Abstract
Background and Objective
Discontinuation of fingolimod ≥2 months before pregnancy is recommended to minimize potential teratogenicity. The magnitude of MS pregnancy relapse risk, particularly severe relapses, after fingolimod cessation is unclear, as is whether this risk is reduced by pregnancy or modifiable factors.
Methods
Pregnancies who stopped fingolimod treatment within 1 year before or during pregnancy were identified from the German MS and Pregnancy Registry. Data were collected through structured telephone-administered questionnaires and neurologists' notes. Severe relapses were defined as a ≥2.0 increase in Expanded Disability Status Scale (EDSS) or new or worsening relapse-related ambulatory impairment. Women who continued to meet this definition 1 year postpartum were classified as reaching the Severe Relapse Disability Composite Score (SRDCS). Multivariable models accounting for measures of disease severity and repeated events were used.
Results
Of the 213 pregnancies among 201 women (mean age at pregnancy onset 32 years) identified, 56.81% (n = 121) discontinued fingolimod after conception. Relapses during pregnancy (31.46%) and the postpartum year (44.60%) were common. Nine pregnancies had a severe relapse during pregnancy and additional 3 during the postpartum year. One year postpartum, 11 of these (6.32% of n = 174 with complete EDSS information) reached the SRDCS. Adjusted relapse rates during pregnancy were slightly higher compared with the year before pregnancy (relapse rate ratio = 1.24, 95% CI 0.91–1.68). Neither exclusive breastfeeding nor resuming fingolimod within 4 weeks of delivery were associated with a reduced risk of postpartum relapses. Most pregnancies relapsed during the first 3 months postpartum (n = 55/204, 26.96%).
Discussion
Relapses during pregnancy after fingolimod cessation are common. Approximately 6% of women will retain clinically meaningful disability from these pregnancy-related, fingolimod cessation relapses 1 year postpartum. This information should be shared with women on fingolimod desiring pregnancy, and optimizing MS treatment with nonteratogenic approaches should be discussed.
Fingolimod (FTY) is an effective and well-tolerated treatment for relapsing-remitting MS, but if discontinued, severe clinical disease activity can return and in November 2018 the FDA issued a warning also included in the patient information.1 Severe relapses might occur between 4% and 26% of patients after 4–16 weeks with the major limitation that most cohorts are small, and the magnitude of the risk is incompletely understood. Pregnancy is believed to be an effective natural treatment and associated with a reduced relapse risk in the third trimester.2,3 However, smaller cohort studies show that relapse and disability progression risk during pregnancy and postpartum are higher in women who received FTY before pregnancy, which has to be stopped due to its potential teratogenicity 2 months before conception.4-6 Concerning case reports of severe rebound relapses and disease reactivation have been reported in the context of pregnancy planning,7-12 but the magnitude of this risk is still unknown.
For an informed decision-making discussion between patients and treating physicians, the knowledge of the frequency of occurrence of severe FTY cessation relapses is essential.
In previous randomized controlled trials and pregnancy studies in the setting of MS, it was common to count any relapse or sustained disability progression.4-6 However, this approach does not reflect the disabling relapses MS patients fear, especially the loss of the lower limb function.13
The purpose of this study, similar to our previous study on the withdrawal of natalizumab,14 therefore, was to describe (1) the absolute risk of severe relapses using a novel, patient-centered definition; (2) persistent disability accrual from these relapses; and (3) the absolute risk of relapses during pregnancy and the postpartum year after FTY cessation. We also examined whether these risks were modified by pregnancy itself, timing of FTY cessation, exclusive breastfeeding (ExBF), and/or resuming FTY immediately postpartum.
Methods
Study Design, Setting, and Participants
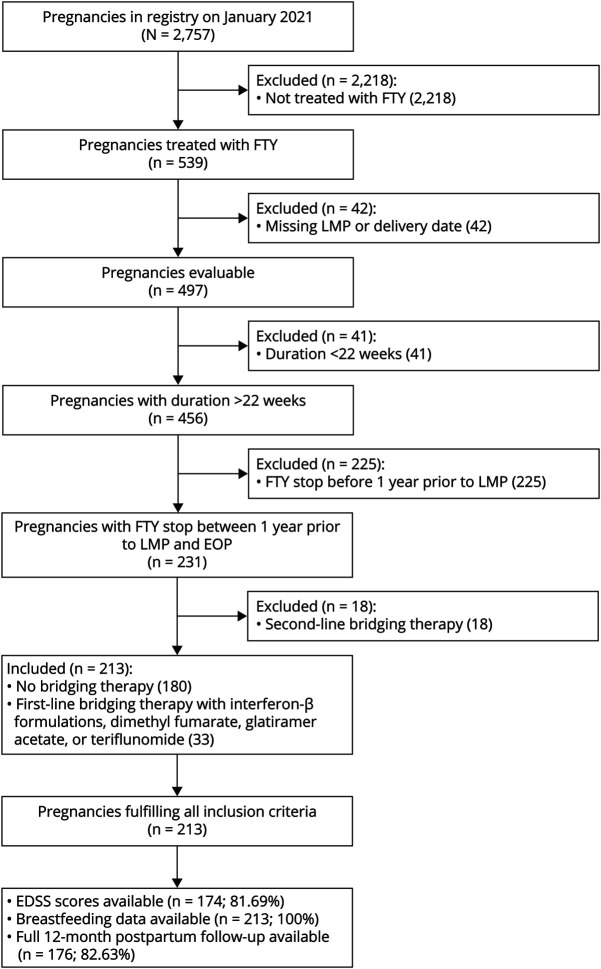
Pregnancies were identified from the German Multiple Sclerosis and Pregnancy Registry on June 8, 2020. Inclusion criteria comprised treatment with FTY stopped within 1 year prior or at any time point during pregnancy, documented last menstrual period (LMP) and delivery date, and pregnancy duration of at least 22 weeks. Pregnancies were excluded if these criteria were not met or they used a second-line disease-modifying therapy (DMT; natalizumab or cell-depleting DMTs) as a prepregnancy bridging therapy (Figure 1). Data were collected through standardized, telephone-administered questionnaires at enrollment, during each remaining trimester, and 1, 3, 6, and 12 months postpartum15; self-reported relapses were confirmed, and Expanded Disability Status Scale (EDSS) obtained from the treating physician. Information for 7 pregnancies was obtained after delivery.
Figure 1. Inclusion Criteria and Eligible Pregnancies in the Cohort.
Depicted are the pregnancies fulfilling the inclusion criteria. EDSS values were reported for 174/81.69% pregnancies for 3 time points (baseline up to 3 months before LMP, third trimester of pregnancy, and postpartum 12 months ± 6 weeks, all at least 30 days after a relapse) from the treating neurologists. Data on breastfeeding behavior including the information when supplemental feeding was introduced in those breastfeeding exclusively for >2 months were available for all 213 pregnancies. EDSS = Expanded Disability Status Scale; EOP = end of pregnancy; FTY = fingolimod; LMP = last menstrual period.
Outcomes
EDSS was obtained from treating neurologists' records as follows: before pregnancy (up to 3 months), last pregnancy trimester, 12 months postpartum period ( ±7 weeks) and ≥30 days before and after a relapse, and, for those with relapses, maximum EDSS during relapse. Relapses were also confirmed by treating neurologists.
Severe relapses until the end of pregnancy or the postpartum year were defined as new or worsening ambulatory impairment to capture clinical meaningful disability end points. We used the Severe Relapse Disability Composite Score (SRDCS)14 defined as any relapse during pregnancy or postpartum leading to (1) an EDSS increase of 2 or more points, (2) new ambulatory impairment in those without significant prepregnancy ambulatory impairment (EDSS increase from ≤3.5 to ≥4.0), or (3) significant worsening ambulatory impairment in those with at least some preexisting ambulatory impairment (EDSS increased from ≤5.5 to ≥6.0, cane or worse, 6.0 to ≥6.5, walker or worse; 6.5 to ≥7.0, wheelchair or worse, 7.0 to ≥8.0, bedbound with some arm function or worse). If EDSS during relapse was missing, relapses were considered nonsevere.
To facilitate the comparison with the existing literature, we conducted additional analyses using the standard definition of disability progression (defined as a worsening of at least 1.5 EDSS points if baseline EDSS = 0, at least 1 point for baseline EDSS 1–5.5, and 0.5 point for baseline EDSS ≥6.0)16 and the outcome “significant clinical worsening” (at least a 2-point increase in patients with an EDSS score of <5.5, or an increase of at least 1 point, in patients with an EDSS score of ≥5.5)17 presented as additional data in eTable 1 (links.lww.com/NXI/A822).
Additional outcomes included the occurrence of any relapse (yes/no), number of relapses during and after pregnancy, during each trimester of pregnancy, and the first year postpartum. Relapse rates were calculated per trimester, defined from LMP+84 days (first trimester), the second trimester lasting for 112 days, and the third trimester ending at delivery.
Exposures and Covariates
ExBF was defined as exclusive breastfeeding for at least 2 months. Nonexclusive breastfeeding (non-ExBF) was defined as introducing supplemental feedings within the first 2 months or no breastfeeding. Early reintroduction of FTY was defined as restarting within 4 weeks postpartum. The use of bridging therapies was defined as introduction of first-line treatments any time after FTY stop but before pregnancy (Figure 1).
To examine whether the timing of FTY cessation influenced pregnancy-associated relapse risks, pregnancies were divided into 2 FTY cessation groups. The washout group defined those where FTY was discontinued at least 60 days and up to 365 days before the LMP or no washout group defined as pregnancies with last FTY intake less than 60 days pre-LMP or during pregnancy.
Statistical Analyses
Analyses were conducted with R version 4.1.2 “Bird Hippie” and RStudio version 1.1.463 with a two-sided significance level of α = 0.05. In addition, 95% confidence intervals (CI) are reported. Descriptive statistics are reported as mean (SD) or median (range) for continuous data and number/denominator (percent) (n/N [%]) for categorical data.
Annualized relapse rates (ARRs) were estimated by multivariable mixed-effects Poisson regression models with a random pregnancy effect to account for the repeated measures approach.
The model estimating ARRs per FTY cessation group across the total observational period was adjusted for the use of bridging therapies, age at pregnancy onset, disease duration, and gestational week at entry into the cohort. In this model, the observational period was divided into 11 time frames (4 quarters of the year prepregnancies, 3 pregnancy trimesters, and 4 quarter of the postpartum year), allowing for the estimation of ARR per time frame and FTY cessation group.
To assess the association of breastfeeding behavior (ExBF vs non-ExBF) and early FTY reintroduction with ARRs in 12 months postpartum, an additional model was constructed. The year postpartum was divided into 4 quarters, and the model was corrected for having had a relapse in pregnancy (yes/no).
An additional analysis, based on the previous model, was conducted comparing a subgroup of the ExBF group, who did not use additional DMTs, to the group with early FTY introduction.
We intended to compare early use of first-line or second-line therapies to early FTY reintroduction or ExBF but lacked sufficient sample to conduct these analyses.
The adjusted ARRs were compared as relapse rate ratios (RRR with 95% CI) extracted from planned linear contrasts and tested for significant difference using t test.
To assess whether the risk of severe relapse-associated disability varied by the FTY cessation group, analyses were restricted to the 174 pregnancies with 3 available EDSS values, and multivariable Firth logistic regression models were applied.18 The models examining severe relapse-associated disability and other definitions of disability accruement (disability progression, significant clinical worsening) were adjusted for age at LMP, disease duration, MS-related disability at baseline, having had a relapse in the year prepregnancy (yes/no), and gestational week at entry.
Standard Protocol Approvals, Registrations, and Patient Consents
The registry is approved by the Institutional Review Board of the Ruhr University Bochum (18-6474-BR), and women give informed consent.
Data Availability
Anonymized data that support the findings of this study will be shared on reasonable request and if compatible with data protection policies.
Results
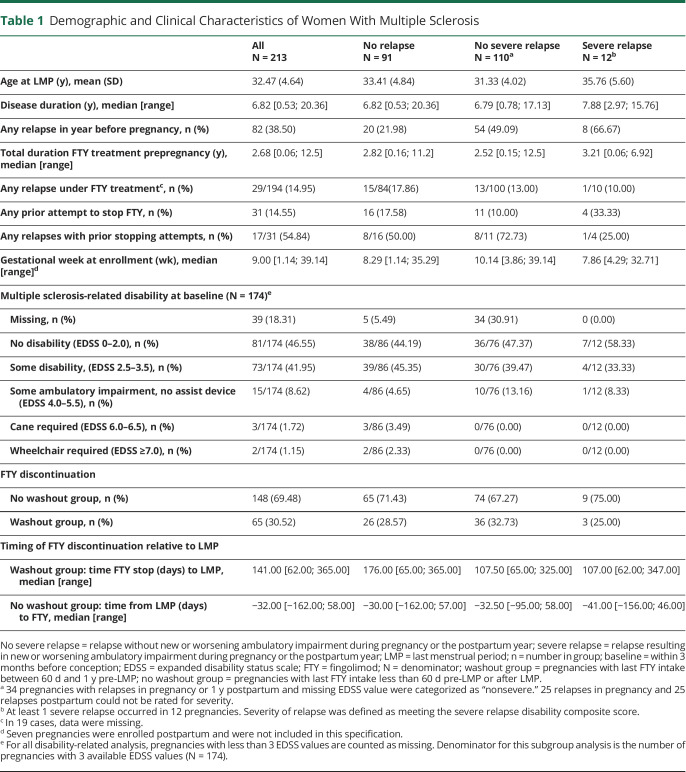
We identified 213 pregnancies in 201 women with a mean age of 32.47 (±4.64) years at the time of conception. FTY discontinuation was most common during the first trimester of pregnancy (n = 114/213, 53.52%); 7/213 (3.29%) stopped during the second trimester. FTY was stopped as recommended >60 days before pregnancy in only 65/213 pregnancies (30.52%; Table 1). In total, 27/213 (12.68%) stopped less than 60 days before LMP. Of 92 pregnancies with FTY cessation before pregnancy, at least 1 relapse occurred between FTY discontinuation and LMP, in 22/65 (33.85%) who stopped >60 days and in 3/27 (11.12%) who stopped <60 days before LMP. 33 pregnancies used a first-line bridging DMT (n = 22, glatiramer acetate, n = 6, beta-interferons, the remaining dimethyl fumarate or IV immunoglobulins) for a median duration of 128 (range 7–452) days (Figure 1). Only 1 woman used 2 different treatments and switched from glatiramer acetate to interferon-β. Bridging therapy was stopped in the first trimester in 28 pregnancies; only 2/33 (6.06%) continued the treatment during the entire pregnancy. In most pregnancies, we observed no significant disability at conception; only 20 of 174 with complete EDSS information (11.49%) presented some ambulatory impairment, of whom 5/174 (2.87%) required a cane or worse (Table 1).
Table 1.
Demographic and Clinical Characteristics of Women With Multiple Sclerosis
Relapses
Clinical characteristics of pregnancies stratified by relapse type are presented in Table 1. During pregnancy and the postpartum year, we observed at least 1 relapse in 122/213 (57.28%) pregnancies, and in 5.63% (n = 12/213), a severe relapse was observed. Pregnancies with at least 1 relapse were conceived by younger women, more likely to have had a relapse in the year before pregnancy and more likely to have had no physical disability at pregnancy onset compared with pregnancies who were relapse-free during the study period. Disease duration, relapses on FTY, timing of FTY stop, prior FTY stopping attempts, and gestational week of joining the registry were similar between pregnancies with or without relapses during the study period. Most pregnancies were registered in the first trimester regardless of relapse type (Table 1).
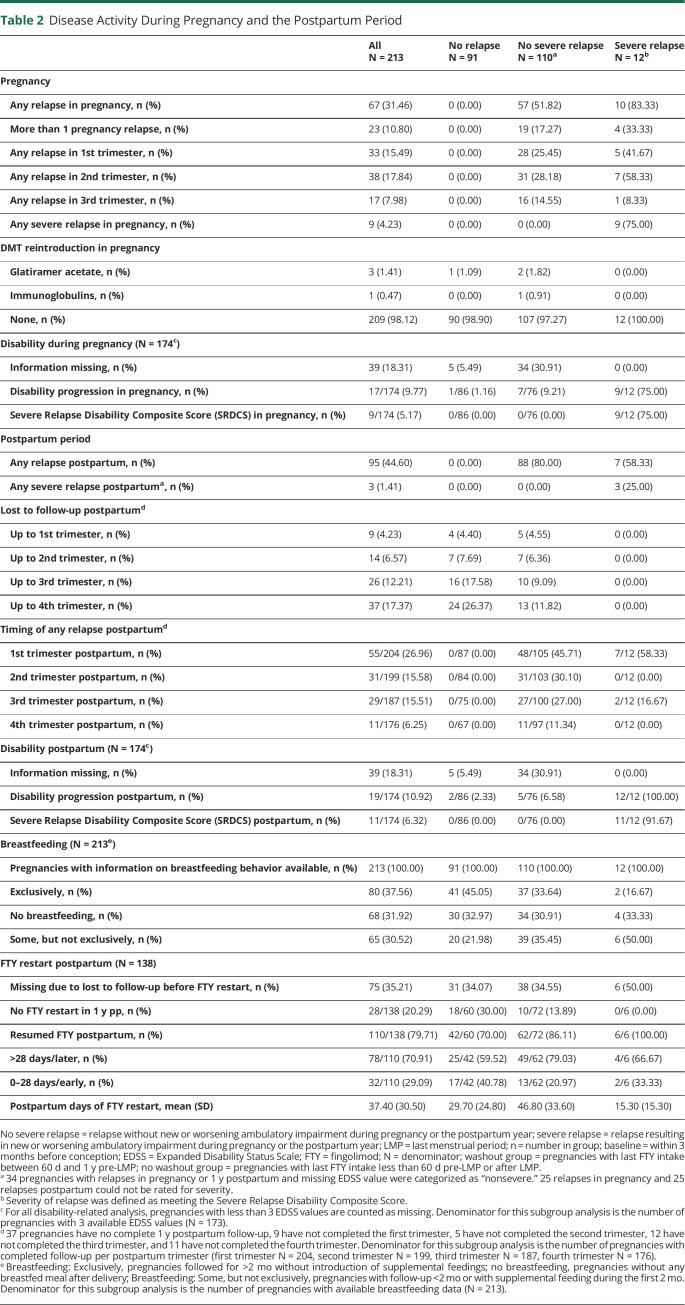
During pregnancy, at least 1 relapse was reported in 67/213 (31.46%) pregnancies, and in 23/213 (10.80%), more than 1 relapse was reported (Table 2). Most postpartum relapses occurred during the first 3 months postpartum. The adjusted annualized relapse rate was not statistically significantly higher during pregnancy compared with the prepregnancy year (RRR 1.24, 95% CI 0.91–1.68), increased in the first 3 months postpartum and returned to prepregnancy rates months 4–12 postpartum (Figure 2). A relapse during pregnancy was associated with an increased ARR postpartum (RRR 1.92, 95% CI 1.36–2.72).
Table 2.
Disease Activity During Pregnancy and the Postpartum Period
Figure 2. Mean Annual Relapse Rate per Time Frame in the Observational Period as Estimated by Zero-Inflated Poisson Regression.
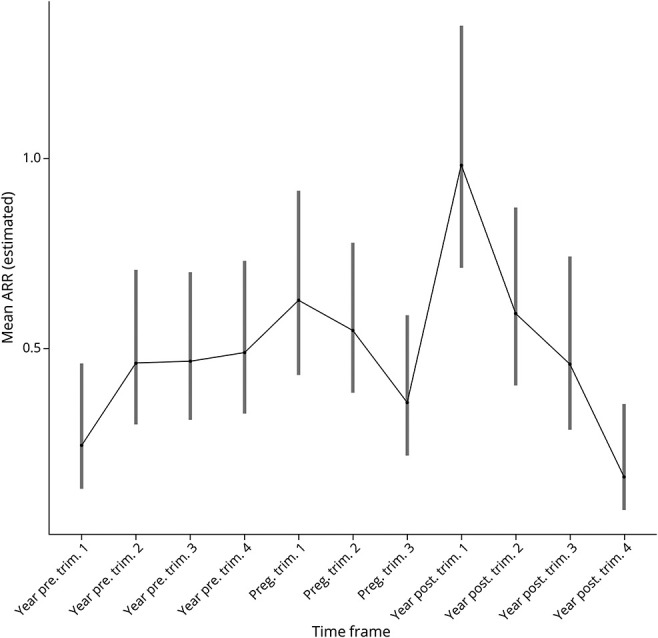
The line depicts the ARR course of the total group. CIs are given for each stratum in each time frame. ARR = annualized relapse rate; CI = 95% confidence interval; preg = pregnancy; Trim = trimester; year pre = year before pregnancy; year post = year postpartum.
Severe Relapses and Disability Accumulation
Severe relapses occurred in 4.23% (n = 9/213) of pregnancies during pregnancy, of whom 8/9 (88.89%) had persistent severe relapse-related disability (measured with SRDCS) by 12 months postpartum (Figure 3). Postpartum, 3/213 (1.41%) additional severe relapses were observed (Table 2, Figure 3).
Figure 3. Disability Development During Pregnancy and Postpartum Using the SRDCS in the 174 Pregnancies With 3 Available EDSS Values.
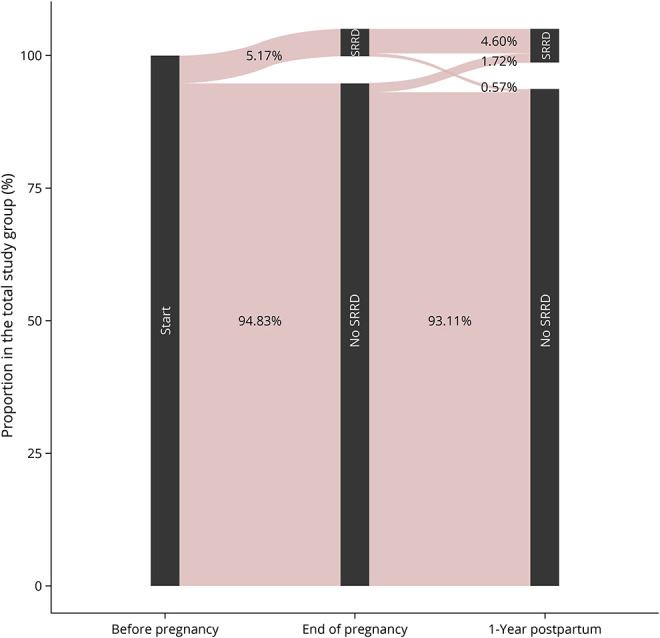
Occurrence of SRDCS at the end of pregnancy and the postpartum year. All pregnancies start before LMP with no information regarding previous SRDCS. The no SRDCS category also contains pregnancies with missing data on the SRDCS. EDSS = Expanded Disability Status Scale; LMP = last menstrual period; SRDCS = Severe Relapse Disability Composite Score.
One or more relapses in the year before pregnancy (adjusted OR [CI]: 5.57 [1.61–22.3]), age at conception (adjusted OR [CI]: 1.19 [1.04–1.36]), and MS-related disability (global test: χ2 9.55, p < 0.001) were associated with risk of severe relapses in the total observation period. The FTY washout group was not significantly associated with the risk of severe relapses (adjusted OR [CI]: 1.00 [0.23–3.73]).
The 5 most severe relapses (EDSS increase ≥3) occurred during pregnancy, including 1 with a Δ EDSS of 4.5 (additional data are listed in eTable 2, links.lww.com/NXI/A822). All women started with a baseline EDSS of 0 or 1.0; the worst documented EDSS during a relapse was 6.0, and this woman recovered to an EDSS of 5.5 at the end of pregnancy and an EDSS of 4.0 by 12 months postpartum. Relapses were treated during pregnancy with high-dose corticosteroid, and 2 women received additional apheresis treatments. None of these women recovered to the baseline EDSS during the postpartum year.
Among the 12 pregnancies with severe relapses during pregnancy or the postpartum period, 11 (6.32% of the 174 pregnancies with complete EDSS information) still had severe relapse-related disability (measured with SRDCS) by the end of the postpartum year (Figure 3). Only 3/174 (1.72%) pregnancies had EDSS worsening independent of a documented relapse at 12 months postpartum compared with prepregnancy. Multiple sclerosis–related disability remained unchanged throughout pregnancy and the postpartum year in most (n = 117/174, 67.24%) of our pregnancies. Alternative analyses using traditional definitions of disability progression (n = 17/174, 9.77%, Table 2) and alternative definitions of significant clinical worsening during pregnancy (n = 10/174, 5.75%; additional data are listed in eTable 1, links.lww.com/NXI/A822) showed a significant association with relapses in the year before pregnancy.
Influence of Timing of Fingolimod Withdrawal
Relapses during pregnancy, particularly the second trimester, were more common in the FTY washout group compared with the no washout group, although neither difference reached statistical significance (pregnancy n = 27/65, 41.54% and n = 40/148, 27.03%; second trimester n = 17/65, 26.15% and n = 21/148, 14.19%, washout and no washout group, respectively; additional data are listed in eTables 3 and 4, links.lww.com/NXI/A822). This increased risk of relapse during pregnancy in the washout group also did not reach statistical significance in adjusted comparison with the no washout group (RRR 1.76, 95% CI 0.91–3.41) or when comparing pregnancy with prepregnancy ARR in the washout group (RRR 1.49, 95% CI 0.90–2.48).
A nonsignificant increase in relapses during the first 3 months postpartum but not later in the postpartum year in the washout compared with the no washout group was also detected (RRR 1.31, 95% CI 0.73–2.33).
Modifiable Risk Factors of Postpartum Relapses
Of the 213 pregnancies, 145 (68.08%) decided to breastfeed, 80 (37.56%) exclusively for at least 2 months (Table 2). 13 breastfed, 8 exclusively, under DMTs (n = 1 interferon-β; n = 1 immunoglobulins; n = 4 glatiramer acetate; n = 2 FTY for few days during weaning; n = 2 ocrelizumab; n = 3 natalizumab). Of 161 pregnancies resuming DMTs in 1 year postpartum, the 68 who decided not to breastfed did so earlier than those who breastfed (median [range] 24.50 [0.0–342] vs 95.0 [−21 to 347], days). ExBF had no significant effect on adjusted relapse rates postpartum, compared with non-ExBF in the total cohort (RRR 0.75, CI 0.5–1.12).
In 32 pregnancies, FTY was reintroduced within 4 weeks of delivery. Very few used a first-line (n = 6 glatiramer acetate, n = 2 dimethyl fumarate, and n = 1 interferon-beta) or another second-line DMT (n = 7 natalizumab, n = 2 ocrelizumab, and n = 1 rituximab), in the early postpartum period. Comparing ExBF without DMT (n = 72) with early FTY reintroduction without any breastfeeding (n = 32), we found no significant difference in relapse rates in any of the postpartum trimesters nor in the total postpartum year (RRR 1.33, CI 0.73–2.42; Figure 4).
Figure 4. Course of Mean Annual Relapse Rates as Estimated by Zero-Inflated Poisson Regression in 1 Year Postpartum in the Subgroup With Exclusive Breastfeeding or Early Restart of FTY.
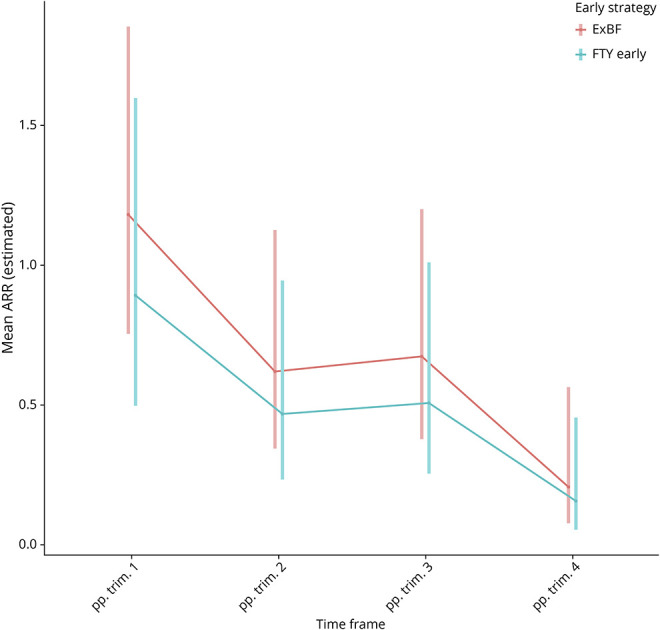
104 pregnancies included (72 pregnancies with exclusive breastfeeding but without DMT reintroduction and 32 pregnancies with early restart of FTY but without breastfeeding). The red line depicts ARR course of the postpartum strategy exclusive breastfeeding (n = 72/69.23%); the turquoise of postpartum strategy early FTY reintroduction (n = 32/30.77%). ARR = annual relapse rate; DMT = disease-modifying therapy; ExBF = exclusive breastfeeding; FTY = fingolimod; pp = postpartum; Trim = trimester.
Of the 10 with other early second-line DMT use, we observed only 1 relapse during the first 6 months pp.
Discussion
Pregnancy or postpartum relapses after FTY cessation were common (57.28%). Using the innovative SRDCS, a patient-centric measure of severe relapses, we observed such severe relapses in 11 (6.32%) women that they retained meaningful disability up to 1 year postpartum. Although the general relapse risk was highest postpartum, most severe (rebound) relapses (75%) occurred during pregnancy. Neither pregnancy itself, early FTY reintroduction during the first 4 weeks postpartum, nor ExBF was associated with a reduced relapse risk. Our study provides relevant information on various maternal risks and should serve as a basis of a risk-benefit discussion between neurologists and women treated with FTY who plan a pregnancy.
To be able to quantify the potential loss of lower limb function from a relapse that patients fear most,13 we recently developed a novel patient-centric, composite definition of SRDCS.14 Existing MS outcome measures incompletely capture this type of severe relapse-related disability. As a result, health care providers are impaired in effectively communicating the risk of severe relapse-related disability to patients. Applying this definition, we found that up to 6.32% of women in our pregnancy registry experienced severe relapses that resulted in irreversible disability at 12 months postpartum. We did not observe catastrophic or life-threatening withdrawal relapses in our study of 213 pregnancies, although case reports related to family planning exist.12 While we cannot provide a precise estimate of catastrophic/life-threatening relapses after FTY cessation for pregnancy, the results of this study estimate it as less than 0.5%—with the limitation, that we capture only pregnant women and not women who stopped FTY as recommended,19 with relapses so severe, that they did not become pregnant in the near future.
Studies in the nonpregnant setting on the effect of FTY withdrawal on residual disability because of severe relapses are sparse and the difference between case reports and cohorts striking. A case report of severe FTY cessation relapse resulting in death led to revision of the FDA label. The severe relapse/rebound risk in the published literature ranges from 4% to 25.8%.20 This wide variation is likely due to differences in definition of rebound or severe relapses and methodological limitations including referral center and other types of selection bias, cross-sectional study design, insufficient follow-up, or heterogeneity in follow-up treatment.20 Of note, the lowest risk (≈4%) for severe relapses (defined as a relapse leading to hospitalization or with incomplete recovery or an EDSS increase ≥ 3 at baseline EDSS = 0, EDSS increase ≥ 2 at baseline EDSS between 1 and 5 or an EDSS increase of 1 at baseline EDSSS >5) was observed in a post hoc analysis of the pivotal trials. It was equally distributed between the FTY and the placebo arm, but here, only a small subcohort of the initial participants had sufficient follow-up time.21
Although pregnancy itself is believed to be protective against MS relapses,22,23 case reports and small cohort studies (study sample between 21 and 75 pregnancies) have reported severe relapses after FTY withdrawal in the context of family planning; 3 smaller cohort studies did not report an EDSS delta or disability progression4,24,25 or grouped NTZ and FTY together,6 making it extremely challenging to directly compare with the results of this study. The largest FTY withdrawal pregnancy study (n = 75) found similar to our study conventional disability progression in 12% of the pregnancies without further stratifying the severity of these relapses.5 In this study, similar to our study, the highest relapse risk during pregnancy was observed during the second trimester and decreased during the third trimester, following natural history studies. Alike our cohort, the relapse rate was similar between pregnancies of the washout or no washout group, and we observed a trend toward fewer relapses in the no washout group.5 Other smaller studies, reported the highest risk during the first24 or second trimester.6 The most likely explanation for these difference is that most of our study subjects were exposed to FTY 2 months before pregnancy or later, and earlier in others,5,25 but only 1 study was large enough to stratify between washout and no washout without giving an exact definition of “washout.”5
Compared with smaller studies, the pregnancy relapse risk in our study (31.46%) was similar to other FTY withdrawal studies in the context of pregnancy (22–50%).4-6,24,25 Similar to our NTZ cohort, pregnancy did not reduce the relapse risk in the adjusted model.
Postpartum, we, like others,4-6,24 observed an increased relapse rate during the first 3 months and found pregnancy relapses as the most important risk factor for postpartum relapses. Others associated higher prepregnancy disease severity, relapses during pregnancy, and longer washout period with an increased postpartum relapse risk.4-6 In contrast to these studies, most pregnancies in our cohort were exposed to FTY and stopped the drug relatively late. ExBF did not significantly affect the risk of postpartum relapses, even if it meant foregoing early resumption of DMTs.
Similar to our NTZ cohort, we found that an early postpartum restart of FTY (or NAT)14 did not reduce the early postpartum relapses risk underlining the immunologic preparation of these early relapses already during late pregnancy where a decline in circulating CD4+IFN-γ producing begins.26 As in higher dose phase 2 studies gadolinium-enhancing lesions seemed to be reduced only 2 months after the beginning of treatment with FTY, we speculate that these early postpartum relapses cannot be affected by early postpartum FTY treatment start.27
It should be noted that the risk of severe relapse-related disability 1 year postpartum in our FTY cessation cohort was lower than in our previously reported natalizumab cessation pregnancy cohort (relapse-related disability measured with SRDCS natalizumab: 12.78% vs FTY: 6.32%, respectively). Although we did not conduct analyses directly comparing these 2 groups of women, we speculate that the lower rate after FTY cessation for pregnancy is due to more complete capture of FTY contacting the registry earlier during pregnancy than natalizumab-treated pregnancies by our registry because of the well-described teratogenicity with FTY but not natalizumab exposure. Another potential explanation is that women treated with natalizumab had higher disease activity before treatment start than those treated with FTY.
Beside the dependency on routine medical records for outcome collection and a potential selection bias, our study has some limitations typical for registry studies. Although EDSS values were available for more than 80% of our participants, we did not collect data on visual or cognitive function and outcomes that were important to patients after ambulatory function.13 In addition, EDSS was not collected in a standardized manner as is performed in clinical studies, suggesting that some of the EDSS changes may be due to inter-rater reliability.28 As we practice a voluntary enrollment at any time point during pregnancy in our registry, potential selection bias toward more aggressive cases cannot be ruled out. However, in contrast to our recently published NTZ cohort, the gestational week of joining the registry was similar between women with severe relapses and those without relapses, although relapses after FTY withdrawal normally occur earlier than those after NTZ withdrawal. We can only speculate that women and physicians being aware of the potential teratogenicity of FTY might fear the risk and therefore contact the registry earlier. Owing to the potential teratogenicity, S1P modulators are recommended to be paused before pregnancy according to their different half-life times and their use during pregnancy is contraindicated.29 As we mostly enroll pregnant women in our registry, we likely underestimate the occurrence of relapses before pregnancy so that women forego pregnancy and can give no risk estimates for this scenario. Our results are restricted to FTY, and it is unknown whether newer S1P modulators exhibit the same risk. Half-life times differ substantially between newer formulations and might shift risks (e.g., lower teratogenicity risk with shorter half-life time but higher risk of relapse occurrence, although rebound was not reported yet). These data are lacking and needed from contemporary cohorts.
Nonetheless, our study has strengths, among them is the relevance of the question, a prospective and longitudinal follow-up, the large sample size, and the development of a novel, patient-centric measure of severe relapse-related disability.
In this study, we found that the risk of relapses and relapse-related disability after FTY cessation for pregnancy is high. Although we did not observe life-threatening relapses with an EDSS >6.0, these relapses are reported in pregnant women after FTY withdrawal and women should be informed about this risk. Although S1P modulators are contraindicated during pregnancy, most of our pregnancies were exposed to FTY—a concerning finding of our study that should reinforce counseling strategies in patients, moreover as the use of depleting agents before pregnancy or the continuation of NTZ show increasingly promising results.30,31
Acknowledgment
The authors thank all the participants of the DMSKW as well as the referring neurologists and MS nurses. The authors also thank the referring neurologists Maria Seipelt, Sylvia Menck, Ulrich Kausch, Ina van Loh, and the team of the MIND MVZ Stuttgart for supporting our registry and providing >3 EDSS values each.
Glossary
- ARR
annualized relapse rate
- CI
confidence intervals
- DMSKW
German Multiple Sclerosis and Pregnancy Registry
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- ExBF
exclusive breastfeeding
- FTY
fingolimod
- LMP
last menstrual period
- Non-ExBF
nonexclusive breastfeeding
- SRDCS
Severe Relapse Disability Composite Score
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Appendix. Authors
Study Funding
This study was supported by the Innovation Fund of the Federal Joint Committee (Grant No. 01VSF17022) and the German Multiple Sclerosis and pregnancy registry is in general partly supported by Almirall, Biogen, Teva Pharma, Novartis, Roche, and Merck. None of the funders had any influence on design, acquisition, analysis or interpretation of data, or writing of the manuscript.
Disclosure
K. Hellwig has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme, Roche, and Teva; has received support for congress participation from Bayer, Biogen, Merck, Roche, Sanofi-Genzyme, and Teva; and has served on scientific advisory boards for Bayer, Biogen, Sanofi, Teva, Roche, Novartis, and Merck. M. Tokic is employed in a project funded by a grant from the Innovation Fund of the Federal Joint Committee. S. Thiel received speakers honoraria from Bayer Healthcare and Biogen GmbH as well as payment for manuscript writing from HEXAL AG. S. Hemat reports no disclosures relevant to the manuscript. N. Timmesfeld has received a grant from the Innovation Fund of the Federal Joint Committee. A.I. Ciplea has received speaker honoraria from Bayer Healthcare, sponsorship for congress participation from Teva, and travel grants from Teva and Novartis. R. Gold has received speaker honoraria and research support from Bayer-Schering Healthcare, Biogen-Idec Germany, Chugai, Eisai, Merck Serono, Nikkiso Pharma, Novartis, Roche, Sanofi-Genzyme, and TEVA; has received consulting honoraria from CSL Behring, Baxter, Janssen, and Talecris; and has stock options in Bayer, Merck, and Roche. A. Langer-Gould reports no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosure.
References
- 1.FDA. FDA warns about severe worsening of multiple sclerosis after stopping the medicine Gilenya (fingolimod). Accessed February 20, 2022. fda.gov/drugs/drug-safety-and-availability/fda-warns-about-severe-worsening-multiple-sclerosis-after-stopping-medicine-gilenya-fingolimod.
- 2.Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and Multiple Sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360. doi: 10.1093/brain/awh152 [DOI] [PubMed] [Google Scholar]
- 3.Hellwig K. Pregnancy in multiple sclerosis. Eur Neurol. 2014;72(suppl. 1):39-42. doi: 10.1159/000367640 [DOI] [PubMed] [Google Scholar]
- 4.Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J. Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology. 2018;90(10):e840-e846. doi: 10.1212/wnl.0000000000005065 [DOI] [PubMed] [Google Scholar]
- 5.Yeh WZ, Widyastuti PA, Van der Walt A, et al. Natalizumab, fingolimod and dimethyl fumarate Use and pregnancy-related relapse and disability in women with multiple sclerosis. Neurology. 2021;96(24):e2989-e3002. doi: 10.1212/wnl.0000000000012084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bsteh G, Algrang L, Hegen H, et al. Pregnancy and multiple sclerosis in the DMT era: a cohort study in Western Austria. Mult Scler J. 2020;26(1):69-78. doi: 10.1177/1352458518816614 [DOI] [PubMed] [Google Scholar]
- 7.Havla JB, Pellkofer HL, Meinl I, Gerdes LA, Hohlfeld R, Kümpfel T. Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol. 2012;69(2):262-264. doi: 10.1001/archneurol.2011.1057 [DOI] [PubMed] [Google Scholar]
- 8.Ghezzi A, Rocca MA, Baroncini D, et al. Disease reactivation after fingolimod discontinuation in two multiple sclerosis patients. J Neurol. 2013;260(1):327-329. doi: 10.1007/s00415-012-6744-7 [DOI] [PubMed] [Google Scholar]
- 9.La Mantia L, Prone V, Marazzi MR, Erminio C, Protti A. Multiple sclerosis rebound after fingolimod discontinuation for lymphopenia. Neurol Sci. 2014;35(9):1485-1486. doi: 10.1007/s10072-014-1800-y [DOI] [PubMed] [Google Scholar]
- 10.Fragoso YD, Adoni T, Gomes S, et al. Severe exacerbation of multiple sclerosis following withdrawal of fingolimod. Clin Drug Investig. 2019;39(9):909-913. doi: 10.1007/s40261-019-00804-6 [DOI] [PubMed] [Google Scholar]
- 11.Członkowska A, Smoliński Ł, Litwin T. Severe disease exacerbations in patients with multiple sclerosis after discontinuing fingolimod. Neurol Neurochir Pol. 2017;51(2):156-162. doi: 10.1016/j.pjnns.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Novi G, Ghezzi A, Pizzorno M, et al. Dramatic rebounds of MS during pregnancy following fingolimod withdrawal. Neurol Neuroimmunol Neuroinflamm 2017;4(5):e377. doi. 10.1212/nxi.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14(7):988-991. doi: 10.1177/1352458508088916 [DOI] [PubMed] [Google Scholar]
- 14.Hellwig K, Tokic M, Thiel S, et al. Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open. 2022;5(1):e2144750. doi: 10.1001/jamanetworkopen.2021.44750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellwig K, Rockhoff M, Herbstritt S, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol. 2015;72(10):1132-1138. doi: 10.1001/jamaneurol.2015.1806 [DOI] [PubMed] [Google Scholar]
- 16.Portaccio E, Moiola L, Martinelli V, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: II: maternal risks. Neurology. 2018;90(10):e832-e839. doi: 10.1212/wnl.0000000000005068 [DOI] [PubMed] [Google Scholar]
- 17.Vidal-Jordana A, Tintoré M, Tur C, et al. Significant clinical worsening after natalizumab withdrawal: predictive factors. Mult Scler J. 2015;21(6):780-785. doi: 10.1177/1352458514549401 [DOI] [PubMed] [Google Scholar]
- 18.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409-2419. doi: 10.1002/sim.1047 [DOI] [PubMed] [Google Scholar]
- 19.Thone J, Thiel S, Gold R, Hellwig K. Treatment of multiple sclerosis during pregnancy - safety considerations. Expert Opin Drug Saf. 2017;16(5):523-534. doi: 10.1080/14740338.2017.1311321 [DOI] [PubMed] [Google Scholar]
- 20.Barry B, Erwin AA, Stevens J, Tornatore C. Fingolimod rebound: a review of the clinical experience and management considerations. Neurol Ther. 2019;8(2):241-250. doi: 10.1007/s40120-019-00160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermersch P, Radue EW, Putzki N, Ritter S, Merschhemke M, Freedman MS. A comparison of multiple sclerosis disease activity after discontinuation of fingolimod and placebo. Mult Scler J Exp Transl Clin. 2017;3(3):2055217317730096. doi: 10.1177/2055217317730096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sepúlveda M, Montejo C, Llufriu S, et al. Rebound of multiple sclerosis activity after fingolimod withdrawal due to planning pregnancy: analysis of predisposing factors. Mult Scler Relat Disord. 2020;38:101483. doi: 10.1016/j.msard.2019.101483 [DOI] [PubMed] [Google Scholar]
- 23.Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73(7):790-794. doi: 10.1001/jamaneurol.2016.0826 [DOI] [PubMed] [Google Scholar]
- 24.Bianco A, Lucchini M, Totaro R, et al. Disease reactivation after fingolimod discontinuation in pregnant multiple sclerosis patients. Neurotherapeutics. 2021;18(4):2598-2607. doi: 10.1007/s13311-021-01106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berenguer-Ruiz L, Gimenez-Martinez J, Palazón-Bru A, Sempere AP. Relapses and obstetric outcomes in women with multiple sclerosis planning pregnancy. J Neurol. 2019;266(10):2512-2517. doi: 10.1007/s00415-019-09450-6 [DOI] [PubMed] [Google Scholar]
- 26.Langer-Gould A, Gupta R, Huang S, et al. Interferon-gamma-producing T cells, pregnancy, and postpartum relapses of multiple sclerosis. Arch Neurol. 2010;67(1):51-57. doi: 10.1001/archneurol.2009.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comi G, O'Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler J. 2010;16(2):197-207. doi: 10.1177/1352458509357065 [DOI] [PubMed] [Google Scholar]
- 28.Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the expanded disability status scale (EDSS) and functional systems (FS) in a multiple sclerosis clinical trial. Neurology 1990;40(6):971-975. doi. 10.1212/wnl.40.6.971 [DOI] [PubMed] [Google Scholar]
- 29.EMA. Gilenya® (fingolimod) - EPAR Summary of product characteristics. Updated August 3, 2021. Accessed February 16, 2015. ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf.
- 30.Kümpfel T, Thiel S, Meinl I, et al. Anti-CD20 therapies and pregnancy in neuroimmunologic disorders: a cohort study from Germany. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e913. doi: 10.1212/nxi.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krysko KM, Graves JS, Dobson R, et al. Sex effects across the lifespan in women with multiple sclerosis. Ther Adv Neurol Disord. 2020;13:1756286420936166. doi: 10.1177/1756286420936166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data that support the findings of this study will be shared on reasonable request and if compatible with data protection policies.