Abstract
Changes to donor availability, collection center capacity, and travel restrictions during the early phase of the COVID-19 pandemic led to routine cryopreservation of most unrelated donor products for hematopoietic transplantation prior to the recipient commencing the conditioning regimen. We investigated the effect of this change on unrelated donor product quality and clinical outcomes. Product information was requested from transplantation centers in Australia and New Zealand and clinical outcome data from the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR). In total, 191 products were collected between April 1, 2021, and September 30, 2021, and most (74%) were from international collection centers. Median post-thaw CD34 recovery was 78% (range 25% to 176%) and median post-thaw CD34 viability was 87% (range 34% to 112%). Median time to neutrophil recovery was 17 days (interquartile range 10 to 24 days), and graft failure occurred in 6 patients (4%). These clinical outcomes were similar to those of “fresh” unrelated donor transplants reported to the ABMTRR in 2019. However, recipient transplantation centers reported problems with 29% of products in the form of damage during transit, low cell dose, inadequate labeling, missing representative samples, or missing documentation. These problems were critical in 7 cases (4%). At last follow-up, 22 products (12%) had not been infused. Routine cryopreservation of unrelated donor hemopoietic progenitor cell products has enabled safe continuation of allogeneic transplant services during the COVID-19 pandemic. However, practices for product tracing, documentation, and transportation can be optimized, and measures to reduce the incidence of unused unrelated donor product are required.
Key Words: COVID-19, Stem cell quality, Cryopreservation, Donor, Engraftment
The rapid global spread of SARS-CoV-2 (COVID-19) in March 2020 led to major changes to stem cell donor availability and product transportation. Transplantation organizations throughout the world responded by recommending cryopreservation of all unrelated donor hemopoietic progenitor cell (HPC) products before commencing recipient conditioning 1, 2, 3, 4. Although necessary to enable continued access to unrelated donors, routine cryopreservation may introduce additional risks to both donor and recipient. Our centers recently reported significant variation in post-thaw CD34 recovery from cryopreserved allogeneic HPC, particularly where products have been subject to prolonged travel times before cryopreservation [5]. Infusion reactions caused by cryopreservation agents and delayed neutrophil or platelet recovery may result [6,7]. Furthermore, collection and cryopreservation of products before the recipient has commenced conditioning increases the risk that the donor product will never be infused and that the donor will be exposed to unnecessary risk [8].
The Australian and New Zealand experience is notable for a low community incidence of COVID-19 in the region during 2020, a historically high reliance on overseas unrelated donors for HPC transplantation, and long travel times to most unrelated donor collection centers. In this context, we investigated the effect of the first 6 months of the COVID-19 pandemic on unrelated donor HPC product acquisition, product quality, and clinical engraftment outcomes.
Methods
We requested HPC product information on unrelated peripheral blood or bone marrow donor HPC collected between April 1, 2020, and September 30, 2020 (“COVID era”), for recipients at all adult and pediatric Australian and New Zealand allogeneic transplantation centers. For comparison, we also requested information regarding neutrophil and platelet recovery for all unrelated peripheral blood or bone marrow recipients in 2018 and 2019 from the Australasian Bone Marrow Transplant Recipient Registry (“pre-COVID era”). Medians were compared using the Mann-Whitney U test, and the effect of categorical variables on binary outcomes was assessed by the chi-squared test. Graft quality was assessed by recipient transplant center laboratories, and included product packing, labelling, accompanying samples and documentation, and CD34 recovery and CD34 viability. CD34 recovery was defined as post-thaw viable CD34+ cell count/pre-cryopreservation viable CD34+ count. CD34 recovery >100% was possible because of CD34 measurement uncertainty and method variation between laboratories. CD34 viability was defined as the percentage of viable events within the total CD34+ gate in flow cytometry assays based on 7-AAD exclusion. Where available, CD34 recovery and viability was assessed using representative samples (“pilot vials”) cryopreserved and shipped alongside the product itself and thawed at the recipient laboratory before transplantation. Neutrophil recovery was defined as the first of 3 consecutive days >0.5 × 109/L and platelet recovery as the first of 7 consecutive days >20 × 109/L unsupported by platelet transfusion. Cumulative incidences of neutrophil and platelet recovery were calculated using death before day 21 as a competing risk. Analysis was performed using SPSS, version 24.0 (IBM, Chicago, IL) and R.
Results
Collected products
Fourteen centers contributed data, representing the majority (82%) of unrelated donor centers in the region. Information was received on a total of 191 HPC products collected for 175 recipients, with 16 patients requiring 2 HPC collections to achieve target cell dose or collection center specification. Of these 191 products, 50 (26%) were collected within Australia or New Zealand, 93 (49%) from Europe, 21 (11%) from the USA, 16 (8%) from the UK and 11 (6%) from other overseas collection centers. The proportion of domestic collections was greater than that observed in 2019 (18%) (ABMDR, personal communication). Three HPC products were bone marrow (HPC[M]), whereas the remainder (98%) were mobilized peripheral blood apheresis products (HPC[A]). Of the 191 products collected, 190 were cryopreserved. Cryopreservation occurred at the collection center (49%), at a regional cryopreservation hub (45%) or at the transplantation center (6%). Of the 50 products collected in Australia and New Zealand, 39 (80%) were cryopreserved at the collection center, 10 (20%) were cryopreserved after transportation to the transplant center, and 1 was infused fresh.
Pre-cryopreservation CD34 enumeration is a mandatory criterion for product release for most donor centers and was provided for all but one product in this study. However, only a minority of processing laboratories provided information on the post-thaw composition of HPC products (CD34 enumeration was available for 46% of products and CD34 viability was available for 32% of products).
In contrast, post-thaw CD34 enumeration was measured by the recipient transplant center for all infused products and for 98% of collected products. Median post-thaw CD34 recovery calculated at the transplantation center was 78% but ranged from 25% to 176%. Eight products (4%) had a CD34 recovery of less than 50%. Median CD34 recovery varied according to where the product had been cryopreserved: collection center (81%, range 25% to 176%), regional cryopreservation hub (75%, range 32% to 133%), or transplantation center (65%, range 54% to 109%), overall P = .038.
Post-thaw CD34 viability as a proportion of all CD34+ events reflects the integrity and quality of the HPC product and was available from the transplantation center for 73% of collected products. The median post-thaw CD34 viability was 87% (range 34% to 112%) and was less than 50% in 4 products (2%). Median CD34 viability also varied according to location of cryopreservation: collection center (84%, range 34% to 98%), regional cryopreservation hub (90%, range 46% to 112%), transplantation center (79%, range 60% to 99%), overall P = .027.
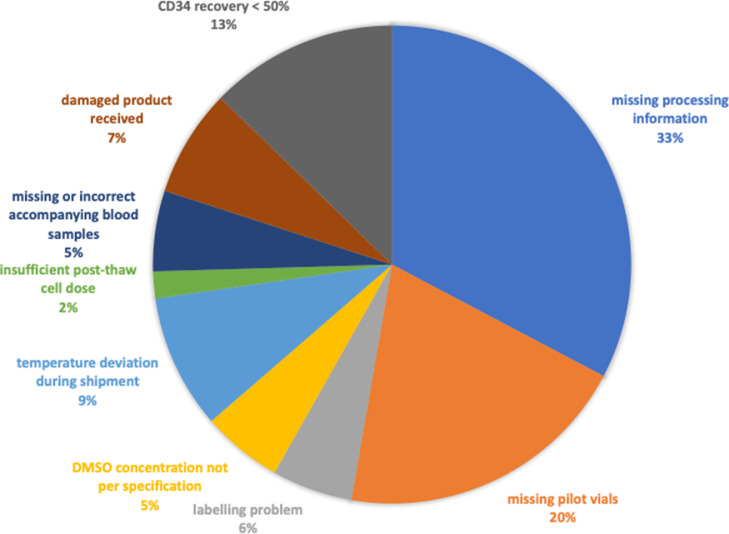
We also collected information regarding mishaps, concerns, or non-conformances relating to the products reported by the transplantation centers, either directly or through Serious Product Events and Adverse Reactions (SPEAR) reports sent to the Australian Bone Marrow Donor Registry (ABMDR). Overall, reports were received regarding 55 (29%) products (Figure 1 ). Process quality issues were most commonly reported (19% of products) and included missing product information (n = 15 [8%]) and missing or incorrect representative product samples (n = 10 [5%]). Of the 9 products that arrived without any representative product samples, 7 also had no post-thaw CD34 enumeration result available from the processing center. Product quality issues were reported for 10% of products, including temperature deviation during transport and low CD34 recovery. Infrequent but potentially critical product non-conformances included damaged product (n = 4), <50% requested cell dose received (n = 1), and product received in multiple (>10) bags resulting in large dimethyl sulfoxide volume (n = 2).
Figure 1.
Product non-conformances or low CD34 recovery reported by recipient transplant center (n = 55, 29% of all products).
Comparison with grafts cryopreserved prior to the COVID pandemic
We have previously reported a median CD34 recovery of 76% (range 6% to 122%) for HPC products cryopreserved for Australian recipients prior to the COVID-19 pandemic (2015 to 2019) [5]. While the median CD34 recovery of products cryopreserved during the first 6 months of the COVID pandemic was similar (78%), the proportion of grafts with very low (<50%) CD34 recovery was actually lower during the pandemic (4% versus 15%, P < .001).
Infused products
A total of 164 products had been infused for 151 patients (86%) by December 31, 2020. Nine (6%) patients underwent transplantation for nonmalignant disease, the remainder for hematological malignancies. Infusion reactions were reported for 25 patients (17%) and were clinically moderate or severe in 5 (3%) and 4 (3%), respectively. Median infused CD34+ cell dose was 5.04 × 106/kg (range 1.6 to 13.7). Neutrophil and platelet recovery are summarized in Table 1 . Three patients (2%) suffered primary graft failure, and 3 (2%) had initial neutrophil recovery followed by secondary graft failure (Table 2 ). No association was observed between neutrophil recovery and infused viable CD34+ cell dose nor CD34 recovery, although a statistically significant association was observed between platelet recovery and infused viable CD34+ cell dose (HR 1.12 per 1.0 × 106/kg CD34+ cell dose, 95% confidence interval 1.01-1.22 [P = .026]) but not CD34 recovery. Of 5 patients who underwent transplantation for severe aplastic anemia, 1 received fresh HPC(M) and the remainder received cryopreserved HPC(A). All achieved engraftment (median time to neutrophil recovery 17 days, range 7-23 days) and remain alive at last follow-up.
Table 1.
Comparison of Patient Characteristics Between Study (“COVID”) Cohort and 2018 to 2019 (“Pre-COVID”) Cohort
| “Pre-COVID” cohort | “COVID” cohort | P | |
|---|---|---|---|
| Total number of patients | 613 | 151 | |
| Age, median (range) | 54 (0-74) | 53 (0-75) | .827 |
| Diagnosis, n (%) | |||
| AML | 262 (43) | 65 (43)* | .768 |
| ALL | 90 (15) | 17 (11) | |
| MDS | 105 (17) | 28 (19) | |
| MPN | 42 (7) | 9 (6) | |
| Lymphoma | 72 (12) | 17 (11) | |
| Bone marrow failure syndromes | 21 (3) | 5 (3) | |
| Immunodeficiency | 13 (2) | 3 (2) | |
| Other | 8 (1) | 3 (2) | |
| ATG given, n (%) | |||
| Yes | 373 (61%) | 99 (70%)† | .038 |
| No | 240 (39%) | 42 (30%) | |
| MTX-based GVHD prophylaxis, n (%) | |||
| Yes | 520 (81%) | 126 (89%)† | .166 |
| No | 93 (15%) | 15 (11%) | |
| Stem cell source, n (%) | |||
| HPC (A) | 552 (90%) | 148 (98%) | .002 |
| HPC (M) | 61 (10%) | 3 (2%) | |
| Regimen intensity, n (%) | |||
| Myeloablative | 230 (38%) | 49 (35%)‡ | .503 |
| Reduced intensity | 383 (63%) | 93 (66%) | |
| Time to neutrophil recovery, median (IQR) | 17 (10-24) | 17 (10 – 24) | .540 |
| Neutrophil recovery by day 28, cumulative incidence (95% confidence interval) | 90 (89-92%) | 92 (90–95%) | .521 |
| Time to platelet recovery, median (IQR) | 20 (9-31) | 21 (8-34) | .123 |
| Platelet recovery by day 60, cumulative incidence (95% confidence interval) | 87 (86-88%) | 92 (89-95%) | .755 |
AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplasia including myeloproliferative/myelodysplastic overlap conditions; MPN, myeloproliferative neoplasms; IQR, interquartile range.
* 4 cases missing data.
†10 cases missing data.
‡9 cases missing data.
Table 2.
Patients With Primary or Secondary Graft Failure
| Case | Patient age | Cell source | Diagnosis | Conditioning regimen | Cryopreservation location | Non-conformance | Viable CD34 recovery⁎ | Post-thaw viability of CD34+ cells† | Infused viable CD34+ cell dose (×106/kg) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | HPC(A) | AML | Flu/Mel (RIC) + ATG | Collection center (domestic) | None | 79% | Not available | 4.37 | Primary graft failure. Alive at day 55 |
| 2 | 57 | HPC(A) | AML | Flu/Mel (RIC) + ATG | Collection center (international) | None | 64% | 74% | 4.8 | Died day 25 of infection without neutrophil recovery |
| 3 | 68 | HPC(A) | AML | Flu/Mel (RIC) + ATG | Collection center (international) | Missing labeling and processing information | 127% | 98% | 7.6 | Neutrophil recovery day 27 then secondary graft failure. |
| 4 | 69 | HPC(A) | AML | Bu/Flu (RIC) + ATG | Collection center (international) | Missing accompanying blood sample | 78% | Not available | 4.1 | Primary graft failure. Died day 174. |
| 5 | 60 | HPC(A) | AML | Flu/TBI (RIC) + ATG | Collection center (international) | Graft arrived partially thawed | 56% | Not available | 2.8 | Neutrophil recovery day 18 then secondary graft failure. |
| 6 | 49 | HPC(A) | AML | TBI + Cy (MAC) | Regional hub (international) | None | 68% | 66% | 3.2 | Neutrophil recovery day 16, then secondary graft failure. |
MAC indicates myeloablative conditioning; RIC, reduced-intensity conditioning.
Post-thaw/pre-crypreservation CD34 count.
Percentage of all CD34+ events in sample that are viable.
Comparison with neutrophil and platelet recovery prior to the COVID-19 pandemic
To assess the effect of routine cryopreservation compared with the use of fresh products prior to the COVID-19 pandemic, we measured graft failure rates, platelet and neutrophil recovery in 613 consecutive patients from the “pre-COVID era” (2018 to 2019). Patient and transplant characteristics are summarized in Table 1. Patients who underwent transplantation during the COVID-19 pandemic were more likely to be recipients of anti-thymocyte globulin (ATG) and HPC(A) products. Neutrophil and platelet recovery were similar in the “pre-COVID era,” and the proportion of patients with primary graft failure was also similar (n = 19 [3%]).
Products not infused
Twenty-seven products had not been infused at clinical data cutoff, which was at least 90 days after the last product was collected. Since December 2020, a further 5 products have been infused, leaving 22 products still not infused after a minimum of 9 months of follow-up. This represents 12% of all products collected during the study period. Of these 22, 4 (18%) were not infused because of a problem with the product, including damaged on arrival (n = 1), missing product representative samples (n = 2), and insufficient cell dose (n = 1). Eight (36%) were not infused because the patient was not ready for transplantation (eg, disease progression or interval illness), whereas a reason was not provided for the remaining 10 (46%) products. In comparison, the ABMDR reported only 3 products not infused after a minimum 12 months of follow-up for the calendar year 2019, out of a total of 339 unrelated donor products (0.9%) (ABMDR, personal communication).
Discussion
Despite the severe disruption to international healthcare and travel industries that occurred at the time, we observed satisfactory post-thaw product quality and neutrophil and platelet recovery for the majority of unrelated donor products collected for Australia and New Zealand recipients during the first 6 months of the COVID-19 pandemic. This observation is a testament to the resourcefulness and commitment of the unrelated HPC donor sector, including registries, collection and processing centers, couriers, recipient transplantation centers, and the donors themselves. A key element in the response to the travel uncertainty imposed by the pandemic was to perform cryopreservation as soon as possible after collection of the product. We observed excellent post-thaw CD34 recovery for products cryopreserved at collection centers or regional cryopreservation hubs (median 81% and 75%, respectively). Furthermore, the products collected during this period had a lower incidence of very low (<50%) CD34 recovery compared with a historical cohort of products cryopreserved before the COVID-19 pandemic, the majority of which had long liquid storage times during travel to Australia [5]. These observations are broadly concordant with those of a large European cryopreservation center [6] and suggest that the strategy of cryopreservation at collection centers or local regional hubs is effective for preserving product quality. Conversely, a strategy of cryopreservation at transplantation centers appears less desirable, possibly because of the longer liquid storage time during transit from the collection center.
Neutrophil recovery appeared similar after infusion of thawed cryopreserved products to that achieved with “fresh” unrelated donor products in 2018 to 2019. This observation is consistent with an earlier report of unrelated donor HPC cryopreservation for transplantation [7] and with a description of recipients of cryopreserved products who received post-transplantation cyclophosphamide for graft-versus-host disease (GVHD) prophylaxis [8]. However, 2 recent CIBMTR publications comparing historical cryopreserved and fresh HPC outcomes for malignancies [9] and severe aplastic anemia [10] reported different findings. Both of these observed delayed neutrophil and platelet recovery and inferior overall survival for recipients of cryopreserved products. Neither study included information regarding post-thaw composition or viability of the cryopreserved products, and it is possible that cryopreservation of the product itself was responsible for these differences. However, it is also possible that the reason for cryopreservation, which before the COVID-19 pandemic was usually due to emergent patient-related issues, may explain inferior patient outcomes. For example, relapse requiring additional chemotherapy or infection requiring intensive therapy may result in the patient being more vulnerable to toxicity or progression after transplantation. Our study is therefore unique in comparing the clinical outcome of “routine” cryopreservation with the results of fresh HPC product infusion. Although the numbers of severe aplastic anemia transplant recipients in our study are too small to make any conclusions, it is somewhat reassuring that these patients were able to undergo transplantation and achieve timely engraftment despite HPC cryopreservation.
Infusion reactions were reported for 17% of patients and were mostly mild and consistent with reactions to cryopreservation additives. A large study of 1269 recipients of cryopreserved autologous HPC reported an incidence of infusion adverse events of 38% [11], whereas another report of infusion reactions in children reported an overall incidence of 36%, with a higher risk after fresh allogeneic compared with cryopreserved autologous product infusion [12]. Differences in cryoprotectant concentration, pre-medication, infusion technique, and adverse event reporting may account for the lower incidence reported in our study.
Although the majority of recipients received satisfactory HPC products and achieved successful clinical engraftment outcomes, our observations highlight some important risks and opportunities for improvement. Receiving transplantation centers reported product non-conformances for a significant proportion (26%) of products, including 7 cases where neither a representative sample nor post-processing information was available to determine the hemopoietic potential of the cryopreserved product, leaving transplantation centers with no way of determining the quality of the cryopreserved product short of thawing the product itself. Transplantation centers need to be able to make an assessment of the hemopoietic potential of a cryopreserved product before the recipient commencing the conditioning regimen, and such evaluations are required to be performed by cell therapy laboratories both before and after processing procedures according to FACT/JACIE standards [13]. The provision of processing information, post-thaw testing, and product representative samples may require increased attention from accreditation and regulatory agencies in the context of ongoing local cryopreservation of unrelated donor HPC products.
This study also observed a lower use of HPC(M) during the COVID-19 pandemic. This change was likely driven by a lack of availability of bone marrow collection facilities at many donor centers during the height of the public health crisis [4]. It is possible that such changes in stem cell source may alter outcomes for patients particularly vulnerable to the risk of GVHD, including children and patients with nonmalignant indications for transplantation. GVHD outcomes were not captured for this study. Although ATG use was more frequent during the COVID pandemic than the preceding 2 years, this is likely due to several centers changing institutional practice just before the pandemic to prescribe ATG as routine GVHD prophylaxis for unrelated donor recipients (personal communication).
Our data also highlight risks to the donor of unused donation during the early phase of the COVID-19 pandemic. The high proportion of products not infused within 9 months of collection (12%) likely reflects a high number of products that will never be infused, and is dramatically different to the incidences of unused product in 2019 reported by the ABMDR (0.9%) and DKMS Germany (0.32%) [14]. This raises ethical concerns for donors who may have suffered not only minor adverse effects and inconvenience associated with stem cell collection, but also potential exposure to COVID-19 as a result of attendance at hospital clinics and collection centers in high-prevalence areas. To address this, some registries have proposed setting up banks of “excess” HPC from young donors with common HLA genotypes and favorable donor/patient weight ratios to provide ready access to cryopreserved product without subjecting additional donors to the risks of stem cell donation [14]. Transplantation centers can also abrogate the risk of unused product by only requesting donation after confirming recipient transplant eligibility and scheduling infusion as close as possible to receipt of product. However, long travel times and the need for quality assessment of the product after arrival mean that some delay between collection and infusion is unavoidable.
Local cryopreservation and delayed recipient conditioning have enabled safe continuation of unrelated donor transplantation in Australia and New Zealand during the COVID-19 pandemic. However, transplantation centers have faced challenges because of damaged product or missing information in a significant proportion of products, and it appears that significant numbers of unrelated donors have undergone unnecessary mobilization and collection procedures for products that will not be infused. These observations support increased attention to quality management systems around cryopreservation and transportation of HPC products and strategies to minimize unnecessary unrelated donor collection.
Acknowledgments
The authors thank Leonie Wilcox and Kamal Aryal from the ABMTRR and Garth Healey from the ABMDR for providing data for this project. The authors are especially grateful to the staff of the ABMDR for working tirelessly to enable patients to access donors during this period.
Financial disclosure: ABMTRR is partly funded by the Arrow Foundation.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: D.P., N.H., V.A., C.H., G.K., D.G., D.M. and P.C. designed the research; D.P. analyzed data; and D.P., N.H., V.A., C.H., G.K., D.G., D.M., P.C., K.M., A.M., C.F., P.J.S., H.T., K.K., M.G., L.B., A.B., E.O'F., D.C., L.P., T.P., and S.T. wrote the report.
Footnotes
Financial disclosure: See Acknowledgments on page 1022.e6.
References
- 1.EBMT. Coronavirus disease COVID-19: EBMT recommendations update April 21, 2020. Available at: https://www.ebmt.org/sites/default/files/2020-04/EBMT_COVID-19-guidelines_v.7.1%282020-04-21%29.pdf. Accessed April 29, 2020.
- 2.BeTheMatch. Donor Product Cryopreservation (updated March 23, 2020). Available at: https://network.bethematchclinical.org/news/nmdp/be-the-match-response-to-covid-19/updates-for-transplant-centers-and-cooperative-registries/#Cryopreservation. Accessed April 29, 2020.
- 3.BMTSANZ. Bone Marrow Transplant Society of Australia and New Zealand COVID19 Consensus Position Statement 15th April 2020 [Updated April 29, 2020]. Available from: https://www.bmtsanz.org.au/bone-marrow-transplant-society-of-australia-and-new-zealand-covid19-consensus-position-statement-15th-april-2020/. Accessed April 29, 2020.
- 4.Auletta JJ, Novakovich JL, Stritesky GL, et al. Meeting the demand for unrelated donors in the midst of the COVID-19 pandemic: rapid adaptations by the National Marrow Donor Program and its network partners ensured a safe supply of donor products. Transplant Cell Ther. 2021;27:133–141. doi: 10.1016/j.jtct.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purtill D, Antonenas V, Chiappini P, et al. Variable CD34+ recovery of cryopreserved allogeneic HPC products: transplant implications during the COVID-19 pandemic. Blood Adv. 2020;4:4147–4150. doi: 10.1182/bloodadvances.2020002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiercinska E, Schlipfenbacher V, Bug G, et al. Allogeneic transplant procurement in the times of COVID-19: Quality report from the central European cryopreservation site. J Transl Med. 2021;19:145. doi: 10.1186/s12967-021-02810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medd P, Nagra S, Hollyman D, Craddock C, Malladi R. Cryopreservation of allogeneic PBSC from related and unrelated donors is associated with delayed platelet engraftment but has no impact on survival. Bone Marrow Transplant. 2013;48:243–248. doi: 10.1038/bmt.2012.118. [DOI] [PubMed] [Google Scholar]
- 8.Hamadani M, Zhang MJ, Tang XY, et al. Graft cryopreservation does not impact overall survival after allogeneic hematopoietic cell transplantation using post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2020;26:1312–1317. doi: 10.1016/j.bbmt.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu JW, Farhadfar N, Murthy H, et al. The effect of donor graft cryopreservation on allogeneic hematopoietic cell transplantation outcomes: a Center for International Blood and Marrow Transplant Research Analysis. Implications during the COVID-19 pandemic. Transplant Cell Ther. 2021;27:507–516. doi: 10.1016/j.jtct.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eapen M, Zhang MJ, Tang XY, et al. Hematopoietic cell transplantation with cryopreserved grafts for severe aplastic anemia. Biol Blood Marrow Transplant. 2020;26(7):e161–e166. doi: 10.1016/j.bbmt.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otrock ZK, Sempek DS, Carey S, Grossman BJ. Adverse events of cryopreserved hematopoietic stem cell infusions in adults: a single-center observational study. Transfusion. 2017;57:1522–1526. doi: 10.1111/trf.14072. [DOI] [PubMed] [Google Scholar]
- 12.Truong TH, Moorjani R, Dewey D, Guilcher GM, Prokopishyn NL, Lewis VA. Adverse reactions during stem cell infusion in children treated with autologous and allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:680–686. doi: 10.1038/bmt.2015.331. [DOI] [PubMed] [Google Scholar]
- 13.Foundation for the Accreditation of Cellular Therapy. FACT-JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration. Seventh Edition. 2018.
- 14.Schmidt AH, Buk D, Platz A, van den Brink MRM. Cryopreservation for all is no option in unrelated stem cell transplantation. Comment on Dholaria B, et al. Securing the Graft During Pandemic: Are We Ready for Cryopreservation for All? Biol Blood Marrow Transplant. 2020;26:e145–e146. doi: 10.1016/j.bbmt.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]