Abstract
Background
Severe fever with thrombocytopenia syndrome virus (SFTSV) is transmitted through tick bites. Ticks are potential vectors for the bacterium Coxiella burnetii that causes Query fever. Here, we analyzed SFTSV and C. burnetii co-infection rates in ticks in rural areas of Jeju Island, South Korea.
Methods
Free ticks were collected from the natural environment of the island between 2016 and 2019, and SFTSV RNA was extracted. Additionally, ribosomal RNA gene sequencing was used to identify Coxiella species.
Results
Haemaphysalis longicornis was the most common tick species followed by H. flava. Tick number gradually increased from April, peaked in August, and was lowest in March. Of all the collected ticks, 82.6% (2,851/3,458) were nymphs, 17.9% (639/3,458) adults, and 0.1% (4/3,458) larvae. SFTSV-infected ticks comprised 12.6% of all ticks; their numbers were the lowest in November–December, increased from January, and were mostly identified in the adult stage during June–August. C. burnetii infections were detected in 4.4% of the SFTSV-infected H. longicornis ticks. C. burnetii co-infection was mainly observed in the nymph stage of H. longicornis, with the highest infection rate in January, followed by December and November.
Conclusion
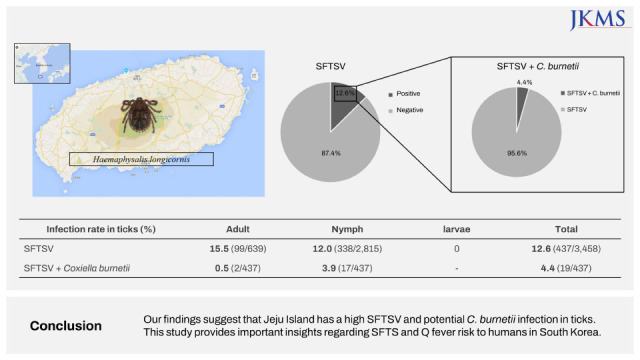
Our findings suggest that Jeju Island has a high SFTSV and potential C. burnetii infection in ticks. This study provides important insights regarding SFTS and Q fever risk to humans in South Korea.
Keywords: Severe Fever With Thrombocytopenia Syndrome Virus, Coxiella burnetii, Q Fever, Ticks, Hemaphysalis longicornis, Jeju-do, South Korea
Graphical Abstract
INTRODUCTION
Ticks transmit various pathogens to humans, including bacteria, viruses, and other parasites, and their populations and geographical ranges are expanding worldwide.1,2,3 The prevalence and transmission of emerging tick-borne diseases are important public health issues. Various tick species in natural environments are vectors of zoonotic diseases, such as anaplasmosis, Lyme borreliosis, coxiellosis [commonly known as Query fever, Q fever), ehrlichiosis, severe fever with thrombocytopenia syndrome (SFTS), tularemia, and relapsing fever.1,2 These tick-borne diseases are difficult to diagnose using commercially available laboratory equipment. Moreover, tick and tick-borne diseases differ based on the geographical region; therefore, their monitoring is important for public health.
In a nationwide tick survey by the Korea Disease Control and Prevention Agency (KDCA) in 2013–2015, Haemaphysalis longicornis was the dominant species (88.9%), followed by H. flava and Ixodes species.4 A recent study reported the following distribution profile of ticks in the South Korean mainland: H. longicornis (89.2%), H. flava (5.9%), Ixodes nipponensis (5.0%), and Amblyomma testudinarium (0.01%). The presence of the following tick-borne pathogens was also reported: Borrelia spp. (32 cases), Babesia microti (7 cases), and SFTS virus (SFTSV, 2 cases).5
Among tick-borne pathogens, SFTSV was first identified in 2009 in China and in 2010 in South Korea.6,7,8 Between 2013 and 2021, 1500 cases were recorded in the National Notifiable Diseases Surveillance System (NNDSS) by KDCA.9 SFTSV is transmitted through the bite of an infected hard tick, 8 and it has been detected in H. longicornis, A. testudinarium, and I. nipponensis.1 SFTS is endemic in South Korea, and its prevalence rate is the highest in Jeju Island (33°0′N, 126°0′E) (17.6/100,000 inhabitants vs. 3.1/100,000 inhabitants in mainland of South Korea).9 Currently, an effective treatment for SFTS is not yet available, highlighting the importance of early diagnosis and prevention.
Query fever (Q fever) is a zoonotic disease caused by the bacterium Coxiella burnetii, which has been reported worldwide. The first case of Q fever in South Korea was reported in 1993,5 747 cases were recorded in NNDSS by KDCA from 2006 to 2021, and only 2 cases were recorded on Jeju Island.9 C. burnetii is transmitted to humans mainly via inhalation of contaminated aerosols from infected animals and consumption of contaminated milk10; however, no clear evidence of transmission of vector-borne diseases from ticks infected with C. burnetti to humans and animals is available. In addition, high seroprevalence of IgM or IgG (Phase I and II antibodies) to C. burnetii (35.7%) was recently reported in the rural population of Jeju Island.11 In addition, SFTS and Q fever coinfection by tick bite was reported in South Korea in 2021.12
Although Q fever cases are rarely reported in this region, SFTSV and C. burnetii infection rates (IRs) in ticks on Jeju Island were analyzed in the present study based on a case of SFTS and Q fever coinfection in South Korea and the high seroprevalence of C. burnetii in agricultural population of this region.11,12
METHODS
Epidemiological and environmental investigation
This study was conducted from January 2016 to February 2019 in the rural areas of Jeju Island, South Korea. Jeju Island is the largest island located off the coast of the Korean Peninsula and is characterized by grazing land and woodlands. The island has a humid, subtropical climate and is warmer than the rest of South Korea (daily mean temperature: 15.8–16.6°C).13 The winter climate is moderate, with temperatures rarely falling below 0°C (32°F).
Free ticks from the natural environment were collected by well-equipped trained researchers; collection did not require ethical approval from any authority. The collection of ticks did not harm the environment and animals in the region. Tick sampling sites were located in the following five rural areas in Jeju Island: Aewol-eup (AW), Seon Hul-ri (SH), Jeo Ji-ri (JJ), Ha Do-ri (HD), and Bo Mok-ri (BM) (Supplementary Fig. 1).
Ticks were manually collected from each site twice, during the first and third weeks, in each month (January–December) by dragging a white cloth through the woodlands for 2 hours. Tick species and their developmental stages were identified morphologically using an Olympus SD-ILK-200–2 stereomicroscope (Olympus Corporation, Tokyo, Japan) (Supplementary Fig. 2). Nucleic acids from all collected H. longicornis were extracted using the QIAamp RNA Mini kit (QIAGEN Inc., Mainz, Germany) according to the manufacturer’s instructions.
SFTSV and C. burnetii in ticks
SFTSV RNA was extracted from each tick using the QIAamp Viral RNA Mini kit (QIAGEN Inc.) according to the manufacturer’s instructions. The extracted RNA was preserved in elution buffer at −70°C until real-time reverse transcription polymerase chain reaction (RT-PCR) of the partial S segment of SFTSV was performed for molecular diagnosis. The real-time RT-PCR mixture contained 8 µL of one-step RT-PCR premix, 7 µL of detection solution, and 5 µL of the RNA template, for a total reaction volume of 20 µL. PCR was performed under the following cycling conditions: 30 minutes at 45°C, 10 minutes at 90°C, and 45 cycles of 15 seconds at 95°C and 30 seconds at 48°C. The real-time RT-PCR products were sequenced using the BigDye Terminator Cycle Sequencing kit (Perkin Elmer Applied Biosystems, Warrington, UK). Phylogenetic analyses of SFTSV partial S segment sequences from ticks were conducted with MEGA6,14 and phylogenetic trees were constructed using the maximum likelihood method. Real-time RT-PCR of the 16S rRNA gene sequence of clone XCP-1 was used to identify Coxiella species in the each SFTSV-infected tick. A phylogenetic tree was constructed based on clone XCP-1 16S rRNA gene sequencing of C. burnetii. Definition of IR for SFTSV in ticks was rate of the number of SFTSV identifications of each the number of total collected ticks, IR for C. burnetii was rate of the number of C. burnetii identification of each the number of SFTSV infected ticks.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Categorical variables are summarized as frequencies and proportions, and continuous variables are summarized as means.
RESULTS
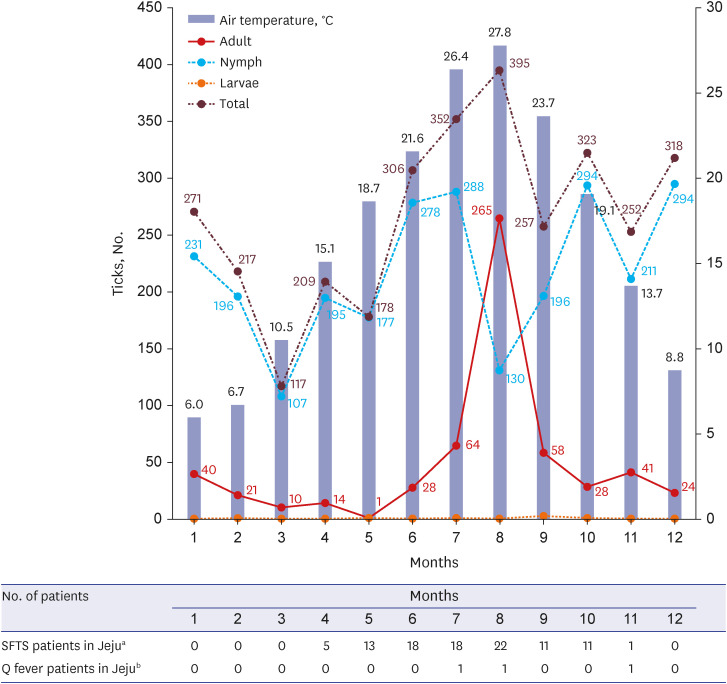
Of the total 3,458 ticks collected, the most prevalent species was H. longicornis (98.4%), followed by H. flava (1.6%). Other species were not found during this study period. The total number of ticks increased gradually from June (8.8%), peaked in July (17.8%), and was lowest in March (3.4%) (Fig. 1). The nymph developmental stage was maintained year-round in Jeju Island. The number of nymph stage ticks decreased in August and September (summer season in South Korea), when adult stage numbers peaked. Of the total collected ticks, 2,851 (82.6%) were nymphs; 639 (17.9%) were adults, among which 59.4% were females; and 4 (0.1%) were larvae. Based on the developmental stage, monthly changes in tick numbers were as follows: 1) the nymph count was highest from June (278/2,815 [9.9%]) to July (288/2,815 [10.2%]) and from October (294/2,815 [10.4%]) to December (294/2,815 [10.4%]); 2) the number of adults was the highest in August (265/639 [41.5%]).
Fig. 1. Monthly trend according to the tick developmental stage and monthly number of patients with SFTSV and Coxiella burnetii in Jeju Island, South Korea.
SFTSV = severe fever with thrombocytopenia syndrome virus.
aNumber of patients with SFTSV between 2013 and 2022; bNumber of patients with C. burnetii between 2006 and 2022.
SFTSV in ticks
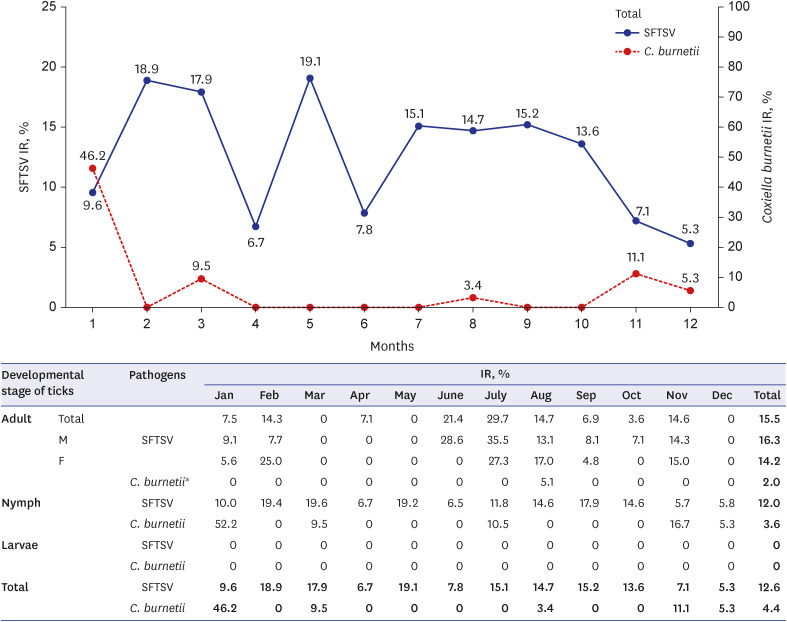
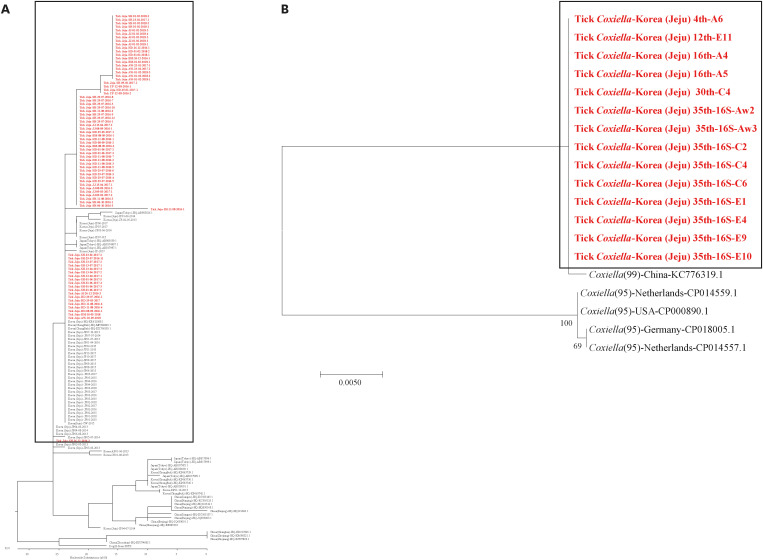
SFTSV-infected ticks were identified annually in the five rural regions, and infection was detected in 12.6% of the collected ticks (437/3,458) (Fig. 2). SFTSV IRs were 15.4% in SH, 12.8% in AW, 12.0% in HD, 10.3% in JJ, and 8.6% in BM. Among the SFTS-infected ticks, highest SFTSV IRs were observed in February (18.9%), March (17.9%), May (19.1%), July (15.1%), August (14.7%), and September (15.2%). Meanwhile, the lowest SFTSV IRs were the observed in April (6.7%), June (7.8%), November (7.1%), and December (5.3%), and increased from January onwards. SFTSV IRs were 16.3% in female adults, 14.2% in male adults, 12.0% in nymphs, and 0% in larvae (Fig. 2). Adult SFTSV-infected ticks were mostly identified in June (21.4%), July (29.7%), and August (14.7%). Therefore, this three-host adult tick species transmits pathogens to humans during this period (June–August) in South Korea. Phylogenetic analysis based on SFTSV partial S segment sequences from ticks in the five rural regions of Jeju Island revealed that they were closely related, but were found to be different from those characterized from patients with SFTS in mainland of South Korea, China, and Japan (Fig. 3A).15
Fig. 2. Monthly IR of SFTSV and Coxiella burnetii in Jeju Island. The blue line indicates SFTSV IR, and the red dot line indicates C. burnetii IR.
aC. burnetii infection in tick was only identified in the female adult stage.
SFTSV = severe fever with thrombocytopenia syndrome virus, Jan = January, Feb = February, Mar = March, Apr = April, Aug = August, Sep = September, Oct = October, Nov = November, Dec = December, M = male, F = female, IR = infection rate.
Fig. 3. Phylogenetic trees of SFTSV and Coxiella burnetii in Jeju Island, South Korea. (A) Phylogenetic tree based on partial SFTSV S segment sequences. The partial S sequences were obtained from ticks collected between June 2016 and January 2019. The partial S sequence data for SFTSV identified in China, South Korea, and Japan were obtained from NCBI/BLAST. (B) Phylogenetic tree based on clone XCP-1 16S rRNA gene sequencing of C. burnetii.
SFTSV = severe fever with thrombocytopenia syndrome virus.
C. burnetii in ticks
C. burnetii infections were detected in 4.4% of the SFTSV-infected H. longicornis ticks (19/437) in all five rural regions. C. burnetii IRs were 5/437 in SH, 3/437 in AW, 4/437 in HD, 4/437 in JJ, and 3/437 in BM. Of the 437 SFTSV-infected ticks, C. burnetii IR was highest in January (12/26), and were rarely identified in other seasons (Fig. 2). The number of C. burnetii-infected ticks, mainly identified in the nymph stage (17/19), was the highest in January (12/19) (Fig. 2). Only two adult C. burnetii-infected ticks were identified in August. The phylogenetic tree based on clone XCP-1 16S C. burnetii rRNA gene sequencing was different from that from the USA and Europe (Fig. 3B).
DISCUSSION
Jeju Island has a subtropical climate and is inhabited by various animals, such as horses, pigs, cows, deer, roe deer, and many wild animals.13 The island’s climate and environment hold multiple benefits for the tick life cycle. H. longicornis has been reported as the most dominant hard tick in Jeju Island, an isolated region of South Korea, and is found to be more extensively distributed in Jeju Island than in other regions of South Korea (98.5% vs. 89%).4,16 According to a previous report, the distribution of different tick species in the temperate region of South Korea in 2019 was as follows: H. longicornis (89%), followed by H. flava (6%), and I. nipponensis (5%).5 In their study, the number of ticks increased gradually from April, peaked in August, and then steeply decreased in October.
In the present study, we observed that the number of H. longicornis increased from June, peaked in August, and then gradually decreased in September. In addition, SFTSV IR was found to be very high, compared to that in the mainland of South Korea.17 The SFTSV IR in ticks co-infected with C. burnetii was 4.4%. SFTSV infection in ticks was identified in the nymph and adult developmental stages in all seasons; however, the IRs were high in the adult stage and during the summer season, which also showed high risk of SFTSV human infection. C. burnetii IR was high in the nymph developmental stage and winter season, and infection was hardly found in the adult stage and in other seasons. H. longicornis ticks, which are distributed in various regions of Jeju Island, are a species of hard ticks and have a three-host life cycle that usually spans 2–3 years.18,19 Adult females drop off the third host, usually a lager herbivore, a carnivore, or a human, to lay eggs after feeding.
During a similar study in Deogyusan National Park of South Korea, H. longicornis (94.2%), H. flava (5.0%), and I. nipponensis (0.8%) were collected between 2016 and 2018. The total SFTSV IR in ticks was 6.0%, of which 5.3% in adults and 6.0% in nymphs.17 The prevalence rate of SFTS in Daejeon region was 3.1 among 100,000 inhabitants, with a mean prevalence rate similar to that observed in South Korea.9
In another study in South Korea, SFTSV IR was 0.04% in larvae, 0.13% in nymphs, 0% in male adults, 0.12% in female adults, and 0.1% in total ticks.17 However, in the present study, SFTSV IR was 16.3% in male adults, 14.2% in female adults, and 12.0% in nymphs in Jeju Island. According to data from the KDCA during 2013–2021, SFTSV infections in Jeju Island occurred mainly during the spring and summer seasons, in contrast with regions in the mainland, where SFTSV incidence was high during autumn.20 Therefore, summer presents a high risk of SFTSV human infection in Jeju Island.
Q fever has been observed in South Korea since 2012, and 573 cases were recorded by KDCA from 2012 to 2021.9 In a previous study, C. burnetii was identified in 1.5% of H. longicornis collected in South Korea.21 However, C. burnetii was identified in 52.4% of 213 ticks collected from horses in Jeju Island,22 compared with the 4.4% in ticks collected from the SFTSV infected ticks in the present study. C. burnetii is transmitted to humans mainly via inhalation of contaminated aerosols from infected animals,10 and the vector capacity of ticks to transmit C. burnetii remains unclear. However, most tick species can transmit C. burnetii to uninfected animals in experimental systems, and C. burnetii has been detected in the midgut, Malphighian tubes, and salivary glands. This indicates the potential risk of tick-borne infection from tick excreta, tick bites, or via direct contact.12,23 Q fever and SFTSV co-infection from a tick bite is possible in humans, with high risk especially during the winter season. C. burnetii-infected ticks were mainly identified in the nymph stage in the present study; hence, human infection with C. burnetii is less likely in Jeju Island as H. longicornis is a three-host tick that attaches to medium-sized wild animals, such as rabbits and raccoons, in the nymph stage and then falls on the ground to develop into the adult stage.24 However, wild animals can become infected through a tick bite, and their excreta can be transmitted to the respiratory tract through the dry winter environment. In addition, according to the phylogenetic tree analysis, C. burnetii in Jeju Island may have formed an endemic area, which may have been imported from a neighboring country, but not America or Europe.
This study has some limitations. First, ticks could not be collected from more varied regions. Second, the S, L, and M RNAs of the SFTSV genome in ticks could not be fully analyzed because only partial S segment analysis was available. Further study is required for the whole-genome analysis of patients with SFTS, animal hosts, and ticks. Third, the samples were collected for a long duration in the most common endemic area, and ticks were collected monthly, as opposed to other studies. Finally, we did not know C. burnetii IR in ticks in natural environment of Jeju Island because of we were performed PCR analysis among the SFTSV-infected H. longicornis ticks. Further study will be necessary to test C. burnetii IR from total collected ticks.
In summary, H. longicornis was the most dominant hard tick in Jeju Island. SFTSV IR in ticks was found to be very high during all seasons, while C. burnetii infection in ticks was detected in the winter season. Our findings suggest that SFTSV and C. burnetii coinfection is possible in humans.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF), the Ministry of Science, ICT, and Future Planning (grant number: NRF-2021R1A2C2091578).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoo JR, Heo ST, Kim M.
- Data curation: Yoo JR, Lee KH.
- Formal analysis: Yoo JR, Lee KH.
- Funding acquisition: Lee KH.
- Investigation: Kang MJ, Kang SY.
- Methodology: Lee KH.
- Supervision: Kim M.
- Visualization: Kim ET.
- Writing - original draft: Yoo JR.
- Writing - review & editing: Lee KH.
SUPPLEMENTARY MATERIALS
Tick sampling regions in Jeju Island, South Korea from June 2016 to January 2019. Black dots indicate sampling sites. The tick sampling regions were located in five rural areas: Aewol-eup (AW), N 33° 45′ 90.0″, E 126° 34′ 24.4″; Seon Hul-ri (SH), N 33° 50′ 82.6″, E 126° 69′ 92.9″; Jeo Ji-ri (JJ), N 33° 34′ 00.2″, E 126° 26′ 45.8″; Ha Do-ri (HD), N 33° 50′ 69.6″, E 126° 89′ 05.3″; and Bo Mok-ri (BM), N 33° 24′ 83.1″, E 126° 60′ 32.2″. This map was extracted from Google (https://www.google.co.kr/maps/?hl=ko).
Microscopic examination of Haemaphysalis longicornis species collected during the study period in Jeju Island, South Korea. (A) Adult female dorsal surface. (B) Adult female ventral surface. (C) Adult male dorsal surface. (D) Adult male ventral surface.
References
- 1.Dehhaghi M, Kazemi Shariat Panahi H, Holmes EC, Hudson BJ, Schloeffel R, Guillemin GJ. Human tick-borne diseases in Australia. Front Cell Infect Microbiol. 2019;9:3. doi: 10.3389/fcimb.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E. Emerging tick-borne diseases. Clin Microbiol Rev. 2020;33(2):e00083-18. doi: 10.1128/CMR.00083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perveen N, Muzaffar SB, Al-Deeb MA. Ticks and tick-borne diseases of livestock in the middle East and North Africa: a review. Insects. 2021;12(1):83. doi: 10.3390/insects12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song B, Lee W, Ju Y. Geographical distribution of Ixodid ticks in the Republic of Korea, 2015. Public Health Wkly Rep. 2017;10(10):239–245. [Google Scholar]
- 5.Seo JW, Han SY, Sung SH, Jung EY, Kim JH, Lee SJ, et al. Survey on tick distribution and tick-borne pathogens in Daejeon and adjacent areas in South Korea. Ticks Tick Borne Dis. 2021;12(4):101711. doi: 10.1016/j.ttbdis.2021.101711. [DOI] [PubMed] [Google Scholar]
- 6.Heo ST, Yoo JR, Lee KH, Ko KS. The first case of non-retrospective clinical identification of severe fever with thrombocytopenia syndrome patient in 2013 in South Korea. J Bacteriol Virol. 2015;45(2):155–158. [Google Scholar]
- 7.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19(11):1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korea Disease Control and Prevention Agency (KDCA) Statistical System of Notifiable Disease Surveillance System (2013–2021) [Updated 2023]. [Accessed April 10, 2022]. https://is.cdc.go.kr .
- 10.Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30(1):115–190. doi: 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo JR, Kim MS, Heo ST, Oh HJ, Oh JH, Ko SY, et al. Seroreactivity to Coxiella burnetii in an agricultural population and prevalence of Coxiella burnetii Iinfection in ticks of a non-endemic region for Q fever in South Korea. Pathogens. 2021;10(10):1337. doi: 10.3390/pathogens10101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Choi YJ, Lee KS, Kim JE, Oh JW, Moon JH. Severe fever with thrombocytopenia syndrome with Q fever coinfection in an 8-year-old girl. Pediatr Infect Dis J. 2021;40(1):e31–e34. doi: 10.1097/INF.0000000000002948. [DOI] [PubMed] [Google Scholar]
- 13.Korea Meteorological Administration (KMA) Jeju’s Climatic Region. [Updated 2023]. [Accessed December 17, 2022]. http://www.kma.go.kr/eng/index.jsp .
- 14.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo JR, Heo ST, Song SW, Bae SG, Lee S, Choi S, et al. Severe fever with thrombocytopenia syndrome virus in ticks and SFTS incidence in humans, South Korea. Emerg Infect Dis. 2020;26(9):2292–2294. doi: 10.3201/eid2609.200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae J. Survey of Severe Fever With Thrombocytopenia Syndrome Virus in Natural Environment and Study on Useful Meterial for Development of Tick Repellent. Incheon, Korea: National Institute of Environmental Research; 2017. pp. 1–329. [Google Scholar]
- 17.Kang JG, Cho YK, Jo YS, Han SW, Chae JB, Park JE, et al. Severe fever with thrombocytopenia syndrome virus in ticks in the Republic of Korea. Korean J Parasitol. 2022;60(1):65–71. doi: 10.3347/kjp.2022.60.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14(8):763–772. doi: 10.1016/S1473-3099(14)70718-2. [DOI] [PubMed] [Google Scholar]
- 19.White SA, Bevins SN, Ruder MG, Shaw D, Vigil SL, Randall A, et al. Surveys for ticks on wildlife hosts and in the environment at Asian longhorned tick (Haemaphysalis longicornis)-positive sites in Virginia and New Jersey, 2018. Transbound Emerg Dis. 2021;68(2):605–614. doi: 10.1111/tbed.13722. [DOI] [PubMed] [Google Scholar]
- 20.Oh H, Yoo JR, Kim M, Heo ST. Current status and infection control of severe fever with thrombocytopenia syndrome in Korea. Korean J Healthc Assoc Infect Control Prev. 2022;27(1):18–27. [Google Scholar]
- 21.Lee JH, Park HS, Jang WJ, Koh SE, Park TK, Kang SS, et al. Identification of the Coxiella sp. detected from Haemaphysalis longicornis ticks in Korea. Microbiol Immunol. 2004;48(2):125–130. doi: 10.1111/j.1348-0421.2004.tb03498.x. [DOI] [PubMed] [Google Scholar]
- 22.Seo MG, Lee SH, Ouh IO, Lee GH, Goo YK, Kim S, et al. Molecular detection and genotyping of Coxiella-like endosymbionts in ticks that infest horses in South Korea. PLoS One. 2016;11(10):e0165784. doi: 10.1371/journal.pone.0165784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duron O, Sidi-Boumedine K, Rousset E, Moutailler S, Jourdain E. The importance of ticks in Q fever transmission: What has (and has not) been demonstrated? Trends Parasitol. 2015;31(11):536–552. doi: 10.1016/j.pt.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Korea Disease Control and Prevention Agency (KDCA) Guideline for control of tick and rodent-borne infectious disease. [Updated 2021]. [Accessed March 21, 2022]. https://is.cdc.go.kr .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tick sampling regions in Jeju Island, South Korea from June 2016 to January 2019. Black dots indicate sampling sites. The tick sampling regions were located in five rural areas: Aewol-eup (AW), N 33° 45′ 90.0″, E 126° 34′ 24.4″; Seon Hul-ri (SH), N 33° 50′ 82.6″, E 126° 69′ 92.9″; Jeo Ji-ri (JJ), N 33° 34′ 00.2″, E 126° 26′ 45.8″; Ha Do-ri (HD), N 33° 50′ 69.6″, E 126° 89′ 05.3″; and Bo Mok-ri (BM), N 33° 24′ 83.1″, E 126° 60′ 32.2″. This map was extracted from Google (https://www.google.co.kr/maps/?hl=ko).
Microscopic examination of Haemaphysalis longicornis species collected during the study period in Jeju Island, South Korea. (A) Adult female dorsal surface. (B) Adult female ventral surface. (C) Adult male dorsal surface. (D) Adult male ventral surface.