Graphical Abstract
Graphical Abstract.
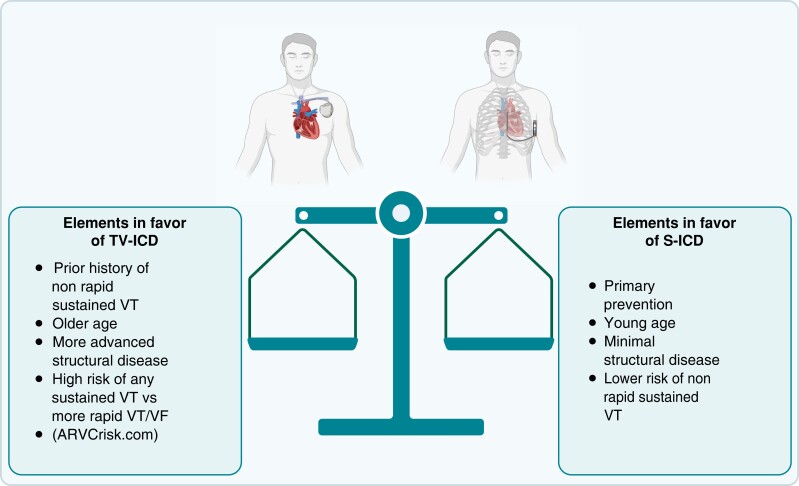
S-ICD, subcutaneous ICD; TV-ICD, transvenous implantable cardioverter defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia. Created with BioRender.com.
This editorial refers to ‘Right ventricular function is a predictor for sustained ventricular tachycardia requiring anti-tachycardic pacing in arrhythmogenic ventricular cardiomyopathy: insight into transvenous vs. subcutaneous implantable cardioverter defibrillator insertion’ by S. Honarbakhsh et al., https://doi.org/10.1093/eupace/euad073.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a significant cause of sudden cardiac death (SCD) in young individuals and athletes, for whom implantable cardioverter defibrillators (ICDs) are the only effective preventive strategy.1 Avoiding SCD while averting potential complications associated with ICD use is a common dilemma faced when treating this population. In recent years, subcutaneous ICDs (S-ICD) have emerged as an alternative to standard transvenous ICDs, with short-term evidence suggesting a lower risk of lead-related complications.2 However, these devices are still limited by their inability to avoid deleterious ICD shocks by delivering effective anti-tachycardia pacing (ATP). Compared with ICD shocks, ATP is associated with a lower risk of hospitalization and mortality, while also averting the psychological burden related to shocks.
To indirectly assess the suitability of an S-ICD in the ARVC population, Honarbakhsh et al.3 analysed an approach to predict events triggering ATP. For this purpose, a small single-centre cohort of 46 ICD recipients diagnosed with ARVC who were followed over 12 years was assembled. The patients were implanted with either a transvenous ICD or an S-ICD, both for primary and secondary prevention purposes. Their primary outcome was defined as the use of ATP for a ventricular arrhythmia (VA), regardless of the efficacy of the ATP. Of important note, ATP delivered in the ventricular fibrillation (VF) zone (ATP during charging) was not counted in the primary outcome. Studied events thus included any ventricular tachycardia (VT) of sufficient duration and rate to trigger treatment between the lowest programmed therapy zone and the programmed VF zone, starting between 230 and 250 b.p.m. The main finding is that severe right ventricular (RV) dysfunction on echocardiogram [Tricuspid annular plane systolicexcursion (TAPSE) <1.3 cm and fractional area change (FAC) of <23%] predicts ATP-triggering VA events.
As this primary finding translates, in part, the VA event rate, a potential explanation can be found in prior literature. It has previously been shown that RV dysfunction is an independent predictor of sustained VA in ARVC,4 but not of the most rapid subset of events (VT >250 b.p.m., VF, sudden cardiac arrest, or death).5 This dichotomy is believed to be related to the different mechanisms of VAs in ARVC. Indeed, slower and stable events are most likely scar dependent, linked to a more advanced structural burden that translates into reduced RV function. On the other hand, the most rapid and unstable events are believed to be, in part, attributable to non-substrate-related mechanisms including interactions between the desmosome and other electrical components of the myocyte. These events can thus occur independently from the underlying substrate.
Three important pitfalls of the analysis potentially increase the strength of the association between RV function and ATP delivery. First, RV function was taken at the time of the event in patients who had ATP vs. at baseline. This most likely introduces a source of bias towards lower RV function in patients who had ATP, given the fact that ARVC is a structurally progressive disease and that the follow-up of this study is long (median of 12 years). Thus, association at the time of ATP and not prediction of the need for ATP can be drawn from this observation.
Secondly, a multivariable model is presented to support the independent value of severe RV dysfunction as a predictor of ATP-triggering events. Importantly, this study is not adequately powered for such analysis. There are only 15 events for 13 predictors, while a minimum of 10 events per predictor is recommended for such a model (overfitting). Lastly, the most significant predictor of events triggering ATP, a prior history of monomorphic VT falling in the VT zone, was not adequately explored beyond the knowledge that the ICD was implanted for secondary prevention. It comes as little surprise that previous VA predicts future events. As they say, past performance predicts future performance!
The outcome used, delivery of ATP, also raises several questions. First, the use of ATP in this retrospective study is highly dependent on device programming. The statement that programming followed HRS programming guidelines implies a certain uniformity for patients who received their devices for primary prevention in recent years. However, device programming in secondary prevention is individualized depending on the prior arrhythmia event. Moreover, given the very long follow-up period (12 years), it can be expected that many patients received their ICD before the first iteration of these guidelines and the MADIT-RIT trial in 2012,6 which changed the practice by deferring intervention, promoting ATP and reducing shocks, particularly at high heart rates.
It is also uncertain why authors chose to predict VT resulting in ATP rather than the successful response to ATP. Triggering of ATP does not necessarily imply responsiveness to ATP, which seems more relevant to answer the underlying question of S-ICD suitability. Of note, the success rate of ATP in this cohort was lower than in previous cohorts. In the present study, the ATP success rate was 80% when excluding rapid events but reduced to 47% when including these events. In a larger multicentre cohort of ARVC patients, the ATP success rate reached 92%, and interestingly, this rate was independent of VT cycle length.7 However, since a slower VT rate is usually an important predictor of ATP response, the lower response to ATP could find its explanation in the high proportion of rapid events falling in the VF zone in this cohort (45 vs. 16–23% in prior literature).5 Device programming also plays a role in the success rate.8
Other interesting data presented include the complication rate of ICDs and suitability for an S-ICD. The reported 7 patients (15%) with inappropriate shocks and 11 (24%) with lead-related complications might seem surprisingly high at first glance. However, it parallels what has been reported in young patients with inherited arrhythmia syndromes and ARVC.9,10 The potential suitability for S-ICD was assessed indirectly with the surface electrocardiogram. The rate of bundle branch block potentially impacting suitability (19.6%) is significantly higher in this cohort than usually seen in this population. In clinical practice, the usual limitation to S-ICD implantation in ARVC lies in the R/T ratio, which often decreases with disease progression.
The question of who should get a transvenous ICD and who should get an S-ICD in ARVC is definitely relevant. In this particular population, the benefits of both approaches must be carefully weighed. Transvenous ICDs offer the benefit of ATP but are associated with more lead-related complications and long-term risk of lead failure and abandonment or extraction, which is a particular concern in this young population. Transvenous ICD use is not without its challenges because significant replacement fibrosis of the RV can complicate lead placement and cause its deterioration with time. On the other hand, S-ICDs reduce the risk related to leads and simplify long-term management but are associated with more inappropriate shocks; moreover, long-term sensing is unproved.2 In a recent report of ARVC patients, S-ICDs have been associated with more body image concerns and a reduced range of motion.11
Patients who are the most likely to benefit from a transvenous system are those with a prior history of sustained VA at a rate that would benefit from ATP. On the other hand, younger age, a low structural burden, and a lower overall risk of sustained VT4 (ARVCrisk.com) favour an S-ICD. In the young population, especially with a mild structural phenotype, and a proportionally high risk of rapid VA5 compared with the total risk of VA,4 an S-ICD can save years of transvenous leads, even in the case where VT potentially benefiting from ATP develops over time. In this case, a change to a transvenous system, VT ablation,12 or the use of a leadless ATP delivering system, which is currently being studied, could be considered. There is definitely not a ‘one size fits all’ approach to implantable device selection in ARVC. In every case, the advantages and disadvantages of these two options should be discussed with the patient and their values and preferences carefully weighed.
Contributor Information
Julia Cadrin-Tourigny, Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 rue Bélanger, Montréal, QC, Canada H1T 1C8.
Andrew D Krahn, Division of Cardiology, University of British Columbia, Vancouver, Canada.
Magdi Saba, Cardiovascular Clinical Academic Group, St George’s University Hospitals NHS Foundation Trust, London, UK.
Funding
J.C.-T. is supported by the Philippa and Marvin Carsley Cardiology Research Chair and the Fondation Institut de Cardiologie de Montreal.
References
- 1. Bosman LP, Nielsen Gerlach CL, Cadrin-Tourigny J, Orgeron G, Tichnell C, Murray Bet al. Comparing clinical performance of current implantable cardioverter-defibrillator implantation recommendations in arrhythmogenic right ventricular cardiomyopathy. Europace 2022;24:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Healey JS, Krahn AD, Bashir J, Amit G, Philippon F, McIntyre WFet al. Perioperative safety and early patient and device outcomes among subcutaneous versus transvenous implantable cardioverter defibrillator implantations: a randomized, multicenter trial. Ann Intern Med 2022;175:1658–65. [DOI] [PubMed] [Google Scholar]
- 3. Honarbakhsh SPA, Monkhouse C, Hunter RJ, Elliott PM, Lambiase PD. Right ventricular function is a predictor for sustained ventricular tachycardia requiring anti-tachycardic pacing in arrhythmogenic ventricular cardiomyopathy: insight into transvenous vs. subcutaneous implantable cardioverter defibrillator insertion. Europace 2023:euad073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale Aet al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2022;43:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cadrin-Tourigny J, Bosman LP, Wang W, Tadros R, Bhonsale A, Bourfiss Met al. Sudden cardiac death prediction in arrhythmogenic right ventricular cardiomyopathy: a multinational collaboration. Circ Arrhythm Electrophysiol 2021;14:e008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JPet al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
- 7. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear Ket al. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol 2014;64:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sterns LD, Auricchio A, Schloss EJ, Lexcen D, Jacobsen L, DeGroot Pet al. Antitachycardia pacing success in implantable cardioverter-defibrillators by patient, device, and programming characteristics. Heart Rhythm 2023;20:190–7. [DOI] [PubMed] [Google Scholar]
- 9. Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AAet al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016;13:443–54. [DOI] [PubMed] [Google Scholar]
- 10. Christensen AH, Platonov PG, Svensson A, Jensen HK, Rootwelt-Norberg C, Dahlberg Pet al. Complications of implantable cardioverter-defibrillator treatment in arrhythmogenic right ventricular cardiomyopathy. Europace 2022;24:306–12. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Gasperetti A, Sears SF, Tichnell C, Murray B, Tandri Het al. Subcutaneous and transvenous defibrillators in arrhythmogenic right ventricular cardiomyopathy: a comparison of clinical and quality-of-life outcomes. JACC Clin Electrophysiol 2022;9:394–402. [DOI] [PubMed] [Google Scholar]
- 12. Gandjbakhch E, Laredo M, Berruezo A, Gourraud JB, Sellal JM, Martins Ret al. Outcomes after catheter ablation of ventricular tachycardia without implantable cardioverter-defibrillator in selected patients with arrhythmogenic right ventricular cardiomyopathy. Europace 2021;23:1428–36. [DOI] [PubMed] [Google Scholar]