Abstract
The common marmoset, Callithrix jacchus, is increasingly being used as the preferred nonhuman primate (NHP) model in biomedical research. Marmosets share several physiological and biological similarities with humans, as a Simiiformes species, and their use in research programs advances knowledge in several fields. Their unique characteristics, such as their small size, high fecundity, and rapid growth, offer additional advances in laboratory settings. This article reviews the developments in experimental disease models using marmosets based on our experience at the Central Institute for Experimental Animals (CIEA) in Japan. The development of genetically modified marmoset models using advanced genome editing technology is attracting researchers, particularly in neuroscience-related fields. In parallel, various marmoset models of human diseases induced by surgery or drug administration have contributed to preclinical and translational studies. Among these are models for Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, spinal cord injury models, a model for type 1 diabetes induced by the combination of partial pancreatectomy and streptozotocin administration, and a hepatic fibrosis model induced by thioacetamide. The development of these models has been supported by refinements in veterinary care, such as the careful design of anesthetic protocols and better understanding of pathogenic microorganisms. In the second part of this review, we present a compilation of practices currently in use at CIEA that provide optimal animal care and enable safe experimentation.
Keywords: anesthesia protocols, disease model, marmoset, microbiology, translational research
Introduction
The common marmoset (Callithrix jacchus), a species of New World monkeys, shares many biological and physiological similarities with humans and is an increasingly valuable laboratory animal model. Several unique traits make marmosets an advantageous model, such as their small size (average body weight: 350 g), easy handling, high fecundity with frequent twin delivery, relatively short life cycle, and rapid sexual maturity (by 12–18 months of age) [1]. Marmoset models have been widely used in biomedical research, particularly in neuroscience, infectious diseases, and preclinical studies for the development of novel drugs and therapies [1, 2]. Recent advances in genetic engineering based on stable assisted reproductive technology have further expanded the usefulness of marmoset models [3, 4].
Since the 1970s, the Central Institute for Experimental Animals (CIEA) in Japan has conducted research and development programs using marmosets as a nonhuman primate (NHP) model to bridge the critical gap between rodent models and humans. In particular, over the last decade, marmoset models of human disease for preclinical research developed at CIEA have included genetically modified models and experimental models induced by drug administration or surgery. Development of these programs has been largely supported by refinements in veterinary care and animal management. In the first part of this article, we review the current status of experimental marmoset models of disease at CIEA; this is followed by a discussion on current anesthetic protocols and microbiome surveys as part of veterinary management of the marmoset colony.
Experimental Disease Models for Translational Research Using Marmosets
Overview of marmoset research at CIEA
Historically, marmosets have been maintained as pets and zoo animals; their use as laboratory animals began in earnest in the 1960s and 1970s [5]. During this period, breeding colonies of common marmosets for laboratory use were founded in the United Kingdom, other European countries, and the United States. CIEA imported 12 species of small NHPs, including marmosets and tamarins, in the 1970s to develop NHP models for biomedical research. Since the introduction of common marmosets in 1976, CIEA has improved husbandry methods and established a breeding colony of this species from 12 marmoset pairs originally imported from the former Imperial Chemical Industries (London, UK) in 1983 [6]. The breeding colony was transferred to a commercial breeder, CLEA Japan (formerly Japan EDM), in 1991. CLEA Japan has maintained the colony since then without crossbreeding with animals from other origins, though they have introduced animals a few times from other colonies of domestic facilities. Animals bred from this colony have been supplied to research institutes in Japan and worldwide, including in Korea and the United States.
Since the introduction of marmosets, CIEA has continued basic research projects for animal care and scientific use, such as husbandry, reproduction, experimental techniques, and veterinary care, and published their outcomes as handbooks for researchers and animal technicians in Japan [7, 8]. Over the last two decades, alongside basic research programs, CIEA has conducted translational biomedical research projects using marmosets, particularly in the fields of developmental biology, magnetic resonance imaging (MRI) applications, and preclinical evaluation of novel therapies. In particular, the development of genetically modified marmosets has been promoted with the advancement of developmental engineering technology [4]. Sasaki and colleagues have established a protocol for stable assisted reproductive technology [9, 10], have developed a method for producing transgenic marmosets using a lentiviral vector, and were the first to report the germline transmission of a transgene in primates [11]. Recently, they proposed technologies for the knockout of target genes and point mutagenesis by genome editing tools and produced novel disease models, including models for immunodeficiency and Alzheimer’s disease [12,13,14].
Experimental disease models for translational research using marmosets
In addition to use in genetically modified (GM) disease models, marmosets have been used in non-GM disease studies [1, 2, 15]. CIEA and collaborating institutes have developed various non-GM disease models induced by surgery and/or drug administration (mentioned below) and models for infectious [16, 17] and spontaneous diseases [18] for preclinical research, as outlined in Table 1.
Table 1. Examples of experimental (non-GM) disease models using marmosets at CIEA and collaborating institutes.
| Category | Disease model | Methods | Research purposes | References |
|---|---|---|---|---|
| Central nervous system | Parkinson’s disease | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration 6-hydroxydopamine injection in the brain | Behavioral pharmacology, preclinical study in drug development, MR imaging | 25,26,27,28,29,30,31 |
| Spinal cord injury | Contusive injury or hemisection | Pathophysiology, stem cell therapy, preclinical study in drug development, MR imaging | 32,33,34 | |
| Multiple sclerosis (experimental autoimmune encephalomyelitis, EAE) | Recombinant human myelin-oligodendrocyte glycoprotein extracellular domain (rhMOG) immunization | Pathophysiology | – | |
| Cerebral ischemia | Middle cerebral artery occlusion | Stem cell therapy | – | |
| Infectious disease | Human T-cell leukemia virus type1 (HTLV-1) | Infection and immune suppression | Pathophysiology | 21 |
| Influenza A | Infection | Pathophysiology | 20 | |
| Others | Myocardial infarction | Ligation of left anterior descending coronary artery | Stem cell therapy | - |
| Hypertrophic scar | Skin incision | Preclinical study of nucleic acid-targeted drugs | 35 | |
| Diabetes mellitus (Type I) | Partial pancreatectomy and streptozotocin (STZ) administration | Stem cell therapy | 36 | |
| Liver fibrosis | Thioacetamide administration | Stem cell therapy | 37 | |
| Glaucoma | Spontaneous (aged) | Pathophysiology | 22 | |
–, unpublished.
Marmosets and humans share the basic plan of nervous system organization, and marmoset models of neurodegenerative disease are valuable for translational research [1]. A Parkinson’s disease (PD) model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration that causes degeneration of dopaminergic neurons in the substantia nigra is a flagship marmoset model. The model has been extensively used in various applications, from basic pathophysiological studies to the preclinical evaluation of novel drugs and therapies worldwide [19, 20], whereas another PD model induced by 6-hydroxydopamine (6-OHDA) injection into dopaminergic neural areas has been used in marmosets as in rodents [21]. Compared with other NHPs, marmosets are particularly suited for behavioral measurements in parkinsonism and for safety management of the MPTP toxicity because of their small body size and abundant motor activity. Ando and colleagues [22, 23] established a simple dosing schedule for MPTP administration to induce PD with subcutaneous injections of 2 or 1 mg/kg/day for three consecutive days. They have also established care protocols for the acute toxic phase that include oral administration of nutrient solution and subcutaneous infusion for hydration, as well as protocols for behavioral measurements, such as automated counting of spontaneous motor activity and dysfunction scoring systems. MPTP-treated marmosets exhibited major signs of PD, such as immobility (decrease of spontaneous motor activity), tremor, muscle rigidity, and postural dysfunction, in conjunction with dopaminergic degeneration of the substantia nigra [22, 24]. Furthermore, in MRI studies of MPTP-treated marmosets, voxel-based morphometry has revealed a decreased local tissue volume in the substantia nigra, and diffusion-tensor imaging demonstrated fiber loss in the nigrostriatal pathway [25, 26]. These findings suggest a novel role for MRI in the clinical diagnosis of PD. Furthermore, in the MPTP model, dyskinesia (involuntary movements of the body), a side effect of long-term dopamine replacement therapy with L-DOPA, was induced by repeated L-DOPA administration (10 mg/kg/day on 3 days/week for 6 weeks) [27].
Marmoset models have further contributed to the preclinical evaluation of novel therapies, such as regenerative medicine research. For example, during the early stages of research and development projects, preparing large amounts of testing materials, such as induced cells, can prove technically and economically challenging. The small body weight of marmosets, equivalent to that of rats (approximately one tenth of that of cynomolgus macaques), can facilitate experiments at a lower cost. Marmoset models of cervical spinal cord injury [28] have been used for the evaluation of regeneration-based therapies using hepatocyte growth factor (HGF) [29] and transplantation of iPS cell-derived neural stem/progenitor cells [30]. Other several experimental disease models for translational research have been developed in marmosets, including a hypertrophic scar [31] model to test nucleic acid-targeting drugs, as well as models for myocardial infarction (Hattori et al., unpublished) and type 1 diabetes mellitus [32] for the preclinical assessment of cell transplantation therapies (Table 1).
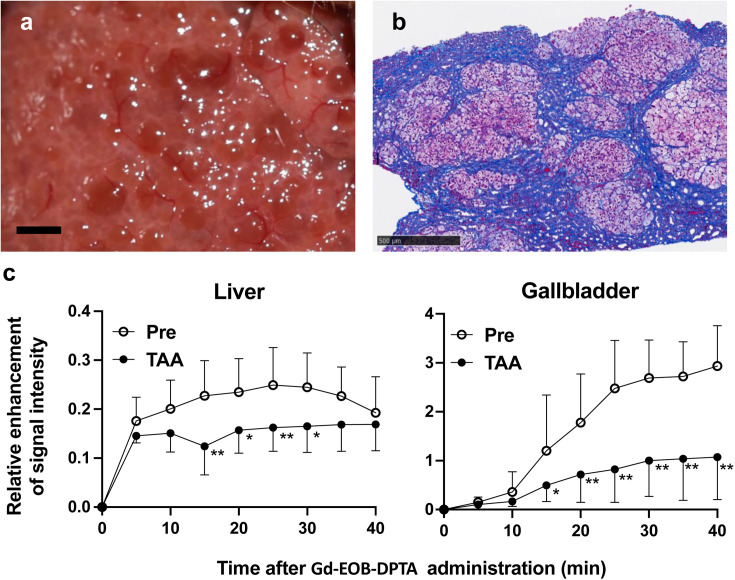
Preclinical models for liver regeneration therapies for cirrhosis would also be useful; however, marmoset models of experimental hepatic fibrosis are not available at this time. We attempted to induce liver fibrosis by administration of thioacetamide (TAA), a common hepatotoxin in rodents, and found that subcutaneous injection (SC) of TAA at doses of 2.5–40 mg/kg two or three times a week for more than 11 weeks caused hepatic fibrosis [33]. In a subsequent study, marked liver fibrotic lesions were induced by adjusting the TAA doses to 30 mg/kg twice a week for an additional period of 12 months (Figs. 1a and b); TAA administration was terminated when acute liver failure was suspected based on weekly monitoring of blood chemistry.
Fig. 1.
Hepatic fibrosis induced by thioacetamide (TAA) in marmosets. a. Nodular liver surface of a marmoset subcutaneously injected with TAA at a dose of 30 mg/kg twice a week for 15 months. Scale bar (black): 2 mm. b. Liver biopsy specimen with Masson’s trichrome stain of a marmoset given the same treatment as in a. Fibrous lesions containing blue-stained collagen were largely located around hepatic lobules. Scale bar (black): 500 µm. c. Relative enhancement (RE) of signal intensity by dynamic contrast-enhanced MRI using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), a hepatocyte-targeted contrast agent, before and 15 months post continuous TAA treatment. RE in the liver and gallbladder at time points after Gd-EOB-DTPA injection was significantly decreased post TAA treatment in marmosets (n=3). Statistical analysis was conducted by Bonferroni’s multiple comparisons test following two-way ANOVA. *P<0.05, **P<0.01.
Furthermore, noninvasive evaluation of hepatic lesions by contrast-enhanced MRI using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), a hepatocyte-targeted contrast agent [34], was tested as an alternative to invasive hepatic biopsy. MRI data were obtained using a 7.0T Biospec 70/16 scanner system (Bruker BioSpin GmbH, Ettlingen, Germany) equipped with actively shielded gradients at a maximum strength of 700 mT/m and an imaging coil with an inner diameter of 60 mm. Dynamic contrast-enhanced MRI was performed with intravenous administration of 0.025 mmol Gd/kg (0.1 ml/kg) of Gd-EOB-DTPA (Primovist, Bayer, Leverkusen, Germany). Three marmosets underwent MRI using T1-weighted fast low angle shot sequences before and 65 weeks after continuous TAA administration using the above protocol. The relative enhancement (RE) of the signal intensity [35, 36] obtained from the regions of interest in the liver and the gallbladder was significantly decreased post TAA administration (Fig. 1c), indicating decreased uptake of Gd-EOB-DTPA in hepatocytes in the context of TAA-induced fibrosis. The protocols for inducing stable liver fibrosis and noninvasive assessment of the hepatic lesion are an attractive option in preclinical research for novel transplantation therapies.
Veterinary Management for Marmosets in Biomedical Research
Research on veterinary management of marmosets at CIEA
In the past 15 years, research in marmoset veterinary care has mainly involved clinical and pathological studies as well as design of anesthetic protocols and microbiological surveys; the latter two topics are presented in detail in the following sections. Our clinical and pathological surveys in the past five years (2017–2021) revealed that the primary spontaneous diseases in marmosets leading to death or euthanasia were marmoset wasting syndrome (MWS), followed by duodenal dilation and neoplasms. This indicates that gastrointestinal (GI) diseases are common in captive marmosets and a major health problem for colonies [37, 38].
MWS is clinically characterized by impaired weight gain, weight loss, muscle atrophy, and alopecia commonly accompanied with anemia and hypoalbuminemia [39, 40]. The etiology remains unknown, but MWS is associated with chronic lymphocytic enteritis [37, 38, 41]. Histological examination of MWS cases at our facility also showed considerable mononuclear cell infiltration in the lamina propria of the small intestinal mucosa.
Recently, our group described “duodenal dilation syndrome” as a novel GI disease characterized by proximal duodenal obstruction and dilation with chronic repetitive vomiting, chronic bloating, and exhaustion, which can cause fatal aspiration pneumonia [42]. Autopsy revealed a narrowing lumen of the distal duodenum due to an ulcer scar or abnormal flexure, suggesting an association with duodenal ulceration, duodenal-colonic adhesion, or cholangitis; however, the cause of onset of the disease is unclear, and similar cases have been found in other colonies [38]. We have established diagnosis methods for duodenal dilation using a combination of radiography and ultrasonography [38], and we will continue to investigate the etiology of the disease and treatment options.
Neoplasms observed in marmosets at CIEA include intestinal lymphomas and small intestinal adenocarcinomas, which are the commonly observed GI tumors in captive colonies [37, 38, 41], as well as rare lung adenocarcinomas [42]. In addition, clinical procedures to maintain the health of the colony have been refined. For example, marmosets have a high risk of fatal blood loss because of their low whole blood volume; an adult marmoset of average weight (350 g) has an estimated circulating blood volume of 24.5 ml, and only 4.9 ml (20% circulating blood volume) of acute blood loss can cause hemorrhagic shock [43]. We have established a protocol for whole blood transfusion, including crossmatching, for marmosets and have demonstrated its efficacy and safety in severe anemia and persistent hemorrhage cases [44].
Anesthesia and analgesia protocols in marmosets
Administration of anesthesia before surgical procedures is crucial to relieve animal pain and distress and performing stable experiments. Anesthetic and analgesic protocols should be optimized for specific animal species and experimental purposes. Diverse anesthetic and analgesic regimes for marmosets have been reviewed recently [45, 46]. In this section, we describe our procedures and some cautionary notes based on our experience at CIEA.
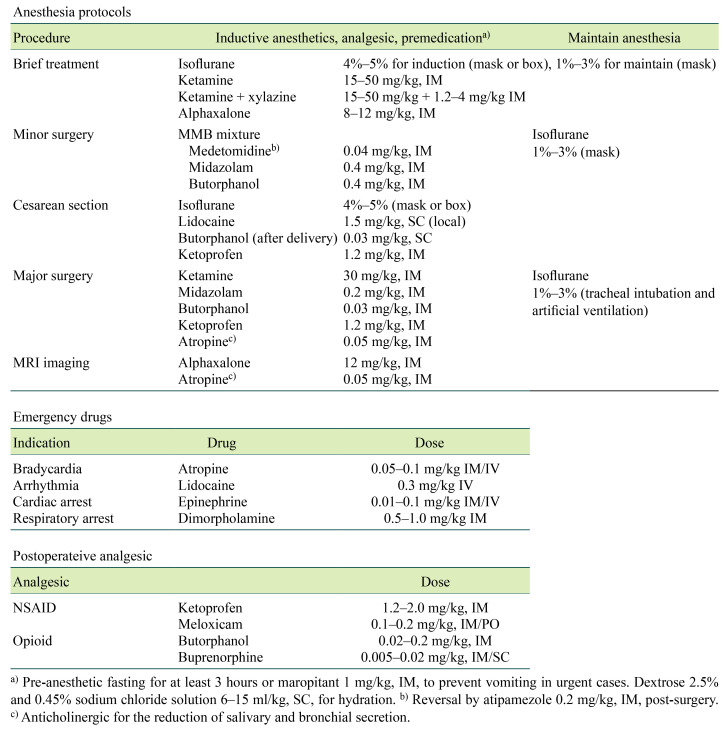
Table 2 lists the anesthetic protocols, including premedication, emergency drugs, and postoperative analgesic doses for marmosets currently in use at CIEA. The small bodies of marmosets and their narrow airways pose challenges to the administration of anesthesia. Particular attention should be given to preventing vomiting because of the considerable risk of death from aspiration. Animals should be routinely fasted before anesthesia (at least 3 h), and the administration of antiemetics (e.g., maropitant) is recommended. To maintain stable respiration, the use of anticholinergics (e.g., atropine) for the reduction of salivary and bronchial secretions, keeping the tongue pulled out to prevent glossoptosis, and careful observation of breathing during anesthesia are recommended. Fluid administration before and during anesthesia is recommended to support cardiovascular function and compensate for fluid losses. For example, 6–15 ml/kg of 2.5% dextrose and 0.45% sodium chloride solution is subcutaneously administered before anesthesia at CIEA. Thermal support during and post anesthesia with a heating device and an intensive care unit chamber is essential because the larger surface area to volume ratio makes marmosets susceptible to hypothermia.
Table 2. Anesthesia and analgesia protocols for marmosets at CIEA.
In addition, a previous report indicated that the administration of anesthetic agents might lead to hypoxemia [47]. Except for minor treatments, inhalation anesthesia supplemented with oxygen and monitoring of the saturation of percutaneous oxygen (SpO2) is recommended. At CIEA, an SpO2 sensor probe for human neonates (e.g., TL-260T multi-site Y probe, Nihon Kohden, Tokyo, Japan) is attached or clipped to the hand, foot, calf, or tail, and a monitoring equipment for human (e.g., OLV-4201, Nihon Kohden) and small animal medicine (e.g., BSM-3592, Nihon Kohden) is used. Other SpO2 sensors designed for pediatric use or rodents are available for marmosets [46]. During major surgery or long anesthesia, the rectal temperature and electrocardiogram of marmosets are monitored in addition to respiration, SpO2, and pulse.
If an anesthetic emergency, such as bradycardia (<120 bpm) or respiratory arrest, is observed, the inhaled anesthetic concentration is lowered, and emergency drugs are administered depending on the situation. Table 2 lists the emergency medications administered at CIEA. The short oral cavity and visible larynx of the marmoset make intratracheal intubation relatively easy, and inhalation anesthesia with a ventilator should be performed in long surgeries to maintain a stable ventilation. At CIEA, feeding tubes (6–8 Fr) for human neonates (Atom Medical, Tokyo, Japan) are intubated as endotracheal tubes at a distance of 4–5 cm from the incisors, and volume control ventilation is performed with a tidal volume of 4–7 ml 30–40 times/min using a ventilator (SN-480, Shinano Manufacturing, Tokyo, Japan).
Induction with injectable agents allows for a smooth transition to anesthesia and provides adequate analgesia and stable maintenance of the anesthetic level when combined with inhalation anesthetics. In the past, ketamine had been mainly used for induction at CIEA. Ketamine is a useful injectable anesthetic agent because of its rapid induction of anesthesia, analgesic effect as a N-methyl-D-aspartate receptor antagonist, and wide safety margin [46]. Combinations of ketamine and α2-adrenergic receptor agonists, such as xylazine, medetomidine, and dexmedetomidine, induce sedation or general anesthesia in marmosets [7, 45, 46]. On the other hand, ketamine has been regulated as a narcotic agent with strict license-based restrictions in Japan since 2007. In our experience, administration of ketamine (30 mg/kg) causes adverse side effects, such as hypersalivation, vomiting, and respiratory arrest, during isoflurane inhalation anesthesia.
A combination of medetomidine, an α2-adrenergic receptor agonist; midazolam, a benzodiazepine; and butorphanol, an opioid (MMB), which has been widely used in mice and other laboratory animals [48, 49], is used as an alternative induction agent (Table 1). Conveniently, butorphanol is known to have an antiemetic effect [50, 51]. The preferred combination of MMB is medetomidine 0.04 mg/kg, midazolam 0.4 mg/kg, and butorphanol 0.4 mg/kg delivered via intramuscular injection (IM). This combination was optimized for marmosets based on doses reported in ring-tailed lemurs [52] and patas monkeys [53]. The administration of atipamezole 0.2 mg/kg IM at the end of surgery reverses the effect of medetomidine and facilitates smooth recovery from anesthesia. In our experience, MMB before isoflurane inhalation has been used in more than 1,000 operations a year in the last 10 years with limited adverse effects, notably, hypersalivation, vomiting, and apnea. Alfaxalone, which has been available in Japan since 2014, and its combinations are also valid options for injectable anesthesia in marmosets [54, 55].
Postoperative analgesia must be provided for both humane and scientific purposes. Analgesic regimens for marmosets reviewed in the literatures help to ensure appropriate pain management; however, there is insufficient information concerning evaluations of the efficacy or pharmacokinetics of analgesic agents in marmosets [46]. At CIEA, the analgesic protocol using nonsteroidal anti-inflammatory drugs (NSAIDs) is ketoprofen 1.2–2 mg/kg IM or meloxicam 0.1–0.2 mg/kg IM/per os administered once daily for three or more days post surgery (Table 2). In cases where potent analgesia is required, for example after a major surgery, opioids, butorphanol (0.02–0.2 mg/kg IM), or buprenorphine (0.005–0.02 mg/kg IM/SC) are administered in addition to NSAIDs as a multimodal approach.
Microbiological surveys in marmosets
Microbiological control is an essential process to maintain the health of the colony, reduce biosafety risks, and obtain reliable scientific results. Although specific pathogen-free colonies have been established in barrier environments [56, 57], marmosets are commonly raised in conventional environments. Marmosets are susceptive to various human pathogens. For example, fatal outbreaks of measles [58] and herpes simplex viruses [59] have been reported. Emphasis should be placed on preventive medical practices against human pathogens, including mandatory health certificates for staff and visitors, showing measles antibody levels and tuberculosis-free status, and restricting admission of individuals suspected of having infectious diseases. Zoonotic risks from marmosets to humans are low in established laboratory animal colonies, as marmosets are not natural hosts of herpes B virus, which is a serious zoonotic pathogen transmitted from macaques to humans [41]. Nevertheless, major zoonotic pathogens that have serious risks among humans and marmosets should be monitored because pathogens can be transmitted by indirect or direct contact with infected humans, NHPs, or other animals. At CIEA, Salmonella spp., Shigella spp., Yersinia spp., and intestinal parasites have been examined in quarantine and periodical examinations. No positive cases of these bacteria or pathogenic parasites, including Entamoeba histolytica, have been found since establishment of the colony.
However, a major source for concern is GI tract diseases, a usual finding in captive marmosets. Opportunistic microbial infections are suspected causes of intestinal lesions, and several pathogens related to diseases have been reported in investigations conducted at marmoset facilities worldwide to understand disease causation [40, 60,61,62]. However, there is limited information available, and microbes harbored by animals depend on their origins and housing environments. A survey was conducted at the CIEA marmoset colony with the aim of identifying pathogens associated with intestinal diseases and improving veterinary care practices, and the rest of this section highlights its main results.
Table 3 lists protozoan, bacterial, and viral pathogens detected from the marmosets at CIEA. Trichomonad protozoa are prevalent intestinal parasites in the colony, and their association with bowel diseases has been evaluated [63]. Trichomonas is a flagellate protozoan parasite that infects the digestive tract and reproductive organs of various mammals, including members of the Callitrichidae family [40]. Identification of protozoan species and reports on pathogenicity in marmoset colonies are largely limited. In our survey [63], morphological characterization and 18S rRNA gene analysis of marmoset fecal samples identified Pentatrichomonas hominis, a nonpathogenic opportunist in the large intestine of various mammalian hosts, including NHPs [40, 64]. The positive rates of trichomonad trophozoites in normal and diarrheal feces were similar in our survey, indicating that P. hominis was not the primary cause of diarrhea or colitis. On the other hand, there tended to be large numbers of these protozoa found in diarrhea feces. Some diarrheal cases with large numbers of P. hominis have been treated successfully with metronidazole, an antitrichomonal and antibacterial agent, suggesting that P. hominis is likely associated with diarrhea, and treatment with metronidazole in diarrhea cases with elevated trichomonad levels can be effective. In a subsequent analysis of the nucleotide sequences, including the internal transcribed spacer regions, we revealed low genetic divergence of P. hominis within our colony and other reported mammal hosts, suggesting that P. hominis can be transmitted among marmosets and other mammals.
Table 3. Microorganisms harbored in common marmosets surveyed at CIEA.
| Microorganisms | Relation with disease | |
|---|---|---|
| Protozoa | Pentatrichomonas hominis | Commensal or diarrhea |
| Bacteria | Enteropathogenic Escherichia coli (EPEC) | Bloody diarrhea |
| Clostridioides difficile | Diarrhea, pseudomembranous colitis (severe) | |
| Clostridium perfringens | Sepsis (rare) | |
| Klebsiella pneumoniae | Sepsis, pneumonia (prevalent in the early years of the colony) | |
| Virus | Callitrichine herpesvirus 3 | Lymphoproliferative disease |
Enteropathogenic Escherichia coli (EPEC) is a common bacterial pathogen in the GI tract of marmosets (Table 3). EPEC positive for the attaching and effacing virulence gene, eae, is a recognized cause of typhlocolitis in marmosets [65,66,67]. Hayashimoto et al. [66] revealed the prevalence of EPEC in bloody diarrhea cases within the CIEA colony, and experimental infection of an EPEC strain (R811) isolated from a marmoset in our facility caused hematochezia with attachment of gram-negative bacilli to epithelial apical membranes and desquamated epithelial cells in the cecum. The recommended treatment for hemorrhagic typhlocolitis at CIEA is the administration of an appropriate antibiotic choice (e.g., enrofloxacin). It should be noted that asymptomatic carriers of EPEC have also been found [66], and management of EPEC in the colony requires further assessment.
Clostridioides (Clostridium) difficile has also been implicated in GI diseases in the CIEA marmoset colony. C. difficile is a gram-positive spore-forming anaerobic bacillus found naturally in the GI tracts of various mammals as well as in soils and the environment [68]. Elevated concentrations of these bacteria produce toxins that cause diarrhea and colitis in the host organism because of an imbalance in intestinal microbiota, and fatal pseudomembranous enterocolitis cases associated with C. difficile infection have been reported in common marmosets and related species [69, 70]. At CIEA, we have used an immunochromatography kit (C. DIFF QUIK CHEK®, Alere, Orland, FL, USA) to detect C. difficile toxins. The clinical presentations of C. difficile enteritis include diarrhea with mucus, acute weight loss, anorexia, and no feces. When signs are observed in the colony, diagnostic screening is performed, and positive cases are treated with appropriate antibiotics, commonly vancomycin or metronidazole. Fecal transplantation can also be a designated treatment strategy for C. difficile infection in marmosets [71].
Among rarely occurring diseases, sepsis and pneumonia cases caused by Klebsiella pneumoniae were prevalent in the early years of the breeding colony, in the 1970s and early 1980s, and vaccination with formaldehyde-killed bacteria was conducted to manage infection [72]. In addition, a sepsis case (nontraumatic gas gangrene) caused by Clostridium perfringens type A has been reported in the colony [73]; sepsis is rare, as C. perfringens is generally considered commensal.
Although current knowledge on viruses endemic to marmosets is limited, Callitrichine herpesvirus 3 (CalHV-3) is a recognized agent that may induce intestinal lymphoproliferative disease or lymphoma [74, 75]. CalHV-3 is a lymphocryptovirus of the Gammaherpesvirinae subfamily and closely related to the human Epstein–Barr virus [75]. The seroprevalence rates of CalHV-3 were 37% and 47% in two captive colonies and 50% in individuals recently captured from the wild, indicating that marmosets are natural hosts for CalHV-3 [76]. We surveyed the prevalence of CalHV-3 in the CIEA colony using polymerase chain reactions to amplify DNA samples from peripheral blood and enlarged lymph nodes of marmosets, with primers targeting major internal repeats designed by Fogg et al. [76]. The three samples from the enlarged lymph nodes and 63% (15/25) of the blood samples tested positive. These results suggest that the virus is endemic to our marmoset colony and may be responsible for the lymphoproliferative disease.
Concluding Remarks
The common marmoset is currently emerging as the NHP species of choice for biomedical research. There is an increasing demand worldwide for marmosets in neuroscience projects to elucidate the organization of brain circuits and as models for neurological disorders, and marmosets are particularly advantageous for genome editing technologies applicable in translational studies [77,78,79]. The recent successful use of marmosets in biomedical studies is an extension of basic research projects for breeding, care, and experimental use since the 1970s. The development of experimental disease and preclinical marmoset models, which was reviewed in this report, has expanded research applications using this species. Furthermore, experimental procedures, such as MRI, anesthesia, and veterinary care and management, including microbiological control of marmoset colonies, have advanced in parallel. To sustain research using the marmoset paradigm, we will continue refining experimental methods and improving veterinary care as well as practicing the principles of the 3Rs (replacement, reduction, and refinement) for animal experimentation.
Acknowledgments
We thank Dr. Nobuhito Hayashimoto, Dr. Masahiko Yasuda, Dr. Takayuki Mineshige, Dr. Yoko Kurotaki, Mr. Norio Okahara, Ms. Tomoko Ishibuchi, Ms. Emi Sasaki, Ms. Chihoko Yamada, Dr. Hanako Morita, Dr. Kenji Kawai, Dr. Yuji Komaki, Dr. Kiyoshi Ando, Dr. Toshio Ito, and all members of CIEA involved in marmoset research. We also thank Dr. Yukihito Ishizaka, Dr. Jun Lu, and Dr. Masayuki Shimoda (National Center for Global Health and Medicine, Tokyo, Japan) for their collaboration with respect to the TAA-induced hepatic fibrosis model. This work was partially supported by a grant for the Platform for Marmoset Research Support project (JP19dm0207068) of the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development (AMED), and a grant for the Differentiation of Mesenchymal Stem Cells to Hepatic Cells by Artificial Transcriptional Factors project (JP18fk0210103) of the Program for Basic and Clinical Research on Hepatitis from AMED.
References
- 1.Fox JG, Marini RP, Wachtman LM, Tardif SD, Mansfield K. The common marmoset in captivity and biomedical research. London: Academic Press, an imprint of Elsevier; 2019. [Google Scholar]
- 2.’t Hart BA, Abbott DH, Nakamura K, Fuchs E. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discov Today. 2012; 17: 1160–1165. doi: 10.1016/j.drudis.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki E. Prospects for genetically modified non-human primate models, including the common marmoset. Neurosci Res. 2015; 93: 110–115. doi: 10.1016/j.neures.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 4.Sasaki E. Development of genetically modified nonhuman primates toward models for translational research. Transl Regul Sci. 2019; 1: 15–23. [Google Scholar]
- 5.National Academies of Sciences E. Medicine, Division on E, Life S, Institute for Laboratory Animal R, Roundtable on S, et al. Care, Use, and Welfare of Marmosets as Animal Models for Gene Editing-Based Biomedical Research: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2019. [PubMed] [Google Scholar]
- 6.Nomura T, Tanioka Y. Characteristics and experimental use of the common marmoset. Tokyo, Japan: Soft Science; 1989. [Google Scholar]
- 7.Tanioka Y, Taniguchi K, Fujino K. Care, reproduction, experimental techniques and anatomy of the common marmoset. Tokyo: Adthree; 1996. [Google Scholar]
- 8.Sasaki E, Inoe T, Kurotaki Y, Miki R. Laboratory Manual for marmoset studies -From handling to the frontline of research- Tokyo: Adthree; 2018. [Google Scholar]
- 9.Park JE, Sasaki E. Assisted reproductive techniques and genetic manipulation in the common marmoset. ILAR J. 2020; 61: 286–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurotaki Y, Sasaki E.Practical reproductive techniques for the common marmoset. J Mamm Ova Res. 2017; 34: 3–12. doi: 10.1274/032.034.0103 [DOI] [Google Scholar]
- 11.Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009; 459: 523–527. doi: 10.1038/nature08090 [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Oiwa R, Kumita W, Henry R, Sakuma T, Ito R, et al. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell. 2016; 19: 127–138. doi: 10.1016/j.stem.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Sasaguri H, Kumita W, Inoue T, Kurotaki Y, Nagata K, et al. A non-human primate model of familial Alzheimer’s disease. bioRxiv. 2020: 2020.2008.2024.264259.
- 14.Kumita W, Sato K, Suzuki Y, Kurotaki Y, Harada T, Zhou Y, et al. Efficient generation of Knock-in/Knock-out marmoset embryo via CRISPR/Cas9 gene editing. Sci Rep. 2019; 9: 12719. doi: 10.1038/s41598-019-49110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003; 53: 383–392. [PubMed] [Google Scholar]
- 16.Iwatsuki-Horimoto K, Nakajima N, Kiso M, Takahashi K, Ito M, Inoue T, et al. The marmoset as an animal model of influenza: infection with A(H1N1)pdm09 and Highly Pathogenic A(H5N1) viruses via the conventional or tracheal spray route. Front Microbiol. 2018; 9: 844. doi: 10.3389/fmicb.2018.00844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirose L, Hiramoto T, Tian Y, Kohara H, Kobayashi S, Nagai E, et al. A pilot study to establish human T-cell leukemia virus type 1 (HTLV-1) carrier model using common marmoset (Callithrix jacchus). J Med Primatol. 2020; 49: 86–94. doi: 10.1111/jmp.12454 [DOI] [PubMed] [Google Scholar]
- 18.Noro T, Namekata K, Kimura A, Azuchi Y, Hashimoto N, Moriya-Ito K, et al. Normal tension glaucoma-like degeneration of the visual system in aged marmosets. Sci Rep. 2019; 9: 14852. doi: 10.1038/s41598-019-51281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippens IHCHM. Marmosets in Neurologic Disease Research: Parkinson’s Disease. In: Fox JG, Marini RP, Wachtman LM, Tardif SD, Mansfield K, editors. The common marmoset in captivity and biomedical research. London: Academic Press, an imprint of Elsevier; 2019. pp. 415–435. [Google Scholar]
- 20.Jenner P. The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology. 2003; 61:(Suppl 3): S4–S11. doi: 10.1212/WNL.61.6_suppl_3.S4 [DOI] [PubMed] [Google Scholar]
- 21.Ando K, Nishime C, Inoue R, Nishinaka E, Kawai K, Urano K, et al. Differential effects of dopaminergic drugs on spontaneous motor activity in the common marmoset following pretreatment with a bilateral brain infusion of 6-hydroxydopamine. Behav Pharmacol. 2017; 28: 670–680. doi: 10.1097/FBP.0000000000000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando K, Obayashi S, Nagai Y, Oh-Nishi A, Minamimoto T, Higuchi M, et al. PET analysis of dopaminergic neurodegeneration in relation to immobility in the MPTP-treated common marmoset, a model for Parkinson’s disease. PLoS One. 2012; 7: e46371. doi: 10.1371/journal.pone.0046371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ando K, Maeda J, Inaji M, Okauchi T, Obayashi S, Higuchi M, et al. Neurobehavioral protection by single dose l-deprenyl against MPTP-induced parkinsonism in common marmosets. Psychopharmacology (Berl). 2008; 195: 509–516. doi: 10.1007/s00213-007-0929-2 [DOI] [PubMed] [Google Scholar]
- 24.Ando K, Inoue T, Hikishima K, Komaki Y, Kawai K, Inoue R, et al. Measurement of baseline locomotion and other behavioral traits in a common marmoset model of Parkinson’s disease established by a single administration regimen of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: providing reference data for efficacious preclinical evaluations. Behav Pharmacol. 2020; 31: 45–60. doi: 10.1097/FBP.0000000000000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hikishima K, Ando K, Komaki Y, Kawai K, Yano R, Inoue T, et al. Voxel-based morphometry of the marmoset brain: In vivo detection of volume loss in the substantia nigra of the MPTP-treated Parkinson’s disease model. Neuroscience. 2015; 300: 585–592. doi: 10.1016/j.neuroscience.2015.05.041 [DOI] [PubMed] [Google Scholar]
- 26.Hikishima K, Ando K, Yano R, Kawai K, Komaki Y, Inoue T, et al. Parkinson Disease: Diffusion MR Imaging to Detect Nigrostriatal Pathway Loss in a Marmoset Model Treated with 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Radiology. 2015; 275: 430–437. doi: 10.1148/radiol.14140601 [DOI] [PubMed] [Google Scholar]
- 27.Ando K, Inoue T, Itoh T. L-DOPA-induced behavioral sensitization of motor activity in the MPTP-treated common marmoset as a Parkinson’s disease model. Pharmacol Biochem Behav. 2014; 127: 62–69. doi: 10.1016/j.pbb.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 28.Iwanami A, Yamane J, Katoh H, Nakamura M, Momoshima S, Ishii H, et al. Establishment of graded spinal cord injury model in a nonhuman primate: the common marmoset. J Neurosci Res. 2005; 80: 172–181. doi: 10.1002/jnr.20435 [DOI] [PubMed] [Google Scholar]
- 29.Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011; 6: e27706. doi: 10.1371/journal.pone.0027706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012; 7: e52787. doi: 10.1371/journal.pone.0052787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igarashi J, Fukuda N, Inoue T, Nakai S, Saito K, Fujiwara K, et al. Preclinical Study of Novel Gene Silencer Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Hypertrophic Scars in a Common Marmoset Primate Model. PLoS One. 2015; 10: e0125295. doi: 10.1371/journal.pone.0125295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan W, Fukuda S, Inoue T, Okochi H, Sasaki E, Shimoda M. Establishment of a diabetes mellitus type 1 model in the common marmoset. Sci Rep. 2019; 9: 14546. doi: 10.1038/s41598-019-51199-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue T, Ishizaka Y, Sasaki E, Lu J, Mineshige T, Yanase M, et al. Thioacetamide-induced hepatic fibrosis in the common marmoset. Exp Anim. 2018; 67: 321–327. doi: 10.1538/expanim.17-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H. A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn Reson Med. 1991; 22: 233–237, discussion 242. doi: 10.1002/mrm.1910220214 [DOI] [PubMed] [Google Scholar]
- 35.Saito S, Moriyama Y, Kobayashi S, Ogihara R, Koto D, Kitamura A, et al. Assessment of liver function in thioacetamide-induced rat acute liver injury using an empirical mathematical model and dynamic contrast-enhanced MRI with Gd-EOB-DTPA. J Magn Reson Imaging. 2012; 36: 1483–1489. doi: 10.1002/jmri.23726 [DOI] [PubMed] [Google Scholar]
- 36.Verloh N, Utpatel K, Haimerl M, Zeman F, Fellner C, Fichtner-Feigl S, et al. Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A histopathologic correlation. Sci Rep. 2015; 5: 15408. doi: 10.1038/srep15408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer JA. Chapter 13 − Diseases of the Gastrointestinal System. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The Common Marmoset in Captivity and Biomedical Research: Academic Press; 2019. pp. 213–230. [Google Scholar]
- 38.Fitz C, Goodroe A, Wierenga L, Mejia A, Simmons H. Clinical Management of Gastrointestinal Disease in the Common Marmoset (Callithrix jacchus). ILAR J. 2020; 61: 199–217. doi: 10.1093/ilar/ilab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rensing S, Oerke AK. Husbandry and Management of New World Species: Marmosets and Tamarins. In: Wolfe-Coote S, editor. The Laboratory Primate Amsterdam: Elsevier; 2005. pp. 145–162. [Google Scholar]
- 40.Potkay S. Diseases of the Callitrichidae: a review. J Med Primatol. 1992; 21: 189–236. doi: 10.1111/j.1600-0684.1992.tb00583.x [DOI] [PubMed] [Google Scholar]
- 41.Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med. 2003; 53: 369–382. [PubMed] [Google Scholar]
- 42.Mineshige T, Inoue T, Yasuda M, Yurimoto T, Kawai K, Sasaki E. Novel gastrointestinal disease in common marmosets characterised by duodenal dilation: a clinical and pathological study. Sci Rep. 2020; 10: 3793. doi: 10.1038/s41598-020-60398-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001; 21: 15–23. doi: 10.1002/jat.727 [DOI] [PubMed] [Google Scholar]
- 44.Yurimoto T, Mineshige T, Shinohara H, Inoue T, Sasaki E. Whole blood transfusion in common marmosets: a clinical evaluation. Exp Anim. 2022; 71: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marini RP, Haupt J. Anesthesia and select surgical procedures. In: Fox JG, Marini RP, Wachtman LM, Tardif SD, Mansfield K, editors. The common marmoset in captivity and biomedical research. London: Academic Press, an imprint of Elsevier; 2019. [Google Scholar]
- 46.Goodroe A, Fitz C, Bakker J. Current topics in marmoset anesthesia and analgesia. ILAR J. 2020; 61: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konoike N, Miwa M, Ishigami A, Nakamura K. Hypoxemia after single-shot anesthesia in common marmosets. J Med Primatol. 2017; 46: 70–74. doi: 10.1111/jmp.12262 [DOI] [PubMed] [Google Scholar]
- 48.Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Kurosawa T. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp Anim. 2013; 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Saito Y, Takeuchi T. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol in rats-strain difference and antagonism by atipamezole. Exp Anim. 2016; 65: 27–36. doi: 10.1538/expanim.15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papastefanou AK, Galatos AD, Pappa E, Lymperis AG, Kostoulas P. The effect of butorphanol on the incidence of dexmedetomidine-induced emesis in cats. Vet Anaesth Analg. 2015; 42: 608–613. doi: 10.1111/vaa.12260 [DOI] [PubMed] [Google Scholar]
- 51.Schurig JE, Florczyk AP, Rose WC, Bradner WT. Antiemetic activity of butorphanol against cisplatin-induced emesis in ferrets and dogs. Cancer Treat Rep. 1982; 66: 1831–1835. [PubMed] [Google Scholar]
- 52.Williams CV, Glenn KM, Levine JF, Horne WA. Comparison of the efficacy and cardiorespiratory effects of medetomidine-based anesthetic protocols in ring-tailed lemurs (Lemur catta). J Zoo Wildl Med. 2003; 34: 163–170. doi: 10.1638/1042-7260(2003)034[0163:COTEAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 53.Kalema-Zikusoka G, Horne WA, Levine J, Loomis MR. Comparison of the cardiorespiratory effects of medetomidine-butorphanol-ketamine and medetomidine-butorphanol-midazolam in patas monkeys (Erythrocebus patas). J Zoo Wildl Med. 2003; 34: 47–52. doi: 10.1638/1042-7260(2003)34[0047:COTCEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 54.Miyabe-Nishiwaki T, Miwa M, Konoike N, Kaneko A, Ishigami A, Natsume T, et al. Evaluation of anaesthetic and cardiorespiratory effects after intramuscular administration of alfaxalone alone, alfaxalone-ketamine and alfaxalone-butorphanol-medetomidine in common marmosets (Callithrix jacchus). J Med Primatol. 2020; 49: 291–299. doi: 10.1111/jmp.12482 [DOI] [PubMed] [Google Scholar]
- 55.Bakker J, Uilenreef JJ, Pelt ER, Brok HP, Remarque EJ, Langermans JA. Comparison of three different sedative-anaesthetic protocols (ketamine, ketamine-medetomidine and alphaxalone) in common marmosets (Callithrix jacchus). BMC Vet Res. 2013; 9: 113. doi: 10.1186/1746-6148-9-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross CN, Austad S, Brasky K, Brown CJ, Forney LJ, Gelfond JA, et al. The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research. Aging (Albany NY). 2017; 9: 2544–2558. doi: 10.18632/aging.101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobbs KR, Clough G, Bleby J. The establishment of specified-pathogen-free marmosets, Callithrix jacchus. Lab Anim. 1977; 11: 29–34. doi: 10.1258/002367777780959184 [DOI] [PubMed] [Google Scholar]
- 58.Levy BM, Mirkovic RR. An epizootic of measles in a marmoset colony. Lab Anim Sci. 1971; 21: 33–39. [PubMed] [Google Scholar]
- 59.Mätz-Rensing K, Jentsch KD, Rensing S, Langenhuyzen S, Verschoor E, Niphuis H, et al. Fatal Herpes simplex infection in a group of common marmosets (Callithrix jacchus). Vet Pathol. 2003; 40: 405–411. doi: 10.1354/vp.40-4-405 [DOI] [PubMed] [Google Scholar]
- 60.Baker DG. Chapter 17 − Parasitic Diseases. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The Common Marmoset in Captivity and Biomedical Research: Academic Press; 2019. pp. 289–303. [Google Scholar]
- 61.Mansfield KG, Fox JG. Chapter 16 − Bacterial Diseases. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The Common Marmoset in Captivity and Biomedical Research: Academic Press; 2019. pp. 265–287. [Google Scholar]
- 62.Mätz-Rensing K, Bleyer M. Chapter 15 − Viral Diseases of Common Marmosets. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The Common Marmoset in Captivity and Biomedical Research: Academic Press; 2019. pp. 251–264. [Google Scholar]
- 63.Inoue T, Hayashimoto N, Yasuda M, Sasaki E, Itoh T. Pentatrichomonas hominis in laboratory-bred common marmosets. Exp Anim. 2015; 64: 363–368. doi: 10.1538/expanim.15-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toft JD, 2nd. The pathoparasitology of the alimentary tract and pancreas of nonhuman primates: a review. Vet Pathol Suppl. 1982; 19:(Suppl 7): 44–92. doi: 10.1177/030098588201907s06 [DOI] [PubMed] [Google Scholar]
- 65.Carvalho VM, Gyles CL, Ziebell K, Ribeiro MA, Catão-Dias JL, Sinhorini IL, et al. Characterization of monkey enteropathogenic Escherichia coli (EPEC) and human typical and atypical EPEC serotype isolates from neotropical nonhuman primates. J Clin Microbiol. 2003; 41: 1225–1234. doi: 10.1128/JCM.41.3.1225-1234.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayashimoto N, Inoue T, Morita H, Yasuda M, Ueno M, Kawai K, et al. Survey and Experimental Infection of Enteropathogenic Escherichia coli in Common Marmosets (Callithrix jacchus). PLoS One. 2016; 11: e0160116. doi: 10.1371/journal.pone.0160116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomson JA, Scheffler JJ. Hemorrhagic typhlocolitis associated with attaching and effacing Escherichia coli in common marmosets. Lab Anim Sci. 1996; 46: 275–279. [PubMed] [Google Scholar]
- 68.Rodriguez C, Taminiau B, Van Broeck J, Delmée M, Daube G. Clostridium difficile in Food and Animals: A Comprehensive Review. Adv Exp Med Biol. 2016; 932: 65–92. doi: 10.1007/5584_2016_27 [DOI] [PubMed] [Google Scholar]
- 69.Rolland RM, Chalifoux LV, Snook SS, Ausman LM, Johnson LD. Five spontaneous deaths associated with Clostridium difficile in a colony of cotton-top tamarins (Saguinus oedipus). Lab Anim Sci. 1997; 47: 472–476. [PubMed] [Google Scholar]
- 70.Armstrong AR, Wünschmann A, Rigatti LH, Klein EC. Clostridium difficile Enterocolitis in a Captive Geoffroy’s Spider Monkey (Ateles geoffroyi) and Common Marmosets (Callithrix jacchus). Vet Pathol. 2019; 56: 959–963. doi: 10.1177/0300985819864307 [DOI] [PubMed] [Google Scholar]
- 71.Yamazaki Y, Kawarai S, Morita H, Kikusui T, Iriki A. Faecal transplantation for the treatment of Clostridium difficile infection in a marmoset. BMC Vet Res. 2017; 13: 150. doi: 10.1186/s12917-017-1070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito T. Klebsiella pneumoniae infection (1). In: Pathology TSoPDa, editor. Color atras of diseases in non-human primates (in Japanese). Tsukuba: Isebu; 2011. pp. 30–31. [Google Scholar]
- 73.Yasuda M, Inoue T, Ueno M, Morita H, Hayashimoto N, Kawai K, et al. A case of nontraumatic gas gangrene in a common marmoset (Callithrix jacchus). J Vet Med Sci. 2016; 77: 1673–1676. doi: 10.1292/jvms.15-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramer JC, Garber RL, Steele KE, Boyson JF, O’Rourke C, Thomson JA. Fatal lymphoproliferative disease associated with a novel gammaherpesvirus in a captive population of common marmosets. Comp Med. 2000; 50: 59–68. [PubMed] [Google Scholar]
- 75.Cho Y, Ramer J, Rivailler P, Quink C, Garber RL, Beier DR, et al. An Epstein-Barr-related herpesvirus from marmoset lymphomas. Proc Natl Acad Sci USA. 2001; 98: 1224–1229. doi: 10.1073/pnas.98.3.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fogg MH, Carville A, Cameron J, Quink C, Wang F. Reduced prevalence of Epstein-Barr virus-related lymphocryptovirus infection in sera from a new world primate. J Virol. 2005; 79: 10069–10072. doi: 10.1128/JVI.79.15.10069-10072.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okano H, Sasaki E, Yamamori T, Iriki A, Shimogori T, Yamaguchi Y, et al. Brain/MINDS: A Japanese National Brain Project for Marmoset Neuroscience. Neuron. 2016; 92: 582–590. doi: 10.1016/j.neuron.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 78.Callaway EM, Dong HW, Ecker JR, Hawrylycz MJ, Huang ZJ, Lein ES, et al. BRAIN Initiative Cell Census Network (BICCN). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature. 2021; 598: 86–102. doi: 10.1038/s41586-021-03950-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Servick K. U.S. labs clamor for marmosets. Science. 2018; 362: 383–384. doi: 10.1126/science.362.6413.383 [DOI] [PubMed] [Google Scholar]