Abstract
Large vessel occlusion stroke due to underlying intracranial atherosclerotic disease (ICAD-LVO) is prevalent in 10 to 30% of LVOs depending on patient factors such as vascular risk factors, race/ethnicity and age. Patients with ICAD-LVO derive similar functional outcome benefit from endovascular thrombectomy as other mechanisms of LVO, but up to half of ICAD-LVO patients re-occlude after revascularization. Therefore, early identification and treatment planning for ICAD-LVO is important given the unique considerations before, during, and after endovascular thrombectomy. In this review of ICAD-LVO, we propose a multistep approach of ICAD-LVO identification, pretreatment and endovascular thrombectomy considerations, adjunctive medications, and medical management. There have been no large-scale randomized controlled trials dedicated to studying ICAD-LVO, therefore this review focuses on observational studies.
Graphical Abstract
Introduction
Stroke is a leading cause of death worldwide and approximately half of those who survive a stroke are chronically disabled, resulting in a public health burden.1 Large vessel occlusion (LVO) occurs in approximately 15% to 30% of ischemic stroke and results in higher morbidity and mortality than other ischemic stroke subtypes.2,3 Intracranial atherosclerotic disease (ICAD) is the underlying cause of ischemic stroke in approximately 40% of Asian, 30% of Black, and 10% of White patients.4-6 Accordingly, LVO due to underlying ICAD (ICAD-LVO) has an estimated prevalence between 10% to 48% of LVO, differing by race/ethnicity and site of arterial occlusion.7,8 Because advanced age and smoking are the main risk factors for atherosclerosis, the rate of ICAD-LVO also differs based on the presence of these vascular risk factors.6,9
Herein, we review literature focusing on identification, pre-procedural management, thrombectomy technique, rescue therapy, and sub-acute management for ICAD-LVO patients.8 There have been no randomized trials dedicated to ICAD-LVO; therefore, the majority of this review focuses on retrospective studies or subgroup analyses. With the proliferation of research in the LVO field, we anticipate that dedicated, rigorous ICAD-LVO studies will be forthcoming.
Definition and Identification of ICAD-LVO
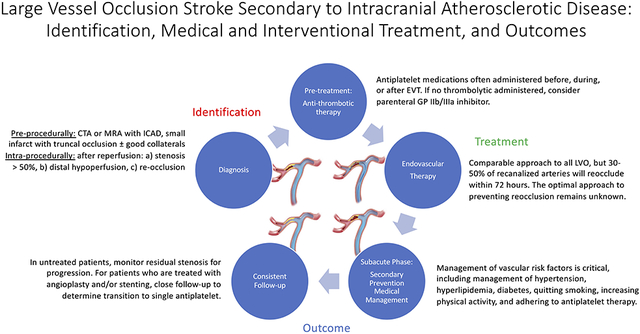
The identification of ICAD-LVO relies on neuroimaging. The time windows for diagnosis of ICAD-LVO can be divided into pre-procedural, intra-procedural, and subacute phases. Characteristic imaging of an ICAD-LVO patient is seen in Figure 1.
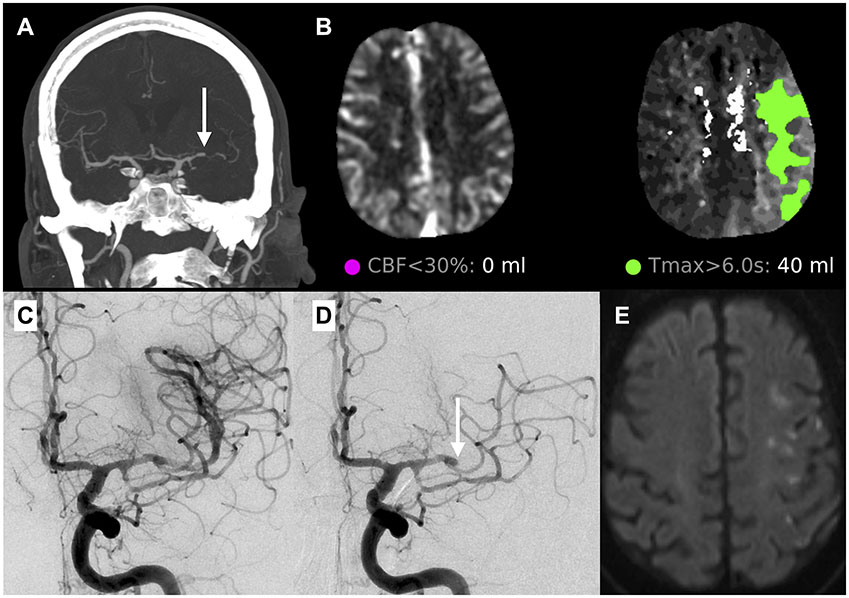
Figure 1.
A case of ICAD-LVO of the left middle cerebral artery (MCA) (panel A, white arrow) showing a truncal type occlusion with adjacent stenosis. CT perfusion (panel B) shows no appreciable core with a moderate sized ischemic penumbra of 40 mL. After one pass of a stentriever, the vessel was recanalized (panel C). However, on a 10-minute follow-up angiogram, there was re-occlusion (panel D, white arrow) and the EVT procedure was terminated. Despite the re-occlusion, the final infarct volume was moderate sized on MRI with scattered areas of ischemic throughout the left MCA territory (panel E).
Pre-Procedural Identification of ICAD-LVO
Pre-procedural imaging can inform the underlying cause of LVO. On computed tomography (CT) or magnetic resonance imaging (MRI), the presence of calcification of the intracranial arteries can be suggestive of ICAD.10 Assessment of collaterals on pre-procedural CT or MR angiography can aid diagnosis. Baek et al. investigated the utility of pre-procedural leptomeningeal collateral status in predicting ICAD-LVO.11 In their study of 40 patients with ICAD-LVO and 186 with cardioembolic LVO, excellent collaterals were found in 52.5% of ICAD-LVO versus 20.4% of non-ICAD patients.11 Furthermore, evaluating core infarct volume at presentation on CT/MRI or perfusion imaging may help. Suh et al. found smaller core infarct volume at presentation among patients with ICAD-LVO compared to cardioembolic LVO (14 mL vs 54 mL, respectively, p<0.001), which is thought to be related to better collateral status in ICAD patients from chronic remodelling.12 Presence of ICAD in other vascular territories may also raise suspicion of ICAD-LVO.
Prior neurovascular imaging, if available, can inform the presence of pre-existing intracranial stenosis (or lack thereof) to support or refute ICAD-LVO. A patient with patent intracranial vasculature within 6 months of LVO presentation would not be expected to have ICAD-LVO, whereas a patient with known severe intracranial stenosis and acute LVO would be highly suspected of having ICAD-LVO. Patients who present with fluctuating symptoms may also raise suspicion for ICAD-LVO as well as those with subacute or chronic borderzone territory infarction on parenchymal imaging.8 In clinical practice, it is difficult to be certain of the presence of ICAD-LVO prior to thrombectomy, particularly in patients who do not present with the above features.
Intra-Procedural Identification of ICAD-LVO
Intra-procedurally, the diagnosis of ICAD-LVO can be made on the first angiographic run or following initial reperfusion. The presence of tapered narrowing proximal to the occlusion is more likely to be found with ICAD-LVO (Figures 1/2).13 In addition, a truncal type occlusion, versus a branching-site occlusion typical of cardioembolic LVO, can be predictive of ICAD because cardioemboli are more likely to cause an occlusion at a bifurcation compared to a plaque which tends to originate from the middle of an arterial segment (Figure 2).
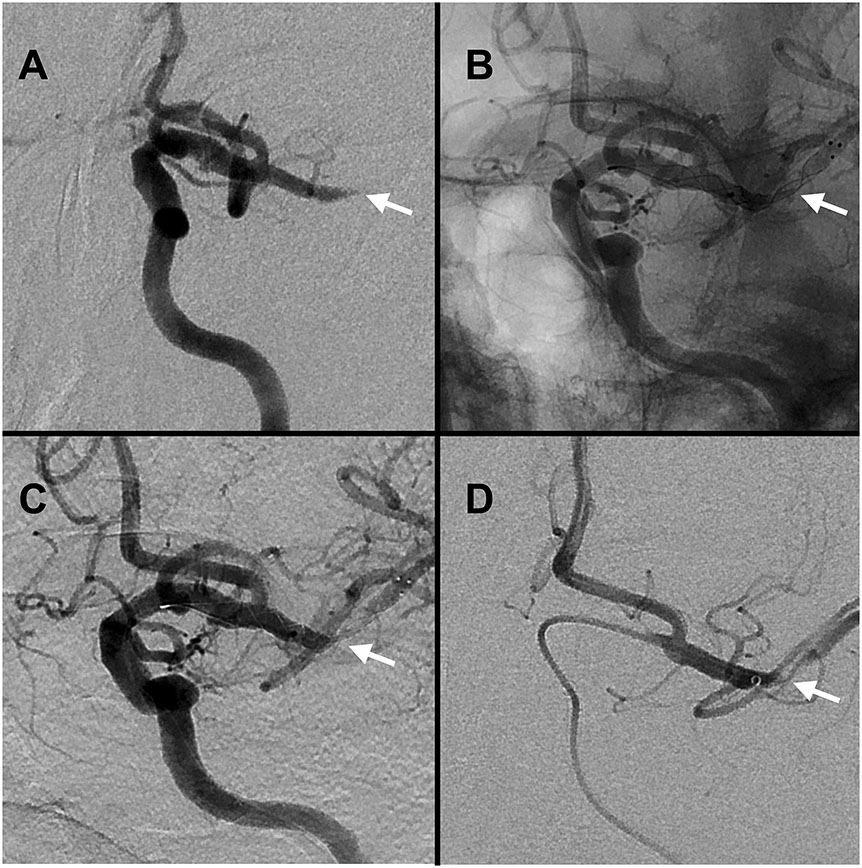
Figure 2.
Case of middle cerebral artery ICAD-LVO on angiography. Panel A: ICAD-LVO tapered occlusion (white arrow). Panels B-C: recanalization with deployed stentriever conforming to the underlying stenosis (white arrow, B unsubtracted, C subtracted). Panel D: post-recanalization angiography confirming ICAD with severe stenosis (black arrow).
Following reperfusion, the presence of any of the following criteria has been proposed as an acceptable definition of ICAD-LVO: a) residual fixed stenosis that measures > 50% (Figure 2D), b) evidence of distal hypoperfusion or watershed infarctions, or c) stenosis with re-occlusion on follow up angiography (Figure 1C).14,15 Re-occlusion is less common in cardioembolic LVO compared to ICAD-LVO (<5% vs. 30-50%, respectively).16-18 Differences between cardioembolic and ICAD-LVO are illustrated in Figure 3. In 2022, the European Stroke Organization defined ICAD-LVO incorporating these concepts, including: 1) absence of atrial fibrillation, 2) absence of CT hyperdense or MRI susceptibility sign, 3) watershed infarction, 4) truncal-type occlusion, 5) residual stenosis when the stent is open or after three passes or 6) early re-occlusion.19
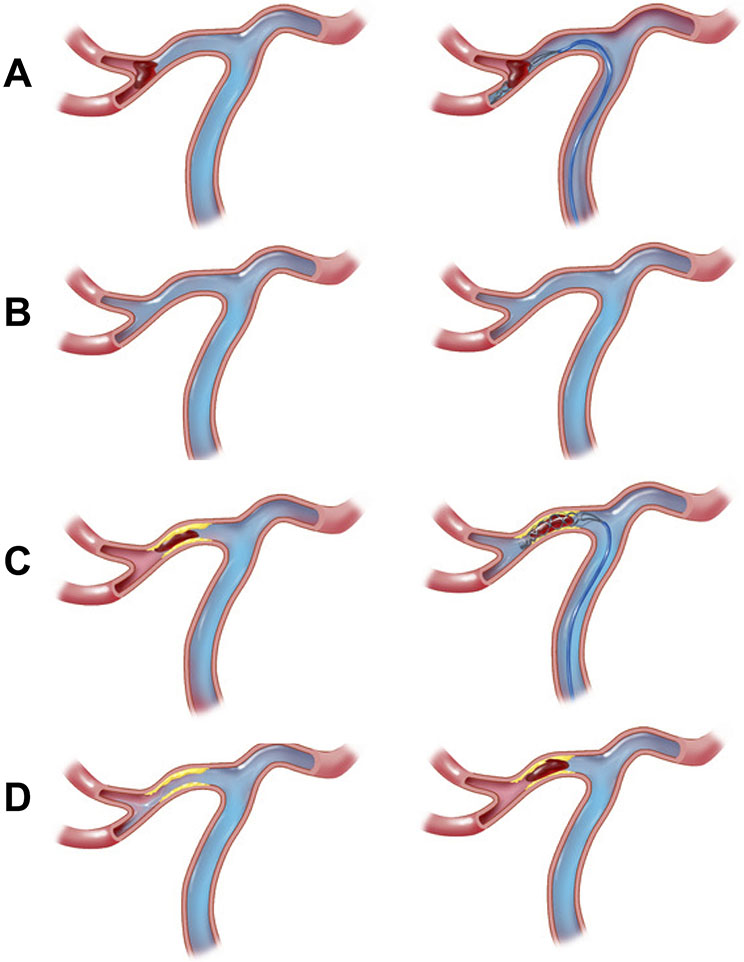
Figure 3.
Middle cerebral artery occlusion angiographic and EVT treatment images. Panels A-B (cardioembolic): a branch segment occlusion characteristic of atrial fibrillation that is successfully recanalized with a stent-retriever on follow-up angiography (panel B, right); Panels C-D (ICAD): a truncal occlusion characteristic of ICAD that is successfully recanalized with a stent-retriever (panel D, left) followed by re-occlusion due to ICAD (panel D, right). Note contrast flow when stentriever is deployed in the inferior division only with embolic LVO (panel A, right) compared to flow in both superior and inferior divisions in ICAD LVO case (panel C, right)
Recent studies explored sonographic imaging modalities such as intravascular ultrasound (IVUS) in ICAD-LVO.20 IVUS can identify intraplaque hemorrhage or ulceration, aid in better understanding ICAD-LVO and potentially stratify high risk patients for rescue angioplasty/stenting.21 Optical Coherence Tomography (OCT), which uses near infrared light, can provide information regarding plaque calcification, lipid content, and cap thickness.21,22
The differential diagnosis for ICAD-LVO includes vasospasm, dissection, and residual embolus.8,23,24 Vasospasm is characterized by a transient focal narrowing in the arterial lumen which may improve with intraarterial vasodilators such as verapamil or nicardipine.25,26 Intracranial dissection, which can be iatrogenic, traumatic, or spontaneous, is recognized when a dissection flap is visualized.27,28 A recalcitrant embolus is suspected when an abrupt cut-off in the vessel is visualized without change following EVT attempts. A change in the angiographic appearance following EVT attempts and the absence of a tapered stenosis are the hallmarks of a sub-occlusive residual embolus.28,29
Subacute Identification of ICAD-LVO
In ICAD-LVO patients who are not treated with EVT or fail recanalization, it may be impossible to identify underlying ICAD. For those who are recanalized, in the absence of diffuse atherosclerotic disease and/or atherosclerotic risk factors, isolated focal stenosis may represent a spontaneous dissection or unilateral idiopathic occlusive disease rather than an atherosclerotic plaque. Nonetheless, isolated focal stenosis may manifest as nascent ICAD and in population-based studies over 1/3 of individuals with ICAD had only one stenotic intracranial artery.30 Other arteriopathies that may cause luminal narrowing, such as reversible vasoconstriction syndrome or inflammatory etiologies such as primary angiitis of the CNS are unlikely to cause LVO as these tend to affect the small- or medium-size rather than large intracranial vessels.
There is emerging data on the use of high-resolution vessel wall MRI (vwMRI) for the characterization of atherosclerotic plaque, dissection, and moyamoya syndrome or disease.31 In moyamoya, the arterial vessel wall is generally not thickened. The outer and inner arterial diameters are concentrically reduced.32,33 In ICAD, there is often eccentric intramural thickening, with a heterogenous, eccentric T1 post-contrast enhancement that relates to the histopathology of the atherosclerotic process. In addition, there can be remodeling whereby the luminal diameter is preserved despite the presence of a large plaque.32 One caveat regarding the use of vwMRI is the association of vessel wall enhancement with EVT, particularly after stentriever use.34 In dissection, a double lumen or intimal flap may be found in up to 75% of patients, as well as an intramural hematoma (bright on T1-weighted sequences) in a similar proportion.35
Additional Approaches to Identifying ICAD-LVO
There are predictive models based on demographics and comorbidities for identifying ICAD-LVO. Zha et al. developed the ISAT (in situ atherosclerotic thrombosis) score for predicting atherosclerosis in acute vertebrobasilar occlusion patients.36 The score assigns 1 point each for: history of hypertension, atrial fibrillation and baseline glucose level ≥ 7.55 mmol/l. The area under the curve (AUC) values for the model were 0.85 and 0.80 in the derivation and validation cohorts, respectively. Liao et al. developed the ABC2D score which assigned 1 point each for: atrial fibrillation, hypertension, neurological deficit, CT hyperdense sign, and diabetes.37 The AUC for ICAD-LVO prediction was 0.88 in both the derivation and validation cohorts. Both studies were limited to Asian populations and neither have been validated prospectively.
Studies evaluating the histological findings of clots retrieved during EVT have reported conflicting results regarding the relationship between clot composition and underlying stroke etiology. While some studies report that atherosclerosis-related clots have a higher percentage of fibrin compared to cardioembolic clots, other studies found a lower percentage of fibrin in atherosclerosis-related clots or no difference at all.38-40 These conflicting results are likely secondary to heterogeneity in clot composition or the lack of unified radiographic criteria for ICAD-LVO diagnosis.
Endovascular Treatment Considerations
Similar to patients with cardioembolic LVO, those with ICAD-LVO can derive benefit from EVT and should receive this treatment based on conventional criteria.41 EVT pre-treatment is a consideration because up to half of ICAD-LVO patients experience re-occlusion.42 Much of the evidence is from non-LVO ICAD trials. In randomized trials of intracranial stenting for symptomatic non-LVO ICAD (SAMMPRIS, VISSIT, and CASSISS), patients were pretreated with dual antiplatelet therapy (DAPT).43-45 In the context of EVT and high suspicion for underlying ICAD-LVO, it may be reasonable to load with DAPT (e.g. aspirin 325 mg and clopidogrel 300 mg) in individuals who are not eligible for or do not receive intravenous thrombolysis (IVT).46 If patients were administered IVT, additional pre-treatment is currently not indicated.41
There are different endovascular approaches to treating ICAD-LVO. The central question is whether to start with a) contact aspiration (CA), b) stentriever (SR), or c) combined approach. In trials of EVT for LVO, no single approach has been shown to be superior, but these studies mostly enrolled cardioembolic LVOs.47,48 In a retrospective study of individuals with ICAD-LVO, 62 had CA and 49 had SR as their first-line EVT approach.25 SR-first achieved a higher rate of successful reperfusion (77.6%) versus CA-first (43.5%) (p=0.001). In the same study, switching to other approaches was more frequent in the CA-first (59.7%) versus SR-first approach (12.2%) (p<0.001).
The potential advantages of SR-first in ICAD-LVO are that SRs may conform their shape to the plaque, helping to establish the ICAD-LVO diagnosis. SRs also have radial force that can create a reperfusion channel, facilitating rescue therapy. With CA, the catheter tip is placed proximal to the occlusion and hence may not be in direct contact with the ICAD-LVO thrombus due to its contact with the plaque. Hence CA first-line for ICAD-LVO may be less effective than strategies that cover the length of the clot, such as SR.8 Furthermore, in patients with ST-segment elevation myocardial infarction, a cardiac analogue of ICAD-LVO, a meta-analysis of 3 RCTs showed that CA thrombectomy did not improve outcomes.49
When TICI 2b or better reperfusion has been achieved and maintained by EVT, most interventionalists will not pursue further treatment. However, a survey of 136 neurointerventionalists in North America revealed that 20% favored angioplasty and/or stenting over medical therapy in the treatment of ICAD-LVO.50 Nonetheless, most interventionalists pursue medical management alone after EVT for ICAD-LVO and randomized trial data of angioplasty and/or stenting for symptomatic ICAD without LVO shows a high rate of peri-procedural complications.43,44 However, there is a rationale for empiric angioplasty and/or stenting after successful TICI ≥ 2b reperfusion,51 based on the high probability of re-occlusion and the association of re-occlusion with unfavorable outcome.52,53
Ultimately, the data supporting the practice of angioplasty and/or stenting following successful thrombectomy is limited.19 Randomized clinical trial data would be needed to change the current practice of conservative management after a successful thrombectomy in ICAD-LVO. The European Stroke Organization guidelines propose that angioplasty and/or stenting could be used as rescue therapy in ICAD-LVO. While rescue therapy also lacks a trial evidence base, it is appealing because the LVO has already demonstrated that it will remain occluded. An international survey found that in the setting of ICAD with persistent basilar artery occlusion, many neurointerventionalists would consider rescue stenting or angioplasty, and would do so after 1, 2, or 3 passes without successful reperfusion (14.7%, 25.1%, and 48.2%, respectively).46
Several studies and meta-analyses have examined rescue therapy in ICAD-LVO. A meta-analysis compared outcomes among ICAD-LVO patients without revascularization treated conservatively versus those treated with rescue therapy and reported worse outcomes and higher mortality rates among ICAD-LVO patients managed conservatively.54 A propensity score matched analysis in a multicenter Asian cohort demonstrated higher rates of good outcome in patients with ICAD-LVO and refractory occlusion undergoing rescue angioplasty and/or stenting.55 A multicenter study in a Western population with ICAD-LVO reported good outcomes with rescue stenting.56 Another multicenter propensity matched analysis of patients with failed reperfusion after EVT indicated that patients who underwent rescue intracranial stenting had more favorable outcomes compared to those who failed reperfusion (acOR 2.31 [95%CI, 1.61-3.32]; p<0.001).57
A number of approaches to rescue therapy can be utilized: balloon angioplasty alone, stent alone (self-expanding or balloon-mounted), or stent with either pre- or post-stent balloon angioplasty.58 Due to its higher radial force, balloon-mounted stenting may be favored over a self-expanding stent for ICAD-LVO based on observational data in symptomatic ICAD patients.59 However, delivery of a BMS is more challenging than a SES due to the stiffness of the balloon on the delivery wire. There is no comparative data available on the different endovascular approaches. Atherosclerotic lesion length and geometry may influence the success of interventions.60 In a SAMMPRIS post hoc analysis, lesion length was the most important predictor of 30-day stroke or mortality in patients undergoing angioplasty and stenting.61 While lesion characteristics cannot be determined pre-EVT in ICAD-LVO, this classification may be possible during EVT based on the balloon response, stentriever expansion (Figure 2B), or post-EVT luminal angiographic appearance (Figure 2C).
In a meta-analysis, the re-occlusion rate following EVT in ICAD-LVO was 37% versus 2.7% without ICAD, with an OR of 23.7 for ICAD-LVO (95% CI, 6.96-80.7).42 No data exists regarding the mean time to re-occlusion following recanalization of ICAD-LVO. Generally, one can wait 10 to 20 minutes following EVT recanalization before assessing for re-occlusion. A proceduralist may look for filling defects suggestive of thrombus. If re-occlusion is observed, additional reperfusion and/or rescue angioplasty/stenting can be considered. Additionally, a flat-panel CT may rule out intracranial hemorrhage. Factors favoring rescue stenting include poor collaterals, large perfusion defects, and smaller size infarctions, while factors precluding stenting include intracranial hemorrhage, large core infarct, or extensive parenchymal contrast staining.
The peri-procedural stroke or death rates in the stenting of symptomatic severe ICAD in the Wingspan Stent System Post Market Surveillance (WEAVE) study were 2.6% in experienced operators using a protocolized approach to treatment.62 More data is needed to understand this element because interventional treatment of ICAD-LVO might be performed at any site capable of EVT, which spans a wide range of interventionalist expertise. An ongoing prospective registry of ICAD-LVO (RESCUE-ICAS) will shed light on some of the questions regarding the imaging characteristics and outcomes among patients managed with medical therapy compared to rescue angioplasty/stenting.63
Acute Phase Antithrombotic Therapy
Antiplatelet management of ICAD-LVO can be challenging. Because ICAD-LVO re-occlusion is usually caused by residual plaque and platelet activation leading to thrombosis,16,17 antiplatelet medications are often administered before, during, or after EVT. The 2019 AHA/ASA guidelines state that for patients treated with IVT, antithrombotic therapy should be delayed for 24 hours. However, initiating antithrombotic therapy within 24 hours after thrombolytic can be considered with conditions for which the benefit of early initiation is judged to outweigh the risk (Class of Recommendation IIb; Level of Evidence B-NR), which pertains to ICAD-LVO.41
Tirofiban is a selective GP IIb/IIIa inhibitor. Retrospective studies reported that LVO patients treated with parenteral tirofiban had a higher recanalization rate, better outcome, lower mortality, and less re-occlusion with residual stenosis, and a lower early re-occlusion rate after emergent angioplasty with or without stenting.64,65 In the RESCUE BT randomized trial (Endovascular Treatment With vs Without Tirofiban for Patients with Large Vessel Occlusion Stroke) trial, IV tirofiban (bolus dose before EVT, followed by 24-hour infusion) did not improve functional outcome compared to placebo. In a subgroup analysis of 435 patients with ICAD-LVO, tirofiban was associated with a favorable mRS shift [aOR 1.40 (95% CI 1.00-1.97); p=0.049] without increasing sICH [7.1% vs. 7.2%; aOR 1.05 (0.49-2.22)].66
Eptifibatide is another intravenous GP IIb/IIIa inhibitor. In a matched study of 81 patients treated with EVT plus pre-EVT eptifibatide vs. EVT alone, eptifibatide was associated with higher rates of successful recanalization (91.3% versus 81.5%; P=0.043) and 3-month mRS 0-2 (53.1% versus 33.3%; P=0.016) without increasing sICH. However, in another study of 50 patients with ICAD-LVO, the recanalization rate was similar between the eptifibatide and non-eptifibatide groups.67 Abciximab is another GP IIb/IIIa inhibitor; however as it is not reversible it is less appealing in an acute stroke context.68 The 2019 AHA/ASA guidelines state that the safety and efficacy of IV GP IIb/IIIa inhibitors during EVT is uncertain (Class of Recommendation IIb; Level of Evidence C-LD).41
Regarding other parenteral antiplatelet agents, there is no published data on IV aspirin in ICAD-LVO. For patients treated with IV alteplase, the ARTIS (Antiplatelet Therapy in Combination with rt-PA Thrombolysis in Ischemic Stroke) trial showed that IV aspirin increased the risk of sICH without benefit on functional outcome.69 Cangrelor is a parenteral platelet P2Y12 receptor antagonist. In a systematic review involving 171 patients with acute LVO, not limited to ICAD-LVO, who received EVT and parenteral cangrelor, the recanalization rate post-EVT day 1 was 89.7% (95% CI, 81.4%–94.6%) and 47.6% (95% CI, 27.4-68.7%) achieved mRS 0-2. The sICH rate was 8.6% (95% CI, 5.0%–14.3%), and the 90-day mortality rate was 22.6% (95% CI, 13.6%–35.2%).70 However, without a comparator group, these results are difficult to contextualize.
In acute ICAD-LVO, enteral antiplatelet therapy has less immediate effect than intravenous medications. In patients treated with rescue intracranial angioplasty/stenting and no intracranial hemorrhage, the benefit of DAPT with aspirin plus clopidogrel might outweigh the risk.71 Ticagrelor, a P2Y12 receptor antagonist, has a faster onset of action than clopidogrel, but no published data are available for ticagrelor in ICAD-LVO. In a retrospective study of patients treated with EVT plus extracranial carotid stenting, ticagrelor plus aspirin versus clopidogrel plus aspirin was associated with an increased risk of sICH.72
Subacute Medical Management of ICAD-LVO
Subacute Antiplatelet Therapy
The first high-quality data on subacute DAPT in patients with ICAD comes from the SAMMPRIS trial.73 SAMMPRIS randomized 451 patients with symptomatic 70-99% ICAD to best medical management compared to best medical management plus angioplasty/stenting. Best medical management consisted of DAPT (aspirin plus clopidogrel) for 90 days, followed by a single agent, high-intensity statin, and lifestyle changes.73 The recurrence rate in medically treated patients was lower than that reported in prior studies and there was no sICH from DAPT in the medical arm.74
In patients with minor stroke or TIA, three randomized trials showed the superiority of DAPT with aspirin and clopidogrel as well as aspirin and ticagrelor for three weeks to three months following stroke compared to aspirin monotherapy.75-77 In subgroup analyses, DAPT appeared to be more effective in patients with intracranial and extracranial arterial stenosis.78 The POINT trial suggested that the efficacy of DAPT was greatest in the first 30 days after which there was an increased risk of major bleeding events without additional benefit.75 The CHANCE trial showed that clopidogrel was only effective in non-CYP2C19 carriers and the CHANCE-2 trial showed the superiority of ticagrelor over clopidogrel in patients with minor stroke and TIA who were carriers of CYP2C19.79 CHANCE-2 was performed in a Chinese population, thus limiting the generalizability of its findings. A post-hoc analysis of the POINT trial did not show that CYP2C19 status modified the effect of clopidogrel.80
Based on the current evidence, it is reasonable to use DAPT with aspirin and clopidogrel or aspirin and ticagrelor for 90-days after successful recanalization of ICAD-LVO, followed by aspirin or another antiplatelet monotherapy. Furthermore, while testing for clopidogrel resistance is reasonable, the clinical utility in populations outside East Asia remains uncertain. In the sub-group of patients with rescue stenting, longer term antiplatelet therapy may be required. In such scenarios, the benefit of longer-duration antiplatelets should be weighed against hemorrhagic risk.81
Blood Pressure Management
For patients receiving IVT and/or EVT, a post treatment blood pressure goal of <180/105 mmHg is recommended by the AHA and European Stroke Organization guidelines.41 When neither treatment is performed, permissive hypertension up to 220/110 mmHg is recommended unless concurrent medical conditions warrant BP lowering.
In the real world, lower BP goals are often targeted, especially with successful EVT.82 Two randomized trials failed to show the benefit of BP lowering after successful EVT.83,84 Worse outcomes were observed in successfully reperfused patients with intensive blood pressure target (i.e., SBP ≤ 120 mm Hg) in the ENCHANTED-2 trial, in which 48% of enrolled patients had ICAD-LVO. Hence this intensive blood pressure target should be avoided. Chronic hypertension, the main contributor to ICAD and stroke risk, causes a right shift in cerebral autoregulation.85 This puts ICAD patients at risk of compromised cerebral perfusion, even with small reductions in systemic BP. Thus, for ICAD-LVO, higher blood pressure targets, even after successful EVT, may be justified in the short term. After successful revascularization, ICAD-LVO are also prone to re-occlusion, reinforcing the considerations for higher blood pressure targets in the subacute phase.16 Additionally, use of acute angioplasty/stenting and antiplatelet medications complicates hemodynamic management,86 warranting flexible approaches tailored to the patient.
Depending on the patient’s clinical course, blood pressure can begin to be lowered in the days after stroke onset and long-term antihypertensive therapy can be instituted. Post-hoc analyses of ICAD patients in WASID and SAMMPRIS demonstrated that a mean SBP <140 mm Hg during long-term follow-up was associated with a lower risk of recurrent stroke and vascular events,43,87,88 but the current AHA guidelines recommend a target SBP of <130 mm Hg. However, there may be a subgroup of ICAD-LVO patients who benefit from a higher target of <140 mm Hg for weeks or months after a stroke given the short-term risk of hemodynamic failure.88-91
Lipid lowering Therapy
An elevated low-density lipoprotein (LDL) is associated with ICAD and increases the risk of stroke recurrence.92 There is strong evidence supporting the use of high-intensity statins among patients with atherosclerotic disease.93 With regards to ICAD specifically, a single center randomized trial showed lower risk of cerebrovascular events and improved perfusion imaging parameters among patients treated with high-intensity statins.94 Recent studies have also shown atherosclerotic plaque stabilization among patients with ICAD using high intensity statins.95 Post hoc analyses of the SAMMPRIS and WASID trials showed lower rates of recurrent strokes among patients with lower LDL.96 The current AHA/ASA and AAN guidelines recommend high-intensity statin therapy to achieve a target LDL of < 70 mg/dL for patients with symptomatic ICAD.89,97
Outcomes in ICAD-LVO and Considerations for Patient Follow-Up
The clinical course and functional outcome of patients with ICAD-LVO is distinct from cardioembolic LVO, although the two conditions may co-exist.98 Because the development of ICAD is a chronic process, collaterals may develop over time. As a result, ICAD-LVO may present with lower severity of symptoms or fluctuating symptoms compared to a cardioembolic LVO in which the symptoms are maximal at onset.8 The German Stroke Registry demonstrated that in a cohort of 2,637 patients cardioembolic LVO was associated with better 90-day functional outcomes compared with large artery atherosclerosis LVO (aOR 1.25, 95% CI 1.01–1.49), which was partially driven by higher reperfusion rates in the cardioembolic group.99 The Endovascular Treatment Key Technique and Emergency WorkFlow Improvement of Acute Ischemic Stroke (ANGEL-ACT) registry in China examined outcomes in 1,635 patients with LVO. Despite higher reperfusion scores in patients with a cardioembolic etiology, the 90-day outcomes were similar between stroke subtypes.100 There is no available data regarding long-term outcomes among patients with ICAD-LVO treated with rescue stenting or medical management.
Further complicating the picture, ICAD is a progressive vascular disease with a higher risk of subsequent ischemic events, even past the 90-day outcome window. After reperfusion of ICAD-LVO, more severe stenosis predicts the risk of recurrent stroke in the territory of the stenotic artery. The one-year rate of recurrent stroke in patients with symptomatic ≥70% stenosis is between 10 to 30%, despite aggressive medical treatment, compared to <10% in those with less severe stenosis.43,101-103 In general, intensive clinical follow-up programs correlate with better patient outcomes.104
Follow-up of ICAD-LVO patients is also determined in part by the treatment approach at the time of the index event. For patients managed with medical therapy or EVT combined with medical therapy, residual stenosis is monitored for progression. Durable complete occlusion may require no imaging follow-up specific to that artery. For patients who are treated with rescue angioplasty and/or stenting, the treated artery requires follow-up imaging for a patient-specific, variable period of time to determine the transition to single antiplatelet therapy. Finally, posterior circulation ICAD may be more prone to both progression and symptoms and should be monitored more closely.8
Conclusion
ICAD-LVO is an increasingly recognized subgroup of LVO with unique diagnostic and therapeutic considerations. Although ICAD-LVO typically presents with less severe stroke, functional outcomes are equivalent to other mechanisms of LVO because of the high rate of re-occlusion and a high rate of recurrent stroke. ICAD-LVO warrants dedicated research to establish an evidence base for individualized treatment decisions.
Sources of Funding:
Dr. de Havenon reports NIH/NINDS funding (K23NS105924).
Non-Standard Abbreviations
- CA
contact aspiration
- DAPT
dual antiplatelet therapy
- EVT
endovascular therapy
- ICAD
intracranial atherosclerotic disease
- LVO
large vessel occlusion
- SR
stentriever
- vwMRI
vessel wall MRI
Footnotes
Disclosures:
Dr. de Havenon reports compensation from Novo Nordisk for consultant services; grants from American Heart Association; stock options in TitinKM; compensation from Integra for consultant services; and stock options in Certus. Dr .Nguyen reports compensation from Medtronic for other services and grants from Society of Vascular and Interventional Neurology. Dr. Mazighi reports compensation from boerhinger-ingelheim for consultant services; compensation from Novo Nordisk for consultant services; and compensation from acticor biotech for consultant services. Dr. Mistry reports employment by University of Cincinnati; grants from National Institute of Health; compensation from RAPID AI for consultant services; and compensation from American Heart Association for consultant services. Dr. Yaghi reports compensation from Medtronic USA, Inc. for other services and employment by Brown University. Dr.Derdeyn reports employment by University of Iowa; compensation from Penumbra, Inc. for data and safety monitoring services; compensation from noNO for data and safety monitoring services; compensation from Silk Road Medical, Inc. for data and safety monitoring services; and stock options in Euphrates Vascular. Dr. Al Kasab reports compensation from Stryker for other services.
References
- 1.Donkor ES. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat 2018;2018:3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waqas M, Rai AT, Vakharia K, Chin F, Siddiqui AH. Effect of definition and methods on estimates of prevalence of large vessel occlusion in acute ischemic stroke: a systematic review and meta-analysis. J. NeuroInterventional Surg 2020;12:260–265. [DOI] [PubMed] [Google Scholar]
- 3.Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, Lucke-Wold N, Carpenter JS. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J. Neurointerventional Surg 2017;9:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri MFK, Johnston SC. Epidemiology of intracranial stenosis. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2009;19 Suppl 1:11S–6S. [DOI] [PubMed] [Google Scholar]
- 5.Bang OY. Intracranial Atherosclerosis: Current Understanding and Perspectives. J. Stroke 2014;16:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee C, Chimowitz MI. Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circ. Res 2017;120:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia B, Ren Z, Mokin M, Burgin WS, Bauer CT, Fiehler J, Mo D, Ma N, Gao F, Huo X, et al. Current Status of Endovascular Treatment for Acute Large Vessel Occlusion in China: A Real-World Nationwide Registry. Stroke. 2021;52:1203–1212. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Lee S-J, Hong JM, Alverne FJAM, Lima FO, Nogueira RG. Endovascular Treatment of Large Vessel Occlusion Strokes Due to Intracranial Atherosclerotic Disease. J. Stroke 2022;24:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011;42:S20–23. [DOI] [PubMed] [Google Scholar]
- 10.Kim YW. Effect of Intracranial Atherosclerotic Disease on Endovascular Treatment for Patients with Acute Vertebrobasilar Occlusion. AJNR Am J Neuroradiol. 2016;37:2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek JH. Utility of Leptomeningeal Collaterals in Predicting Intracranial Atherosclerosis-Related Large Vessel Occlusion in Endovascular Treatment. J Clin Med. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh HI. Imaging Predictors for Atherosclerosis-Related Intracranial Large Artery Occlusions in Acute Anterior Circulation Stroke. J Stroke. 2016;18:352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Kasab S, Almallouhi E, Spiotta AM. Rescue Endovascular Treatment for Emergent Large Vessel Occlusion With Underlying Intracranial Atherosclerosis: Current State and Future Directions. Front. Neurol. [Internet] 2021. [cited 2022 Oct 5];12. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2021.734971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y. Prevalence and Outcomes of Symptomatic Intracranial Large Artery Stenoses and Occlusions in China. Stroke. 2014;45:663–669. [DOI] [PubMed] [Google Scholar]
- 15.Baek J-H. Outcomes of Endovascular Treatment for Acute Intracranial Atherosclerosis Related Large Vessel Occlusion. Stroke. 2018;49:2699–2705. [DOI] [PubMed] [Google Scholar]
- 16.Tsang ACO. Thrombectomy Outcomes of Intracranial Atherosclerosis-Related Occlusions. Stroke. 2019;50:1460–1466. [DOI] [PubMed] [Google Scholar]
- 17.Yin NS. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke. 2010;41:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang DH. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37:350–5. [DOI] [PubMed] [Google Scholar]
- 19.Psychogios M, Brehm A, López-Cancio E, Marco De Marchis G, Meseguer E, Katsanos AH, Kremer C, Sporns P, Zedde M, Kobayashi A, et al. European Stroke Organisation guidelines on treatment of patients with intracranial atherosclerotic disease. Eur. Stroke J 2022;7:XLII–LXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zacharatos H, Hassan AE, Qureshi AI. Intravascular Ultrasound: Principles and Cerebrovascular Applications. AJNR Am. J. Neuroradiol 2010;31:586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlin-Premrl D, Sharma R, Campbell BCV, Mocco J, Opie NL, Oxley TJ. Advanced Imaging of Intracranial Atherosclerosis: Lessons from Interventional Cardiology. Front. Neurol. [Internet] 2017. [cited 2023 Jan 27];8. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2017.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaraja V, Kalra A, Puri R. When to use intravascular ultrasound or optical coherence tomography during percutaneous coronary intervention? Cardiovasc. Diagn. Ther 2020;10:1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek J-H, Kim BM. Angiographical Identification of Intracranial, Atherosclerosis-Related, Large Vessel Occlusion in Endovascular Treatment. Front. Neurol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang OY. Endovascular Therapy for Acute Ischemic Stroke of Intracranial Atherosclerotic Origin-Neuroimaging Perspectives. Front Neurol. 2019;10:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo J. Immediate effects of first-line thrombectomy devices for intracranial atherosclerosis-related occlusion: stent retriever versus contact aspiration. BMC Neurol; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchikawa H. Vasospasm as a major complication after acute mechanical thrombectomy with stent retrievers. J. Clin. Neurosci 2019;64:163–168. [DOI] [PubMed] [Google Scholar]
- 27.Debette S. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14:640–654. [DOI] [PubMed] [Google Scholar]
- 28.Abdalla RN. Refractory Stroke Thrombectomy: Prevalence, Etiology, and Adjunctive Treatment in a North American Cohort. AJNR Am J Neuroradiol. 2021;42:1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BM. Causes and Solutions of Endovascular Treatment Failure. J Stroke. 2017;19:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao Y, Guallar E, Suri FK, Liu L, Zhang Y, Anwar Z, Mirbagheri S, Xie YJ, Nezami N, Intrapiromkul J, et al. MR Imaging Measures of Intracranial Atherosclerosis in a Population-based Study. Radiology. 2016;280:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Havenon A, Chung L, Park M, Mossa-Basha M. Intracranial vessel wall MRI: a review of current indications and future applications. Neurovascular Imaging. 2016;2:10. [Google Scholar]
- 32.Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, Xiao XL. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett. 2015;584:77–82. [DOI] [PubMed] [Google Scholar]
- 33.Jiang T, Perry A, RG D Jr, Zipfel GJ, Derdeyn CP. Intracranial atherosclerotic disease associated with moyamoya collateral formation: histopathological findings. J Neurosurg. 2013;May;118(5):1030–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasab SA, Bathla G, Varon A, Roa JA, Sabotin R, Raghuram A, Chaorong W, Hasan DM, Turan TN, Chatterjee R, et al. High-resolution vessel wall imaging after mechanical thrombectomy. Neuroradiol. J. 2021;34:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryu J, Lee KM, Kim H-G, Choi SK, Kim EJ. Diagnostic Performance of High-Resolution Vessel Wall Magnetic Resonance Imaging and Digital Subtraction Angiography in Intracranial Vertebral Artery Dissection. Diagn. Basel Switz 2022;12:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zha M. A Pre-interventional scale to predict in situ atherosclerotic thrombosis in acute vertebrobasilar artery occlusion patients. Front. Neurol 2021;12:648081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao G. A simple score to predict atherosclerotic or embolic intracranial large-vessel occlusion stroke before endovascular treatment. J. Neurosurg 2022;1:1–8. [DOI] [PubMed] [Google Scholar]
- 38.Kim SK. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am J Neuroradiol. 2015;36:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khismatullin RR. Quantitative Morphology of Cerebral Thrombi Related to Intravital Contraction and Clinical Features of Ischemic Stroke. Stroke. 2020;51:3640–3650. [DOI] [PubMed] [Google Scholar]
- 40.Niesten JM. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 42.Tsang ACO, Orru E, Klostranec JM, Yang I-H, Lau KK, Tsang FCP, Lui WM, Pereira VM, Krings T. Thrombectomy Outcomes of Intracranial Atherosclerosis-Related Occlusions. Stroke. 2019;50:1460–1466. [DOI] [PubMed] [Google Scholar]
- 43.Chimowitz MI, Lynn MJ, Derdeyn CP. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, Gupta R, Kirshner H, Megerian JT, Lesko J, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;24-31;313(12):1240–8. [DOI] [PubMed] [Google Scholar]
- 45.Gao P, Wang T, Wang D, Liebeskind DS, Shi H, Li T, Zhao Z, Cai Y, Wu W, He W, et al. CASSISS Trial Investigators. Eff. Stenting Plus Med. Ther. Vs Med. Ther. Alone Risk Stroke Death Patients Symptomatic Intracranial Stenosis CASSISS Randomized Clin. Trial JAMA. 2022;9;328(6):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein P, Herning A, Drumm B, Raymond J, Abdalkader M, Siegler JE, Chen Y, Huo X, Schonewille WJ, Liu X, et al. Basilar Artery Occlusion Thrombectomy Technique: An International Survey of Practice Patterns†. Stroke Vasc. Interv. Neurol 0:e000642. [Google Scholar]
- 47.Lapergue B. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER Randomized Clinical Trial. JAMA. 2017;318:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;10. [DOI] [PubMed] [Google Scholar]
- 49.Jolly SS, James S, Džavík V, Cairns JA, Mahmoud KD, Zijlstra F, Yusuf S, Olivecrona GK, Renlund H, Gao P, et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation. 2017;135:143–152. [DOI] [PubMed] [Google Scholar]
- 50.Lazaro TT, Hoang AN, Cotton PC, Dang HQ, Tanweer O, Raper DMS. Management strategies of unanticipated intracranial stenosis during mechanical thrombectomy for acute stroke: A survey of academic neurointerventionalists. Interv. Neuroradiol. J. Peritherapeutic Neuroradiol. Surg. Proced. Relat. Neurosci 2022;15910199221110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowry R, Parker SA, Yamal J-M, Hwang H, Appana S, Rangel-Gutierrez N, Wu T-C, Rajan SS, Grotta JC. Time to Decision and Treatment With tPA (Tissue-Type Plasminogen Activator) Using Telemedicine Versus an Onboard Neurologist on a Mobile Stroke Unit. Stroke. 2018;49:1528–1530. [DOI] [PubMed] [Google Scholar]
- 52.Mosimann PJ, Kaesmacher J, Gautschi D, Bellwald S, Panos L, Piechowiak E, Dobrocky T, Zibold F, Mordasini P, El-Koussy M, et al. Predictors of Unexpected Early Reocclusion After Successful Mechanical Thrombectomy in Acute Ischemic Stroke Patients. Stroke. 2018;49:2643–2651. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira R, Correia MA, Marto JP, Carvalho Dias M, Mohamed GA, Nguyen TN, Nogueira RG, Aboul-Nour H, Marin H, Bou Chebl A, et al. Reocclusion after successful endovascular treatment in acute ischemic stroke: systematic review and meta-analysis. J. Neurointerventional Surg 2022;jnis-2022-019382. [DOI] [PubMed] [Google Scholar]
- 54.Almallouhi E, Murad MH, Chalhoub R, Kicielinski KP, Lena J, Brennan EA, Zaidat O, de Havenon A, Spiotta AM, Al Kasab S. Rescue Endovascular Treatment of Patients With Emergent Large Vessel Occlusion Attributed to Intracranial Atherosclerosis: A Systematic Review and Meta-Analysis. Stroke Vasc. Interv. Neurol 0:e000510. [Google Scholar]
- 55.Peng F, Wan J, Liu W, Huang W, Wang L, Qiu T, Yang S, Shi Q, Zhang S, Zeng G, et al. Efficacy and safety of rescue stenting following failed mechanical thrombectomy for anterior circulation large vessel occlusion: propensity score analysis. J. NeuroInterventional Surg 2020;12:271–273. [DOI] [PubMed] [Google Scholar]
- 56.Al Kasab S, Almallouhi E, Alawieh A, Wolfe S, Fargen KM, Arthur AS, Goyal N, Dumont T, Kan P, Kim J, et al. Outcomes of Rescue Endovascular Treatment of Emergent Large Vessel Occlusion in Patients With Underlying Intracranial Atherosclerosis: Insights From STAR. J. Am. Heart Assoc 2021;10:e020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohammaden MH, Haussen DC, Al-Bayati AR, Hassan A, Tekle W, Fifi J, Matsoukas S, Kuybu O, Gross BA, Lang MJ, et al. Stenting and Angioplasty in Neurothrombectomy: Matched Analysis of Rescue Intracranial Stenting Versus Failed Thrombectomy. Stroke. 2022;53:2779–2788. [DOI] [PubMed] [Google Scholar]
- 58.Lee JS. Endovascular Treatment of Large Vessel Occlusion Strokes Due to Intracranial Atherosclerotic Disease. J Stroke. 2022;24:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hassan AE, Khalil M, Desai S, Tekle WG. Resolute onyx stent more effective than wingspan stent at preventing procedural complications and long-term restenosis. Interv. Neuroradiol. J. Peritherapeutic Neuroradiol. Surg. Proced. Relat. Neurosci 2022;15910199221104632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miao Z, Song L, Liebeskind DS, Liu L, Ma N, Wang Y, Mo D, Gao F, Zhao X, Dong K, et al. Outcomes of tailored angioplasty and/or stenting for symptomatic intracranial atherosclerosis: a prospective cohort study after SAMMPRIS. J. NeuroInterventional Surg 2015;7:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaghi S, Khatri P, de Havenon A, Yeatts S, Chang AD, Cutting S, Grory BM, Burton T, Jayaraman MV, McTaggart RA, et al. Peri-procedural stroke or death in stenting of symptomatic severe intracranial stenosis. J. NeuroInterventional Surg 2020;12:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, Song SS, Yu W, null null, Feng L, et al. WEAVE Trial. Stroke. 2019;50:889–894. [DOI] [PubMed] [Google Scholar]
- 63.MD SAK. Registry of Emergent Large veSsel oCclUsion duE to IntraCranial AtherosclerosiS [Internet]. clinicaltrials.gov; 2022. [cited 2022 Dec 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT05403593 [Google Scholar]
- 64.Sun X, Zhang H, Tong X, Gao F, Ma G, Miao Z. Effects of Periprocedural Tirofiban vs. Oral Antiplatelet Drug Therapy on Posterior Circulation Infarction in Patients With Acute Intracranial Atherosclerosis-Related Vertebrobasilar Artery Occlusion. Front Neurol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baek BH, Yoon W, Lee YY, Kim SK, Kim JT, Park MS. Intravenous Tirofiban Infusion After Angioplasty and Stenting in Intracranial Atherosclerotic Stenosis-Related Stroke. Stroke. 2021;52:1601–1608. [DOI] [PubMed] [Google Scholar]
- 66.Investigators RBT, Qiu Z, Li F, Sang H, Luo W, Liu S, Liu W, Guo Z, Li H, Sun D. Effect of Intravenous Tirofiban vs Placebo Before Endovascular Thrombectomy on Functional Outcomes in Large Vessel Occlusion Stroke: The RESCUE BT Randomized Clinical Trial. JAMA. 2022;328:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma G, Sun X, Cheng H, Burgin WS, Luo W, Jia W, Liu Y, He W, Geng X, Zhu L. Combined Approach to Eptifibatide and Thrombectomy in Acute Ischemic Stroke Because of Large Vessel Occlusion: A Matched-Control Analysis. Stroke. 2022;53:1580–1588. [DOI] [PubMed] [Google Scholar]
- 68.Lee JS, Hwang Y-H, Sohn S-I. Factors Contributing to an Efficacious Endovascular Treatment for Acute Ischemic Stroke in Asian Population. Neurointervention. 2021;16:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zinkstok SM, Roos YB, A. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380:731–737. [DOI] [PubMed] [Google Scholar]
- 70.Marnat G, Finistis S, Delvoye F, Sibon I, Desilles JP, Mazighi M, Gariel F, Consoli A, Rosso C, Clarencon F. Safety and Efficacy of Cangrelor in Acute Stroke Treated with Mechanical Thrombectomy: Endovascular Treatment of Ischemic Stroke Registry and Meta-analysis. AJNR Am J Neuroradiol. 2022;43:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS ∣ European Heart Journal ∣ Oxford Academic [Internet]. [cited 2022 Dec 11];Available from: https://academic.oup.com/eurheartj/article/39/3/213/4095043 [Google Scholar]
- 72.Bucke P, Aguilar Perez M, AlMatter M, Hellstern V, Bazner H, Henkes H. Functional Outcome and Safety of Intracranial Thrombectomy After Emergent Extracranial Stenting in Acute Ischemic Stroke Due to Tandem Occlusions. Front Neurol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (sammpris): The final results of a randomised trial. Lancet. 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaturvedi S, Turan TN, Lynn MJ, Derdeyn CP, Fiorella D, Janis LS. Do patient characteristics explain the differences in outcome between medically treated patients in sammpris and wasid? Stroke. 2015;46:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N. Engl. J. Med 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 77.Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, et al. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N. Engl. J. Med 2016;375:35–43. [DOI] [PubMed] [Google Scholar]
- 78.Amarenco P, Denison H, Evans SR H A J S K, M. Ticagrelor added to aspirin in acute nonsevere ischemic stroke or transient ischemic attack of atherosclerotic origin. Stroke. 2020;51:3504–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Zhao X, Lin J, Li H, Johnston SC, Lin Y. Association between cyp2c19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. Jama. 2016;316:70–78. [DOI] [PubMed] [Google Scholar]
- 80.Meschia JF, Walton RL, Farrugia LP, Ross OA, Elm JJ, Farrant M. Efficacy of clopidogrel for prevention of stroke based on cyp2c19 allele status in the point trial. Stroke. 2020;51:2058–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diener H-C, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht H-J, MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet Lond. Engl 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 82.Mistry EA, Mayer SA, Khatri P. Blood Pressure Management after Mechanical Thrombectomy for Acute Ischemic Stroke: A Survey of the StrokeNet Sites. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc 2018;27:2474–2478. [DOI] [PubMed] [Google Scholar]
- 83.Yang P, Song L, Zhang Y, Zhang X, Chen X, Li Y, Sun L, Wan Y, Billot L, Li Q, et al. Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open-label, blinded-endpoint, randomised controlled trial. Lancet Lond. Engl 2022;400:1585–1596. [DOI] [PubMed] [Google Scholar]
- 84.Mazighi M, Richard S, Lapergue B, Sibon I, Gory B, Berge J, Consoli A, Labreuche J, Olivot J-M, Broderick J, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021;20:265–274. [DOI] [PubMed] [Google Scholar]
- 85.Ruland S, Aiyagari V. Cerebral autoregulation and blood pressure lowering. Hypertens. Dallas Tex 1979. 2007;49:977–978. [DOI] [PubMed] [Google Scholar]
- 86.Wu C, Chang W, Wu D, Wen C, Zhang J, Xu R, Liu X, Lian Y, Xie N, Li C, et al. Angioplasty and/or stenting after thrombectomy in patients with underlying intracranial atherosclerotic stenosis. Neuroradiology. 2019;61:1073–1081. [DOI] [PubMed] [Google Scholar]
- 87.Chimowitz MI, Lynn MJ, Howlett-Smith H. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. [DOI] [PubMed] [Google Scholar]
- 88.Feng X, Chan KL, Lan L. Translesional Pressure Gradient Alters Relationship Between Blood Pressure and Recurrent Stroke in Intracranial Stenosis. Stroke. 2020;51:1862–1864. [DOI] [PubMed] [Google Scholar]
- 89.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke [Internet]. 2021. [cited 2022 Aug 10];52. Available from: https://www.ahajournals.org/doi/10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 90.Flusty B, Havenon A, Prabhakaran S, Liebeskind DS, Yaghi S. Intracranial Atherosclerosis Treatment: Past, Present, and Future. Stroke. 2020;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amin-Hanjani S, Turan TN, Du X. Higher Stroke Risk with Lower Blood Pressure in Hemodynamic Vertebrobasilar Disease: Analysis from the VERiTAS Study. J Stroke Cerebrovasc Dis. 2017;26:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gutierrez J, Turan TN, Hoh BL, Chimowitz MI. Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2022;21:355–368. [DOI] [PubMed] [Google Scholar]
- 93.Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha J-K, Ducrocq G, Giroud M, et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med 2020;382:9–19. [DOI] [PubMed] [Google Scholar]
- 94.Zhou P, Lu Z, Gao P, Wang P, Cao Z, Zhang G, Wang S, Feng Y, Wang P. Efficacy and safety of intensive statin therapy in Chinese patients with atherosclerotic intracranial arterial stenosis: a single-center, randomized, single-blind, parallel-group study with one-year follow-up. Clin. Neurol. Neurosurg 2014;120:6–13. [DOI] [PubMed] [Google Scholar]
- 95.Chung J-W, Cha J, Lee MJ, Yu I-W, Park M-S, Seo W-K, Kim ST, Bang OY. Intensive Statin Treatment in Acute Ischaemic Stroke Patients with Intracranial Atherosclerosis: a High-Resolution Magnetic Resonance Imaging study (STAMINA-MRI Study). J. Neurol. Neurosurg. Psychiatry 2020;91:204–211. [DOI] [PubMed] [Google Scholar]
- 96.Turan TN, Nizam A, Lynn MJ, Egan BM, Le N-A, Lopes-Virella MF, Hermayer KL, Harrell J, Derdeyn CP, Fiorella D, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turan TN, Zaidat OO, Gronseth GS, Chimowitz MI, Culebras A, Furlan AJ, Goldstein LB, Gonzalez NR, Latorre JG, Messé SR, et al. Stroke Prevention in Symptomatic Large Artery Intracranial Atherosclerosis Practice Advisory: Report of the AAN Guideline Subcommittee. Neurology. 2022;98:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JS, Hong JM, Lee KS, Suh HI, Demchuk AM, Hwang Y-H, Kim BM, Kim JS. Endovascular Therapy of Cerebral Arterial Occlusions: Intracranial Atherosclerosis versus Embolism. J Stroke Cerebrovasc Dis. 2015;24:2074–2080. [DOI] [PubMed] [Google Scholar]
- 99.Tiedt S, Herzberg M, Küpper C, Feil K, Kellert L, Dorn F, Liebig T, Alegiani A, Dichgans M, Wollenweber FA. Stroke Etiology Modifies the Effect of Endovascular Treatment in Acute Stroke. Stroke. 2020;51:1014–1016. [DOI] [PubMed] [Google Scholar]
- 100.Huo X, Sun D, Raynald J B T X W A M N G F M D M G. Endovascular Treatment in Acute Ischemic Stroke with Large Vessel Occlusion According to Different Stroke Subtypes: Data from ANGEL-ACT Registry. Neurol Ther. 2022;11:151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hurford R, Wolters FJ, Li L, Lau KK, Kuker W, Rothwell PM. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: A population-based cohort study. Lancet Neurol. 2020;19:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. [DOI] [PubMed] [Google Scholar]
- 103.Prabhakaran S, Liebeskind DS, Cotsonis G, Nizam A, Feldmann E, Sangha RS, Campo-Bustillo I, Romano JG, MYRIAD Investigators. Predictors of Early Infarct Recurrence in Patients With Symptomatic Intracranial Atherosclerotic Disease. Stroke. 2021;52:1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmadi M, Laumeier I, Ihl T, Steinicke M, Ferse C, Endres M. A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (inspire-tms): An open-label, randomised controlled trial. Lancet Neurol. 2020;19:49–60. [DOI] [PubMed] [Google Scholar]