Abstract
Introduction
Genetic variation in Cytochrome P450 2A6 (CYP2A6), the major nicotine metabolizing enzyme, is associated with nicotine dependence and smoking cessation. Nicotine dependence severity also predicts smoking cessation. Our goals were to determine how CYP2A6 variation and nicotine dependence alter smoking cessation, and whether dependence could refine CYP2A6-based treatment recommendations.
Aims and Methods
Adult smokers treated for 12 weeks with placebo, nicotine patch, or varenicline (NCT01314001) were grouped as CYP2A6 normal (n = 567) or slow (n = 432) nicotine metabolizers based on a CYP2A6 weighted genetic risk score. Fagerström test for nicotine dependence scores were measured at baseline and biochemically verified smoking cessation was assessed at end of treatment.
Results
Dependence neither mediated nor moderated an association between CYP2A6 variation and smoking cessation overall, within any treatment arm, or after stratifying by ancestry (n = 591 European, n = 408 African ancestry) or sex (n = 444 women, n = 555 men). In within-treatment analyses, the mediation effect odds ratio (OR) ranged from 0.95 to 1.00 and the bias-corrected 95% confidence interval contained 1. Moderation (i.e. interaction) effect ORs ranged from 0.88 to 1.61 (p = .397–.828). For CYP2A6 normal metabolizers, quit rates on varenicline were similar for those with high (41.1%) and low (43.4%) dependence, while quit rates were lower for those with high versus low dependence on both patch (16.5 vs. 29.7%) and placebo (8.9 vs. 18.5%). CYP2A6 slow metabolizers with high versus low dependence had lower quit rates in all three treatment arms.
Conclusions
Although nicotine dependence severity neither mediated nor moderated CYP2A6 associations with smoking cessation, incorporating information on dependence may optimize the choice of smoking cessation treatment aid in CYP2A6 normal and slow metabolizers.
Implications
Variation in CYP2A6 and nicotine dependence severity alter smoking cessation success. Our findings suggest that while nicotine dependence severity is unlikely to mediate or moderate CYP2A6 associations with cessation, incorporating patient information on both CYP2A6 and nicotine dependence severity may lead to improved smoking cessation strategies.
Introduction
Nicotine is the major psychoactive compound in cigarettes; it promotes dependence and chronic smoking.1 While ~70% of smokers express a desire to quit, many attempts are often required before successful smoking cessation is achieved.2 Twin studies show a 50%–60% heritability estimate for smoking cessation.3 Genetic variation in Cytochrome P450 2A6 (CYP2A6), which encodes the major nicotine-inactivating enzyme, is associated with the rate of nicotine clearance and numerous smoking behaviors including smoking cessation outcomes.4 CYP2A6 inactivates nicotine to cotinine5 and further metabolizes cotinine to 3ʹhydroxycotinine.6 The nicotine metabolite ratio (NMR; ratio of 3ʹhydroxycotinine to cotinine) is a biomarker of CYP2A6 activity, where higher NMR indicates faster CYP2A6 activity and nicotine clearance.7 Smokers with normal (vs. slow) CYP2A6 activity, measured by CYP2A6 genetics or the NMR biomarker, smoke more cigarettes, inhale more deeply, experience greater nicotine reinforcement, and have a higher risk for lung cancer.4,8–11 Variation in CYP2A6 activity is also associated with smoking cessation outcomes; for example, normal (vs. slow) metabolizers have lower quit rates on the nicotine patch,12,13 but show relatively higher quit success on varenicline (vs. nicotine patch).14
Nicotine dependence severity is also a robust predictor of smoking cessation outcomes; in general, greater nicotine dependence severity is associated with reduced cessation success.15 Moreover, variation in CYP2A6 and the NMR is associated with nicotine dependence in some studies,8,11,16 and genetic variation may interact with nicotine dependence severity to alter smoking cessation outcomes.15 Smoking cessation outcomes also differ by ancestry and by sex/gender. For example, compared to European ancestry (EA) smokers, African ancestry (AA) smokers have lower smoking cessation success.17 In terms of sex and/or gender differences, compared to placebo, women have relatively lower quit success on bupropion and the nicotine patch versus men, but equivalent quit success on varenicline.18
We recently created ancestry-specific CYP2A6 weighted genetic risk scores (wGRSs) in EA and in AA smokers by combining previously characterized and novel CYP2A6 variants discovered in genome-wide association studies of the NMR (>95% of hits mapped to the CYP2A6 locus on chromosome 19).19,20 These wGRSs capture ~30%–35% of the variability in the NMR and replicate NMR-based associations with smoking cessation treatment outcomes in both EA and AA smokers.19,20
Although there are associations between CYP2A6 variation and nicotine dependence,8,11,16CYP2A6 variation and smoking cessation,12–14,21 and nicotine dependence and smoking cessation,15 the mechanism(s) by which CYP2A6 variation and nicotine dependence alter smoking cessation is unclear; here we investigated mediation and moderation as two potential mechanisms in EA and AA smokers. To advance our goal of developing personalized smoking cessation approaches, we additionally examined whether information on nicotine dependence severity could be used to refine CYP2A6-based treatment recommendations.
Methods
Study Participants
This was a secondary analysis of data from the pharmacogenetics of nicotine addiction treatment-2 clinical trial (NCT01314001) in 1246 adult smokers (≥10 cigarettes/day) randomized 1:1:1 to placebo, nicotine patch, or varenicline based on their NMR, which was measured from blood samples collected at trial baseline.14 All participants received behavioral counseling. The current analyses were restricted to individuals of EA and AA ancestry, which was determined using multidimensional scaling in combination with HapMap 3 data and visualization; genetic ancestries were >95% concordant with self-reported ancestries.22,23 Sex was determined using information from the X and Y chromosomes.22,23 Participants who have provided written informed consent for DNA collection and genotyping were analyzed, for a final analytic sample of n = 999 participants. Procedures were approved by institutional review boards (IRBs) at all clinical sites and at the University of Toronto.14
CYP2A6 wGRS Determination and Grouping Strategy
A CYP2A6 wGRS comprising a combination of selected known functional CYP2A6 alleles and variants tagging independent signals from genome-wide association studies of the NMR was previously constructed separately for EA and AA participants.19,20 An individual’s overall CYP2A6 wGRS was computed by summing the number of effect alleles weighted by their unstandardized effects on the NMR.19,20 Participants were grouped as normal or slow CYP2A6 metabolizers based on a CYP2A6 wGRS cut point of 2.140 in EA (normal: wGRS ≥2.140; slow: wGRS <2.140) and 2.089 in AA (normal: wGRS ≥2.089; slow: wGRS <2.089) which optimally dichotomizes normal and slow CYP2A6 metabolizers; the wGRSs replicated NMR-based clinical outcomes alone or with both ancestries together.20
Measurement of Nicotine Dependence and Smoking Cessation
Nicotine dependence was measured at baseline using the Fagerström test for nicotine dependence (FTND; possible score range: 0–10), with higher scores indicating greater severity of dependence. Biochemically verified (carbon monoxide ≤8 ppm = abstinent; carbon monoxide >8 ppm = still smoking) 7-day point prevalence abstinence was assessed at week 12 (i.e. end of treatment).14 Participants were analyzed as intention to treat; those with carbon monoxide values >8 ppm and those lost to follow-up were considered to be smoking.14
Mediation Path Analysis
We assessed an indirect effect of the CYP2A6 wGRS (coded as 0 = slow metabolism, 1 = normal metabolism) on smoking cessation (coded as 1 = quit, 0 = still smoking) through raw FTND score. The indirect effect is the product of two unstandardized linear regression coefficients: The first is the effect of X (CYP2A6 wGRS group) on the mediator (FTND score) (i.e. path a), and the second is the effect of the mediator on Y (smoking cessation) (i.e. path b) in a model controlling for X. The direct effect measured the effect of X on Y (i.e. path c’) after controlling for the mediation effect (a*b) and covariates. We analyzed the three treatment arms together (controlling for ancestry, sex, age, and treatment arm) and separately (controlling for ancestry, sex, and age). We also conducted within-treatment analyses stratified by ancestry (controlling for sex and age) and by sex (controlling for ancestry and age). Model #4 in PROCESS (version 4.0; written by Dr. Andrew Hayes), a macro implementation of moderation and mediation analyses for statistical package for social sciences (SPSS) (version 27; Armonk, NY: IBM Corp.), was used for analyses.24 A bias-corrected bootstrapping method was used to model the indirect effect and 10 000 replications were used to estimate the 95% confidence intervals. The total effect (path c) was calculated separately in SPSS using logistic regression models (controlling for covariates). Analysis code is available upon request.
Moderation (i.e. Interaction) Analysis
We also tested whether nicotine dependence severity interacts with the CYP2A6 wGRS to influence smoking cessation (coded as 1 = quit, 0 = still smoking) using logistic regression. In the overall analysis, the main effects of CYP2A6 wGRS (coded as 0 = slow metabolism, 1 = normal metabolism), FTND (dichotomized as low and high dependence: ≤5 vs. >5, coded as 0 and 1, respectively), and treatment (coded as 0 = placebo, 1 = nicotine patch, and 2 = varenicline), as well as, all 2-way interaction terms and the 3-way interaction term, were included. Models controlled for ancestry, sex, and age. We also examined possible CYP2A6 wGRS*FTND interactions in the three treatment arms separately. To identify potential subgroup differences, we performed ancestry- and sex-stratified analyses.
We also examined potential interactions between nicotine dependence severity and treatment on smoking cessation (coded as 1 = quit, 0 = still smoking) within CYP2A6 normal and slow metabolizers using logistic regression. Models included main effects of FTND (dichotomized as low and high dependence: ≤5 vs. >5, coded as 0 and 1, respectively) and treatment (coded as 0 = placebo, 1 = nicotine patch, and 2 = varenicline), and the treatment*FTND interaction term. Covariates included ancestry, sex, and age.
Mediation and Moderation Analyses Using the Heaviness of Smoking Index
In smokers screened for participation in this trial, the NMR (CYP2A6 activity biomarker) was shown to be associated with another measure of nicotine dependence, the heaviness of smoking index (HSI).25 Thus, we also tested whether the HSI mediates or moderates associations between CYP2A6 variation and smoking cessation. The HSI (possible score range: 0–6) was calculated by summing two FTND items: Time to first cigarette and cigarettes/day.25 HSI was used as the mediator (raw HSI score) in an analysis of the overall group (controlling for ancestry, sex, age, and treatment arm), and as the moderator (dichotomized as low and high dependence: ≤3 vs. >3, coded as 0 and 1, respectively) in analyses conducted in the three treatment arms separately (controlling for ancestry, sex, and age).
Results
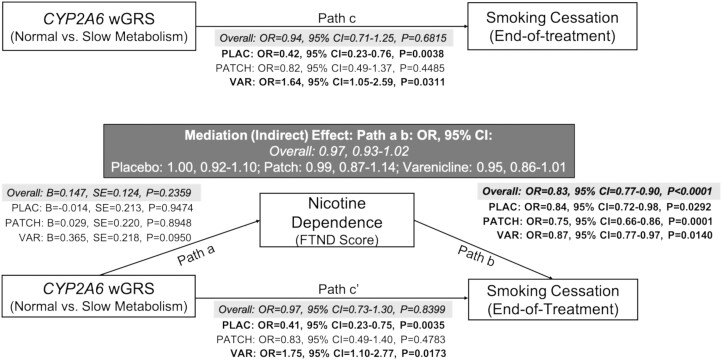
Characteristics of the final analytic sample are shown in Supplementary Table S1. We found no support for a mediating effect of FTND on an association between the CYP2A6 wGRS and smoking cessation in the overall group, within any of the treatment arms, or when stratifying by ancestry or sex (Figure 1; Supplementary Table S2). Similarly, we found no evidence that FTND moderates an association between the CYP2A6 wGRS and smoking cessation in the overall group, within any of the treatment arms, or when stratifying by ancestry or sex (Supplementary Figure S1, Table S3). Substituting HSI for FTND did not yield a significant mediating effect in the overall group (Indirect effect: Path a b: OR = 0.96, 95% CI = 0.90, 1.01). Similarly, no moderating effects of HSI have been observed: The CYP2A6 wGRS*HSI interaction ORR on cessation was 1.30 (95% CI = 0.47 to 3.61), 1.05 (95% CI = 0.34 to 3.23), and 0.72 (95% CI = 0.22 to 2.34) in the varenicline, nicotine patch, and placebo arms, respectively.
Figure 1.
Nicotine dependence severity did not mediate an association between the CYP2A6 wGRS and smoking cessation at end of treatment in the PNAT2 clinical trial. This model shows the relationship between the CYP2A6 weighted genetic risk score and smoking cessation success at end-of-treatment via nicotine dependence severity measured at baseline using FTND. The (a) pathway shows the association between the CYP2A6 wGRS and FTND score. The (b) pathways show the association between FTND score and smoking cessation success. The mediating effect of FTND on smoking cessation success is shown in the (a b) pathway (i.e. indirect effect pathway) and is shaded in dark gray. The (c) pathway shows the direct effect of the CYP2A6 wGRS on smoking cessation success adjusted for covariates, while the (cʹ) pathway shows the direct effect of the CYP2A6 wGRS on smoking cessation success adjusted for covariates and the indirect effect. Statistically significant effects (at p < .05) are bolded. Ancestry, sex, and age were included as covariates in the treatment-stratified analyses (n = 320 PLAC; n = 332 PATCH; n = 347 VAR). The overall analysis (n = 999) additionally controlled for treatment; results are shaded in light gray. Abbreviations: wGRS = weighted genetic risk score, FTND = Fagerström Test for Nicotine Dependence, OR = Odds Ratio, CI = confidence interval, PLAC = Placebo, VAR = Varenicline.
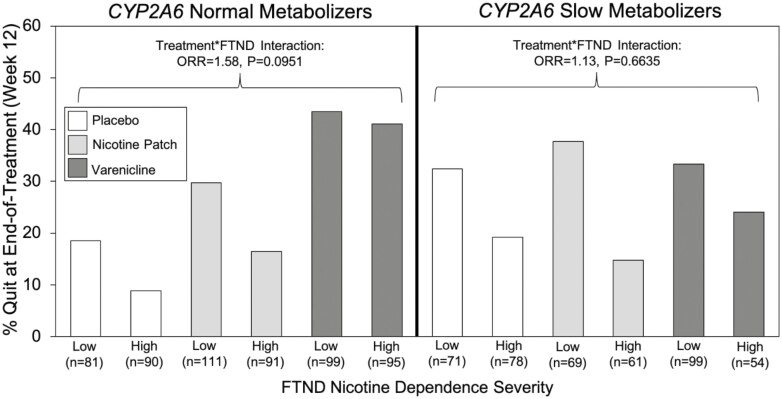
Within CYP2A6 normal metabolizers, there were significant main effects of treatment and FTND on cessation, as well as a trending treatment*FTND interaction effect (Supplementary Table S4, Figure 2). For CYP2A6 normal metabolizers, the overall quit rates on varenicline, nicotine patch, and placebo were 42.3%, 23.8%, and 13.5%, respectively. Varenicline was similarly efficacious in those with high (41.1%) and low (43.4%) dependence, while quit rates were lower for those with high versus low dependence on both patches (16.5 vs. 29.7%) and placebo (8.9 vs. 18.5%) (Figure 2, Supplementary Figure S1). Within CYP2A6 slow metabolizers, there was a significant main effect of FTND on cessation, but no main effect of treatment or treatment*FTND interaction effect (Supplementary Table S4, Figure 2). Within CYP2A6 slow metabolizers, the overall quit rates on varenicline, nicotine patch, and placebo were 30.1%, 26.9%, and 25.5%, respectively. Quit rates were lower for those with high versus low dependence on varenicline (24.1 vs. 33.3%), nicotine patch (14.8 vs. 37.7%), and placebo (19.2 vs. 32.4%) (Figure 2, Supplementary Figure S1).
Figure 2.
There was a trending interaction effect between treatment and nicotine dependence severity on smoking cessation at end-of-treatment in CYP2A6 normal metabolizers from the PNAT2 clinical trial. Full results from the moderation analysis are available in Supplementary Table S4. Abbreviations: FTND = Fagerström Test for Nicotine Dependence; ORR = ratio of odds ratios.
Discussion
In this secondary analysis of data from the pharmacogenetics of nicotine addiction treatment-2 (PNAT2) clinical trial, we found no evidence that nicotine dependence severity mediates or moderates associations between CYP2A6 and smoking cessation success overall, on varenicline, on nicotine patches, or on placebo. While we did not detect a significant association between the CYP2A6 wGRS and nicotine dependence in this sample, the effect was in the anticipated direction (i.e. normal CYP2A6 metabolizers had higher FTND (β = 0.147) and HSI (β = 0.125) scores). The inconsistent association between the NMR (CYP2A6 activity biomarker) and dependence in clinical samples may be because of the selection of a more highly dependent sample with modest variation in nicotine dependence scores. Our study also tested whether information on baseline nicotine dependence severity could refine smoking cessation treatment recommendations for CYP2A6 normal and slow metabolizers. In this trial, we previously showed that varenicline was more efficacious than nicotine patches for normal, but not slow, metabolizers.14 Our current findings support the use of varenicline as a frontline cessation therapy in CYP2A6 normal metabolizers regardless of dependence level (Figure 2). Within CYP2A6 slow metabolizers, quit rates on placebo (i.e. behavioral counseling), nicotine patch, and varenicline was similar, but consistently lower in those with high (vs. low) dependence. In general, CYP2A6 slow metabolizers experience a more favorable side effect profile with nicotine patch therapy compared to varenicline.14 Together these findings support the use of behavioral counseling with or without nicotine patch therapy in CYP2A6 slow metabolizers; however, those with high dependence would likely benefit from additional cessation support.
Strengths of our study include the use of both mediation and moderation modeling, the use of two measures of nicotine dependence (FTND and HSI), sex- and ancestry-stratified analyses, and the evaluation of multiple cessation treatments. Our mediation models satisfied the temporality requirement, as CYP2A6 genotype is determined before birth, nicotine dependence severity was measured at the trial baseline, and abstinence was measured at 12 weeks. A limitation of our study is the relatively small sample for subgroup analyses. Future studies could incorporate rare functional CYP2A6 variants, which improves the prediction of CYP2A6 metabolizer status particularly among individuals of AA.26
In conclusion, we have provided preliminary evidence suggesting that nicotine dependence severity is unlikely to mediate or moderate associations between CYP2A6 variation and smoking cessation success. Our findings also provide initial support for incorporating information on nicotine dependence severity to optimize the choice of smoking cessation treatment in CYP2A6 normal and slow metabolizers. Optimized smoking cessation strategies in turn will reduce the enormous morbidity and mortality associated with cigarette smoking.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Meghan J Chenoweth, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Caryn Lerman, Department of Psychiatry and USC Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA, USA.
Jo Knight, Data Science Institute, Lancaster University Medical School, Lancaster, UK; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Rachel F Tyndale, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Funding
This work was funded by a Canadian Institutes of Health Research (CIHR) Catalyst T2023973 grant, a CIHR Foundation FDN-154294 grant, a CIHR Project PJY-159710 grants, a National Institutes of Health (NIH) PGRN DA020830 grant, a Canada Research Chair in Pharmacogenomics (Tyndale), and the Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health (CAMH).
Declaration of Interests
R. F. Tyndale has consulted for Quinn Emanuel and Ethismos. The other authors declare no conflicts of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
MJC and RFT were responsible for the study concept and analysis plan. CL and RFT oversaw the design of the original study. MJC performed data analysis. MJC, CL, JK, and RFT interpreted the findings. MJC, CL, JK, and RFT obtained funding for the study. MJC and RFT drafted the manuscript and revised it based on coauthor comments. All authors critically reviewed the manuscript and approved the final version for publication.
Data Availability
Because of the type of informed consent given by student participants, individual-level data cannot be made publicly available. Analysis code is available upon request.
References
- 1. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaiton M, Diemert L, Cohen JE, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6(6):e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J.. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet. 2006;9(1):64–72. [DOI] [PubMed] [Google Scholar]
- 4. Chenoweth MJ, Tyndale RF.. Pharmacogenetic optimization of smoking cessation treatment. Trends Pharmacol Sci. 2017;38(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakajima M, Yamamoto T, Nunoya K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24(11):1212–1217. [PubMed] [Google Scholar]
- 6. Nakajima M, Yamamoto T, Nunoya K, et al. Characterization of CYP2A6 involved in 3’-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277(2):1010–1015. [PubMed] [Google Scholar]
- 7. Dempsey D, Tutka P, JacobP, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. [DOI] [PubMed] [Google Scholar]
- 8. Sofuoglu M, Herman AI, Nadim H, Jatlow P.. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37(6):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C.. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9(4):511–518. [DOI] [PubMed] [Google Scholar]
- 10. Chenoweth MJ, Novalen M, HawkLW, Jr, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103(17):1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–608. [DOI] [PubMed] [Google Scholar]
- 14. Lerman C, Schnoll RA, HawkLW, Jr, et al. ; PGRN-PNAT Research Group. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leventhal AM, Lee W, Bergen AW, et al. Nicotine dependence as a moderator of genetic influences on smoking cessation treatment outcome. Drug Alcohol Depend. 2014;138:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kubota T, Nakajima-Taniguchi C, Fukuda T, et al. CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J. 2006;6(2):115–119. [DOI] [PubMed] [Google Scholar]
- 17. Nollen NL, Mayo MS, Cox LS, et al. Factors that explain differences in abstinence between black and white smokers: a prospective intervention study. J Natl Cancer Inst. 2019;111(10):1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA. Sex differences in smoking cessation pharmacotherapy comparative efficacy: a network meta-analysis. Nicotine Tob Res. 2017;19(3):273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Boraie A, Taghavi T, Chenoweth MJ, et al. Evaluation of a weighted genetic risk score for the prediction of biomarkers of CYP2A6 activity. Addict Biol. 2020;25(1):e12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Boraie A, Chenoweth MJ, Pouget JG, et al. Transferability of ancestry-specific and cross-ancestry CYP2A6 activity genetic risk scores in African and European populations. Clin Pharmacol Ther. 2021;110(4):975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu DF, Hinks LJ, Morton NE, Day IN.. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet. 2000;64(Pt 5):383–390. [DOI] [PubMed] [Google Scholar]
- 22. Chenoweth MJ, Cox LS, Nollen NL, et al. Analyses of nicotine metabolism biomarker genetics stratified by sex in African and European Americans. Sci Rep. 2021;11(1):19572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chenoweth MJ, Ware JJ, Zhu AZX, et al. ; PGRN-PNAT Research Group. Genome-wide association study of a nicotine metabolism biomarker in African American smokers: impact of chromosome 19 genetic influences. Addiction. 2018;113(3):509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 2nd ed. New York: The Guilford Press; 2018. [Google Scholar]
- 25. Schnoll R, George TP, Hawk LW, Cinciripini P, Wileyto P, Tyndale RF. The relationship between the nicotine metabolite ratio and three self-reported measures of nicotine dependence across sex and race. Psychopharmacology. 2014;231(12):2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El-Boraie A, Tanner JA, Zhu AZX, et al. Functional characterization of novel rare CYP2A6 variants and potential implications for clinical outcomes. Clin Transl Sci. 2022;15:204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the type of informed consent given by student participants, individual-level data cannot be made publicly available. Analysis code is available upon request.