Abstract
Tanapox is a rarely diagnosed zoonosis known to be endemic to equatorial Africa. All previously reported human cases were acquired within 10° north or south of the Equator, most recently 19 years ago. We describe a human case of tanapox in South Africa (24° south of the Equator). Expanded surveillance for this pathogen is warranted.
Keywords: Yatapoxvirus, Tanapoxvirus, South Africa, zoonotic virus, zoonoses, viruses, light microscopy, electron microscopy, PCR, DNA sequencing, vector-borne infections
Tanapox is a rarely diagnosed zoonosis endemic to equatorial Africa. Only 4 exported cases from Africa involving either human or nonhuman primates have been reported (Table). Initial human outbreaks, in 1957 and 1962, were recorded from the Tana River Valley of Kenya, and the etiologic agent was subsequently isolated and described as Tanapox virus (TANV) (genus Yatapoxvirus, family Poxviridae) in 1965 (1). Subsequently, the occurrence of tanapox in research laboratory primates imported into the United States (2,3) led to a serologic survey of 12 nonhuman primate species from Kenya, Ethiopia, Cameroon, Côte d’Ivoire, Liberia, and Senegal; seropositivity was detected in all species surveyed in those countries (8). Therefore, nonhuman primates across equatorial Africa were surmised to be the natural reservoirs of TANV and humans incidental hosts (3,8). On the basis of the overlap between human tanapox cases and the geographic ranges of selected nonhuman primates, an ecologic niche model predicted that tanapox could be found from Somalia to Senegal, with the most southerly range above the Tropic of Capricorn (9).
Table. History of recorded tanapox cases in humans and nonhuman primates, 1957–2004.
| Year | Location of exposure | Epidemiologic description | Reference |
|---|---|---|---|
| 1957 |
Ngau, Kenya (Tana River Valley) |
Several Wapakomo school children diagnosed with tanapox |
(1) |
| 1962 |
Between Garissa and Garsen, Kenya (Tana River Valley) |
About 50 case-patients from the Wapakomo tribe |
(1) |
| 1965–1966* |
Holding facilities of primate supplier, USA |
Infected macaques from the same supplier, distributed to 3 primate research centers in Oregon, California, and Texas, USA |
(2–4) |
| 1966–1968† |
Laboratory-acquired |
Several laboratory workers in Oregon and California became infected after handling of laboratory macaques |
(2–4) |
| 1971† |
Laboratory-acquired |
Human volunteer was inoculated with tanapox virus, and clinical progression of the disease was monitored and recorded |
(1) |
| 1979–1983 |
Mongala, Democratic Republic of Congo (then Zaire) |
A total of 357 cases reported, of which 264 were confirmed by laboratory testing |
(3) |
| 1999 |
Bagamoyo, Tanzania |
Traveler from Germany diagnosed with tanapox upon return from Tanzania |
(5) |
| 2002† |
Sierra Leone |
Person from Sierra Leone admitted to hospital in New York, USA, 2 weeks after arrival from Sierra Leone |
(6) |
| 2004 | Republic of Congo | Volunteer working with chimpanzees has onset of tanapox; only diagnosed after return to USA | (7) |
*Initially identified as Yaba-like disease virus; subsequent research indicated homology with Tanapoxvirus.†Date of report (date of actual case not published).
The epidemiology and natural ecology of tanapox is largely unknown, but previous reports indicate that all infected humans are equally affected, regardless of age group and sex. Serologic surveys conducted in Tana River communities indicated 16.3% prevalence in 1971 and 9.2% in 1976 (10). Human-to-human transmission is rare, and although transmission from nonhuman primates to humans by contact or inoculation has been noted under laboratory conditions, natural human infections are more likely to be acquired by mechanical transmission from contaminated mouthparts of hematophagous arthropods (2–4). This vector theory arose because of the synchronicity between tanapox outbreaks and the increased arthropod activity associated with seasonal high temperatures, high rainfall, and flooding in the riparian areas in which surveillance was done (1,3,9). The similarities in the distribution and incidence of TANV and West Nile virus antibodies in serum samples collected in the Tana River Valley in 1971 led to the suggestion that both viruses are transmitted in the same way (i.e., by a culicine mosquito, probably a species of Mansonia) (1,3).
In humans, tanapox typically manifests with 1 or 2 characteristic, nodular skin lesions that are large, raised, umbilicated, and painful and generally ulcerate without becoming pustular (in contrast to lesions observed in most other poxvirus infections) (1,3,7). The lesions may be associated with localized lymphadenopathy, and their gradual development is preceded by a mild, short-lived febrile illness, with possible pruritus and myalgia leading to prostration and headaches (1–3,5,6). Histologically, lesions are restricted to the epithelial layers, with cells containing eosinophilic cytoplasmic inclusions and vacuolated nuclei (1,2,7). TANV virions are poxlike but cannot be distinguished microscopically from the other species in the genus (e.g., Yaba monkey tumor virus) or from Orthopoxvirus virions (3,7). Clinical differential diagnoses have included cutaneous anthrax, other poxvirus infections, sporotrichosis, Mycobacterium marinum infection, spotted fever group rickettsial infections, tropical ulcers, insect bites, and scabies (3,7,9). The disease is self-limiting, with no recorded fatalities (3). We describe a case of tanapox in South Africa, 19 years after the last published report of human tanapox (7).
The Study
We obtained written consent from the patient in this study and received ethics clearance from the Faculty of Health Sciences of University of the Witwatersrand, Johannesburg (approval no. M210752). During February 2–6, 2022, a 61-year old woman served as a volunteer in Kruger National Park (KNP), South Africa. She stayed in a tented bush camp along the banks of the Sand River, ≈20 km from the town of Skukuza (24°59′43′′ S, 31°35′34′′E) (Figure 1; Appendix Figure 1). The woman noted large numbers of arthropods (e.g., spiders, insects, and ticks) around the camp site and reported being bitten on several occasions on various body parts (especially on her hands, shoulders, arms, and back). Because of heavy rains, trails were overgrown, so bushwalks resulted in scratches on her arms, and many ticks were found on her clothing. During February 7–9, she continued her visit to KNP as a guest in air-conditioned accommodation and had no further direct contact with vegetation. She reported no direct contact with primates, although vervet monkeys (Chlorocebus pygerythrus) are seen near the camps.
Figure 1.
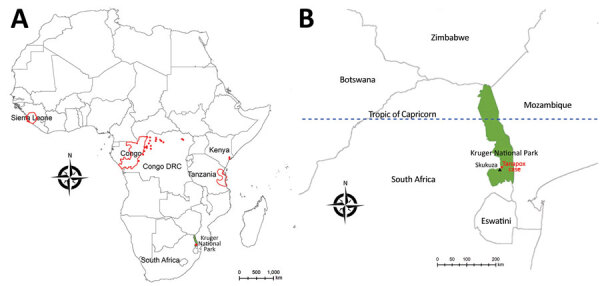
Geographic distribution of recorded human cases of tanapox. A) Locations of previous tanapox cases reported in the literature. Red dots indicate cases acquired locally; red outlines indicate regions of countries visited by travelers to Africa. B) Location of the case acquired in Kruger National Park, South Africa, 2022. Green shading shows the park’s location; black triangle indicates town of Skukuza.
Two days after her return to urban Johannesburg, she experienced pruritus at the base of her thumb on the dorsal side of her right hand and noticed a pale blister forming there, followed 2 days later by another blister on the side of her left hand. Initially, both papules were round and white with erythematous edges (Appendix Figure 2, panel A), but they became dome-shaped, firm, smooth, umbilicated nodules 12–15 mm in size (Appendix Figure 2, panels B, C). A third lesion formed on the woman’s mid-upper back but was perforated through chafing from clothing. About 3 days after the appearance of the first lesion, the woman reported feeling unwell, fatigued, and feverish and had severe headaches. No lymphadenopathy was recorded. The lesions were persistently painful and hypersensitive, but none were cystic or became pustular. Instead, they became ulcerated, open, and dry (Appendix Figure 2, panel D), and all 3 resolved over a period of 6 weeks, leaving slight discoloration. After discovering the third lesion, the woman sought medical attention. Differential diagnoses included allergies, cellulitis, erysipelas, pyoderma gangrenosum, or granulomas caused by foreign bodies or insect bites (e.g., mango fly bites).
Histopathologic examination of a lesion biopsy indicated a possible pox infection, given the presence of acidophilic intracytoplasmic inclusion bodies and cellular vacuolation (Figure 2, panel A). The lesion was confined to the epithelial layers with many cells that had vacuolated nuclei, and we observed ballooning cells in the deeper epithelial layer (Figure 2, panel B). Subsequently, we took an additional biopsy for electron microscopy and 2 lesion swab specimens for molecular characterization. After routine processing (Appendix), we observed numerous brick-shaped virions 287 nm x 221 nm with distinct surface tubules and generally an outer membrane layer (capsular form) (Figure 2, panel C). We used 1 dry swab sample for PCR analysis (Appendix), and partial sequence analysis indicated clustering with available TANV sequences (Appendix Figure 3). Attempts at full genomic sequencing and virus isolation (from the second swab specimen) were unsuccessful, possibly because of the limited clinical material available.
Figure 2.
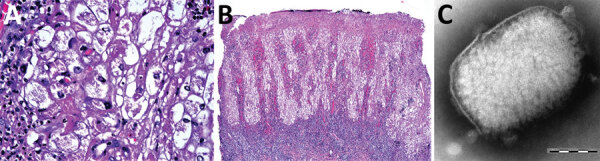
Diagnostic light and electron microscopy of tanapox lesion biopsies from a case-patient, South Africa, 2022. A) High-power photomicrograph of initial skin biopsy, showing prominent vacuolation of epidermal keratinocytes, granular intracytoplasmic inclusions, and intranuclear pseudoinclusions. Hematoxylin and eosin stain; original magnification ×400. B) Low-power photomicrograph of initial skin biopsy, showing a superficially eroded hyperplastic epidermis, with cytoplasmic pallor and a dense underlying superficial dermal lymphoid infiltrate. Hematoxylin and eosin stain; original magnification ×40. C) Negatively stained tanapox virus virion with surface tubules evident beneath the remains of the surrounding membrane. Virion dimensions were 159–327 nm × 186–289 nm. Scale bar indicates 100 nm.
Recent surveys of the mosquito distribution in southern Africa have found that 2 culicine genera (Culex and Mansonia) comprise 91% of the mosquito population in the town of Skukuza (11). Weather conditions at the time of the case exposure were conducive to vector replication; recent rainfall was up to 147% higher than the average annual cumulative total (12), and ambient temperatures were 100°F–104°F (38°C–40°C) (13). In terms of virus reservoirs, a limitation of Monroe et al.’s model (9) was that the restricted range of recorded human cases determined the exclusion of many other primates with extensive geographic ranges. Our report extends this range beyond the most southerly predictions of the model, which increases the pool of potential reservoir hosts.
Conclusions
The clinical findings for this reported case were in keeping with previous clinical reports of tanapox, and the diagnosis was supported by histopathology, electron microscopy, and molecular analyses. The importance and continuing relevance of histopathology and microscopy to the diagnosis and investigation of zoonotic disease were clearly illustrated. Given that tanapox is a vectorborne disease, many drivers, including anthropogenic destruction of wildlife habitats, environmental instability, and global climate change, may influence its emergence (14). Improved surveillance, including studies relating to the ecology and epidemiology of TANV in vectors, hosts, and humans, is warranted.
Additional information about tanapox, South Africa, 2022.
Acknowledgments
We thank Nosihle Msomi for her support in sequencing and sequencing analysis performed in this study.
Biography
Dr. Birkhead is a medical scientist in the electron microscopy laboratory of the National Institute for Communicable Diseases, a division of the National Health Laboratory Service, in Johannesburg, South Africa. Her primary research interests include the use of ultrastructural information for diagnoses of and research on human infectious diseases.
Footnotes
Suggested citation for this article: Birkhead M, Grayson W, Grobbelaar A, Msimang V, Moolla N, Mathee A, et al. Tanapox, South Africa, 2022. Emerg Infect Dis. 2023 Jun [date cited]. https://doi.org/10.3201/eid2906.230326
These first authors contributed equally to this article.
References
- 1.Downie AW, Taylor-Robinson CH, Caunt AE, Nelson GS, Manson-Bahr PEC, Matthews TCH. Tanapox: a new disease caused by a pox virus. BMJ. 1971;1:363–8. 10.1136/bmj.1.5745.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downie AW, España C. A comparative study of Tanapox and Yaba viruses. J Gen Virol. 1973;19:37–49. 10.1099/0022-1317-19-1-37 [DOI] [PubMed] [Google Scholar]
- 3.Jezek Z, Arita I, Szczeniowski M, Paluku KM, Kalisa R, Nakano JH. Human tanapox in Zaire: clinical and epidemiological observations on cases confirmed by laboratory studies. Bull. World Health Organ. 1985;63:1027–35. [PMC free article] [PubMed]
- 4.Downie AW, España C. Comparison of Tanapox virus and Yaba-like viruses causing epidemic disease in monkeys. J Hyg (Lond). 1972;70:23–32. 10.1017/S0022172400022051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croitoru AG, Birge MB, Rudikoff D, Tan MH, Phelps RG. Tanapox virus infection. Skinmed. 2002;1:156–7. 10.1111/j.1540-9740.2002.01778.x [DOI] [PubMed] [Google Scholar]
- 6.Stich A, Meyer H, Köhler B, Fleischer K. Tanapox: first report in a European traveller and identification by PCR. Trans R Soc Trop Med Hyg. 2002;96:178–9. 10.1016/S0035-9203(02)90295-6 [DOI] [PubMed] [Google Scholar]
- 7.Dhar AD, Werchniak AE, Li Y, Brennick JB, Goldsmith CS, Kline R, et al. Tanapox infection in a college student. N Engl J Med. 2004;350:361–6. 10.1056/NEJMoa031467 [DOI] [PubMed] [Google Scholar]
- 8.Downie AW. Serological evidence of infection with Tana and Yaba pox viruses among several species of monkey. J Hyg (Lond). 1974;72:245–50. 10.1017/S0022172400023445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monroe BP, Nakazawa YJ, Reynolds MG, Carroll DS. Estimating the geographic distribution of human Tanapox and potential reservoirs using ecological niche modeling. Int J Health Geogr. 2014;13:34. 10.1186/1476-072X-13-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axford JS, Downie AW. Tanapox. A serological survey of the lower Tana River Valley. J Hyg (Lond). 1979;83:273–6. 10.1017/S0022172400026061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornel AJ, Lee Y, Almeida APG, Johnson T, Mouatcho J, Venter M, et al. Mosquito community composition in South Africa and some neighboring countries. Parasit Vectors. 2018;11:331. 10.1186/s13071-018-2824-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SANparks Scientific Services. Data and information resources: Kruger climate and rainfall [cited 2023 Mar 27]. https://www.sanparks.org/scientific-services/wp-content/uploads/2022/01/December.pdf
- 13.Meteoblue. Weather history and climate archive [cited 2023 Mar 27]. https://www.meteoblue.com/en/weather/historyclimate/weatherarchive/skukuza_south-africa_954955
- 14.Carlson CJ, Albery GF, Merow C, Trisos CH, Zipfel CM, Eskew EA, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607:555–62. 10.1038/s41586-022-04788-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about tanapox, South Africa, 2022.


