Abstract
Aims
Arrhythmogenic right ventricular cardiomyopathy (ARVC) patients develop ventricular arrhythmias (VAs) responsive to anti-tachycardia pacing (ATP). However, VA episodes have not been characterized in accordance with the device therapy, and with the emergence of the subcutaneous implantable cardioverter defibrillator (S-ICD), the appropriate device prescription in ARVC remains unclear. Study aim was to characterize VA events in ARVC patients during follow-up in accordance with device therapy and elicit if certain parameters are predictive of specific VA events.
Methods and results
This was a retrospective single-centre study utilizing prospectively collated registry data of ARVC patients with ICDs. Forty-six patients were included [54.0 ± 12.1 years old and 20 (43.5%) secondary prevention devices]. During a follow-up of 12.1 ± 6.9 years, 31 (67.4%) patients had VA events [n = 2, 6.5% ventricular fibrillation (VF), n = 14], 45.2% VT falling in VF zone resulting in ICD shock(s), n = 10, 32.3% VT resulting in ATP, and n = 5, 16.1% patients had both VT resulting in ATP and ICD shock(s). Lead failure rates were high (11/46, 23.9%). ATP was successful in 34.5% of patients. Severely impaired right ventricular (RV) function was an independent predictor of VT resulting in ATP (hazard ratio 16.80, 95% confidence interval 3.74–75.2; P < 0.001) with a high predictive accuracy (area under the curve 0.88, 95%CI 0.76–1.00; P < 0.001).
Conclusion
VA event rates are high in ARVC patients with a majority having VT falling in the VF zone resulting in ICD shock(s). S-ICDs could be of benefit in most patients with ARVC with the absence of severely impaired RV function which has the potential to avoid consequences of the high burden of lead failure.
Keywords: Ventricular tachycardia, Arrhythmogenic ventricular cardiomyopathy, Transvenous implantable defibrillator, Subcutaneous implantable defibrillator
Graphical Abstract
Graphical Abstract.
What’s new?
This is the first study that has characterized the ventricular tachycardia (VT) episodes in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) in accordance with the device therapy received and identified predictors of VT resulting in anti-tachycardia pacing (ATP).
This study has shown that VT as opposed to ventricular fibrillation (VF) was the most common ventricular arrhythmia (VA) and predominantly fell in the VF zone resulting in implantable cardioverter defibrillator (ICD) shock(s).
The patients that had VT resulting in ATP more commonly had PKP2 gene mutation, severely dilated and impaired right ventricular (RV) function compared to those without VT resulting in ATP. Severely impaired RV function was an independent predictor of VT resulting in ATP with a strong predictive accuracy.
This can be used to guide transvenous ICD prescription to patients with ARVC and severely impaired RV function and consider subcutaneous ICD implantation in the remaining patients to avoid the risk of lead failure.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy associated with a high risk of ventricular arrhythmias (VAs) and sudden cardiac death (SCD).1–3 Therefore, the optimal prescription of implantable cardioverter defibrillator (ICD) type, i.e. transvenous vs. subcutaneous, in these patients is fundamentally important. Research has focused on developing predictive models for VA in patients with ARVC to aid in the prescription of ICDs.2,4 However, there is currently no clear guidance on what type of ICD would be most appropriate for these patients.
Due to the high risk of ventricular tachycardia (VT) in these patients, transvenous ICDs are commonly implanted on the grounds that it enables anti-tachycardia pacing (ATP) as a treatment for the VT and avoids shocks.5 It has, however, been established that transvenous ICDs are at risk of lead failure which is particularly more common in younger patients,6,7 which applies to ARVC patients in whom the median age at cardiac arrest has been reported to be around 35–40 years old.8,9 Furthermore, ICD-related complications are particularly high in ARVC patients.10,11
The subcutaneous ICD (S-ICD) was designed to prevent long-term transvenous ICD lead complications, as it relies on a subcutaneous lead. However, it cannot provide ATP. Therefore, there are limitations with both devices with regard to management of VT in patients with ARVC when the initial implant decision is made. At the time of writing, leadless systems are emerging but not established as an adjunct to S-ICD implantation and have cost implications as a combined S-ICD and leadless ATP pacemaker vs. transvenous ICD.
Even though patients with ARVC are at risk of VT, the characteristics of the VT as defined by the device therapy have not been well established. It remains unclear whether patients with ARVC predominantly rely on ATP from the device or ICD shocks, and if so, which clinical markers predict ATP. This study aimed to characterize the VA events during follow-up in accordance with the device therapy delivered and ultimately provide potential guidance for which ICD system, i.e. transvenous vs. S-ICD, would be most beneficial to ARVC patients.
Methods
Study design
This is a single-centre cohort study involving retrospective analysis of prospectively collated registry including patients with a diagnosis of ARVC. From the ARVC registry, all patients that had an ICD implanted were identified. The aim of the study was to determine the VA event rates in these patients and characterize patients and events in accordance with the therapy delivered by the device and to determine clinical markers that predict VT resulting in ATP. The study complied with the Declaration of Helsinki and was registered and endorsed by the Barts Health NHS Trust Clinical Effectiveness Unit (registration ID: 13052).
Data collection
Baseline characteristics were collected using electronic records. Data on anti-arrhythmic drug use at the time of the VA events, echocardiogram, and device findings were also collected.
Electrocardiogram findings
Serial 12-lead electrocardiograms (ECGs) were reviewed during the patient’s follow-up. Due to the progressive nature of ARVC, the aim was to ensure any changes in ECG parameters were identified and that ECG parameters at the time of the VA events were utilized for the analysis. Electrocardiogram findings reviewed included the presence of bundle branch block (BBB) defined as a QRS duration of >120 ms. R-wave amplitude was recorded in the lead with the largest T-wave amplitude to determine the R:T ratio and proportion of patients with a R:T ratio of >4. The presence of T-wave inversion in all the inferior leads (II, III, and aVF) was also evaluated.
Echocardiogram findings
Echocardiogram findings consisted of biventricular size, biventricular function, and the presence of right ventricular aneurysm. Echocardiogram findings were reviewed by two independent operators to ensure consensus. Due to the progressive nature of ARVC, serial echocardiograms were reviewed to ensure changes during follow-up were recorded. In those with a VA event, echocardiogram findings at the time of the VA event were used for the analysis. Right ventricular (RV) function and dimensions were determined through a combination of manual assessment including diameter and volume measurements, the use of fractional area change (FAC) and tricuspid annular plane systolic excursion (TAPSE) measurements. TAPSE and FAC were used as both have shown to effectively correlate with RV assessment on magnetic resonance imaging (MRI).12 Severe RV impairment was defined as a TAPSE <1.3 cm and FAC of <23%.12 Severe RV dilatation was defined when the RV size exceeded the left ventricular (LV) size with a ratio of <113 and two operators agreed that the RV was severely dilated based on RV size measurements and visual assessment. Left ventricular function was evaluated by a combination of visual assessment and the use of Simpson’s biplane ejection fraction (EF) measurement. Severe LV impairment was defined as an EF of ≤35%.
Genetic mutation
The presence of gene mutation and type of gene mutation was reviewed in all patients.
Device data
Device data consisted of age at the time of device implant, indication, type of device, and programming with regard to VA therapies. VA events during follow-up were recorded and were defined as either ventricular fibrillation (VF) or sustained VT. If a VA event occurred, the device therapy delivered to treat this and the symptoms in relation to the VA event were recorded. The percentage of ventricular pacing during follow-up was also documented. Data on device complications including device-related infection, lead failure, and inappropriate therapy were collected. Patients that were treated with at least one ATP during follow-up for VT in the VT zone, successful or not, with the exclusion of ATP in the VF zone, were included in the VT resulting in ATP cohort.
Follow-up
Follow-up for patients was defined as the follow-up from the time of device implantation. All patients had a combination of both remote and face-to-face follow-up in the device clinic. Any patients with a follow-up of less than 2 years were excluded from the analysis.
Predictive model for ventricular tachycardia resulting in anti-tachycardia pacing
Differences in clinical markers in patients with VT resulting in ATP and those without VT resulting in ATP were determined. The predictive power and diagnostic accuracy of these markers with regard to slow VT resulting in ATP were determined. Time to event was used for the analysis.
Statistical analysis
All statistical analyses were performed using SPSS (IBM SPSS Statistics, Version 25 IBM Corp, NY, USA). Continuous variables are displayed as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Categorical variables are presented as a number and a percentage. Fisher’s exact test was used for the comparison of nominal variables. The Student’s t-test or its non-parametric equivalent Mann–Whitney was used for comparison of continuous variables. One-way analysis of variance (ANOVA) was performed to compare parameters during multigroup comparison. Cox proportional hazard ratio was used to determine predictors of VT resulting in ATP during follow-up. The mean restricted survival was determined for these predictors, defined as the average survival from slow VT resulting in ATP from time of ICD implant to first event rate or latest follow-up if no events. Receiver operating characteristic (ROC) analysis was performed to determine the association between the clinical markers and VT resulting in ATP during follow-up. Area under the curve (AUC) was determined. Sensitivity, specificity, positive predictive value, and negative predictive value were also determined. Kaplan–Meier survival curves were plotted, and log rank was calculated.
Results
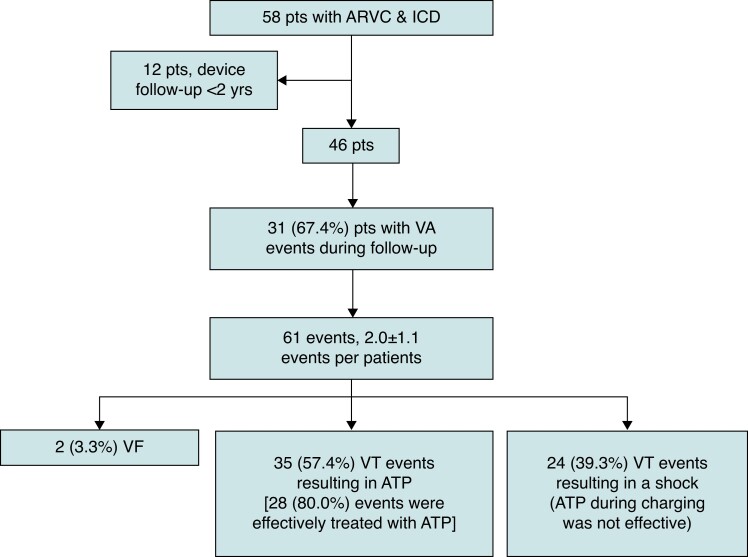
The registry consisted of an ARVC cohort of 296 patients. Out of these patients, 58 patients had an ICD implanted. Out of these patients, 12 (20.7%) patients were excluded as they had less than 2 years device follow-up (Figure 1). In the remaining 46 patients, an ICD was implanted in 20 (43.5%) and 26 (56.5%) patients for secondary and primary prevention, respectively. The average age was 54.0 ± 12.1 years and 28 (60.9%) were male. A proportion of patients had severely impaired RV systolic function (13/46, 28.3%) and LV systolic function (7/46, 15.2%).
Figure 1.
Consort flow diagram for the study. ARVC, arrhythmogenic right ventricular cardiomyopathy; ATP, anti-tachycardia pacing; ICD, implantable cardioverter defibrillator; pts, patients; VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia; yrs, years.
Baseline characteristics, indication for ICD implantation, and type of ICD are demonstrated in Table 1. Two of the patients that had a dual-chamber ICD implanted had this upgrade to a cardiac resynchronization therapy defibrillator (CRT-D) during follow-up due to the development of severe LV impairment, left BBB, and heart failure symptoms. The patients with S-ICDs had no VA during follow-up. The age at device implantation was 40.6 ± 12.6 years. None of the 46 patients had any initial Class I or IIa pacing indication and did not have ventricular pacing during follow-up. A dual-chamber ICD was implanted to aid in supraventricular tachycardia (SVT)/VT discrimination.
Table 1.
Baseline characteristics
| Baseline characteristics | Cohort n = 46 |
|---|---|
| Age years mean ± SD | 54.0 ± 12.1 |
| Male n (%) | 28 (60.9) |
| Diabetes mellitus n (%) | 3 (6.5) |
| Hypertension n (%) | 13 (28.3) |
| TIA/CVA n (%) | 0 (0) |
| Ischaemic heart disease n (%) | 1 (2.2) |
| Cardiac surgery n (%) | 0 (0) |
| Gene status n (%) | 35 (76.1) |
| DSP n (%) | 13 (37.1) |
| PKP2 n (%) | 13 (37.1) |
| DES n (%) | 1 (2.9) |
| DSG2 n (%) | 7 (20.0) |
| RYR2 n (%) | 1 (2.9) |
| Left ventricular involvement n (%) | 22 (47.8) |
| Left ventricular EF mean ± SD | 49.5 ± 11.2 |
| Severe ventricular impairment | |
| Right ventricle n (%) | 13 (28.3) |
| Left ventricle n (%) | 7 (15.2) |
| Severe ventricular dilatation | |
| Right ventricle n (%) | 12 (26.1) |
| Left ventricle n (%) | 4 (8.7) |
| Right ventricle aneurysm n (%) | 2 (4.3) |
| Bundle branch block n (%) | 9 (19.6) |
| T-wave inversion all inferior leads n (%) | 10 (21.7) |
| R:T ratio >4 in the inferior leads n (%) | 41 (89.1) |
| Atrial fibrillation n (%) | 8 (17.4) |
| Current anti-arrhythmic drugs n (%) | 28 (60.9) |
| Sotalol n (%) | 18 (64.3) |
| Flecainide n (%) | 5 (17.9) |
| Amiodarone n (%) | 5 (17.9) |
| Current rate-controlling drugs n (%) | 23 (50.0) |
| Bisoprolol n (%) | 22 (95.7) |
| Propanolol n (%) | 1 (4.3) |
| ICD indication | |
| Secondary prevention n (%) | 20 (43.5) |
| Syncope and sustained VT n (%) | 13 (65.0) |
| Cardiac arrest | 7 (35.0) |
| Primary prevention n (%) | 26 (56.5) |
| Non-sustained VT and FMH SCD | 9 (34.6) |
| Non-sustained VT and syncope | 5 (19.2) |
| Syncope and +VT stim | 5 (19.2) |
| Non-sustained VT and severe LV impairment | 6 (23.1) |
| Syncope and severe RV impairment | 1 (3.8) |
| ICD type | |
| Dual chamber | 29 (63.0) |
| Single chamber | 13 (28.3) |
| CRT-D | 2 (4.3) |
| S-ICD | 2 (4.3) |
DES, desmin; DSG2, desmoglein-2; DSP, desmoplakin; EF, ejection fraction; FMH, family history; PKP2, plakophilin 2; RYR2, ryanodine receptor 2; SCD, sudden cardiac death; TIA/CVA, transient ischaemic attack/cerebrovascular attack; VT, ventricular tachycardia.
Implantable cardioverter defibrillator programming
All patients had a VF zone programmed between 230 and 250 b.p.m. depending on the device manufacturer and in accordance with guidelines.14 All patients had a burst of ATP programmed in the VF zone that was delivered during ICD charging. All patients had a fast VT zone programmed which started from 190 to 240 b.p.m. In this zone, the programming consisted of two deliveries of ATP either as burst or ramp followed by a shock if the ATP did not effectively terminate the VT. An additional VT2 zone with ATP only was programmed in 16 patients. The VT2 zone was programmed 10–20 b.p.m. <VT rate.14 The programming zone was between 140 and180 b.p.m. as guided by the documented VT rate. All the 16 patients had a secondary prevention indication for the ICD. All patients had a VT monitor zone programmed. Brady programming was set between 40 and 60 b.p.m. The ICD programming maintained consistent during follow-up in all patients except two. In these two patients, the VT2 zone was lowered because of documented VT below the therapy zone.
Ventricular arrhythmia events
The follow-up after device implantation was 12.1 ± 6.9 years. During the follow-up, 31 out of the 46 (67.4%) patients had VA events. The 5-year incidence rate of VA events was 17.5%. Out of the 31 patients, 2 (6.5%) patients had VF resulting in an ICD shock(s), 14 (45.2%) patients had only fast VT resulting in ICD shock(s), 10 (32.3%) patients had only VT resulting in ATP, and 5 (16.1%) patients had both VT resulting in ATP and ICD shock(s) (see Supplementary material online, Table S1). Two out of the 10 patients that had only VT resulting in ATP also had one episode each of VT that fell in the monitor zone which spontaneously terminated and was not associated with any symptoms. The VF events started with VF.
There were 61 VA events in total that resulted in device therapy. The number of VA events per patient during follow-up was 2.0 ± 1.1 (1–4 VA events). The age at the time of the first VA event was 46.6 ± 12.8 years. The time of VA event post ICD implant was 6.9 ± 6.2 years. The VA events were predominantly due to sustained VT (59/61, 96.7%) with only 2 (2/61, 3.3%) VF events. Out of 59 VT events, 35 (59.3%) resulted in ATP from the device and 24 (40.7%) events resulted in ICD shock(s) as the cycle length of the VT event fell in the VF zone.
Out of the 46 patients, 8 (17.4%) patients had endocardial and epicardial VT ablations due to recurrent VT resulting in device therapy. Out of these 8 patients, 2 patients had 2 VT ablations and 1 had 4 VT ablations.
Differences in parameters between patients that had VA events and those that did not are demonstrated in Supplementary material online, Table S2. There were no differences in parameters between these two cohorts. Multigroup comparison with the groups allocated in accordance with device therapy is demonstrated in Supplementary material online, Table S3.
Anti-tachycardia pacing efficacy
Out of the 35 events in 15 patients of sustained VT that resulted in ATP, 22 (62.9%) events in 6 (40.0%) patients were associated with syncope/pre-syncope.
Out of the 35 events in 15 patients, 7 (20.0%) events in 5 (33.3%) patients were not effectively treated with ATP and the patient subsequently had an ICD shock. Therefore, ATP effectively treated 28 (80.0%) events in 10 (66.7%) patients. When reviewing the 29 patients that had VT events and including the ATP delivered prior to the ICD shock, ATP was effective in terminating the VT event in 34.5% of patients (10/29).
Fast ventricular tachycardia events
Out of the 24 events in 14 patients of sustained fast VT that fell in the VF zone from the start, 21 (87.5%) events in 13 (92.9%) patients were associated with syncope/pre-syncope. The average VT rate was 242 ± 6.5 b.p.m.
In all patients, the ATP delivered whilst the ICD was charging was not effective resulting in a shock that effectively terminated the VT episode. With 6 (25%) events, the VT reinitiated and resulted in a further shock. Out of these 6 events with 5 (83.3%) events, the VT episode continued to reinitiate resulting in multiple shocks from the device (3–20 shocks).
The use of anti-arrhythmic drugs at the time of the VA events in those patients that had effective ATP (n = 10) vs. those patients that had an ICD shock because ATP was not effective (n = 5) or the VT fell in the VF zone (n = 14) were not different (8/10, 80.0% vs. 15/19, 78.9%; P = 1.00).
Device complications
Out of the 46 patients, 19 (41.3%) patients had device-related complications. This included inappropriate shocks for atrial fibrillation/supraventricular tachycardia (7/19, 36.8%), RV lead failure with noise on the RV lead and suspected RV lead fracture (11/19, 57.9%), and device erosion (1/19, 5.3%). All the RV lead failures and the device erosion resulted in the extraction of the existing system and implantation of a new system. In these 12 patients, a new transvenous ICD system was implanted in 11 patients and 1 patient had an S-ICD implanted. No device-related complications occurred in the 2 patients with S-ICDs.
Predictors of ventricular tachycardia resulting in anti-tachycardia pacing
Several parameters were evaluated to determine if they were predictive of VT resulting in ATP during follow-up. The VT resulting in ATP cohort consisted of 15 patients. The without VT resulting in ATP cohort consisted of 31 patients. Severely impaired RV function and RV size were significantly more common in patients with VT resulting in ATP compared to patients without VT resulting in ATP (Table 2).
Table 2.
The differences in parameters between patients with VT resulting in ATP vs. patients without VT resulting in ATP during follow-up
| Proposed risk factors | VT resulting in ATP n = 15 | No VT resulting in ATP n = 31 | P-value |
|---|---|---|---|
| Age mean ± SD | 55.5 ± 13.3 | 53.2 ± 11.4 | 0.59 |
| Age at diagnosis mean ± SD | 41.6 ± 13.8 | 40.2 ± 12.0 | 0.35 |
| Male n (%) | 11 (73.3) | 17 (54.8) | 0.34 |
| Diabetes mellitus n (%) | 0 (0) | 3 (9.7) | 0.54 |
| Hypertension n (%) | 5 (33.3) | 8 (25.8) | 0.73 |
| Ischaemic heart disease n (%) | 1 (6.7) | 0 (0) | 1.00 |
| Atrial fibrillation n (%) | 2 (13.3) | 6 (19.4) | 0.33 |
| Bundle branch block n (%) | 2 (13.3) | 7 (22.6) | 0.70 |
| Anti-arrhythmic drugs n (%) | 10 (66.7) | 18 (58.1) | 0.75 |
| Sotalol n (%) | 8 (80.0) | 10 (55.6) | 0.21 |
| Flecainide n (%) | 2 (20.0) | 3 (16.7) | 1.00 |
| Amiodarone n (%) | 0 (0) | 5 (27.8) | 0.16 |
| Left ventricular function ≥55% n (%) | 8 (53.3) | 16 (51.6) | 1.00 |
| Left ventricular involvement n (%) | 7 (46.7) | 15 (48.4) | 1.00 |
| Severe ventricular impairment n (%) | |||
| RV function n (%) | 12 (80.0) | 1 (3.2) | <0.001 |
| LV function n (%) | 3 (20.0) | 4 (12.9) | 0.67 |
| Severely ventricular dilatation n (%) | <0.001 | ||
| RV size n (%) | 9 (60.0) | 3 (9.7) | <0.001 |
| LV size n (%) | 2 (13.3) | 2 (6.5) | 0.59 |
| Right ventricle aneurysm n (%) | 1 (6.7) | 1 (3.2) | 1.00 |
| Gene status n (%) | 14 (93.3) | 21 (67.7) | 0.07 |
| DSP n (%) | 2 (14.3) | 11 (52.4) | 0.03 |
| PKP2 n (%) | 10 (71.4) | 3 (14.3) | 0.001 |
DSP, desmoplakin; LV, left ventricle; PKP2, plakophilin 2; RV, right ventricle.
PKP2 gene mutation
Patients with PKP2 gene mutation had a lower mean restricted survival from VT resulting in ATP compared to patients without (11.4 years 95%CI 7.3–15.4 vs. 22.1 years 95%CI 17.4–26.9; P = 0.02).
In the univariate analysis, PKP2 gene mutation showed a strong association with VT resulting in ATP during follow-up (HR 3.44, 95%CI 1.19–9.94; P = 0.02) (Table 3). PKP2 gene mutation showed a high diagnostic accuracy for predicting VT resulting in ATP during follow-up (see Supplementary material online, Table S4).
Table 3.
Multivariate analysis of the parameters that were predictive of VT treated with ATP during follow-up
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Predictive markers | P-value | HR ratio (95%CI) | P-value | HR ratio (95%CI) |
| Age at ARVC diagnosis | 0.26 | 1.03 (0.98–1.07) | — | — |
| Male | 0.37 | 1.80 (0.50–6.47) | — | — |
| Anti-arrhythmic drugs | 0.80 | 1.15 (0.39–3.45) | — | — |
| Bundle branch block | 0.76 | 1.27 (0.28–5.84) | — | — |
| Genetic mutation | 0.20 | 0.03 (0.00–4.78) | — | — |
| DSP gene mutation | 0.94 | 1.06 (0.24–4.78) | — | — |
| PKP2 gene mutation | 0.02 | 3.44 (1.19–9.94) | — | — |
| Secondary prevention device | 0.47 | 1.48 (0.51–4.27) | — | — |
| Severely impaired RVͲ function | <0.001 | 16.80 (3.74–75.52) | <0.001 | 16.80 (3.74–75.52) |
| Severely dilated RV | 0.007 | 4.49 (1.49–13.51) | — | — |
| Severely impaired LVŦ function | 0.84 | 1.13 (0.32–4.09) | — | — |
| Severely dilated LV | 0.29 | 2.29 (0.49–10.63) | — | — |
| RV aneurysm | 0.48 | 2.09 (0.27–16.38) | — | — |
DSP, desmoplakin; LVŦ, left ventricle; PKP2, plakophilin 2; RVͲ, right ventricle.
Right ventricular size
Patients with severely dilated RV had a lower mean restricted survival compared to patients without (4.2 years 95%CI 3.6–4.9 vs. 5.5 years 95%CI 5.0–6.0; P = 0.002).
In the univariate analysis, severely dilated RV showed a strong association with VT resulting in ATP during follow-up (HR 4.49, 95%CI 1.49–13.51; P = 0.007) (Table 3). Severely dilated RV had a strong diagnostic accuracy for predicting VT resulting in ATP during follow-up (see Supplementary material online, Table S4). A majority of patients that had severely dilated RV size also had severely impaired RV function (11/12, 91.7%).
Right ventricular function
Patients with severe RV impairment had a lower mean restricted survival compared to patients without (4.1 years 95%CI 5.2–8.4 vs. 6.2 years 95%CI 3.4–4.8; P < 0.001).
In the univariate analysis, severely impaired RV function showed a strong association with VT resulting in ATP during follow-up (HR 16.80, 95%CI 3.74–75.52; P < 0.001) (Table 3). Severely impaired RV function showed a high diagnostic accuracy for predicting VT resulting in ATP during follow-up (see Supplementary material online, Table S4).
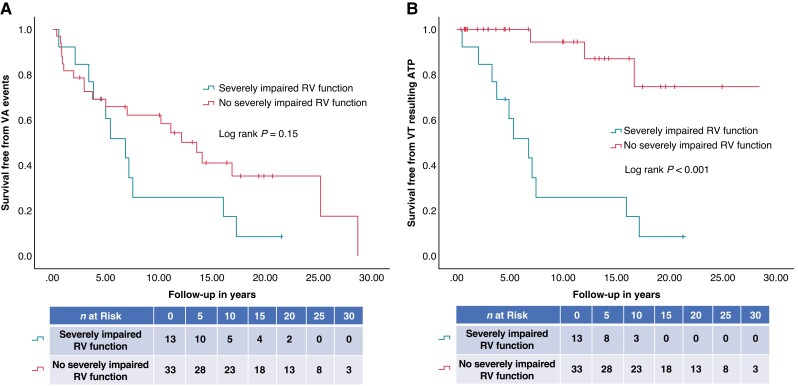
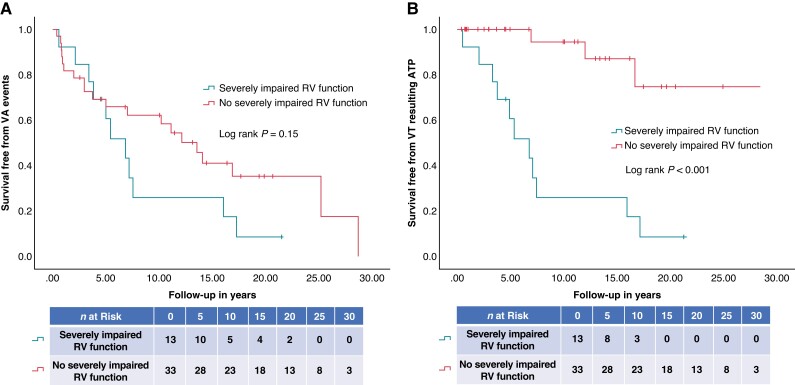
In the multivariate analysis, severely impaired RV function was shown to be the only independent predictor of VT resulting in ATP during follow-up (HR 16.80, 95%CI 3.74–75.52; P < 0.001) (Table 3). Differences in survival free from VA events and VT resulting in ATP in patients with severely impaired RV function are demonstrated in representative Figure2A and B.
Figure 2.
(A, B) Kaplan–Meier survival curves of the survival free from (A) VA events and (B) VT resulting in ATP in patients with severe RV impairment compared to patients without severe RV impairment. ATP, anti-tachycardia pacing; RV, right ventricular; VA, ventricular arrhythmia; VT, ventricular tachycardia.
In the absence of severely impaired RV function, a majority of patients that had a VA event had this due to fast VT that fell in the VF zone and was treated with ICD shock(s) (15/18, 83.3%).
Subcutaneous implantable cardioverter defibrillator suitability
A majority of patients did not have BBB (37/46, 80.4%). Out of the 46 patients, 10 (21.7%) patients had T-wave inversion in all the inferior leads (II, III, and aVF). The R:T ratio was >4 in 41 (89.1%) patients. Out of the 33 patients without severe RV impairment, 27 (81.8%) patients did not have BBB, T-wave inversion in the inferior leads, and R:T ratio was >4 and would potentially be suitable for S-ICD (see Supplementary material online, Figure S1).
Discussion
This is the first study that has characterized the VT episodes in patients with ARVC in accordance with the device therapy received and identified predictors for VT resulting in ATP. This study has demonstrated several important findings:
VA events are frequent in patients with ARVC with more than two-thirds of the patients experiencing at least one VA event during follow-up.
VT as opposed to VF was the most common VA and predominantly fell in the VF zone resulting in an ICD shock(s).
Patients with VT resulting in ATP more commonly had PKP2 gene mutation, severely dilated and impaired RV function compared to those without VT resulting in ATP. Severely impaired RV function was an independent predictor of VT resulting in ATP with a strong predictive accuracy. This can be used to guide transvenous ICD prescription to patients with ARVC and severely impaired RV function.
The majority of the ARVC patients does not have BBB, T-wave inversion in the inferior leads, and R:T ratio <4 potentially making S-ICD implantation feasible in these patients.
Subcutaneous implantable cardioverter defibrillator implantation could be considered in patients without severe RV impairment and sustained slow monomorphic VT.
Ventricular arrhythmia events during follow-up
This study supports the findings of other studies2,3,8 that patients with ARVC are at risk of VA events. This study did not demonstrate a significantly higher rate of VA events in patients with PKP2 gene mutation, DSP gene mutation, severely impaired ventricular function, severely dilated ventricular size, and secondary prevention device.
Predictors for ventricular tachycardia resulting in anti-tachycardia pacing
Even though a lot of focus has been on identifying predictive parameters of VA events in patient with ARVC and to guide prescription of ICD, there has been less emphasis on determining the most suitable ICD device in these patients. Currently, due to the high risk of VT in patients with ARVC, transvenous ICDs are predominantly implanted as they can deliver ATP. However, it is well recognized that, particularly in young patients, there is a high risk of lead failures from these devices resulting in the need for further interventions.6,7,10,11 This is demonstrated in this study whereby 23.9% of patients had lead failure and required extraction of their existing device and implantation of a new ICD system. Even though a similar proportion of patients in this cohort had VT resulting in ICD shocks as it fell in the VF zone and VT resulting in ATP, the patients with VT resulting in ATP had predominantly severely impaired RV function. Thereby, in the absence of severely impaired RV function, patients that had VA events predominantly experienced rapid VT resulting in ICD shocks.
A previous study reported a high rate of VT resulting in ATP in patients with ARVC,15 which is contrary to the findings of this study. RV function and size were not assessed in this cohort. Based on the higher VA event rates in this cohort (10.5 events per patient), it is suggestive that this is a more advanced diseased cohort compared to the cohort in this study. It is known that with progression of ARVC, the risk of VA events increases. Therefore, it is possible to speculate that patients in this cohort were more likely to have RV impairment and dilatation. As shown in this study, these were predictors of VT resulting in ATP which could account for the higher rate of VT resulting in ATP. RV dysfunction has already been shown to be a predictor of life-threatening arrhythmias including SCD in patients with ARVC3 and shown to be a major risk factor for arrhythmic events in patients with ARVC.4,16 Severe RV dysfunction is also one of the Class I indications for an ICD in patients with ARVC in accordance with four stratification algorithms.17 In this study, it has also been shown that RV dysfunction is also an independent predictor of VT resulting in ATP.
We note that PKP2 mutation patients had a higher burden of VT resulting in ATP. PKP2 mutations play an important role in promoting fibrosis as central players in the coordination of desmosome-dependent TGF-β1/p38 MAPK signalling.18 In early phase of ARVC before significant structural changes develop, VA and SCD occur at high heart rates during stress, exercise, or sports activity (increased adrenergic drive) due to the initial reduction in conduction reserve and the steep restitution slope of action potential duration in the tissue.19 In contrast, during later phases of ARVC when structural changes and fibro-fatty replacement advances, VAs are more common at lower heart rates as a monomorphic VT. The advanced structural changes promote greater conduction delay at longer coupling intervals to prevent engagement of the proximal portion of the action potential restitution curve reducing the possibility of VT degenerating into VF which occurs in the early subclinical phase. Therefore, the VA in ARVC may arise from different mechanisms as the disease progresses with the development of structural changes over time. This has important implications for device prescription as illustrated here.
Subcutaneous implantable cardioverter defibrillator suitability
A large proportion of the patients in this study had VT falling in the VF zone resulting in an ICD shock from the device. As a result, these patients could effectively be managed with an S-ICD which would have an equivalent VF shock zone programming as a transvenous ICD. Prior to consideration of S-ICD implantation, ECG screening is required to ensure appropriate R-wave identification and to avoid T-wave oversensing. However, there are several ECG parameters including T-wave inversion in the inferior leads,20 BBB,21 and R:T ratio <322 that have been shown to predict the failing of S-ICD screening. The presence of these parameters was infrequent in this cohort. This emphasizes that even though patients would require pre-implantation S-ICD screening, it is likely based on the ECG screening data a large proportion of these patients would have potentially passed S-ICD screening and be suitable for an S-ICD. Studies have shown that S-ICDs are effective in treating VA events in patients with ARVC but that strategies need to be implemented to overcome the risk of inappropriate shocks.23,24
Furthermore, it has been shown that VA events frequently self-terminate,25 and as shown in this study, a majority of patients with VT resulting in successful ATP was asymptomatic. Thereby, all the VT episodes in this cohort might not have required ATP therapy.
ARVC is a progressive disease, so patients can develop later ventricular impairment as fibro-fatty replacement evolves. As shown in this study, severe RV impairment was an independent predictor of VT resulting in ATP. Therefore, if patients have an S-ICD implanted because of the absence of severe RV impairment, there is a risk that patients would develop RV impairment during follow-up which will put them at risk of VT that would benefit from ATP. In this situation, the S-ICD would not be effective to manage these episodes. Currently, this would require S-ICD removal and implantation of a transvenous ICD. However, even though this would require further intervention, an S-ICD approach would allow preservation of vasculature and minimize the risk of transvenous ICD lead failure and repeat procedures to address this at least in the first period of ICD therapy in these young patients. At the time of writing, with advancement in S-ICDs and the development of a leadless pacemaker (Empower modular pacing system, Boston Scientific) which communicates with the S-ICD and thereby enables ATP delivery, there is the possibility that if patients become at risk of or develop VT which is likely to be pace-terminable, a leadless pacemaker can be implanted.
Limitations
One of the main limitations of this study is the small cohort of patients. However, the patient cohort size is compatible with other published ARVC studies. The average follow-up in this study was about 13 years. Even though this emphasizes a long-term follow-up, due to the progressive nature of ARVC, a longer follow-up would have allowed the impact of disease progression to be better assessed. In this study, only cases with a minimum device follow-up of 2 years were included in the study. Patients’ suitability for S-ICDs in this study was determined utilizing ECG data that have previously been shown to correlate to passing S-ICD screening. To accurately determine whether these patients would be suitable for an S-ICD, S-ICD screening would still be required. This was not performed in this study. This study was an observational study and thereby faces the limitations seen with observational studies. The findings from this study provide the grounds for performing a randomized controlled trial to further evaluate different ICD device types in patients with ARVC.
Conclusions
ARVC patients are at high risk of VA events particularly VT resulting in ATP and VT falling in the VF zone resulting in an ICD shock. Severely impaired RV function was shown to be an independent predictor of VT resulting in ATP and demonstrated a strong predictive accuracy. In the absence of severely impaired RV function, a majority of patients had VT falling in the VF zone resulting in ICD shocks. These findings indicate that in the absence of severely impaired RV function, patients may effectively have an S-ICD implanted that would effectively provide the shock therapy to treat VA without the additional risk of lead failure which was shown in this study to be particularly high in these patients.
Supplementary Material
Contributor Information
Shohreh Honarbakhsh, The Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS trust, West Smithfield, London WC1 8BE, UK; William Harvey Research Institute, Queen Mary’s University of London, London, E1, UK.
Alexander Protonotarios, The Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS trust, West Smithfield, London WC1 8BE, UK.
Christopher Monkhouse, The Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS trust, West Smithfield, London WC1 8BE, UK.
Ross J Hunter, The Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS trust, West Smithfield, London WC1 8BE, UK.
Perry M Elliott, The Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS trust, West Smithfield, London WC1 8BE, UK.
Pier D Lambiase, The Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS trust, West Smithfield, London WC1 8BE, UK.
Supplementary material
Supplementary material is available at Europace online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Marcus FI, Abidov A. Arrhythmogenic right ventricular cardiomyopathy 2012: diagnostic challenges and treatment. J Cardiovasc Electrophysiol 2012;23:1149–53. [DOI] [PubMed] [Google Scholar]
- 2. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale Aet al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agbaedeng TA, Roberts KA, Colley L, Noubiap JJ, Oxborough D. Incidence and predictors of sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy: a pooled analysis. Europace 2022;24:1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017;376:1489–90. [DOI] [PubMed] [Google Scholar]
- 5. Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WOet al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation 2004;110:2591–6. [DOI] [PubMed] [Google Scholar]
- 6. Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider Set al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation 2007;115:2474–80. [DOI] [PubMed] [Google Scholar]
- 7. Koneru JN, Jones PW, Hammill EF, Wold N, Ellenbogen KA. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J Am Heart Assoc 2018;7:e007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale Aet al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2022;43:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jorda P, Bosman LP, Gasperetti A, Mazzanti A, Gourraud JB, Davies Bet al. Arrhythmic risk prediction in arrhythmogenic right ventricular cardiomyopathy: external validation of the arrhythmogenic right ventricular cardiomyopathy risk calculator. Eur Heart J 2022;43:3041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schinkel AF. Implantable cardioverter defibrillators in arrhythmogenic right ventricular dysplasia/cardiomyopathy: patient outcomes, incidence of appropriate and inappropriate interventions, and complications. Circ Arrhythm Electrophysiol 2013;6:562–8. [DOI] [PubMed] [Google Scholar]
- 11. Wichter T, Paul M, Wollmann C, Acil T, Gerdes P, Ashraf Oet al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy: single-center experience of long-term follow-up and complications in 60 patients. Circulation 2004;109:1503–8. [DOI] [PubMed] [Google Scholar]
- 12. Pavlicek M, Wahl A, Rutz T, de Marchi SF, Hille R, Wustmann Ket al. Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr 2011;12:871–80. [DOI] [PubMed] [Google Scholar]
- 13. Schneider M, Binder T. Echocardiographic evaluation of the right heart. Wien Klin Wochenschr 2018;130:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stiles MK, Fauchier L, Morillo CA, Wilkoff BL. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm 2020;17:e220–8. [DOI] [PubMed] [Google Scholar]
- 15. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear Ket al. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol 2014;64:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burri H, Starck C, Auricchio A, Biffi M, Burri M, D'Avila Aet al. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace 2021;23:983–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosman LP, Nielsen Gerlach CL, Cadrin-Tourigny J, Orgeron G, Tichnell C, Murray Bet al. Comparing clinical performance of current implantable cardioverter-defibrillator implantation recommendations in arrhythmogenic right ventricular cardiomyopathy. Europace 2022;24:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubash AD, Kam CY, Aguado BA, Patel DM, Delmar M, Shea LDet al. Plakophilin-2 loss promotes TGF-beta1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J Cell Biol 2016;212:425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JEet al. Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J 2012;33:1942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groh CA, Sharma S, Pelchovitz DJ, Bhave PD, Rhyner J, Verma Net al. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2014;11:1361–6. [DOI] [PubMed] [Google Scholar]
- 21. Campbell M, Moore JP, Sreeram N, von Alvensleben JC, Shah A, Batra Aet al. Predictors of electrocardiographic screening failure for the subcutaneous implantable cardioverter-defibrillator in children: a prospective multicenter study. Heart Rhythm 2018;15:703–7. [DOI] [PubMed] [Google Scholar]
- 22. Olde Nordkamp LRA, Warnaars JLF, Kooiman KM, de Groot JR, Rosenmoller B, Wilde AAMet al. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol 2014;25:494–9. [DOI] [PubMed] [Google Scholar]
- 23. Orgeron GM, Bhonsale A, Migliore F, James CA, Tichnell C, Murray Bet al. Subcutaneous implantable cardioverter-defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia: a transatlantic experience. J Am Heart Assoc 2018;7:e008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Migliore F, Viani S, Bongiorni MG, Zorzi A, Silvetti MS, Francia Pet al. Subcutaneous implantable cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy: results from an Italian multicenter registry. Int J Cardiol 2019;280:74–9. [DOI] [PubMed] [Google Scholar]
- 25. Burke MC, Aasbo JD, El-Chami MF, Weiss R, Dinerman J, Hanon Set al. 1-year prospective evaluation of clinical outcomes and shocks: the subcutaneous ICD post approval study. JACC Clin Electrophysiol 2020;6:1537–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.