diet-induced obesity in rodent models and is also observed in humans with obesity (18–24). In experimental models of brain injury, gliosis often begins as a neuroprotective response, but sustained glial cell activation ultimately contributes to neurotoxicity and disease progression (17). Previous work has demonstrated that hypothalamic astrogliosis increases transiently in rodents over the first few weeks of HFD feeding and then recurs later when obesity is established (18). The combination of ORX-induced hypogonadism and HFHS or HFD for 4 wk synergistically increased hypothalamic astrogliosis but not microgliosis. Peripheral administration of T in ORX mice completely abolished hypothalamic gliosis induced by 4 wk of diet, consistent with prior studies showing that T can reduce reactive gliosis after brain injury in the cortex and hippocampus (26, 27, 66). The fact that the astrogliosis induced by hypogonadism plus diet was observed with different diet compositions [high-fat diet (HFD) and high-fat, high-sucrose diet with added cholesterol (HFHS)], different mouse models (normolipidemic WT and hyperlipidemic AAV-mPCSK9 treated), and both primary and secondary hypogonadism suggests that adding T deficiency to HFD feeding likely amplifies gliosis via direct androgen action on MBH glia or neighboring neurons. However, T deficiency alone fails to induce hypothalamic astrogliosis in mice maintained on chow diet either 5 days or 8–10 wk after ORX (67), suggesting that a synergistic interaction between central androgen signaling and diet-induced inflammation provokes glial activation.
Reactive astrogliosis is a universal response of astrocytes to diverse insults including trauma, infection, aging, and obesogenic diets. However, transcriptomic analyses have demonstrated the existence of different subtypes of reactive gliosis (68). Interestingly, hypogonadism in combination with HFD feeding produces a transcriptomic profile that combines upregulation of inflammatory pathways and downregulation of metabolic pathways such as glycolysis, adipogenesis, and oxidative phosphorylation. Links between inflammation and changes in cellular metabolism in astrocytes have been demonstrated in aging rodents (60, 69), suggesting that astrocytes lacking androgen signaling might have acceleration of age-related processes induced by an obesogenic diet. Although growing evidence has emerged supporting a role of hypothalamic neuronal populations in hepatic lipid homeostasis (70–72) and leukocyte shifts (73), the potential involvement of astrocytes remains largely untested. Since astrocytes regulate neuronal metabolism and synaptic activity, the robust astrocytic inflammatory response induced by testosterone deficiency and obesogenic diet likely modifies the behavior of neighboring hypothalamic neurons that regulate systemic metabolism. Future studies targeting hypothalamic astrocytes will clarify their role in the peripheral changes observed in ORX-HFHS animals.
Although most efforts have focused on peripheral mechanisms by which T and androgen receptor signaling affect CVD risk, a few previous studies have provided support for a CNS role in lipid metabolism, hematopoiesis, and atherosclerosis (70, 73, 74). Central administration of T and DHT in ORX-HFHS mice was sufficient to prevent not only the hypothalamic astrogliosis but also some signs of peripheral inflammation, supporting the hypothesis that central androgen receptor signaling is a component of a protective mechanism to limit CVD risk. Although T and DHT had comparable effects on hypothalamic gliosis and liver inflammation, T did not reduce leukocytic markers of inflammation and monocytosis. These steroids differ, among other aspects, in their potency to activate androgen receptors and their susceptibility to enzymatic conversion to estrogens. Specifically, T is 10 times less potent than DHT in activating androgen receptors, and it can be metabolized by aromatase to 17β-estradiol. Therefore, one could speculate that central estrogen receptor signaling promotes monocytosis in ORX mice or a higher dose of T is required to prevent it. Future analyses of glial cell-specific androgen and/or estrogen receptor knockout mice would test these possibilities and provide a platform to identify glial targets for therapies to reduce hypogonadism-associated CVD risk.
The clinical relevance of these findings is supported by a prior MRI-based study showing that MBH gliosis is negatively associated with plasma concentrations of both free and total testosterone (25). Extending from this previous study, we provide the first evidence in humans of a positive association between hypothalamic gliosis and LDL-cholesterol, which is a strong independent predictor of CVD (75). In contrast, no relationship was observed between MBH gliosis and circulating HDL-cholesterol or TG. This correlation between gliosis and cholesterol levels adds to those previously described between gliosis and BMI (positive), insulin resistance (positive), and plasma testosterone (negative) (24, 25). Together, these findings suggest a connection between sex steroid signaling, gliosis, and metabolic dysfunction in obese subjects that provides a rationale for further mechanistic studies to clarify these links and identify potential targets for CVD risk reduction.
DATA AVAILABILITY
The code to reproduce the results is available on the GitHub repository https://github.com/novonordisk-research/ORX_HFD_Astrocytes_TRAPseq. Data will be made available upon reasonable request.
SUPPLEMENTAL MATERIALS
Supplemental Fig. S1: http://doi.org/10.6084/m9.figshare.22064813.
Supplemental Fig. S2: http://doi.org/10.6084/m9.figshare.22064810.
Supplemental Fig. S3: http://doi.org/10.6084/m9.figshare.22064819.
Supplemental Fig. S4: https://doi.org/10.6084/m9.figshare.22064822.
Supplemental Fig. S5: http://doi.org/10.6084/m9.figshare.22064825.
Supplemental Fig. S6: http://doi.org/10.6084/m9.figshare.22064816.
Supplemental Fig. S7: http://doi.org/10.6084/m9.figshare.22064828.
Supplemental Data S1: http://doi.org/10.6084/m9.figshare.22065005.
GRANTS
This work was supported by a Scientist Development Award from the American Heart Association (AHA; 16SDG27010018), by Pilot and Feasibility Awards from the Diabetes Research Center (DRC; P30 DK017047) and University of Washington Medicine Diabetes Institute (UWMDI), and by K01HL153205 to M.D.D., by R01DK119754 to J.P.T., by R35HL150754 to K.E.B., and by 1R01DK121756 to J.E.K. In addition, services and support were provided by the Nutrition Obesity Research Center (DK035816) and Diabetes Research Center (DK017047) at the University of Washington.
DISCLOSURES
T. Monfeuga, A. Chandran, and T. H. Meek are employees of Novo Nordisk Ltd. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.D.D., K.B.R., E.A.S., K.E.B., and J.P.T. conceived and designed research; M.D.D., J.M.F., R.D.F., A.C., E.L., and I.V. performed experiments; M.D.D., T.M., S.J.M., and J.E.K. analyzed data; M.D.D., T.M., S.J.M., J.E.K., T.H.M., E.A.S., K.E.B., and J.P.T. interpreted results of experiments; M.D.D., T.M., S.J.M., and I.V. prepared figures; M.D.D. drafted manuscript; M.D.D., T.M., S.J.M., J.E.K., J.M.F., R.D.F., A.C., K.B.R., T.H.M., E.A.S., K.E.B., and J.P.T. edited and revised manuscript; M.D.D., T.M., S.J.M., J.E.K., J.M.F., R.D.F., A.C., E.L., I.V., K.B.R., T.H.M., E.A.S., K.E.B., and J.P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance provided by Farah Kramer and Shari Wang at the University of Washington and many discussions with members of the J. P. Thaler and M. W. Schwartz laboratories.
REFERENCES
- 1. Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab 93: 2042–2049, 2008. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 2. Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, Forti G, Mannucci E, Maggi M. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 165: 687–701, 2011. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 3. Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam Study. J Clin Endocrinol Metab 87: 3632–3639, 2002. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 4. Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis 210: 232–236, 2010. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 5. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60: 762–769, 2006. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, Ljunggren Ö, Vandenput L, Mellström D, Tivesten Å. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men: the MrOS (Osteoporotic Fractures in Men) Study in Sweden. J Am Coll Cardiol 58: 1674–1681, 2011. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 7. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med 363: 109–122, 2010. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, Lin Y. L, Goodwin JS. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 48: 1138–1144, 2014. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubois V, Laurent MR, Jardi F, Antonio L, Lemaire K, Goyvaerts L, Deldicque L, Carmeliet G, Decallonne B, Vanderschueren D, Claessens F. Androgen deficiency exacerbates high-fat diet-induced metabolic alterations in male mice. Endocrinology 157: 648–665, 2016. doi: 10.1210/en.2015-1713. [DOI] [PubMed] [Google Scholar]
- 10. Harada N, Hanaoka R, Horiuchi H, Kitakaze T, Mitani T, Inui H, Yamaji R. Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci Rep 6: 23001, 2016. doi: 10.1038/srep23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourghardt J, Wilhelmson AS, Alexanderson C, De Gendt K, Verhoeven G, Krettek A, Ohlsson C, Tivesten Å. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology 151: 5428–5437, 2010. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- 12. Hatch NW, Srodulski SJ, Chan HW, Zhang X, Tannock LR, King VL. Endogenous androgen deficiency enhances diet-induced hypercholesterolemia and atherosclerosis in low-density lipoprotein receptor-deficient mice. Gend Med 9: 319–328, 2012. doi: 10.1016/j.genm.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Senmaru T, Fukui M, Okada H, Mineoka Y, Yamazaki M, Tsujikawa M, Hasegawa G, Kitawaki J, Obayashi H, Nakamura N. Testosterone deficiency induces markedly decreased serum triglycerides, increased small dense LDL, and hepatic steatosis mediated by dysregulation of lipid assembly and secretion in mice fed a high-fat diet. Metabolism 62: 851–860, 2013. doi: 10.1016/j.metabol.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 14. Kelly DM, Sellers DJ, Woodroofe MN, Jones TH, Channer KS. Effect of testosterone on inflammatory markers in the development of early atherogenesis in the testicular-feminized mouse model. Endocr Res 38: 125–138, 2013. doi: 10.3109/07435800.2012.735307. [DOI] [PubMed] [Google Scholar]
- 15. Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 5: 673–681, 2009. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- 16. Yu IC, Lin HY, Liu NC, Sparks JD, Yeh S, Fang LY, Chen L, Chang C. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-κB-mediated PTP1B expression. Diabetes 62: 411–423, 2013. doi: 10.2337/db12-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248, 2014. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. [Erratum in J Clin Invest 122: 778, 2012]. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9: 2124–2138, 2014. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao Y, Ottaway N, Schriever SC, Legutko B, García-Cáceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschöp MH, Yi CX. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62: 17–25, 2014. doi: 10.1002/glia.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab 26: 185–197.e3, 2017. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 6: 366–373, 2017. doi: 10.1016/j.molmet.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, Morton GJ, Thaler JP. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat Commun 8: 14556, 2017. doi: 10.1038/ncomms14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schur EA, Melhorn SJ, Oh SK, Lacy JM, Berkseth KE, Guyenet SJ, Sonnen JA, Tyagi V, De Leon MR, Webb MF, Gonsalves ZT, Fligner CL, Schwartz MW, Maravilla KR. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 23: 2142–2148, 2015. [Erratum in Obesity (Silver Spring) 30: 1520, 2022]. doi: 10.1002/oby.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berkseth KE, Rubinow KB, Melhorn SJ, Webb MF, De Leon MR, Marck BT, Matsumoto AM, Amory JK, Page ST, Schur EA. Hypothalamic gliosis by MRI and visceral fat mass negatively correlate with plasma testosterone concentrations in healthy men. Obesity (Silver Spring) 26: 1898–1904, 2018. doi: 10.1002/oby.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci 25: 3039–3046, 2007. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Estrada J, del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res 628: 271–278, 1993. doi: 10.1016/0006-8993(93)90964-O. [DOI] [PubMed] [Google Scholar]
- 28. Hussain R, Ghoumari AM, Bielecki B, Steibel J, Boehm N, Liere P, Macklin WB, Kumar N, Habert R, Mhaouty-Kodja S, Tronche F, Sitruk-Ware R, Schumacher M, Ghandour MS. The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain 136: 132–146, 2013. doi: 10.1093/brain/aws284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflammation 11: 162, 2014. doi: 10.1186/s12974-014-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A 3rd, Kirk EA, O’Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 28: 685–691, 2008. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowen RS, Knab AM, Hamilton AT, McCall JR, Moore-Harrison TL, Lightfoot JT. Effects of supraphysiological doses of sex steroids on wheel running activity in mice. J Steroids Horm Sci 3: 110, 2012. doi: 10.4172/2157-7536.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keil KP, Mehta V, Branam AM, Abler LL, Buresh-Stiemke RA, Joshi PS, Schmitz CT, Marker PC, Vezina CM. Wnt inhibitory factor 1 (Wif1) is regulated by androgens and enhances androgen-dependent prostate development. Endocrinology 153: 6091–6103, 2012. doi: 10.1210/en.2012-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro G, Allard C, Morford JJ, Xu W, Liu S, Molinas AJ, Butcher SM, Fine NH, Blandino-Rosano M, Sure VN, Yu S, Zhang R, Münzberg H, Jacobson DA, Katakam PV, Hodson DJ, Bernal-Mizrachi E, Zsombok A, Mauvais-Jarvis F. Androgen excess in pancreatic β cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 3: e98607, 2018. doi: 10.1172/jci.insight.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moghadami S, Jahanshahi M, Sepehri H, Amini H. Gonadectomy reduces the density of androgen receptor-immunoreactive neurons in male rat’s hippocampus: testosterone replacement compensates it. Behav Brain Funct 12, 2016. doi: 10.1186/s12993-016-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serra C, Bhasin S, Tangherlini F, Barton ER, Ganno M, Zhang A, Shansky J, Vandenburgh HH, Travison TG, Jasuja R, Morris C. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology 152: 193–206, 2011. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem 377: 990–1002, 2003. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 37. Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res 41: 1020–1026, 2000. doi: 10.1016/S0022-2275(20)32045-9. [DOI] [PubMed] [Google Scholar]
- 38. Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, , et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res 47: D766–D773, 2019., doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon: fast and bias-aware quantification of transcript expression using dual-phase inference. Nat Methods 14: 417–419, 2017. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32: 3047–3048, 2016. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Park J, Susztak K, Zhang NR, Li M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat Commun 10, 2019. doi: 10.1038/s41467-018-08023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, la Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular architecture of the mouse nervous system. Cell 174: 999–1014.e22, 2018. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol 33: 495–502, 2015. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R. Integrated analysis of multimodal single-cell data. Cell 184: 3573–3587.e29, 2021., doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Love MI, Soneson C, Hickey PF, Johnson LK, Pierce NT, Shepherd L, Morgan M, Patro R. Tximeta: Reference sequence checksums for provenance identification in RNA-seq. PLoS Comput Biol 16: e1007664, 2020. doi: 10.1371/journal.pcbi.1007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pimentel H, Bray NL, Puente S, Melsted P, Pachter L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat Methods 14: 687–690, 2017. doi: 10.1038/nmeth.4324. [DOI] [PubMed] [Google Scholar]
- 47. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27: 1739–1740, 2011. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst 1: 417–425, 2015. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov M, Sergushichev A. Fast gene set enrichment analysis (Preprint). bioRxiv 060012, 2021. doi: 10.1101/060012. [DOI]
- 51. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 52. Strachan E, Hunt C, Afari N, Duncan G, Noonan C, Schur E, Watson N, Goldberg J, Buchwald D. University of Washington Twin Registry: poised for the next generation of twin research. Twin Res Hum Genet 16: 455–462, 2013. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, Webb MF, de Leon MR, Grabowski TJ, Leibel RL, Schur EA. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr 107: 145–154, 2018. doi: 10.1093/ajcn/nqx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, D’Agostino R, Marcovina S, Dabelea D. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: The SEARCH for diabetes in youth case-control study. Diabetes Care 32: 416–420, 2009. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bjørklund MM, Hollensen AK, Hagensen MK, Dagnaes-Hansen F, Christoffersen C, Mikkelsen JG, Bentzon JF. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res 114: 1684–1689, 2014. doi: 10.1161/CIRCRESAHA.114.302937. [DOI] [PubMed] [Google Scholar]
- 56. Prenner SB, Mulvey CK, Ferguson JF, Rickels MR, Bhatt AB, Reilly MP. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis 236: 244–250, 2014. doi: 10.1016/j.atherosclerosis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat Protoc 9: 1282–1291, 2014. doi: 10.1038/nprot.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bellesi M, de Vivo L, Tononi G, Cirelli C. Effects of sleep and wake on astrocytes: Clues from molecular and ultrastructural studies. BMC Biol 13, 2015. doi: 10.1186/s12915-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115, 2018. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112: 1785–1788, 2003. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park B, Lee YJ. Inverse association of testosterone and sex hormone binding globulin levels with leukocyte count in middle-aged and elderly men. Aging Male 21: 176–181, 2018. doi: 10.1080/13685538.2018.1477934. [DOI] [PubMed] [Google Scholar]
- 64. Brand JS, van der Schouw YT, Dowsett M, Folkerd E, Luben RN, Wareham NJ, Khaw KT. Testosterone, SHBG and differential white blood cell count in middle-aged and older men. Maturitas 71: 274–278, 2012. doi: 10.1016/j.maturitas.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 65. Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc 3: 91–107, 2019. doi: 10.1210/js.2018-00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. García-Estrada J, Luquín S, Fernández AM, Garcia-Segura LM. Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. Int J Dev Neurosci 17: 145–151, 1999. doi: 10.1016/S0736-5748(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 67. McQueen JK, Wright AK, Arbuthnott GW, Fink G. Astrocytes immunoreactive for glial fibrillary acidic protein (GFAP) are increased in the mediobasal hypothalamus in hypogonadal (hpg) mice. Mol Cell Neurosci 3: 473–481, 1992. doi: 10.1016/1044-7431(92)90059-B. [DOI] [PubMed] [Google Scholar]
- 68. Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410, 2012. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13: 1059–1067, 2014., doi: 10.1111/acel.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Perez-Tilve D, Hofmann SM, Basford J, Nogueiras R, Pfluger PT, Patterson JT, Grant E, Wilson-Perez HE, Granholm NA, Arnold M, Trevaskis JL, Butler AA, Davidson WS, Woods SC, Benoit SC, Sleeman MW, Dimarchi RD, Hui DY, Tschöp MH. Melanocortin signaling in the CNS directly regulates circulating cholesterol. Nat Neurosci 13: 877–882, 2010. doi: 10.1038/nn.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wiedmer P, Chaudhary N, Rath M, Yi CX, Ananthakrishnan G, Nogueiras R, Wirth EK, Kirchner H, Schweizer U, Jonas W, Veyrat-Durebex C, Rohner-Jeanrenaud F, Schürmann A, Joost HG, Tschöp MH, Perez-Tilve D. The HPA axis modulates the CNS melanocortin control of liver triacylglyceride metabolism. Physiol Behav 105: 791–799, 2012. doi: 10.1016/j.physbeh.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566: 383–387, 2019. doi: 10.1038/s41586-019-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stafford JM, Yu F, Printz R, Hasty AH, Swift LL, Niswender KD. Central nervous system neuropeptide Y signaling modulates VLDL triglyceride secretion. Diabetes 57: 1482–1490, 2008. doi: 10.2337/db07-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, , et al. Heart Disease and Stroke Statistics—2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018]. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: http://doi.org/10.6084/m9.figshare.22064813.
Supplemental Fig. S2: http://doi.org/10.6084/m9.figshare.22064810.
Supplemental Fig. S3: http://doi.org/10.6084/m9.figshare.22064819.
Supplemental Fig. S4: https://doi.org/10.6084/m9.figshare.22064822.
Supplemental Fig. S5: http://doi.org/10.6084/m9.figshare.22064825.
Supplemental Fig. S6: http://doi.org/10.6084/m9.figshare.22064816.
Supplemental Fig. S7: http://doi.org/10.6084/m9.figshare.22064828.
Supplemental Data S1: http://doi.org/10.6084/m9.figshare.22065005.
Data Availability Statement
The code to reproduce the results is available on the GitHub repository https://github.com/novonordisk-research/ORX_HFD_Astrocytes_TRAPseq. Data will be made available upon reasonable request.