Sphingolipids, including ceramide, glucosylceramide, and gangliosides, have gained traction as cell-autonomous inducers of insulin resistance. β-Hexosaminidase is a heterodimeric enzyme comprising hexosaminidase A (HEXA) and hexosaminidase B (HEXB). The enzyme is canonically found within lysosomes, where it functions to break down GM2 gangliosides into GM3 gangliosides. In this issue of Diabetes, Montgomery et al. (1) identify endocrine functions of circulating HEXA that improve glucose homeostasis by enhancing glucose uptake into skeletal muscle. Their work highlights HEXA as a biomarker for type 2 diabetes and nonalcoholic fatty liver disease and reveals a novel role for the secreted enzyme in modulating glucose uptake into muscle. Collectively, the work suggests that metabolic stress triggers hepatic production of HEXA, which works as a hepatokine to restructure signaling platforms within detergent-resistant lipid domains in muscle.
Muscle accounts for 80% of postprandial glucose uptake, and understanding the mechanisms that impair glucose transport into muscle is critical to defining the mechanisms that govern the onset of type 2 diabetes. Montgomery et al. (1) elucidates new cross talk between the liver and muscle that is governed by hepatic secretion of hexosaminidase, a sphingolipid metabolizing enzyme, which can trigger enhancements in muscle glucose uptake. Their work suggests a role for the protein as a biomarker for nonalcoholic fatty liver disease and type 2 diabetes. Moreover, their findings suggest that HEXA is a bona fide hepatokine along the lines of fibroblast growth factor 21 (2), as both are secreted in response to metabolic stress and can prompt changes to the metabolic programs of their target tissues.
When energy intake exceeds energy expenditure, excess lipid deposition in adipose tissue allows for efficient accommodation of caloric surplus. When lipid storage capacity is surpassed for a long period, ectopic lipid accumulation occurs in other tissues less suited for lipid storage, such as skeletal muscle and liver. This results in a lipotoxic stress driven by the excess overaccumulation of lipid metabolites that impair cellular function. Biosynthesis of sphingolipids, a complex class of metabolically active lipids containing an amino acid-derived sphingoid backbone, is invariably stimulated by circulating factors (cytokines, fatty acids, and glucocorticoids) associated with obesity (3). Ceramide, the base structure of complex sphingolipids, can incorporate an acyl group, phosphate, choline, or a chain of sugars (glucose, galactose, galactosamine, sialic acid, etc.). The latter modification gives rise to an incredibly diverse family of hexosylceramide derivatives, including gangliosides, which are important in cellular recognition. Genetic and pharmacological strategies for disrupting the biosynthesis of ceramide, glucosylceramide, or GM3 gangliosides have all shown glycemic benefit in mouse models of obesity and diabetes (4).
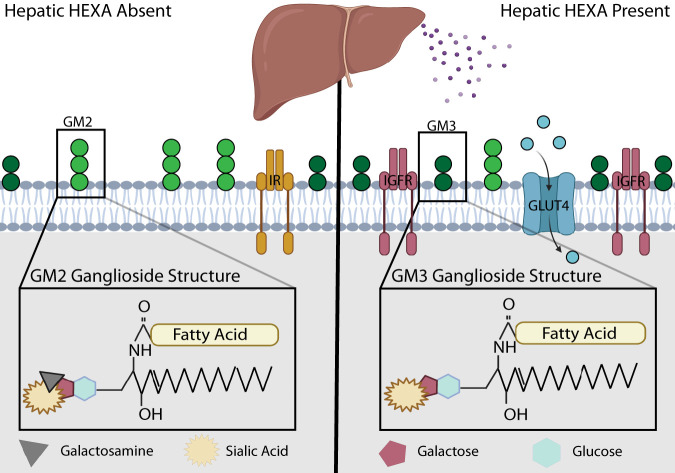
Molecular characterization of inborn errors in metabolism has propelled our understanding of ganglioside lipids and the enzymes that metabolize them. Multiple lysosomal storage diseases are characterized by genetic deficiencies in the lysosomal breakdown of gangliosides. Two such diseases, Tay-Sachs disease and Sandhoff disease, are characterized by GM2 ganglioside overaccumulation, which is driven by loss-of-function mutations in the HEXA or HEXB gene, respectively (5). These separately encoded subunits of the β-hexosaminadase catalyze the degradation of GM2 ganglioside to GM3 ganglioside by hydrolyzing GM2’s terminal galactosamine moiety (Fig. 1); loss of HEXA or HEXB function is associated with neurological defects. In recent years, HEXA, which contains an N-terminal signal peptide characteristic of secreted proteins, has emerged as a circulating biomarker that positively correlates with cardiovascular disease (6), type 1 diabetes (7), liver cholestasis (8), and cirrhosis (9). In this issue, Montgomery et al. (1) further demonstrates that HEXA correlates with nonalcoholic fatty liver disease, increasing stepwise during progression from fatty liver disease to nonalcoholic fatty liver disease. Moreover, HEXA increases in insulin-resistant subjects and in individuals with type 2 diabetes. Liver biopsy specimens revealed increased transcription of HEXA, which was positively correlated with glycemia and hepatic steatosis. Mouse models of diabetes and hepatic steatosis show similar trends, with 6- and 20-fold increases in serum HEXA levels of diet-induced obese mice and genetically obese Leprdb/db mice.
Figure 1.
HEXA remodels signaling platforms in muscle. Detergent-resistant domains are enriched with sphingolipids and cholesterol, aiding the function and clustering of the membrane-bound receptors and nutrient transporters enriched within these domains. HEXA is a liver-derived enzyme that catalyzes the conversion of GM2 ganglioside to GM3 ganglioside via the cleavage of N-acetyl-d-galactosamine from GM2. HEXA enhances the abundance of insulin-like growth factor receptors 1 and 2 (IGFR) within detergent-resistant muscle cell membranes while displacing insulin receptors. The net effect of HEXA is an increase in glucose uptake driven by IGF-mediated translocation of glucose transporter 4 (GLUT4) to the muscle plasma membrane. GLUT4 activation allows glucose uptake into skeletal muscle and decreases serum glucose concentration. In vivo, HEXA stimulates improved glucose tolerance with less need for insulin.
Gangliosides are primarily trafficked and localized to the cell membrane, where their distinguishing oligosaccharide chains facilitate cell-to-cell recognition. Gangliosides are heavily enriched in detergent-resistant membrane domains, where their presence may recruit or repel membrane proteins (5). Mechanistic examination of the role of GM3 ganglioside on insulin action in adipocytes pinpointed altered localization of insulin receptor to these caveolin-enriched domains (10). In murine studies of rodents lacking GM3 synthase, genetic ablation of this GM3-producing enzyme protects mice from high-fat-diet–induced glucose intolerance and improved glucose uptake into muscle during hyperinsulinemic-euglycemic conditions (11). Moreover, our work has demonstrated that overexpression of glucosylceramide synthase (UGCG) in myotubes effectively disrupts insulin signaling and suggests that both ceramide and glucosylceramide derivatives can impair insulin action via distinct regulatory mechanisms (12).
The role of sphingolipids as metabolically active intermediates or second messengers is not new. Several cytokines, including tumor necrosis factor-α, trigger sphingomyelinase activation to drive the rapid regeneration of ceramide (4). Conversely, adiponectin triggers the rapid degradation of ceramide and formation of sphingosine-1-phosphate by activating ceramidase activities endogenous to its receptors (4). To our knowledge, a ligand-gated remodeling of glucosylceramide derivatives has not been described.
Montgomery et al. (1) identify a novel regulatory action of hepatic HEXA, the α-subunit of hexosaminidase, in influencing glucose uptake into skeletal muscle and whole-body glucose homeostasis. Work with liver-specific HEXA overexpression (HEXA adeno-associated virus) effectively demonstrates that hepatic HEXA is sufficient to drive circulating HEXA and glycemic changes. Furthermore, radiolabeled 2-deoxyglucose pointed to changes in skeletal muscle metabolism as a driver of this effect. Consistent with HEXA’s known function as an enzyme that converts GM2 to GM3 ganglioside, GM3 content in skeletal muscle increases upon administration of liver-tropic HEXA adeno-associated virus. At the same time, other lipid species, including ceramides and other gangliosides, remained unchanged. Complementary studies in cultured myotubes stably overexpressing GLUT4 strengthen the argument that HEXA administration improves glucose metabolism by enhancing glucose uptake into skeletal muscle. In vivo delivery of HEXA decreased circulating levels of insulin, suggesting a decreased biological need for insulin; although this is consistent with improved insulin sensitivity, molecular analyses revealed an interesting twist.
Detailed analysis revealed that HEXA appears to facilitate glucose uptake in response to insulin-like growth factor 1 (IGF-1). Proteomic and Western blot analyses show that HEXA induces the abundance of IGF receptors 1 and 2 while displacing insulin receptor from detergent-resistant membrane fractions. Indeed, IGF-1 potently increased glucose uptake in HEXA-treated myotubes, and knockdown of IGF receptor 1 abolished HEXA-induced increases in glucose uptake. Importantly, HEXA-mediated improvements are absent from catalytically inactive mutants of HEXA or when raft domains are chemically disrupted. Collectively, these findings suggest that secretion of hepatic HEXA regulates blood glucose by remodeling the detergent-resistant raft domains in muscle, which allows for the enhanced colocalization of IGF receptors and additional machinery required to stimulate glucose transport within these domains. In turn, recombinant HEXA administration or viral transduction of HEXA in liver or muscle stimulates the translocation of intracellular vesicles containing GLUT4 to the plasma membrane of muscle to enhance glucose uptake.
The observations presented by Montgomery et al. (1) merit further investigation to understand 1) the mechanisms by which HEXA expression increases in response to metabolic stress, 2) the mechanisms regulating HEXA secretion and cellular targeting, 3) the metabolic itinerary of HEXA secretion and cellular targeting, and 4) the complexing of HEXA to other subunits, including HEXB and GM2 activator protein, which aid enzymatic activity. Concerns that increasing liver HEXA enhances very-low-density lipoprotein secretion may be blocking the therapeutic utility of HEXA (13). Similarly, an enhanced GM3 abundance in adipocytes, which rely more exclusively on insulin receptor homodimers, could blunt suppression of lipolysis. Thus, concerns with respect to hyperlipidemia and differing roles of HEXA in multiple tissues will need to be evaluated in future work.
Article Information
Funding. The authors received research support from the National Institutes of Health (DK115824, DK116888, and DK116450 to S.A.S. and DK112826 and DK130296 to W.L.H.), the American Diabetes Association (to W.L.H.), the Margolis Foundation (to W.L.H.), and the National Science Foundation (to F.M.B.).
Duality of Interest. S.A.S. is a consultant, cofounder, and shareholder in Centaurus Therapeutics, LLC. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 715.
References
- 1. Montgomery MK, Bayliss J, Nie S, et al. Liver-secreted hexosaminidase A regulates insulin-like growth factor signaling and glucose transport in skeletal muscle. Diabetes 2023;72:715–727 [DOI] [PubMed] [Google Scholar]
- 2. Adams AC, Coskun T, Rovira AR, et al. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLoS One 2012;7:e38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 2021;18:701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metab 2019;1:1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandhoff K, Harzer K. Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J Neurosci 2013;33:10195–10208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hultberg B, Isaksson A, Lindgren A, Israelsson B, Brattström L. β-Hexosaminidase isoenzymes A and B in middle-aged and elderly subjects: determinants of plasma levels and relation to vascular disease. Ann Clin Biochem 1996;33:432–437 [DOI] [PubMed] [Google Scholar]
- 7. Hultberg B, Isaksson A, Agardh E, Agardh CD. Plasma β-hexosaminidase isoenzymes A and B exhibit different relations to blood glucose levels in a population of type 1 diabetic patients. Scand J Clin Lab Invest 1995;55:723–728 [DOI] [PubMed] [Google Scholar]
- 8. Hultberg B, Pålsson B, Isaksson A, Masson P. β-Hexosaminidase in bile and plasma from patients with cholestasis. Liver 1995;15:153–158 [DOI] [PubMed] [Google Scholar]
- 9. Pérez LF, Casal JA, Rojas P, Tutor JC. Relationship between plasma ammonia concentration and β-N-acetylhexosaminidase isoenzyme activities in liver cirrhosis. Clin Chem Lab Med 2000;38:1237–1241 [DOI] [PubMed] [Google Scholar]
- 10. Kabayama K, Sato T, Saito K, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A 2007;104:13678–13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamashita T, Hashiramoto A, Haluzik M, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A 2003;100:3445–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem 2014;289:723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montgomery MK, Taddese AZ, Bayliss J, Nie S, Williamson NA, Watt MJ. Hexosaminidase A (HEXA) regulates hepatic sphingolipid and lipoprotein metabolism in mice. FASEB J 2021;35:e22046. [DOI] [PubMed] [Google Scholar]