Abstract
Parvovirus B19 is the causative agent of “fifth disease” of childhood. It has been implicated in a variety of conditions, including unsuccessful pregnancy and rheumatoid arthritis, and is a potential contaminant of blood products. There has been little study of immunity to parvovirus B19, and the exact nature of the protective humoral and cell-mediated immune response is unclear. Immune responses to purified virus capsid proteins, VP1 and VP2, were examined from a cohort of recently infected children and compared with responses from long-term convalescent volunteers. The results demonstrate that antibody reactivity is primarily maintained against conformational epitopes in VP1 and VP2. The unique region of VP1 appears to be a major target for cell-mediated immune responses, particularly in recently infected individuals. We confirm that antibody reactivity against linear epitopes of VP2 is lost shortly after infection but find no evidence of the proposed phenotypic switch in either the subclass of parvovirus B19-specific antibody or the pattern of cytokine production by antigen-specific T cells. The dominant subclass of specific antibody detected from both children and adults was immunoglobulin G1. No evidence was found for interleukin 4 (IL-4) or IL-5 production by isolated lymphocytes from children or adults. In contrast, lymphocytes from convalescent adults produced a typical type 1 response associated with high levels of IL-2 and gamma interferon (IFN-γ). However, we observed a significant (P < 0.001) deficit in the production of IFN-γ in response to VP1 or VP2 from lymphocytes isolated from children. Taken together, these results imply that future parvovirus B19 vaccines designed for children will require the use of conformationally preserved capsid proteins incorporating Th1 driving adjuvants. Furthermore, these data suggest novel mechanisms whereby parvovirus B19 infection may contribute to rheumatoid arthritis and unsuccessful pregnancy.
Human parvovirus B19 (B19V) causes the common childhood illness known as “fifth disease” or erythema infectiosum. While the symptoms are generally mild, there are a variety of conditions under which infection has more severe outcomes. In the immunocompromised or patients with underlying hemolytic disorders, such as sickle-cell disease and hereditary spherocytosis, infection with B19V can result in an acute aplastic crisis or in chronic anemia (39, 53). During pregnancy, the virus can be transmitted transplacentally from an infected mother to the fetus and can cause spontaneous abortion or fetal anemia (9). Direct infection of the fetus can result in nonimmune hydrops fetalis. B19V has also been linked to arthritis in adults and children (41). It has been estimated that 60% of women with symptomatic disease manifest arthropathy (53). The symptoms generally subside within 3 weeks, but about 20% of affected women suffer a persistent or recurring arthropathy. At present there is no effective vaccine available either for women of child-bearing age or for the general population.
B19V is a small, nonenveloped, single-stranded DNA virus classified as an erythrovirus. The virus replicates in human erythroid progenitor cells of the bone marrow and blood, inhibiting erythropoiesis (54). Infection with B19V is common, and upwards of 60 to 70% of the population is seropositive by adulthood (8). Transmission most commonly occurs by personal contact via aerosol or respiratory secretions; however, contaminated blood products may also be a source of iatrogenic transmission.
The B19V capsid consists of an 83-kDa minor structural protein, VP1, and a 53-kDa major structural protein, VP2. VP2 makes up about 95% of the total capsid, with VP1 making up the remainder (38). The sequences of the two proteins are colinear, with VP2 being identical to the carboxyl terminus of VP1; however, VP1 contains an additional 227 amino acids unique to the amino-terminal end.
Although little is known about the protective immune response against B19V in humans, specific antiviral antibody is considered the major mechanism of protection. This is based on the circumstantial evidence that high-dose immunoglobulin therapy is sometimes beneficial for infected patients (23, 43). This treatment does not work in all cases, and no data is available on the actual protective level of B19V immunoglobulin G (IgG), although levels greater than 6 IU are thought to be protective (44). It has been previously shown that a time-dependent change in antibody response occurs against viral capsid proteins by an unknown mechanism (47). It is characterized by a loss of antibody specificity against linear viral epitopes of VP1 and VP2 and also by a proposed antibody subclass switch from IgG3 to IgG4. It has been speculated that this switch is caused by an underlying alteration in the type of CD4+ T-cell response; however, there has been very little examination of this response in humans (16, 51). It is accepted that in response to antigen, T helper (Th) cells secrete cytokines, which are involved in regulatory functions or can mediate direct activity against invading viruses. The current paradigm is that Th cells can be subdivided into at least three populations according to the pattern of cytokines secreted on activation. Th1 cells secrete interleukin 2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor beta, Th2 cells secrete IL-4 and IL-5 (34), and the recently described regulatory subset (Tr1) produces IL-10 (1, 13, 33, 40). The Th cell subset classification also correlates with a functional dichotomy. Th1 cells are the principal effectors of proinflammatory reactions, delayed-type hypersensitivity, and cell-mediated immunity against intracellular pathogens. The main Th1 cytokine, IFN-γ, enhances the differentiation of CD8+ T lymphocytes into activated cytotoxic T leukocytes, activates macrophages to phagocytose and destroy microbial pathogens, and has a direct antiviral effect (30, 49). The precise natures of the protective humoral and cell-mediated responses to B19V are unknown, but such understanding is central to the rational design of a protective vaccine and will also influence the choice of a vaccine delivery vehicle.
In the present study, we provide the first systematic examination of the humoral and cell-mediated immune responses from cohorts of recently infected children and from seropositive and seronegative adult volunteers. This study provides the first characterization of the patterns of antigen-specific cytokine induction in this infection and correlates these responses with an analysis of the subclass of antigen-specific antibody induced in children and adults. The data challenge the existence of a Th1-to-Th2 phenotypic switch following B19V infection but demonstrate a deficient IFN-γ response to the virus in recently infected children.
MATERIALS AND METHODS
Patients.
Heparinized blood samples (n = 19) were obtained 3 months after a confirmed outbreak of B19V infection at a national school from children between ages 7 and 11 who had displayed symptoms of fifth disease. These samples were labelled A1 to A19. Blood samples were also taken from a panel of healthy adult volunteers with no recent record of B19V or other infection (n = 26). These samples were identified numerically with a prefix of “B” or “V.” Samples that tested positive from this cohort of adults are considered to indicate past infection and are referred to as “convalescent” in the text. Consent was obtained from all volunteers or their guardians prior to sample collection.
Antigens.
B19V recombinant VP1 and VP2 proteins were expressed in the baculovirus expression system using Spodoptera frugiperda (Sf9) insect cells (6, 7). VP1 was prepared by detergent lysis of recombinant cells, removal of soluble protein by centrifugation, and solubilization by 6 M guanidinium chloride. VP2 was purified by lysing recombinant cells and precipitating the protein with ammonium sulphate using a modification of previously described methods (12). Purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis. Both preparations were dialyzed into 50 mM carbonate buffer, pH 9.6, and the pH was neutralized before use in T-cell proliferation and cytokine production assays. For determinations of IgG reactivity by enzyme immunoassay (EIA) against nonconformational or linear epitopes, VP1 and VP2 preparations were linearized with 5 mM dithiothreitol–0.1% (wt/vol) sodium dodecyl sulfate prior to microplate coating. Antigens were stored at −20°C until use.
Antibody assays.
Plasma obtained from blood samples was used to examine the B19V IgG reactivity to the capsid proteins. Total B19V-specific IgG for conformational VP2 (VP2-N) was determined by commercial EIA (Biotrin International, Dublin, Ireland). Linear VP1 (VP1-D) and VP2 (VP2-D) were also determined by EIA. Briefly, microtiter plates (Nalge Nunc International, Roskilde, Denmark) were coated overnight at 4°C with either VP1-D or VP2-D. Plates were washed and then blocked for 2 h before incubation for 1 h at 20°C with plasma samples, diluted in 0.05% (wt/vol) Tween 20–phosphate-buffered saline. After four washes, wells were treated with horseradish peroxidase-conjugated anti-human IgG (Dako A/S, Glostrup, Denmark) for 30 min. After further washing, this complex was detected by the addition of tetramethyl benzidine substrate for 10 min. The reaction was terminated with the addition of 1 N sulfuric acid. Absorbance was measured at 450 and 630 nm. Results are expressed as index values (I.V.) representing specimen absorbance divided by cutoff absorbance. The cutoff was established as the absorbance 2 standard deviations greater than the mean absorbance obtained from a panel of B19V-negative samples. An I.V. of less than 1.0 was considered seronegative. VP2-N EIA reactivity was calibrated against the international standard for B19V-specific antibody (15). IgG reactivity to conformationally intact VP1 (VP1-N) was determined by a commercial qualitative immunofluorescent assay. The degree of fluorescence was graded on a scale of 0 to 4 according to the manufacturer's instructions (Biotrin International).
IgG subclass assays.
Microtiter plates were coated with VP1 and VP2 and incubated with plasma samples as described for total B19V IgG EIA. IgG subclasses were detected by incubation at 20°C for 1 h with murine monoclonal antibodies specific for human IgG1, IgG2, IgG3, or IgG4 (Serotec, Ltd., Oxford, United Kingdom). Following a wash step, horseradish peroxidase-conjugated anti-mouse IgG (Bio-Rad Laboratories, Hertfordshire, United Kingdom) was added to wells. The complex was then detected by addition of tetramethyl benzidine substrate and terminated by adding 1 N sulfuric acid. The absorbance was measured at 450 and 630 nm. I.V. were calculated as before.
T-cell proliferation assay.
Peripheral blood mononuclear cells (PBMC) from adults or children were isolated by density gradient centrifugation as previously described (42). PBMC (2 × 106 cells/ml) were cultured in triplicate with purified recombinant VP1 (10 μg/ml) or VP2 (10 μg/ml) for 72 h. Cells cultured with medium alone or with a combination of phytohemagglutinin (PHA) (2 μg/ml) served as negative and positive controls, respectively. For the final 4 h of culture, cells were labeled with 1 μCi of [3H]thymidine before harvesting as previously described (42). Background values for negative control samples were typically between 200 and 600 cpm and always less than 1,500 cpm. Results are expressed as stimulation indices (S.I.), representing the proliferative response for test samples divided by the response obtained from the negative control sample.
Analysis of cytokine production.
Supernatants were removed from PBMC cultures after 24 h to determine the concentration of IL-2 and after 72 h to determine the IFN-γ, IL-4, and IL-5 concentrations as previously described (42). Briefly, IL-2 release was assessed by the ability of culture supernatants to support the proliferation of the IL-2-dependent cell line CTLL-2. Concentrations of IL-4, IL-5, and IFN-γ were determined by EIA using commercially available antibodies (PharMingen, San Diego, Calif.). Concentrations were determined by comparing either the proliferation or the absorbance at 405 nm for test samples with a standard curve for recombinant cytokines of a known potency and concentration.
Statistical methods.
Cytokine secretion data from different treatment groups were compared by use of the Student t test.
RESULTS
Antibodies against conformational epitopes of VP1 and VP2 persist, and those against nonconformational determinants are lost.
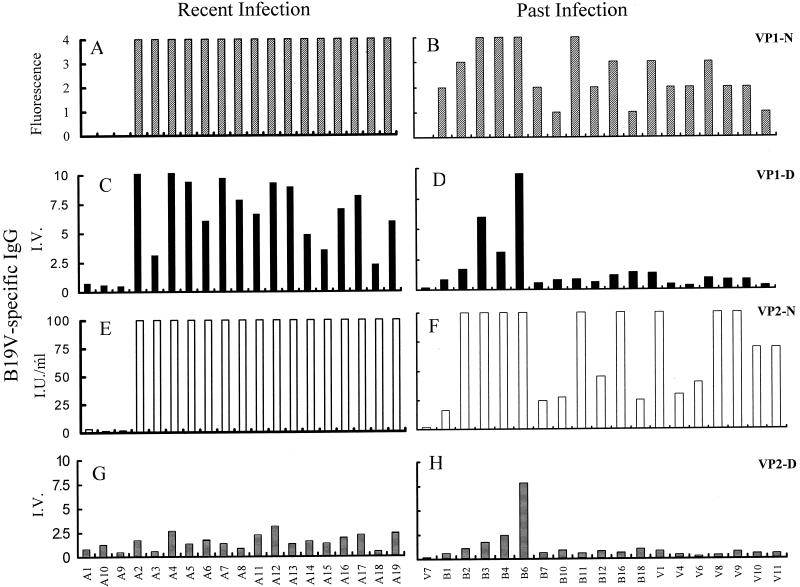
Previous serological studies have suggested that antibody responses against linear, but not conformational, epitopes of the B19V capsid protein VP2 are lost during convalescence. If correct, these findings have serious implications for peptide-based vaccines. In the present study, we examined this phenomenon using individual samples in well-characterized, standardized assay systems. A strong antibody response against B19V was observed in 16 of 19 specimens taken from children within either 3 months of infection or 3 months of presentation with symptoms of infection (Fig. 1A). The humoral response against B19V in this cohort of recently infected children is dominated by the presence of antibodies directed against conformational epitopes on VP1 and VP2 and against linear epitopes present on the unique region of VP1. Specifically, all 16 seropositive children exhibited intense immunoreactivity against epitopes on conformationally intact VP1-N and linearized VP1-D (mean I.V. = 7.14 ± 2.6) (Fig. 1C). Samples from these children also displayed >100 IU of B19V IgG/ml directed against VP2-N (Fig. 1E). The observed reactivity against VP2-D (mean I.V. = 1.67 ± 0.7) (Fig. 1G) confirms both the poor immunogenicity of linear epitopes present in VP2 and that the observed immunoreactivity with VP1-D is indeed directed against the 227-amino-acid VP1-unique region. No viral DNA was found in any specimen tested (data not shown), and all children made a full recovery from B19V infection.
FIG. 1.
Total B19V IgG reactivity against VP1 and VP2 in plasma from recently infected children (A, C, E, and G) or previously infected (convalescent) adults (B, D, F, and H). Results shown are the responses against conformationally intact VP1-N (hatched bars) determined by immunofluorescence assay and graded according to the manufacturer's criteria (0, negative; 1, weak positive; 2, intermediate positive; 3 to 4, strong positive) (A and B), against linearized VP1-D (solid bars) determined by EIA and expressed as I.V. (C and D), against conformational VP2-N (open bars) determined by EIA and expressed as international units per milliliter (E and F), and against linearized VP2-D (horizontal bars) determined by EIA and expressed as I.V. (G and H).
The antibody response against B19V capsid antigens was also determined from a panel of either seronegative (n = 8) or long-term convalescent, seropositive (n = 18) volunteer donors. There is a persistence of antibody reactivity directed against conformational epitopes on VP1-N and VP2-N (mean amount of B19V IgG = 91.8 ± 63 I.U./ml) (Fig. 1B and F). However, the data also illustrate a significant loss of immunoreactivity against VP1-D (mean I.V. = 1.73 ± 2.36) (Fig. 1D) compared to that for recently infected individuals (P = 0.001). Indeed, 11 of 18 (61%) exhibited no immunoreactivity against linear epitopes on the VP1-unique region even in the presence of significant reactivity against VP1-N and VP2-N. The expected minimal immunoreactivity against VP2-D was observed (Fig. 1H) (mean I.V. = 1.2 ± 1.7). This value was not significantly different from values for the antibody response against VP2-D in recently infected children (P = 0.306). Thus, the antibody response generated by infection against linear or nonconformational epitopes of B19V does not persist with time.
The humoral response against B19V is dominated by IgG1.
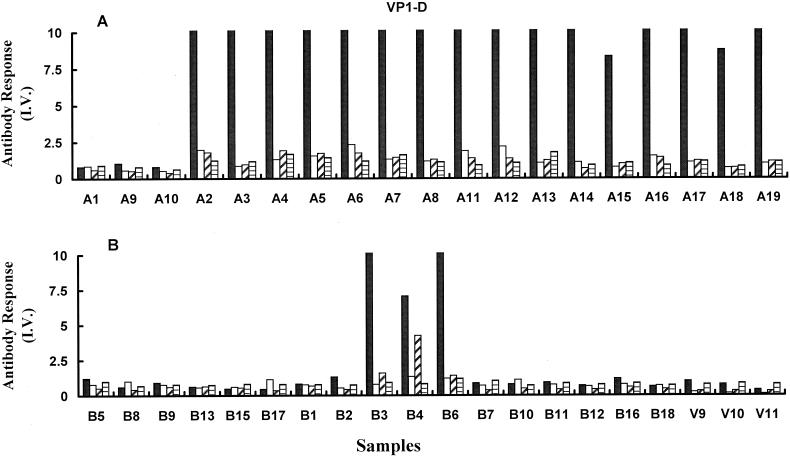
The subclasses of B19V-specific antibody from recently infected children or convalescent adults were measured to examine the possibility of an IgG subclass switch in the response against this virus with time. In the group of 16 B19V IgG-positive samples from recently infected children (Fig. 2A), the specific response against VP1-D was dominated by IgG1 (mean I.V. = 23.5 ± 8.7). The observed levels of IgG2, IgG3, and IgG4 were 1.37 ± 0.5, 1.3 ± 0.37, and 1.2 ± 0.29, respectively. No significant difference was observed between the IgG3 and IgG4 subclass reactivities in this group. Little reactivity of any antibody subclass was detected against VP1-D in plasma from convalescent volunteers (Fig. 2B), confirming the loss of antibody reactivity against nonconformational epitopes that was previously observed (Fig. 1B). Only three samples (B3, B4, and B6) showed any reactivity to the denatured capsid antigen, and these corresponded to patients with the strongest total B19V IgG response against VP1-D.
FIG. 2.
B19V IgG subclass reactivity against linearized VP1 (VP1-D). The results were determined by EIA for B19V-specific IgG1 (solid bars), IgG2 (open bars), IgG3 (diagonal shading), and IgG4 (horizontal shading). Results are plotted as I.V. for recently infected children (A) and convalescent adults and uninfected volunteers (B).
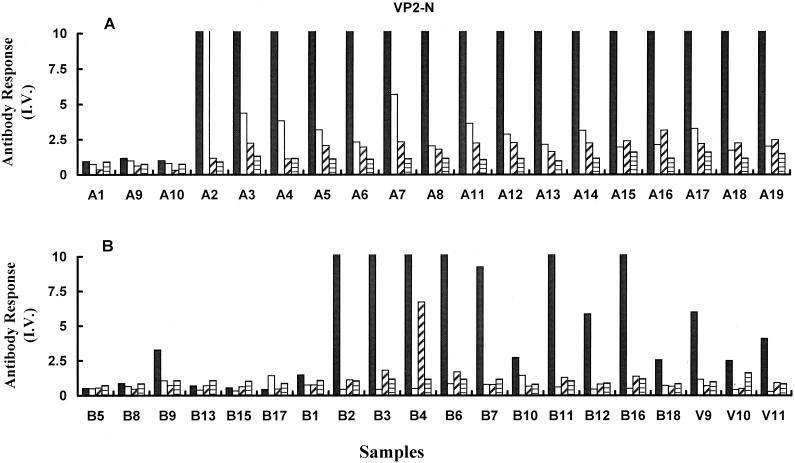
The subclass profile of the antibody response directed against VP2-N was also examined for recently infected children and convalescent adults. In both groups, IgG1 dominated the subclass profile (Fig. 3). Recently infected children showed a strong IgG2 response (3.5 ± 2.4), which was not detected in long-term convalescent samples. Children's samples also displayed significantly greater levels of IgG3 than IgG4 directed against VP2-N (P = 0.001). However, no statistically significant difference was observed between IgG3 and IgG4 subclass reactivities for convalescent individuals (P = 0.1), nor was any evidence found for a subclass switch in reactivity in this group.
FIG. 3.
B19V IgG subclass reactivity against VP2-N. The results were determined by EIA for B19V-specific IgG1 (solid bars), IgG2 (open bars), IgG3 (diagonal shading), and IgG4 (horizontal shading). Results are plotted as I.V. for recently infected children (A) and convalescent adults and uninfected volunteers (B).
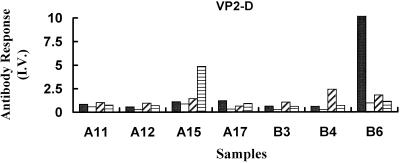
As with VP1-D, the IgG subclass reactivity against VP2-D was negligible for the majority of specimens tested from either recently infected children or convalescent adults (Fig. 4 and data not shown). Only one sample from a recently infected child, A15 (IgG4 I.V. = 4.9), and two samples from seropositive adults, B4 (IgG3 I.V. = 2.4) and B6 (IgG1 I.V. = 28), showed any reactivity against VP2 in this form (Fig. 4). The presence of detectable IgG4 in specimen A15 not only confirms the functionality of the IgG4 immunoassay but cautions against the use of pooled serum samples for interpreting the IgG subclass responses from different cohorts of patients.
FIG. 4.
B19V IgG subclass reactivity against VP2-D. The results were determined by a specific EIA for B19V-specific IgG1 (solid bars), IgG2 (open bars), IgG3 (diagonal shading), and IgG4 (horizontal shading) only for samples with a high level of total IgG reactivity against linear VP2. Results are expressed as I.V.
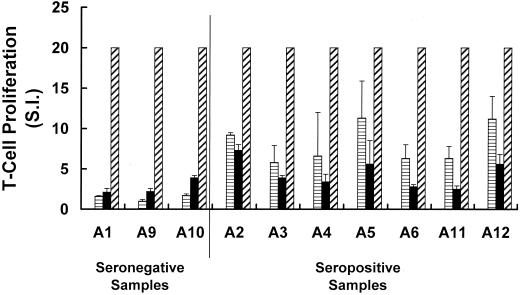
VP1 is a major antigenic target for T cells from recently infected children.
There has been little study of the T-cell response induced by B19V infection in humans. Although all children included in the present study had displayed clinical symptoms of infection, three of the samples showed no proliferative T-cell response against VP1 and little or no response to VP2 (Fig. 5). These three samples were also negative for IgG and IgM against the capsid proteins VP1 and VP2 (Fig. 1A and data not shown). The remaining children tested all showed a specific proliferative T-cell response against VP1, and T cells in samples from most children also recognized VP2 (Fig. 5 and data not shown). Since CD4+ T cells recognize short linear peptide sequences and since VP1 and VP2 are colinear, this suggests that the T-cell response is targeted against at least one site in the unique region of VP1 corresponding to the 227 amino acids of the unique region of VP1. However, further T-cell epitopes exist outside this region. This is suggested by the observed proliferative responses to VP2 by lymphocytes from seropositive children and adults (Fig. 5 and data not shown).
FIG. 5.
Proliferative responses of PBMC from children recently infected with B19V. Cells were stimulated in vitro with purified VP1 (horizontal shading), VP2 (solid bars), or either medium alone (open bar) or PHA (diagonal shading) as a negative or positive control, respectively. Results are given as the S.I. and are the mean (± the SE) of at least two experiments performed in triplicate. For clarity the results for some positive controls have been truncated at an S.I. of 20.
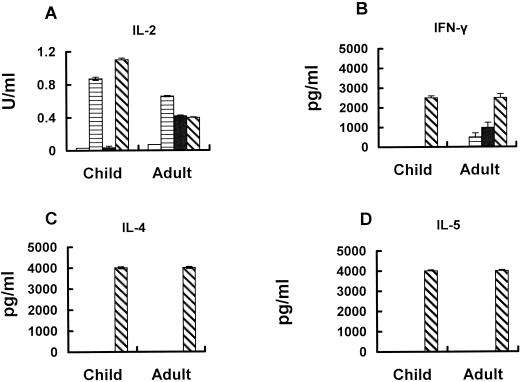
Children fail to mount an IFN-γ response against B19V.
A switch in the profile of cytokine production by B19V-specific T cells has been suggested to explain some of the features of B19V infection in humans. To examine this hypothesis, the production of the type 1 cytokines IFN-γ and IL-2 or the type 2 cytokines IL-4 and IL-5 was examined. Lymphocytes isolated from recently infected children or from adults seropositive for B19V did not secrete IL-4 or IL-5 following antigen stimulation (Fig. 6 and data not shown). This strongly suggests that there is no switch to a Th2 pattern of cytokine expression during convalescence from B19V infection. In fact, seropositive adults displayed a potent Th1 response, with high levels of IFN-γ (Table 1) and IL-2 (mean ± the standard error (SE) = 0.25 ± 0.1 U/ml for VP1 stimulation; 0.43 ± 0.01 U/ml for VP2 stimulation). Lymphocytes from recently infected children also produced IL-2 following VP1 stimulation (mean ± SE = 0.12 ± 0.05 U/ml) relative to seronegative children (mean ± SE = 0.037 ± 0.005 U/ml) (Fig. 6). In contrast, antigen-specific IFN-γ production could not be detected from T cells of these children when stimulated with either VP1 or VP2, despite strong T-cell proliferative responses (Table 1). This result was significantly different (P < 0.001) from the strong IFN-γ responses seen from lymphocytes isolated from seropositive adults that had been stimulated with either VP1 or VP2. Notably, mitogen-stimulated controls gave detectable levels of IFN-γ, indicating that this was not due to an intrinsic deficit in this population.
FIG. 6.
Comparison of B19V antigen-specific cytokine responses by PBMC from a recently infected child and a seropositive convalescent adult. Cells were stimulated in vitro with purified VP1 (horizontal shading), VP2 (solid bars), or either medium alone (open bars) or PHA (diagonal shading) as a negative or positive control, respectively. IL-2 (A) was measured by specific bioassay, and IFN-γ (B), IL-4 (C), and IL-5 (D) were measured by EIA. Results are the mean concentration of cytokine (± the SE) detected in at least two experiments performed in triplicate.
TABLE 1.
IFN-γ secretion by PBMC from recently infected children or adults in response to B19V capsid proteins
| Source of samples | B19V-specific IFN-γ productiona (pg/ml [mean ± SE]) in response to:
|
No. of samples | |
|---|---|---|---|
| VP1 | VP2 | ||
| Children | <80 | <80 | 16 |
| Adults | 610 ± 348 | 1,765 ± 825 | 7 |
IFN-γ responses by PBMC isolated from recently infected children or B19V-seropositive convalescent adults following in vitro stimulation with either purified VP1 or VP2 (10 μg/ml).
DISCUSSION
The present study provides the first comprehensive analysis of both humoral and cell-mediated immunity against B19V infection in humans. The data show the necessity of determining IgG reactivity against B19V VP2 to confirm past infection and the extent of antibody loss against B19V VP1 and VP2 linear epitopes and illustrates that the unique sequence of the capsid protein VP1 is a major target of the cell-mediated immune response. Analysis of the pattern of cytokine production following antigen stimulation of lymphocytes from recently infected children and convalescent adults shows no evidence of a Th1-to-Th2 switch; likewise, no alteration in the profile of the antibody subclass was detected. However, our results do show that recently infected children fail to mount an IFN-γ response to this virus. This immunological deficit has implications for the pathology of the childhood infection and for the design of future vaccines.
Little is known about the nature of the antibody or cellular response against recent B19V infection in children. Our analysis of B19V serology in the recently infected children demonstrates that levels of capsid-specific VP2 IgG exceed 100 IU/ml within 3 months of infection and that high levels of VP1 IgG reactivity are also attained in this time period. A number of recent reports have shown that these antibody responses against conformational epitopes on VP1 and VP2 persist following B19V infection, whereas humoral responses directed against linear epitopes of both VP1 and VP2 are lost (21, 47). Although we observed a similar phenomenon, our results indicate that 61% of convalescent specimens may lack antibodies directed against linear epitopes on the VP1-unique region. This finding not only means that B19V diagnostics which do not contain conformationally intact capsid epitopes may be unreliable but also has serious implications for vaccine design. The data suggest that vaccines based on short peptide sequences corresponding to regions of the capsid proteins may not induce a protective or persistent immunological memory. This is consistent with previous work from different viral systems demonstrating the importance of conformational antigenic determinants in protective immunity (20, 29).
A variety of mechanisms have been invoked to explain why reactivity against linear epitopes is lost, including the induction of T-cell anergy or tolerance (16). However, these mechanisms do not adequately explain why there is a selective loss of reactivity against the linear epitopes of the capsid proteins. A more likely explanation centers on the induction of B-cell memory. B-cell memory is maintained by the persistence of antigen on specialized follicular dendritic cells. This involves the deposition of antigen as immune complexes on the surface of the follicular dendritic cells, a process that preserves the conformational integrity of the antigen (11, 35, 48). We propose that this competitive situation would favor the more avid recognition by B cells expressing surface Ig directed against conserved conformational epitopes, and hence antibodies against linear epitopes would tend to be lost from the repertoire.
Franssila et al. also reported that there is a subclass switch for antibody against the VP1-unique region from IgG3 during acute infection to IgG4 during convalescence and proposed that this was due to a switch from a Th1 response to a Th2 response (16). In other infections and model systems, members of our group and others have reported a broadening of cytokine responses with time (2, 3, 27). These have involved initial responses dominated by either type 1 or type 2 cytokines and have been due to a variety of factors, including subclinical repeat infection and variables in the initial priming (2, 3, 27). However, in the present study, we found no evidence of a switch in either the humoral or cell-mediated immune compartments. Although it is possible that a phenotypic switch in reactive antibody or the pattern of cytokine production may have occurred prior to 3 months postinfection, previous work has suggested that this did not occur until at least 3 to 4 months postinfection (16). Furthermore, the absence of the IgG4 subclass in all children's specimens suggests that it is unlikely that a previous phenotypic switch has occurred. The dominant subclass of antibody specific for B19V detected from recently infected children or long-term convalescent adults was IgG1. IgG1 and IgG3 are associated with Th1 responses in humans, and the lack of specific IgG1 is a feature of parasite-driven Th2 responses (22, 36). The high levels of B19V-specific IgG1 in convalescent plasma detected in this study are not consistent with a switch to a Th2 response. Significant levels of IgG3 were detected in samples from recently infected children in this study. This is consistent with a recent study by Bostic et al. (5) and with the well-documented significance of opsonizing and complement-fixing IgG3 in providing protection against other viral infections (4, 18, 19, 46). The IgG2 response following B19V infection appears to be directed mainly against nonlinear epitopes on VP2 and to decline with time. The mechanisms governing IgG2 induction are thought to involve a factor other than IFN-γ (45), although the reasons for the decline observed in this study are not clear. IgG4 is the human IgG subclass most closely associated with Th2-driven immune responses (14, 22, 36); however, with one exception, little or no B19V-specific IgG4 was detected for either children or adults.
The elucidation of the protective immune response to B19V is a prerequisite to the design of an effective vaccine. Initial efforts to explore the nature of the cellular immune response to B19V were unsuccessful (24). However, more recent attempts at studying T-cell proliferation using recombinant B19V antigens have proved more informative (50). The present study allowed the comparison of specimens taken from individuals with clinical as well as serological evidence of recent B19V infection. T cells from recently infected children proliferated strongly in response to VP1 (Fig. 5). Since the responses to VP2 were weaker, this suggests that the unique region of VP1, corresponding to the 227 amino acids at the amino-terminal end, is a major but not exclusive target for T-cell recognition and antibody responses generated by infection. This hypothesis could be addressed by using peptides corresponding to regions of VP1 and VP2 to map the T-cell epitopes of B19V (28). The relationship between sites of B-cell recognition and T-cell recognition on complex antigens is not straightforward; nevertheless, our observation for children that these sites reside in close proximity on the B19V capsid is in accordance with previous studies. We have previously shown that in poliovirus, there is a proximity between neutralizing antibody sites 1 and 3 and sequences recognized by T cells (17, 25, 28), suggesting that specific antibody may protect adjacent T-cell epitopes during antigen processing. Proliferative responses by T cells from adults were generally weaker than those observed in children, probably reflecting a waning in immunity since infection. In accordance with the results of von Poblotzki et al., significant proliferative responses against both VP1 and VP2 were detected in seropositive adult samples (50). It is not clear why the proliferative response should broaden with time, but this may represent subclinical exposure to B19V.
Although enhanced IL-1β, IL-6, and IFN-γ mRNA production has been observed during acute B19V infection in a single patient (51), there has been no characterization to date of the pattern of cytokine induction following B19V infection in humans. In the present study, we found no evidence of the proposed Th2 response to purified B19V antigens in long-term convalescent subjects; rather, the response in these adult volunteers was dominated by IL-2 and IFN-γ, which is typical of the Th1 responses induced by many viral infections (28, 29). Th1 responses have well-documented roles in antiviral immunity; however, such responses are not always beneficial. The pathological damage witnessed during rheumatoid arthritis is also associated with type 1 responses. Interestingly, B19V infection has recently been linked to both rheumatoid and juvenile arthritis. Another condition in which type 1 responses are considered detrimental is pregnancy. Pregnancy is associated with a profound suppression of Th1-mediated immunity (52). This is thought to protect the fetus from maternal mechanisms of allograft rejection. Infection of pregnant mothers by B19V is a well-documented abortifacient (9). Undoubtedly, many of these cases are due to direct infection of the fetus or to nonimmune mechanisms; however, our data suggest that infection of immunologically naïve, pregnant women by B19V would induce a virus-specific Th1 response. This type of response may not be compatible with carriage of the fetus to term and may contribute to a B19V-induced immune-mediated fetal loss.
Although we found no evidence of a Th1-to-Th2 switch following B19V infection, we did observe a significant deficit in the IFN-γ response against capsid proteins in recently infected children. It has been previously shown that production of IFN-γ by neonatal CD4+ and CD8+ T cells is markedly lower than that by analogous adult cell populations (26). This is supported by studies of primary herpes simplex virus infection, with which there is a significantly reduced IFN-γ response by T cells from infants compared to that of cells from adults in the first 3 to 6 weeks postinfection (10). However, other data show that under certain conditions, neonates and infants can display a memory Th1-type response of a magnitude similar to that observed later in life (32). Our data show that the deficit in IFN-γ production is not an intrinsic feature of the T-cell population from recently infected children, since mitogen-specific responses are equivalent to those seen in adults. It may be that other deficiencies, either in the antigen-presenting cell populations or in expression of costimulatory signals, contribute to this deficit (37).
Whatever the mechanism underlying the antigen-specific deficit in IFN-γ responses observed from these children, our data have important implications for the design of vaccines against B19V. It may be that candidate B19V vaccines, designed for childhood immunization, will need to be formulated with adjuvants or delivery vehicles which favor the induction of Th1 responses, such as IL-12 or the mycobacterium-derived trehalose dimycolate, rather than traditional adjuvants, such as aluminium salts, which tend to induce Th2-type responses (31).
ACKNOWLEDGMENTS
This work was funded by the Irish Health Research Board. B. P. Mahon is a Wellcome Trust/HRB New Blood Fellow.
We thank Biotrin International, Dublin, Ireland, for providing infected Sf9 cells expressing recombinant VP1 and VP2.
REFERENCES
- 1.Asseman C, Powrie F. Interleukin 10 is a growth factor for a population of regulatory T cells. Gut. 1998;42:157–158. doi: 10.1136/gut.42.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausiello C M, Lande R, Urbani F, La Sala A, Stefanelli P, Salmaso P, Mastrantonio P, Cassone A. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect Immun. 1999;67:4064–4071. doi: 10.1128/iai.67.8.4064-4071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard A, Mahon B P, Watkins J, Redhead K, Mills K H G. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck O E. Distribution of virus antibody activity among human IgG subclasses. Clin Exp Immunol. 1981;43:625–632. [PMC free article] [PubMed] [Google Scholar]
- 5.Bostic J R, Brown K E, Young N S, Koenig S. Quantitative analysis of neutralizing immune responses to human parvovirus B19 using a novel reverse transcriptase polymerase chain reaction based assay. J Infect Dis. 1999;179:619–626. doi: 10.1086/314648. [DOI] [PubMed] [Google Scholar]
- 6.Brown C S, Salimans M M M, Noteborn M H M, Harro T W. Antigenic parvovirus B19 coat proteins VP1 and VP2 produced in large quantities in a baculovirus expression system. Virus Res. 1990;15:197–212. doi: 10.1016/0168-1702(90)90028-a. [DOI] [PubMed] [Google Scholar]
- 7.Brown C S, Van Lent J W M, Vlak J M, Spaan W J W. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J Virol. 1991;65:2702–2706. doi: 10.1128/jvi.65.5.2702-2706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown K E. Human parvovirus B19 epidemiology and clinical manifestations. In: Anderson L J, Young N S, editors. Human parvovirus B19. Vol. 20. Basel, Switzerland: Karger; 1997. pp. 42–60. [Google Scholar]
- 9.Brown T A, Anand A, Richie L D, Clewley L P, Reid T M S. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984;ii:1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- 10.Burchett S K, Corey L, Mohan K M, Westall J, Ashley R, Wilson C B. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J Infect Dis. 1992;165:813–818. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 11.Burton G F, Masuda A, Heath S L, Smith B A, Tew J G, Szakal A K. Follicular dendritic cells (FDC) in retroviral infection: host/pathogen perspectives. Immunol Rev. 1997;156:185–197. doi: 10.1111/j.1600-065x.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 12.Casal J I. Use of parvovirus-like particles for vaccination and induction of multiple immune responses. Biotechnol Appl Biochem. 1999;29:141–150. [PubMed] [Google Scholar]
- 13.Chakraborty N G, Li L, Sporn J R, Kurtzman S H, Ergin M T, Mukherji B. Emergence of regulatory CD4+ T cell response to repetitive stimulation with antigen-presenting cells in vitro: implications in designing antigen-presenting cell-based tumor vaccines. J Immunol. 1999;162:5576–5583. [PubMed] [Google Scholar]
- 14.de Martino M, Rossi M E, Azzari C, Chiarelli F, Galli L, Vierucci A. Low IgG3 and high IgG4 subclass levels in children with advanced human immunodeficiency virus-type 1 infection and elevated IgE levels. Ann Allergy Asthma Immunol. 1999;83:160–164. doi: 10.1016/S1081-1206(10)62629-4. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson M, Walker D, Cohen B. Report of a collaborative study to establish the international standard for parvovirus B19 serum IgG. Biologicals. 1997;25:283–288. doi: 10.1006/biol.1997.0098. [DOI] [PubMed] [Google Scholar]
- 16.Franssila R, Soderlund M, Brown S B, Spaan W J M, Seppala I, Hedman K. IgG response to human parvovirus B19 infection. Clin Diagn Virol. 1996;6:41–49. doi: 10.1016/0928-0197(96)00156-0. [DOI] [PubMed] [Google Scholar]
- 17.Graham S, Wang E C Y, Jenkins O, Borisiewicz S K. Analysis of the T-cell response to picornaviruses: identification of T-cell epitopes of poliovirus. J Virol. 1993;67:1627–1637. doi: 10.1128/jvi.67.3.1627-1637.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta C K, Leszczynski J, Gupta R K, Siber G R. IgG subclass antibodies to human cytomegalovirus (CMV) in normal human plasma samples and immune globulins and their neutralizing activities. Biologicals. 1996;24:117–124. doi: 10.1006/biol.1996.0015. [DOI] [PubMed] [Google Scholar]
- 19.Julkunen I, Ukkonen P, Stenvik M, Hovi T, Renkonen L, Makela O. Proportions of immunoglobulin isotypes in paralytic poliomyelitis and after vaccination. J Clin Immunol. 1987;7:319–326. doi: 10.1007/BF00915554. [DOI] [PubMed] [Google Scholar]
- 20.Katrak K, Mahon B P, Minor P D, Mills K H G. Cellular and humoral immune responses to poliovirus in mice: a role for helper T cells in heterotypic immunity to poliovirus. J Gen Virol. 1991;72:1093–1098. doi: 10.1099/0022-1317-72-5-1093. [DOI] [PubMed] [Google Scholar]
- 21.Kerr S, O'Keeffe G, Kilty C, Doyle S. Undenatured parvovirus B19 antigens are essential for the accurate detection of parvovirus B19 IgG. J Med Virol. 1999;57:179–185. doi: 10.1002/(sici)1096-9071(199902)57:2<179::aid-jmv16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Kurniawan-Atmadja A, Sartono E, Partono F, Yazdanbakhsh M, Maizels R. Specificity of predominant IgG4 antibodies to adult and microfilarial stages of Brugia malayi. Parasite Immunol. 1998;20:155–162. [PubMed] [Google Scholar]
- 23.Kurtzman G, Frickhofen N, Kimball J, Jenkins D W, Nienhuis A W, Young N S. Pure red-cell aplasia of 10 years' duration due to persistent parvovirus B19 infection and its cure with immunoglobulin therapy. N Engl J Med. 1989;321:519–523. doi: 10.1056/NEJM198908243210807. [DOI] [PubMed] [Google Scholar]
- 24.Kurtzman G J, Cohen B J, Field A M, Oseas R, Blaese R M, Young N S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Investig. 1989;84:1114–1123. doi: 10.1172/JCI114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeClerc C, Deriaud E, Mimic V, van der Werf S. Identification of a T cell epitope adjacent to neutralizing antibody site 1 of poliovirus type 1. J Virol. 1991;65:711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis D B, Yu C C, Meyer J, English B K, Kahn S J, Wilson C B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Investig. 1991;87:194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahon B P, Brady M T, Mills K H G. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long term immunity in children. J Infect Dis. 2000;181:2087–2091. doi: 10.1086/315527. [DOI] [PubMed] [Google Scholar]
- 28.Mahon B P, Katrak K, Mills K H G. Antigenic sequences of poliovirus recognized by T cells: serotype-specific epitopes on VP1 and VP3 and cross-reactive epitopes on VP4 defined by using CD4+ T-cell clones. J Virol. 1992;66:7012–7020. doi: 10.1128/jvi.66.12.7012-7020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahon B P, Katrak K, Nomoto A, Macadam A J, Minor P D, Mills K H G. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med. 1995;181:1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahon B P, Mills K H G. Interferon-γ mediated immune effector mechanisms against Bordetella pertussis. Immunol Lett. 1999;68:213–217. doi: 10.1016/s0165-2478(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 31.Mahon B P, Moore A, Johnson P, Mills K H G. Approaches to new vaccines. Crit Rev Biotechnol. 1998;18:257–282. doi: 10.1080/0738-859891224167. [DOI] [PubMed] [Google Scholar]
- 32.Marchant A, Goetghebuer T, Ota M O, Wolfe I, Ceesay S J, De Groote D, Corrah T, Bennett S, Wheeler J, Huygen K, Aaby P, McAdam K P, Newport M J. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 33.Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 34.Mossman T R, Cherwinski C H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 35.Nayak B P, Agarwal A, Nakra P, Rao K V. B cell responses to a peptide epitope. VIII. Immune complex-mediated regulation of memory B cell generation within germinal centers. J Immunol. 1999;163:1371–1381. [PubMed] [Google Scholar]
- 36.Nicolas L, Langy S, Plichart C, Deparis X. Filarial antibody responses in Wuchereria bancrofti transmission area are related to parasitological but not clinical status. Parasite Immunol. 1999;21:73–80. doi: 10.1046/j.1365-3024.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 37.Nonoyama S, Penix L A, Edwards C P, Lewis D B, Ito S, Aruffo A, Wilson C B, Ochs H D. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Investig. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozawa K, Ayub J, Yu-Shu H, Kurtzman G, Shimada T, Young N. Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol. 1987;61:2395–2406. doi: 10.1128/jvi.61.8.2395-2406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattison J R, Jones S E, Hodgson J. Parvovirus infections and hypoplastic crises in sickle cell anaemia. Lancet. 1981;i:64. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- 40.Read S, Mauze S, Asseman C, Bean A, Coffman R, Powrie F. CD38+ CD45RB(low) CD4+ T cells: a population of T cells with immune regulatory activities in vitro. Eur J Immunol. 1998;28:3435–3447. doi: 10.1002/(SICI)1521-4141(199811)28:11<3435::AID-IMMU3435>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Reid D M, Reid T M S, Brown T, Rennie R A N. Human parvovirus associated arthritis: a clinical and laboratory description. Lancet. 1985;i:422–425. doi: 10.1016/s0140-6736(85)91146-8. [DOI] [PubMed] [Google Scholar]
- 42.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Grothefors L, Øymar K, Miller E, Storsacter J, Mills K H G. Distinct Th cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz T F, Roggendorf M, Hottentrager B, Modrow S, Deinhardt F, Middledorp J. Immunoglobulins in the prophylaxis of parvovirus B19 infection. J Infect Dis. 1990;162:1214. doi: 10.1093/infdis/162.5.1214. [DOI] [PubMed] [Google Scholar]
- 44.Searle K, Guillard C, Enders G. Parvovirus B19 diagnosis in pregnant women—quantification of IgG antibody levels (I.U./ml) with reference to the international parvovirus B19 standard serum. Infection. 1997;25:32–34. doi: 10.1007/BF02113504. [DOI] [PubMed] [Google Scholar]
- 45.Servet-Delprat C, Bridon J M, Djossou O, Yahia S A, Banchereau J, Bri'ere F. Delayed IgG2 humoral response in infants is not due to intrinsic T or B cell defects. Int Immunol. 1996;8:1495–1502. doi: 10.1093/intimm/8.10.1495. [DOI] [PubMed] [Google Scholar]
- 46.Snowden J A, Milford-Ward A, Cookson L J, McKendrick M W. Recurrent lymphocytic meningitis associated with hereditary isolated IgG subclass 3 deficiency. J Infect. 1993;27:285–289. doi: 10.1016/0163-4453(93)92184-x. [DOI] [PubMed] [Google Scholar]
- 47.Soderlund M, Brown S C, Spaan W J M, Hedman L, Hedman K. Epitope type-specific IgG responses to capsid proteins VP1 and VP2 of human parvovirus B19. J Infect Dis. 1995;172:1431–1436. doi: 10.1093/infdis/172.6.1431. [DOI] [PubMed] [Google Scholar]
- 48.Tew J G, Wu J, Qin D, Helm S, Burton G F, Szakal A K. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 49.Torre D, Ferrario G, Bonetta G, Perversi L, Tambini R, Speranza F. Effects of recombinant human gamma interferon on intracellular survival of Bordetella pertussis in human phagocytic cells. FEMS Immunol Med Microbiol. 1994;9:183–188. doi: 10.1111/j.1574-695X.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 50.von Poblotzki A, Gerdes C, Reischl U, Wolf H, Modrow S. Lymphoproliferative responses after infection with human parvovirus B19. J Virol. 1996;70:7327–7330. doi: 10.1128/jvi.70.10.7327-7330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner A, Goronzy J, Matteson E, Weyland C. Systemic monocyte and T cell activation in a patient with human parvovirus B19 infection. Mayo Clin Proc. 1995;70:261–265. doi: 10.4065/70.3.261. [DOI] [PubMed] [Google Scholar]
- 52.Wegmann T G, Lin H, Guilbert L, Mossmann T R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 53.Woolf A D, Campion G V, Chishick A, Wise S, Cohen B J, Kloeida P T, Caul O, Dieppe P A. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153–1156. [PubMed] [Google Scholar]
- 54.Young N, Harrison M, Moore J, Mortimer P, Humphries R K. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Investig. 1984;74:2024–2032. doi: 10.1172/JCI111625. [DOI] [PMC free article] [PubMed] [Google Scholar]