Abstract
Itaconate, produced by aconitate decarboxylase 1 (ACOD1), which is encoded by immune-responsive gene 1 (Irg1), is one of the metabolites derived from the tricarboxylic acid cycle. It has been reported that exogenous itaconate plays an anti-inflammatory role in the progression of multiple diseases and pathological processes, including activated macrophage, ischemia-reperfusion injury, and acute lung injury. However, the role and specific mechanism of endogenous itaconate in endotoxemia-induced acute lung injury (ALI) remain unclear. The animal model of ALI in wild-type and Irg1−/− mice was constructed by LPS intraperitoneal injection. Ultrahigh-performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS) analysis was performed to measure the quantity of endogenous itaconate. The protective effect of itaconate was investigated by the behavioral assessment and the levels of inflammatory cytokines. Acute lung injury was assessed by hematoxylin and eosin staining, total protein in BALF, and Evans blue leakage. Western blotting was used to detect the IRG1 expression and autophagic protein in the lung. We demonstrated that IRG1 was highly expressed in ALI and that endogenous itaconate was produced simultaneously and was 100 times higher. Using Irg1−/− mice, we found that endogenous itaconate was likely to exert an anti-inflammatory effect by activating NRF2 and promoting autophagy. Furthermore, autophagy was restrained by LPS but enhanced by 4-octyl itaconate (4-OI) pretreatment. Our study illustrated that a deficiency of IRG1/Itaconate aggravates ALI and that the IRG1/itaconate pathway protects against ALI. The protective mechanisms could be related to the facilitation of autophagy. Such findings may provide a theoretical foundation for the treatment of endotoxemia-induced ALI.
Keywords: acute lung injury, autophagy, inflammation, itaconate, metabolite
Introduction
Acute lung injury (ALI), which is a life-threatening disease in intensive care units, refers to a series of manifestations involving progressive hypoxemia, dyspnea, and increased work of breathing [1]. Multiple diseases and pathological factors, such as trauma, pneumonia, sepsis, and endotoxemia, result in ALI with high morbidity and mortality [2]. Treatments include lung-protective mechanical ventilation, administration of a neuromuscular blockade, prone positioning, and extracorporeal membrane oxygenation [3]. To date, the specific therapy for endotoxemia-induced ALI has needed to be further explored.
Autophagy is a conserved catabolism pathway in which lysosomes degrade most of the cytoplasmic content and realize the process of resource recycling. However, the role of autophagy is still controversial in quite a few diseases, including ALI [4, 5]. On one hand, appropriate autophagy can be induced by many pathological conditions, like ischemia/reperfusion, tumors, hypoxia, septic acute kidney injury, oxidative stress, and inflammation [6,7,8,9,10,11], and then alleviate the processes above. On the other hand, excessive autophagy results in autophagic cell death (type II cell death). Autophagy promotes lung injury in sepsis [5]. Therefore, further research is required to confirm how autophagy regulates endotoxemia-induced ALI.
Itaconate, known as methylene-succinic acid, is an endogenous metabolite previously proposed to have an anti-inflammatory effect and is synthesized by the decarboxylation of cis-aconitate in the mitochondrial matrix. The decarboxylation is mediated by an aconitate decarboxylase (ACOD1, also called IRG1), which is encoded by immune-responsive gene 1 (Irg1) [12]. A suitable cell-permeable itaconate derivative, 4-octyl itaconate (4-OI), is hydrolyzed to itaconate by intracellular esterases [13]. Nowadays, studies have reported that itaconate plays an anti-inflammatory role or reduces oxidative stress in various diseases and pathological processes, such as macrophage activation, ischemia-reperfusion injury, osteoclast-related diseases, pulmonary fibrosis, renal fibrosis, abdominal aortic aneurysm, and early rheumatoid arthritis [14,15,16,17,18,19,20].
In this study, we aimed to investigate the role of itaconate in endotoxemia-induced ALI progression and to evaluate the underlying mechanism based on the expression of autophagic proteins. Our study may reveal itaconate as a promising candidate for preventative therapy in ALI.
Materials and Methods
Materials
Lipopolysaccharide (from Escherichia coli, 055: B5) and itaconate (>99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and 4-octyl itaconate (≥98%) was the product of Cayman Chemical (Ann Arbor, MI, USA). Itaconic acid-13C5, D4 was purchased from Toronto Research Chemicals (Toronto, ON, Canada). ELISA kits for determination of mouse tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) were purchased from NeoBioscience Technology Co. (Shenzhen, China). The BCA protein assay kit was the product of Thermo Fisher Scientific (Waltham, MA, USA).
Animals
Male 6- to 10-week-old C57BL/6J mice were provided by the Laboratory Animal Center of Chongqing Medical University (Chongqing, China). Male 6- to 10-week-old C57BL/6J-Irg1−/− mice were from Jackson Laboratory. The mice were maintained for 7 days before experimentation and kept in a specific pathogen-free room. They were given food and water ad libitum and subjected to a 12/12 h light/dark schedule. All animal experiments were reviewed and approved by the Animal Care and Use Committee of Chongqing Medical University.
ALI model and itaconate intervention
C57BL/6J mice were randomly divided into four groups: the control group, 4-OI group, ALI group, and ALI + 4-OI group. Irg1−/− mice were randomly divided into three groups: the control group, ALI group, and ALI + 4-OI group. Two hours before injury (ALI induced with 15 mg/kg LPS), mice were administered 4-OI (50 mg/kg, dissolved in 95% olive oil and 5% dimethyl sulfoxide; DMSO) in the ALI + 4-OI group. Animals were sacrificed 18 h after administration of LPS and tissue was harvested.
Behavioral assessment score
Wild-type mice were observed and evaluated by the mouse assessment score [21]. Scores were the total sums of the points for the following four categories of physical appearance: coat, with smooth=1, mild ruffling=2, and significant ruffling=3; activity, with normal=1, moving slowly without stimulation=2, moving only with stimulation=3, and minimal movement with stimulation=4; respiration, with normal=1, labored=2, and irregular=3; and posture, with moving or resting normally=1 and huddled=2.
Histologic analysis
The left lungs were washed in normal saline and fixed in10% paraformaldehyde for 48 h. Microphotographs of hematoxylin and eosin (HE)-stained sections were obtained for conventional morphological evaluation using a light microscope (Leica Microsystems, Wetzlar, Germany). Based on a method described previously [22], lung sections were blindly scored. Briefly, the histological changes were graded on a scale of 0–4 (0, normal; 1, slight; 2, moderate; 3, strong; 4, severe) for the following pathological characters: congestion, edema, inflammation, and hemorrhage. The histology scores for the four parameters were totaled.
ELISA
Based on the manufacturer’s instructions, ELISA kits were used to determine the plasma of tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6; Neobioscience, Shenzhen, China). The samples were diluted to an appropriate concentration with a diluent included with the ELISA kit before determination. The concentrations of TNF-α or IL-6 were calculated using the standard curve.
Collection and analysis of bronchoalveolar lavage of fluid (BALF)
All mice were euthanized by a lethal overdose of sodium pentobarbital. A tracheal cannula was inserted into each mouse, ice-cold PBS (1 ml) was injected, and bronchoalveolar lavage (BAL) was performed via the cannula. The first lavage was collected, and the supernatant was isolated by centrifugation (1,000 g, 5 min, 4°C).
Determination of Evans-blue leakage
As previously reported with slight modifications [23], 1 h before measuring the degree of pulmonary vascular leakage, Evans blue dye (80 mg/kg) was intravenously injected into the mice. The animals were transcardially perfused free of blood with PBS (containing 5 units per milliliter of heparin) via thoracotomy. The left lung was homogenized in N,N-Dimethyl formamide (1 ml/100 g tissue), incubated for 48 h at 60°C, and centrifuged at 5,000 × g for 15 min. The optical density of the supernatant was detected spectrophotometrically at 620 nm. The concentration of Evans blue dye was calculated using the standard curve.
Itaconate identification and quantification
Mouse lungs were used for itaconate identification and quantification as described previously, with a slight modification [18]. Briefly, 20 mg of lung was homogenized with 500 ml of ice-cold methanol/water (100%, v/v) at 30 Hz for 2 min and centrifuged at 12,000 rpm at 4°C for 15 min. Itaconic acid-13C5, D4 and itaconic acid were added to the methanol as the internal standard and for building the standard curve, respectively. The collected supernatant was analyzed using an Agilent 1290 Infinity II liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled to an Agilent 6470 triple quadruple mass spectrometer (Agilent Technologies). Using an Agilent 1260 Infinity HPLC system, 5 µl of the prepared sample was injected into the column (ACE Excel, 2.1 mm × 100 mm, EXL-1010-1002U, Advanced Chromatography Technologies, Aberdeen, UK), and the device was set at 22°C. The flow rate was set at 0.2 ml/min, and sample analysis was completed at 1 min. Other conditions were as follows: solvent system, water (0.1% formic acid): methanol; gradient program, 85:15 V/V at 0 min, 85:15 V/V at 0.5 min, 5:95 V/V at 3.0 min, 5:95 V/V at 5.0 min, 85:15 V/V at 5.1 min, 85:15 V/V at 7.0 min. Mass spectrometric detection was performed in a negative ionisation mode with an Agilent 6470 triple quadrupole tandem mass spectrometer equipped with an Agilent Jet Stream electrospray ionization source. The mass detector parameters were set as follows: gas temperature, 300°C; gas flow, 8 l/min; nebulizer pressure, 35 psi; and capillary voltage, +500 V.
Western blot analysis
Lung tissue was lysed in RIPA lysis buffer with protease inhibitor cocktail and centrifuged at 16,000 g for 10 min, and the supernatant was collected. Then, a bicinchoninic acid assay kit (Thermo Fisher Scientific) was employed to determine protein contents. The protein draws were separated by 6%, 10%, or 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride. The membranes were incubated with the blocking buffer for 1 h at room temperature and then incubated with the primary antibodies. The primary antibodies used were anti-IRG1 (1:1,000, 17805, CST), anti-SQSTM1/p62 (1:1,000, 5114T, CST), anti-LC3A/B (1:1,000, 12741T, CST), anti-β-actin (1:5,000, bs-0061R, Bioss). The membranes were incubated with the primary antibodies overnight at 4°C, followed by washing 5 times in phosphate-buffered saline Tween (PBS-T) for 5 min. They were then incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h and then washed 5 times for 5 min in PBS-T. Finally, Amersham ECL Select Western Blotting Detection Reagent was used for visualization.
Statistical analysis
All experimental data are presented as the mean ± SD. The quantitative data were analyzed using one-way ANOVA, followed by Tukey’s post hoc test. Student’s t-test was used for comparisons between two independent groups.
Results
IRG1 and itaconate production are increased in endotoxemic mouse lung tissue
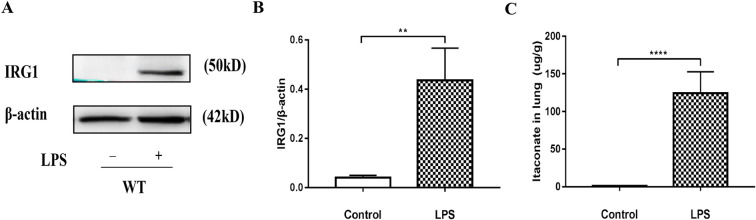
An ALI model was built was built to explore the role of endogenous itaconate. Then, we examined the expression of IRG1 in mice and the expression levels of itaconate in the lungs of ALI mice in the control group and the ALI group. The results proved that IRG1 in ALI mice was apparently activated (Figs. 1A and B) and that the production of itaconate was simultaneously increased by more than 100 times (Fig. 1C).
Fig. 1.
The expression levels of IRG1 and itaconate were increased in WT mice. (A, B) The expression levels of IRG1 in the control group and LPS group were analyzed by Western blot. (C) The levels of itaconate in lung tissue of the control group and LPS group were detected by UPLC-MS/MS. **P<0.01, ****P<0.0001. LPS, lipopolysaccharide; ALI, acute lung injury; IRG1, aconitate decarboxylase; WT, wild type; UPLC-MS/MS, ultrahigh-performance liquid chromatography-tandem mass spectroscopy.
Deficiency of IRG1/itaconate aggravates ALI
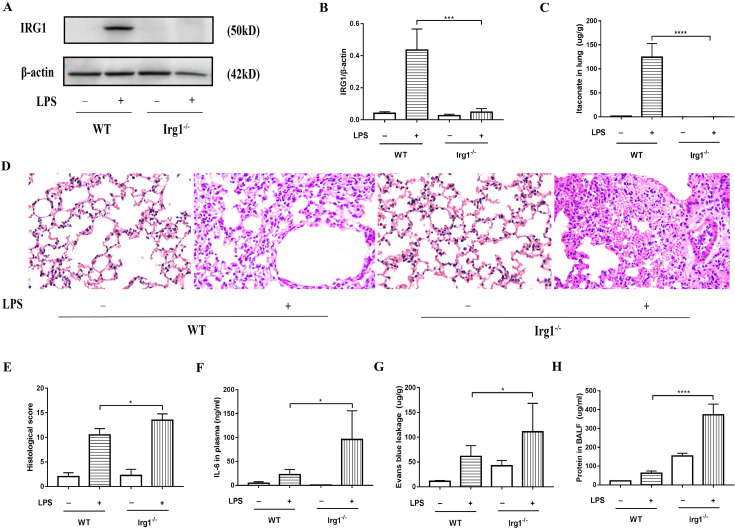
In order to explore the effect of itaconate on ALI, we examined the histological changes, inflammation, and alveolar exudation after LPS treatment. The expression of IRG1 and level of endogenous itaconate were extremely low in Irg1−/− mice (Figs. 2A–C), even in the LPS group, which indicated that the Irg1−/− mice lacked of IRG1/itaconate. The pathological results shows that the Irg1−/− mice lacking itaconate suffered more damage than the wild-type mice (Figs. 2D and E), had a higher level of the pro-inflammatory cytokine IL-6 in plasma (Fig. 2F), and exuded more protein in lungs (Figs. 2G and H). These findings illustrated that the IRG1/itaconate pathway inhibited ALI and prevented inflammation from being further aggravated.
Fig. 2.
Deficiency of IRG1 and itaconate exacerbated histological changes, inflammation, and alveolar exudation. (A, B) The expression of IRG1 in lung tissue was analyzed by Western blot. (C) The levels of itaconate in lung tissue were detected by UPLC-MS/MS. (D) The plasma levels of IL-6 were determined by ELISA. (E, F) Histological score was determined by hematoxylin and eosin (HE) staining (400×). (G) Comparison of Evans Blue leakage in lung tissue. (H) Comparison of protein concentration in BALF. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. ALI, acute lung injury; IRG1, aconitate decarboxylase; UPLC-MS/MS, ultrahigh-performance liquid chromatography-tandem mass spectroscopy; IL-6, interleukin 6; BALF, bronchoalveolar lavage fluid.
4-OI attenuates the inflammatory response and lung injury in ALI mice
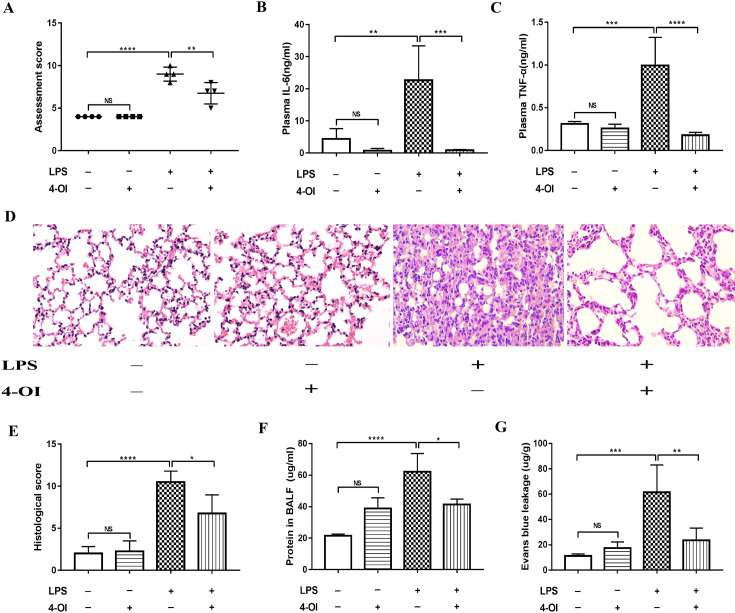
To confirm the protective effect of itaconate in ALI mice, we performed a behavior assessment and estimated the inflammatory response after pretreatment with exogenous itaconate 4-OI. We found that 4-OI reduced assessment scores in ALI mice (Fig. 3A). Moreover, 4-OI reduced the induction of IL-6 and TNF-α (Figs. 3B and C), indicating that 4-OI has a potential anti-inflammatory effect.
Fig. 3.
4-OI relieved histological changes and alveolar exudation in WT mice. (A) The physical appearance of the mice was evaluated by assessment scores. (B, C) The plasma levels of IL-6 and TNF-α were determined by ELISA. (D, E) The histological score was determined by hematoxylin and eosin staining (400×). (F) Comparison of protein concentration in BALF. (G) Comparison of Evans Blue leakage in lung tissue. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. 4-OI, 4-octyl itaconate; LPS, lipopolysaccharide; WT, wild type; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha; ELISA, enzyme-linked immunosorbent assay; BALF, bronchoalveolar lavage fluid.
To evaluate the severity of lung injury, we performed HE staining. The histological results showed that LPS caused severe inflammatory histological changes in lung tissue, including immune cell infiltration, alveolar wall damage, and lung congestion (Figs. 3D and E). However, 4-OI pretreatment significantly reversed these changes. In addition, the total protein in BALF and Evans blue leakage were also used as indicators to assess lung damage. Pretreatment with 4-OI apparently reduced protein exudation and vascular permeability (Figs. 3F and G). These findings provided further support for itaconate potentially reducing acute lung injury.
Endogenous itaconate is involved in the process of autophagy regulation
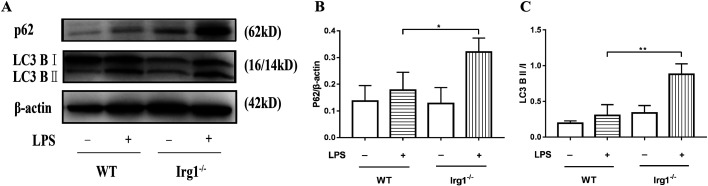
In order to explore the protective mechanism of itaconate in ALI, the autophagy levels were studied in Irg1−/− mice after LPS treatment. Western blotting showed that P62 and LC3B II/I in the lungs of Irg1−/− mice were further increased compared with WT mice (Fig. 4). These results support the lack of IRG1/itaconate inhibition of autophagy, which is equivalent to IRG1/itaconate promoting autophagy.
Fig. 4.
The lack of endogenous itaconate raised the P62 expression and LC3B II/I ratio in the lungs of ALI mice. (A–C) The P62 expression and LC3B II/I ratio in the lungs of ALI mice were detected by Western blot. *P<0.05, **P<0.01. LPS, lipopolysaccharide; ALI, acute lung injury; WT, wild type; Irg1, immune-responsive gene 1.
4-OI promotes autophagy
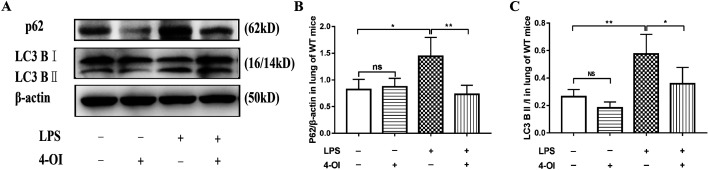
In order to obtain further evidence concerning IRG1/itaconate inhibition of autophagy, the autophagy levels were studied in WT mice after 4-OI pretreatment. The Western blot results for P62 and LC3B II/I showed that LPS suppressed autophagy but that 4-OI pretreatment enhanced autophagy (Fig. 5). All in all, when acute lung injury occurred, autophagy was restrained, whereas IRG/itaconate activated autophagy, thereby exerting a protective effect on the lungs.
Fig. 5.
4-OI suppressed the P62 expression and LC3B II/I ratio in WT mice. (A–C) The P62 expression and LC3B II/I ratio in the lungs of WT mice were detected by Western blot. *P<0.05, **P<0.01, ***P<0.001. 4-OI, 4-octyl itaconate; LPS, lipopolysaccharide; ALI, acute lung injury; WT, wild type.
Discussion
ALI is a serious lung disease, and there is no specific treatment for it currently. Therefore, it is important to investigate its potential pathogenesis, as this is conducive to seeking effective therapeutic strategies. In this study, we showed the protective effect of itaconate and the potential mechanisms in ALI. We found that 4-OI pre-administration mitigated the functional impairment and pathological alteration of endotoxemia-induced acute lung injury, which was accompanied by reduced inflammation and augmented autophagy. Similar to wild-type mice, a more significant effect occurred in Irg1−/− mice. The most crucial discovery is that endogenous itaconate exerted anti-inflammatory effects by enhancing autophagy, thereby protecting ALI mice from heavier damage.
In recent years, itaconate has been found to be a metabolite involved in regulating the phenotype and function of immune cells, and an anti-inflammatory effect of itaconate was found first in the process of activating macrophages with LPS [13]. It is known that the use of itaconate to interfere with LPS-induced macrophages involves four pathways in the anti-inflammatory process: inhibiting SDH to reduce ROS production [14], activating NRF2/KEAP1 and ATF3 [13, 24], and restraining TET DNA dioxygenases [25]. Some recent studies have shown that itaconate has a protective effect on ALI, and preliminary mechanism studies have been conducted to confirm its exact anti-inflammatory effects. Nevertheless, the mechanism of endogenous itaconate in ALI needs to be explored. Our study preliminarily confirmed that endogenous itaconate was 100 times higher in the lungs of LPS-induced mice, which is consistent with the previously found up-regulation of macrophages activated by LPS [13]. In addition, in the absence of endogenous itaconate, the organ damage induced by LPS is significantly aggravated, which indicates that endogenous itaconate is likely to participate in processes of lung tissue damage and repair by regulating macrophages.
Autophagy to promote cell survival has been extensively studied. P62, a protein degraded by autophagy, accumulates when autophagy is impaired. An increased LC3-II level is a crucial indicator of autophagy. P62 and LC3-II levels in the lung were raised when ALI occurred (Figs. 4 and 5). This showed that autophagic flux was blocked by an obstructive merge of autophagosomes and lysosomes. It also suggested that the increase of LC3-II was not caused by increased autophagosome formation but rather damaged clearance of autophagosomes. There is some research proving that autophagy inhibition alleviates lung injury, which is consistent with our results [26, 27]. Besides, our research proved that 4-OI can reduce the autophagic proteins LC3-II and P62, which were enhanced by knockout of Irg1 (Fig. 4), indicating that itaconate in ALI acts as an autophagy activator. A similar role has been shown for 4-OI in osteoarthritis [28]. Itaconate facilitates autophagy and protects against ALI, which emphasizes that blockage of autophagic flux contributed to the pathogenesis of ALI. Inflammation plays an equally important role in ALI. It can be regulated by autophagy [29], and autophagy disorder leads to expanded inflammation. Itaconate prohibited inflammatory expansion (Figs. 2 and 3) by enhancing autophagy in our research, suggesting that impairment of autophagic flux may contribute to the outburst of inflammation and that itaconate can be a physiological protective factor for ALI.
A few studies on LPS-induced ALI related to the autophagic mechanism, in general, autophagy in LPS-induced ALI confers a cytoprotective role [30]. Jia et al. described that the autophagy inducer rapamycin alleviated LPS-induced ALI [31]. LPS-induced ALI was aggravated by the autophagy inhibitor 3-MA [4]. Mahli et al. elucidated that autophagic flux was blocked by chloroquine, an autophagy inhibitor decreasing autophagosome-lysosome fusion [32], which induced the accumulation of LC3-II and p62 expressions [26]. TFEB (transcription factor EB) serves as a master regulator of autophagy-lysosomal biogenesis response. The latest research demonstrated that itaconate is a lysosome inducer through the activation of TFEB to promote antibacterial innate immunity [33]. This process of itaconate-induced TFEB nuclear localization was mediated by directly alkylating TFEB at Cys270 and antagonizing mTOR-mediated phosphorylation and cytosolic retention. Besides, there is evidence that TFEB is involved in the transcriptional regulation of starvation-induced autophagy, with a significant increase in the number of autophagosomes and high expression of lysosomal and autophagic genes [34]. Trehalose was reported to be effective in mitigating several diseases where autophagy is dysfunctional. Recently, Jeong et al. found that trehalose could trigger TFEB activation and nuclear translocation and then regulate the autophagy-lysosome system [35]. Therefore, we speculated that itaconate might induce lysosomal biogenesis by activating TFEB to increase autophagosome-lysosome fusion and then facilitate autophagy flux.
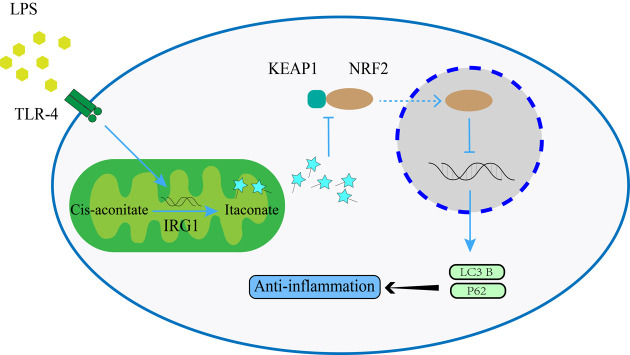
Owing to the fact that in ALI 4-OI activates NRF2 [36,37,38] and the fact that the P62-Keap1-NRF2 signalling pathway exists [39, 40] the P62 expression is induced by 4-OI pretreatment. However, our results showed that P62 expression was suppressed by itaconate (Fig. 4). We speculate that P62 production is increased but by less than the degradation caused by the clearance of autolysosomes. We inferred that endogenous itaconate promotes autophagy by activating NRF2, thereby producing an anti-inflammatory effect when ALI occurs (Fig. 6).
Fig. 6.
Schematic illustration of the anti-inflammatory effects of itaconate mediated by autophagy in the modulation of ALI. Lipopolysaccharide promotes endogenous itaconate through the activation of Irg1. Itaconate assists in the dissociation of KEAP1 and NRF2, and NRF2 thereby inhibits autophagic genes such as P62 and LC3 to downregulate inflammation.
We also explored other potential mechanisms for effects of itaconate on ALI other than inflammation and autophagy. The total protein in BALF and Evans Blue dye leakage can be used to evaluate albumin exudation of pulmonary vessels, representing the pulmonary vascular endothelial barrier. Itaconate relieves LPS-induced vascular barrier dysfunction (Figs. 2 and 3), which is further damaged by knockout of Irg1, indicating that itaconate may affect ALI by protecting lung endothelial cells. Some authors have reported that autophagy in endothelial cells was activated by LPS [41], whereas others have reported that it was inhibited by autophagy inhibitors such as chloroquine and that this aggravated LPS-induced ALI [42]. To date, none of the published articles have studied how itaconate regulates endothelial cells. This will be the next principal goal of our group.
Nevertheless, limitations in this research still exist. We cannot be sure whether autophagy is the dominant factor in the IRG1/itaconate pathway ameliorating ALI. In addition, the mechanism of the effect of itaconate on pulmonary cells in ALI mice has not been further explored.
Conclusion
Taken together, this study indicates that both exogenous itaconate and endogenous itaconate have anti-inflammatory and alleviating lung injury effects against ALI, which may be attributed to inhibition of the release of inflammatory cytokines. These effects are mediated by suppressing autophagy. To some extent, the findings contribute to elucidating the mechanism of acute lung injury and underscoring the therapeutic potential of itaconate for infectious diseases.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Jing-Huan Qiu carried out experiments and wrote the manuscript; Li Zhang conceived the ideas and analyzed data; Qiu-Hong Zhang and Ke-Xin Li contributed to some experiments; Kun Chen and Ke-Rui Fan provided critical materials; Yu Jiang contributed to the manuscript revision and designed the research; Gang Liu supervised and designed the research. All the authors approved the final version of the manuscript.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Chongqing (No. cstc2020jcyj-msxmX0254), Chongqing Municipal Public Health Bureau of Chongqing People’s Municipal Government (2022MSXM006), and Graduate Innovative Special Fund Projects of Chongqing (CYS20222; CYS21248). We are very grateful to the College of Pharmacy, Chongqing Medical University, for its support.
References
- 1.Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Probl Surg. 2020; 57: 100777. doi: 10.1016/j.cpsurg.2020.100777 [DOI] [PubMed] [Google Scholar]
- 2.Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol. 2015; 209: 52–58. doi: 10.1016/j.resp.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Kim WY, Hong SB. Sepsis and Acute Respiratory Distress Syndrome: Recent Update. Tuberc Respir Dis (Seoul). 2016; 79: 53–57. doi: 10.4046/trd.2016.79.2.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y, Zhang L, Jiang Y, Dai J, Tang L, Liu G. Emodin reactivated autophagy and alleviated inflammatory lung injury in mice with lethal endotoxemia. Exp Anim. 2019; 68: 559–568. doi: 10.1538/expanim.19-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Chen H, Xiaoyin M, Yang G, Hu Y, Xie K, et al. Autophagy Activation Improves Lung Injury and Inflammation in Sepsis. Inflammation. 2019; 42: 426–439. doi: 10.1007/s10753-018-00952-5 [DOI] [PubMed] [Google Scholar]
- 6.Zhang DM, Zhang T, Wang MM, Wang XX, Qin YY, Wu J, et al. TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic Biol Med. 2019; 137: 13–23. doi: 10.1016/j.freeradbiomed.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 7.Chung C, Seo W, Silwal P, Jo EK. Crosstalks between inflammasome and autophagy in cancer. J Hematol Oncol. 2020; 13: 100. doi: 10.1186/s13045-020-00936-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021; 6: 76. doi: 10.1038/s41392-020-00453-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhu J, Liu Z, Shu S, Fu Y, Liu Y, et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021; 38: 101767. doi: 10.1016/j.redox.2020.101767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Z, Lim J, Wang Q, Purtell K, Wu S, Palomo GM, et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy. 2020; 16: 917–931. doi: 10.1080/15548627.2019.1644076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020; 32: 44–55.e6. doi: 10.1016/j.cmet.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013; 110: 7820–7825. doi: 10.1073/pnas.1218599110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018; 556: 113–117. doi: 10.1038/nature25986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016; 24: 158–166. doi: 10.1016/j.cmet.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi Z, Deng M, Scott MJ, Fu G, Loughran PA, Lei Z, et al. Immune-Responsive Gene 1/Itaconate Activates Nuclear Factor Erythroid 2-Related Factor 2 in Hepatocytes to Protect Against Liver Ischemia-Reperfusion Injury. Hepatology. 2020; 72: 1394–1411. doi: 10.1002/hep.31147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Zhang B, Pan X, Huang H, Xie Z, Ma Y, et al. Octyl-itaconate inhibits osteoclastogenesis by suppressing Hrd1 and activating Nrf2 signaling. FASEB J. 2019: fj201900887RR. doi: 10.1096/fj.201900887RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian F, Wang Z, He J, Zhang Z, Tan N. 4-Octyl itaconate protects against renal fibrosis via inhibiting TGF-β/Smad pathway, autophagy and reducing generation of reactive oxygen species. Eur J Pharmacol. 2020; 873: 172989. doi: 10.1016/j.ejphar.2020.172989 [DOI] [PubMed] [Google Scholar]
- 18.Song H, Xu T, Feng X, Lai Y, Yang Y, Zheng H, et al. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. EBioMedicine. 2020; 57: 102832. doi: 10.1016/j.ebiom.2020.102832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly R, Blackburn G, Best C, Goodyear CS, Mudaliar M, Burgess K, et al. Changes in plasma itaconate elevation in early rheumatoid arthritis patients elucidates disease activity associated macrophage activation. Metabolites. 2020; 10: 241. doi: 10.3390/metabo10060241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogger PP, Albers GJ, Hewitt RJ, O’Sullivan BJ, Powell JE, Calamita E, et al. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. 2020; 5: eabc1884. doi: 10.1126/sciimmunol.abc1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, Denzel M, et al. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol. 2012; 188: 854–863. doi: 10.4049/jimmunol.1100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagiwara S, Iwasaka H, Matumoto S, Hidaka S, Noguchi T. Effects of an angiotensin-converting enzyme inhibitor on the inflammatory response in in vivo and in vitro models. Crit Care Med. 2009; 37: 626–633. doi: 10.1097/CCM.0b013e3181958d91 [DOI] [PubMed] [Google Scholar]
- 23.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004; 169: 1245–1251. doi: 10.1164/rccm.200309-1258OC [DOI] [PubMed] [Google Scholar]
- 24.Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018; 556: 501–504. doi: 10.1038/s41586-018-0052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LL, Morcelle C, Cheng ZL, Chen X, Xu Y, Gao Y, et al. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat Cell Biol. 2022; 24: 353–363. doi: 10.1038/s41556-022-00853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahli A, Saugspier M, Koch A, Sommer J, Dietrich P, Lee S, et al. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut. 2018; 67: 746–756. [DOI] [PubMed] [Google Scholar]
- 27.Dong W, He B, Qian H, Liu Q, Wang D, Li J, et al. RAB26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy. 2018; 14: 1677–1692. doi: 10.1080/15548627.2018.1476811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X, Shan H, Bai J, Gao T, Chen B, Shen Z, et al. Four-octyl itaconate improves osteoarthritis by enhancing autophagy in chondrocytes via PI3K/AKT/mTOR signalling pathway inhibition. Commun Biol. 2022; 5: 641. doi: 10.1038/s42003-022-03592-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011; 469: 323–335. doi: 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Chen Y, Zhang P, Lin P, Xie N, Wu M. Protective Features of Autophagy in Pulmonary Infection and Inflammatory Diseases. Cells. 2019; 8: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia X, Cao B, An Y, Zhang X, Wang C. Rapamycin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting IL-1β and IL-18 production. Int Immunopharmacol. 2019; 67: 211–219. doi: 10.1016/j.intimp.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 32.Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018; 14: 1435–1455. doi: 10.1080/15548627.2018.1474314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Chen C, Yang F, Zeng YX, Sun P, Liu P, et al. Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mol Cell. 2022; 82: 2844–2857.e10. doi: 10.1016/j.molcel.2022.05.009 [DOI] [PubMed] [Google Scholar]
- 34.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011; 332: 1429–1433. doi: 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong SJ, Stitham J, Evans TD, Zhang X, Rodriguez-Velez A, Yeh YS, et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy. 2021; 17: 3740–3752. doi: 10.1080/15548627.2021.1896906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, Wu Y, Jin S, Sun J, Wan BB, Zhang J, et al. Itaconate ameliorates methicillin-resistant Staphylococcus aureus-induced acute lung injury through the Nrf2/ARE pathway. Ann Transl Med. 2021; 9: 712. doi: 10.21037/atm-21-1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Chen X, Zhang H, Xiao J, Yang C, Chen W, et al. 4-Octyl Itaconate Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting Oxidative Stress and Inflammation. Drug Des Devel Ther. 2020; 14: 5547–5558. doi: 10.2147/DDDT.S280922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He R, Liu B, Xiong R, Geng B, Meng H, Lin W, et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022; 8: 43. doi: 10.1038/s41420-021-00807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv H, Yang H, Wang Z, Feng H, Deng X, Cheng G, et al. Nrf2 signaling and autophagy are complementary in protecting lipopolysaccharide/d-galactosamine-induced acute liver injury by licochalcone A. Cell Death Dis. 2019; 10: 313. doi: 10.1038/s41419-019-1543-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong L, Deng J, Zhou X, Cai B, Zhang B, Chen X, et al. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 2021; 12: 928. doi: 10.1038/s41419-021-04227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu C, et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy. 2016; 12: 2286–2299. doi: 10.1080/15548627.2016.1230584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, Zhou J, Ye LC, Li J, Wu Z, Li Y, et al. Autophagy maintains the integrity of endothelial barrier in LPS-induced lung injury. J Cell Physiol. 2018; 233: 688–698. doi: 10.1002/jcp.25928 [DOI] [PubMed] [Google Scholar]