Abstract
Intrahepatic islet transplantation for type 1 diabetes is limited by the need for multiple infusions and poor islet viability posttransplantation. The development of alternative transplantation sites is necessary to improve islet survival and facilitate monitoring and retrieval. We tested a clinically proven biodegradable temporizing matrix (BTM), a polyurethane-based scaffold, to generate a well-vascularized intracutaneous “neodermis” within the skin for islet transplantation. In murine models, BTM did not impair syngeneic islet renal-subcapsular transplant viability or function, and it facilitated diabetes cure for over 150 days. Furthermore, BTM supported functional neonatal porcine islet transplants into RAG-1−/− mice for 400 days. Hence, BTM is nontoxic for islets. Two-photon intravital imaging used to map vessel growth through time identified dense vascular networks, with significant collagen deposition and increases in vessel mass up to 30 days after BTM implantation. In a preclinical porcine skin model, BTM implants created a highly vascularized intracutaneous site by day 7 postimplantation. When syngeneic neonatal porcine islets were transplanted intracutaneously, the islets remained differentiated as insulin-producing cells, maintained normal islet architecture, secreted c-peptide, and survived for over 100 days. Here, we show that BTM facilitates formation of an islet-supportive intracutaneous neodermis in a porcine preclinical model, as an alternative islet-transplant site.
Article Highlights
Human and porcine pancreatic islets were transplanted into a fully vascularized biodegradable temporizing matrix (Novosorb) that creates a unique intracutaneous site outside of the liver in a large-animal preclinical model.
The intracutaneous prevascularized site supported pancreatic islet survival for 3 months in a syngeneic porcine-transplant model.
Pancreatic (human and porcine) islet survival and function were demonstrated in an intracutaneous site outside of the liver for the first time in a large-animal preclinical model.
Introduction
Islet transplantation is an effective treatment for selected patients with type 1 diabetes, whereby allogeneic human islets, isolated from deceased organ donors, are infused via the portal vein into the liver of recipients with type 1 diabetes with severe metabolic instability and hypoglycemic unawareness (1–3). One of the major limitations to wider application of this technology is the site of implantation; up to 60% of the transplanted islet mass is lost after infusion into the liver (4). This transplant site requires access to the portal circulation by cannulation of the portal vein, either by direct venipuncture or by a transhepatic approach to embolize the islet cells into the hepatic vasculature. The hepatic microcirculation is characterized by low oxygen tension, high levels of endotoxin, and active immunity, which further compromise islet function (5,6). Furthermore, islets are prothrombogenic cell clusters and, therefore, anticoagulation at the time of transplantation is required to reduce the potential for the instant blood-mediated inflammatory reaction. This increases the risk of anticoagulation-related complications (7). Retrieval of transplanted cells is impossible and renders the potential use of newer alternative sources of insulin-secreting tissues, such as stem cell–derived or xenogeneic islets, extremely hazardous (8). Thus, the quest for alternative extrahepatic transplant sites represents a significant target for the islet-transplant field.
A variety of extrahepatic alternative sites have been proposed, including intramuscular, intrathymic, intramedullary long bone marrow (9), and the subcutaneous site (10). Of these proposed sites, the skin proffers the advantages of ease of accessibility, clinical monitoring and imaging, diagnostic biopsy, and even excision of the grafted tissues. The skin has shown promise as an extrahepatic alternative site in experimental rodent models (11); however, its major limitation is the relative hypoxic nature of the dermis and underlying fat (12). The biodegradable temporizing matrix (BTM) is a wholly synthetic, bilayer replacement scaffold developed for use in major burn injury (13–21). The “dermal” component is a 2-mm-thick, open-cell polyurethane foam (marketed as NovoSorb) designed to biodegrade predominantly by hydrolysis. A nonbiodegradable polyurethane film (seal) is bonded to the upper surface and functions as a “pseudo-epidermis,” protecting the wound from the external environment; reduces evaporative water loss; and minimizes wound contraction. After full integration of the dermal component, the seal bond is weakened and the superficial polyurethane film can be peeled away to expose the highly vascularized tissue bed below. Critical to the success of the BTM in wound management is the creation of a highly vascularized “neodermal” intracutaneous space, which, unlike normal dermis, contains loosely folded and whorled collagen bundles, allowing space for other cell types to be implanted (15,16). In this study, using multiple in vitro and in vivo preclinical models, we describe the successful transplantation, survival, and function of murine and porcine islets transplanted into the NovoSorb polyurethane material, revealing the potential of an intracutaneous BTM site as a novel alternative site for islet transplantation.
Research Design and Methods
NovoSorb Biodegradable Temporizing Matrix (BTM with polyurethane film seal) and samples of NovoSorb foam matrix lacking the superficial seal, were provided by PolyNovo Biomaterials Pty. Ltd. (Port Melbourne, Victoria, Australia). The unsealed foam was used for all experiments where the matrix was transplanted internally (i.e., in all subkidney capsule transplant experiments in mice (22) and all in vitro culture assays), where the function of the surface seal was not required.
Glucose-Stimulated Insulin Secretion Assay and In Vitro Islet Viability
NovoSorb foam 3-mm disks presoaked with complete medium (RPMI medium, 10% FCS) to ensure saturation were transferred to 24-well plates and loaded with islets. Complete medium (1 mL) was gently added to wells and islets were incubated for 3 days at 37°C and 5% CO2. Islets were recovered from the NovoSorb foam for analysis of viability and glucose-stimulated insulin secretion assay.
Mouse Transplant Models
Mouse studies were approved by the Garvan Institute’s Animal Ethics Committee (approval no. ARA 17_27). Male RAG-1−/− mice (6–10 weeks old) were used for subkidney capsule experiments and diabetes was induced with alloxan. Diabetes was determined as blood glucose ≥16 mmol/L on 2 consecutive days measured by Free-Style Lite glucometer and Abbott Diabetes Care test strips after tail tipping. For the surgeries, a left-flank surgical incision was made to access the kidney, as previously described (23,24). Using a 21-gauge needle, an insertion point made in the kidney capsule allowed for insertion of 3 mm × 3 mm NovoSorb foam, positioned under the capsule on the kidney parenchyma surface, followed by wound closure. At the determined time after placement, a second surgery was performed to access the kidney. The integrated NovoSorb foam was readily visualized on the kidney surface. As described above, a second small incision was made in the kidney capsule, facilitating the islet bolus infusion, using a Hamilton syringe, into the NovoSorb foam. Pressure from the capsule on the foam holds the placed islets in position. The kidney is recessed and the wound is closed.
Two-Photon In Vivo Microscopy
For in vivo imaging studies of host- and islet-BTM interactions, a murine model was used whereby male Lysozyme M Cre mice were crossed with male ROSAmT/mG mice (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/; The Jackson Laboratories). In this model, macrophages, polymorphonucleocytes, and some dendritic cells express GFP, and blood and lymphatic vessels express membrane tdTomato. NovoSorb foam vascularization was analyzed with a Zeiss 7MP 2-photon microscope (Carl Zeiss) with a W Plan-Apochromat ×20/1.0 digital image correlation (ultraviolet) visible-infrared water-immersion objective.
Neonatal Islet Preparation
Porcine studies were conducted in Good Laboratory Practice–accredited animal facilities and approved by the local animal ethics committees (South Australian Health and Medical Research Institute [SAHMRI], approval no. SAM206). Westran neonatal islet cell clusters (NICCs) and Large White × Landrace neonatal porcine islets (NPIs) were isolated from 2- to 7-day-old pig pancreata by dissection and collagenase digestion using standard protocols (25,26). Briefly, piglets were anesthetized using ketamine/xylazine (0.75 mL of ketamine and 0.25 mL of xylazine). Their pancreata were surgically harvested and subsequently finely dissected into 2-mm pieces in Hanks’ balanced salt solution (Gibco). The fragments were digested with Collagenase type V (Sigma) for 12–14 min at 37°C before filtration through a sterile mesh and washing twice (300g) in Hanks’ balanced salt solution with 1% porcine serum. After culture in 150 mm × 20 mm Petri dishes with Hams F-10 medium at 37°C and 5% CO2 for 6 days, cells were counted. Media changes were performed every 3 days. After culture, cells were counted relative to size to determine their islet equivalent quantification (IEQ). Subsequently, these cells were transplanted into the intracutaneous site created after BTM implantation into adult pigs (weight, 20–50 kg).
Creation of an Intracutaneous Transplant Site using NovoSorb BTM
BTM was transplanted onto the back flank of adult pigs as previously described (13). For this study, either standard 2-mm-thick or a customized 4-mm-thick BTM was engrafted to create the intracutaneous islet transplantation site. In four wounds (40 mm × 40 mm), skin and subcutaneous tissue were removed to the level of the panniculus adiposus. This was performed under general anesthesia induced by intramuscular ketamine (1 mg/10 kg) and maintained by 2.5% isoflurane via endotracheal intubation. The flank and back hair was clipped and then shaved prior to wound creation. Four wound sites were prepared (two on each flank), equidistant (4 cm) from the spine, with 4 cm between ipsilateral flank wounds. All marked wound margins were infiltrated with 0.25% bupivacaine with 1:400,000 adrenaline, providing 12 h of postoperative pain relief and vasoconstriction minimizing local blood loss. Preemptive fentanyl transdermal patches were applied to the ear 24 h prior to surgery (delivering the medication at 2 μg/kg/h) for improved control of postoperative pain. These patches were replaced at the time of surgery to provide another 72 h of postoperative pain relief. In addition, buprenorphine (Temgesic) 0.3–0.5 mg/kg was administered by intramuscular injection during anesthetic recovery, and Carprofen 2 mg/kg was available when required for breakthrough pain suspected by the husbandry staff in the research facility.
The wounds were dressed with Acticoat (Smith & Nephew Ltd., Hull, UK) to reduce shear and minimize the risk of infection; the dressing was held in place by Hypafix (BSN Medical GmBH, Hamburg, Germany). A cotton wool/fabric “combine” dressing was applied before customized fabric jackets were fitted to protect the sites. Dressing changes were performed every 2–7 days for the duration of each experiment.
Pig Islet Transplantation in Large Animals
Female Large White × Landrace pigs (Sus scrofa; initial weight, 25–30 kg) were used in allogeneic and xenogeneic transplant experiments, and female Westran (inbred) pigs were used for syngeneic islet transplants. Standard diet, ad libitum water, and general animal care standard operating procedures were followed throughout the study. All animals were fasted overnight before surgery. Prophylactic antibiotic therapy consisted of intramuscular Co-Amoxyclav (Clavulox, 3.5 mL) postsurgery and Clavulox 250-mg tablets (2 tablets twice daily) throughout the postoperative transplant period. Those animals receiving allogeneic or xenogeneic transplants were placed on an immunosuppressive regimen consisting of tacrolimus (Prograf 0.25 mg/kg; Astellas) and mycophenolate mofetil (CellCept 500 mg twice daily; Roche), which commenced 1–2 weeks prior to islet transplantation. Therapeutic drug monitoring (Roche Immunoassay) for tacrolimus was performed to determine drug levels.
BTM was integrated over 17–25 days prior to islet transplantation. Islets were pelleted in polyethylene tubing (internal diameter, 0.58 mm; external diameter, 0.965 mm) or loaded into a catheter, prior to the removal of the nonbiodegradable seal from each wound site (delamination). Using a 1-mm steel rod, a channel was created to enable insertion of the islet-loaded cannula. A Hamilton syringe was used to slowly deposit islets into the created channel. A split skin graft was taken from adjacent skin and placed over the wound at the completion of the islet transplant procedure (Fig. 1C).
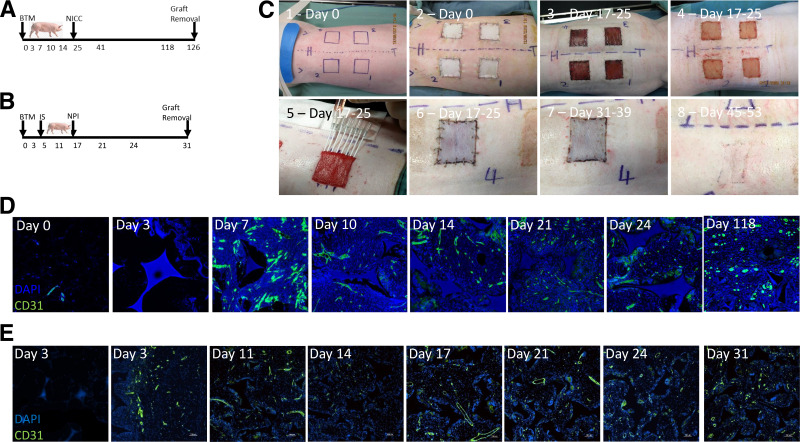
Figure 1.
Large White × Landrace, preclinical, large-animal model of intracutaneous islet transplantation and vascular development. A: Westran pigs were engrafted with BTM followed by a syngeneic islet transplant of NICCs for 101 days prior to graft removal. B: Immunosuppressed Large White × Landrace pigs were transplanted with allogeneic NPIs after BTM engraftment with graft removal 14 days posttransplant. C: The key surgical time points of this preclinical, porcine, intracutaneous islet-transplant model, beginning with marking out the BTM wound sites (image 1, day 0), wound creation and engraftment with four 40 mm × 40 mm × 2 mm BTM foams (image 2, day 0), BTM graft vascularization (image 3, days 17–25), delamination of the seal at days 17–25 (image 4), intracutaneous transplant of 15,000 NICCs or NPIs into one graft site in each pig (image 5, days 17–25), skin grafting of the transplant site (image 6, days 17–25), removal of the staples (image 7, days 31–39), and the transplant site 4 weeks after skin grafting (image 8, days 45–53). D and E: The remaining three sites of the Westran (syngeneic) (D) and Large White × Landrace (allogeneic) (E) pigs were serially biopsied to document the course of vascularization at the time points, indicated by staining with CD31 (green fluorescence) out to day 118 or day 31 after BTM engraftment, respectively. IS, immunosuppressive drug therapy.
Syngeneic Islet Transplantation
NICCs were isolated from Westran piglets by the isolation center at Westmead Hospital (26) (Sydney, New South Wales, Australia) and transported to Adelaide, where they were either transplanted that day or placed in culture overnight prior to transplantation the following day. Islets (7,000–15,000 IEQ total per intracutaneous site) were washed with saline and loaded into cannulas, as described above, before transplantation into Westran recipients weighing 40–50 kg. Saline alone was used as a transplant control. Animals were humanely killed at 101 days posttransplantation for the long-term survival studies.
Allogeneic Islet Transplantation
Large White × Landrace NPIs were isolated and cultured as previously described (25) prior to transplantation (2,500–40,000 IEQ total per intracutaneous site) into immunosuppressed adult recipients (weight, 20–40 kg). The NPI grafts were harvested after a 14-day engraftment period and assessed for survival and revascularization.
Xenogeneic Islet Transplantation
Research-consented human islets (SAHMRI approval no. SAM206) isolated at St. Vincent’s Institute (Melbourne, Victoria, Australia) were transported to Adelaide (South Australia), where they were cultured for a maximum of 6 days in CMRL complete medium supplemented with 2 mmol/L GlutaMax, 10 mmol/L HEPES, penicillin-streptomycin, and 10% human albumin (Albumex 20; Red Cross of Australia) prior to transplantation, as described above. Animals were humanely killed 7–28 days posttransplantation.
Immunofluorescence
All tissues were fixed with 10% buffered formalin for at least 3 days prior to processing. Samples were paraffin embedded and 5-μm sections were processed for histological analysis. Sections were deparaffinized and rehydrated prior to heat-mediated antigen retrieval (Citrate buffer, pH 6). Sections were incubated with Guinea pig anti–human insulin/glucagon antibody (AB7842; Abcam, Cambridge, UK) overnight as a primary antibody alone at 2 μg/mL at 4°C. Insulin staining was visualized with secondary goat anti–guinea pig conjugated to Rhodamine fluorochrome (Jackson ImmunoResearch, West Grove, PA) diluted 1:200 and incubated at room temperature in the dark for 1 h. Otherwise, sections were costained with cross-reactive rabbit anti–mouse CD31 as a primary antibody overnight at 4°C (1:50 dilution of Ab28364; Abcam). A horseradish peroxidase–conjugated goat anti-rabbit polyclonal (1:2,000) was used as the secondary antibody before undergoing signal amplification with Tyramide Signal Amplification Kit per the manufacturer’s instructions (Molecular Probes, ThermoFisher).
Glucose-Stimulation Insulin Secretion Assay
Islets were handpicked into basal Krebs buffer (3 mmol/L glucose) at 37°C. Groups of 10 islets were then transferred into fresh basal Krebs buffer (3 mmol/L glucose) at 37°C for 1 h, and the supernatant was collected. Islets were resuspended in a 20 mmol/L glucose Krebs buffer (high) at 37°C for 1 h. Porcine insulin was analyzed using ALPCO Human Ultrasensitive Insulin ELISA (catalog 80-INSHUU-E01.1). Human c-peptide was analyzed using Roche immunoassay (SA Pathology). For intravenous glucose tolerance testing, glucose was infused intravenously (0.5 g/kg body weight) over 1 min and blood glucose measurements taken immediately before infusion and at 30 min and 60 min postinfusion.
Vascular-Density Quantification
For particle analysis of CD31 (vascular density) immunofluorescence staining, images were taken on a Zeiss confocal microscope at ×20 magnification. Tissue blocks were randomly selected and six representative images from each section (n = 30) were analyzed using ImageJ (with FIJI plugins) (National Institutes of Health, Bethesda, MD) to determine CD31-positive pixels per total image area (%).
Data and Resource Availability
All data generated or analyzed during this study will be made available in full upon request to the lead authors.
Results
Culture of Islets in the Presence of NovoSorb Foam Does Not Affect Viability or Function
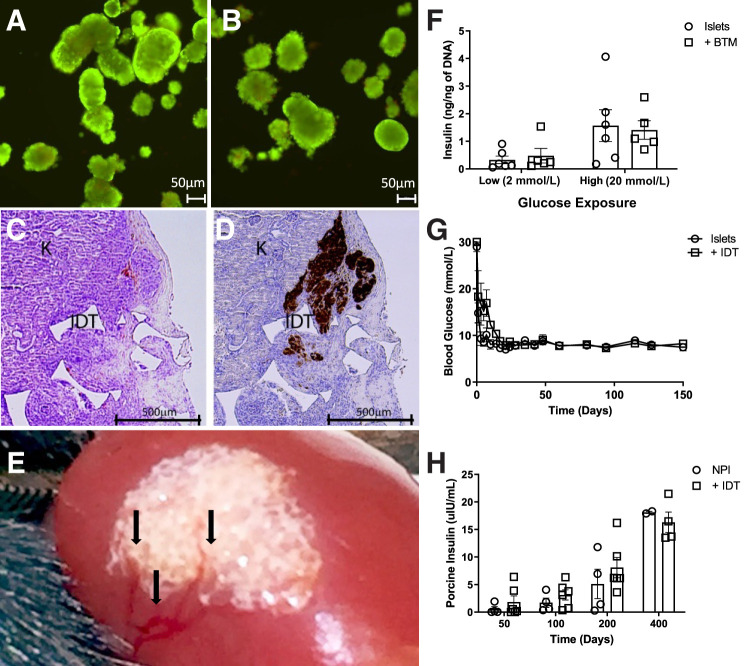
To assess the effect of BTM on islet viability and function in vitro, murine and human pancreatic islets were cultured within NovoSorb foam for 72 h. The islets were then extracted for analysis. Islet viability was determined using annexin and propidium iodide staining. No difference was observed with islets cultured in the presence of NovoSorb foam (Fig. 2A and 2B), nor was there any deleterious effect on islet function, as measured by glucose-stimulated insulin secretion assay (Fig. 2F).
Figure 2.
NovoSorb BTM and NovoSorb foam are nontoxic for islets and do not affect viability or function. A, B, and F: NovoSorb foam had no observable negative impact on islet viability and function as determined by annexin and propidium iodide staining (A: cultured islets only, n = 6; B: islets cocultured with NovoSorb foam, n = 5); and glucose-stimulated insulin secretion (F). Syngeneic mouse islets were transplanted into diabetic recipients under the naked subkidney capsule or the subkidney capsule preimplanted with NovoSorb foam (n = 3 per group). G: Recipient mice showed equally good recovery and long-term maintenance (>100 days) of euglycemia posttransplantation with either islets alone or islets transplanted into the subkidney capsule prepared with NovoSorb foam (IDT). C and D: Histological analysis of NovoSorb/islet grafts at 30 days post-transplantation show islets entwined with the polymer foam, evident normal islet architecture, structural stability of NovoSorb (IDT) (C) and insulin reactivity (D). K, kidney tissue. NPIs were transplanted into the naked subkidney capsule or the subkidney capsule preimplanted with NovoSorb foam of normoglycemic RAG-1−/− mice (n = 4 per group). H: NovoSorb was surgically transplanted under the kidney capsule 30 days prior to NPI transplantation. E: Macroscopic evidence of vascular development, driven by the presence of NovoSorb under the kidney capsule, is clearly visible. These studies showed long-term and increasing porcine insulin production through time to 400 days (H). Error bars: mean ± SD.
Islet Transplant Into Pre-engrafted NovoSorb Foam Under the Renal Capsule Supports Long-term Viability and Function
Islet transplantation under the renal capsule is considered an optimal site for measuring engraftment and function and, therefore, is suitable to test for potential negative effects of the NovoSorb foam on islet engraftment and function per se. Accordingly, murine islets were transplanted under the kidney capsule of alloxan-induced diabetic, immune-deficient RAG-1−/− mice with or without preengrafted NovoSorb foam. Both control and treated mice showed prompt cure, with restoration of normal blood glucose to over 150 days (Fig. 2G). Figure 2C shows the appearance under light microscopy of the NovoSorb foam under the kidney capsule with insulin staining within the NovoSorb foam containing islet graft (Fig. 2D). Long-term islet function within the NovoSorb foam graft was then assessed using NPIs transplanted under the kidney capsule, with or without preengrafted NovoSorb foam, into immune-deficient RAG-1−/− mice. Serial blood samples were taken over the course of 400 days and showed increasing levels of porcine insulin, and indicated no deleterious effect of the NovoSorb foam on long-term islet maturation and function (Fig. 2H). Macroscopic examination of the murine kidney–NovoSorb foam graft shows visible vessels (black arrows in Fig. 2E) growing over the surface of the implanted foam.
Impact of NovoSorb Foam on Neovascularization
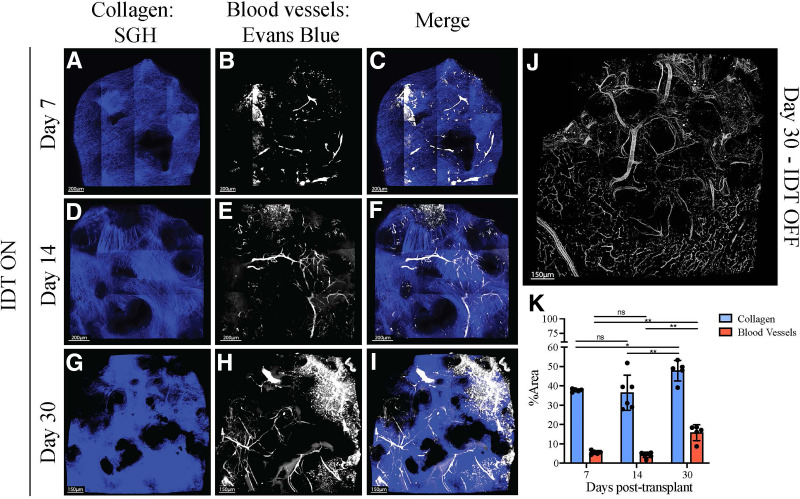
The proangiogenic characteristics of NovoSorb foam posttransplantation under the kidney capsule were investigated by in vivo 2-photon microscopy. Recipient mice engrafted with NovoSorb foam were examined at days 7, 14, and 30. Collagen staining (blue) at the three time points (Fig. 3A, 3D, and 3G) indicates a progressive, incremental increase over the period studied. Vessel formation, as depicted by Evans Blue dye, reveals formation of immature microvessels by day 7 (white areas in Fig. 3B), microvessel maturation at day 14 (Fig. 3E), and further microvessel maturation along with formation of larger vessels at day 30 (Fig. 3H and Supplementary Video 1). The combined images in Fig. 3C, 3F, and 3I illustrate both collagen and blood vessel development over time. Figure 3J shows the dense vascular development that occurred on the surface of the kidney immediately below the NovoSorb foam at day 30, clearly highlighting the ability of the NovoSorb scaffold to promote vascularization in this model. Collagen formation and vessel development are relatively constant at days 7 and 14; however there is a significant increase in both collagen and vessel mass from day 14 to day 30, suggesting that transplantation of islets in this model is more likely to be optimal at the later time point (Fig. 3K; Supplementary Video 1).
Figure 3.
Improved vascularization induced by NovoSorb foam/BTM shown in real time in vivo 2-photon microscopy (mouse). To assess the impact of NovoSorb (IDT) on tissue remodeling in vivo through time, transgenic mice (LysGFPtdTomTg) expressing GFP+ myeloid cells and blood and lymphatic vessels expressing tdTomato red were used. A, D, and G: NovoSorb was transplanted under the kidney capsule of the transgenic mice, and 2-photon microscopy performed at days 7 (A), 14 (D), and 30 (G) demonstrates blue collagen formation under the kidney capsule, with increasing density of blue over time. B, E, and H: At 7 days (B), 14 days €, and 30 days (H), white blood vessel formation is seen, with increasing vascular formation over time. C, F, and I: Images taken at 7 days (C), 14 days (F), and 30 days (I) illustrate the merged collagen density with vascular changes indicating synchronous formation of loose whorls of collagen and vasculature with time. Black voids in all images are the NovoSorb polymer under the kidney capsule. J: A composite low-power (×40) view of the formed vasculature under the kidney capsule at the interface of the kidney tissue and the NovoSorb (IDT) foam, with vessels staining white. K: Quantitative analysis of collagen formation and vessel development through time (days 7, 14, and 30).
In separate experiments, vascular development was assessed in both the syngeneic (no immunosuppression; Fig. 3D) and the allogeneic (with immunosuppression; Fig. 1E) porcine models. Vessel formation was evident by day 3 (in the allogeneic model) and day 7 (in the syngeneic model) and persisted through to the experimental end points of day 31 and day 118, respectively. Clear evidence of vascularity at day 118 indicated long-term persistence of the newly formed vascularity within the skin (Fig. 1D). Vascular development kinetics in the presence of immunosuppression was similar to that observed in the syngeneic model.
Intracutaneous Transplant of Porcine and Human Islets Into Preengrafted BTM
To test the intracutaneous site for islet transplantation, the following three large-animal models were used: 1) syngeneic intracutaneous NICC transplantation (Fig. 1A) (Westran NICC to adult Westran recipient without immune suppression) (23); 2) allogeneic intracutaneous NPI transplantation (Fig. 1B) (Large White × Landrace NPI to adult Large White × Landrace recipient with immune suppression) (24); and 3) xenogeneic intracutaneous human islet transplantation (Fig. 4G) (human cadaveric islet to Large White × Landrace pigs with immune suppression).
Figure 4.
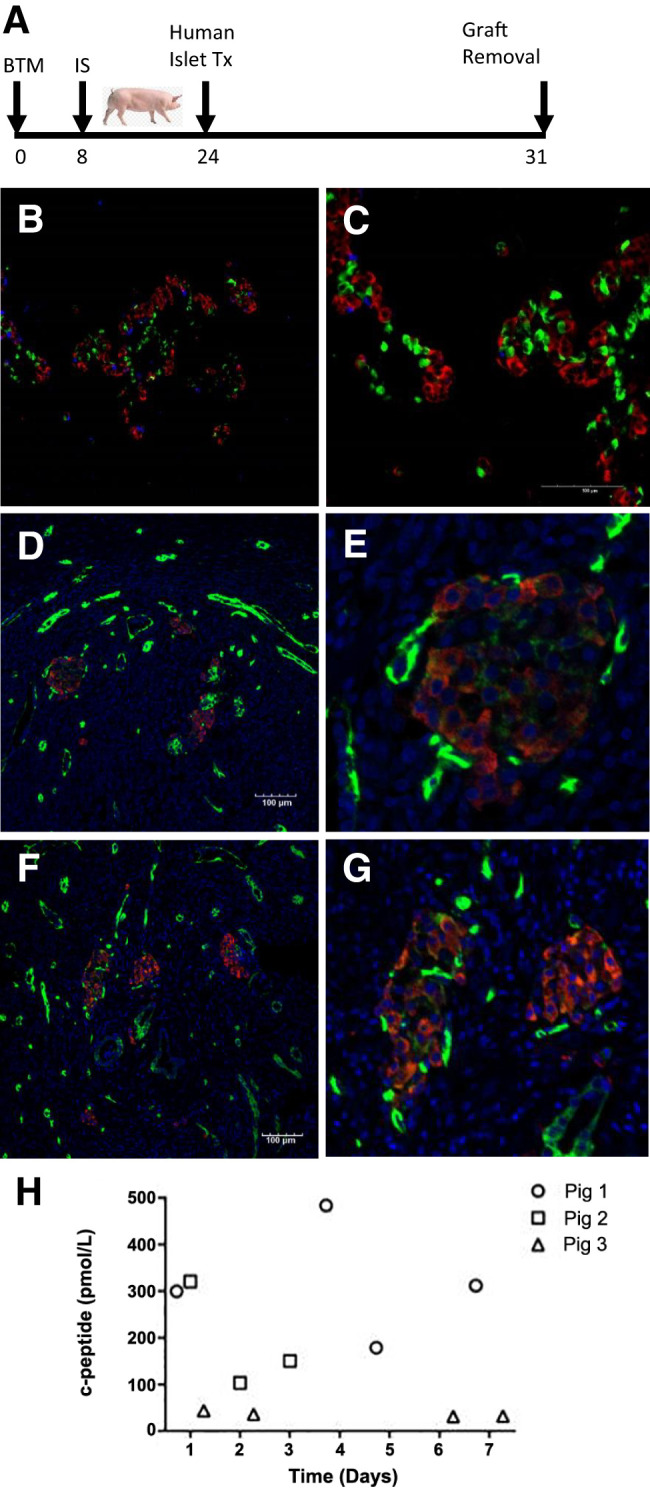
Human pancreatic islets transplanted into the intracutaneous site in Large White × Landrace pigs under immunosuppression are viable after 7 days. G: A schematic of 62,500 IEQ human xenogeneic islets transplanted into an intracutaneous graft site in a Large White × Landrace pig after 24 days of BTM engraftment. Immunosuppression with tacrolimus and mycophenylate mofetil was initiated 16 days prior to islet transplant, and the entire graft site was collected 7 days later. A and B: Triple-islet hormone staining clearly identifies cells positive for insulin (red), glucagon (green), and somatostatin (purple) within the islet graft. C and E: Vascularized human islets in the intracutaneous site are depicted (C and E). D and F: The presence of intraislet vasculature in high-powered images, with insulin-positive cells (red) and CD31-positive vasculature (green). H: The human c-peptide levels, from random serum samples, in three immune-suppressed pigs after xenogeneic intracutaneous islet transplantation across multiple time points out to 7 days after transplantation.
Syngeneic Porcine Islets’ Long-term Survival and Integration With the Vasculature Created Within the Intracutaneous Transplantation Site
To evaluate the long-term viability of islets transplanted into the intracutaneous site without the need for immunosuppression, 15,000 NICCs were transplanted into adult Westran recipients (syngeneic donor and recipient; Fig. 1A). Because Westran NICC mature over 90 days in vivo, grafts were left in situ for 101 days before retrieval. In the absence of immunosuppression, intracutaneously transplanted Westran NICCs were detected at 101 days after engraftment by the presence of insulin- and glucagon-positive cells (n = 2; Fig. 5F–I). Despite the low number of NICCs initially transplanted, we were able to clearly identify their presence across multiple sections within the 40 mm × 40 mm × 2 mm transplantation site. Insulin-positive NICCs can be seen adjacent to CD31-positive vasculature within the intracutaneous site at 101 days posttransplantation (Fig. 5K).
Figure 5.
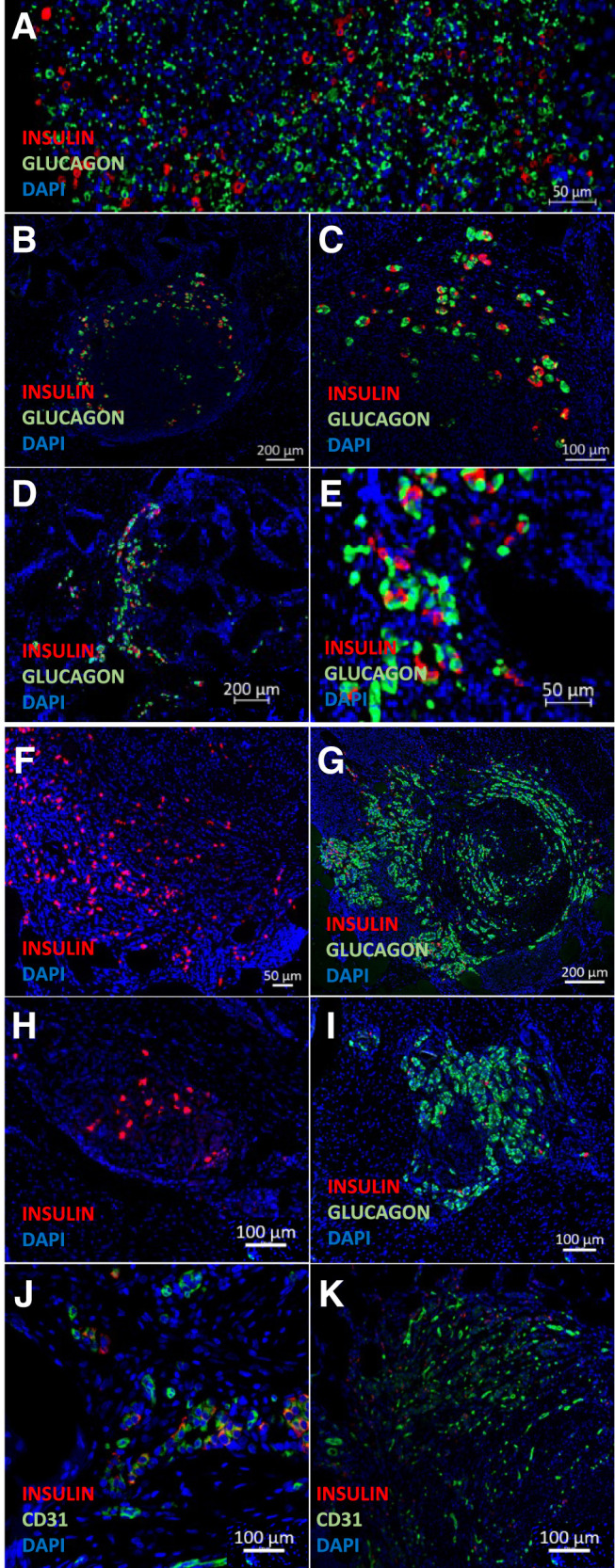
Normal islet hormone expression and viability after intracutaneous transplantation of syngeneic (NICC) islets for 101 days or allogeneic (NPI) islets for 2 days and 14 days. BTM grafts were harvested at day 101 (NICCs) or days 2 and 14 (NPIs) and processed for insulin, glucagon, and CD31 staining. The nuclear stain, DAPI (blue); porcine insulin-positive (red) and glucagon-positive (green) cells of NPI at 2 days posttransplantation (A) and 14 days after transplantation in pig 1 (B and C) and pig 2 (D and E), and NICCs 101 days posttransplantation into Westran pig 1 (F and G) and pig 2 (H and I). J and K: NPIs on day 14 posttransplantation (J) and NICCs on day 101 posttransplantation (K) both showing the presence of insulin-positive (red) cells adjacent to CD31-positive (green) vasculature within the intracutaneous transplantation site.
Allogeneic Porcine Islets Survive and Integrate With the Vasculature Created Within the Intracutaneous Site of Immunosuppressed Pigs
To assess the ability of islets to engraft into an intracutaneous site in a manner analogous to human islet transplantation, ABO blood group–compatible allogeneic NPIs (20,000 IEQ) from Large White × Landrace donor pigs were transplanted using the cannula technique (Fig. 1C days 17–25) into Large White × Landrace recipient pigs (Fig. 1B). Recipient pigs received oral tacrolimus and mycophenylate mofetil immunosuppression, monitored by commercial immunoassay, and demonstrated comparable serum levels to conventional human intrahepatic islet–allograft recipients (data not shown). Histological analysis of graft sites removed at days 2 (Fig. 5A) and 14 posttransplantation (n = 2; Fig. 5B–E) readily identified allogeneic porcine islet allografts integrated within the intracutaneous site, with insulin- and glucagon-positive cells visible within the transplanted material. Interaction between the NPIs and the intracutaneous site vasculature at day 14 is shown in Fig. 5J, with insulin-positive NPIs (red) immediately adjacent to CD31-positive vasculature (green).
Xenogeneic Human Islets Transplanted Into the Intracutaneous Site Are Viable and Functional
To confirm that human islets were viable in the intracutaneous site created after 24 days of BTM integration, human xenogeneic islets (62,500 IEQ) were transplanted into a Large White × Landrace adult pig treated with oral immunosuppression. The islet graft was harvested 7 days later (Fig. 4G). Triple-hormone-positive human islets (Fig. 4A and 4B) were clearly visible within the graft site, and dual staining with insulin and CD31 revealed integration of islets with the intracutaneous vasculature (Fig. 4C–F). To examine human islet function in the intracutaneous site, three Large White × Landrace pigs were transplanted intracutaneously with human cadaveric islets under immunosuppression (oral tacrolimus and mycophenylate mofetil). Islet function was determined by measurement of human c-peptide out to 7 days posttransplantation. C-peptide was detected from random serum samples in all three pigs at multiple time points (Fig. 4H), with levels ranging between 30 and 500 pmol/L.
Discussion
The skin as a potential site for β-cell replacement has been considered since 1894, when P. Watson Williams first unsuccessfully attempted to implant fragments of sheep pancreas into the skin of a patient with type 1 diabetes (27). Although the skin is a hostile environment for cell transplantation, Pepper et al. have used foreign-body reaction induced by a 2-cm nylon catheter to create a vascularized and fibrotic subcutaneous space, which was able to support islet cell implantation in rodent models (11,30). Although islet function did not equal that observed for subrenal capsule implantation (28), those pioneering rodent studies provide support for using skin as a potential site for islet implantation. Here, we provide a novel approach to overcome the limitations of the skin as a transplantation site by creating a fully artificial intracutaneous neodermal site using a biodegradable polyurethane matrix. Until now, the skin has been a disappointing site for islet transplantation, because of poor vasculature and the inability to maintain an appropriate microenvironment for islet survival and function. The clinical advantage of NovoSorb BTM is its proven ability to promote functional vascularization of the implantation site, enabling transplanted islets not only to survive but able to sample the blood delivered by afferent arterioles from the systemic circulation to detect serum glucose levels and subsequently deliver insulin into the efferent venular outflow channels back to the systemic circulation. This novel intracutaneous site can support mouse and porcine islet engraftment, survival, and long-term function in mouse models of diabetes. In the large preclinical porcine model, intracutaneous islet transplants survived over the long term with evidence of early function. Translation of intracutaneous islet transplantation to the clinic would require a BTM graft approximately 3 cm × 5 cm to accommodate the ≤3 mL of purified islet tissue typically transplanted.
Generation and testing of islet encapsulation devices and related technologies are advancing through preclinical stages and are recognized as an engineering approach to overcome the need for heavy immunosuppression but also to facilitate new sources of β-cells (8). Development of coatings and structures using synthetic polyethylene glycol–hydrogel macrodevice systems (31), conformal coating strategies (31), nanoporous polymer-thread technology (32), as well as unique device structures (33) offers opportunities to advance durable β-cell replacement therapies (34). The intracutaneous islet transplant procedure used here differs from approaches designed to house islets internally and that are surgically placed under the skin. These devices are typically not designed to biodegrade and can be seen as foreign to the body for the entirety they remain in situ. The BTM is completely biodegradable, such that once it has driven the vascular development and supported islet engraftment in the early posttransplant period, it is completely degraded and excreted from the body. By 12 months posttransplant, there is little to no BTM material remaining within the transplant site. In the generation of an intracutaneous neodermis using the BTM, an extensive vascular network of small arterioles, capillaries, and venules is created within the dermal compartment, which subsequently is capable of fully supporting a split skin graft. Using this technique, severely burn-injured individuals have been able to survive previously catastrophic burn injury (19,35,36). Additionally, the synthetic nature of the matrix does not provide a culture medium for organisms, which allows its use to be extended to the management of wounds after severe cutaneous or subcutaneous infection (e.g., necrotizing fasciitis) (37) and nonburn trauma (21). During the development of the polyurethane material, the formation of a highly vascularized dermal layer was observed, with loose, whorling bundles of collagen, allowing skin grafts placed on top of the newly created dermal layer to survive. We hypothesized that this rich vascular bed, and the space afforded by loose collagen and edema in the intracutaneous neodermis, might adequately support islet viability and function.
The BTM studied here is a fully biodegradable polyurethane matrix, which underwent 14 years of preclinical and clinical development culminating in regulatory approval in several jurisdictions, including 510k U.S. Food and Drug Administration approval and use of the material in wounds in the U.S. (13–18). The product used a nondegradable, polyurethane, removable seal, which prevents evaporative water loss from the wound, inhibiting the signal for fibrosis. Once the dermal foam component is fully integrated, the seal delaminates easily by gentle teasing. The superficial aspect of the underlying polymer separates to the level of the bond, allowing exposed polymer on the superficial surface of the neodermis to retract back into the newly created tissue. This ensures that all of the seal, and the bond, is removed during delamination, leaving a refreshed wound bed. The NovoSorb biodegradable polyurethane has been designed to maintain physical strength and structure for 3 months postapplication. After this time, progressive hydrolysis of the material results in matrix degradation into products that are easily excreted (CO2, water) or used (lysine). Larger molecular residue is absorbed by macrophages or giant cells, leaving an intact blood vasculature, which has shown persistence in human biopsy specimens out to 5 years. Most of this process is completed by 12 months, and certainly by 18 months there is no histological evidence of its implantation.
There are several strengths to this study, including the demonstration of long-term islet survival in a robust, syngeneic, large-animal model and islet survival in the background of systemic immunosuppression in both the allogeneic and xenogeneic porcine models with evidence of early function, as demonstrated by the detection of serum c-peptide, in the xenogeneic transplant group, thus supporting further translational development toward curative models. Another major strength of the study is that the porcine model represents the animal skin model most similar to humans. Unlike rodents and nonhuman primates, pigs lack a panniculus carnosus (i.e., subcutaneous muscle layer under hair-bearing skin); instead, they possess a panniculus adiposus, a structure in common with human skin in all but a few locations. However, pig models also pose significant challenges (e.g., maintaining immune suppression, husbandry issues, dietary considerations) that make curative studies challenging. Nonetheless, given the physiological and architectural similarities of porcine and human skin, the current data would be supportive of future work using BTM to facilitate a novel, alternative site for islet transplantation.
A current limitation of the study relates to the distribution and delivery of islets into the intracutaneous site. It is recognized that islets need to be within 200 μm of a blood supply for viability; hence, the cannula route of administration used in this study, although appropriate for demonstration of proof of principle and function on implanted islets, is not yet optimized to deliver an islet mass necessary for insulin independence in large animals. Because the external diameter of the cannula was 500 μm, islets in the central core were potentially rendered ischemic. Further experimentation to optimally deliver islets in proximity to vasculature is required. In addition, the dose of islets transplanted in the preclinical porcine models was not intended to be curative; only the xeno-islet transplant group was rendered diabetic to look for early evidence of function, and the islet dose for all three models was governed by the islet isolation yield achieved.
Nevertheless, these studies show proof of principle in large-animal models that pancreatic islets may be successfully transplanted into an intracutaneous site mirroring human skin. Further characterization of this site with regard to potential reduction in instant blood-mediated inflammatory reaction should also be performed. The advantage of being able to visualize the islet graft, biopsy specimen, or to completely remove the cellular transplant makes this site a safe option for transplantation of stem cell and xenogeneic sources of insulin-secreting tissue with the potential for translation into clinical practice via this alternative transplant site.
Article Information
Funding. This work was supported by Juvenile Diabetes Research Foundation International (SRA-2016-257-S-B) and the Hospital Research Foundation, South Australia.
Duality of Interest. P.T.C. and J.E.G. are stakeholders in Beta Cell Technology, a research and development company. J.E.G. is the inventor of the NovoSorb BTM and remains a shareholder in PolyNovo Biomaterials Pty. Ltd. (the manufacturers of NovoSorb BTM). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.R.-C., D.P., S.N.W., C.J.D., J.J., J.N., and S.K. performed experiments and reviewed the manuscript. T.L. and T.W.K. performed human islet isolation. W.H. and P.J.O. provided Westran pigs and performed Westran pig isolation islet isolation. G.K. provided neonatal porcine islets. T.C., J.B., and D.C. performed 2-photon in vivo microscopy. D.R.-C., C.J.D., S.T.G., J.E.G., and P.T.C. designed the experiments and wrote and reviewed the manuscript. P.T.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the International Pancreas and Islet Transplantation Association meeting, Lyon, France, 2–5 July 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22276984.
D.R.-C., S.N.W., and D.P. share first authorship.
S.T.G., C.J.D., and P.T.C. share senior authorship.
References
- 1. O’Connell PJ, Holmes-Walker DJ, Goodman D, et al.; Australian Islet Transplant Consortium . Multicenter Australian trial of islet transplantation: improving accessibility and outcomes. Am J Transplant 2013;13:1850–1858 [DOI] [PubMed] [Google Scholar]
- 2. Hering BJ, Clarke WR, Bridges ND, et al.; Clinical Islet Transplantation Consortium . Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marathe CS, Drogemuller CJ, Marathe JA, et al. Islet cell transplantation in Australia: screening, remote transplantation, and incretin hormone secretion in insulin independent patients. Horm Metab Res 2015;47:16–23 [DOI] [PubMed] [Google Scholar]
- 4. Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. β-Cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 5. Cowley MJ, Weinberg A, Zammit NW, et al. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant 2012;21:2063–2078 [DOI] [PubMed] [Google Scholar]
- 6. Cantley J, Walters SN, Jung MH, et al. A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant 2013;22:2147–2159 [DOI] [PubMed] [Google Scholar]
- 7. Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 2005;54:1755–1762 [DOI] [PubMed] [Google Scholar]
- 8. Odorico J, Markmann J, Melton D, et al. Report of the key opinion leaders meeting on stem cell-derived beta cells. Transplantation 2018;102:1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cantarelli E, Melzi R, Mercalli A, et al. Bone marrow as an alternative site for islet transplantation. Blood 2009;114:4566–4574 [DOI] [PubMed] [Google Scholar]
- 10. Anazawa T, Okajima H, Masui T, Uemoto S. Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg 2018;3:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 2015;33:518–523 [DOI] [PubMed] [Google Scholar]
- 12. Evans NT, Naylor PF. The systemic oxygen supply to the surface of human skin. Respir Physiol 1967;3:21–37 [DOI] [PubMed] [Google Scholar]
- 13. Li A, Dearman BL, Crompton KE, Moore TG, Greenwood JE. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J Burn Care Res 2009;30:717–728 [DOI] [PubMed] [Google Scholar]
- 14. Greenwood JE, Li A, Dearman BL, Moore TG. Evaluation of NovoSorb novel biodegradable polymer for the generation of a dermal matrix part 2: in-vivo studies. Wound Pract Res 2010;18:24–34. [Google Scholar]
- 15. Greenwood JE, Dearman BL. Split skin graft application over an integrating, biodegradable temporizing polymer matrix: immediate and delayed. J Burn Care Res 2012;33:7–19 [DOI] [PubMed] [Google Scholar]
- 16. Dearman BL, Stefani K, Li A, Greenwood JE. “Take” of a polymer-based autologous cultured composite “skin” on an integrated temporizing dermal matrix: proof of concept. J Burn Care Res 2013;34:151–160 [DOI] [PubMed] [Google Scholar]
- 17. Wagstaff MJD, Schmitt BJ, Coghlan P, Finkemeyer JP, Caplash Y, Greenwood JE. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: a pilot study. Eplasty 2015;15:e13. [PMC free article] [PubMed] [Google Scholar]
- 18. Wagstaff MJD, Schmitt BJ, Caplash Y, Greenwood JE. Free flap donor site reconstruction: a prospective case series using an optimized polyurethane biodegradable temporizing matrix. Eplasty 2015;15:e27. [PMC free article] [PubMed] [Google Scholar]
- 19. Greenwood JE, Wagstaff MJ, Rooke M, Caplash Y. Reconstruction of extensive calvarial exposure after major burn injury in 2 stages using a biodegradable polyurethane matrix. Eplasty 2016;16:e17. [PMC free article] [PubMed] [Google Scholar]
- 20. Wagstaff MJ, Caplash Y, Greenwood JE. Reconstruction of an anterior cervical necrotizing fasciitis defect using a biodegradable polyurethane dermal substitute. Eplasty 2017;17:e3. [PMC free article] [PubMed] [Google Scholar]
- 21. Damkat-Thomas L, Greenwood JE, Wagstaff MJD. A synthetic biodegradable temporising matrix in degloving lower extremity trauma reconstruction: a case report. Plast Reconstr Surg Glob Open 2019;7:e2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grey ST, Longo C, Shukri T, et al. Genetic engineering of a suboptimal islet graft with A20 preserves beta cell mass and function. J Immunol 2003;170:6250–6256 [DOI] [PubMed] [Google Scholar]
- 23. Malle EK, Zammit NW, Walters SN, et al. Nuclear factor κB-inducing kinase activation as a mechanism of pancreatic β cell failure in obesity. J Exp Med 2015;212:1239–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zammit NW, Tan BM, Walters SN, et al. Low-dose rapamycin unmasks the protective potential of targeting intragraft NF-κB for islet transplants. Cell Transplant 2013;22:2355–2366 [DOI] [PubMed] [Google Scholar]
- 25. Jimenez-Vera E, Davies S, Phillips P, O’Connell PJ, Hawthorne WJ. Long-term cultured neonatal islet cell clusters demonstrate better outcomes for reversal of diabetes: in vivo and molecular profiles. Xenotransplantation 2015;22:114–123 [DOI] [PubMed] [Google Scholar]
- 26. Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest 1996;97:2119–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Connell PJ, Hawthorne WJ, Simond D, et al. Genetic and functional evaluation of the level of inbreeding of the Westran pig: a herd with potential for use in xenotransplantation. Xenotransplantation 2005;12:308–315 [DOI] [PubMed] [Google Scholar]
- 28. Stokes RA, Simond DM, Burns H, et al. Transplantation sites for porcine islets. Diabetologia 2017;60:1972–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watson-Williams P. Notes on diabetes treated with extract and by grafts of sheep’s pancreas. BMJ 1894;2:1303–1304 [Google Scholar]
- 30. Pepper AR, Pawlick R, Gala-Lopez B, et al. Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device. Transplantation 2015;99:2294–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weaver JD, Headen DM, Hunckler MD, Coronel MM, Stabler CL, García AJ. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018;172:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. An D, Chiu A, Flanders JA, et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad Sci USA 2018;115:E263–E272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smink AM, Skrzypek K, Liefers-Visser JAL, et al. In vivo vascularization and islet function in a microwell device for pancreatic islet transplantation. Biomed Mater 2021;16:035036 DOI: 10.1088/1748-605X/abf5ec [DOI] [PubMed] [Google Scholar]
- 34. Brusko TM, Russ HA, Stabler CL. Strategies for durable β cell replacement in type 1 diabetes. Science 2021;373:516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greenwood JE. The evolution of acute burn care - retiring the split skin graft. Ann R Coll Surg Engl 2017;99:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenwood JE, et al. Experience with a synthetic bilayer biodegradable temporising matrix in significant burn injury. Burns Open 2018;2:17–34 [Google Scholar]
- 37. Marcus JDW, et al. Biodegradable temporising matrix (BTM) for the reconstruction of defects following serial debridement for necrotising fasciitis: A case series. Burns Open 2019;3:12–30 [Google Scholar]