Abstract
Inflammation plays an important role in the pathogenesis of diabetic retinopathy (DR). To precisely define the inflammatory mediators, we examined the transcriptomic profile of human retinal endothelial cells exposed to advanced glycation end products, which revealed the neutrophil chemoattractant chemokine CXCL1 as one of the top genes upregulated. The effect of neutrophils in the alteration of the blood-retinal barrier (BRB) was further assessed in wild-type C57BL/6J mice intravitreally injected with recombinant CXCL1 as well as in streptozotocin-induced diabetic mice. Both intravitreally CXCL1-injected and diabetic animals showed significantly increased retinal vascular permeability, with significant increase in infiltration of neutrophils and monocytes in retinas and increased expression of chemokines and their receptors, proteases, and adhesion molecules. Treatment with Ly6G antibody for neutrophil depletion in both diabetic mice as well as CXCL1-injected animals showed significantly decreased retinal vascular permeability accompanied by decreased infiltration of neutrophils and monocytes and decreased expression of cytokines and proteases. CXCL1 level was significantly increased in the serum samples of patients with DR compared with samples of those without diabetes. These data reveal a novel mechanism by which the chemokine CXCL1, through neutrophil recruitment, alters the BRB in DR and, thus, serves as a potential novel therapeutic target.
Article Highlights
Intravitreal CXCL1 injection and diabetes result in increased retinal vascular permeability with neutrophil and monocyte recruitment.
Ly6G antibody treatment for neutrophil depletion in both animal models showed decreased retinal permeability and decreased cytokine expression.
CXCL1 is produced by retinal endothelial cells, pericytes, and astrocytes.
CXCL1 level is significantly increased in serum samples of patients with diabetic retinopathy.
CXCL1, through neutrophil recruitment, alters the blood-retinal barrier in diabetic retinopathy and, thus, may be used as a therapeutic target.
Introduction
Recent evidence indicates that low-grade chronic inflammation in the retina leads to alteration of the blood-retinal barrier (BRB) resulting in retinal vascular leakage in diabetes (1). The inflammatory phenotype in the diabetic retina is a complex cellular process regulated by cross-communication between retinal microvascular cells (pericytes and endothelial cells), Muller cells, astrocytes, retinal neurons, circulating monocytes, and microglia (2). In a previous study, we showed that there is increased monocyte trafficking into the extravascular retinal tissues and microglia activation that leads to increased vascular permeability and altered junctional proteins (3). Additionally, the role of vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), Ang2, CCL2 (C-C motif chemokine ligand 2), and cathepsin D has been established in in vitro and in vivo studies on BRB alteration in diabetic retinopathy (DR) (3–7).
Because VEGF is a potent vasopermeability factor implicated in the pathogenesis of diabetic macular edema (DME) (8), intravitreal anti-VEGF therapy is currently the first-line treatment in patients with vision-threatening DME (9,10). However, the response to anti-VEGF drugs is widely variable, with only 27–38% of patients responding well (11,12). The persistent retinal edema seen in the significant proportion of patients with DME in spite of anti-VEGF injections could be due to inflammatory cytokines and chemokines other than the VEGF molecule.
Our overall goal was to identify the role of inflammatory mediators in BRB breakdown in diabetes by examining a greater dynamic range for quantifying transcript expression in human retinal endothelial cells (HRECs) and precisely define novel targeted therapies in DME. In this study, we probed the gene expression pattern generated by chronic hyperglycemia, which was mimicked by treating the cells with advanced glycation end products (AGEs) to understand how these genotypic changes contribute to phenotypic changes using in vitro and in vivo functional validation.
Research Design and Methods
Cell Culture
Microvascular HRECs and human retinal pericytes (Cell Systems, Kirkland, WA) between passages 5 and 8 were grown on fibronectin-coated dishes in MCDB-131 growth medium supplemented with FBS (10%), epidermal growth factor (10 ng/mL), hydrocortisone (1 μg/mL), EndoGro (0.2 mg/mL), heparin (0.09 mg/mL), penicillin (100 units/mL), streptomycin (100 μg/mL), and Fungizone (0.25 μg/mL; VEC Technologies, Rensselaer, NY). Human retinal astrocytes were grown on poly-L-lysine–coated plates (1 mg/mL) in astrocyte growth medium. Human brain microglia cells were grown in microglia medium (ScienCell Research Laboratories, Carlsbad, CA). Human neutrophils (iQ Biosciences, Berkeley, CA) were grown in RPMI 1640 growth medium supplemented with 10% FBS. Cell cultures were maintained at 37°C with 5% CO2/95% humidity.
AGE Treatment
AGEs are an important biochemical formation in diabetes. Therefore, to mimic the diabetic environment, HRECs were treated with AGEs free of endotoxin (cat. no. 121800; Sigma-Aldrich) for 96 h (250 μg/mL) (13,14) before experiments. Cells grown in growth medium without AGE treatment were used as a control.
Construction of rRNA-Depleted RNA Sequencing Libraries
Total RNA was extracted from cells using the RNeasy Mini Kit together with on-column DNAse treatment (Qiagen, Germantown, MD) as per the manufacturer protocol. RNA quality was assessed using the 2100 Bioanalyzer (Agilent Technologies; Santa Clara, CA) before library preparation, and only samples with RNA integrity >9 were used for library construction. RNA concentration was determined by Qubit 2.0 Fluorometer (Invitrogen) using the RNA Assay Kit (Thermo Fisher Scientific, Waltham, MA). One to 3 μg total RNA was rRNA depleted using the RiboMinusTM Eukaryote System v2 (Thermo Fisher Scientific) following manufacturer protocols. The rRNA-depleted RNA (10–500 ng) was used to construct a library using the Ion Total RNASeq v2 Kit (Life Technologies) following the manufacturer protocol. The library was quality checked using the Agilent DNA High Sensitivity Chip on the Agilent Bioanalyzer, and quantitative PCR was run to ascertain the library concentration. Samples were further diluted to 50–100 pmol/L and pooled in equimolar concentrations followed by being loaded into the Ion Chef for emulsion, enrichment, and chip loading. The chips were sequenced on the Ion S5 XL System (Thermo Fisher Scientific).
RNA Sequencing Alignment and Differential Expression
Data were initially trimmed using Trimmomatic38, Homo sapiens. The University of California Santa Cruz (UCSC) hg19 human genome from iGenome (UCSC Genome Browser Gateway) was used as the reference genome. The raw library was aligned using TMAP (v5.2.25) to a BED file containing nonoverlapping exons from the UCSC genome hg19. Exon counts were calculated using HT-Seq (v0.6.0), and gene counts were generated by summing counts across exons. Samples were normalized for library size using edgeR, and low-expressing genes were excluded from the final analysis. Principal component analysis was performed using edgeR and DESeq. edgeR was used for the differential expression (crosswise comparison between the groups). Comparison was made between the AGE-treated group and control group using the glm method, with an adjusted P value cutoff of 0.05 and a minimum requirement of twofold change (15).
Biological Pathway Analysis
Molecular functions and pathways were compiled from differentially expressed genes (DEGs) using the KEGG database. After submission of a file containing gene lists, fold change (≥2.0-fold change or ≤2.0-fold cutoff), and P values, enrichment analysis and network interaction were performed using the stepwise process.
Ly6G Antibody Intraperitoneal and Recombinant CXCL1 Intravitreal Injections
Male C57BL/6J mice (The Jackson Laboratory) age between 4 and 6 weeks were used for all in vivo validation experiments. All animal studies were consistent with and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were performed in accordance with University of New Mexico Health Sciences Center animal care and use guidelines. We used 10 animals per treatment group.
For a period of 10 days, 50 μg 1A8 Ly6G (lymphocyte antigen 6 complex locus G6D) antibody diluted in InVivoPure pH 7.0 Dilution Buffer (Bio X Cell) was injected intraperitoneally (i.p.) daily. For intraocular injections, these pretreated animals received 5 μL sterile water (vehicle control) or 5 μL recombinant CXCL1 (C-X-C motif chemokine ligand 1; 100 ng per eye; PeproTech, Rocky Hill, NJ) under isoflurane anesthesia. Animals were euthanized 24 h after intraocular injections, and retinas were isolated for experiments.
For the induction of diabetes, male C57BL/6J mice received five daily consecutive i.p. injections of streptozotocin (STZ; 50 mg/kg per day) in 10 mmol/L citrate buffer (pH 4.5). Animals with plasma glucose concentrations >250 mg/dL 24–48 h after STZ injection were considered diabetic and used in the study. Blood glucose level and body weight were monitored regularly. Animals received insulin (0.5 IU) as needed to maintain body weight. Age-matched nondiabetic animals received citrate buffer alone and were used as comparison controls. For neutrophil depletion experiments, 50 μg 1A8 Ly6G antibody was administered i.p. to the animals every other day for 15 days starting at 10 weeks of diabetes.
Flow Cytometric Analysis of Infiltrated Neutrophils and Monocytes
Retinas from all treated groups were digested with 0.5 mg/mL collagenase D (Roche/Sigma-Aldrich, St Louis, MO) for 30 min at 37°C and 100 mg/mL DNase I and 1 mg/mL RNAse inhibitor in enzyme-free cell dissociation buffer (Life Technologies, Carlsbad, CA). The tissue digest was then filtered through a 70-μm cell strainer and washed with DMEM with 5% FBS for 5 min at 400g at 4°C. The supernatant was carefully removed, and the digested tissue pellet was resuspended in DMEM with 5% FBS to form a single-cell suspension. Cell viability was determined by trypan blue staining method. Viable single-cell suspension was then surface stained using fluorescently conjugated monoclonal antibodies CD11b-PE, Ly6G (Gr-1)-Alexa Fluor700, and Ly6C-APC-eFluor780 (eBioscience, San Diego, CA) for 30 min at 4°C. The cell suspension was washed with DMEM with 5% FBS for 5 min at 400g at 4°C, followed by resuspension in DMEM with 5% FBS. The Attune NxT System was used to sort and analyze the cell suspension.
RT-PCR
Total RNA was isolated from mouse retinas and treated cells using the RNeasy Mini Kit (Qiagen) and converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific), followed by gene expression analysis of specific genes using TaqMan assay probes (Life Technologies) in the 7500 ABI Fast Realtime PCR System (Applied Biosystems, Foster City, CA). Relative levels of mRNA were determined by the comparative cycle threshold method, with normalization to 18S mRNA.
Western Blot
Mouse retinas were lysed in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors followed by centrifugation at 12,000 rpm for 10 min at 4°C. Protein concentration was determined by Pierce BCA Protein Assay as per the manufacturer protocol. Equal amounts of protein were loaded and separated in precast TGX gels (Bio-Rad, Hercules, CA), transferred to nitrocellulose membranes, and probed with anti-rabbit albumin antibody (Abcam, Eugene, OR), followed by incubation with fluorescently labeled secondary antibody. Protein levels were normalized to β-tubulin (Abcam). Bands were visualized using the LI-COR Biosciences Odyssey Imaging System (Lincoln, NE), and band intensity was measured using Image J software.
Electrical Cell-Substrate Impedance Sensing
Monolayer permeability was determined using the electrical cell-substrate impedance sensing (ECIS) system from Applied Biophysics (Troy, NY). HRECs (1 × 105) were plated into fibronectin-coated multiwell chambers (8W10E+) and grown for 16 h until maximum resistance was attained (1,200 Ω). Cells were then treated with appropriate conditions, and resistance was observed for up to 18 h. Resistance values for multiple wells were normalized to an identical starting resistance value and averaged and presented as normalized resistance over time.
ELISA
Quantification of CXCL1 in human serum samples from patients with DR (n = 10 [n = 8 patients with DME and n = 2 patients with proliferative DR) and control participants without diabetes (n = 10; BioIVT, Baltimore, MD, USA) was determined by ELISA (Abcam) as per the manufacturer protocol.
Statistical Methods
For all quantitative experiments, statistical analyses were performed with an unpaired Student t test or a one-way ANOVA (Prism4 software; GraphPad). Differences indicated by ANOVA were compared by the Newman-Keuls test. The value of P < 0.05 was considered significant.
Data and Resource Availability
The data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
RNA Sequencing Shows Differential Gene Expression in HRECs Exposed to AGEs
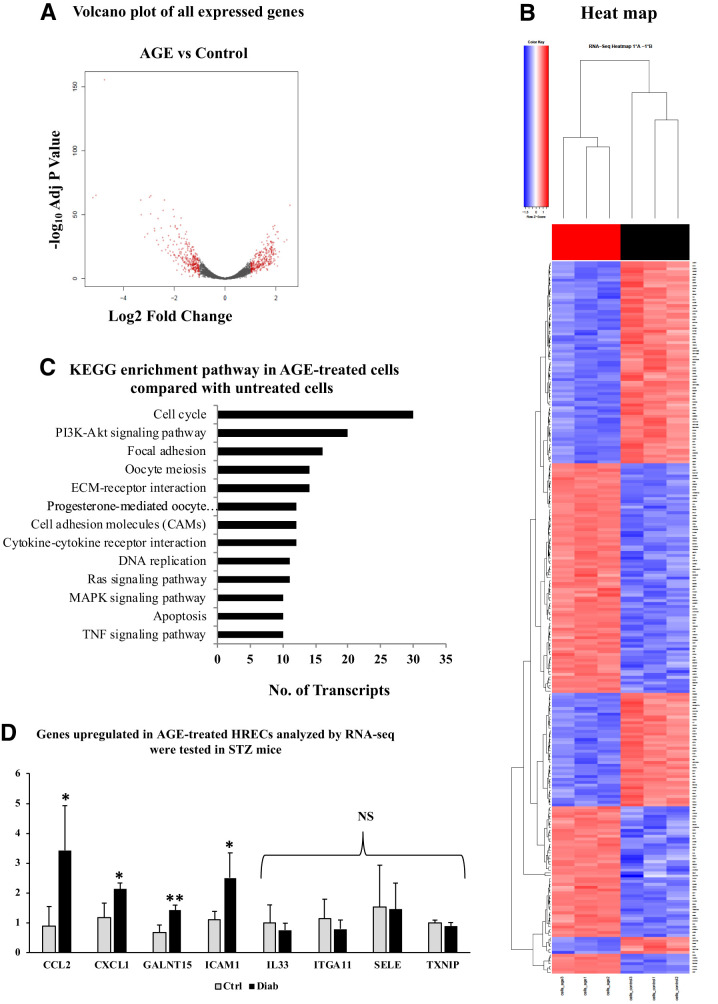
RNA sequencing on HRECs exposed to 250 μg AGE for 96 h revealed that 2,978 genes were significantly downregulated and 3,586 genes were upregulated in AGE-treated cells compared with untreated cells. The volcano plot describing the significantly up- and downregulated genes in AGE-treated cells versus control cells is shown in Fig. 1A. A hierarchically clustered heat map revealed a significantly different expression pattern between AGE-treated cells with untreated (control) cells (Fig. 1B).
Figure 1.
A: Volcano plot of fold change of transcripts derived using edgeR. AGE-treated HRECs compared with control. Red circles at the left side of the plot indicate downregulated genes, and those at the right side indicate upregulated genes. B: Hierarchical cluster heat map of DEGs in AGE-treated and control (untreated) cells. C: KEGG signaling enrichment pathways with genes of fold change of 2 and P value of ≤0.05 in AGE-treated retinal endothelial cells compared with control. D: RT-PCR validation of top 10 upregulated genes from the RNA sequencing (RNA-seq) data on STZ-induced diabetic (2 months of diabetes) and nondiabetic mouse retinas (n = 5 each). Relative gene expression levels of CCL2, CXCL1, GALNT15, and ICAM1 were significantly increased in the retinas of diabetic mice in comparison with those of nondiabetic mice. mRNA levels of IL33, ITGA11, SELE, and TXNIP did not show any significant change in the diabetic retinas compared with nondiabetic retinas. All genes were normalized to 18S. Data are presented as mean ± SD. Diab, positive for diabetes; ECM, extracellular matrix; MAPK, mitogen-activated protein kinase; NS, nonsignificant; TNF, tumor necrosis factor. *P < 0.05, **P < 0.01.
Functional Pathway and Genes by Protein Function Show Top 10 Upregulated Genes
Further dissection of these DEGs using the KEGG database revealed that 13 pathways were enriched (Fig. 1C). Among these, many of the pathways were predominantly associated with DR pathogenesis and disease progression, such as PI3K-Akt signaling, cytokine-cytokine receptor interaction, cell adhesion molecules, extracellular matrix–receptor interaction, adherens junction, tight junction, and focal adhesion. Table 1 shows the top 10 down- and upregulated genes in the different treatment groups. Many of the upregulated genes in the treatment group are related to inflammation and leukostasis, such as SELE (selectin E), CCL2, CXCL1, and ICAM1 (intercellular adhesion molecule 1), which are related to DR pathogenesis. The high distribution of the genes and pathways toward inflammation shows that chronic hyperglycemia leads to the formation of AGEs that participate in the triggering of inflammatory signals, which paves the way for the development and worsening of DR.
Table 1.
Top 10 down- and upregulated genes in AGE-treated retinal endothelial cells compared with untreated cells
| ID | Symbol | Fold change | P | Adjusted P |
|---|---|---|---|---|
| Downregulated | ||||
| NM_014762 | DHCR24 | 0.170596 | 5.34E−61 | 5.69E−58 |
| NM_001001557 | GDF6 | 0.186286 | 2.43E−33 | 4.40E−31 |
| NM_001190481 | CLSPN | 0.199349 | 1.33E−31 | 1.99E−29 |
| NM_001190819 | ORC1 | 0.228472 | 9.77E−24 | 6.56E−22 |
| NM_021062 | HIST1H2BB | 0.235626 | 3.01E−34 | 5.97E−32 |
| NM_005192 | CDKN3 | 0.236398 | 1.85E−19 | 7.69E−18 |
| NM_001255 | CDC20 | 0.236876 | 5.86E−40 | 1.78E−37 |
| NM_003535 | HIST1H3J | 0.240679 | 1.06E−11 | 1.84E−10 |
| NM_005733 | KIF20A | 0.242997 | 1.07E−39 | 3.04E−37 |
| NM_021066 | HIST1H2AJ | 0.249445 | 1.34E−21 | 7.19E−20 |
| Upregulated | ||||
| NM_000450 | SELE | 36.68773 | 3.45E−67 | 5.89E−64 |
| NM_054110 | GALNT15 | 33.79375 | 2.20E−69 | 9.37E−66 |
| NM_002982 | CCL2 | 26.76679 | 3.75E−160 | 3.20E−156 |
| NM_020163 | SEMA3G | 9.93811 | 4.66E−65 | 5.75E−62 |
| NM_033439 | IL33 | 9.836069 | 1.84E−53 | 1.31E−50 |
| NM_181482 | LDLRAD4 | 8.915839 | 1.46E−35 | 3.18E−33 |
| NM_001004439 | ITGA11 | 8.254615 | 4.06E−38 | 1.12E−35 |
| NM_006472 | TXNIP | 7.839528 | 1.60E−67 | 3.42E−64 |
| NM_001511 | CXCL1 | 7.620618 | 3.93E−54 | 3.05E−51 |
| NM_000201 | ICAM1 | 7.5609 | 6.04E−69 | 1.72E−65 |
Gene Validation in Diabetic Mouse Retinas Reveals Upregulated CCL2, CXCL1, GALNT15, and ICAM1 Genes
We then chose to cross-validate the top 10 upregulated genes from the transcriptomic analysis, comparing the retinas of mice that had STZ-induced diabetes for 2 months against nondiabetic mouse retinas. Of the 10 genes, two did not show any expression. Of the remaining eight genes, four showed significantly increased expression in the diabetic retinas compared with the nondiabetic mouse retinas: CCL2 (P < 0.0001), CXCL1 (P < 0.05), GALNT15 (polypeptide N-acetylgalactosaminyltransferase 15; P < 0.01), and ICAM1 (P < 0.05) (Fig. 1D). For further functional validation of the genes, we narrowed our analysis down to CXCL1, a chemokine that is a chemoattractant for neutrophils and has effects on endothelial cells in an autocrine manner. Our laboratory has already shown the impact of CCL2, which attracts monocytes, on retinal vascular permeability alteration (3).
CXCL1 in Combination With Interleukin-1α Shows Increased Permeability in Endothelial Cells In Vitro
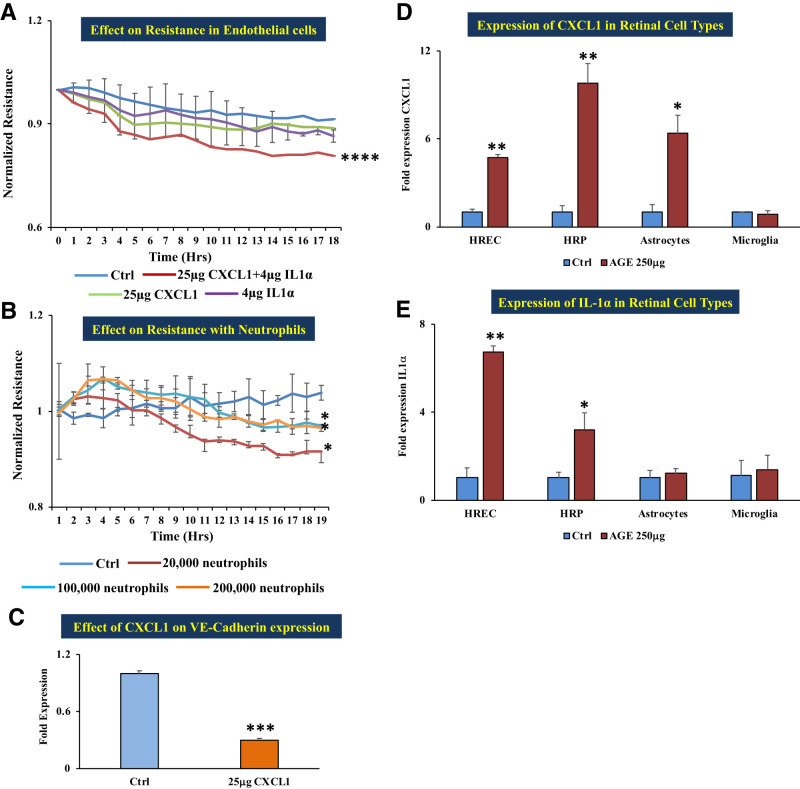
The ECIS system to measure changes in electrical resistance revealed that HRECs treated with CXCL1 alone or with interleukin-1α (IL-1α) alone did not show any change in cell monolayer resistance. However, cells treated with CXCL1 and IL-1α in combination showed significantly decreased electrical resistance (which implies increased permeability) compared with untreated cells (P < 0.0001) (Fig. 2A). Human neutrophils pretreated with 250 μg AGE for 24 h and then layered on the HREC monolayer showed increased permeability in a dose-dependent manner (20,000 to 200,000 cells per well) in comparison with untreated cells (Fig. 2B). The decreased resistance observed in the HREC monolayer treated with the CXCL1–IL-1α combination was correlated with significantly decreased VE-cadherin gene expression compared with untreated cells (Fig. 2C).
Figure 2.
In vitro functional studies of the effect of CXCL1 and neutrophils. A: HRECs treated with a combination of 25 μg recombinant CXCL1 plus 4 μg IL-1α showed significantly decreased resistance compared with cells grown on normal growth medium; cells treated with 4 μg IL-1α alone did not show any significant change in resistance in comparison with untreated cells (control). B: Coculture of HRECs with 250 μg AGE–pretreated human neutrophils of different numbers: 20,000, 100,000, and 200,000 cells showed a significant decrease in the monolayer resistance in comparison with untreated cells (control). Decreased resistance implies increased permeability. C: mRNA expression of adherent junction molecule VE-cadherin was significantly decreased with 25 μg recombinant CXCL1 treatment compared with untreated cells (control). D: mRNA expression of CXCL1 in different retinal cells in treatment with 250 μg AGE–treated HRECs, human retinal pericytes (HRPs), and human retinal astrocytes (HRAs) were significantly increased in comparison with cells grown on respective normal growth medium (control), except microglia. E: mRNA expression of IL-1α in different retinal cells in treatment with 250 μg AGE. HRECs and HRPs were significantly increased in comparison with cells grown on respective normal growth medium (control), except HRAs and microglia. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
CXCL1 Is Produced by Endothelial Cells, Pericytes, and Astrocytes
We treated four retinal cell types (endothelial cells, pericytes, astrocytes, and microglia) with 250 μg AGE. CXCL1 expression was significantly increased in endothelial cells (P < 0.01), pericytes (P < 0.01), and astrocytes (P < 0.05) compared with expression in untreated cells, whereas microglial cells showed no significant change in expression of CXCL1 (Fig. 2D). IL-1α expression was significantly increased in endothelial cells (P < 0.01) and pericytes (P < 0.05) compared with expression in untreated cells, whereas astrocytes and microglial cells showed no significant change (Fig. 2E).
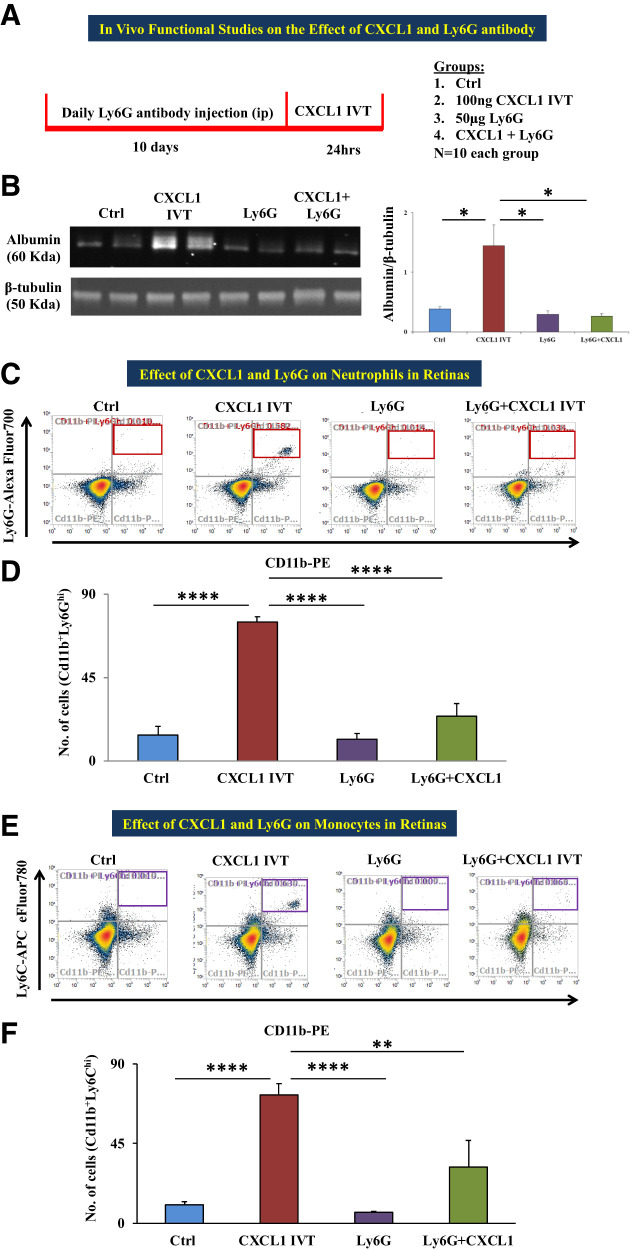
Intravitreal CXCL1 Injection Leads to Increased Retinal Vascular Permeability and Infiltration of Neutrophils and Monocytes in Retinas
Our in vitro studies using neutrophils and recombinant CXCL1 led to further investigation of the role of CXCL1 in an in vivo model. We used four different groups: 1) vehicle-treated animals (control), 2) animals receiving intravitreal injection of recombinant CXCL1 (CXCL1 IVT), 3) animals treated with Ly6G antibody for 10 days (Ly6G), and 4) Ly6G antibody–pretreated animals receiving intravitreal injection of recombinant CXCL1 (Ly6G + CXCL1 IVT).
Vascular Permeability and Immune Cell Infiltration Assessment
Retinas of CXCL1 IVT mice had a significantly increased level of albumin (P = 0.05) as measured by Western blot in comparison with those of control animals. Albumin band intensity was significantly less in Ly6G + CXCL1 IVT (P < 0.05) groups in comparison with CXCL1 IVT mice (Fig. 3A). We checked immune cell infiltration of immune cells, in particular, neutrophils (Cd11b+/Ly6Ghi) and monocytes (Cd11b+/Ly6Chi), using flow cytometric analysis. The numbers of neutrophils and monocytes were significantly increased in the retinas of CXCL1-treated animals compared with in vehicle-injected mice (P < 0.0001 for both neutrophils and monocytes). For Ly6G-pretreated animals, CXCL1 injection did not cause the infiltration of neutrophils or monocytes (Fig. 3C and E). Representative flow cytometric dot plots for neutrophils and monocytes of all the conditions are given in Fig. 3B and D.
Figure 3.
In vivo functional studies of the effect of CXCL1 and Ly6G antibody. A: A schematic representation of the experimental design and groups are depicted. B: Representative Western blot images of albumin and β-tubulin; the right-hand side histogram shows band intensity of albumin, a marker to determine vascular permeability, which showed significantly increased level in the retinas of the animals that received an intravitreal injection of 5 μL of 100 ng recombinant CXCL1 (CXCL1 IVT) compared with animals that received vehicle injection (distilled water); the albumin level was significantly decreased in the retinas of animals treated with 50 μg Ly6G or Ly6G + CXCL1 IVT in comparison with CXCL1 IVT animals. C: Representative dot plots of flow cytometric analysis for neutrophils (gated for Cd11b+/Ly6Ghi in red box at the top right quadrant) of differently treated animal groups: control (vehicle), CXCL1 IVT, Ly6G, and Ly6G + CXCL1 IVT. D: Total number of neutrophils was significantly increased in CXCL1 IVT animal retinas compared with vehicle-injected animal retinas; the neutrophil count was significantly decreased in Ly6G or Ly6G + CXCL1 IVT in comparison with CXCL1 IVT animals. E: Flow cytometric dot plots depict the gating of monocytes (Cd11b+Ly6Chi in purple box at the top right quadrant) in the four different animal groups as mentioned above. F: Monocyte count was significantly increased in the retinas of the CXCL1 IVT group compared with control retinas. Retinas of Ly6G- or Ly6G + CXCL1 IVT–treated groups had a significantly lower monocyte count in comparison with CXCL1 IVT animals. Data are presented as mean ± SD. *P = 0.05, **P < 0.01, ****P < 0.0001.
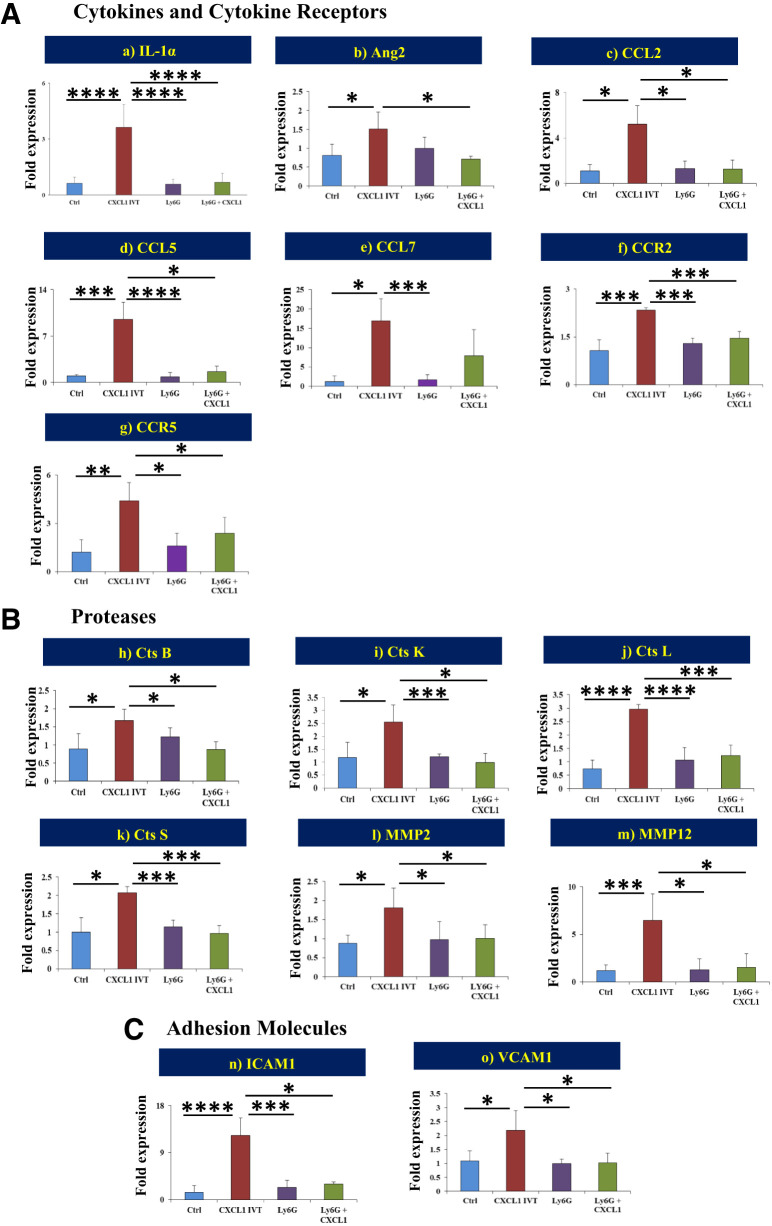
mRNA Expression of Proinflammatory Genes and Proteases After Intravitreal CXCL1 Injection
It is well recognized that vascular permeability and immune cell infiltration are mediated by the orchestration of certain inflammatory genes and their receptors, adhesion molecules, and proteases. The intravitreal injection of CXCL1 significantly increased the mRNA expression of cytokines and cytokine receptors IL-1α (P < 0.0001), Ang2 (P < 0.05), CCL2 (P < 0.01), CCL5 (P < 0.001), CCL7 (P < 0.05), CCR2 (P < 0.05), and CCR5 (P < 0.001) (Fig. 4Aa–g); proteases cathepsin B (P < 0.05), cathepsin K (P < 0.01), cathepsin S (P < 0.05), MMP2 (P < 0.005), and MMP12 (P < 0.001) (Fig. 4Bh–m); and adhesion molecules ICAM1 (P < 0.0001) and VCAM1 (P < 0.01) (Fig. 4Cn and o) compared with vehicle injection.
Figure 4.
Gene expression levels of proinflammatory cytokines, receptors, proteases, and adhesion molecules. mRNA expression levels of multiple genes from cytokines and cytokine receptors (A) [IL-1α (a), Ang2 (b), CCL2 (c), CCL5 (d), CCL7 (e), CCR2 (f), and CCR5 (g)], proteases (B) [cathepsin (Cts) B (h), Cts K (i), Cts L (j), Cts S (k), MMP2 (l), and MMP12 (m)], and adhesion molecules (C) [ICAM1 (n) and VCAM1 (o)] were statistically compared between the different treatment groups: 1) control versus CXCL1 IVT, 2) CXCL1 IVT versus Ly6G, and 3) CXCL1 IVT versus Ly6G + CXCL1 IVT. Data are presented as mean ± SD. *P < 0.05, ***P < 0.001, ****P < 0.0001.
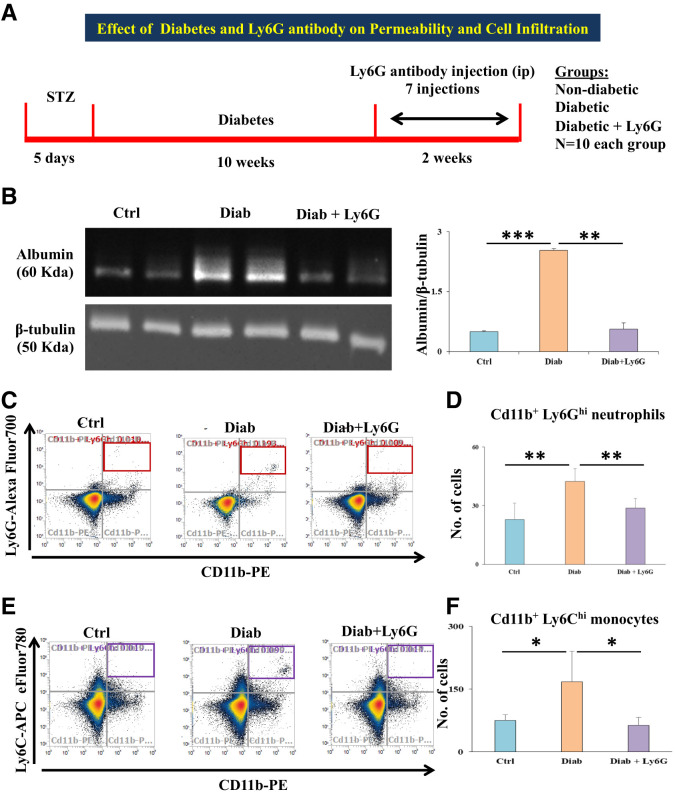
Diabetic Animals Show Increased Retinal Vascular Permeability and Infiltration of Neutrophils and Monocytes
Vascular Permeability and Immune Cell Infiltration Assessment
Retinas of diabetic animals had a significantly increased albumin level, showing increased vascular permeability, compared with those of nondiabetic animals (P = 0.0002), and treatment with Ly6G antibody significantly reduced the albumin level in diabetic animals (P < 0.01) (Fig. 5A). Retinas with diabetes alone showed high neutrophils (Cd11b+/Ly6Ghi; P < 0.01) (Fig. 5C) and high monocytes (Cd11b+/Ly6Chi; P < 0.05) (Fig. 5E) compared with nondiabetic controls. Ly6G antibody treatment in diabetic animals significantly reduced the infiltration of neutrophils (P < 0.01) and monocytes (P < 0.05). Representative flow cytometric dot plots for neutrophils and monocytes of all the conditions are given in Fig. 5B and D.
Figure 5.
A: Schematic representation of the experimental design and groups are depicted. B: Representative Western blot images of albumin and β-tubulin; the histogram on the right-hand side shows significantly increased albumin level in the retinas of diabetic animals compared with that in nondiabetic animals. Meanwhile, the albumin level was significantly decreased in diabetic + Ly6G-treated animal retinas compared with diabetic animal retinas. C: Representative dot plots of flow cytometric analysis for neutrophils (gated for Cd11b+/Ly6Ghi in red box at the top right quadrant) of different experimental groups: control (vehicle), diabetic, and diabetic + Ly6G. D: Total number of neutrophils was significantly increased in the retinas of diabetic animals compared with nondiabetic animal retinas; the neutrophil count was significantly decreased in Ly6G antibody–treated diabetic animal retinas compared with retinas of diabetic animals that did not receive this treatment. E: Representative dot plots of flow cytometric analysis for monocytes (gated for Cd11b+/Ly6Chi in purple box at the top right quadrant) of different experimental groups. F: Total number of monocytes was significantly increased in the retinas of diabetic animals compared with nondiabetic (control) animal retinas. Ly6G antibody treatment in diabetic animals significantly reduced the influx of both monocytes compared with in diabetic animals that did not receive this treatment. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Diab, positive for diabetes.
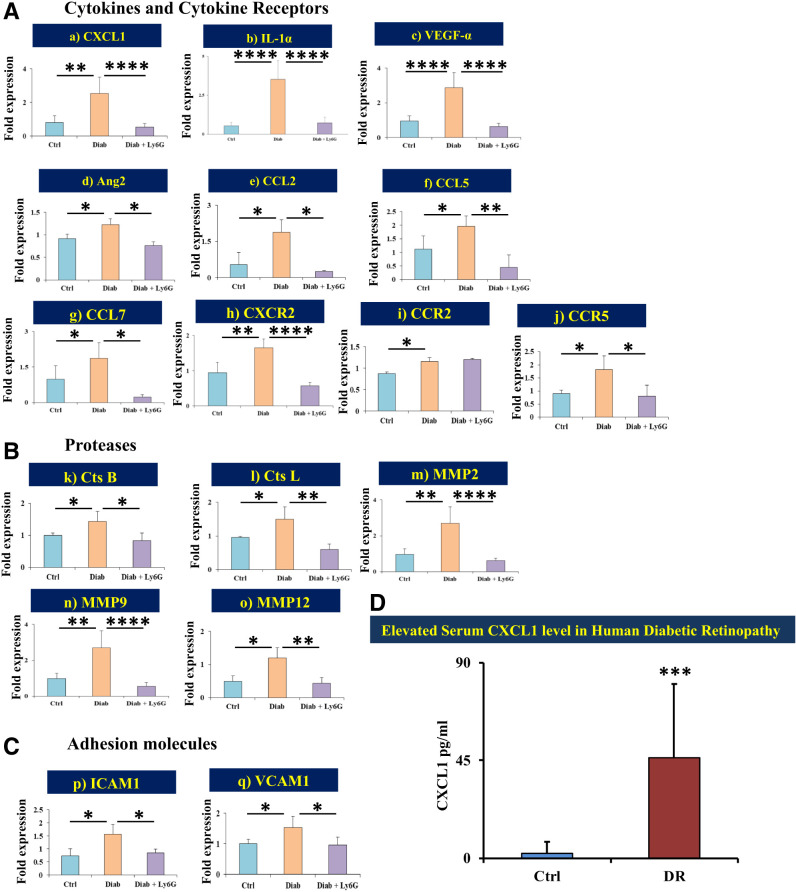
mRNA Expression of Proinflammatory Genes and Proteases in Retinas of Diabetic Mice
Cytokines and cytokine receptors CXCL1 (P < 0.01), IL-1α (P < 0.0001), VEGFα (P < 0.0001), Ang2 (P < 0.05), CCL2 (P < 0.05), CCL5 (P < 0.05), CCL7 (P < 0.05), CXCR2 (P < 0.01), CCR2 (P < 0.05), and CCR5 (P < 0.05) (Fig. 6Aa–j); proteases cathepsin B (P < 0.05), cathepsin L (P < 0.05), MMP2 (P < 0.005), MMP9 (P < 0.005), and MMP12 (P < 0.05) (Fig. 6Bj–o); and adhesion molecules ICAM1 (P < 0.05) and VCAM1 (P < 0.05) (Fig. 6Cp and q) were significantly upregulated in the retinas of diabetic animals compared with those of nondiabetic animals.
Figure 6.
Gene expression levels of proinflammatory cytokines, receptors, proteases, and adhesion molecules. mRNA expression levels of multiple genes from cytokines and cytokine receptors (A) [CXCL1 (a), IL-1α (b), VEGF-α (c), Ang2 (d), CCL2 (e), CCL5 (f), CCL7 (g), CXCR2 (h), CCR2 (i), and CCR5 (j)], proteases (B) [cathepsin (Cts) B (k), Cts L (l), MMP2 (m), MMP9 (n), and MMP12 (o)], and adhesion molecules (C) [ICAM1 (p) and VCAM1 (q)] were statistically compared between the different treatment groups: 1) control versus diabetes and 2) diabetic versus diabetic + Ly6G. D: Human serum CXCL1. Age-adjusted mean values of CXCL1 level measured by ELISA from human serum samples showed a significantly elevated level in patients with DR compared with participants without diabetes. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Diab, positive for diabetes.
Neutrophils Are Essential in Alteration of the BRB and Expression of Proinflammatory Genes
Ly6G antibody was administered i.p. to deplete animals of neutrophils (16,17). Retinal vascular permeability was significantly reduced in animals treated with Ly6G+ CXCL1 IVT (P < 0.05) in comparison with CXCL1 IVT (Fig. 3A and B). For Ly6G-pretreated animals, CXCL1 IVT injection did not cause the infiltration of neutrophils or monocytes (Fig. 3C and E) in retinas. Ly6G antibody had a profound effect in significantly reducing expression levels of cytokines and cytokine receptors, proteases, and adhesion molecules in the retinas (Fig. 4A–C). In diabetic animals, Ly6G antibody treatment significantly reduced albumin leakage in retinas (P < 0.01) (Fig. 5A and B) and also significantly reduced the infiltration of neutrophils (P < 0.01) and monocytes (P < 0.05) (Fig. 5C and E). Diabetic animals exposed to Ly6G antibody treatment showed significantly lower expression of cytokines and cytokine receptors, proteases, and adhesion molecules, with the exception of CCR2 (Fig. 6A–C). These results strongly suggest that neutrophils are essential in BRB alteration in both CXCL1 IVT–treated and diabetic mice.
Increased Serum Level of CXCL1 Is Seen in Patients With DR
We measured the serum level of CXCL1 in patients with DR (n = 10 [n = 8 patients with DME and n = 2 patients with proliferative DR) and control participants without diabetes (n = 10) by ELISA. Serum samples from patients with DR had a significantly higher level of CXCL1 (46.3 ± 33.9 pg/mL) compared with those from healthy participants (2.3 ± 5.3 pg/mL; P < 0.001) (Fig. 6D).
Discussion
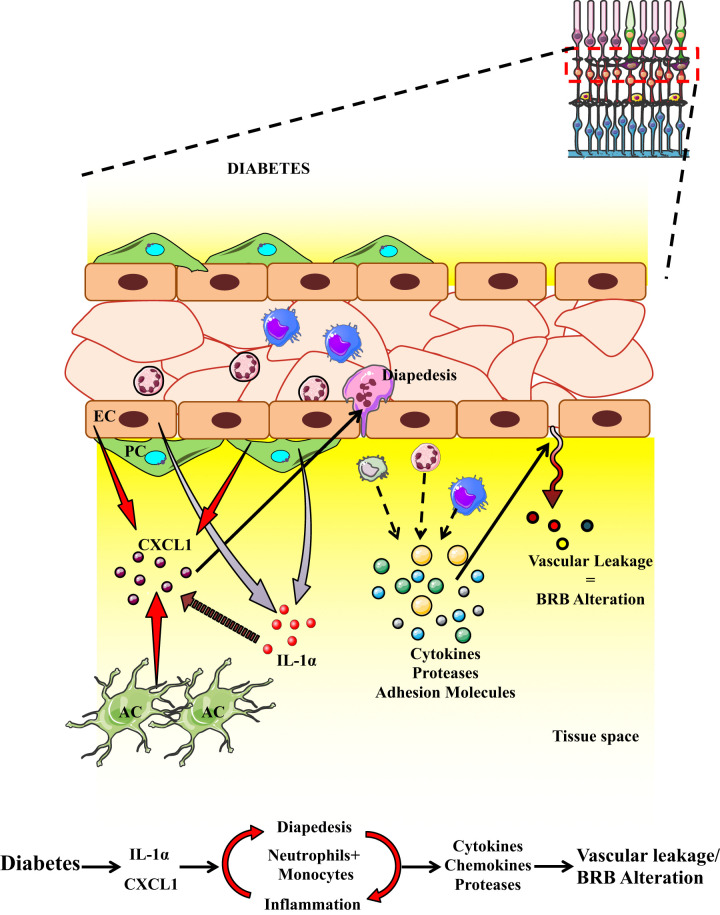
In the current study, the transcriptomic profiling of retinal endothelial cells treated with AGEs revealed upregulated genes and pathways related to inflammation and leukostasis. Further validation of the top 10 upregulated genes showed significant increases in CCL2, CXCL1, ICAM1, and GALNT15 in retinal tissues of diabetic animals. Of the four upregulated genes, we chose to study CXCL1 and its effects on vascular permeability and BRB alteration, because the other two genes CCL2 and ICAM1 have been well studied by our group and others (3,18–20). GALNT15 is a protein modification gene. Both animals intravitreally injected with CXCL1 and diabetic animals showed significantly increased retinal vascular permeability, with infiltration of neutrophils and monocytes in retinas, as well as increased expression of chemokines and receptors, proteases, and adhesion molecules. Ly6G antibody treatment for neutrophil depletion in both in vivo models showed significantly decreased retinal vascular permeability, decreased infiltration of neutrophils and monocytes, and decreased expression of cytokines and proteases. The role of CXCL1 in DR was further confirmed by the significantly elevated level of CXCL1 in the serum of human patients with DR. Thus, our work confirms that CXCL1 plays a significant role in neutrophil and monocyte infiltration and alteration of the BRB in diabetes, and targeting the chemokine CXCL1 may be a robust strategy in patients with DME (Fig. 7).
Figure 7.
Schematic diagram highlighting the effect of CXCL1 and neutrophils. Increased neutrophil and monocyte presence leads to expression of chemokines, cytokines, and proteases, which leads to increased endothelial permeability and alteration of the BRB.
We performed next-generation transcriptomic analysis of retinal endothelial cells sequenced at a high depth, which enabled quantification of DEGs with high confidence and allowed us to uncover novel genes that play an important role in endothelial dysfunction and BRB alteration. Our recent study of transcriptomics in retinas from diabetic animals validated one of the downregulated genes, Notch 3, and identified its role in pericyte dysfunction in diabetes (14). Other transcriptomic studies in STZ animal models have shown physiologically relevant disease-associated pathways, such as inflammation, apoptosis, microvasculature formation, and glucose metabolism (21). A few human studies have also used transcriptomic analysis to reveal key regulators in DR (22,23).
CXCL1 belongs to the CXC family of chemokines (24). It is a functional homolog of human IL-8 and functions in the recruitment and mobilization of neutrophils to the site of infection (25,26). CXCL1 acts via receptor CXCR1 or CXCR2 on neutrophils (27). Our in vitro data show the effect of CXCL1 or neutrophils on vascular permeability in retinal endothelial cells. Although CXCL1 independently reduced the gene expression of VE-cadherin, the endothelial permeability changes required the participation of IL-1α, a known activator of neutrophil migration (28). These functional changes in the cell behavior dictated by CXCL1 and IL-1α were well correlated with the expression pattern of both cytokines on retinal endothelial cells and pericytes when exposed to AGEs. Our study additionally shows neutrophils are one of the primary sources of IL-1α, as evidenced by our ECIS experiments, where AGE-pretreated neutrophils induced vascular permeability on the endothelial monolayer, and sheds light on the coordination of CXCL1–IL-1α in inflammation-mediated vascular permeability. In an animal model, Michael et al. (28) showed the involvement of IL-1α in inducing the production of CXCL1 in a paracrine fashion required for neutrophil transendothelial migration, leading to the breakdown of the blood-brain barrier.
Our in vivo data from diabetic animals treated with Ly6G antibody show a significant decrease in vascular permeability and infiltration of neutrophils and monocytes in diabetic retinas as well as retinas after intravitreal CXCL1 injection in wild-type mice. These functional changes correlated with the molecular changes in the form of decreased expression of proinflammatory cytokines and receptors, especially CXCL1, IL-1α, and VEGF, and proteases and adhesion molecules, resulting in increased vascular permeability. All these findings from Ly6G experiments indicate that neutrophils are essential in the alteration of the BRB in DR. Neutrophils are complex cells with multifunctional capability, including innate immune response, regulation of acute injury and repair, cancer, autoimmunity, and chronic inflammation (29). The role of neutrophils in DR has been shown by the presence of an increased number of polymorphonuclear leukocytes in the retinas of diabetic monkeys (30). Furthermore, neutrophil elastase (31,32) and IL-17α (33) have been shown to contribute to vascular leakage in DR, and neutrophil inhibitory factor has been shown to inhibit early stages of DR (34). Although previous studies have emphasized the role of neutrophils in DR, we took the novel approach of transcriptomics of in vitro retinal endothelial cells and found that CXCL1 is one of the top upregulated genes. Our findings provide strong evidence to conclude that the chemokine CXCL1 is a strong mediator in the retinal vascular permeability in BRB alteration in DR and, therefore, can be targeted as a therapeutic strategy.
Ly6G is a component of the myeloid differentiation antigen Gr-1, together with Ly6C, and is a good marker for detection of peripheral neutrophils, monocytes, and granulocytes. Although the currently experimental evidence shows that Ly6G antibody specifically depletes neutrophils alone (17), we found that in addition to neutrophils, it also decreased monocyte count. Neutrophils have been described to regulate the release of chemoattractants, which helps in the recruitment of other immune cells, in particular, monocytes/macrophages (35). Thus, neutrophils and monocytes/macrophages work together closely to enhance the immune response and regulate other key immune cells, which further leads to downstream inflammatory processes (36).
In summary, we provide evidence that the chemokine CXCL1, through neutrophil recruitment, plays an important role in the alteration of the BRB in DR. By targeting CXCL1, it is possible to inhibit the triggering of the inflammatory mechanism and the downstream cascade of cytokine release and retinal vascular leakage. Recently, at least two clinical trials reported the use of an oral CXCR1/CXCR2 inhibitor in breast cancer (37) and COVID-19 pneumonia (38). A comparative study of the efficacy of CXCL1 inhibition and standard anti-VEGF therapy will be the logical next step in evaluating the efficacy and potential translational impact of CXCL1 targeting in DME. With the emerging advancements in antibody engineering for therapeutics, a combination therapy of anti-CXCL1 and anti-VEGF drugs for the treatment of DR is highly achievable and may offer a novel therapy for DME.
Article Information
Acknowledgments. The authors thank the Analytical and Translational Genomics Shared Resource of the University of New Mexico for helping with RNA sequencing analysis.
Funding. This study was supported by grants from the National Eye Institute, National Institutes of Health (RO1 EY028606-A1 and EY022327), and a VA Merit Review Award (I0BX005348).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.M. wrote the manuscript. F.M., G.A., and A.P.C. conducted the experiments and interpreted the data. F.M. and A.D. designed and conducted the study and contributed to discussion and approval of the manuscript. A.P.C. contributed to the review and editing of the manuscript. A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, 1–7 May 2021, and the ARVO Annual Meeting, Denver, CO, 1–4 May 2022.
References
- 1. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 2011;30:343–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 2015;122:1375–1394 [DOI] [PubMed] [Google Scholar]
- 3. Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 2014;9:e108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 2007;56:2380–2387 [DOI] [PubMed] [Google Scholar]
- 5. Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci 2011;52:3784–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monickaraj F, McGuire PG, Nitta CF, Ghosh K, Das A. Cathepsin D: an Mϕ-derived factor mediating increased endothelial cell permeability with implications for alteration of the blood-retinal barrier in diabetic retinopathy. FASEB J 2016;30:1670–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monickaraj F, McGuire P, Das A: Cathepsin D plays a role in endothelial-pericyte interactions during alteration of the blood-retinal barrier in diabetic retinopathy. FASEB J 2018;32:2539–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002;133:70–77 [DOI] [PubMed] [Google Scholar]
- 9. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017;237:185–222 [DOI] [PubMed] [Google Scholar]
- 10. Bressler NM, Beaulieu WT, Maguire MG, et al.; Diabetic Retinopathy Clinical Research Network . Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol 2018;195:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baker CWGA, Glassman AR, Beaulieu WT, et al.; DRCR Retina Network . CR t: Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA 2019;321:1880–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown DM, Nguyen QD, Marcus DM, et al.; RIDE and RISE Research Group . Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120:2013–2022 [DOI] [PubMed] [Google Scholar]
- 13. Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, Kanda T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-β by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging 2013;34:1902–1912 [DOI] [PubMed] [Google Scholar]
- 14. Rangasamy S, Monickaraj F, Legendre C, et al. Transcriptomics analysis of pericytes from retinas of diabetic animals reveals novel genes and molecular pathways relevant to blood-retinal barrier alterations in diabetic retinopathy. Exp Eye Res 2020;195:108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov 2016;6:176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis RW 4th, Snyder E, Miller J, et al. Luminol chemiluminescence reports photodynamic therapy-generated neutrophil activity in vivo and serves as a biomarker of therapeutic efficacy. Photochem Photobiol 2019;95:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015;522:345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monickaraj F, Oruganti SR, McGuire P, Das A. A potential novel therapeutic target in diabetic retinopathy: a chemokine receptor (CCR2/CCR5) inhibitor reduces retinal vascular leakage in an animal model. Graefes Arch Clin Exp Ophthalmol 2021;259:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 2001;158:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 21. Kandpal RP, Rajasimha HK, Brooks MJ, et al. Transcriptome analysis using next generation sequencing reveals molecular signatures of diabetic retinopathy and efficacy of candidate drugs. Mol Vis 2012;18:1123–1146 [PMC free article] [PubMed] [Google Scholar]
- 22. Skol AD, Jung SC, Sokovic AM, et al.; DCCT/EDIC Study group . Integration of genomics and transcriptomics predicts diabetic retinopathy susceptibility genes. eLife 2020;9:e59980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang JH, Wong RCB, Liu GS. Retinal transcriptome and cellular landscape in relation to the progression of diabetic retinopathy. Invest Ophthalmol Vis Sci 2022;63:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geoffrey R, Pugh-Humphreys P, Thomson AW. Cytokines and their receptors as potential therapeutic targets. In The Cytokine Handbook. Thompson A, Ed. San Diego, CA, Academic Press, Inc., 1994, pp. 525–566 [Google Scholar]
- 25. Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 1989;84:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parkunan SM, Randall CB, Astley RA, Furtado GC, Lira SA, Callegan MC. CXCL1, but not IL-6, significantly impacts intraocular inflammation during infection. J Leukoc Biol 2016;100:1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sawant KV, Poluri KM, Dutta AK, et al. Chemokine CXCL1 mediated neutrophil recruitment: role of glycosaminoglycan interactions. Sci Rep 2016;6:33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michael BD, Bricio-Moreno L, Sorensen EW, et al. Astrocyte- and neuron-derived CXCL1 drives neutrophil transmigration and blood-brain barrier permeability in viral encephalitis. Cell Rep 2020;32:108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev 2019;99:1223–1248 [DOI] [PubMed] [Google Scholar]
- 30. Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA. Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes 2005;54:1534–1542 [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Lessieur EM, Saadane A, Lindstrom SI, Taylor PR, Kern TS. Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy. Diabetologia 2019;62:2365–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lessieur EM, Liu H, Saadane A, et al. Neutrophil-derived proteases contribute to the pathogenesis of early diabetic retinopathy. Invest Ophthalmol Vis Sci 2021;62:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sigurdardottir S, Zapadka TE, Lindstrom SI, et al. Diabetes-mediated IL-17A enhances retinal inflammation, oxidative stress, and vascular permeability. Cell Immunol 2019;341:103921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Veenstra AA, Tang J, Kern TS. Antagonism of CD11b with neutrophil inhibitory factor (NIF) inhibits vascular lesions in diabetic retinopathy. PLoS One 2013;8:e78405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chertov O, Ueda H, Xu LL, et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med 1997;186:739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar V. Dendritic cells in sepsis: potential immunoregulatory cells with therapeutic potential. Mol Immunol 2018;101:615–626 [DOI] [PubMed] [Google Scholar]
- 37. Goldstein LJ, Perez RP, Yardley D, et al. A window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res 2020;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meizlish ML, Pine AB, Bishai JD, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv 2021;5:1164–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]