Summary
Adult-born cells, arriving daily into the rodent olfactory bulb, either integrate into the neural circuitry or get eliminated. However, whether these two populations differ in their morphological or functional properties remains unclear. Using longitudinal in vivo two-photon imaging, we monitored dendritic morphogenesis, odor-evoked responsiveness, ongoing Ca2+ signaling, and survival/death of adult-born juxtaglomerular neurons (abJGNs). We found that the maturation of abJGNs is accompanied by a significant reduction in dendritic complexity, with surviving and subsequently eliminated cells showing similar degrees of dendritic remodeling. Surprisingly, ∼63% of eliminated abJGNs acquired odor responsiveness before death, with amplitudes and time courses of odor-evoked responses similar to those recorded in surviving cells. However, the subsequently eliminated cell population exhibited significantly higher ongoing Ca2+ signals, with a difference visible even 10 days before death. Quantitative supervised machine learning analysis revealed a relationship between the abJGNs’ activity and survival probability, with low neuronal activity being supportive for survival.
Keywords: adult neurogenesis, in vivo imaging, calcium signaling, survival, death, dendritic morphology, odor-evoked response, endogenous activity, dendritic pruning, machine learning
Highlights
-
•
After early growth, abJGNs reduce the dendritic complexity during maturation
-
•
Eliminated and surviving abJGNs show similar degrees of dendritic remodeling
-
•
Eliminated and surviving abJGNs respond similarly to odorant stimulus
-
•
Low levels of ongoing Ca2+ signaling are beneficial for the survival of abJGNs
In this article, Su and colleagues report that in vivo ∼20% of adult-born juxtaglomerular neurons (abJGNs) of the mouse olfactory bulb are eliminated after their birth. Surviving and eliminated abJGNs show similar degrees of dendritic remodeling and respond similarly to odorant stimuli. However, low activity levels of abJGNs are associated with higher survival probability.
Introduction
Adult neurogenesis provides adult brains with an additional level of plasticity and cognitive flexibility, reportedly important for odor-dependent learning, memory encoding, spatial, temporal, or odor pattern separation, and emotional control (Alonso et al., 2012; Anacker and Hen, 2017). The subgranular zone of the dentate gyrus generates hippocampal granule cells (GCs) and the subventricular zone (SVZ) of the lateral ventricle generates adult-born cells (ABCs) of the olfactory bulb (OB) (Ming and Song, 2011; Denoth-Lippuner and Jessberger, 2021). Neuronal progenitors generated in the SVZ migrate through the rostral migratory stream (RMS) into the OB, where ∼90% become GCs, whereas 5%–10% become juxtaglomerular neurons (JGNs) (Alvarez-Buylla and García-Verdugo, 2002; Bonfanti and Peretto, 2011). It takes 4–6 days for ABCs to migrate from the SVZ into the core of the OB, where they start a saltatory (i.e., “stop-and-go”-like) radial migration (Liang et al., 2016). GCs stop at the end of the radial migration phase; adult-born juxtaglomerular neurons (abJGNs) speed up and, after arrival into the glomerular layer, switch from the radial to the long-distance lateral migration, lasting until the end of the pre-integration phase (∼3–4 weeks after ABCs’ birth) (Li et al., 2023; Liang et al., 2016).
Morphogenesis patterns also differ between OB ABCs. While adult-born GCs (abGCs) start to grow dendritic trees after arrival at the final destination and gain stable dendritic morphology ∼30 days after birth (Sailor et al., 2016), abJGNs migrate within the glomerular layer with elaborated albeit constantly changing dendritic trees (Kovalchuk et al., 2015). At the population level, abJGNs’ dendritic trees increased their complexity 10–45 days after birth, reaching a steady state thereafter (Livneh et al., 2009; Mizrahi, 2007). Even after maturation, the structural dynamics of dendrites and spines of both cell types remain high (Mizrahi, 2007; Sailor et al., 2016).
Thousands of ABCs migrate into the OB every day but many of them are eliminated by apoptosis (Biebl et al., 2005; Petreanu and Alvarez-Buylla, 2002; Whitman and Greer, 2007), but see (Platel et al., 2019). According to bromodeoxyuridine (BrdU) incorporation experiments, the elimination of ABCs happens 15–45 days after birth (Petreanu and Alvarez-Buylla, 2002; Whitman and Greer, 2007). Many factors, including intracellular signaling pathways (e.g., pro- and anti-apoptotic Bcl-2 family, cAMP response element-binding protein [CREB]), neurotrophic and growth factors, hormones, and neurotransmitters (GABA, glutamate, serotonin, acetylcholine) were shown to influence the survival of ABCs (Benn and Woolf, 2004; Fomin-Thunemann and Garaschuk, 2021; Khodosevich et al., 2013).
Survival of ABCs seems to depend on odor sensation, as enriched odor exposure and olfactory discrimination learning promote, while odor deprivation (by closing one nostril or ablating olfactory sensory neurons) inhibits the survival of ABCs (Alonso et al., 2006; Sawada et al., 2011; Sultan et al., 2011). During a critical period (∼2–4 weeks after birth) ABCs are most sensitive to sensory experience (Alonso et al., 2006; Mouret et al., 2008; Yamaguchi and Mori, 2005). Since this period corresponds to the pre-integration phase during which ABCs accomplish their migration and start receiving synaptic inputs, it has been postulated that ABCs are eliminated because they “fail to integrate” into the pre-existing neural circuitry (Lin et al., 2010; Turnley et al., 2014), but this hypothesis was never tested directly. Besides, it remains unclear whether surviving and eliminated ABCs differ in morphological or functional properties. In this study, we employed longitudinal in vivo single-cell tracking and ratiometric two-photon Ca2+ imaging to test whether the surviving and the subsequently eliminated abJGNs differ in terms of dendritic morphology and endogenous or sensory-driven Ca2+ signaling. We also examined whether abJGNs are eliminated because they fail to integrate into the pre-existing neural circuitry.
Results
Longitudinal in vivo imaging of abJGNs’ fate
For the identification of abJGNs in longitudinal experiments, we used the red-green-blue (RGB) cell marking approach (Liang et al., 2016). The retroviral RMS injection (Figure 1A) labels all subtypes of abJGNs and RGB marking provides each cell with a defined color identity, enabling accurate tracking of migrating cells (Kovalchuk et al., 2015; Liang et al., 2016). The RGB-labeled abJGNs were visualized through a cranial window and their position as well as dendritic morphology were monitored daily from 12 days post-viral injection (DPI 12) until DPI 45 (Video S1; Figures 1B and 1C). For detecting cell elimination, we relied on our previous results, showing that cells rarely move after being stable for more than 4 days (Figure 4 in Liang et al. [2016]), and routinely left a 30- to 50-μm-wide margin to the border of the field of view (FOV) to avoid confusing cell death with cell migration out of the FOV. At DPI 12–22, abJGNs were scored as eliminated only if cell debris was found in the position where there was an abJGN (upper panel in Figure 1D). After DPI 22, cells were also scored as eliminated if they were present within the FOV at DPI 12, stayed in the same position at DPI 18–22 (at least 4 days), and then disappeared from the dorsal surface of the bulb under the entire cranial window without visible debris (lower panel in Figure 1D). The debris of cells expressing mCherry together with other fluorescent proteins was often colored red (magenta arrow in Figure 1D, upper panel), likely due to the higher resistance of mCherry to proteolysis and degradation (Costantini et al., 2015). According to the above criteria, RGB-labeled abJGNs were classified as surviving (i.e., staying in the same FOV from DPI 12 to 45), eliminated, or uncertain (disappearing from the FOV without fulfilling the above criteria) cells. To avoid false-positive results, we excluded the uncertain cells from further analyses. This likely underestimated the fraction of eliminated cells reported in this study (Video S1).
Figure 1.
Longitudinal tracking of abJGNs' fate
(A and B) Experimental setup (A) and timeline (B).
(C) Maximum intensity projection (MIP) images (0–100 μm, step 2 μm) of RGB+ abJGNs taken at DPI 12 in red, green, and blue channels.
(D) Upper: MIP images (34–54 μm, step 2 μm) showing 1 eliminated cell (magenta arrow) and 2 surviving cells (green arrows) in the same FOV at 4 different time points, as indicated. Red cell debris is visible at DPI 23 and 24. Lower: MIP images (12–52 μm, step 2 μm) showing 1 eliminated and 1 surviving abJGN in the same FOV at 5 different time points.
(E and F) Boxplots showing the median (per mouse) fractions of surviving (E) and eliminated (F) abJGNs at DPI 12–45 (n = 12 mice).
(G) Graph showing the fate of RGB+ abJGNs present within the given FOV at DPI 12 (taken as 100%).
(H and I) Graphs showing cumulative fractions of eliminated (H) and uncertain (I) cells at DPI 12–45 (n = 12 mice).
According to the above criteria, 61.90% ± 11.29% (per mouse) of 528 analyzed abJGNs were classified as surviving (Figure 1E, n = 12 mice), 18.45% ± 12.89% as eliminated (Figure 1F), and the rest as uncertain cells. The cells’ dwell time within the FOVs was short at DPI 12–25 and substantially longer at later time points (Figure 1G), with the cumulative fraction of eliminated cells increasing almost linearly from DPI 12 to 34 (Figure 1H, 91 cells, n = 12 mice) and the cumulative fraction of uncertain cells (Figure 1I) mirroring that of migrating cells in our previous study (see Figure 7 in Liang et al. [2016]). In total, 91.21% of eliminated abJGNs (83/91 cells) died between DPI 12 and DPI 28, with no cells being eliminated after DPI 34. These data are consistent with a previous study showing that the elimination of abJGNs after DPI 28 is minimal (Sawada et al., 2011). Together, our in vivo imaging data documented the death of at least ∼20% of abJGNs. The death was cell age dependent, happening within the first 34 days after birth.
Longitudinal analyses of dendritic development of abJGNs
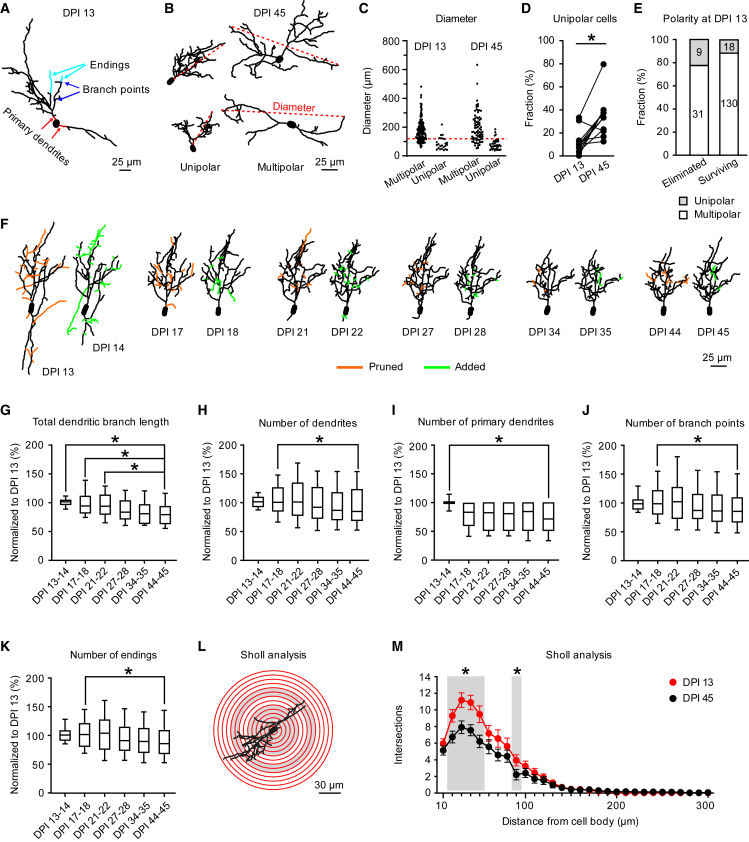
To identify features that may predict the fate of abJGNs, we followed individual cells over time first focusing on dendritic morphology. We analyzed (Note S1) total dendritic branch length (TDBL) as well as the number of dendrites, primary dendrites, branch points, and dendritic endings (Figure 2A). Based on the number of primary dendrites, abJGNs were classified as either unipolar or multipolar cells (Figure 2B). As ∼95% of OB glomeruli have a diameter of <120 μm (Royet et al., 1988), the vast majority of multipolar cells were also multi-glomerular (MGCs), whereas almost all unipolar cells likely were periglomerular cells (PGCs) projecting to a single glomerulus (Kosaka and Kosaka, 2011; Nagayama et al., 2014) (Figure 2C). The fraction of unipolar abJGNs increased significantly from DPI 13 to 45 (Figure 2D, p = 4.9 × 10−3, Wilcoxon signed-rank test, n = 11 mice), suggesting that either multipolar cells pruned their primary dendrites to become unipolar or more multipolar cells were eliminated at DPI 13–45. Out of all abJGNs with documented dendritic morphology, 22.5% (9/40 cells, n = 11 mice) of eliminated and 12.16% of surviving cells (18/148 cells, n = 12 mice) had a unipolar morphology at DPI 13. The observed difference, however, did not reach the level of statistical significance (Figure 2E, p = 0.16, chi-squared test with Yates' correction), suggesting that the unipolarity of the dendritic tree is not beneficial for abJGN’s survival.
Figure 2.
Dendritic development of abJGNs
(A) MIP image of a reconstructed abJGN (DPI 13), illustrating the analyzed morphological parameters. Primary dendrites are color-coded red, some branch points blue and some dendritic endings cyan.
(B) MIP images of two unipolar and two multipolar cells at DPI 45. Red dashed lines mark the diameters of reconstructed dendritic trees.
(C) Dot plot of the dendritic tree diameters of multipolar and unipolar cells (DPI 13: n = 177 multipolar and 24 unipolar cells; DPI 45: 81 multipolar and 35 unipolar cells, 11 mice). Red dashed line marks 120 μm.
(D) Connected dot graph showing the fractions (per mouse) of unipolar cells at DPI 13 and DPI 45 (p = 4.9 × 10−3, Wilcoxon signed-rank test; n = 11 mice).
(E) Bar graph showing the fractions of unipolar and multipolar cells among the eliminated (left) and surviving (right) cells (p = 0.16, chi-squared test with Yates' correction). Cell polarity was evaluated at DPI 13.
(F) Representative reconstructions of the same cell at 12 different time points (groups of 2 consecutive time points, DPI 13–45). Colors highlight pruned (orange) and added (green) dendrites for each group.
(G–K) Boxplots illustrating the normalized TDBL (G) and the numbers of dendrites (H), primary dendrites (I), branch points (J), and endings (K). For each cell, the data were normalized to the respective value measured at DPI 13 (p < 0.05, Friedman test followed by Dunn’s multiple comparisons test; n = 34 cells, 8 mice).
(L) Schematic of the Sholl analysis.
(M) Graph comparing the mean numbers of intersections with Sholl spheres for cells imaged at DPI 13 (red) and DPI 45 (black). Error bars: SEM. Gray zones in (M) mark the regions with significant differences between the two age groups (∗p < 0.05, Wilcoxon signed-rank test; n = 34 cells, 8 mice).
Next, we analyzed the dendritic morphology of stable surviving abJGNs (cells that did not change their position at DPI 13–45; 69.62% ± 12.12% of all surviving cells, n = 12 mice) at 12 different time points (Figure 2F). For each cell, the data were normalized to the respective value measured at DPI 13, and the means of two neighboring time points were used for statistics shown in Figures 2G–2K. Surprisingly, all morphological parameters of stable surviving abJGNs including the median (per mouse) TDBL as well as number of dendrites, primary dendrites, branch points, and endings declined with cells’ maturation (Figures 2G–2K, n = 34 cells, 8 mice). The lower number of primary dendrites is consistent with the higher fraction of unipolar cells seen at DPI 45 (Figure 2D). Concordantly, Sholl analysis revealed a significant reduction of the dendritic arbor complexity at DPI 45 compared with DPI 13 (Figures 2L and 2M).
To analyze the turnover of dendritic branches, we conducted quantitative four-dimensional structural plasticity analyses (4DSPA) using published protocols (Gonçalves et al., 2016). For each stable surviving abJGN, the degree of dendritic remodeling (i.e., the fractions of pruned or added dendritic endings) was analyzed at DPI 13–14, 17–18, 21–22, 27–28, 34–35, and 44–45 (Figure 2F), being high at early time points and decreasing over time (Figure S1). Consistent with the literature (Livneh and Mizrahi, 2011; Mizrahi, 2007), even at DPI 44–45 abJGNs remained structurally dynamic (Figures 2F and S1).
Based on their morphology at DPI 45, we classified the abJGNs into PGCs (43.75% ± 10.00%), MGCs (46.67% ± 20.83%), and cells with unclear morphology (6.25% ± 18.75%; n = 11 mice). Note a relatively high fraction of MGCs cells among abJGNs, which stop migrating at DPI 13. Both stable surviving PGCs and MGCs (Figure S2, n = 13 PGCs, 7 mice; n = 14 MGCs, 5 mice) exhibited a significant reduction in the TDBL and the number of primary dendrites as well as a decrease in the degree of dendritic remodeling from DPI 13 to 45. Thus, despite distinct dendritic morphology, adult-born PGCs and MGCs share similar patterns of dendritic development.
In a subset of experiments, we also followed the dendritic morphology of stable surviving abJGNs from DPI 9 to 13. Dendritic trees of stable surviving abJGNs grew rapidly at DPI 9–13, increasing, for example, the TDBL by 35.21% and the number of dendrites by 68.42% (Figure 3, n = 15 cells, 4 mice). We assured that settings, optimized for the longitudinal imaging (i.e., high photomultiplier gain, low laser power), did not underestimate the dendritic complexity (Figure S3).
Figure 3.
Initial period of rapid dendritic growth in surviving abJGNs at DPI 9–13
(A) Sample reconstructions of abJGNs at DPI 9.
(B–F) Boxplots summarizing the normalized TDBL (B) and the numbers of dendrites (C), primary dendrites (D), branch points (E), and endings (F) in surviving abJGNs at DPI 9–13. For each cell, the data were normalized to the respective values measured at DPI 13 (∗p < 0.05 for all comparisons, Friedman test followed by Dunn’s multiple comparisons test; n = 15 JGNs, 4 mice).
(G) Graph showing the mean numbers of intersections with Sholl spheres at DPI 9 (green) and DPI 13 (red). Gray zone marks the region with significant differences between the two age groups (∗p < 0.05, Wilcoxon signed-rank test; n = 15 JGNs, 4 mice). Error bars: SEM.
Together, our data revealed that, in stable surviving abJGNs, dendritic growth was restricted to a short time window (DPI 9–13). Thereafter (DPI 13–45), the size and complexity of the dendritic tree decreased significantly. At early developmental stages, abJGNs underwent both extensive dendritic pruning and addition. The degree of dendritic remodeling decreased during development, with adult-born PGCs and MGCs sharing similar developmental patterns.
Similar levels of dendritic complexity and morphological plasticity in eliminated and surviving abJGNs
Immature neurons are eliminated by apoptosis (Biebl et al., 2005; Yuan and Yankner, 2000) and the time from the initiation of apoptosis to its completion usually takes less than 24 h (Cellerino et al., 2000; Elmore, 2007). To determine whether the morphological features can distinguish subsequently eliminated from surviving cells, we took the day of cell death as a reference point and analyzed the morphology of eliminated abJGNs 0–24 h (DBD 1), 24–48 h (DBD 2), and 48–72 h (DBD 3) before death. The addition and pruning of dendritic endings between two neighboring time points were assessed by the 4DSPA approach. The data obtained were normalized to the respective median values of all (at least 4) surviving cells of the same subtype (i.e., either PGC- or MGC-like; unequivocal determination of subtype is possible only in the mature state) recorded in the same mouse at the same time point (Figure 4A). Interestingly, even 1–2 days before death, the subsequently eliminated cells continued to prune and add dendritic endings and we found no significant difference between eliminated and surviving abJGNs for all the morphological parameters analyzed (Figures 4B–4H, p > 0.05 for all comparisons, Wilcoxon signed-rank test; n = 10 eliminated and 63 surviving cells, 6 mice). Thus, the complexity of the dendritic tree and the remodeling dynamics cannot predict the subsequent elimination of abJGNs.
Figure 4.
Dendritic morphology cannot predict the fate of abJGNs
(A) Sample reconstructions of eliminated (gray) and corresponding surviving (black) abJGNs from the same mice at DBD 2.
(B–F) Boxplots showing the normalized TDBL (B) and the numbers of dendrites (C), primary dendrites (D), branch points (E), and endings (F) measured at DBDs 3, 2, and 1.
(G and H) Normalized fractions of pruned (G) and added (H) dendritic endings at DBD 3-2 and DBD 2-1. Data obtained in subsequently eliminated cells were normalized to the respective median value obtained in corresponding surviving cells of the same type recorded in the same animal at the same time point (p > 0.05, Wilcoxon signed-rank test; n = 10 eliminated and 63 surviving cells, 6 mice).
Can odor responsiveness predict abJGNs’ fate?
Since the critical period of ABC’s elimination corresponds to the pre-integration phase, it has been postulated that ABCs die because they fail to integrate into the pre-existing neural circuitry (Lepousez and Lledo, 2011; Lin et al., 2010; Turnley et al., 2014). We took the ability to acquire odor responsiveness as the readout of abJGN’s functional integration and tested whether odor responsiveness protects abJGNs from being eliminated. To this end, migrating ABCs in the RMS were labeled by lentivirus encoding the FRET-based ratiometric Ca2+ indicator Twitch-2B (Thestrup et al., 2014). Since it is difficult to determine the identity of migrating abJGNs without unique color tags, our longitudinal Ca2+ imaging started at DPI 18 (Figure 5A), after the majority of abJGNs complete their lateral migration (Figure 7A in Liang et al. [2016]). We focused on the cells, which did not change their position during the first three consecutive imaging sessions (DPI 18, 20, and 22), referred to as stable abJGNs (Figure 5B). Focusing on stable, post-migratory abJGNs also helped to avoid late-coming cells (yellow arrow in Figure 5B), arriving in the OB at a low rate after lentiviral injection in the RMS (Mizrahi, 2007; Wallace et al., 2017). Under these recording conditions, 94.12% ± 7.10% (median per mouse) of Twitch-2B+ abJGNs were stable cells (Figure 5C, n = 533 cells, 11 mice), which were imaged further every second day (Figure 5A).
Figure 5.
Fate of abJGNs is not determined by their odor responsiveness
(A) Experimental timeline.
(B) MIP (63–135 μm, step 3 μm) images showing stable (blue arrows), unstable (red arrows), and late-coming (orange arrow) Twitch-2B+ abJGNs on DPI 18–22.
(C) Boxplot showing the fraction of stable cells at DPI 18–22 (n = 11 mice).
(D) Boxplot showing the fractions of cells eliminated at DPI 22–44 in Twitch-2B+ cell and RGB+ cell groups (p = 0.97, Mann-Whitney test; n = 11 and 12 mice, respectively).
(E) Sample image (average of 140 consecutive frames) of a Twitch-2B+ abJGN at DPI 24.
(F) Fluorescence intensity changes of mCerulean3 and cpVenusCD and the Twitch-2B ratio recorded from the area delineated in (E) in response to a 4-s-long application of the 7-odorant mixture (see experimental procedures).
(G) Boxplot showing the fraction of odor-responding cells at DPI 20–32 (n = 11 mice).
(H) Bar graphs showing the fractions of odor-responding and non-responding cells among the eliminated and surviving cells (p = 0.74, chi-squared test with Yates' correction).
(I and J) Boxplots showing the normalized maximum ΔR/R amplitude (I) (p = 0.49, Wilcoxon signed-rank test) and the AUC (J) (p = 0.23, one-sample t test). The median amplitudes of subsequently eliminated cells were normalized to the median amplitudes of corresponding surviving cells recorded in the same animal at the same time point (n = 12 odor-responding eliminated and 112 odor-responding surviving cells, 8 mice).
Similar to the criteria described for RGB-labeled cells (see above), Twitch-2B+ abJGNs were scored as eliminated either when the cell debris was identified or when stable cells disappeared after DPI 22 (Figure S4). The median (per mouse) fraction of Twitch-2B+ abJGNs eliminated from DPI 22 to 44 was 6.25% ± 5.24% (n = 11 mice) and thus similar to the fraction (5.10% ± 9.87%, n = 12 mice) of RGB+ abJGNs eliminated during the same period (Figure 5D, p = 0.97, Mann-Whitney test). To test whether longitudinal imaging enhances the elimination of abJGNs, we compared the fractions of surviving cells in our RGB+ and Twitch-2B+ groups to a dataset from our laboratory (Li et al., 2023), in which Twitch-2B+ abJGNs were imaged only at two time points: either at DPI 14 and 25 or at DPI 25 and 45. We did not detect any significant difference both at DPI 14–25 (two time points group: 62.50% ± 23.41%, n = 5 mice; RGB+ group: 61.18% ± 24.24%, n = 12 mice; p = 0.65, Mann-Whitney test) and DPI 25–45 (two time points group: 80.00% ± 20.76%, n = 5 mice; RGB+ group: 96.29% ± 7.33%, n = 12 mice; Twitch-2B+ group: 94.74% ± 4.48%, n = 11 mice; p = 0.07, Kruskal-Wallis test), suggesting that our longitudinal imaging did not promote the elimination of abJGNs.
Odor responsiveness of Twitch-2B+ abJGNs was tested by applying the mixture of either three or seven odorants (see experimental procedures) in front of the snout of anesthetized mice. Both odor mixtures cause broad activation of dorsal OB glomeruli (Kovalchuk et al., 2015; Livneh et al., 2014), thereby increasing the probability to recruit odor-responding abJGNs. Under these experimental conditions, 58.00% ± 17.44% (median per mouse, n = 281 cells, 11 mice) of abJGNs showed odor-evoked responses (Figures 5E–5G). The odor-evoked responsiveness of 19 out of 25 cells was tested before their elimination. Of these, 63.16% (12/19 cells, 10 mice) showed odor-evoked Ca2+ signals before death (Figure 5H). Thus, abJGNs can be eliminated despite the acquired odor responsiveness. The fraction of odor-responding cells among eliminated abJGNs (63.16%) was not significantly different from that among surviving abJGNs (56.37%, 146/259 cells, 11 mice) (Figure 5H, p = 0.74, chi-squared test with Yates' correction), suggesting that acquired odor responsiveness per se cannot protect abJGNs from being eliminated.
To investigate whether eliminated and surviving abJGNs responded differently to odor stimulation, we analyzed (Note S1) the maximum ΔR/R amplitude and the area under the curve (AUC) of odor-evoked Ca2+ transients. The median values of eliminated cells were normalized to the median values of corresponding surviving cells recorded from the same mice at the same time points. There was no significant difference between eliminated and surviving cells in terms of the maximum ΔR/R amplitude (Figure 5I, p = 0.49, Wilcoxon signed-rank test) and the AUC (Figure 5J, p = 0.23, one-sample t test; n = 12 odor-responding eliminated and 112 odor-responding surviving cells, 8 mice). Thus, the properties of odor-evoked responses did not correlate with the fate of abJGNs.
Distinct ongoing Ca2+ signaling in eliminated and surviving abJGNs
Next, we examined whether the level or pattern of ongoing Ca2+ signaling, measured as changes in the cells’ Twitch-2B ratio, can predict the fate of abJGNs (Figure 6). To do so, we analyzed activity patterns of subsequently eliminated cells at DBDs 10, 8, 6, 4, and 2 and compared them with activity patterns of surviving abJGNs recorded in the same mice at the same time points. Previously, we have documented the ubiquitous presence of spontaneous Ca2+ transients in the Twitch-2B+ abJGNs and shown, using a voltage-gated Na+ channel blocker tetrodotoxin, that Twitch-2B+ abJGNs can be considered active (i.e., spiking) when their Twitch-2B ratio is above 2.4 and non-active (i.e., electrically silent) when their Twitch-2B ratio is below 2.0 (Fomin-Thunemann et al., 2020; Maslyukov et al., 2018). Here, we used this knowledge to analyze the following six parameters (Note S1): basal Twitch-2B ratio, maximum Twitch-2B ratio, maximum ΔR/R amplitude of ongoing Ca2+ transients, the fraction of time spent in the active state (Twitch-2B ratio >2.4), AUC/second (Figure 6E), and the fraction of time spent in the silent state (Twitch-2B ratio <2.0).
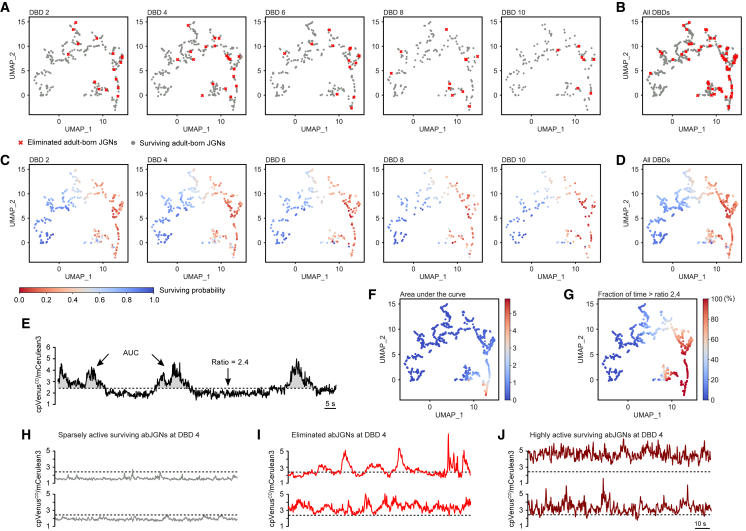
Figure 6.
Surviving probability and ongoing activity patterns in surviving and eliminated abJGNs
(A and B) UMAP visualization of surviving and eliminated abJGNs at different DBDs.
(C and D) Same UMAP visualization with color coding for the surviving probability of abJGNs at different DBDs as predicted by the logistic regression classification model (Note S1).
(E) Graph illustrating the ongoing activity of one abJGN and parameters used for analyses.
(F and G) Distribution of most predictive variables according to the classification model: AUC (F) and the fraction of time spent above 2.4 (G).
(H–J) Sample Twitch-2B ratio traces, recorded from surviving abJGNs located in the lower left (H) and lower right (J) corners of the UMAP and subsequently eliminated abJGNs (I).
The Uniform Manifold Approximation and Projection (UMAP) technique allowed to visualize our six-dimensional data space at different DBDs (Figure 6A). We used the concatenated data from all DBDs (Figure 6B) to train the logistic regression (LR) classification model to predict the survival probability of abJGNs (Note S1). Projected at the same UMAP, the predictions of the model revealed a clear trend toward lower survival probability when moving from the low left to the low right UMAP corner (Figures 6C and 6D). No significant difference was observed for the distributions of predicted probability values at different DBDs (p > 0.05 for all comparisons, Wilcoxon signed-rank test with Bonferroni correction). A similar trend in prediction was also found with the Naive Bayes classifier (Note S1). According to the LR classification model, the AUC and the fraction of time spent in the active state were the most predictive variables. Interestingly, these (Figures 6F and 6G) as well as the other analyzed parameters (maximum Twitch-2B ratio and the fraction of time spent in the silent state, not shown) showed a clear trend toward an increase in abJGNs’ activity from the low left to the low right UMAP corner (Figures 6F–6H). Together, these data reveal a relationship between the abJGNs’ activity and survival probability, with lower activity being supportive for survival.
To examine the stability of ongoing Ca2+ signaling in surviving and subsequently eliminated abJGNs, we took DBD 6 as a reference and calculated, for all parameters under study, the differences between values measured at DBD 6 and at subsequent time points (Figure S5). Both for the surviving and eliminated abJGNs, the individual measured values scattered around zero. The median values for surviving cells were remarkably similar in the three recording sessions (Figure S5; p > 0.05 for all comparisons, Friedman test). For eliminated abJGNs, we did observe an increase in the basal (Figure S5A) and maximum (Figure S5B) Twitch-2B ratio and the maximum ΔR/R amplitude (Figure S5C) at DBD 2 compared with DBDs 4 and 6, probably reflecting the apoptosis-related increase in the intracellular free Ca2+ concentration ([Ca2+]i [Cellerino et al., 2000; Linden et al., 2005]) in some subsequently eliminated cells. However, none of the differences reached the level of statistical significance (Figure S5; p > 0.05 for all comparisons, Wilcoxon signed-rank test; n = 8 eliminated and 138 surviving cells, 5 mice).
It was suggested that fluctuations rather than a steady-state increase in [Ca2+]i are needed to drive the phosphorylation of nuclear CREB, a key mediator of the development and survival of ABCs (Giachino et al., 2005; Jagasia et al., 2009; Li et al., 2016, 2023). However, we found no significant difference in fractions of cells with fluctuating [Ca2+]i between the eliminated and surviving abJGNs at all time points (DBDs 6, 4, and 2) tested (Figure S6; p > 0.05 for all comparisons, chi-squared test with Yates' correction). These data suggest that the absolute levels of [Ca2+]i rather than the lack of fluctuations promote the subsequent cell death.
Discussion
Using longitudinal in vivo imaging, we have characterized the morphological and functional properties of subsequently eliminated and surviving abJGNs. According to our conservative estimate (Video S1), subsequently eliminated cells comprise at least 20% of abJGNs. The elimination is largely accomplished by the end of the pre-integration phase, with no abJGNs being eliminated after DPI 34. Surprisingly, stable surviving abJGNs grew their dendritic trees only during a brief initial period (DPI 9–13) and spent the majority of the maturation time (from DPI 13 onward) reducing and refining their dendritic arbors. Despite the distinct morphological appearance, adult-born PGCs and MGCs showed similar patterns of dendritic development. This knowledge could not have been anticipated based on the previous population-based data, describing the net dendritic growth of abJGNs as the major hallmark of their maturation (Livneh et al., 2009, 2014; Mizrahi, 2007). Dendritic morphogenesis occurred similarly in surviving and eliminated JGNs. Importantly, around 63% of the subsequently eliminated abJGNs acquired odor responsiveness before their death, similar to their surviving counterparts. Finally, the results of LR analysis revealed a relationship between the abJGNs’ activity and survival probability, with low levels of ongoing Ca2+ signaling being beneficial for survival. This finding contrasts the previous concept, associating enhanced neuronal activity with increased survival of ABCs (Lin et al., 2010).
BrdU incorporation experiments revealed the subsequent death of ∼50% of ABCs (Petreanu and Alvarez-Buylla, 2002; Yamaguchi and Mori, 2005) but a recent study cast doubt on this finding (Platel et al., 2019). Using tamoxifen-inducible RFP expression in SVZ stem cells and counting red OB cells once a week at DPI 7–56 showed the death of only 1.5% of abJGNs and 5.9% of abGCs. Similar data obtained in tdTomato+ abGCs, labeled by lentivirus RMS injection, prompted a conclusion that the death of ABCs is caused by the toxic high doses of BrdU (Platel et al., 2019). Although our fraction (18.45%) of eliminated abJGNs is also 2.7 times lower than found using BrdU, giving room to BrdU-mediated toxicity, it is still 10 times higher than that reported by Platel et al. (2019). One likely reason is the population-based nature of all previous studies. Indeed, when imaging many (20–170) red cells once a week (Platel et al., 2019), it is difficult to account for vivid cell migration (Liang et al., 2016), the substitution of dying cells by late-coming ABCs (Sawada et al., 2011), etc. Consistently, longitudinal every other day in vivo imaging of abGCs revealed ∼21% of eliminated abGCs at DPI 24–56 (∼0.7%/day decline in total cell number; Sailor et al., 2016). Together with our data, this shows that considerable fractions of ABCs are eliminated after the arrival at their destination layers. For abJGNs, the period of cell elimination seems to start somewhat earlier than assumed previously (Petreanu and Alvarez-Buylla, 2002), at DPI 12 at the latest, and last until DPI 34 only.
Immature ABCs migrating tangentially in the RMS or radially in the deeper layers of the OB have a polarized morphology: a long leading and a short trailing process (Doetsch et al., 1997; Kaneko et al., 2017). AbGCs stop and settle down at the end of radial migration (Liang et al., 2016) and start growing the dendritic trees (Petreanu and Alvarez-Buylla, 2002; Sailor et al., 2016). The abJGNs, however, switch to lateral migration, migrating and growing their dendritic trees simultaneously (Kovalchuk et al., 2015; Liang et al., 2016). The relationship between the two processes remains unclear. We show now that abJGNs, which settle down by DPI 13, reduce dendritic complexity during the subsequent development, pruning even primary dendrites (Figure 2). Although surprising and counterintuitive at first glance, these data suggest that, while migrating, abJGNs spread their dendrites around to sense activity patterns of many neighboring glomeruli. Once the parent glomeruli have been found, the cells settle down and start to prune unnecessary dendrites, going beyond their parent glomeruli. This hypothesis explains the higher odor responsiveness but lower odor-selectivity of immature abJGNs, compared with their mature counterparts (Livneh et al., 2014). This concept might also apply to the maturation of abGCs, which show overshooting, albeit to a much lesser extent, dendritic complexity (Sailor et al., 2016); higher odor responsiveness and lower odor selectivity (Wallace et al., 2017).
We find that ∼63% of abJGNs respond to odorants before death (Figure 5H). This number is likely underestimated, as instead of determining for each abJGN the odorant activating its parent glomerulus (Kovalchuk et al., 2015), we applied a mixture of odorants broadly activating dorsal glomeruli (Kovalchuk et al., 2015; Livneh et al., 2014) to increase the throughput. Thus, the acquisition of odor responsiveness does not prevent cell death. The parameters of odor-evoked responses analyzed in this study (maximum response amplitude and AUC), were also not able to differentiate between surviving and subsequently eliminated cells. It cannot, however, be excluded that other properties (e.g., odor selectivity or the stability of odor responses) differ in these two groups of cells. Here, we took the cell’s odor responsiveness as a readout of its functional integration into neural circuitry, but whether eliminated odor-responsive cells were synaptically connected remains unclear. PSD95-GFP puncta are considered a good proxy for putative synapses (Kelsch et al., 2008, 2009; Livneh et al., 2009). Already at DPI 12 dendrites of abJGNs were shown to be abundantly decorated by such puncta and the number of puncta increased with maturation (Livneh et al., 2009). We assume, therefore, that odor-responsive abJGNs eliminated after DPI 12 likely were synaptically connected before their elimination.
Many eliminated abJGNs exhibited elevated ongoing Ca2+ signaling over a prolonged time (Figures 6 and S5) and, according to our LR model, the surviving probability of abJGNs decreased with an increase in abJGNs activity. These findings are in contrast with the literature, suggesting that enhancement of neuronal activity via NaChBac overexpression promotes survival of immature abGCs in the OB (Lin et al., 2010) but not in the hippocampus (Sim et al., 2013). The exact reason for this discrepancy remains to be discovered but it suggests that different types of ABCs might possess different survival strategies. Besides, we analyzed the activity of ABCs eliminated after DPI 18, while in Lin et al. (2010) the NaChBac expression started at ABCs’ birth. Thus, timing may also affect the outcome.
Although we hypothesized that abJGNs die due to the loss of fluctuations in [Ca2+]i, needed for optimal activation of the CREB-mediated survival program (Li et al., 2009, 2016), the vast majority of subsequently eliminated abJGNs maintained [Ca2+]i fluctuations even at DBD 2, right before undergoing apoptosis (Figure S6). While some subtle fluctuation features, which are difficult to extract due to the inhomogeneity of activity patterns in individual abJGNs, might differ between the surviving and eliminated cells, our current data suggest that the death of abJGNs is triggered by the sustained increase in the overall level of [Ca2+]i. Here, mitochondria might be an important player. A sustained increase in [Ca2+]i causes a sustained increase in the Ca2+ content of the endoplasmic reticulum (ER) (Garaschuk et al., 1997) and might promote a direct (via the mitochondrial Ca2+ uniporter or voltage-dependent anion channel) or an ER-mediated (via mitochondria-associated ER membranes) sustained Ca2+ accumulation in the mitochondria (Celsi et al., 2009; Rizzuto et al., 2012). The latter can trigger oxidative stress and the release of proapoptotic factors (e.g., cytochrome c, apoptotic inducing factor, procaspase 9, endonuclease G) into the cytosol (Calvo-Rodriguez et al., 2020; Giorgi et al., 2012).
Taken together, our study points to ongoing Ca2+ signaling as a key player influencing the fate of abJGNs. The latter can integrate both the cell-intrinsic firing and the influence of the steady-state odor environment reaching either a pro- or an anti-apoptotic level. Because some odorants increase and others decrease the levels of ongoing activity in JGNs (Homma et al., 2013), this model reconciles the seemingly opposing findings showing that odor enrichment either decreases (Khodosevich et al., 2013) or increases (Forest et al., 2019; Rey et al., 2012; Rochefort et al., 2002) the survival of abJGNs.
Experimental procedures
Resource availability
Corresponding author
Resource inquiries should be directed to and will be fulfilled by corresponding author, Olga Garaschuk (olga.garaschuk@uni-tuebingen.de).
Materials availability
This study did not generate new unique reagents.
Animals
Experiments complied with biometrical planning and institutional animal welfare guidelines and were approved by the government of Baden-Württemberg, Germany. C57BL/6 mice (3–4 months old) of either sex were kept in pathogen-free conditions at 22°C and 60% air humidity, 12-h light/dark cycle, with ad libitum access to food and water. Males were maintained individually, females in groups of 3–5 mice.
Cranial window implantation and stereotaxic viral injection
A cranial OB window was implanted as described previously (Liang et al., 2016); 4 weeks later a retrovirus 1:1:1 mixture encoding mCherry, Venus, and Cerulean or lentivirus encoding the Twitch-2B (Thestrup et al., 2014) was stereotaxically injected (3.0 mm anterior, ±0.82 mm lateral from bregma, and 3.0 mm ventral from dura) into the RMS and a custom-made titanium holder was glued to the skull. Analgesic carprofen (5 μg/g BW, Pfizer, Berlin, Germany) was injected subcutaneously for 3 consecutive days, and antibiotic enrofloxacin (Baytril, 1:100 v/v, Bayer, Leverkusen, Germany) was applied in drinking water for 10 days.
Longitudinal in vivo two-photon imaging
RGB+ abJGNs were imaged (Olympus Fluoview 1000, Olympus, Tokyo, Japan) in awake (position) or anesthetized (morphology; 2% isoflurane for induction, 0.8%–1.2% for maintenance) head-fixed mice. 3D stacks (512 × 512 pixels, Kalman filter 2, step 3 μm) were acquired daily at DPI 12–35 and DPI 44–45. Cerulean and mCherry were excited at 800 nm, Venus at 970 nm. Emitted light was split at 570 nm and filtered at 630/92 nm (Cerulean, Venus) and 500 nm short-pass (mCherry). Sparse labeling, blood vessel patterns, irregularities of the dura and the distance between the stable RGB+ cells helped to identify the same cells across the imaging sessions.
In vivo two-photon Ca2+ imaging
At DPI 5–11, mice were habituated to the setup by daily fixations lasting 5 (at the beginning) to 40 min (at the end of training). Ongoing Ca2+ signals in awake head-fixed mice were sampled at 6.67 Hz by exciting Twitch-2B+ at 890 nm, splitting the emitted light at 515 nm and filtering with a 475/64 nm (mCerulean) and a 500 nm long-pass (cpVenusCD) filters.
Odor-evoked Ca2+ signals were measured in mice (36–37°C, 130–170 BPM), anesthetized with an i.p. injection of midazolam (5 mg/kg BW), medetomidine (0.5 mg/kg BW), and fentanyl (0.05 mg/kg BW). Mixtures consisting of equal parts of either three (isoamyl acetate, 2-hexanone, and ethyl tiglate) or seven (ethyl-acetate, butanal, pentanal, ethyl tiglate, propanal, methyl-propionate, and ethyl-butyrate) odorants (1.7% of saturated vapor) were delivered for 4 s in front of the mouse’s snout via a custom-made flow dilution olfactometer (Homma et al., 2013). Note that under these conditions the fractions of odor-responding/-non-responding abJGNs are similar to that measured in awake mice (p = 0.36, chi-squared test; Fomin-Thunemann et al., 2020).
Statistical analyses
Unless indicated, all data are shown as median ± interquartile range. Two-tailed statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, CA). The normality of data distribution was tested with the Shapiro-Wilk test. Differences were considered significant if p < 0.05.
Author contributions
O.G. initiated and conceived the study. X.S. and Y.K. performed the experiments. X.S. analyzed the data. N.M., F.K., and M.C. contributed to data analyses. X.S. and O.G. wrote the manuscript. All authors have approved the final version of the manuscript.
Acknowledgments
We thank J.T. Goncalves and Y.T. Ching for help with 4DSPA analysis, E. Zirdum, A. Weible, and K. Schoentag for technical assistance, K. Li and K. Figarella for viral production, N. Asavapanumas for help with analysis of ongoing activity, A. Gohl, J. Mueck, M. Knecht, and S. Wang for help with morphological analysis, S. Jessberger, Y. Liang, and K. Li for comments on the manuscript. This work was funded by DFG (GA 654/14-1 to O.G.) and DAAD (91586010 to X.S.).
Conflict of interests
The authors declare no competing interests.
Published: April 27, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.03.018.
Supplemental information
Data and code availability
Data and code can be accessed on https://github.com/GaraschukLab/abJGN-Interneuron-Survival-Classification.
References
- Alonso M., Viollet C., Gabellec M.M., Meas-Yedid V., Olivo-Marin J.C., Lledo P.M. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J. Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M., Lepousez G., Wagner S., Bardy C., Gabellec M.M., Torquet N., Lledo P.M. Activation of adult-born neurons facilitates learning and memory. Nat. Neurosci. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., García-Verdugo J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility-linking memory and mood. Nat. Rev. Neurosci. 2017;18:335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn S.C., Woolf C.J. Adult neuron survival strategies - slamming on the brakes. Nat. Rev. Neurosci. 2004;5:686–700. doi: 10.1038/nrn1477. [DOI] [PubMed] [Google Scholar]
- Biebl M., Winner B., Winkler J. Caspase inhibition decreases cell death in regions of adult neurogenesis. Neuroreport. 2005;16:1147–1150. doi: 10.1097/00001756-200508010-00003. [DOI] [PubMed] [Google Scholar]
- Bonfanti L., Peretto P. Adult neurogenesis in mammals - a theme with many variations. Eur. J. Neurosci. 2011;34:930–950. doi: 10.1111/j.1460-9568.2011.07832.x. [DOI] [PubMed] [Google Scholar]
- Calvo-Rodriguez M., Hou S.S., Snyder A.C., Kharitonova E.K., Russ A.N., Das S., Fan Z., Muzikansky A., Garcia-Alloza M., Serrano-Pozo A., et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020;11:2146. doi: 10.1038/s41467-020-16074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellerino A., Galli-Resta L., Colombaioni L. The dynamics of neuronal death: a time-lapse study in the retina. J. Neurosci. 2000;20:RC92. doi: 10.1523/JNEUROSCI.20-16-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celsi F., Pizzo P., Brini M., Leo S., Fotino C., Pinton P., Rizzuto R. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim. Biophys. Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini L.M., Baloban M., Markwardt M.L., Rizzo M.A., Guo F., Verkhusha V.V., Snapp E.L. A palette of fluorescent proteins optimized for diverse cellular environments. Nat. Commun. 2015;6:7670. doi: 10.1038/ncomms8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoth-Lippuner A., Jessberger S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 2021;22:223–236. doi: 10.1038/s41583-021-00433-z. [DOI] [PubMed] [Google Scholar]
- Doetsch F., García-Verdugo J.M., Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomin-Thunemann N., Garaschuk O. Role of serotonin in modulating the development and function of adultborn neurons in the olfactory bulb. Neural Regen. Res. 2022;17:1253–1254. doi: 10.4103/1673-5374.327337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomin-Thunemann N., Kovalchuk Y., Fink S., Alsema A., Mojtahedi N., Zirdum E., Garaschuk O. Unique functional properties of mature adult-born neurons in the mouse olfactory bulb. Stem Cell Rep. 2020;15:1333–1346. doi: 10.1016/j.stemcr.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest J., Moreno M., Cavelius M., Chalençon L., Ziessel A., Sacquet J., Richard M., Didier A., Mandairon N. Short-term availability of adult-born neurons for memory encoding. Nat. Commun. 2019;10:5609. doi: 10.1038/s41467-019-13521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O., Yaari Y., Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J. Physiol. 1997;502 ( Pt 1):13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C., De Marchis S., Giampietro C., Parlato R., Perroteau I., Schütz G., Fasolo A., Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Baldassari F., Bononi A., Bonora M., De Marchi E., Marchi S., Missiroli S., Patergnani S., Rimessi A., Suski J.M., et al. Mitochondrial Ca2+ and apoptosis. Cell Calcium. 2012;52:36–43. doi: 10.1016/j.ceca.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J.T., Bloyd C.W., Shtrahman M., Johnston S.T., Schafer S.T., Parylak S.L., Tran T., Chang T., Gage F.H. In vivo imaging of dendritic pruning in dentate granule cells. Nat. Neurosci. 2016;19:788–791. doi: 10.1038/nn.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma R., Kovalchuk Y., Konnerth A., Cohen L.B., Garaschuk O. In vivo functional properties of juxtaglomerular neurons in the mouse olfactory bulb. Front. Neural Circuits. 2013;7:23. doi: 10.3389/fncir.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R., Steib K., Englberger E., Herold S., Faus-Kessler T., Saxe M., Gage F.H., Song H., Lie D.C. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N., Sawada M., Sawamoto K. Mechanisms of neuronal migration in the adult brain. J. Neurochem. 2017;141:835–847. doi: 10.1111/jnc.14002. [DOI] [PubMed] [Google Scholar]
- Kelsch W., Lin C.W., Lois C. Sequential development of synapses in dendritic domains during adult neurogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:16803–16808. doi: 10.1073/pnas.0807970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W., Lin C.W., Mosley C.P., Lois C. A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J. Neurosci. 2009;29:11852–11858. doi: 10.1523/JNEUROSCI.2406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodosevich K., Lazarini F., VonEngelhardt J., Kaneko H., Lledo P.M., Monyer H. Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron. 2013;79:1136–1151. doi: 10.1016/j.neuron.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Kosaka K. Interneurons” in the olfactory bulb revisited. Neurosci. Res. 2011;69:93–99. doi: 10.1016/j.neures.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y., Homma R., Liang Y., Maslyukov A., Hermes M., Thestrup T., Griesbeck O., Ninkovic J., Cohen L.B., Garaschuk O. In vivo odourant response properties of migrating adult-born neurons in the mouse olfactory bulb. Nat. Commun. 2015;6:6349. doi: 10.1038/ncomms7349. [DOI] [PubMed] [Google Scholar]
- Lepousez G., Lledo P.-M. Life and death decision in adult neurogenesis: in praise of napping. Neuron. 2011;71:768–771. doi: 10.1016/j.neuron.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Li B., Tadross M.R., Tsien R.W. Sequential ionic and conformational signaling by calcium channels drives neuronal gene expression. Science. 2016;351:863–867. doi: 10.1126/science.aad3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Figarella K., Su X., Kovalchuk Y., Gorzolka J., Neher J.J., Mojtahedi N., Casadei N., Hedrich U.B.S., Garaschuk O. Patterned endogenous activity controls migration, morphogenesis and survival of adult-born neurons in the mouse olfactory bulb. Cell. Mol. Life Sci. 2023;80:98. doi: 10.1007/s00018-023-04753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang C., Takemori H., Zhou Y., Xiong Z.Q. TORC1 regulates activity-dependent CREB-target gene transcription and dendritic growth of developing cortical neurons. J. Neurosci. 2009;29:2334–2343. doi: 10.1523/JNEUROSCI.2296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Li K., Riecken K., Maslyukov A., Gomez-Nicola D., Kovalchuk Y., Fehse B., Garaschuk O. Long-term in vivo single-cell tracking reveals the switch of migration patterns in adult-born juxtaglomerular cells of the mouse olfactory bulb. Cell Res. 2016;26:805–821. doi: 10.1038/cr.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.W., Sim S., Ainsworth A., Okada M., Kelsch W., Lois C. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron. 2010;65:32–39. doi: 10.1016/j.neuron.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R., Martins R.A.P., Silveira M.S. Control of programmed cell death by neurotransmitters and neuropeptides in the developing mammalian retina. Prog. Retin. Eye Res. 2005;24:457–491. doi: 10.1016/j.preteyeres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Livneh Y., Mizrahi A. Long-term changes in the morphology and synaptic distributions of adult-born neurons. J. Comp. Neurol. 2011;519:2212–2224. doi: 10.1002/cne.22625. [DOI] [PubMed] [Google Scholar]
- Livneh Y., Feinstein N., Klein M., Mizrahi A. Sensory input enhances synaptogenesis of adult-born neurons. J. Neurosci. 2009;29:86–97. doi: 10.1523/JNEUROSCI.4105-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y., Adam Y., Mizrahi A. Odor processing by adult-born neurons. Neuron. 2014;81:1097–1110. doi: 10.1016/j.neuron.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Maslyukov A., Li K., Su X., Kovalchuk Y., Garaschuk O. Spontaneous calcium transients in the immature adult-born neurons of the olfactory bulb. Cell Calcium. 2018;74:43–52. doi: 10.1016/j.ceca.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A. Dendritic development and plasticity of adult-born neurons in the mouse olfactory bulb. Nat. Neurosci. 2007;10:444–452. doi: 10.1038/nn1875. [DOI] [PubMed] [Google Scholar]
- Mouret A., Gheusi G., Gabellec M.M., De Chaumont F., Olivo-Marin J.C., Lledo P.M. Learning and survival of newly generated neurons: when time matters. J. Neurosci. 2008;28:11511–11516. doi: 10.1523/JNEUROSCI.2954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S., Homma R., Imamura F. Neuronal organization of olfactory bulb circuits. Front. Neural Circuits. 2014;8:98. doi: 10.3389/fncir.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L., Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel J.C., Angelova A., Bugeon S., Wallace J., Ganay T., Chudotvorova I., Deloulme J.C., Béclin C., Tiveron M.C., Coré N., et al. Neuronal integration in the adult mouse olfactory bulb is a non-selective addition process. Elife. 2019;8:e44830. doi: 10.7554/eLife.44830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey N.L., Sacquet J., Veyrac A., Jourdan F., Didier A. Behavioral and cellular markers of olfactory aging and their response to enrichment. Neurobiol. Aging. 2012;33:626.e9–626.e23. doi: 10.1016/j.neurobiolaging.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Rochefort C., Gheusi G., Vincent J.D., Lledo P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J.P., Souchier C., Jourdan F., Ploye H. Morphometric study of the glomerular population in the mouse olfactory bulb: numerical density and size distribution along the rostrocaudal axis. J. Comp. Neurol. 1988;270:559–568. doi: 10.1002/cne.902700409. [DOI] [PubMed] [Google Scholar]
- Sailor K.A., Valley M.T., Wiechert M.T., Riecke H., Sun G.J., Adams W., Dennis J.C., Sharafi S., Ming G.L., Song H., et al. Persistent structural plasticity optimizes sensory information processing in the olfactory bulb. Neuron. 2016;91:384–396. doi: 10.1016/j.neuron.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M., Kaneko N., Inada H., Wake H., Kato Y., Yanagawa Y., Kobayashi K., Nemoto T., Nabekura J., Sawamoto K. Sensory input regulates spatial and subtype-specific patterns of neuronal turnover in the adult olfactory bulb. J. Neurosci. 2011;31:11587–11596. doi: 10.1523/JNEUROSCI.0614-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S., Antolin S., Lin C.W., Lin Y., Lois C. Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J. Neurosci. 2013;33:7928–7940. doi: 10.1523/JNEUROSCI.1571-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S., Rey N., Sacquet J., Mandairon N., Didier A. Newborn neurons in the olfactory bulb selected for long-term survival through olfactory learning are prematurely suppressed when the olfactory memory is erased. J. Neurosci. 2011;31:14893–14898. doi: 10.1523/JNEUROSCI.3677-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thestrup T., Litzlbauer J., Bartholomäus I., Mues M., Russo L., Dana H., Kovalchuk Y., Liang Y., Kalamakis G., Laukat Y., et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat. Meth. 2014;11:175–182. doi: 10.1038/nmeth.2773. [DOI] [PubMed] [Google Scholar]
- Turnley A.M., Basrai H.S., Christie K.J. Is integration and survival of newborn neurons the bottleneck for effective neural repair by endogenous neural precursor cells? Front. Neurosci. 2014;8:29. doi: 10.3389/fnins.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J.L., Wienisch M., Murthy V.N. Development and refinement of functional properties of adult-born neurons. Neuron. 2017;96:883–896.e7. doi: 10.1016/j.neuron.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M.C., Greer C.A. Adult-generated neurons exhibit diverse developmental fates. Dev. Neurobiol. 2007;67:1079–1093. doi: 10.1002/dneu.20389. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. USA. 2005;102:9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Yankner B.A. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code can be accessed on https://github.com/GaraschukLab/abJGN-Interneuron-Survival-Classification.