Summary
Stem cells can undergo continuous self-renewal and meanwhile retain the stemness capability to differentiate to mature functional cells. However, it is unclear whether the proliferation property can be segregated from the stemness in stem cells. The intestinal epithelium undergoes fast renewal, and the Lgr5+ intestinal stem cells (ISCs) are essential to maintain homeostasis. Here, we report that methyltransferase-like 3 (Mettl3), a critical enzyme for N6-methyladenosine (m6A) methylation, is required for ISCs maintenance as its deletion results in fast loss of stemness markers but has no effect on cell proliferation. We further identify four m6A-modified transcriptional factors, whose ectopic expression can restore stemness gene expression in Mettl3−/− organoids, while their silencing leads to stemness loss. In addition, transcriptomic profiling analysis discerns 23 genes that can be segregated from the genes responsible for cell proliferation. Together, these data reveal that m6A modification sustains ISC stemness, which can be uncoupled from cell proliferation.
Keywords: intestinal stem cells, Lgr5, stemness, proliferation, Mettl3, m6A modification
Graphical abstract
Highlights
-
•
Mettl3 is required for homeostatic maintenance of the intestinal epithelium
-
•
Mettl3 deletion impairs stemness but has no effect on proliferation in ISCs
-
•
Twenty-three ISC signature genes are sifted to represent stemness
-
•
Four m6A-modified TFs can sufficiently restore ISC stemness impaired by Mettl3 KO
Based on impaired stemness but maintained proliferation of intestinal stem cells induced by Mettl3 knockout, Chen and colleagues uncover the critical role of Mettl3 in intestinal homeostasis, segregate stemness from proliferation, and narrow down 23 genes that are specifically identified as intestinal stem cell signature genes. Four screened m6A-modified transcriptional factors can restore ISC stemness through binding to their promoter.
Introduction
Stem cells have the abilities to continuously self-renew themselves and to differentiate to mature functional cells under certain conditions (Cai et al., 2004; Ivanova et al., 2002; Post and Clevers, 2019; Ramalho-Santos et al., 2002). However, it is unclear whether the proliferation property can be segregated from the stemness at the molecular level. The intestinal epithelium undergoes fast renewal, and the Lgr5-GFP-marked intestinal stem cells (ISCs) are essential to maintain the intestinal epithelium homeostasis by refreshing themselves and simultaneously supplying progenitors that divide and migrate up to the villus as they mature into absorptive or secretory epithelial cells, while a subset of progenitor cells undergo differentiation into Paneth cells residing with stem cells at the bottom of the crypts (Barker, 2014; Clevers, 2013; Qi and Chen, 2015). The Lgr5+ ISCs are a great model for investigation of tissue stem cells (Clevers, 2013). By comparing mRNAs of Lgr5+ ISCs and their daughters, 384 stem cell signature genes were identified, which contain many genes associated with proliferation (Munoz et al., 2012).
m6A methylation, the most abundant modification in eukaryotic mRNAs, regulates a variety of biological processes (He and He, 2021; Zaccara et al., 2019). m6A modification is catalyzed by a multicomponent methyltransferase complex, called “writer,” consisting of the methyltransferase-like 3 (METTL3), methyltransferase-like 14, Wilms’ tumor 1-associating protein, vir-like m6A methyltransferase associated , and RNA binding motif protein 15 (Deng et al., 2018; Zaccara et al., 2019). This modification is dynamic and reversible and can be removed by demethylases (erasers) such as FTO and ALKBH5. m6A-modified RNAs are recognized by a variety of proteins (readers), which regulate RNA stability, splicing, nuclear export, or translation (Deng et al., 2018; Zaccara et al., 2019). Furthermore, m6A modification can regulate transcription (Shi et al., 2019; Wang et al., 2014a, 2014b).
m6A RNA methylation and its associated methyltransferase METTL3 have been shown to modulate stem cell differentiation, tissue development, and tumor progression. m6A RNA methylation is required for differentiation of embryonic stem cells and neural stem cells (Batista et al., 2014; Cao et al., 2020; Geula et al., 2015; Yoon et al., 2017), while m6A modification has also been shown to be crucial for self-renewal of hematopoietic stem cells (Vu et al., 2017; Weng et al., 2018; Zhang et al., 2017), glioblastoma stem cells (Cui et al., 2017), and embryonic neural stem cells (Wang et al., 2018). METTL3 has been shown to promote the colorectal cancer progression (Li et al., 2019; Shen et al., 2020; Zhu et al., 2020), and YTH N6-methyladenosine RNA binding protein F1 (YTHDF1) is critical for the ISC maintenance during regeneration and tumorigenesis (Han et al., 2020). However, the role of METTL3 in the maintenance of ISCs during homeostasis remains unknown.
In this study, we show that METTL3 is required for ISC maintenance during intestinal epithelium homeostasis, and its deletion results in impaired stemness but not proliferation. Transcriptomic profiling analysis discerns 23 stemness genes that are specifically expressed in ISCs and can be segregated from the genes responsible for cell proliferation. We have further identified four transcriptional factors whose mRNAs undergo METTL3-mediated m6A modification, and their ectopic expression can restore stemness gene expression in Lgr5+ ISCs.
Results
Mettl3 is required for small intestine maintenance
To examine whether RNA m6A modification plays a role in the homeostatic maintenance of the intestinal epithelium, we first examined the expression pattern of the m6A transferase Mettl3. Immunohistochemical staining and q-PCR revealed that Mettl3 was enriched at the base of crypts (Figures S1A and S1B). To explore the role of Mettl3 in the intestinal epithelium, we generated inducible Mettl3 conditional knockout (KO) Villin-CreERT2;Mettl3fl/fl (Mettl3-KO) mice (Figures S1C and S1D). Tamoxifen-induced deletion of Mettl3 resulted in a rapid-onset body weight loss and death in ∼7–11 days (Figures S1E and S1F). Morphologically, the small intestine of Mettl3-KO mice displayed an enlarged crypts zone at 6 days post-tamoxifen treatment (dpt), and the crypt structure disappeared with progressive shortened villus at 8 dpt (Figure S1G). Consistently, the organoids derived from Mettl3-KO mouse crypts, upon 4-hydroxytamoxifen (4-OHT)-induced Mettl3 deletion, displayed an impaired growth ability, increased dying cells, and reduced budding (Figures S1H–S1J). These data indicate a critical role of Mettl3 in the maintenance of the intestinal epithelium homeostasis.
Mettl3 deletion leads to loss of intestinal stem cells but has no effect on cell proliferation
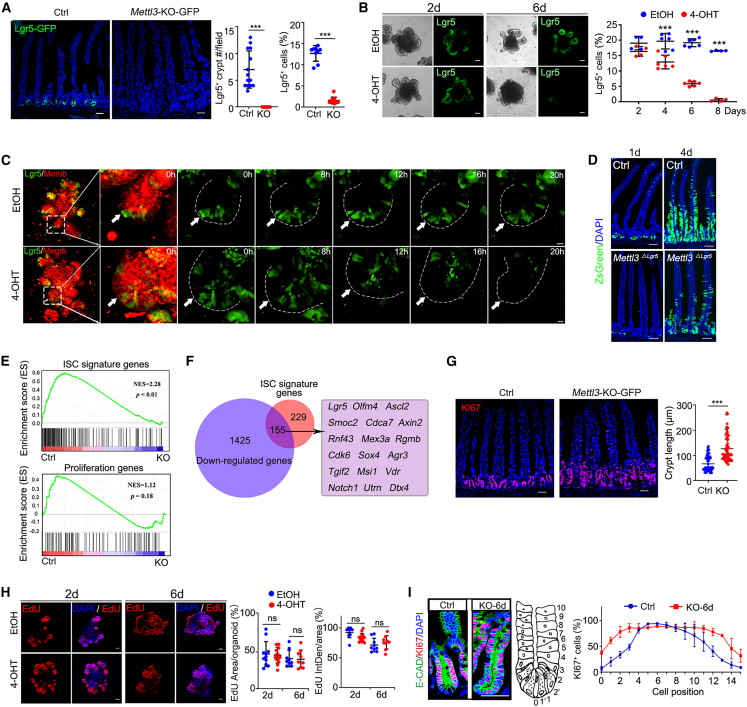
To dissect the function of Mettl3, we crossed Lgr5-EGFP-IRES-CreERT2 mice with Villin-CreERT2;Mettl3fl/fl mice to obtain Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl (Mettl3-KO-GFP) mice, which could label stem cells and analyze the transcriptome of ISCs. To rule out the possible non-specific effect of tamoxifen on mice or organoids, we treated Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2 mice with tamoxifen or Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2-derived organoids with 4-OHT compared with controls, and we found that tamoxifen and 4-OHT did not cause any phenotypic changes (Figure S2). Mettl3 KO significantly decreased Lgr5-GFP ISCs in the small intestine at 4 dpt, which was almost undetectable at 6 dpt as revealed by fluorescence-activated cell sorting (FACS) analysis and immunostaining (Figures 1A and S3A). Similar data were observed in the colon and the organoids derived from the Mettl3-KO-GFP mice (Figures 1B, S3B, and S3C). Mettl3 deletion-induced disappearance of Lgr5+ stem cells could be captured in the organoid by time-lapsed confocal microscopy (Figures 1C and S3D and Movie S1). The ISC marker OLFM4 was no longer detected at the crypt base of KO mice at 8 dpt (Figure S3E). In line with it, lineage tracing revealed that Mettl3 deletion resulted in reduction of Lgr5+ cells (Figures 1D and S3F). These data indicate that Mettl3 plays a critical role in stem cell maintenance.
Figure 1.
Mettl3 deletion disrupts intestinal stem cells but not cell proliferation
(A and B) Representative images and quantification of Lgr5-GFP+ cells in the jejunum of control and Mettl3-KO-GFP mice at 6 dpt (A) or organoids at 2 or 6 dpt (B). N = 3 mice per group, n = 15 fields per group (A). Data from three independent experiments were combined and shown (B).
(C) Time-lapse imaging of Lgr5-GFP+ organoid after EtOH or 4-OHT treatment. The organoid boundary was marked with the dotted line; see also Movie S1.
(D) Lineage tracing of Lgr5+ cells in the jejunum of control (Lgr5-CreERT2;Rosa26loxp-stop-loxp-ZsGreen) and Mettl3ΔLgr5 (Mettl3fl/fl;Lgr5-CreERT2;Rosa26loxp-stop-loxp-ZsGreen) mice at 1 or 4 dpt.
(E) GSEA of RNA-seq data from control and Mettl3-KO-GFP mice at 6 dpt.
(F) Venn diagram displaying the overlap between the ISC signature and the downregulated genes in Mettl3-KO-GFP Lgr5+ cells at 6 dpt.
(G) Representative images of KI67+ cells and quantification of crypt length in the control and Mettl3-KO-GFP jejunum at 6 dpt. N = 3 mice per group, n > 80 crypts per group.
(H) Representative images and quantification of EdU+ cells in organoids at 2 or 6 dpt. IntDen: integrated density. N > 9 organoids per group from three independent experiments.
(I) Representative images and quantification showing KI67+ cell position in control and Mettl3-KO-GFP crypts at 6 dpt. N = 3 mice per group, n = 15 fields per group. Organoids cultured from crypts of Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl mice were treated with EtOH or 4-OHT (B, C, H). Data represent mean ± SD. ∗∗∗p < 0.001, unpaired two-tailed t test (A, G), two-way ANOVA (B, H, I). Scale bars: 10 μm (C), 50 μm (A, B, D, G, H, and I). Nuclei were counter-stained with DAPI. See also Figures S1–S4 and Table S1.
Organoids derived from Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl mice were imaged every 1 h from day 4.5 to day 6.5 after being treated with EtOH (left) or 4-OHT (right) and were labeled with CellMask (orange plasma membrane stain, red). Video shows the change of Lgr5-GFP cells over time from 0 to 20 h.
RNA-seq analysis revealed that Mettl3 deletion resulted in reduced expression of most of the stem cell signature genes (Munoz et al., 2012) in ISCs from Mettl3-KO-GFP mice, including Lgr5, Olfm4, Ascl2, and Smoc2 (Figures 1E, 1F, and S3G), which were confirmed by q-PCR (Figure S3H). Interestingly, we found that Mettl3 deletion did not disrupt cell proliferation (Figures 1G–1I, S3I, and S3J). In fact, the longer crypts with more proliferative cells were detected in Mettl3-KO-GFP mice, and consistently, more crypt epithelial cells and Lgr5+ ISCs were detected in the G2/M and S phase (Figure S3K). We also detected cell death at the base of crypt in KO-4d and KO-6d mice (Figure S3L). Cell differentiation was slightly affected by Mettl3 KO as goblet cells, enteroendocrine cells, and Paneth cells were transiently increased at 6 dpt, but all intestinal cell types reduced at 8 dpt (Figures S4A and S4B), which was consistent with higher expression of the differentiation genes in the KO-4d Lgr5+ cells (Figures S4C and S4D). Taken together, these results indicate that Mettl3 deletion deteriorates the ISC stemness but not cell proliferation.
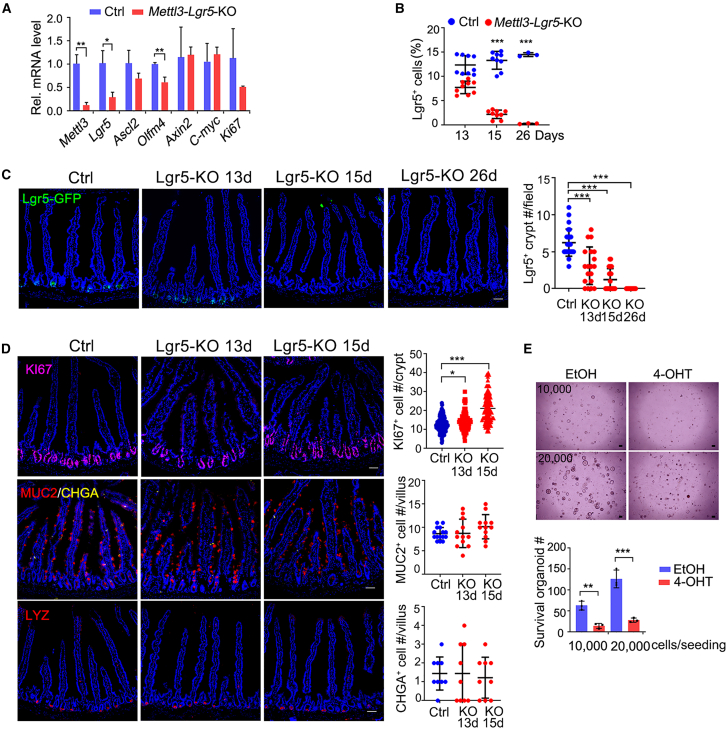
To uncover the role of Mettl3 in ISCs, we generated a stem cell-specific Mettl3 KO mouse, Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl (Mettl3-Lgr5-KO). As expected, the expression of stem cell signature genes, including Lgr5 and Olfm4, was decreased in Mettl3-Lgr5-KO ISCs (Figure 2A). Similar to the results in Mettl3-KO-GFP mice, a significant decrease of Lgr5+ ISCs was detected in Mettl3-Lgr5-KO mice at 26 dpt (Figures 2B and 2C). KI67+ cells were observed in Mettl3-Lgr5-KO mice at 15 dpt, and differentiated cells remained similar (Figure 2D). To test the self-renewal ability of Lgr5+ ISCs in vitro (Sato et al., 2009), we seeded with an equal number of single Lgr5+ ISCs and induced Mettl3 deletion by 4-OHT at the seeding time. Fewer organoids with Mettl3 KO indicated that the colony forming potential was compromised in Mettl3-KO ISCs (Figure 2E).
Figure 2.
Mettl3 is essential for the stemness maintenance in Lgr5+ intestinal stem cells
(A) q-PCR analysis of Mettl3, the ISCs marker genes (Lgr5, Ascl2, Olfm4, Axin2), and the proliferation marker gene Ki67 in FACS-sorted Lgr5-GFP+ cells from control and Mettl3-Lgr5-KO mice at 13 dpt. N = 3 mice per group.
(B) Quantitation of Lgr5-GFP+ cells in control and Mettl3-Lgr5-KO mice at the indicated time. N = 3 mice per group.
(C) Representative immunofluorescence images and quantification of Lgr5-GFP in the jejunum of control and Mettl3-Lgr5-KO mice at the indicated time. N = 3 mice per group, n = 9–15 fields per group.
(D) Representative immunofluorescence images and quantification of KI67 staining, MUC2 staining, CHGA staining, and LYZ staining in the jejunum of control and Mettl3-Lgr5-KO mice at the indicated time. N = 3 mice per group, n = 9–15 fields per group.
(E) Representative images (top) and quantification (bottom) of intestinal organoids cultured from sorted Lgr5-GFP+ cells derived from Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl mice after being treated with EtOH or 4-OHT for 5 days. The numbers of seeded cells were 10,000 and 20,000. Data from three independent experiments were combined and are shown. All the data represent mean ± SD. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, two-way ANOVA (A, B, E), one-way ANOVA (C, D). Scale bars: 50 μm (C, D), 100 μm (E). Nuclei were counter-stained with DAPI. See also Table S2.
Mettl3 deletion downregulates the stem cell signature genes
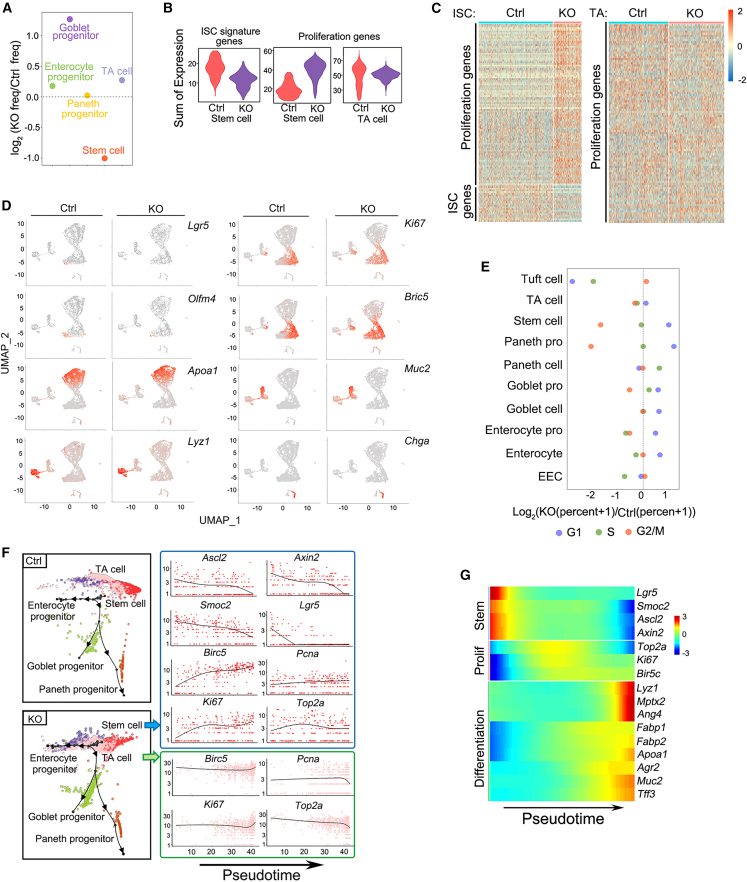
To monitor the cell lineage alteration upon Mettl3 deletion, we performed single-cell RNA-seq of small intestinal epithelial cells from control and Mettl3-KO-GFP mice at 4 dpt. Quality control selected 7,865 cells (4,467 control cells and 3,398 KO cells) for further analysis. By unsupervised clustering combined with cell-known marker genes, we identified distinct clusters, including stem cells (Lgr5, Olfm4, and Ascl2), transient amplifying (TA) cells (Ki67, Tubb5, and Birc5), goblet cells (Tff3, Agr2, and Muc2), Paneth cells (Lyz1, Ang4, and Mptx2), enteroendocrine cells (Chga, Chgb, and Sct), enterocytes (Apoa1, Fabp1, and Fabp2), tuft cells (Dclk1, Gfi1b, and Rgsl3), and progenitors (Figures S5A–S5C). Comparison of the abundance of each cell type unveiled a decrease of ISCs and an increase of goblet progenitor cells in the KO-4d epithelium (Figures 3A and S5D), consistent with a transient increase of goblet cells in KO-6d (Figures S4A and S4B). In line with it, Mettl3 deletion reduced the expression of stem cell signature genes but elevated proliferation gene levels in ISCs (Figures 3B–3D) and resulted in more stem cells in the G2/M phase (Figure 3E). To deduce the dynamic change of gene expression, we used the PAGA Tree (Dyno) and DDRTree (Monocle 2) to derive pseudotime trajectories, designating high expression of ISC markers as the start point and mature lineage as the end (Figures 3F and S5E). Stem cell signature genes, like Ascl2, Axin2, Smoc2, and Lgr5, declined along the pseudotime, while the proliferation genes Ki67, Pcna, Birc5, and Top2a increased gradually in KO stem cells (Figures 3F and S5F). Differentiation genes were enriched in Mettl3-KO cells (Figure 3G). These results together suggest that Mettl3 deficiency disrupts the stemness program in ISCs.
Figure 3.
Mettl3 deletion results in downregulation of the stem cell signature genes
(A) Dot plot depicting the relative frequency of stem cells, TA cells, and progenitor cells in control and Mettl3-KO-GFP mice at 4 dpt, revealed by scRNA-seq.
(B) Violin plots showing the metagene expression levels per single cell of the stem cell signature genes and proliferation genes in stem cells and TA cells of control and Mettl3-KO-GFP mice at 4 dpt revealed by scRNA-seq.
(C) Expression heatmap of the stem cell signature genes and proliferation genes in stem cells (left) and TA cells (right) in control and Mettl3-KO-GFP mice.
(D) Expression of the stem cell marker genes (Lgr5, Olfm4), the proliferation marker genes (Ki67, Birc5), and the differentiation cell marker genes (Apoa1, Lyz1, Muc2, Chga) in intestinal epithelium from control and Mettl3-KO-GFP mice were plotted by UMAP. Color from gray to red indicates relative expression levels from low to high.
(E) Dot plot depicts the relative percent of the cells in the G1, G2/M, and S phase in control and Mettl3-KO-GFP mice.
(F) Pseudotime analysis of stem cells, TA cells, and progenitor cells in control and Mettl3-KO-GFP mice at 4 dpt based on scRNA-seq (left). The expression of stem cell markers (Ascl2, Axin2, Smoc2, and Lgr5) and proliferation markers (Birc5, Top2a, Ki67, and Pcna) in stem cells and TA cells of Mettl3-KO-GFP mice was plotted along the pseudotime axis (right).
(G) Heatmap showing the transcription trends of marker genes of stem cells, TA cells, and differentiated cells along the pseudotime. Color scale represents Z score normalized expression levels. The pseudotime line contains all control and Mettl3-KO-GFP cells with the control cell cluster as the starting point. See also Figure S5.
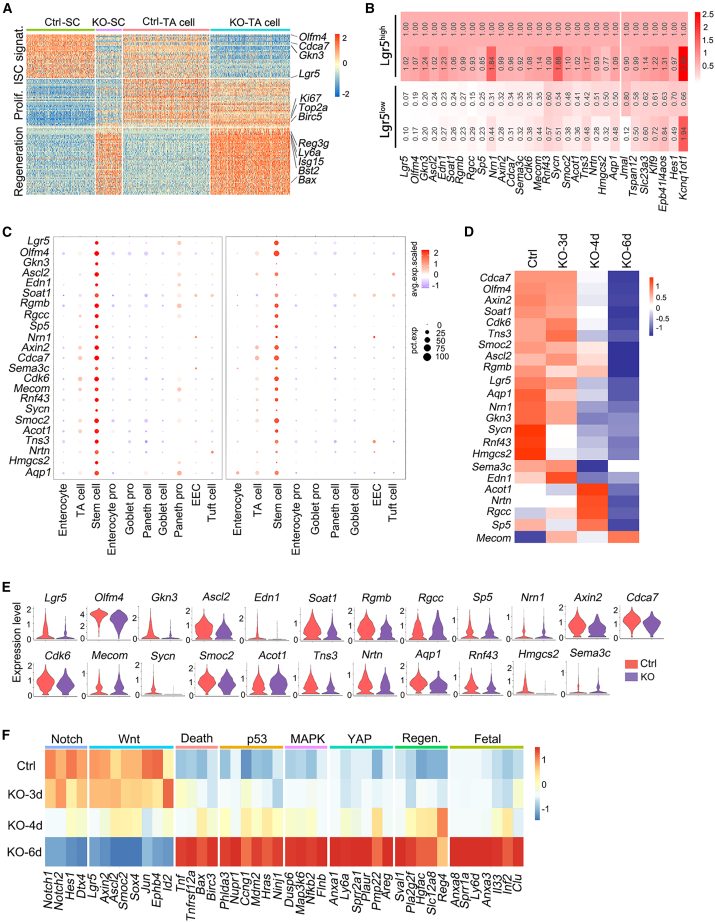
Stem cells have been defined to have the capacity to renew themselves and the ability to generate differentiated daughter cells (Ivanova et al., 2002; Post and Clevers, 2019; Ramalho-Santos et al., 2002). Great efforts have been taken to search for the genes responsible for these stem cell properties, but these kinds of genes are still elusive (Post and Clevers, 2019; Vogel, 2003). The above data showed that Mettl3 KO reduced the expression of the stem cell markers, but not proliferation genes, suggesting that the program to maintain stemness could be uncoupled from proliferation. By comparing their expression in Lgr5+ ISCs and their daughter cells, 384 genes enriched in Lgr5+ ISCs were together defined as Lgr5+ ISC signature genes (Munoz et al., 2012), but some of these genes are associated with proliferation. To separate the stemness genes from the proliferation genes, we characterized the differentially expressed genes in stem cells and TA cells from control or Mettl3-KO-GFP mice at single-cell level and found that these genes could be divided into three groups: the ISC signature genes (Munoz et al., 2012) and genes involved in proliferation (Tirosh et al., 2016) and regeneration (Liu and Chen, 2020; Wang et al., 2019) (Figure 4A). The ISC signature genes, such as Lgr5, Olfm4, Cdca7, and Gkn3, were downregulated in Mettl3-KO stem cells, while the proliferation genes did not change much in stem cells. Some of the ISC signature genes were found in TA cells as they may be related to cell proliferation, like Cenpf (Munoz et al., 2012). The regeneration genes were elevated in KO cells, probably due to the cell state associated with death-induced stress (Li et al., 2010). To better define the genes associated with stemness and exclude the ones related to proliferation, we analyzed the stem cell signature genes in our bulk RNA-seq and single-cell RNA sequencing (scRNA-seq) as well as the data from Gu et al. (Gu et al., 2021), and we found that 23 genes, including the previously described ISC-specific genes Lgr5, Ascl2, and Olfm4, were highly enriched in Lgr5high cells, and most of them decreased in Lgr5low cells and Mettl3-KO stem cells (Figures 4B–4E), suggesting that these 23 genes could be defined as the stemness genes to mark ISCs.
Figure 4.
Identification of the stemness genes via bulk RNA-seq and scRNA-seq analysis
(A) Heatmap of differentially expressed genes in stem cells and TA cells of control and Mettl3-KO-GFP mice at 4 dpt based on scRNA-seq.
(B) RNA-seq analysis of the relative expression of genes, which were enriched in Lgr5high cells in control mice.
(C) scRNA-seq analysis showing the gene expression level (color scale) and expressing cells (point diameter) in each cell cluster of the intestinal epithelium. All of these genes were sifted out of the gene list (B) showing specific expression in stem cells in our scRNA-seq data (left) and published ileum scRNA-seq data (right).
(D) Expression heatmap of the filtered 23 stemness genes in Lgr5+ ISCs of control and Mettl3-KO-GFP mice at 3, 4, and 6 dpt, based on bulk RNA-seq.
(E) Violin plots showing the expression of the 23 stemness genes in stem cells of control and Mettl3-KO-GFP mice.
(F) Expression heatmap of different pathway genes in Lgr5+ ISCs of control and Mettl3-KO-GFP mice at 3, 4, and 6 dpt, based on bulk RNA-seq. See also Figure S5.
To explore the signaling events and/or factors that regulate the ISC stemness, we compared the differentially expressed genes in Lgr5+ ISCs from control and Mettl3-KO-GFP mice at different time points post-tamoxifen injection (Figure S5G). Mettl3 KO led to a decreased expression of the genes involved in Wnt and Notch signaling (Figures 4F and S5H), consistent with the critical role of these two signaling pathways in ISC maintenance (Clevers, 2013; Qi and Chen, 2015; Zhu et al., 2021). Most of the stemness genes were further downregulated in Mettl3-KO ISCs at later time points (Figure 4D), while the genes related to cell death, P53, MAPK, YAP, regeneration, and fetal signature genes were upregulated (Figures 4F and S5H). These changes indicate that Mettl3 deletion leads to loss of stemness but not proliferation and enhanced regeneration and cell death.
ASCL2, KLF9, FOSB, and PURB collaborate to regulate stemness gene expression
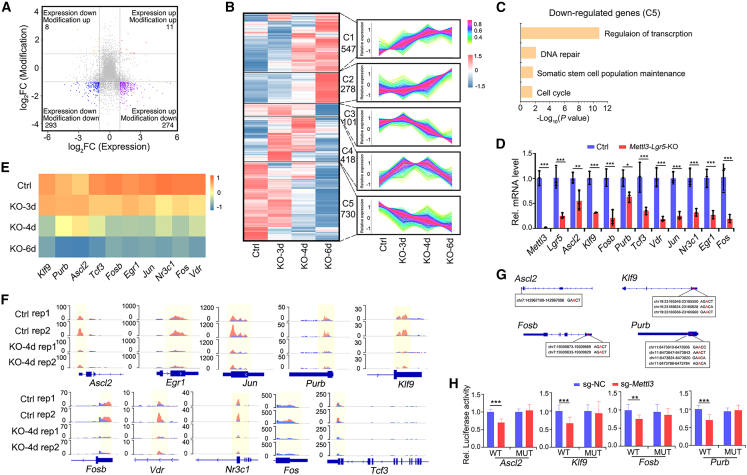
To identify potential targets regulated by METTL3-mediated m6A modification, we performed methylated RNA immunoprecipitation (MeRIP)-seq in Lgr5high ISCs and found that 2,074 transcripts showed a reduced m6A modification in Lgr5high ISCs from Mettl3-KO-GFP mice. Most of the targets contained the common m6A motif “RRACH (GGACU)” in the protein-coding region and 3′ UTR, especially enriched near the stop codon (Figures S6A–S6C). In the 2,074 transcripts, 831 were downregulated (clusters 3 and 5) in Mettl3-KO ISCs, and among the 293 with more than 2-fold decrease in expression, 66 were transcriptional factors (TFs) (Figures 5A, 5B, and S6D). Interestingly, the genes in cluster 5 reduced in Mettl3-KO Lgr5+ ISCs at 3 dpt were also involved in transcription regulation (Figure 5C). To identify the TFs responsible for the impaired stemness, we focused on the TFs that are m6A modified and downregulated at early time points upon Mettl3 deletion. By the combinatory analysis of bulk RNA-seq and MeRIP-seq data and q-PCR confirmation, we sifted out 10 TFs (Figures 5D–5F).
Figure 5.
Identification of transcriptional factors that promote stemness
(A) Distribution of the genes that exhibited different m6A modification and gene expression levels in control or Mettl3-KO-GFP Lgr5high cells (|log2FC|≥1; p < 0.05). The gene number in each category is shown.
(B) The expression of the genes with reduced m6A modification in Mettl3-KO-GFP Lgr5+ cells were divided into five modules according to their expression dynamics. The change trend in each module is shown in the line chart on the right.
(C) Functional enrichment analysis of the genes whose expression was downregulated at 3 dpt from the module C5 in (B).
(D) Expression of Mettl3, Lgr5, and the 10 TFs (Ascl2, Klf9, Fosb, Purb, Tcf3, Vdr, Jun, Nr3c1, Egr1, Fos) in FACS-sorted Lgr5high cells from control and Mettl3-Lgr5-KO mice at 4 dpt by q-PCR. N = 3 mice per group.
(E) Expression heatmap of the 10 TF candidates in Lgr5+ ISCs from control and Mettl3-KO-GFP mice, based on bulk RNA-seq.
(F) Integrative Genomics Viewer (IGV) tracks displaying MeRIP-seq reads along the indicated mRNAs in Lgr5high ISCs of control and Mettl3-KO-GFP mice. Blue reads are from input libraries and red reads from anti-m6A immunoprecipitation libraries. The y axis represents the CPM (count per million) of genes. The yellow boxes of the tracks depict the positions of m6A peaks.
(G) Representative m6A modification sites at the genomic loci of Ascl2, Klf9, Fosb, and Purb.
(H) Fetal human colon cells (FHC cells) were transfected with WT or MUT 3′ UTR luciferase reporter, as well as with control sgRNA or Mettl3 sgRNA, and luciferase activity was determined 48 h later. NC: control. MUT: mutation all sites (A to T). Data from three independent experiments were combined and are shown. The data represent mean ± SD. ∗∗∗p < 0.001, ∗∗p < 0.01, two-way ANOVA (D and H). See also Figure S6 and Tables S2–S4.
Among them, Ascl2, Klf9, Fosb, and Purb (AKFP) are the top TFs that might regulate the expression of the 384 ISC signature genes (Munoz et al., 2012) and the 23 stemness genes. Consistently, prediction of the binding sites of AKFP in the genome with JASPAR, TRANFAC, and HOMER revealed more than 4,000 possible target genes that are mainly enriched for stem cell signature genes and Wnt and Notch signaling (Figures S6E–S6G). To further address whether METTL3-mediated m6A binding sites of 3′ UTR are required for RNA stability modulation, we analyzed the m6A modification sequence through MeRIP-seq (Figure 5G), performed the AKFP 3′ UTR-reporter luciferase assay and found that Mettl3 KO decreased the activity of the luciferase construct containing 3′ UTR (Figure 5H). Mutation of all these sites (A to T) rendered resistance to the effect of Mettl3 KO. Therefore, METTL3 regulates the stability of these TF transcripts dependent on its m6A methyltransferase activity.
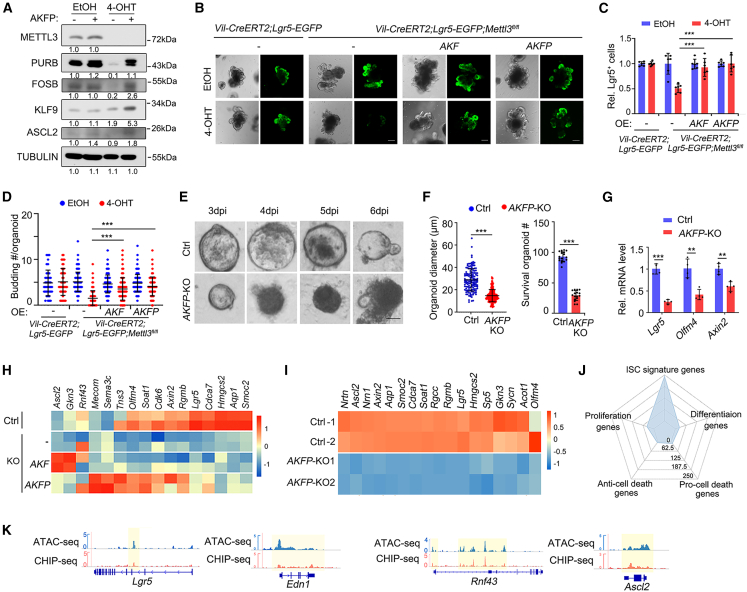
To determine whether these TFs are the key factors controlling ISC stemness, we performed rescue experiments in organoids derived from the crypts of Mettl3-KO-GFP mice by ectopically expressing AKFP and knockout assay in the organoids derived from the crypts of Rosa26loxp-stop-loxp-Cas9-EGFP mice. As shown in Figures 6A–6D, expression of AKF (Ascl2, Klf9, and Fosb) and AKFP was able to restore Lgr5+ ISCs and budding number of Mettl3-KO-GFP organoids. Conversely, AKFP-KO organoids resulted in reduced cell survival rate, downregulated ISC signature genes, and subsequent cell death (Figures 6E–6G). Transcriptome profile analysis revealed that only AKFP could better rescue some of the 23 stemness genes (Figure 6H). In contrast, ectopic expression of individual factors only partially restored morphology, survival rate, budding, and Lgr5+ ISC number in Mettl3-KO-GFP organoids (Figures S6H–S6J). Furthermore, AKFP-KO organoids exhibited decreased 23 stemness genes expression (Figure 6I). These results suggest that AKFP, but not any single one, can restore Lgr5+ ISCs to a certain extent.
Figure 6.
ASCL2, KLF9, FOSB, and PURB collaborate to regulate stemness gene expression
(A) Immunoblots of ASCL2, KLF9, FOSB, and PURB protein expression in organoids derived from the crypts of Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl mice infected with control retrovirus or AKFP-expressed retrovirus at day 5 after EtOH or 4-OHT treatment for 2 days. TUBULIN, loading control.
(B) Representative morphology and Lgr5+ cells of organoids derived from the crypts of Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl mice, which were infected with retrovirus expressing AKF (Ascl2, Klf9, and Fosb) or AKFP (Ascl2, Klf9, Fosb, and Purb) and then treated with EtOH or 4-OHT for 2 days and subjected for analysis at day 5.
(C and D) Relative Lgr5+ cell number (C) and budding number (D) of organoid in (A). Data from three independent experiments were combined and are shown (C). N > 100 organoids per group from three independent experiments (D).
(E) Representative morphology of organoids derived from the crypts of Villin-CreERT2;Rosa26loxp-stop-loxp-Cas9-EGFP mice at indicated times, which were infected with recombinant AAV to knock out AKFP or control recombinant AAV.
(F) Organoid diameter (left) of control or AKFP-KO organoid at day 3 and survival organoid number (right) at day 4. N > 100 organoids per group from four independent experiments.
(G) q-PCR shows gene expression of control and AKFP-KO organoids at day 4. Data from four independent experiments were combined and are shown.
(H and I) Stemness gene expression of AKF and AKFP overexpression organoids in (A) and AKFP-KO organoids in (E).
(J) Function characteristics distribution of all the predicted target genes of KLF9, ASCL2, FOSB, and PURB.
(K) ChIP-seq and ATAC-seq tracks of Fosb binding to the stem cell signature genes Lgr5, Rnf43, Edn1, Ascl2. The data represent mean ± SD. ∗∗∗p < 0.001, ∗∗p < 0.01, two-way ANOVA (C, D, and G), unpaired two-tailed t test (F). Scale bar: 100 μm (A, E). AKF: Ascl2, Klf9, and Fosb; AKFP: Ascl2, Klf9, Fosb, and Purb. See also Figure S6 and Table S2.
We further predicted the possible downstream target genes of these TFs and found that the stem cell signature genes were enriched (Figures 6J and S6K). In agreement with it, based on the binding motif of FOSB (Figure S6L), FOSB bound to around 36% of predicated stem cell signature genes as showed by ChIP-seq (Figure S6M), for instance, the stemness genes Lgr5, Edn1, Ascl2, and Rnf43 but not the proliferation genes Ki67 and Birc5 (Figures 6K and S6N). These binding sites were correlated with the chromatin accessibility as shown by assay for transposase-accessible chromatin with sequencing (ATAC-seq) analysis. These data together indicate that METTL3-mediated m6A modification is critical for stabilization of AKFP transcripts, whose protein products then regulate stemness gene expression to maintain Lgr5+ ISCs.
Discussion
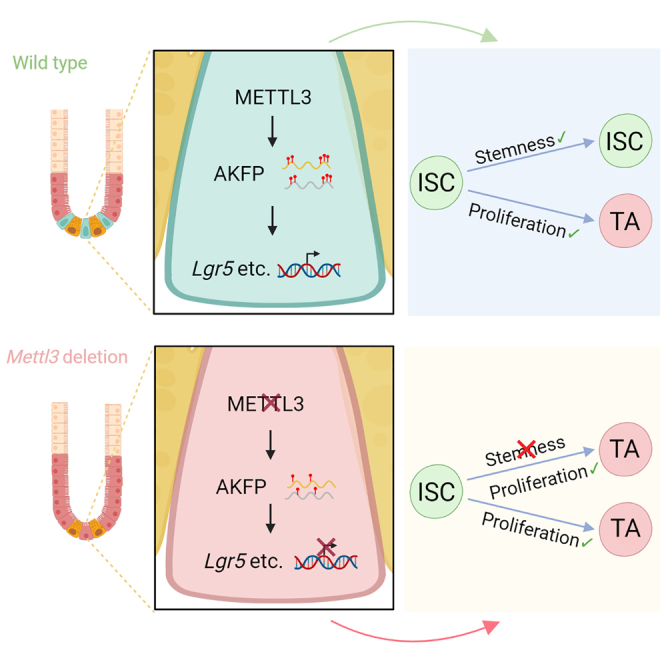
In this study, we showed that the m6A methyltransferase Mettl3 is essential for the homeostatic maintenance of the small intestinal epithelium. m6A modification has emerged as a crucial regulation in tissue development and stem cell fate determination (Batista et al., 2014; Cao et al., 2020; Geula et al., 2015; Yoon et al., 2017), while the overall impact of this modification on stem cells is complex (Cui et al., 2017; Vu et al., 2017; Wang et al., 2018; Weng et al., 2018; Zhang et al., 2017), due to diversified targets and different regulations on the targets. Several studies have reported the regulatory role of m6A modification in the intestinal epithelium: METTL3-mediated m6A modification has been shown to promote colorectal cancer development by activating glycolysis (Shen et al., 2020), stabilizing SOX2 mRNA (Li et al., 2019) or CCNE2 mRNA (Zhu et al., 2020); the m6A reader YTHDF1 mediates Wnt-driven ISC maintenance during regeneration and tumorigenesis by enhancing the translation of TCF7L2 (Han et al., 2020). Here, we demonstrate that conditional KO of Mettl3 in the intestinal epithelium results in rapid loss of Lgr5+ ISCs, enhanced cell death, and mild effects on differentiation, but it has no influence on proliferation. Loss of Lgr5+ ISCs is very likely due to loss of their stem cell identity and accelerated cell death.
Stem cells are capable of proliferation, self-maintenance, and differentiation toward mature cells. At the time point when the stemness markers in ISCs disappear upon Mettl3 KO or Mettl3 Lgr5-KO, the proliferation genes still remain in both Mettl3-deficient ISCs and TA cells. Owing to the low labeling efficiency of Lgr5-EGFP-IRES-CreERT2 mice (Barker, 2014; Sato et al., 2009), no significant change of differentiation genes was observed in Mettl3-Lgr5-KO mice, which is different from the phenotype of Mettl3-KO mice. However, we did observe the impaired stemness phenotype in the GFP-labeling cells, which co-expressed CreERT2, and as a result, Mettl3 was deleted in these cells upon tamoxifen treatment. Our sc-RNA-seq analysis also showed that Mettl3 deletion reduced the expression of stem cell signature genes but elevated expression of the genes related to proliferation and differentiation in the ISCs cluster. Previous studies have attempted to determine the stemness signature for embryonic stem cells and adult stem cells by comparison of transcriptional profiling between stem cells and differentiated cells (Cai et al., 2004; Ivanova et al., 2002; Munoz et al., 2012; Ramalho-Santos et al., 2002). However, those stem cell signatures contain many genes related to cell proliferation. We show here that the program to control stemness gene expression can be uncoupled from the one to regulate proliferation.
Furthermore, we identified four TFs (Ascl2, Klf9, Fosb, and Purb), whose mRNA is stabilized by METTL3-mediated m6A modification. m6A generally promotes mRNA degradation (Shi et al., 2019; Wang et al., 2014a, 2014b), but we found that loss of m6A modification was correlated with reduced levels of these TF transcripts. How m6A stabilizes mRNA is unclear: it could be due to inappropriate splicing, as being proposed that m6A modification can regulate gene expression by affecting alternative splicing (Xu et al., 2017). These four TFs control the expression of stemness genes but not proliferation genes in ISCs. Furthermore, AKFP-overexpressing organoids could maintain Lgr5-GFP+ cells number but did not suppress cell death; it is most likely that the m6A-modified death genes are not regulated by AKFP. It would be worthwhile to explore the mechanism underlying the m6A disruption-triggered cell death in the future. Our findings that the stemness program can be uncoupled from the proliferation program in ISCs also imply that similar mechanisms may also exist in other types of stem cells.
Experimental procedures
Resource availability
Corresponding author
Ye-Guang Chen (ygchen@tsinghua.edu.cn).
Material availability
All reagents generated in this study are available from the corresponding author.
Mice
Lgr5-EGFP-IRES-CreERT2 mice were obtained from the Jackson Laboratory. Villin-CreERT2 mice were obtained from Dr. Sylvie Robine (Institute Curie-CNRS, Paris), Mettl3fl/fl mice from Dr. Wei Li (Institute of Zoology, CAS), Lgr5-CreERT2 and Rosa26loxp-stop-loxp-ZsGreen mice from Dr. Xiao-Dong Wang (National Institute of Biological Sciences, Beijing), and Rosa26loxp-stop-loxp-Cas9-EGFP mice from Dr. Jianwei Wang (Tsinghua University). All mice were performed as previously described (Qi et al., 2017). For Mettl3-KO, Mettl3fl/fl mice were crossed with Villin-CreERT2 mice. The Vil-CreERT2;Mettl3fl/fl mice and littermate mice (Mettl3fl/fl) were intraperitoneally injected with four consecutive daily doses of 20 mg/mL tamoxifen (TAM) (Sigma, T5648-5G) in sunflower oil. To monitor the Lgr5-GFP+ ISCs, the littermate mice, whose genotypes were Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl, were treated with oil or tamoxifen, as control and Mettl3-KO-GFP, respectively. For Mettl3-Lgr5-KO, the littermate mice, whose genotypes were Lgr5-EGFP-IRES-CreERT2;Mettl3w/w or Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl, were injected with tamoxifen for 5 days, as control and Mettl3-Lgr5-KO, respectively. For lineage tracing, control (Lgr5-CreERT2;Rosa26loxp-stop-loxp-ZsGreen) mice and Mettl3ΔLgr5 (Mettl3fl/fl;Lgr5-CreERT2;Rosa26loxp-stop-loxp-ZsGreen) mice were intraperitoneally injected with a single dose of 20 mg/mL TAM. The animals used in this study are summarized in Table S1.All animal studies were performed in accordance with the relevant guidelines and under the approval of the Institutional Animal Care and Use Committee of Tsinghua University.
Intestinal crypt and Lgr5+ ISC isolation and organoids culture
Intestinal crypts were isolated and cultured as previously described (Qi et al., 2017). Briefly, the enriched intestinal crypts were isolated and embedded in Matrigel and seeded on plate. The ENR medium (advanced DMEM/F12 supplemented with penicillin/streptomycin, GlutaMAX, N2, B27, and N-acetylcysteine containing 50 ng/mL EGF, 100 ng/mL Noggin, and 500 ng/mL R-spondin1) was added and refreshed every 2 days.
To purify single Lgr5+ cells, isolated intestinal crypts were treated with TrypLE (Gibco, 12604021). After passing through a 40-μm cell strainer (BD, 352340), staining with propidium iodide (Sigma, P4170), Lgr5+ cells were sorted using MoFlo Astrios EQ (BeckMan). Lgr5+ cells were embedded in Matrigel and seeded on a 48-well plate. The cells were cultured with expansion medium (ENR medium plus 6.67 μM blebbistatin, 2.5 μM CHIR-99021, 10% Wnt3a conditional medium) plus 1 μM Jagged1 (Zhang et al., 2020).
Virus production and organoid infection
Retrovirus and recombinant adeno-associated virus (AAV) were produced as previously described (Koo et al., 2011; Li et al., 2021). Before virus infection, organoids were cultured with the expansion medium plus 10 mM nicotinamide for 2 days. Then, the organoids were digested with TrypLE (Gibco, 12604021) and re-suspended with the expansion medium plus 10 μg/mL polybrene (Macgene, MC032) containing virus. We added 250 μL of expansion medium plus polybrene containing cells and virus on the pre-solidified Matrigel and incubated overnight at 37°C. The next day, we removed the medium and washed the virus with warm PBS. Then, we overlaid 10 μL Matrigel and cultured the organoids with expansion medium.
For retrovirus infection, we changed the medium with ENR plus 2 μg/mL puromycin 2 days post-infection. After the organoids grew normally under the puromycin selection, protein expression was induced with 4-OHT (0.5 μM, Sigma, H7904). For recombinant AAV, Villin-CreERT2;Rosa26loxp-stop-loxp-Cas9-EGFP organoids were pretreated with 4-OHT to induce Cas9 expression.
Immunoblotting
Cells were lysed in RIPA buffer (Beyotime, P0013B) with protease inhibitors (Roche, 04693132001) and PMSF (Beyotime, ST506). After quantification using a BCA protein assay kit (Beyotime, P0012S), 40 μg of total protein was separated with 7.5% SDS-PAGE under denaturing conditions and was transferred to nitrocellulose membranes (PALL, 66485). The membranes were blocked and then incubated with the primary antibody overnight at 4°C, followed by incubation with anti-rabbit or anti-mouse conjugated antibodies. Antibodies are listed in the supplemental experimental procedures.
Immunofluorescence and histological staining
Immunofluorescence and histological staining were performed as previously described (Qi et al., 2017). H&E staining was performed according to the manufacturer instructions (Beyotime, C0205S). Briefly, the sections were dewaxed by conventional procedures and stained with hematoxylin for 2 min and eosin for 20 s. Alcian blue staining was used to detect goblet cells, and slides were stained with Alcian blue for 15 min and nuclear fast red for 1 min (Baso, BA4087B). All sections were visualized with a confocal microscope (Olympus, FV3000) or a slide scanning system (Jiang Feng, KF-PRO-120).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Paraformaldehyde-fixed paraffin-embedded tissues were subjected to cell death assessment using the In Situ Cell Death detection kit (Roche, 11684795910).
Organoid time-lapse imaging
To mark the cell membrane, 1.25 μg/mL CellMask Orange Plasma Membrane Stain (Thermo Fisher Scientific, C10045) was added to the medium 12 h before imaging. Confocal time-lapse imaging was performed with Nikon AX R Confocal Microscope System by resonant scanner and Nikon NIS-Elements Denoise.ai software. Different organoids were selected and imaged every 1 h from day 4.5 to day 6.5. A volume of 200–300 μm was gained every 3 μm along the z axis. To exclude autofluorescence of dead cells, only organoids containing clear buds were selected to build surfaces for statistics. Surfaces at different times marking Lgr5-GFP signals, value of fluorescence intensity, and surface volume were computed automatically by the Imaris software at the same parameters. The average fluorescence intensity value was calculated as the total fluorescence intensity at each time point divided by the surface volume at the starting point.
BrdU and EdU labeling
Mice were given a single intraperitoneal injection of 100 μL BrdU (BD Pharmingen BrdU Flow Kits, BD, 552598) 2 or 24 h before intestine isolation. BrdU staining was performed with the BrdU flow kit (BD Pharmingen). For cell cycle analysis, the epithelium was stained with EpCam-PE, BrdU-APC, and 7-AAD 2 h later after a single intraperitoneal injection of BrdU. Cell cycle was analyzed by FACS. EdU labeling was performed by following the manufacturer’s instruction (Click-iT EdU Imaging Kit, Invitrogen, C10339).
RNA extraction and quantitative RT-PCR (q-PCR)
Total RNA from tissue or sorted cells was extracted with RNeasy Mini Kit (QIAGEN, 74104) or TRIzol Reagent (Thermo Fisher Scientific, 15596026), respectively. RNA was reverse-transcribed into cDNA using ReverTra Ace-α kit (TOYOBO, FSK-101). q-PCR was carried out with NovoStart SYBR qPCR Super-Mix Plus (Novoprotein, E096-01A) on LightCycle 480II PCR system (Roche). All primer sequences are shown in Table S2 and were purchased from Sangon Biotech.
RNA-seq
Total RNA was extracted with RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. Bulk RNA-seq was performed using the Illumina Hiseq X Ten and analyzed as previously described (Pertea et al., 2016).
MeRIP-seq
Total RNA was extracted with RNeasy Mini Kit (QIAGEN, 74104). mRNA was purified using Dynabeads mRNA Purification kit (Invitrogen, 61006). Purified mRNA was fragmented into ∼100 nucleotides by RNA Fragmentation Reagents (Ambion, AM8740) and immunoprecipitation with m6A antibody (NEB, E1610S), followed by RNA extraction using RNA clean&concentrator-5 (ZYMO RESEARCH, 1013). Data analysis is described in the supplemental experimental procedures in detail.
ATAC-seq
For mini-ATAC-seq, the library was constructed with TruePrep DNA library Prep Kit V2 for Illumina (Vazyme, TD 202 and TD502). Subsequently, the library was purified with AMPure XP beads (Beckman, A63881) and sequenced with Illumina Novaseq 6000. HISAT2 was used to align the sequences to the mouse genome and generate bam files. After being deprived of PCR duplicates using Picard tools, Deeptools (3.3.1) bamCoverage was used to generate bigwig files from bam files. MACS2 (v2.2.5) was used for peak calling and to generate bed files from aligned reads.
scRNA-seq
Single-cell suspensions from the intestinal epithelium of Mettl3-KO-GFP-4d and control mice were sorted by FACS and re-suspended at a density of 1,000 cells per μL (n = 3 mice per group). About 8,000 cells were loaded into each reaction. RNA transcripts from single cells were uniquely barcoded and reverse-transcribed. scRNA-seq libraries were generated using the Chromium Single Cell 3′ Reagent Ki V3 (10× Genomics). The libraries were sequenced as paired-end with Illumina Novaseq 6000.
Raw reads were aligned to the GRCm38/mm10 mouse genome, and Cell Ranger (v3.1.0) was used to estimate unique molecular identifiers. Raw aligned features were loaded and processed using the Seurat package (v4.0.2) in R version 4.0.5. Low-quality cells were filtered if they expressed no more than 200 genes or with more than 20% of mitochondrial genes. More bioinformation analysis is described in the supplemental experimental procedures.
Dual luciferase reporter assay
The 3′ UTR fragments of Ascl2, Fosb, Klf9, and Purb containing enriched m6A motif (RRACH) and their respective A-to-T mutant fragments were cloned into pGL3-CMV firefly luciferase vector. FHCs (fetal human colon cells) were seeded and co-transfected with pGL3-CMV-WT-3′UTR or pGL3-CMV-mut-3′UTR, Renilla luciferase plasmid, and px458-sgNC/Mettl3-1 using Lipo2000 (Invitrogen, 11668019). The relative luciferase activity was determined using the Dual-Luciferase Reporter Assay System and Microplate Chemiluminescence Meter (Berthold, LB960) 48 h later. The sequences are shown in Tables S3 and S4.
Statistical analysis
All experiments were carried out with at least three independent experiments. Exact numbers of mice, fields, and organoids are shown in the figure legends. Statistical analysis was performed with Graphpad Prism v8.0. All data shown in graphs represent mean ± SD as indicated in the figure legends. Unpaired two-tailed t test, one-way ANOVA, two-way ANOVA, and Log rank test were used to compare differences, as indicated in the figure legends.
Author contributions
Y.L., M.H., X.W., and Y.-G.C conceived the experiments and wrote the manuscript. Y.L., M.H., Z.L., and S.L. performed the experiments, and X.W. analyzed the data.
Acknowledgments
We thank Drs. Sylvie Robine, Wei Li, Xiao-Dong Wang, and Jianwei Wang for mice, Dr. Wei Xie for the advice on ATAC-seq, and Dr. Meng Xu and Dr. Yun-Gui Yang for the advice on MeRIP-seq. We are grateful to Mengxian Zhang and Huidong Liu for critical comments and valuable review. This work was supported by grants from the National Key Research and Development Program of China (2017YFA0103601) and the National Natural Science Foundation of China (31988101 and 31730056 to Y.G.C.; 31900550 to Y.L.).
Conflict of interests
The authors declare no competing financial interests.
Published: April 6, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.03.007.
Supplemental information
Data and code availability
The RNA-seq data, scRNA-seq data, MeRIP-seq data, ATAC-seq data, and ChIP-seq data generated in this study are publicly available through the Gene Expression Omnibus (GEO) with the accession code GSE186917. The scRNA-seq data GSE148693 was used to confirm the 23 stem genes (Gu et al., 2021). All codes that enable the main steps of the analysis and data are available from the corresponding author under request.
References
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Batista P.J., Molinie B., Wang J., Qu K., Zhang J., Li L., Bouley D.M., Lujan E., Haddad B., Daneshvar K., et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Weiss M.L., Rao M.S. In search of "stemness". Exp. Hematol. 2004;32:585–598. doi: 10.1016/j.exphem.2004.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Zhuang Y., Chen J., Xu W., Shou Y., Huang X., Shu Q., Li X. Dynamic effects of Fto in regulating the proliferation and differentiation of adult neural stem cells of mice. Hum. Mol. Genet. 2020;29:727–735. doi: 10.1093/hmg/ddz274. [DOI] [PubMed] [Google Scholar]
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Cui Q., Shi H., Ye P., Li L., Qu Q., Sun G., Sun G., Lu Z., Huang Y., Yang C.G., et al. m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Su R., Weng H., Huang H., Li Z., Chen J. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula S., Moshitch-Moshkovitz S., Dominissini D., Mansour A.A., Kol N., Salmon-Divon M., Hershkovitz V., Peer E., Mor N., Manor Y.S., et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- Gu W., Wang H., Huang X., Kraiczy J., Singh P.N.P., Ng C., Dagdeviren S., Houghton S., Pellon-Cardenas O., Lan Y., et al. SATB2 preserves colon stem cell identity and mediates ileum-colon conversion via enhancer remodeling. Cell Stem Cell. 2021;29:101–115.e10. doi: 10.1016/j.stem.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Yan S., Wei S., Xiang J., Liu K., Chen Z., Bai R., Sheng J., Xu Z., Gao X. YTHDF1-mediated translation amplifies Wnt-driven intestinal stemness. EMBO Rep. 2020;21 doi: 10.15252/embr.201949229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P.C., He C. m(6) A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 2021;40 doi: 10.15252/embj.2020105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N.B., Dimos J.T., Schaniel C., Hackney J.A., Moore K.A., Lemischka I.R. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Koo B.K., Stange D.E., Sato T., Karthaus W., Farin H.F., Huch M., van Es J.H., Clevers H. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods. 2011;9:81–83. doi: 10.1038/nmeth.1802. [DOI] [PubMed] [Google Scholar]
- Li F., Huang Q., Chen J., Peng Y., Roop D.R., Bedford J.S., Li C.Y. Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration. Sci. Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Hu P.S., Zuo Z., Lin J.F., Li X., Wu Q.N., Chen Z.H., Zeng Z.L., Wang F., Zheng J., et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol. Cancer. 2019;18:112. doi: 10.1186/s12943-019-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., He Y., Peng J., Su Z., Li Z., Zhang B., Ma J., Zhuo M., Zou D., Liu X., et al. Mutant Kras co-opts a proto-oncogenic enhancer network in inflammation-induced metaplastic progenitor cells to initiate pancreatic cancer. Nat. Cancer. 2021;2:49–65. doi: 10.1038/s43018-020-00134-z. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen Y.G. Intestinal epithelial plasticity and regeneration via cell dedifferentiation. Cell Regen. 2020;9:14. doi: 10.1186/s13619-020-00053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz J., Stange D.E., Schepers A.G., van de Wetering M., Koo B.K., Itzkovitz S., Volckmann R., Kung K.S., Koster J., Radulescu S., et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post Y., Clevers H. Defining adult stem cell function at its simplest: the ability to replace lost cells through mitosis. Cell Stem Cell. 2019;25:174–183. doi: 10.1016/j.stem.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Qi Z., Chen Y.G. Regulation of intestinal stem cell fate specification. Sci. China Life Sci. 2015;58:570–578. doi: 10.1007/s11427-015-4859-7. [DOI] [PubMed] [Google Scholar]
- Qi Z., Li Y., Zhao B., Xu C., Liu Y., Li H., Zhang B., Wang X., Yang X., Xie W., et al. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat. Commun. 2017;8 doi: 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M., Yoon S., Matsuzaki Y., Mulligan R.C., Melton D.A. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Shen C., Xuan B., Yan T., Ma Y., Xu P., Tian X., Zhang X., Cao Y., Ma D., Zhu X., et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol. Cancer. 2020;19:72. doi: 10.1186/s12943-020-01190-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wei J., He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., 2nd, Treacy D., Trombetta J.J., Rotem A., Rodman C., Lian C., Murphy G., et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. Stem cells. 'Stemness' genes still elusive. Science. 2003;302:371. doi: 10.1126/science.302.5644.371a. [DOI] [PubMed] [Google Scholar]
- Vu L.P., Pickering B.F., Cheng Y., Zaccara S., Nguyen D., Minuesa G., Chou T., Chow A., Saletore Y., MacKay M., et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G., et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chiang I.L., Ohara T.E., Fujii S., Cheng J., Muegge B.D., Ver Heul A., Han N.D., Lu Q., Xiong S., et al. Long-term culture captures injury-repair cycles of colonic stem cells. Cell. 2019;179:1144–1159.e15. doi: 10.1016/j.cell.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Toth J.I., Petroski M.D., Zhang Z., Zhao J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Yue M., Wang J., Kumar S., Wechsler-Reya R.J., Zhang Z., Ogawa Y., Kellis M., Duester G., Zhao J.C. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat. Neurosci. 2018;21:195–206. doi: 10.1038/s41593-017-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H., Huang H., Wu H., Qin X., Zhao B.S., Dong L., Shi H., Skibbe J., Shen C., Hu C., et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22:191–205.e9. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Yang Y., Feng G.H., Sun B.F., Chen J.Q., Li Y.F., Chen Y.S., Zhang X.X., Wang C.X., Jiang L.Y., et al. Mettl3-mediated m(6)A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–1114. doi: 10.1038/cr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K.J., Ringeling F.R., Vissers C., Jacob F., Pokrass M., Jimenez-Cyrus D., Su Y., Kim N.S., Zhu Y., Zheng L., et al. Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell. 2017;171:877–889.e17. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccara S., Ries R.J., Jaffrey S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- Zhang C., Chen Y., Sun B., Wang L., Yang Y., Ma D., Lv J., Heng J., Ding Y., Xue Y., et al. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu Y., Chen Y.G. Generation of 3D human gastrointestinal organoids: principle and applications. Cell Regen. 2020;9:6. doi: 10.1186/s13619-020-00040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Hu J., Xi R. The cellular niche for intestinal stem cells: a team effort. Cell Regen. 2021;10:1. doi: 10.1186/s13619-020-00061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Si Y., Xu J., Lin Y., Wang J.Z., Cao M., Sun S., Ding Q., Zhu L., Wei J.F. Methyltransferase like 3 promotes colorectal cancer proliferation by stabilizing CCNE1 mRNA in an m6A-dependent manner. J. Cell Mol. Med. 2020;24:3521–3533. doi: 10.1111/jcmm.15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organoids derived from Villin-CreERT2;Lgr5-EGFP-IRES-CreERT2;Mettl3fl/fl mice were imaged every 1 h from day 4.5 to day 6.5 after being treated with EtOH (left) or 4-OHT (right) and were labeled with CellMask (orange plasma membrane stain, red). Video shows the change of Lgr5-GFP cells over time from 0 to 20 h.
Data Availability Statement
The RNA-seq data, scRNA-seq data, MeRIP-seq data, ATAC-seq data, and ChIP-seq data generated in this study are publicly available through the Gene Expression Omnibus (GEO) with the accession code GSE186917. The scRNA-seq data GSE148693 was used to confirm the 23 stem genes (Gu et al., 2021). All codes that enable the main steps of the analysis and data are available from the corresponding author under request.