Abstract
Objectives
Myasthenia gravis (MG) is a classic autoantibody‐mediated disease in which pathogenic antibodies target postsynaptic membrane components, causing fluctuating skeletal muscle weakness and fatigue. Natural killer (NK) cells are heterogeneous lymphocytes that have gained increasing attention owing to their potential roles in autoimmune disorders. This study will investigate the relationship between the distinct NK cell subsets and MG pathogenesis.
Methods
A total of 33 MG patients and 19 healthy controls were enrolled in the present study. Circulating NK cells, their subtypes and follicular helper T cells were analysed by flow cytometry. Serum acetylcholine receptor (AChR) antibody levels were determined by ELISA. The role of NK cells in the regulation of B cells was verified using a co‐culture assay.
Results
Myasthenia gravis patients with acute exacerbations had a reduced number of total NK cells, CD56dim NK cells and IFN‐γ‐secreting NK cells in the peripheral blood, while CXCR5+ NK cells were significantly elevated. CXCR5+ NK cells expressed a higher level of ICOS and PD‐1 and a lower level of IFN‐γ than those in CXCR5− NK cells and were positively correlated with Tfh cell and AChR antibody levels. In vitro experiments demonstrated that NK cells suppressed plasmablast differentiation while promoting CD80 and PD‐L1 expression on B cells in an IFN‐γ‐dependent manner. Furthermore, CXCR5− NK cells inhibited plasmablast differentiation, while CXCR5+ NK cells could more efficiently promote B cell proliferation.
Conclusion
These results reveal that CXCR5+ NK cells exhibit distinct phenotypes and functions compared with CXCR5− NK cells and might participate in the pathogenesis of MG.
Keywords: CXC chemokine receptor 5, humoral immune response, myasthenia gravis, natural killer cells
In this study, we found that circulating CXCR5⁺ natural killer cells are expanded in the myasthenia gravis patients. Different NK cell subtypes exhibit distinct effects on B cell differentiation. CXCR5⁻ NK cells suppress plasmablast differentiation while CXCR5⁺ NK cells more efficiently promote B cell proliferation.
Introduction
Myasthenia gravis (MG) is a classic autoantibody‐mediated disease in which pathogenic antibodies target postsynaptic membrane components, causing fluctuating skeletal muscle weakness and fatigue. 1 MG has an annual incidence of 8–10 cases per 1 million individuals and a prevalence of 150–250 cases per 1 million individuals. 1 , 2 The incidence and prevalence of MG have been on the rise in recent years, which might be attributed to the growth of the ageing population in conjunction with increased longevity, changes in lifestyle and advances in diagnostics and treatments.
Natural killer (NK) cells are known to be lymphocytes of the innate immune system that function as cytotoxic effector cells in the defence against malignant or virally infected cells. 3 In recent years, however, with the progress in the field of NK cell biology and understanding of NK cell function, NK cells are now recognised to play critical roles in immune regulation. 3 The modulatory effects of NK cells on immune reactions are complex. In experimental autoimmune encephalomyelitis (EAE) models, diminishing recruitment of NK cells to the central nervous system exacerbates pathology and neurological dysfunction, suggesting a protective role of NK cells in neuroinflammation. 4 Regarding experimental autoimmune myasthenia gravis (EAMG), NK cells have been shown to promote or restrain disease progression. 5 , 6 Understanding of the NK cell function in the immune response has been further prompted by recent data describing their capacity to modulate different immune cells. Cook et al. 7 recently reported that NK cells inhibited B cell responses by suppressing the activities of follicular helper T (Tfh) cells, thereby preventing humoral immunity in lymphocytic choriomeningitis virus infections. NK cells also exhibit direct cytolytic effects on Tfh cells 6 , 8 and can suppress the proliferation and antibody (Ab) affinity maturation of B cells. 9 , 10
The human NK cell repertoire is remarkably diverse and comprises many disparate lineages. 11 , 12 CD56, an isoform of the human neural cell adhesion molecule used as a general marker for human NK cells, divides these lymphocytes into two populations. Most human circulating NK cells (90%) with low CD56 expression are known as CD56dim NK cells. These cells are mostly CD16 positive, showing high cell‐killing ability. 13 In contrast, the minority population of circulating CD56bright NK cells (10%) is generally considered cytokine‐producing regulatory cells. 13 Moreover, NK cells are known to produce a variety of cytokines depending on the inflammatory microenvironment, such as IFN‐γ, IL‐4 and IL‐10, and exert profound effects on the immune response. 3 , 14 , 15 Our previous results revealed a specific subpopulation of NK cells expressing CXCR5 and highlighted the different roles of CXCR5+ NK cells and CXCR5− NK cells in EAMG. 6 However, whether and how these different NK cell subtypes are relevant to the pathogenesis of MG remains unclear.
Results
Demographical data and clinical characteristics of study subjects
A total of 52 patients (19 controls and 33 patients with MG) were included in the study. Based on the quantitative myasthenia gravis (QMG) score, 20 patients were classified into the aggravated MG group and 13 were classified into the remitted MG group. Sex and age were not different among the three groups. The control group contained 12 (63%) male individuals with a mean age of 45.79 ± 12.82 years (range, 31–73 years). The mean age of patients in the aggravated MG group was 53.20 ± 14.88 years (range, 18–74 years), with 11 (55%) males. Of the subjects analysed in the remitted MG group, 7 (54%) were male, with a mean age of 52.85 ± 13.99 years (range, 33–74 years).
Furthermore, we differentiated patients with myasthenia according to their clinical features (muscle involvement and disease progression), age of onset (early or late onset), antibodies to the acetylcholine receptor (AChR; seropositive or seronegative) and thymic pathology (normal thymic, thymic hyperplasia or thymoma). Among the aggravated patients, 7 (35%) had early‐onset MG, 19 (95%) had AChR antibodies and 7 (35%) had thymic hyperplasia or thymoma. In the remitted group, 6 (46%) patients had early‐onset MG, 12 (92%) had AChR antibodies and 7 (54%) had thymic hyperplasia or thymoma. According to the Osserman classification, the fractures were type I in 7 (35%), type IIa in 1 (5%) and type IIb in 12 (60%) patients with aggravated MG, and type I in 1 (8%), type IIA in 2 (15%) and type IIB in 10 (77%) patients with remitted MG. The QMG score was significantly higher (worse) in the aggravated MG group (mean ± SD, 12.70 ± 6.47) than in the remitted MG group (mean ± SD, 5.15 ± 2.38; P < 0.001). All patients on immunosuppressive medication at the time of venesection are summarised in Table 1. Of note, the MG patients receiving immunosuppressants mainly belong to the remitted group, which may be a confounding factor when immunophenotyping patients.
Table 1.
Demographic and clinical characteristics of MG patients and healthy controls
| Control | Aggravated MG | Remitted MG | P‐value | |
|---|---|---|---|---|
| Number | 19 | 20 | 13 | – |
| Age, years, Mean ± SD | 45.79 ± 12.82 | 53.20 ± 14.88 | 52.85 ± 13.99 | 0.204 |
| Sex, male, n (%) | 12 (63) | 11 (55) | 7 (54) | 0.824 |
| Early‐onset (< 50 years), n (%) | – | 7 (35) | 6 (46) | 0.717 |
| Thymoma or hyperplasia, n (%) | – | 7 (35) | 7 (54) | 0.472 |
| Anti‐AChR Ab (+), n (%) | – | 19 (95) | 12 (92) | 1.000 |
| QMG score, Mean ± SD | – | 12.70 ± 6.47 | 5.15 ± 2.38 | < 0.001 |
| Modified Osserman classification | – | 0.144 | ||
| Type I, n (%) | – | 7 (35) | 1 (8) | – |
| Type IIa, n (%) | – | 1 (5) | 2 (15) | – |
| Type IIb, n (%) | – | 12 (60) | 10 (77) | – |
| Type III, IV or V, n (%) | – | 0 (0) | 0 (0) | – |
| Medication | ||||
| Pyridostigmine bromide | – | 11 (55) | 6 (46) | 0.619 |
| ± Glucocorticoids | – | 2 (10) | 6 (46) | 0.002 |
| ± Immunosuppressants | – | 0 (0) | 6 (46) | < 0.001 |
Notes: P > 0.05, no statistically significant difference between groups. Immunosuppressants include azathioprine, tacrolimus or cyclophosphamide.
AChR, acetylcholine receptor; MG, myasthenia gravis; QMG score, quantitative MG score; –, not applicable.
NK cell percentage and IFN‐γ production were decreased in the peripheral blood of patients with aggravated MG
Many studies have reported that several autoimmune diseases are associated with impaired NK cell proportion or function. 3 , 13 However, the changes in NK cells in MG remain controversial. Since multiple subsets of NK cells have been clearly identified and the complexity of NK cells is increasingly recognised, we analysed diverse NK cell subsets in the blood of controls and patients with MG. The gating strategy for the total NK cells (defined as CD3−CD19−CD56+) is shown in Figure 1a. The percentage of total NK cells in the peripheral blood of the aggravated MG group was significantly lower than that of the remitted and control groups (Figure 1a). The percentage of CD56bright NK cells in patients in the aggravated group showed an increasing trend compared to that in the control group. In the remitted group, the percentage of CD56bright NK cells was significantly lower than that in the aggravated MG group (Figure 1b). The percentage of CD56dim NK cells showed opposite changes across the different groups (Figure 1b). The percentage of IFN‐γ‐positive NK cells in the peripheral blood of patients with aggravated MG was significantly lower than that in the control group, whereas no significant difference was observed between the control and remission groups (Figure 1c and d). Meanwhile, no significant differences were observed in the percentages of IL‐10‐ or IL‐17‐producing NK cells among the three groups (Figure 1c, e and f).
Figure 1.
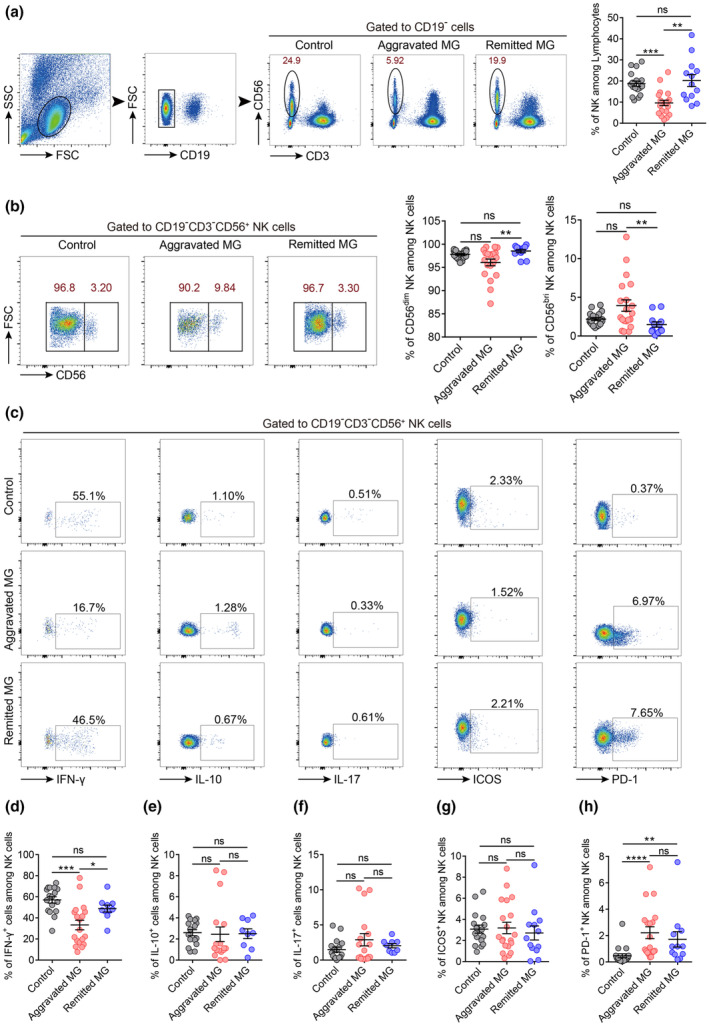
NK cell percentage and IFN‐γ production were decreased in the peripheral blood of patients with aggravated MG. (a) Gating strategy, representative dot plots and percentages of NK cells (CD3−CD19−CD56+) from the peripheral blood of control (n = 18), aggravated MG (n = 20) and remitted MG groups (n = 13). (b) Representative dot plots and percentages of CD56bright (CD3−CD19−CD56bright) and CD56dim (CD3−CD19−CD56dim) NK cell subsets among NK cells in controls (n = 19), aggravated MG (n = 20) and remitted MG (n = 12). (c) Representative dot plots showing the expression of IFN‐γ, IL‐10, IL‐17, ICOS and PD‐1 in NK cells. (d–f) Percentages of IFN‐γ‐, IL‐10‐ or IL‐17‐positive cells among NK cells in the control (n = 17, 16 and 17, respectively), aggravated MG (n = 19, 17 and 17, respectively) and remitted MG (n = 10, 9 and 9, respectively). (g, h) Percentages of ICOS+ and PD‐1+ NK cells among NK cells in controls (n = 17 and 19, respectively), aggravated MG (n = 20 and 19, respectively) and remitted MG (n = 13 and 12, respectively). Data are presented as the mean ± SEM. The Kruskal–Wallis test followed by Dunn's multiple comparisons was used. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001, ns, not significant.
Considerable research has shown that aberrantly expressed costimulatory molecules are closely related to the occurrence of autoimmune diseases. Imbalances in the expression of related molecules in immune cells may also be involved in the initiation and progression of MG. To this end, we examined the expression of costimulatory molecules, such as ICOS and PD‐1, on the surface of NK cells. Specifically, no significant differences were observed in the expression of ICOS on human circulating NK cells among the different groups (Figure 1c and g). PD‐1 expression on NK cells was higher in patients with aggravated MG than in controls, whereas in the remitted group, a decreasing trend in PD‐1 expression on NK cells was observed compared to the aggravated group although this was not statistically significant (Figure 1h).
CXCR5 + NK cells exhibited different phenotypes compared to CXCR5 − NK cells
Our previous study demonstrated the presence of CXCR5+ NK cells in the spleen, peripheral blood and lymph nodes of rats and the different roles of CXCR5−/+ NK cells in regulating EAMG. 6 However, whether these specialised subtypes of NK cells are present in human peripheral blood is unknown. We validated the existence of CXCR5+ NK cells in human circulation, which confirmed and expanded our previous findings (Figure 2a). To clarify the functional heterogeneity among CXCR5−/+ NK cell subgroups, we analysed the surface and intracellular molecules expressed by CXCR5− and CXCR5+ NK cells. As shown in Figure 2b and c, there was no difference in CD56 expression between the two groups of cells. CXCR5+ NK cells had higher expression levels of ICOS, PD‐1 and FasL but a lower expression level of NKG2D than those in CXCR5− NK cells (Figure 2c). To analyse the cytotoxicity of CXCR5−/+ NK cell subsets, we measured intracellular granzyme B and perforin contents via flow cytometry. The results revealed that CXCR5+ NK cells had decreased expression of granzyme B and perforin compared with the negative population (Figure 2c). In addition, compared with CXCR5− NK cells, the expression level of IFN‐γ in CXCR5+ NK cells significantly decreased, while IL‐10 and IL‐17 expression did not differ (Figure 2c).
Figure 2.
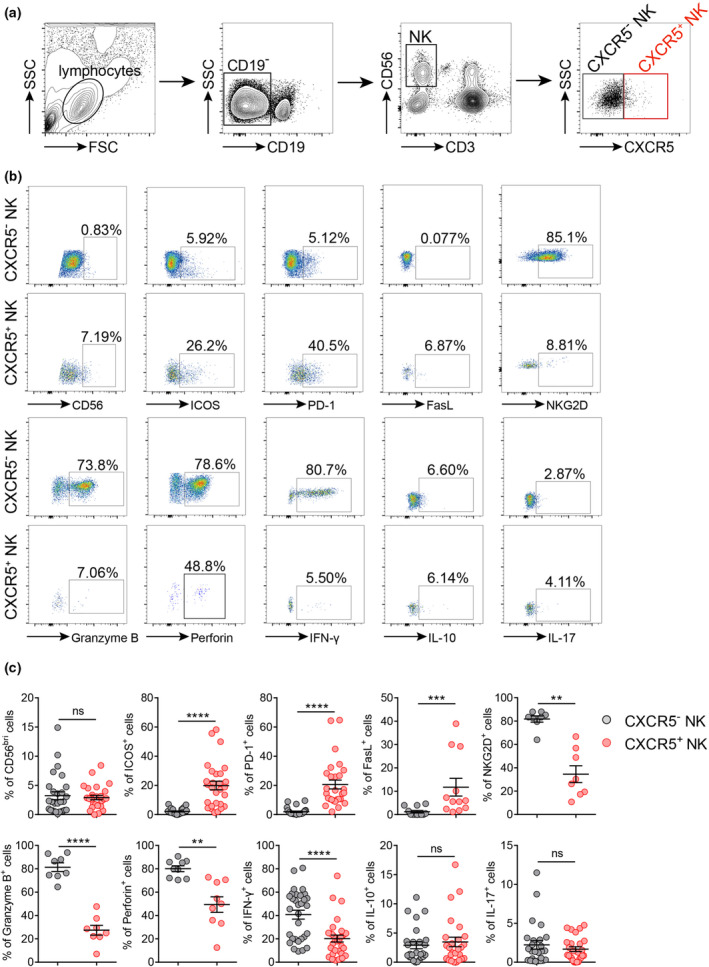
Phenotypic differences between CXCR5− and CXCR5+ NK cells in human peripheral blood. (a) Gating strategy of CXCR5− and CXCR5+ NK cells from human peripheral blood. (b) Representative dot plots showing the different expressions of CD56, ICOS, PD‐1, FasL, NKG2D, IFN‐γ+, IL‐10+, IL‐17+, Granzyme B and Perforin in CXCR5− and CXCR5+ NK cells. (c) Percentages of CD56bright, ICOS+, PD‐1+, FasL+, NKG2D+, IFN‐γ+, IL‐10+, IL‐17+, Granzyme B+ and Perforin+ in cells with CXCR5− and CXCR5+ NK cells (n = 28 for CD56, ICOS and PD‐1; n = 12 for FasL; n = 8 for NKG2D and granzyme B; n = 9 for perforin; n = 31 for IFN‐γ and n = 26 for IL‐10 and IL‐17, respectively). Data are presented as the mean ± SEM. The Wilcoxon matched‐pairs signed rank test or the paired t‐test was used when applicable. **P < 0.01, ***P < 0.001 and ****P < 0.0001, ns, not significant.
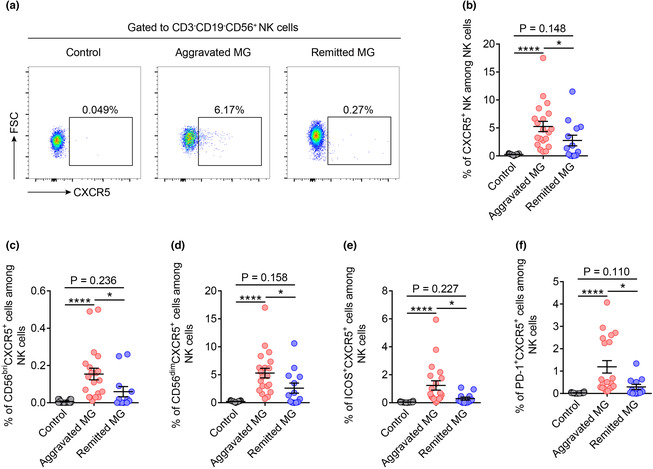
CXCR5 + NK cells were expanded in the peripheral blood of patients with aggravated MG
These data prompted us to explore the potential relationship between CXCR5+ NK cells and MG disease activity. Representative flow cytometry plots of CXCR5+ NK cells in different patient groups are shown in Figure 3a. We found that the CXCR5+ NK cells in peripheral blood were nearly absent in the controls. Patients with aggravated MG had a significantly higher percentage of CXCR5+ NK cells than that in patients in the remission and control groups (Figure 3b). Similarly, an increased number of CD56brightCXCR5+, CD56dimCXCR5+, ICOS+CXCR5+ and PD‐1+CXCR5+ NK cells in patients with aggravated MG was also observed (Figure 3c–f).
Figure 3.
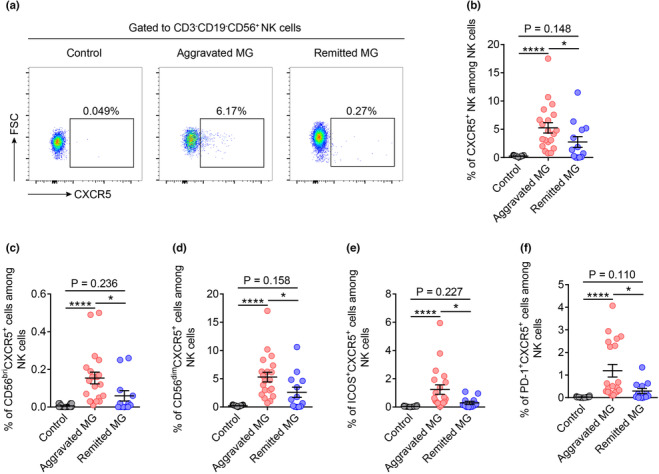
CXCR5+ NK cells were expanded in the peripheral blood of patients with aggravated MG. (a) Representative flow cytometric dot plots of CXCR5+ NK cells from the peripheral blood of control, aggravated MG patients and remitted MG patients. (b) Percentages of CXCR5+ NK cells among circulating total NK cells in controls (n = 19), aggravated MG (n = 20) and remitted MG (n = 13). (c–f) Percentages of CD56brightCXCR5+, CD56dimCXCR5+, ICOS+CXCR5+ and PD‐1+CXCR5+ NK cells among circulating total NK cells in controls (n = 18 or 19), patients with aggravated MG (n = 20) and patients with remitted MG (n = 12 or 13). Data are presented as the mean ± SEM. The Kruskal–Wallis test followed by Dunn's multiple comparisons was used. *P < 0.05 and ****P < 0.0001.
Patients with aggravated MG had increased Tfh cell proportion and decreased Tfr cell proportion in peripheral blood
Recent studies have highlighted the pivotal function of Tfh cells in promoting and maintaining germinal centre reactions in autoimmune pathogenesis, whereas T follicular regulatory (Tfr) cells are thought to block the function of Tfh cells, reduce GC reaction and inhibit Ab responses. 16 , 17 We, therefore, examined the proportion of Tfh and Tfr cells via flow cytometry. The Tfh cells were gated as CD4+CXCR5+ and Tfr cells as CD4+CXCR5+Foxp3+. Our data suggest that the percentage of Tfh cells increased significantly in the peripheral blood of patients with aggravated MG compared with the controls (Figure 4a). This cell type showed decreasing trends in the remitted group versus the aggravated group (Figure 4a). Patients with aggravated MG showed significantly higher percentages of ICOS+ Tfh cells among CD4+ T cells or lymphocytes than that in patients in the remitted and control groups (Figure 4b). We did not observe a significant difference in the proportion of PD‐1+ Tfh cells among CD4+ T cells among the three groups (Figure 4c). However, the percentage of PD‐1+ Tfh cells in lymphocytes was higher in the aggravated MG group than that in the control group (Figure 4c). The percentage of Tfr cells in the aggravated MG group was lower than that in the control group, whereas the reduced Tfr cell proportion was restored in the remitted MG group (Figure 4d). Finally, the ratio of Tfh to Tfr increased in the aggravated group compared to both the control and remitted groups (Figure 4d). We also checked the frequency of CD19+ cells in each group and found that there was no difference between the control group and the aggravated MG group, while the frequency of CD19+ cells was decreased in remitted MG group (Supplementary figure 1a).
Figure 4.
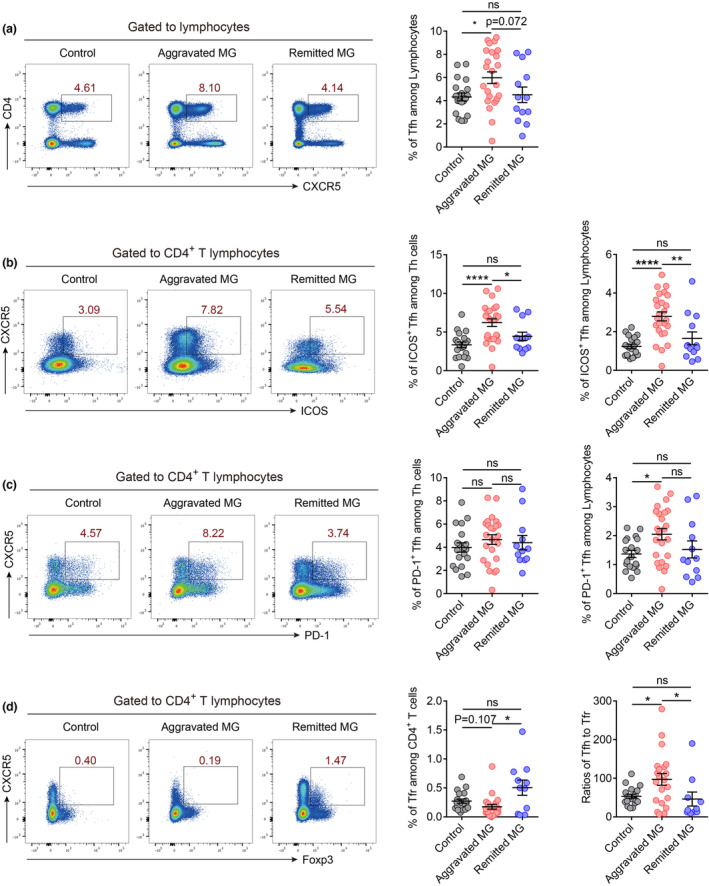
Patients with aggravated MG had increased Tfh cell proportion and decreased Tfr cell proportion in peripheral blood. (a) Percentages of Tfh cells (CD4+CXCR5+) among human peripheral blood lymphocytes in controls (n = 19), aggravated MG (n = 24) and remitted MG (n = 13). (b) Percentages of ICOS+ Tfh (CD4+CXCR5+ICOS+) cells among human peripheral blood CD4+ T cells or total lymphocytes in controls (n = 19), aggravated MG (n = 25) and remitted MG (n = 13). (c) Percentages of PD‐1+ Tfh cells (CD4+CXCR5+PD‐1+) among human peripheral blood CD4+ T cells or total lymphocytes in controls (n = 19), aggravated MG (n = 24) and remitted MG (n = 12). (d) Percentages of Tfr cells (CD4+CXCR5+Foxp3+) among CD4+ T cells and ratios of Tfh cells to Tfr cells in controls (n = 19), aggravated MG (n = 21) and remitted MG (n = 12). Data are presented as the mean ± SEM. The Kruskal–Wallis test followed by Dunn's multiple comparisons was used. *P < 0.05, **P < 0.01 and ****P < 0.0001, ns, not significant.
Circulating CXCR5 + NK cells were positively correlated with Tfh cells and serum AChRAb concentrations in patients with MG
To further investigate the potential function of CXCR5+ NK cells in MG pathogenesis, we performed correlation analysis between NK cells or CXCR5+ NK cells and Tfh cells, ICOS+ Tfh cells, Tfh/Tfr ratios or AChRAb levels. We observed a negative correlation between the proportions of NK cells and Tfh cells (Figure 5a). Similarly, a slightly negative correlation was observed between the proportions of ICOS+ Tfh cells and NK cells (Figure 5b). In contrast, positive correlations between Tfh cell proportions, ICOS+ Tfh cell proportions or Tfh/Tfr ratios and CXCR5+ NK cells were observed in the peripheral blood of MG patients (Figure 5c–e). In addition, AChRAb concentrations positively correlated with the proportion of CXCR5+ NK cells (Figure 5f). However, we did not observe a positive correlation between the Tfh cells and AChR antibodies (Figure 5g). We observed that the ratios of Tfh to Tfr cells were positively correlated with AChR antibody concentrations (Figure 5h), although the correlation is weak (P = 0.025). These results indicate CXCR5+ NK cells might act as a positive regulator in the MG humoral response.
Figure 5.
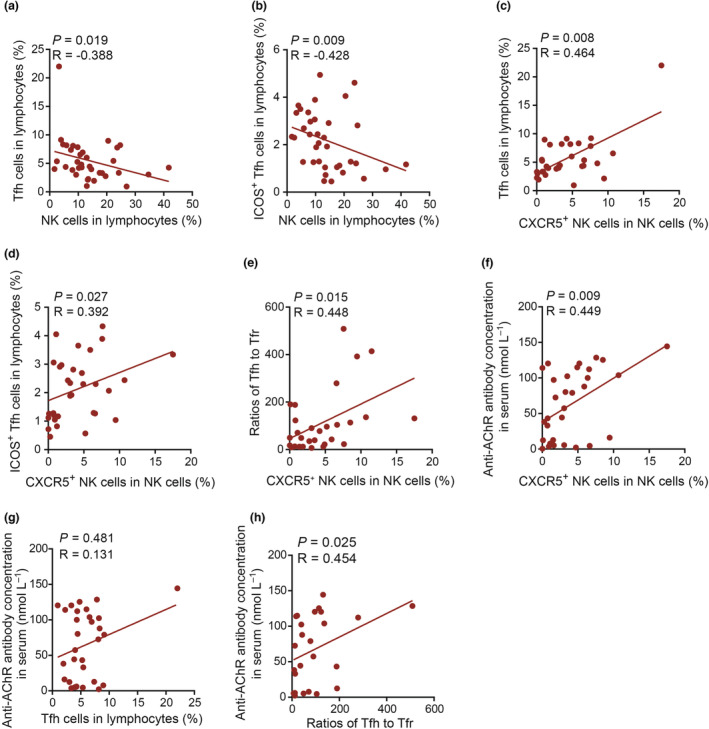
Circulating CXCR5+ NK cells were positively correlated with Tfh cells and serum AChRAb concentrations in patients with MG. (a, b) Correlation analysis of the percentages of NK cells and Tfh or ICOS+ Tfh cell among lymphocytes (n = 36). (c, d) Correlation analysis of the percentages of CXCR5+ NK cells among NK cells and the percentages of Tfh or ICOS+ Tfh cells among lymphocytes (n = 32). (e) Correlation analysis of the percentages of CXCR5+ NK cells among NK cells and Tfh/Tfr ratios (n = 29). (f) Correlation analysis of the percentages of CXCR5+ NK cells among NK cells and serum AChRAb concentrations (n = 32). (g) Correlation analysis of the percentages of Tfh cells with serum AChRAb concentrations (n = 31). (h) Correlation analysis of the Tfh‐to‐Tfr ratios with serum AChRAb concentrations (n = 25). The Spearman correlation test was used. A P‐value of < 0.05 was considered statistically significant.
CXCR5 + and CXCR5 − NK cells exert distinct effects on B cell differentiation
Our previous study illustrated the immunomodulatory effects of NK cells on Tfh cells. 6 , 18 However, the regulation of B cell function by NK cells remains elusive. To address this issue, mouse splenic B cells (CD3−CD19+) were purified and co‐cultured with NK cells (CD3−CD19−NK1.1+) in the presence of lipopolysaccharide (LPS), anti‐CD40 Ab, IL‐4 and IL‐15 for 4 days. According to our previous studies, these NK cells mainly contained CXCR5− NK cells (approximately 90%). As shown in Figure 6a, the surface levels of CD80 and PD‐L1 on B cells significantly increased after co‐culture with NK cells. NK cells dramatically inhibited plasmablast (CD19+CD138+) differentiation (Figure 6b). Consistently, IgG levels decreased in the supernatants of B cells co‐cultured with NK cells (Figure 6b). Interestingly, blocking IFN‐γ with monoclonal Ab reversed these effects (Figure 6a and b).
Figure 6.
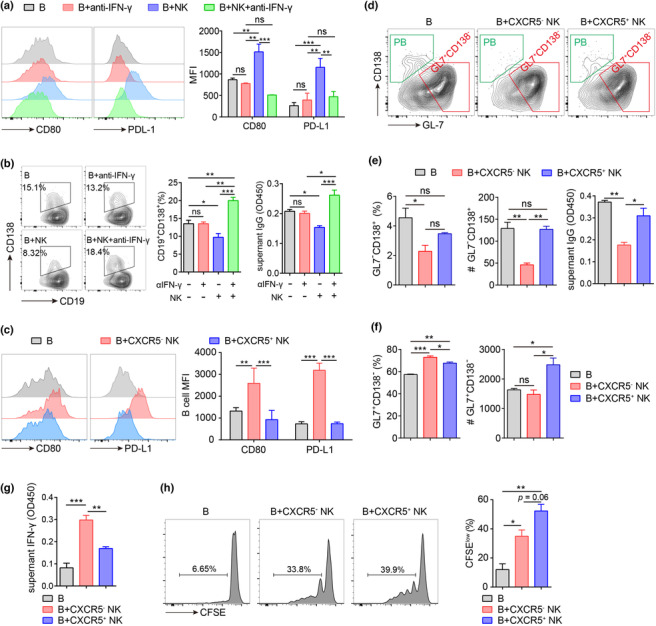
CXCR5+ NK promoted B cell proliferation more vigorously compared to CXCR5− NK cells. (a,b) Murine splenic B cells were cultured alone or with NK cells (B: NK = 5: 1) for 4 days in the presence or absence of IL‐4 (5 ng mL−1), anti‐CD40 Ab (1 μg mL−1), LPS (1 μg mL−1) and IL‐15 (20 ng mL−1) plus anti‐IFN‐γ Ab (4 μg mL−1). (a) Expression of CD80 and PDL‐1 was determined by flow cytometry. (b) Percentages of plasmablasts (CD19+CD138+) among B cells determined by flow cytometry, and supernatant IgG levels determined by ELISA. (c–h) Murine splenic B cells were cultured alone or with CXCR5− or CXCR5+ NK cells (B: NK = 5: 1) for 4 days in the presence of IL‐4 (5 ng mL−1), anti‐CD40 Ab (1 μg mL−1), LPS (1 μg mL−1) and IL‐15 (20 ng mL−1). (c) Expression of CD80 and PD‐L1 on B cells determined by flow cytometry. (d) Representative plots of activated B cells (CD19+GL7+CD138−) and plasmablasts (CD19+GL7−CD138+) among CD19+ B cells. (e) Percentages and cell number of plasmablasts (CD19+ GL7−CD138+) and the supernatant IgG levels (OD450) in different groups. (f) Percentages and cell number of activated B cells (CD19+GL7+CD138−) in different groups. (g) Supernatant IFN‐γ levels (OD450) in different groups. (h) Murine splenic B cells were stained with CFSE before co‐culturing with CXCR5− or CXCR5+ NK cells. Shown are the representative histograms and percentages of proliferative B cells (CFSElow). Data are shown from three independent experiments. MFI, mean fluorescent intensity; #, cell number; shown are the mean ± SEM values. One‐way analysis of variance was applied. *P < 0.05, **P < 0.01 and ***P < 0.001, ns, not significant.
According to CXCR5 expression, human peripheral NK cells can be divided into CXCR5+ and CXCR5− NK cells, which exhibit distinct phenotypes. Compared with that in CXCR5− NK cells, the expression level of IFN‐γ in CXCR5+ NK cells significantly decreased (Figure 2c), suggesting the possibility that CXCR5+ and CXCR5− NK cells might exert distinct effects on B cell differentiation. To address this issue, mouse splenic B cells were purified and co‐cultured with CXCR5+ and CXCR5− NK cells in the presence of LPS, anti‐CD40 Ab, IL‐4 and IL‐15 for 4 days. Interestingly, CXCR5− NK cells but not CXCR5+ NK cells stimulated the expression of CD80 and PD‐L1 on B cells (Figure 6c). Additionally, CXCR5− NK cells could more efficiently inhibit plasmablast cell differentiation compared with CXCR5+ NK cells, as lower percentage and number of plasmablasts were observed in CXCR5− NK cell groups (Figure 6d and e). Consistently, CXCR5− NK cells but not CXCR5+ NK cells suppressed Ab production of B cells (Figure 6e). Although both CXCR5+ and CXCR5− NK cells upregulated the percentage of CD19+CD138−GL‐7+ B cells, only CXCR5+ NK cells increased the absolute number of CD19+CD138−GL‐7+ B cells (Figure 6f). Indeed, the concentration of IFN‐γ was higher in CXCR5− NK cell culture supernatants than in CXCR5+ NK cell culture supernatants (Figure 6g). To determine why there were more CD19+CD138−GL‐7+ B cells when co‐cultured with CXCR5+ NK cells, we performed cell proliferating assays. Interestingly, B cells proliferated more vigorously when co‐cultured with CXCR5+ NK cells than with CXCR5− NK cells (Figure 6h). All of these results indicate that CXCR5− and CXCR5+ exert distinct effects on B cell function. CXCR5− NK cells could more efficiently inhibit plasmablast cell differentiation, while CXCR5+ NK cells are more likely to promote activated B cell expansion.
Discussion
Myasthenia gravis is an organ‐specific autoimmune disease mediated by Abs targeting AChR or related molecules at the neuromuscular junction. 1 Abnormal ectopic germinal centre formation in the thymus is traditionally considered to be involved in the pathogenesis of MG. 19 , 20 Dysregulation in immune tolerance, autoreactive B cell survival and activation and pathogenic autoantibody production have been proposed as the critical nodes in the onset of MG. 20 However, the mechanisms that drive and regulate ectopic germinal centre responses in the thymus that can lead to the development of autoreactive B cells and autoantibody production are not fully understood. NK cells are important innate immune lymphocytes equipped with potent cytolytic and immunoregulatory activities, accounting for 5–20% of peripheral blood lymphocytes in humans. 3 , 13 Although many efforts have been made, their roles in MG remain unclear. In this study, the percentages of NK cells and their subtypes in the peripheral blood of patients with MG were dysregulated compared to those of healthy volunteers. In addition, dysregulated NK cells returned to near‐normal levels when disease symptoms were controlled after medication. These results indicate that dysregulated NK cells and their subtypes may contribute to the pathogenesis of MG.
NK cells have been shown to either augment or inhibit humoral response. 5 , 6 , 7 Shi et al. 5 demonstrated that NK cells promoted humoral response by augmenting Th1 responses and suppressing TGF‐β production in mice with EAMG. In an LCMV infection model, NK cells inhibited Tfh cell differentiation and subsequent Ab production. 7 The inhibitory effects of NK cells on humoral response were replicated in alum‐adjuvanted hapten–protein conjugate vaccination 9 and rat EAMG model. 6 In these experimental settings, NK cells were thought to modulate the humoral response in a T cell‐dependent manner, through either Th1 or Tfh cells. 5 , 6 , 7 , 9 In this study, we assessed the direct modulation of NK cells on B cell activation and differentiation. When mouse B cells were isolated and co‐cultured with NK cells, plasmablast differentiation was significantly inhibited. However, some studies revealed augmented effects of NK cells on B lymphocyte differentiation and Ab production, whereas others showed the opposite results. 10 These discrepancies might be attributed to differences in the experimental methodology, which may affect both B cell and NK cell functions. Interestingly, when co‐cultured with NK cells, B cells exhibited more activated phenotypes, characterised by higher expression levels of CD80 and PD‐L1. These results indicate that NK cells may skew activated B cells from committing to plasmablasts to inflammatory effector B cells, a phenomenon mimicked by IFN‐γ under certain circumstances. 21
IFN‐γ is a multifaceted cytokine produced by diverse cells, including T, NK and dendritic cells. It can either augment or suppress autoimmunity, which is dependent on the specific disease and the timing, location and intensity of IFN‐γ. 22 IFN‐γ was initially reported as an inhibitor of B cell responses caused by inhibition of IgM production. 23 However, it can also induce B cell proliferation when stimulated with anti‐CD40 Ab. 23 IFN‐γ plays an indispensable role in TLR7‐accelerated SLE autoimmunity by promoting germinal centre response and autoantibody production, which involves signalling by the transcription factors STAT1 and T‐bet in B cells. 24 Indeed, IFN‐γ may exert different effects on B cell fate in different microenvironments. Stone et al. 21 demonstrated that IFN‐γ signalling not only induced Blimp1 expression in B cells but also initiated an inflammatory gene programme that, if not restrained, prevented the formation of Ab‐secreting cells. This reveals a finely tuned adaptation of the B cell response to IFN‐γ. NK cells are one of the major IFN‐γ‐producing cell subtypes, especially during the initial phase of the immune response. NK cell‐derived IFN‐γ has been proven to affect dendritic cell activation and T cell polarisation. 25 , 26 , 27 , 28 Interestingly, the percentage of IFN‐γ‐positive NK cells in MG blood samples was lower than that in control samples. When co‐cultured with B cells, IFN‐γ derived from NK cells suppressed plasmablast differentiation. These results indicate that a reduced number of IFN‐γ‐positive NK cells might contribute to the pathogenesis of MG.
IFN‐γ derived from NK cells also promotes CD80 and PD‐L1 expression in B cells. Current notions indicate that PD‐L1 negatively regulates Tfh cell differentiation and thus humoral immunity. 29 , 30 , 31 , 32 PD‐L1 expressed on follicular B cells suppresses the migration of Tfh cells to B cell follicles. 29 Furthermore, PD‐L1hi B cells were proposed as a new subtype of regulatory B cells apart from the traditional Breg cells characterised by IL‐10 production. 31 Adoptive transferring of these PD‐L1hi B cells reduced disease severity in EAE. 31 Based on these observations, it can be postulated that NK cells could also inhibit humoral response by driving B cells to express PD‐L1 in an IFN‐γ‐dependent manner. Whether PD‐L1 exerts negative feedback on B cell activation, proliferation or differentiation should also be determined.
NK cells are a heterogeneous cell subtype. Conventionally, human peripheral blood NK cells are divided into CD56bright and CD56dim NK cells based on the cell surface expression of CD56. 3 , 13 The CD56dim NK population is further divided into CD56dimCD57− and CD56dimCD57+ subsets according to differences in CD57 expression. 12 However, recent works by Horowitz et al. uncovered more heterogeneity in NK cell phenotypes than previously described. 11 With the discovery of new NK cell subsets, more questions are raised regarding the accurate function of each subset. Herein, we defined a novel subtype of NK cells in humans and mice characterised by the expression of CXCR5 and thus named CXCR5+ NK cells. CXCR5+ NK cells exhibit distinct phenotypes compared with CXCR5− NK cells, as they express higher levels of ICOS, PD‐1 and FASL and lower levels of ING‐γ, NKG2D, granzyme B and perforin. Patients with MG showed abnormal accumulation of CXCR5+ NK cells in the peripheral blood.
CXCR5 is a chemokine receptor initially identified to be expressed by B cells. Guided by the chemokine CXCL13, which is enriched in B cell follicles and the germinal centre, B cells are recruited to these territories. Along with the expanding research, an increasing number of cell subtypes have been found to express CXCR5, including but not limited to Tfh cells, 33 follicular helper natural killer T (NKT) cells, 34 , 35 Tfr cells 36 and follicular cytotoxic CD8+ T cells. 37 , 38 These CXCR5‐positive cell subtypes are equipped with the capacity to migrate to B cell follicles and germinal centres and modulate humoral response through sophisticated mechanisms. In germinal centres, activated B cells must integrate the complex array of microenvironmental cues provided by these CXCR5‐positive cell subtypes during the processes of class‐switch recombination, proliferation and differentiation. 21 For example, Tfh cells express high levels of ICOS and PD‐1 in addition to CXCR5. 33 Interaction of ICOS and ICOSL expressed by B cells promotes Ab affinity maturation and plasma cell differentiation. 39 Meanwhile, PD‐1 has been shown to regulate the survival and selection of B cells in the germinal centre and long‐lived plasma cell generation. 40
Similar to Tfh cells and follicular helper NKT cells, CXCR5+ NK cells also express high levels of ICOS and PD‐1, suggesting that this new NK cell subtype may also play critical roles in regulating humoral reactions. Interestingly, positive correlations between CXCR5+ NK cells and Tfh cells were observed in both patients with MG and EAMG rats, 6 which indicates that CXCR5+ NK cells may act as a positive modulator in humoral reactions. Essentially, CXCR5+ NK cells boost Tfh cell differentiation in vitro in our previous study. 6 Here, we found that CXCR5+ NK cells promoted B cell proliferation more vigorously than CXCR5− NK cells. Based on the similarity between CXCR5+ NK cells and other well‐characterised Tfh cell subsets with respect to both phenotype and function, we propose that CXCR5+ NK cells may be a new follicular helper subset that specialises in providing help to B cells or other immune cells localised in lymphoid follicles.
In conclusion, NK cells and their subtypes were disrupted in the peripheral blood of patients with MG. Different NK cell subtypes exhibit distinct effects on B cell differentiation, that is, CXCR5− NK cells suppress plasmablast differentiation while CXCR5+ NK cells could more efficiently promote B cell proliferation. CXCR5+ NK cells might be a new follicular helper subset specialised in providing help to B cells or other immune cells localised in lymphoid follicles. The identification of CXCR5+ NK cells as the modulator of B cell homeostasis might be of significance to research exploring potential intervening targets for humoral response in both vaccination and autoimmune diseases.
Methods
Patients
Patients with MG were recruited from either the outpatient or inpatient departments of the Department of Neurology, First Affiliated Hospital of Shandong First Medical University, between July 2018 and August 2020. Age‐ and sex‐matched volunteers from the physical examination centre were selected as controls. MG diagnosis was based on internationally recognised criteria. Exclusion criteria for patients were as follows: (1) history of other autoimmune disorders, except for the present MG; (2) chronic infections including hepatitis, HIV or tuberculosis; (3) severe systematic disease, such as tumors (hepatic or renal) or cardiac dysfunction and haematopoietic system diseases; (4) pregnant and lactating; (5) received blood purification therapy or intravenous immunoglobulin treatment within 3 months before the enrolment; and (6) age < 18 years or > 75 years.
Patients were divided into the following groups: aggravated MG, remitted MG and control. The inclusion criterion for the aggravated MG group was patients who complained of apparent aggravation or relapse of myasthenia symptoms, and the QMG scores of patients with generalised MG (GMG) and ocular MG (OMG) were greater than 10 and 3, respectively. In the remitted MG group, patients reported significant relief in symptoms and simultaneously achieved a QMG score of < 8 for patients with GMG and 1 for patients with OMG. The inclusion criteria for the control group were subjects with no history of autoimmune disease, no long‐term use of immune suppressors/regulators or glucocorticoid hormones and no acute events such as acute myocardial infarction or acute cerebral infarction within the past 6 months.
The sample sizes were determined as described in our previous study. In brief, the formula used was as follows: , where n is the minimum sample size required, , , respectively, represent the mean and standard deviation of the ‐th group; , is the number of groups; , and ‐values are available from the ‐value table for the sample size estimates of multi‐sample comparison. The sample size was calculated only for the primary outcome, and 8 was calculated to obtain the minimal sample size.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University. All enrolled patients were informed of the study protocol.
Demographic and clinical information collection
Demographic and clinical data were collected from medical records and self‐reports. A total of 52 subjects were included in this study. Of these, 20 patients were included in the aggravated MG group (11 males and 9 females, mean age 53.20 ± 14.88 years), 13 in the remitted MG group (7 males and 6 females, mean age 52.85 ± 13.99 years) and 19 in the control group (12 males and 7 females, mean age 45.79 ± 12.82 years). No significant differences in age or sex were observed among the three groups (P > 0.05). MG subtyping was based on the clinical data of age of onset, pattern of muscle involvement, thymic pathology and the modified Osserman classification. Detailed demographic and clinical information are shown in Table 1.
Clinical evaluation of patients with MG
The severity of illness was assessed using the QMG scores issued by the MG Foundation of America in 2000.
Sample processing
Venous blood samples were collected in ethylenediaminetetraacetic acid anticoagulant tubes (BD Biosciences, CA, USA) and coagulation‐promoting tubes (BD Biosciences). Peripheral blood mononuclear cells (PBMCs) were isolated from anticoagulated blood via Ficoll density gradient centrifugation (Dakewe, Shenzhen, China) and washed twice with phosphate‐buffered saline (PBS) within 3 h. The blood in the coagulation‐promoting tube was allowed to stand for 1 h at room temperature and then centrifuged to extract serum.
Immunostaining and flow cytometry analysis
For cell surface marker staining, fresh isolated human PBMCs were incubated with 0.5% bovine serum albumin (Sigma‐Aldrich, MO, USA) in PBS for 5 min and stained in the dark at 4°C for 30 min with different combinations of the following antibodies: BV421‐anti‐CD3 (UCHT1; BD Biosciences), peridinin chlorophyll protein (PerCP)/Cyanine5.5‐anti‐CD4 (RPA‐T4; eBioscience, CA, USA), allophycocyanin (APC)‐anti‐CXCR5 (MU5UBEE; eBioscience), fluorescein‐5‐isothiocyanate (FITC)‐anti‐CD4 (RPA‐T4; BioLegend, CA, USA), phycoerythrin (PE)/Cy7‐anti‐CXCR5 (J252D4; BioLegend), PE/Cy7‐anti‐ICOS (C398.4A; BioLegend), PE‐anti‐PD‐1 (EH12.2H7; BioLegend), PerCP/Cyanine5.5‐anti‐CD19 (HIB19; BioLegend), FITC‐anti‐CD56 (MEM‐188; BioLegend, CA, USA), PE‐anti‐FasL (NOK‐1; BioLegend) and APC‐anti‐NKG2D (1D11; BioLegend). Appropriate isotype controls were used as negative controls to ensure the accuracy of the results.
For intracellular cytokine staining, PBMCs were stimulated with a cell stimulation cocktail plus protein transport inhibitors (eBioscience), which contains phorbol 12‐myristate 13‐acetate, ionomycin, Brefeldin A and monensin, in cell culture medium for 4 h at 37°C. The stimulated cells were then collected and washed, and surface markers were stained as described above. Paraformaldehyde (2%) and a permeabilisation wash buffer (eBioscience) were used for cell fixation and permeabilisation, respectively, according to the manufacturer's instructions. Thereafter, PE‐anti‐IFN‐γ (4S.B3; eBioscience), PE‐anti‐IL‐17 (eBio64CAP17; eBioscience), PE‐anti‐IL‐10 (JES3‐9D7; BioLegend), PE‐anti‐granzyme B (GB11; BD Biosciences) and APC‐anti‐perforin (dG9; BioLegend) were added and incubated for 30 min at 4°C to detect the respective antigen.
For intracellular Foxp3 staining, the cells were stained with CD4 and CXCR5 antibodies, fixed and permeabilised with Foxp3‐staining buffer set (eBioscience) and incubated with PE‐anti‐Foxp3 (150D; BioLegend) for 40 min at room temperature. Data were acquired via flow cytometry using FACSAria II/III (BD Biosciences) and analysed using FlowJo v10 (FlowJo, LLC).
Cell sorting and co‐culture
C57BL/6J mice were purchased from the Vital River Corporation (Beijing, China) and maintained under specific pathogen‐free conditions. All procedures were approved by the Ethics Committee of First Affiliated Hospital of Shandong First Medical University. To isolate NK and B cells, mouse spleen was minced through a 70‐ μm cell strainer and lysed with red blood cell lysis buffer to obtain a mononuclear cell suspension. Staining was then performed with the following monoclonal antibodies: PerCP anti‐mouse CD19 (BioLegend), APC anti‐mouse CD3 (BioLegend), FITC anti‐mouse NK1.1 (BioLegend) and PE/Cy7 anti‐mouse CXCR5 (eBioscience). NK, CXCR5− NK, CXCR5+ NK and B cells were purified via fluorescence‐activated cell sorting (FACS). The purity of each subset was routinely greater than 90%, as confirmed by flow cytometry.
For co‐culture assay, purified B cells (2 × 105) were co‐cultured with NK, CXCR5− NK or CXCR5+ NK cells (4 × 104) in 96‐well plates containing RPMI1640 supplemented with 10% heat‐inactivated foetal bovine serum (Gibco, Invitrogen, CA, USA) and 1% penicillin–streptomycin (Gibco) and incubated in a 5% CO2 humidified incubator at 37 °C. LPS (1 μg mL−1; Sigma‐Aldrich), anti‐mouse CD40 Ab (1 μg mL−1; 1C10; Invitrogen), mouse IL‐4 (5 ng mL−1; PeproTech, NJ, USA) and mouse IL‐15 (20 ng mL−1; PeproTech) were added to the culture medium to activate B or NK cells. To block IFN‐γ, 4 μg mL−1 neutralising anti‐IFN‐γ Ab (XMG1.2; BioLegend) was added to the culture medium. After culturing for the indicated times, the cells were harvested and stained with the following antibodies: PerCP‐anti‐mouse CD19 (6D5; BioLegend), PE‐anti‐mouse CD80 (16‐10A1; BioLegend), APC‐anti‐mouse PDL‐1 (10F.9G2; BioLegend), AF488‐anti‐mouse GL7 (GL7; BioLegend) and APC‐anti‐mouse (281–2; BioLegend). Data were acquired by flow cytometry using a FACSAria III (BD Biosciences) and analysed using FlowJo v10 (FlowJo).
Enzyme‐linked immunosorbent assay (ELISA)
Serum AChRAb concentrations were quantified by ELISA using a commercial AChR Autoantibody ELISA Kit (RSR Ltd., Cardiff, UK). Briefly, AChRAbs in patient serum bind to similar sites on the AChR (coated on ELISA plate wells) as various reconstituted AChR monoclonal antibodies (rAChR MAb, labelled with biotin). A competitive inhibition reaction occurred between AChRAb and rAChR mAb‐biotin with the pre‐coated AChR. Biotin‐specific conjugates and streptavidin peroxidase were then added to each well. Tetramethylbenzidine was added after incubation and extensive washing. The reaction was terminated using a stop solution, and the absorbance at 450 nm was measured using an ELISA plate reader. The higher the concentration of AChRAb in the test serum, the greater the inhibition of rMAb‐biotin binding, resulting in a reduced final absorbance at 450 nm. The AChR Ab levels of patient samples were obtained after setting the negative control value to 0.2 nmol L−1 and constructing a standard curve.
Statistical analysis
Statistical analyses were performed using SPSS (version 25.0; SPSS Inc., IBM, USA), and figures were drawn using GraphPad Prism 7.0 (GraphPad Software Inc., USA). The independent samples t‐test and the Mann–Whitney U‐test were applied to normally and non‐normally distributed data respectively. For paired samples, a parametric paired t‐test or a nonparametric Wilcoxon test was used according to the data distribution. Categorical variables were analysed using the chi‐square (χ 2 ) test. Pearson's or Spearman's correlation tests were used to determine correlations. All results are expressed as the mean ± standard error of the mean (SEM), and statistical significance was set at P < 0.05.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82071345, 82101421), Academic Promotion Programme of Shandong First Medical University (2019QL013) and Natural Science Foundation of Shandong Province, China (ZR2020MH142).
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Meng‐Ru Ge: Data curation; formal analysis; investigation; methodology; project administration; resources; software; validation; visualization; writing – original draft; writing – review and editing. Chun‐Lin Yang: Data curation; formal analysis; investigation; methodology; resources; software; validation; visualization; writing – original draft; writing – review and editing. Tao Li: Data curation; formal analysis; investigation; methodology; visualization. Tong Du: Data curation; formal analysis; investigation; methodology. Peng Zhang: Data curation; formal analysis; investigation; methodology. Xiao‐Li Li: Data curation; investigation; methodology. Ying‐Chun Dou: Resources; supervision; validation; writing – review and editing. Rui‐Sheng Duan: Conceptualization; formal analysis; funding acquisition; project administration; resources; supervision; writing – original draft; writing – review and editing.
Supporting information
Supplementary figure 1
Data availability statement
All data will be made available by the corresponding author upon reasonable request.
References
- 1. Gilhus NE. Myasthenia gravis. N Engl J Med 2016; 375: 2570–2581. [DOI] [PubMed] [Google Scholar]
- 2. Carr AS, Cardwell CR, McCarron PO, McConville J. A systematic review of population based epidemiological studies in myasthenia gravis. BMC Neurol 2010; 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kucuksezer UC, Aktas Cetin E, Esen F et al. The role of natural killer cells in autoimmune diseases. Front Immunol 2021; 12: 622306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hertwig L, Hamann I, Romero‐Suarez S et al. CX3CR1‐dependent recruitment of mature NK cells into the central nervous system contributes to control autoimmune neuroinflammation. Eur J Immunol 2016; 46: 1984–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi FD, Wang HB, Li H et al. Natural killer cells determine the outcome of B cell‐mediated autoimmunity. Nat Immunol 2000; 1: 245–251. [DOI] [PubMed] [Google Scholar]
- 6. Yang CL, Zhang P, Liu RT et al. CXCR5‐negative natural killer cells ameliorate experimental autoimmune myasthenia gravis by suppressing follicular helper T cells. J Neuroinflammation 2019; 16: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol 2015; 98: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H. Mobilization of natural killer cells inhibits development of collagen‐induced arthritis. Proc Natl Acad Sci USA 2011; 108: 14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rydyznski CE, Cranert SA, Zhou JQ et al. Affinity maturation is impaired by natural killer cell suppression of germinal centers. Cell Rep 2018; 24: 3367–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Commes T, Clofent G, Jourdan M, Bataille R, Klein B. Human natural killer cells suppress the proliferation of B cells. Immunol Lett 1990; 24: 57–61. [DOI] [PubMed] [Google Scholar]
- 11. Horowitz A, Strauss‐Albee DM, Leipold M et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013; 5: 208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez‐Verges S, Milush JM, Pandey S et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK‐cell subset. Blood 2010; 116: 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Day J, Souza‐Fonseca Guimaraes F, Wicks IP, Louis C. Natural killer cells in inflammatory autoimmune diseases. Clin Transl Immunol 2021; 10: e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higuma‐Myojo S, Sasaki Y, Miyazaki S et al. Cytokine profile of natural killer cells in early human pregnancy. Am J Reprod Immunol 2005; 54: 21–29. [DOI] [PubMed] [Google Scholar]
- 15. Mehrotra PT, Donnelly RP, Wong S et al. Production of IL‐10 by human natural killer cells stimulated with IL‐2 and/or IL‐12. J Immunol 1998; 160: 2637–2644. [PubMed] [Google Scholar]
- 16. Huang Y, Chen Z, Wang H et al. Follicular regulatory T cells: a novel target for immunotherapy? Clin Transl Immunol 2020; 9: e1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Y, Zou L, Liu YC. T follicular helper cells, T follicular regulatory cells and autoimmunity. Int Immunol 2016; 28: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu RT, Li W, Guo D et al. Natural killer cells promote the differentiation of follicular helper T cells instead of inducing apoptosis in myasthenia gravis. Int Immunopharmacol 2021; 98: 107880. [DOI] [PubMed] [Google Scholar]
- 19. Cron MA, Maillard S, Villegas J et al. Thymus involvement in early‐onset myasthenia gravis. Ann N Y Acad Sci 2018; 1412: 137–145. [DOI] [PubMed] [Google Scholar]
- 20. Lefeuvre CM, Payet CA, Fayet OM et al. Risk factors associated with myasthenia gravis in thymoma patients: the potential role of thymic germinal centers. J Autoimmun 2020; 106: 102337. [DOI] [PubMed] [Google Scholar]
- 21. Stone SL, Peel JN, Scharer CD et al. T‐bet transcription factor promotes antibody‐secreting cell differentiation by limiting the inflammatory effects of IFN‐γ on B cells. Immunity 2019; 50: 1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu X, Ivashkiv LB. Cross‐regulation of signaling pathways by interferon‐γ: implications for immune responses and autoimmune diseases. Immunity 2009; 31: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vazquez MI, Catalan‐Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015; 74: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chodisetti SB, Fike AJ, Domeier PP et al. Type II but not type I IFN signaling is indispensable for TLR7‐promoted development of autoreactive B cells and systemic autoimmunity. J Immunol 2020; 204: 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ge MQ, Ho AW, Tang Y et al. NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza a infection by IFN‐γ and perforin‐dependent mechanisms. J Immunol 2012; 189: 2099–2109. [DOI] [PubMed] [Google Scholar]
- 26. Lopez‐Yglesias AH, Burger E, Camanzo E et al. T‐bet‐dependent ILC1‐ and NK cell‐derived IFN‐γ mediates cDC1‐dependent host resistance against Toxoplasma gondii . PLoS Pathog 2021; 17: e1008299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walwyn‐Brown K, Guldevall K, Saeed M et al. Human NK cells lyse Th2‐polarizing dendritic cells via NKp30 and DNAM‐1. J Immunol 2018; 201: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryan EJ, Harenberg A, Burdin N. The canarypox‐virus vaccine vector ALVAC triggers the release of IFN‐γ by natural killer (NK) cells enhancing Th1 polarization. Vaccine 2007; 25: 3380–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD‐1 controls follicular T helper cell positioning and function. Immunity 2018; 49: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cubas RA, Mudd JC, Savoye AL et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 2013; 19: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD‐L1hi B cells are critical regulators of humoral immunity. Nat Commun 2015; 6: 5997. [DOI] [PubMed] [Google Scholar]
- 32. Buermann A, Romermann D, Baars W, Hundrieser J, Klempnauer J, Schwinzer R. Inhibition of B‐cell activation and antibody production by triggering inhibitory signals via the PD‐1/PD‐ligand pathway. Xenotransplantation 2016; 23: 347–356. [DOI] [PubMed] [Google Scholar]
- 33. Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 2019; 50: 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang PP, Barral P, Fitch J et al. Identification of Bcl‐6‐dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol 2011; 13: 35–43. [DOI] [PubMed] [Google Scholar]
- 35. Tonti E, Fedeli M, Napolitano A et al. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4+ T cell help. J Immunol 2012; 188: 3217–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fonseca VR, Ribeiro F, Graca L. T follicular regulatory (Tfr) cells: dissecting the complexity of Tfr‐cell compartments. Immunol Rev 2019; 288: 112–127. [DOI] [PubMed] [Google Scholar]
- 37. Yu D, Ye L. A portrait of CXCR5+ follicular cytotoxic CD8+ T cells. Trends Immunol 2018; 39: 965–979. [DOI] [PubMed] [Google Scholar]
- 38. Chen Y, Yu M, Zheng Y et al. CXCR5+PD‐1+ follicular helper CD8 T cells control B cell tolerance. Nat Commun 2019; 10: 4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu D, Xu H, Shih C et al. T‐B‐cell entanglement and ICOSL‐driven feed‐forward regulation of germinal Centre reaction. Nature 2015; 517: 214–218. [DOI] [PubMed] [Google Scholar]
- 40. Good‐Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD‐1 regulates germinal center B cell survival and the formation and affinity of long‐lived plasma cells. Nat Immunol 2010; 11: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1
Data Availability Statement
All data will be made available by the corresponding author upon reasonable request.


