Summary
Human retinal organoid transplantation could potentially be a treatment for degenerative retinal diseases. How the recipient retina regulates the survival, maturation, and proliferation of transplanted organoid cells is unknown. We transplanted human retinal organoid-derived cells into photoreceptor-deficient mice and conducted histology and single-cell RNA sequencing alongside time-matched cultured retinal organoids. Unexpectedly, we observed human cells that migrated into all recipient retinal layers and traveled long distances. Using an unbiased approach, we identified these cells as astrocytes and brain/spinal cord-like neural precursors that were absent or rare in stage-matched cultured organoids. In contrast, retinal progenitor-derived rods and cones remained in the subretinal space, maturing more rapidly than those in the cultured controls. These data suggest that recipient microenvironment promotes the maturation of transplanted photoreceptors while inducing or facilitating the survival of migratory cell populations that are not normally derived from retinal progenitors. These findings have important implications for potential cell-based treatments of retinal diseases.
Keywords: photoreceptor cell, pluripotent stem cell, hereditary retinal diseases, cell invasion, cell motility, transcriptome, neural progenitor
Highlights
-
•
Transplanted retinal organoids can generate unexpected nonretinal cells
-
•
These cells include astrocytes and brain/spinal-like (BSL) precursors
-
•
The astrocytes and BSL cells show long-range transretinal migration
-
•
Photoreceptor maturation in retinal organoids is accelerated in the subretinal space
To study how the recipient retina regulates the survival, maturation, and proliferation of exogenous transplanted cells, Singh and colleagues transplanted human retinal organoids into photoreceptor-deficient mice alongside cultured controls. They found unexpected cells that migrated long distances in the retina and identified them as astrocytes and brain/spinal-like precursors. Their findings suggest that recipient microenvironmental cues may play a role in in regulating the fate, survival, and spatial distribution of transplanted cells.
Introduction
Transplantation of immature retinal cells, such as photoreceptor precursor cells and retinal progenitor cells, has the potential to restore function to the degenerated or dysfunctional human retina. Vision loss may be retarded or reversed by direct cellular integration or cellular material transfer (Bartsch et al., 2008; Gouras et al., 1994; Kalargyrou et al., 2021; Kwan et al., 1999; Lamba et al., 2009; MacLaren et al., 2006; Ortin-Martinez et al., 2021; Pearson et al., 2012, 2016; Santos-Ferreira et al., 2016; Singh et al., 2013, 2016; Yang et al., 2010). Stem cell-derived retinal organoids (Assawachananont et al., 2014; Capowski et al., 2019; Eiraku et al., 2011; Eldred et al., 2018; Gonzalez-Cordero et al., 2013; Kaewkhaw et al., 2015; Mandai et al., 2017; Nakano et al., 2012; Shirai et al., 2016; Tucker et al., 2013; Wahlin et al., 2017; Zhong et al., 2014) are potential sources of renewable and standardized donor cells for therapy. Successful transplantation of these cells will require more advanced understanding of the interactions between donor cells and the recipient microenvironment. Here we address this challenge by transplanting human retinal organoid-derived donor cells subretinally into recipient mice and examining how the recipient microenvironment affects donor cells.
A substantial body of data in multiple animal models supports the regenerative potential of non-migratory donor photoreceptor precursor-derived cells that mature in the recipient subretinal space, spurring experiments in large animals (Aboualizadeh et al., 2020; Ghosh et al., 2004; Klassen et al., 2007; Shirai et al., 2016; Tu et al., 2019; Zhou et al., 2011) en route to clinical studies (Berger et al., 2003; Das et al., 1999; Humayun et al., 2000; jCyte and California Institute for Regenerative, 2020; Liu et al., 2017; ReNeuron, 2022). Aside from the non-migratory cells, migratory donor cells in the inner retinal layers overlying the graft have been observed (Foik et al., 2018; Hambright et al., 2012; Klassen et al., 2004; Lin et al., 2018; Luo et al., 2014; McLelland et al., 2018; Thomas et al., 2021; Yamasaki et al., 2022; Yang et al., 2002; Zou et al., 2019), which do not appear to be essential for the therapeutic mechanism. These observations raised concerns that long-range migration of donor cells beyond the graft margins may trigger immune exposure and invasive tissue damage. In the few studies of photoreceptor transplantation in nonhuman primate recipients (Shirai et al., 2016; Tu et al., 2019), migratory cells have occasionally been observed (Tu et al., 2019), raising the possibility that it may also occur in humans. Studying the migration of donor cells may also illuminate strategies to regulate their spatial targeting, as with retinal ganglion cell homing in response to an induced chemokine gradient (Soucy et al., 2021). Here, we assess the effects of the recipient retinal microenvironment on the maturation and migratory behavior of transplanted cells derived from human retinal organoids. We molecularly define the identity of the migratory cells that depart from, and the non-migratory cells that remain in, the subretinal graft.
Whereas cell movement is not readily observed in the normal and pathological adult retina, retinal progenitor cells and their descendants undergo considerable radial migration and limited tangential migration during neurogenesis (Poggi et al., 2005; Turner and Cepko, 1987; Turner et al., 1990). In contrast, other CNS cells, including GABAergic neural precursors in the telencephalon (Park et al., 2002) and optic nerve-derived astrocytes in the retina (Paisley and Kay, 2021), undergo long-distance tangential migration. Similarly, stem cells and their progeny migrate following transplantation into the CNS (Liu et al., 2000; Scheffler et al., 2003; Srivastava et al., 2006; Tabar et al., 2005). Thus, each developmental or transplantation context differentially regulates migration.
The origin, heterogeneity, spatial distribution, and proliferative capacity of migratory donor cells derived from retinal sources have not been characterized. Migratory cells can arise from human retinal organoids (Foik et al., 2018; McLelland et al., 2018; Thomas et al., 2021; Tu et al., 2019; Yamasaki et al., 2022; Zou et al., 2019) and human fetal retina (Lin et al., 2018; Zhou et al., 2015), suggesting that migratory behavior is not unique to donor cells obtained through pluripotent stem cell culture. Migratory donor cells were observed in mouse, rat, cat, and nonhuman primate retinas, and in normal and degenerative retinas (Hambright et al., 2012; Klassen et al., 2004; Luo et al., 2014; McLelland et al., 2018; Singh et al., 2019; Tu et al., 2019; Yang et al., 2002; Zhu et al., 2018), suggesting that this phenomenon is common across recipient species and independent of retinal health. A few migratory cells were reported to express GFAP (Yang et al., 2002; Zhu et al., 2018), progenitor markers (e.g., PAX6, Nestin) (Luo et al., 2014; Zhu et al., 2018) or neuronal markers (e.g., MAP2, β-tubulin3) (Seiler et al., 2014), but not mature photoreceptor markers (Foik et al., 2018; Hambright et al., 2012; McLelland et al., 2018; Seiler et al., 2017; Thomas et al., 2021; Tu et al., 2019; Yamasaki et al., 2022; Zhu et al., 2018; Zou et al., 2019). Gasparini et al., in a study of the influence of the murine retinal microenvironment on the morphological maturation of transplanted donor human cone photoreceptors, also observed migratory cells but did not molecularly characterize these cells (Gasparini et al., 2022). Our studies use an unbiased approach to molecularly characterize the migratory and non-migratory cells that arise from human retinal organoids following transplantation into mice.
To understand how the recipient microenvironment influences donor cells, we transplanted the micro-dissected multilayered retinal fragments from human retinal organoids into mouse retinas and used imaging and single cell transcriptomics to characterize the identities, maturity, and migratory activities of graft-derived cells. We identified donor-derived migratory astrocytes and brain/spinal cord-like neural precursors that do not normally arise from retinal progenitor cells, and found that non-migratory photoreceptors matured more rapidly in the subretinal space. Our findings highlight a key strength of organoid-derived cell transplantation in promoting photoreceptor maturation and a potential weakness in the expansion and widespread tangential migration of a population of migratory astrocytes and brain/spinal cord-like neural precursor cells.
Results
Subretinally transplanted human cells migrate from or remain in the subretinal space
To determine how the recipient subretinal space affects donor cells, we differentiated human retinal organoids, dissected them into retinal fragments and transplanted them into recipient mice, and later assessed donor cell position, fate, and maturity. To generate recipient mice, we crossed and bred mice with immune deficiency and retinal degeneration (Figure S1). These C3H/HeJ-Pde6bRd1/Rd1(Rd1) and NOD.Cg-Prkdcscid/J (NOD/Scid) double mutant mice are termed Rd1/NS. To generate donor cells, we used H9 human embryonic stem cells (hESCs) carrying a reporter that is expressed in both rod and cone photoreceptors (CRX:tdTomato) (Phillips et al., 2018). We used a gravity aggregation approach to differentiate stem cells into retinal organoids with robust generation of photoreceptors (Eldred et al., 2018; Nakano et al., 2012; Wahlin et al., 2017). On day 134 of organoid culture, we micro-dissected the human retinal organoids and transplanted the fragments into the subretinal space of recipient eyes (n = 16 eyes). Four and a half months later, we evaluated the transplants.
As homozygosity for the Rd1 allele causes virtually all photoreceptors to degenerate by adulthood in mice (Bowes et al., 1990), distinct recipient outer nuclear and outer plexiform layers were not observed but the inner nuclear, inner plexiform, retinal ganglion cell (RGC), and retinal nerve fiber layers (collectively, the “inner retina”) were present.
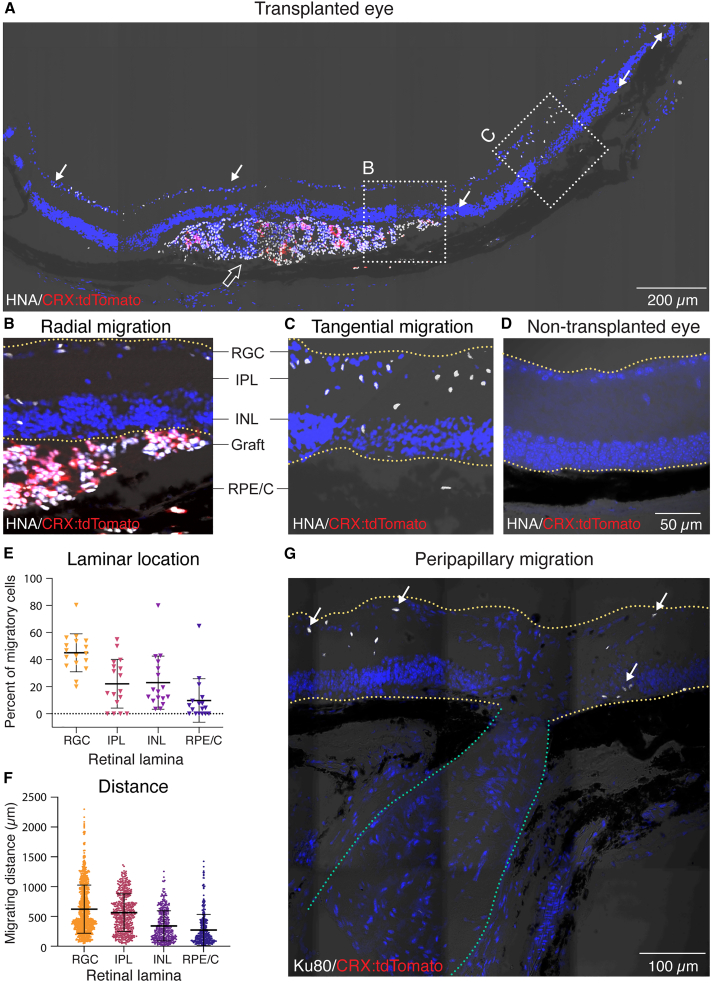
We determined the positions of donor cells relative to the subretinal transplantation site. We identified all human donor cells on the basis of immunolabeling for human nuclear antigen (HNA) or human ATP-dependent DNA helicase 2 subunit (Ku80 protein). We identified human donor photoreceptors on the basis of transgenic expression of CRX:tdTomato (Figure 1A). We observed two main classes of donor cells: (1) human cells in the recipient subretinal space (“non-migratory cells”) that included both photoreceptor or non-photoreceptor cells (Figure 1A) and (2) human cells within the recipient inner retina (“migratory cells”) that were not photoreceptors (Figure 1A), suggesting that this population had migrated from the graft. Migratory cells were observed in the recipient inner retinal layers overlying the graft (“radial migration”; Figure 1B), whereas others had migrated away from and beyond the edges of the graft (“tangential migration”; Figure 1C). Migratory cells traveled into all retinal layers, including the retinal ganglion cell layer, inner plexiform layer (IPL), inner nuclear layer (INL), and retinal pigment epithelium/choroid (RPE/C) layer (Figure 1E). Of the tangential migratory cells (n = 2,378 cells), 98.9% were within 1,500 μm of the edges of the graft. The remaining 1.1% traveled beyond 1,500 μm and were located exclusively in the RGC layer (Figure 1F). We detected several migratory human cells in the regions flanking the optic nerve (“peripapillary migration”; Figure 1G) but none in the optic nerve itself. We next sought to molecularly classify the fates of these non-migratory and migratory cells.
Figure 1.
Subretinally transplanted human cells migrate from, or remain in, the subretinal space
(A) IHC staining of human nuclear antigen (HNA) showed migratory (arrows) and non-migratory (empty arrow) human cells in the mouse retina. Transplanted photoreceptors were identified by the expression of CRX:tdTomato reporter.
(B and C) Migratory cells were detected overlying the graft (radial migration) and beyond the graft edge (tangential migration).
(D) A non-transplanted mouse eye showed negative staining for HNA and CRX:tdTomato.
(E) Relative abundance of migratory human cells in different recipient retinal laminae.
(F) Migrating distance of human cell nuclei from the graft edge in different retinal laminae.
(G) Migratory Ku80+ human cells found in the regions flanking the optic nerve (peripapillary migration). White arrows showed representative migratory human cells.
Data were collected from 5 eyes. Statistical data were presented as mean ± SD. Yellow lines in (B), (C), (D), and (G) denote the boundaries of the recipient retina; green lines in (G) denote the optic nerve.
Subretinal microenvironment facilitates the proliferation, differentiation and/or survival of retinal and nonretinal cells
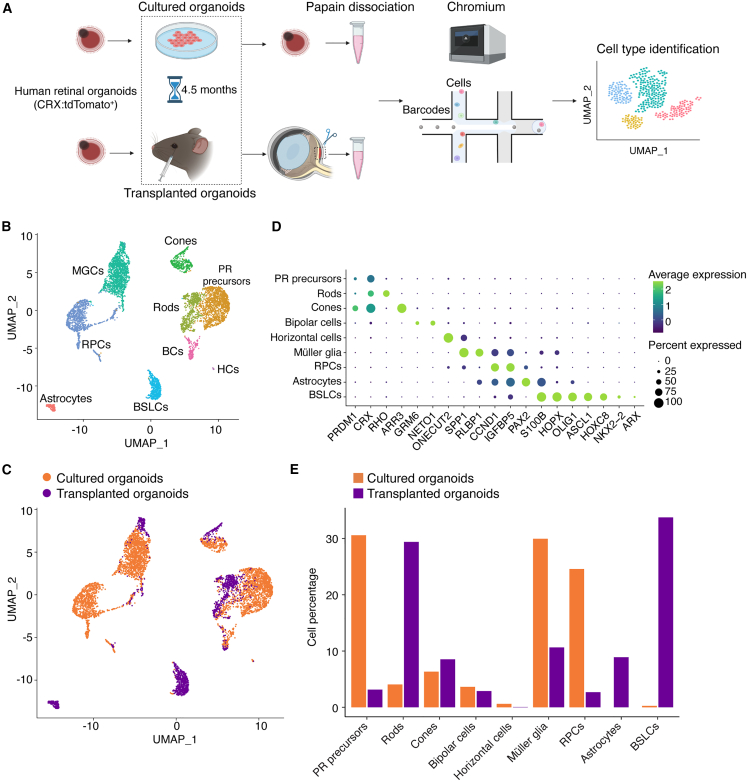
To determine how the recipient subretinal microenvironment affects the gene expression and the specification of the migratory and non-migratory donor cells, we conducted single-cell RNA sequencing (scRNA-seq) on cells from human CRX:tdTomato+ retinal organoids transplanted and matured in vivo (“transplanted organoids”) and from age-matched CRX:tdTomato+ retinal organoids that were maintained in vitro (“cultured organoids”) (Figure 2A). We analyzed a total of 5,831 human cells that were recovered from the transplanted (1,561 cells) and cultured (4,270 cells) organoids. We identified retinal cell types including retinal progenitor cells (RPCs), photoreceptor precursor cells, rods, cones, bipolar cells, horizontal cells, and Müller glia on the basis of their gene expression profiles (Figures 2B–2D). The quantities of cones, bipolar cells, and horizontal cells were similar in the transplanted and cultured organoids. In contrast, retinal progenitor cells, photoreceptor (PR) precursor cells, and Müller glia were more abundant in cultured organoids, whereas rods were more abundant in transplanted organoids (Figure 2E). The smaller populations of RPCs and photoreceptor precursor cells and larger population of rods in the transplanted organoids suggest that the recipient microenvironment promotes the survival and/or maturation of exogenous photoreceptor cells.
Figure 2.
Subretinal microenvironment facilitates the proliferation, differentiation and/or survival of retinal and nonretinal cells
(A) Schematic showing the in vivo and in vitro conditions of the donor cells analyzed by single-cell RNA sequencing (scRNA-seq).
(B and C) scRNA-seq identified nine transcriptionally distinct cell clusters from the pool of transplanted and cultured retinal organoid cells (n = 5,831 cells).
(D) Dot plots of marker gene expression in the identified cell clusters. Color scale indicates the average gene expression; Dot size represents the percent of positively expressing cells.
(E) The relative abundance of various cell types in transplanted and cultured retinal organoids.
In addition to these cell types, we identified two cell clusters that could not be ascribed solely to known retinal-derived cell fates. The cells in one cluster expressed genes that are broadly expressed in retinal and other CNS progenitors such as ASCL1 (Figure 2D) and HES6 (Figure S2). They also expressed genes that are not normally detected in the developing retina including NKX2-2 and ARX, both of which are prominently expressed in telencephalic and/or diencephalic neural progenitors, as well as HOXC8, whose expression is normally restricted to the developing spinal cord (Figure 2D). On the basis of this gene expression profile, we designated the cells in this cluster as “brain and spinal cord-like” (BSL) cells. BSL cells comprised approximately 1% of cells in the cultured organoids but were more than 30 times more abundant in the transplanted organoids (Figure 2E). Cells in the second cluster expressed markers characteristic of retinal astrocytes, such as PAX2 and S100B (Figure 2D). Normally, retinal astrocytes are born in the optic nerve head and migrate into the retina. Strikingly, astrocytes were entirely absent in the cultured organoids but constituted approximately 8% of cells in the transplanted organoids (Figure 2E). These data suggest that microenvironmental cues in the host retina allow the proliferation, differentiation and/or survival of cell types that are not normally derived from retinal progenitors.
Donor-derived migratory cells include astrocytes and brain/spinal cord-like neural precursors
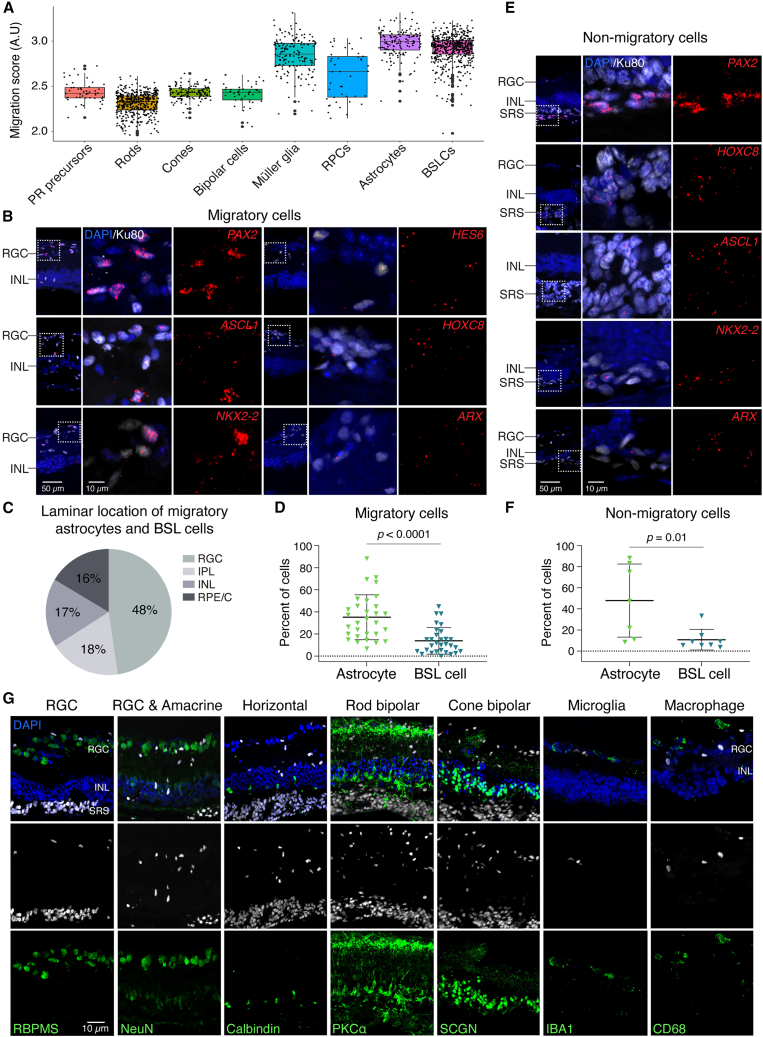
Next, we connected the cell types identified by scRNA-seq analysis to their migratory or non-migratory properties. We computationally aggregated and scored the expression of genes associated with cell motility and migration by Gene Ontology (GO) classification (Data S1). We found that astrocytes and BSL cells had the highest average migration scores (Figure 3A), suggesting that these were the observed migratory cells.
Figure 3.
Donor-derived migratory cells include astrocytes and brain/spinal cord-like neural precursors
(A) Astrocytes and BSL cells show the highest migration score among the cell types identified in transplanted retinal organoids (n = 3 eyes).
(B) RNAScope showed migratory cells expressing markers of astrocytes (PAX2, HES6) and BSL cells (ASCL1, HOXC8, NKX2-2, and ARX).
(C) Relative abundance of migratory astrocytes and BSL cells in different recipients’ retinal laminae.
(D) Quantification of migratory astrocytes and BSL cells in migratory human cells (n = 3 or 4 eyes).
(E and F) RNAScope staining (E) and quantification (F) of non-migratory astrocytes and BSL cells in the subretinal space (SRS) (n = 4 eyes).
(G) Migratory cells negatively expressed markers of RGC (RBPMS, NeuN), amacrine cells (NeuN), horizontal cells (calbindin), rod bipolar cells (PKCα), cone bipolar cells (SCGN), microglia (IBA1), and macrophage (CD68). KU80- and HNA-labeled human cells.
Statistical data were presented as mean ± SD.
To test this hypothesis, we examined expression of genes including PAX2 (astrocyte marker), and HES6, ASCL1, HOXC8, NKX2-2, and ARX (BSL markers), that identify the migratory cells. We observed expression of these genes in migratory cells derived from hESCs (Figures 3B, 3C, and S2). To validate these observations in progeny of a different pluripotent cell lines, we stained for PAX2, HOXC8, NKX2-2 in specimens from recipient eyes that were transplanted with human induced pluripotent stem cell (hiPSC)-derived retinal organoid cells. In this context, approximately four months post-transplantation into adult Rd1/NS recipient mice, we detected PAX2, HOXC8, NKX2-2 in migratory cells (Figure S3). We have not detected the gene expression of human astrocyte (PAX2) and BSL (HES6, ASCL1, HOXC8, NKX2-2, ARX) in non-transplanted Rd1/NS control mice (Figure S4). The hESC-derived and hiPSC-derived cells expressing these genes were located in all recipient retinal layers, suggesting that astrocytes and BSL cells derived from both types of pluripotent cells possessed migratory capacity. Among migratory cells, human astrocytes were more abundant than BSL cells (Figure 3D). Among non-migratory cells that remained in the subretinal space within the graft, we also observed astrocytes and BSL cells (Figures 3E and 3F), suggesting that expression of astrocytes or BSL cell-related genes alone was insufficient for migration.
Migratory cells were almost exclusively CRX:tdTomato–, consistent with these cells being astrocytes or BSL cells and not photoreceptor cells (see Figure 1A). Rare CRX:tdTomato+ cells were detected in the RPE/Choroid layer and were possibly misplaced photoreceptors (see Figure 1A). Human migratory cells did not express established markers of RGCs, amacrine cells, horizontal cells, rod and cone bipolar cells, microglia, or macrophages (Figures 3G and S2).
Together, these data suggest that some but not all donor human astrocytes and BSL cells were migratory cells whereas donor photoreceptors and other retinal neurons were non-migratory.
Actively proliferating cells are rare among migratory and non-migratory donor cells
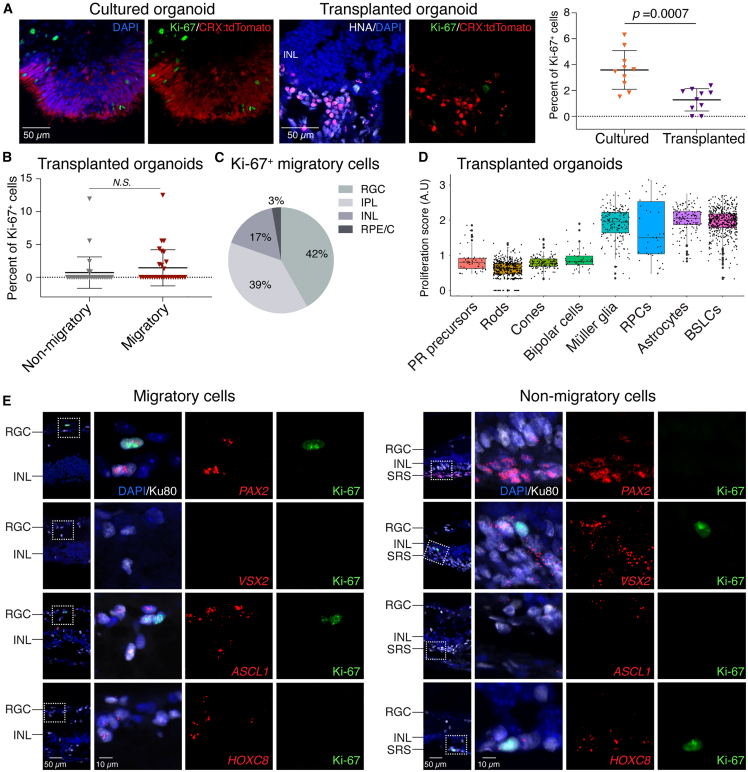
Migratory cells, especially if they are proliferative, may negatively impact the recipient. To determine the influence of the recipient microenvironment on the proliferation of migratory and non-migratory donor cells, we examined expression of the proliferation marker protein Ki-67. As expected, expression of Ki-67 was rarely observed in cultured retinal organoids and was significantly less abundant in transplanted organoids (Figure 4A). The smaller population of proliferating cells in the transplanted organoids suggests that the in vivo microenvironment may promote maturation of postmitotic photoreceptor cells.
Figure 4.
Actively proliferating cells are rare among migratory and non-migratory donor cells
(A) IHC staining showed that Ki-67+ proliferating cells were rare in cultured organoids (n = 4 in one batch) and significantly less in transplanted organoids (n = 3 eyes).
(B) Ki-67+ cells were rare among non-migratory and migratory cells in transplanted retinal organoids (n = 5 eyes).
(C) Relative abundance of migratory Ki-67+ cells in different retinal laminae.
(D) ScRNA-seq analysis showed the highest proliferation score in Müller glia, retinal progenitor cells (RPCs), astrocytes, and BSL cells among the identified cell types in transplanted retinal organoids (n = 3 eyes).
(E) Histological assay showed sparse Ki-67+ cells in migratory astrocytes (PAX2+), BSL cells (ASCL1+), and non-migratory RPCs (VSX2+) and BSL cells (HOXC8+) (n = 4 eyes).
Statistical data were presented as mean ± SD.
In eyes with transplanted organoids, 0.7% of non-migratory cells and 1.4% of migratory cells expressed Ki-67 (Figure 4B), and the difference between these values was not statistically significant. We observed that the few Ki-67+ migratory cells occupied all laminae of the recipient retina (Figure 4C).
To identify the proliferating cells, we developed a proliferation scoring system by computationally aggregating the expression level of proliferation-associated genes (Data S2). We found that astrocytes, Müller glia, RPCs, and BSL cells showed the highest proliferation score (Figure 4D), suggesting that these cells were proliferating. To test this hypothesis, we examined expression of Ki-67 in PAX2+ (astrocytes), VSX2+ (RPCs), and ASCL1+ and HOXC8+ (BSL) cells. Precise quantification was impractical because of the rarity of double-positive cells. Nevertheless, we found a few migratory PAX2+ astrocytes, and very few migratory ASCL1+ BSL cells, that were Ki-67+. Proliferating Ki-67+/VSX2+ RPCs remained in the subretinal space (Figure 4E).
Taken together, these data suggest that migratory proliferating donor human cells are rare and are mostly astrocytes, and that non-migratory proliferating cells are rare and are mostly RPCs.
In contrast to migrating cells, the subretinal microenvironment induces accelerated maturation of non-migratory rods and cones
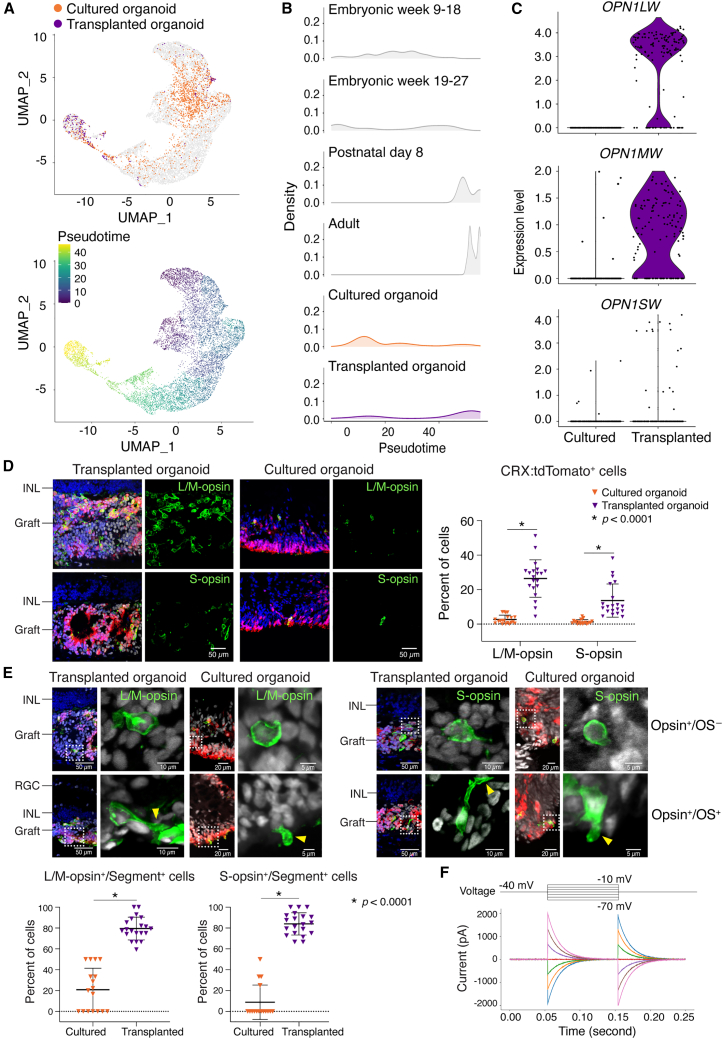
Our scRNA-seq analysis suggested that the recipient subretinal space promotes photoreceptor maturation (see Figure 2E). To test this hypothesis, we assessed cone maturation. We evaluated the gene expression profiles of cones from transplanted organoids and cultured organoids using pseudotime analysis, comparing these cells to published datasets of embryonic, postnatal, and adult cones isolated directly from human retina (Lu et al., 2020). The transcriptional profiles suggested that the cones from transplanted organoids resembled adult cones, whereas the cones from cultured organoids more closely resembled embryonic cones (Figures 5A and 5B). Expression of mature cone-specific genes were consistently higher in transplanted than in cultured cones (Figure S5), including all three cone opsins (OPN1LW, OPN1MW, and OPN1SW) (Figure 5C). The proportions of CRX:tdTomato+ cells that expressed L/M-opsin or S-opsin were significantly higher in transplanted organoids (L/M-opsin+, 26.4%; S-opsin+, 13.7%) compared with cultured organoids (L/M-opsin+, 2.7%; S-opsin+, 1.3%) (Figure 5D). Similarly, the fraction of L/M-opsin+ or S-opsin cells+ with inner or outer segments (segment+) was significantly higher in transplanted organoids than cultured organoids (Figure 5E). We measured the intrinsic electrical properties of a transplanted human cone cell and found large capacitance currents (∼2 nA), indicating the presence of relatively large cell membrane areas that are normally observed in mature cones (Figure 5F).
Figure 5.
The subretinal microenvironment induces accelerated maturation of non-migratory cones
(A) Uniform manifold approximation and projection (UMAP) plots embedded the pseudotime maturation trajectories of cone photoreceptors in transplanted (n = 3 eyes) and cultured retinal organoids (n = 2 form the same batch as the transplanted organoids), comparing to normal human cone development. Cells are colored by cell type (top) and pseudotime (bottom).
(B) Ridge plots showed the transcriptional maturation of transplanted and cultured cone photoreceptors.
(C) Violin plots showed significantly more OPN1LW, OPN1MW, and OPN1SW expression in transplanted than cultured retinal organoids.
(D) Histological analysis of L/M-opsin+ or S-opsin+ photoreceptors in transplanted (n = 4 eyes) and cultured retinal organoids (n = 4 in one batch).
(E) Representative L/M-opsin+ or S-opsin+ cone photoreceptors with (OS+, yellow arrow heads) or without (OS−) outer segments. Quantification showed the fraction of L/M-opsin+ or S-opsin+ cells with inner/outer segment (segment+) in transplanted (n = 4 eyes) and cultured retinal organoids (n = 4 in one batch).
(F) Single-cell patch-clamp recording of a transplanted human cone photoreceptor.
Statistical data were presented as mean ± SD.
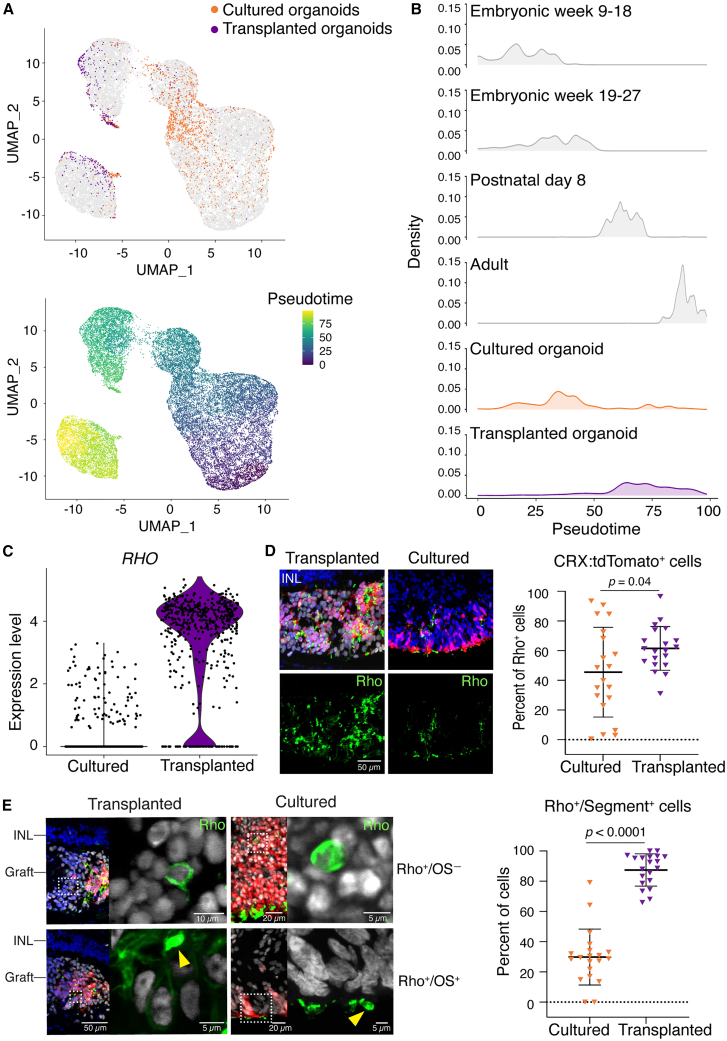
Next, we evaluated rod maturation in transplanted organoids and cultured organoids. As with cones, gene expression and pseudotime analysis suggested that rods from transplanted organoids resembled adult rods, whereas rods from cultured organoids resembled embryonic rods (Figures 6A and 6B). Expression of RHO (Figure 6C) and other rod-specific genes (Figure S5) was higher in rods from transplanted organoids than cultured organoids. The proportions of CRX:tdTomato+ cells that expressed Rho were significantly higher in transplanted organoids (61.5%) compared with cultured organoids (45.5%) (Figure 6D). Similarly, the fraction of Rho+ cells with inner or outer segments (segment+) was significantly higher in transplanted organoids (87.4%) than cultured organoids (29.8%) (Figure 6E).
Figure 6.
The subretinal microenvironment induces accelerated maturation of non-migratory rods
(A) UMAP plots embedded the pseudotime maturation trajectories of rod photoreceptors in transplanted (n = 3 eyes) and cultured retinal organoids (n = 2 from the same batch as the transplanted organoids), comparing to human rod development. Cells were colored by cell type (top) and pseudotime (bottom).
(B) Ridgeline plot showed the transcriptional maturation of transplanted and cultured rod photoreceptors.
(C) Violin plots showed more RHO gene expression in transplanted than cultured retinal organoids.
(D) IHC analysis showed the percent of Rho+ cells in transplanted (n = 4 eyes) than cultured retinal organoids (n = 4 in one batch).
(E) Representative images showed Rho+ rod photoreceptors with (OS+, yellow arrow heads) or without (OS−) outer segments. Quantification showed the fraction of Rho+ cells with inner/outer segment (segment+) in transplanted (n = 4 eyes) than cultured retinal organoids (n = 4 in one batch).
Statistical data were presented as mean ± SD.
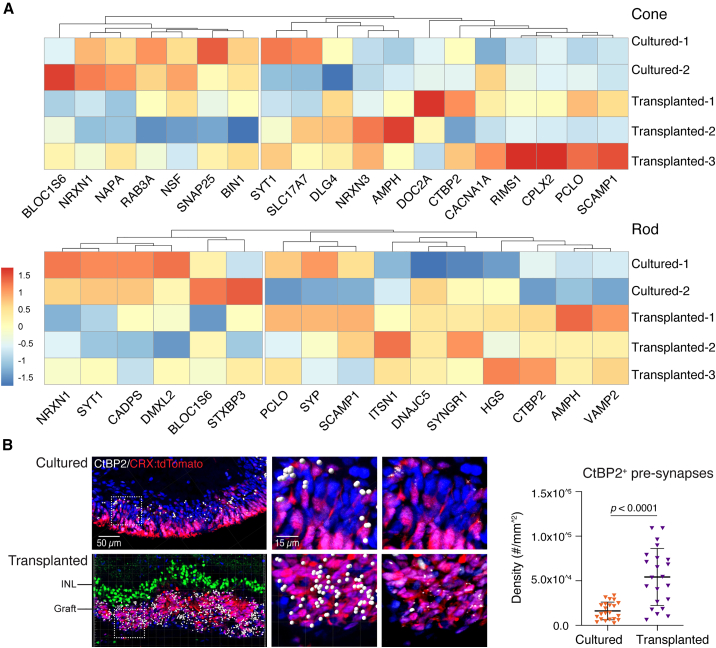
Finally, we investigated general features of photoreceptor maturity. Expression of certain synaptic proteins was upregulated in cones and rods in transplanted CRX:tdTomato+ organoids compared with cultured CRX:tdTomato+ organoids (Figure 7A). In CRX:tdTomato+ donor photoreceptors, the number of CtBP2+ puncta, which marks synaptic ribbons, was significantly higher in cells from transplanted organoids compared with cultured retinal organoids (Figure 7B). These data suggest that the recipient subretinal space promotes maturation of rods and cones more effectively than the in vitro microenvironment of cultured organoids.
Figure 7.
Expression of synaptic proteins is upregulated in transplanted photoreceptors
(A) Heatmaps of synaptic gene expression in transplanted and cultured retinal organoids.
(B) IHC test showed CtBP2+ ribbons synapse in photoreceptors in transplanted (n = 4 eyes) than cultured retinal organoids (n = 4 in one batch). CtBP2+ pre-synapses are highlighted with white dots using Imaris (version 9.1), raw images are on the right panel. IHC staining of SCGN (green) showed the recipient bipolar layer.
Statistical data were presented as mean ± SD.
Discussion
In these studies, we observed two major differences between cells from donor retinal organoids transplanted into mice and cells from chronologically equivalent retinal organoids maintained in culture. The transplanted cells were maintained in the degenerative recipient subretinal space for several months, therefore simulating conditions directly relevant for cell-based therapies for photoreceptor dystrophy. The most prominent and unexpected difference was the observation of migratory donor astrocytes and BSL cells in the transplanted cell population. Astrocytes and BSL cells underwent radial migration into, and long-distance tangential migration along, all retinal laminae (apart from the outer nuclear layer of photoreceptor cells that was absent in the degenerate recipients). The migratory astrocytes and BSL cells were generally non-proliferative, although graft-derived retinal progenitors showed proliferation without migration. In contrast to these migratory cells, transplanted photoreceptors, inner retinal neurons, and Müller glia were non-migratory and remained in the subretinal transplant site. The second major difference between transplanted and cultured organoids pertained to photoreceptor maturity. On the basis of gene expression and morphology, transplanted rods and cones were more mature than photoreceptors from cultured organoids. These data expand our understanding of photoreceptor and non-photoreceptor development in transplanted retinal organoids and highlight the importance of unbiased approaches to cell fate identification and spatial tracking following organoid transplantation.
We considered the possibility that cellular material transfer (Ortin-Martinez et al., 2017; Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2014, 2016) of human nucleus-related antigens may have confounded the histological detection of human cells. We believe that the migratory cells in this report are bona fide human cells for five reasons. First, all the cells expressing astrocyte and BSCL cell markers were identified by scRNA-seq as being human cells by expression of many different human mRNAs from those of mouse. Second, we used human-specific RNA probes for RNAScope assays used to detect migrating cells. Third, we used two human nuclear-specific antibodies—HNA and Ku80, which target different human nuclear antigens—and found identical features of migration of HNA+ and Ku80+ cells. Fourth, the uniform and strong intensity of histological staining (HNA and Ku80), essentially iso-intense with the subretinal human cells, also implies that these cells are of human rather than mouse origin. Last, the migratory HNA+ and Ku80+ cells were generally located at considerable distances from the subretinal donor cell mass, arguing against cellular material transfer which tends to occur among neighboring cells with the potential for direct cell-cell membrane contact.
The migratory astrocytes and BSL cells from transplanted organoids display molecular profiles distinct from cells present in mature cultured organoids. The astrocytes express PAX2, which normally delineates optic stalk-derived astrocytes in vivo (Wahlin et al., 2021). PAX2 is also detected in retinal progenitors in early-stage retinal organoids but is undetectable at later stages (Cowan et al., 2020; Lu et al., 2020). Moreover, cultured retinal organoids have not been reported to generate astrocytes in vitro. The BSL cells express ASCL1, HOXC8, NKX2-2, and ARX. NKX2-2- and ARX-expressing cells are found in very early-stage retinal organoids, but are not detected after 60 days in culture (Lu et al., 2020). HOXC8 expression is normally restricted to the posterior spinal cord and is entirely absent from developing human retina and retinal organoids (Cowan et al., 2020; Lu et al., 2020). Though PAX2+ astrocytes and ARX+ telencephalic interneurons undergo long-distance tangential migration in vivo (Paisley and Kay, 2021; Park et al., 2002), astrocyte or BSL identity was not sufficient to induce migration of graft-derived cells, as many astrocytes and BSL cells remained localized in the subretinal space. Our experiments lacked the temporal resolution to determine whether transplantation induced trans-differentiation of cells that initially adopted retinal identity or selectively promoted the proliferation and/or survival of small numbers of residual astrocytes and BSL cells that are present at the time of transplantation.
Prior publications have shown migratory transplanted cells, but their capacity to proliferate and migrate long distances were not known. Seiler and colleagues noted migratory human donor cells six months after transplantation of early-stage hESC-derived retinal organoids in the retinal degeneration nude rat (McLelland et al., 2018). Using LMNB2 to identify human donor cells, Lamba and colleagues reported occasional migratory human induced pluripotent stem cell (iPSC)-derived PAX6+ and GFAP+ cells at 2 months post-transplantation (Zhu et al., 2018). In another study, migratory cells were seen just seven days after subretinal delivery of human fetal CD29+/SSEA1+ donor cells (Lakowski et al., 2018), suggesting that migration occurs soon after transplantation. Whether the early-migratory cells are the same as those observed months later is unknown. In the wild-type cat (Singh et al., 2019), enhanced immunosuppression appeared to lead to greater cell migration, suggesting a role of immune cells in restricting this process. It is not known whether migratory donor cells negatively affect recipient retinal function or if depletion of astrocyte/BSL precursor cells is required prior to transplantation for maximal therapeutic efficacy.
The cues in the recipient microenvironment that promote cell migration and photoreceptor maturation are not known. Multiple cell-extrinsic cues regulate cell specification in human retinal organoids. Dynamic regulation of thyroid hormone and retinoic acid signaling specifies cone subtypes in human retinal organoids (Eldred et al., 2018; Hadyniak et al., 2021). Though the roles of these cues in the subretinal microenvironment following transplantation is not understood, they potentially regulate photoreceptor specification and maturation.
In conclusion, we found that the murine subretinal microenvironment affects human stem cell-derived retinal organoid cells in two distinct ways. First, the recipient microenvironment facilitates the differentiation and/or survival of cell populations of organoid-derived astrocytes and BSL cells that are capable of radial and tangential migration. Second, the recipient microenvironment promotes the maturation of organoid-derived rod and cone photoreceptors that remain in the subretinal space. These results may inform future research on the consequences of donor cell migration in transplant recipients, methods to purify retinal organoid-derived cells, and pharmacological strategies to accelerate the maturation of donor photoreceptor cells. A deeper understanding of these issues may help to guide the development of safe and effective clinical treatment involving the replacement or augmentation of human stem cell-derived retinal photoreceptor cells for the purposes of restoring visual function.
Experimental procedures
Resource availability
Corresponding author
The data that support the findings of this study are available from the corresponding authors Mandeep S. Singh (singhcorrespauth@gmail.com), Seth Blackshaw (sethblackshaw@gmail.com), and Robert J. Johnston (robertjohnston@jhu.edu), upon reasonable request.
Materials availability
This study did not generate new unique materials.
The use of human stem cells was approved by the Johns Hopkins ISCRO (ISCRO00000249) and the University of Colorado Office of Regulatory Compliance. All animal experiments were carried out in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All procedures were approved by the Johns Hopkins University Animal Care and Use Committee (approval M016M17).
Detailed experimental methods are provided in the supplemental information.
Author contributions
M.S.S., S.B., and R.J.J. conceived the experiments. Y.V.L., C.P.S., A.S., G.J.K., S.E.H., K.A.H., T.J.C., L.D.O., Z.J., K.V.L., M.F.-B., and S.A.-D. performed the experiments. All the authors contributed to data analysis and approved the final manuscript.
Acknowledgments
This work was funded by the following funding: National Eye Institute (NEI) R01EY033103 (M.S.S.), Foundation Fighting Blindness (M.S.S.), Stein Innovation Award from Research to Prevent Blindness (S.B.), the Shulsky Foundation (M.S.S.), the Joseph Albert Hekimian Fund (M.S.S.), the Juliette RP Vision Foundation (Y.L.), Gates Frontiers Fund (M.V.C.-S.), The Solich Fund (M.V.C.-S.), CellSight Development Fund (M.V.C.S.), Research to Prevent Blindness (unrestricted grant to the Wilmer Eye Institute at Johns Hopkins University, the Clean Energy Institute at Baylor College of Medicine, and the Department of Ophthalmology, University of Colorado), NEI Core Grant EY001765, Visual Sciences Training grant 2T32EY007143 (C.P.S.), National Science Foundation (NSF) DGE-1746891 (K.A.H.), NIH F31EY029157 (S.E.H.), and NEI R01EY030872 (R.J.J.). We thank Dr. David M. Gamm (University of Wisconsin Hospitals) for kindly providing CRX:tdTomato cell line. We thank Dr. Thomas Vincent Johnson III (Johns Hopkins University) for antibody gifts. We thank Dr. Donald J. Zack (Johns Hopkins University) and José Sahel (University of Pittsburgh) for their helpful comments on this paper. We thank Rhonda Griebe and Mary Ellen Pease (Johns Hopkins University) for their kind technical assistance. We thank Wendy Yap for comments on the manuscript. We also thank Brittney Wick, Matthew Speir, and Maximilian Haeussler with their assistance with hosting the data on the UCSC Cell Browser.
Conflict of interests
M.S.S. is/was a paid advisor to Revision Therapeutics, Johnson & Johnson, Third Rock Ventures, Bayer Healthcare, Novartis Pharmaceuticals, W. L. Gore & Associates, Deerfield, Trinity Partners, Kala Pharmaceuticals, Janssen, and Acucela. M.S.S. has received sponsored research support from Bayer for other research. S.B. receives research support from Genentech, is a co-founder and shareholder in CDI Labs, LLC, and is/was a consultant for Third Rock Ventures and Tenpoint Therapeutics. M.S.S. and R.J.J are co-founders and shareholders in Agnos Therapeutics. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. M.S.S., S.B., J.Q., R.J.J., Y.V.L., C.P.S., M.V.C.-S., M.F.-B., S.A.-D., and K.V.L. are named as inventors on patents or patent applications assigned to their respective universities.
Published: May 9, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.04.004.
Contributor Information
Robert J. Johnston, Jr., Email: robertjohnston@jhu.edu.
Seth Blackshaw, Email: sethblackshaw@gmail.com.
Mandeep S. Singh, Email: singhcorrespauth@gmail.com.
Supplemental information
Data and code availability
The raw scRNA-seq data and count matrices generated during this study can be accessed at GEO accession number GSE197847. The merged count matrices and cell metadata are also available for download at https://github.com/csanti88/transplant_human_organoid_retina_2022. Interactive queries of individual gene expression patterns can be performed on the UCSC cell browser at https://xeno-hesc-retina.cells.ucsc.edu (Speir et al., 2021).
References
- Aboualizadeh E., Phillips M.J., McGregor J.E., DiLoreto D.A., Jr., Strazzeri J.M., Dhakal K.R., Bateman B., Jager L.D., Nilles K.L., Stuedemann S.A., et al. Imaging transplanted photoreceptors in living nonhuman primates with single-cell Resolution. Stem Cell Rep. 2020;15:482–497. doi: 10.1016/j.stemcr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., Sasai Y., Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2:662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch U., Oriyakhel W., Kenna P.F., Linke S., Richard G., Petrowitz B., Humphries P., Farrar G.J., Ader M. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp. Eye Res. 2008;86:691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Berger A.S., Tezel T.H., Del Priore L.V., Kaplan H.J. Photoreceptor transplantation in retinitis pigmentosa: short-term follow-up. Ophthalmology. 2003;110:383–391. doi: 10.1016/S0161-6420(02)01738-4. [DOI] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L.C., Applebury M.L., Farber D.B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Capowski E.E., Samimi K., Mayerl S.J., Phillips M.J., Pinilla I., Howden S.E., Saha J., Jansen A.D., Edwards K.L., Jager L.D., et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 2019;146:dev171686. doi: 10.1242/dev.171686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.S., Renner M., De Gennaro M., Gross-Scherf B., Goldblum D., Hou Y., Munz M., Rodrigues T.M., Krol J., Szikra T., et al. Cell types of the human retina and its organoids at single-cell resolution. Cell. 2020;182:1623–1640.e34. doi: 10.1016/j.cell.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T., del Cerro M., Jalali S., Rao V.S., Gullapalli V.K., Little C., Loreto D.A., Sharma S., Sreedharan A., del Cerro C., Rao G.N. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: results of a long-term safety study. Exp. Neurol. 1999;157:58–68. doi: 10.1006/exnr.1998.6992. [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Eldred K.C., Hadyniak S.E., Hussey K.A., Brenerman B., Zhang P.W., Chamling X., Sluch V.M., Welsbie D.S., Hattar S., Taylor J., et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science. 2018;362:eaau6348. doi: 10.1126/science.aau6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foik A.T., Lean G.A., Scholl L.R., McLelland B.T., Mathur A., Aramant R.B., Seiler M.J., Lyon D.C. Detailed visual cortical responses generated by retinal sheet transplants in rats with severe retinal degeneration. J. Neurosci. 2018;38:10709–10724. doi: 10.1523/JNEUROSCI.1279-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S.J., Tessmer K., Reh M., Wieneke S., Carido M., Völkner M., Borsch O., Swiersy A., Zuzic M., Goureau O., et al. Transplanted human cones incorporate into the retina and function in a murine cone degeneration model. J. Clin. Invest. 2022;132:e154619. doi: 10.1172/JCI154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh F., Wong F., Johansson K., Bruun A., Petters R.M. Transplantation of full-thickness retina in the rhodopsin transgenic pig. Retina. 2004;24:98–109. doi: 10.1097/00006982-200402000-00014. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cordero A., West E.L., Pearson R.A., Duran Y., Carvalho L.S., Chu C.J., Naeem A., Blackford S.J.I., Georgiadis A., Lakowski J., et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Du J., Kjeldbye H., Yamamoto S., Zack D.J. Long-term photoreceptor transplants in dystrophic and normal mouse retina. Invest. Ophthalmol. Vis. Sci. 1994;35:3145–3153. [PubMed] [Google Scholar]
- Hadyniak S.E., Eldred K.C., Brenerman B., Hussey K.A., McCoy R.C., Sauria M.E.G., Kuchenbecker J.A., Neitz M., Neitz J., Taylor J., Johnston R.J. Temporal regulation of green and red cone specification in human retinas and retinal organoids. bioRxiv. 2021 doi: 10.1101/2021.03.30.437763. Preprint at. [DOI] [Google Scholar]
- Hambright D., Park K.Y., Brooks M., McKay R., Swaroop A., Nasonkin I.O. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol. Vis. 2012;18:920–936. [PMC free article] [PubMed] [Google Scholar]
- Humayun M.S., de Juan E., Jr., del Cerro M., Dagnelie G., Radner W., Sadda S.R., del Cerro C. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000;41:3100–3106. [PubMed] [Google Scholar]
- jCyte I., California Institute for Regenerative M. Safety and efficacy of intravitreal injection of human retinal progenitor cells in adults with retinitis pigmentosa. 2020. https://ClinicalTrials.gov/show/NCT03073733
- Kaewkhaw R., Kaya K.D., Brooks M., Homma K., Zou J., Chaitankar V., Rao M., Swaroop A. Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and gene networks. Stem Cell. 2015;33:3504–3518. doi: 10.1002/stem.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalargyrou A.A., Basche M., Hare A., West E.L., Smith A.J., Ali R.R., Pearson R.A. Nanotube-like processes facilitate material transfer between photoreceptors. EMBO Rep. 2021;22:e53732. doi: 10.15252/embr.202153732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H., Kiilgaard J.F., Zahir T., Ziaeian B., Kirov I., Scherfig E., Warfvinge K., Young M.J. Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients. Stem Cell. 2007;25:1222–1230. doi: 10.1634/stemcells.2006-0541. [DOI] [PubMed] [Google Scholar]
- Klassen H.J., Ng T.F., Kurimoto Y., Kirov I., Shatos M., Coffey P., Young M.J. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest. Ophthalmol. Vis. Sci. 2004;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- Kwan A.S., Wang S., Lund R.D. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp. Neurol. 1999;159:21–33. doi: 10.1006/exnr.1999.7157. [DOI] [PubMed] [Google Scholar]
- Lakowski J., Welby E., Budinger D., Di Marco F., Di Foggia V., Bainbridge J.W.B., Wallace K., Gamm D.M., Ali R.R., Sowden J.C. Isolation of human photoreceptor precursors via a cell surface marker panel from stem cell-derived retinal organoids and fetal retinae. Stem Cell. 2018;36:709–722. doi: 10.1002/stem.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba D.A., Gust J., Reh T.A. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B., McLelland B.T., Mathur A., Aramant R.B., Seiler M.J. Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration. Exp. Eye Res. 2018;174:13–28. doi: 10.1016/j.exer.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Qu Y., Stewart T.J., Howard M.J., Chakrabortty S., Holekamp T.F., McDonald J.W. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl. Acad. Sci. USA. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen S.J., Li S.Y., Qu L.H., Meng X.H., Wang Y., Xu H.W., Liang Z.Q., Yin Z.Q. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res. Ther. 2017;8:209. doi: 10.1186/s13287-017-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Shiau F., Yi W., Lu S., Wu Q., Pearson J.D., Kallman A., Zhong S., Hoang T., Zuo Z., et al. Single-cell analysis of human retina identifies evolutionarily conserved and species-specific mechanisms controlling development. Dev. Cell. 2020;53:473–491.e9. doi: 10.1016/j.devcel.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Baranov P., Patel S., Ouyang H., Quach J., Wu F., Qiu A., Luo H., Hicks C., Zeng J., et al. Human retinal progenitor cell transplantation preserves vision. J. Biol. Chem. 2014;289:6362–6371. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Mandai M., Fujii M., Hashiguchi T., Sunagawa G.A., Ito S.I., Sun J., Kaneko J., Sho J., Yamada C., Takahashi M. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice (vol 8, pg 69, 2017) Stem Cell Rep. 2017;8:1112–1113. doi: 10.1016/j.stemcr.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland B.T., Lin B., Mathur A., Aramant R.B., Thomas B.B., Nistor G., Keirstead H.S., Seiler M.J. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest. Ophthalmol. Vis. Sci. 2018;59:2586–2603. doi: 10.1167/iovs.17-23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Ortin-Martinez A., Tsai E.L.S., Nickerson P.E., Bergeret M., Lu Y., Smiley S., Comanita L., Wallace V.A. A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cell. 2017;35:932–939. doi: 10.1002/stem.2552. [DOI] [PubMed] [Google Scholar]
- Ortin-Martinez A., Yan N.E., Tsai E.L.S., Comanita L., Gurdita A., Tachibana N., Liu Z.C., Lu S., Dolati P., Pokrajac N.T., et al. Photoreceptor nanotubes mediate the in vivo exchange of intracellular material. EMBO J. 2021;40:e107264. doi: 10.15252/embj.2020107264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisley C.E., Kay J.N. Seeing stars: development and function of retinal astrocytes. Dev. Biol. 2021;478:144–154. doi: 10.1016/j.ydbio.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.T., Wu J., Rao Y. Molecular control of neuronal migration. Bioessays. 2002;24:821–827. doi: 10.1002/bies.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A., Barber A.C., Rizzi M., Hippert C., Xue T., West E.L., Duran Y., Smith A.J., Chuang J.Z., Azam S.A., et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A., Gonzalez-Cordero A., West E.L., Ribeiro J.R., Aghaizu N., Goh D., Sampson R.D., Georgiadis A., Waldron P.V., Duran Y., et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016;7:13029. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.J., Jiang P., Howden S., Barney P., Min J., York N.W., Chu L.F., Capowski E.E., Cash A., Jain S., et al. A novel approach to single cell RNA-sequence analysis facilitates in silico gene reporting of human pluripotent stem cell-derived retinal cell types. Stem Cell. 2018;36:313–324. doi: 10.1002/stem.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi L., Vitorino M., Masai I., Harris W.A. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J. Cell Biol. 2005;171:991–999. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReNeuron L. Safety and tolerability of hRPC in retinitis pigmentosa. 2022. https://ClinicalTrials.gov/show/NCT02464436
- Santos-Ferreira T., Llonch S., Borsch O., Postel K., Haas J., Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 2016;7:13028. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler B., Schmandt T., Schröder W., Steinfarz B., Husseini L., Wellmer J., Seifert G., Karram K., Beck H., Blümcke I., et al. Functional network integration of embryonic stem cell-derived astrocytes in hippocampal slice cultures. Development. 2003;130:5533–5541. doi: 10.1242/dev.00714. [DOI] [PubMed] [Google Scholar]
- Seiler M.J., Aramant R.B., Jones M.K., Ferguson D.L., Bryda E.C., Keirstead H.S. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:1079–1092. doi: 10.1007/s00417-014-2638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler M.J., Lin R.E., McLelland B.T., Mathur A., Lin B., Sigman J., De Guzman A.T., Kitzes L.M., Aramant R.B., Thomas B.B. Vision recovery and connectivity by fetal retinal sheet transplantation in an immunodeficient retinal degenerate rat model. Invest. Ophthalmol. Vis. Sci. 2017;58:614–630. doi: 10.1167/iovs.15-19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai H., Mandai M., Matsushita K., Kuwahara A., Yonemura S., Nakano T., Assawachananont J., Kimura T., Saito K., Terasaki H., et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. USA. 2016;113:E81–E90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Aslam S.A., Duncan I.L., Cramer A.O., Barnard A.R., MacLaren R.E. Cell fusion following photoreceptor transplantation into the non-degenerate retina. Invest. Ophth. Vis. Sci. 2014;55:3989. [Google Scholar]
- Singh M.S., Balmer J., Barnard A.R., Aslam S.A., Moralli D., Green C.M., Barnea-Cramer A., Duncan I., MacLaren R.E. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 2016;7:13537. doi: 10.1038/ncomms13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Charbel Issa P., Butler R., Martin C., Lipinski D.M., Sekaran S., Barnard A.R., MacLaren R.E. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA. 2013;110:1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K., Occelli L.M., Binette F., Petersen-Jones S.M., Nasonkin I.O. Transplantation of human embryonic stem cell-derived retinal tissue in the subretinal space of the cat eye. Stem Cells Dev. 2019;28:1151–1166. doi: 10.1089/scd.2019.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy J., Oswald J., Baranov P.Y. SDF1 directs donor retinal ganglion cell migration into the retina following allotransplantation in mice. Invest. Ophthalmol. Vis. Sci. 2021;62:2782. [Google Scholar]
- Speir M.L., Bhaduri A., Markov N.S., Moreno P., Nowakowski T.J., Papatheodorou I., Pollen A.A., Raney B.J., Seninge L., Kent W.J., Haeussler M. UCSC cell browser: visualize your single-cell data. Bioinformatics. 2021;37:4578–4580. doi: 10.1093/bioinformatics/btab503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A.S., Shenouda S., Mishra R., Carrier E. Transplanted embryonic stem cells successfully survive, proliferate, and migrate to damaged regions of the mouse brain. Stem Cell. 2006;24:1689–1694. doi: 10.1634/stemcells.2005-0531. [DOI] [PubMed] [Google Scholar]
- Tabar V., Panagiotakos G., Greenberg E.D., Chan B.K., Sadelain M., Gutin P.H., Studer L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat. Biotechnol. 2005;23:601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Thomas B.B., Lin B., Martinez-Camarillo J.C., Zhu D., McLelland B.T., Nistor G., Keirstead H.S., Humayun M.S., Seiler M.J. Co-grafts of human embryonic stem cell derived retina organoids and retinal pigment epithelium for retinal reconstruction in immunodeficient retinal degenerate royal college of surgeons rats. Front. Neurosci. 2021;15:752958. doi: 10.3389/fnins.2021.752958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H.-Y., Watanabe T., Shirai H., Yamasaki S., Kinoshita M., Matsushita K., Hashiguchi T., Onoe H., Matsuyama T., Kuwahara A., et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–574. doi: 10.1016/j.ebiom.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B.A., Mullins R.F., Streb L.M., Anfinson K., Eyestone M.E., Kaalberg E., Riker M.J., Drack A.V., Braun T.A., Stone E.M. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D.L., Cepko C.L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner D.L., Snyder E.Y., Cepko C.L. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Wahlin K.J., Cheng J., Jurlina S.L., Jones M.K., Dash N.R., Ogata A., Kibria N., Ray S., Eldred K.C., Kim C., et al. CRISPR generated SIX6 and POU4F2 reporters allow identification of brain and optic transcriptional differences in human PSC-derived organoids. Front. Cell Dev. Biol. 2021;9:764725. doi: 10.3389/fcell.2021.764725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin K.J., Maruotti J.A., Sripathi S.R., Ball J., Angueyra J.M., Kim C., Grebe R., Li W., Jones B.W., Zack D.J. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Sci. Rep. 2017;7:766. doi: 10.1038/s41598-017-00774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Tu H.Y., Matsuyama T., Horiuchi M., Hashiguchi T., Sho J., Kuwahara A., Kishino A., Kimura T., Takahashi M., Mandai M. A Genetic modification that reduces ON-bipolar cells in hESC-derived retinas enhances functional integration after transplantation. iScience. 2022;25:103657. doi: 10.1016/j.isci.2021.103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Seiler M.J., Aramant R.B., Whittemore S.R. Differential lineage restriction of rat retinal progenitor cells in vitro and in vivo. J. Neurosci. Res. 2002;69:466–476. doi: 10.1002/jnr.10320. [DOI] [PubMed] [Google Scholar]
- Yang Y., Mohand-Said S., Léveillard T., Fontaine V., Simonutti M., Sahel J.A. Transplantation of photoreceptor and total neural retina preserves cone function in P23H rhodopsin transgenic rat. PLoS One. 2010;5:e13469. doi: 10.1371/journal.pone.0013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Gutierrez C., Xue T., Hampton C., Vergara M.N., Cao L.H., Peters A., Park T.S., Zambidis E.T., Meyer J.S., et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Wang W., Liu Y., Fernandez de Castro J., Ezashi T., Telugu B.P.V.L., Roberts R.M., Kaplan H.J., Dean D.C. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cell. 2011;29:972–980. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P.-Y., Peng G.-H., Xu H., Yin Z.Q. c-Kit⁺ cells isolated from human fetal retinas represent a new population of retinal progenitor cells. J. Cell Sci. 2015;128:2169–2178. doi: 10.1242/jcs.169086. [DOI] [PubMed] [Google Scholar]
- Zhu J., Reynolds J., Garcia T., Cifuentes H., Chew S., Zeng X., Lamba D.A. Generation of transplantable retinal photoreceptors from a current good manufacturing practice-manufactured human induced pluripotent stem cell line. Stem Cells Transl. Med. 2018;7:210–219. doi: 10.1002/sctm.17-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T., Gao L., Zeng Y., Li Q., Li Y., Chen S., Hu X., Chen X., Fu C., Xu H., Yin Z.Q. Organoid-derived C-Kit/SSEA4 human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nat. Commun. 2019;10:1205. doi: 10.1038/s41467-019-08961-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw scRNA-seq data and count matrices generated during this study can be accessed at GEO accession number GSE197847. The merged count matrices and cell metadata are also available for download at https://github.com/csanti88/transplant_human_organoid_retina_2022. Interactive queries of individual gene expression patterns can be performed on the UCSC cell browser at https://xeno-hesc-retina.cells.ucsc.edu (Speir et al., 2021).