Abstract
Babesiosis is a globally distributed parasitic infection caused by intraerythrocytic protozoa. The full spectrum of neurologic symptoms, the underlying neuropathophysiology, and neurologic risk factors are poorly understood. Our study sought to describe the type and frequency of neurologic complications of babesiosis in a group of hospitalized patients and assess risk factors that might predispose patients to neurologic complications. We reviewed medical records of adult patients who were admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, during January 2011–October 2021 with laboratory-confirmed babesiosis. More than half of the 163 patients experienced >1 neurologic symptoms during their hospital admissions. The most frequent symptoms were headache, confusion/delirium, and impaired consciousness. Neurologic symptoms were associated with high-grade parasitemia, renal failure, and history of diabetes mellitus. Clinicians working in endemic areas should recognize the range of symptoms associated with babesiosis, including neurologic.
Keywords: Babesia microti, babesiosis, neurology, parasites, United States
Babesiosis is an emerging parasitic infection with global distribution. The infection is caused by intraerythrocytic protozoa of the genus Babesia. During the past 2 decades, the incidence of babesiosis has increased, particularly in the northeastern and northern midwestern United States. The Centers for Disease Control and Prevention reported an increased babesiosis incidence in Connecticut, USA, from 2011 (2.1 cases/100,000 persons) to 2019 (9 cases/100,000 persons), more than 10 times the incidence reported nationally during that time period (1). More than 100 species of Babesia have been described in wild and domestic animals. The predominant species causing human disease in the United States is B. microti (1–3). The disease is transmitted primarily through the bite of an infected ixodid tick, which is capable of transmitting several pathogens at the same time, including Borrelia burgdorferi, the cause of Lyme disease (2–5). Babesiosis is less commonly transmitted via blood transfusion, organ transplantation, or through the placenta (2,3,6).
Although most persons with babesiosis experience nonspecific influenza-like symptoms, more severe and prolonged disease can occur in persons >50 years of age; those who are immunocompromised due to asplenia, cancer, or HIV/AIDS or who are receiving immunosuppressive drugs; and those who have chronic heart, lung, renal, or liver disease (2,7,8). Severe infection is associated with high-grade parasitemia and organ failure (e.g., acute respiratory distress syndrome, congestive heart failure, severe hemolytic anemia, or renal failure) and death (2,8–11). Little has been published about babesiosis-induced central nervous system dysfunction (12,13). Neurologic complications include headache, syncope, neuropathy, retinal nerve infarcts, and altered state of consciousness (9,12,14–20). The full spectrum of neurologic complications and underlying pathophysiology are poorly understood, as are factors that predispose patients to neurologic complications.
We conducted this study to investigate the type and frequency of neurologic complications of babesiosis in a group of hospitalized patients and to assess risk factors that predispose patients to neurologic complications. We hypothesized that patients with a diagnosis of babesiosis commonly experience neurologic system manifestations and that those symptoms are most frequent in patients with severe babesiosis. Accordingly, we conducted a retrospective medical record review of all adult patients admitted to Yale-New Haven Hospital (YNHH) in New Haven, Connecticut, USA, during 2011–2021 with laboratory-confirmed babesiosis.
Methods
Design and Setting
The sample population included all adult patients (≥18 years of age) admitted to YNHH during January 2011–October 2021 with a diagnosis of babesiosis. Patients were required to have Babesia parasites present on thin blood smear or amplification of B. microti DNA by PCR to be included. Eligible patients were identified in collaboration with the Yale Center for Clinical Investigation Joint Data Analytics Team.
Data Collection
We obtained approval for study procedures from the Yale University Human Investigation Committee before data collection (HIC protocol #2000030420). We performed comprehensive medical record reviews and systematically abstracted study variables by using a standardized medical questionnaire (21). We recorded demographic and clinical variables, including the following underlying conditions and comorbidities: chronic cardiac, pulmonary, renal, or hepatic conditions; dementia; diabetes mellitus; hypertension; malignancy; migraine; seizure; stroke; and immunocompromised status.
We recorded all neurologic symptoms and complications documented at the time of hospital admission: acute cerebrovascular disease, acute syncope, ataxia/gait disturbance, confusion/delirium, facial droop, focal weakness, headache, impaired consciousness, nerve pain, tremor, language deficit, vision impairment, vertigo, and seizure. The neurologic symptoms and complications we recorded were then independently confirmed by 2 neurologists. We defined confusion/delirium as a deficit in mental status characterized by disorientation, bewilderment, or difficulty following commands (22). We categorized impaired consciousness (i.e., diminished arousal and response to stimulation) on the basis of severity using the Glasgow Coma Scale, classifying symptoms as either lethargy, obtundation, stupor, or coma (23). Lethargy is a mild reduction in alertness, obtundation is a moderate reduction in alertness, stupor is a condition of deep sleep in which the patient can only be aroused by vigorous external stimulation, and coma refers to a complete lack of motor response to any stimuli from the external environment (23,24).
We abstracted key laboratory variables related to the severity of infection. We computed median laboratory values and interquartile ranges (IQRs) on the basis of either the recorded minimum or maximum laboratory value, as appropriate. Laboratory parameters included peak parasitemia, minimum hematocrit and platelet count, and maximum blood urea nitrogen, creatinine, aspartate aminotransferase and alanine aminotransferase. We calculated glomerular filtration rate by using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation (25).
Statistical Analysis
We performed statistical analyses by using SAS Studio 3.8 (SAS Institute Inc.). We summarized demographic characteristics of the sample by using appropriate descriptive statistics. We categorized patients according to peak parasitemia (group 1, <1.0%; group 2, 1.0% −10.0%; group 3, >10.0%) (Table 1). For patients with peak thin blood smear results reported as <1%, we used a value of 0.9% in calculations. We compared those subgroups to determine if neurologic manifestations were more frequent in patients with high parasitemia. Given the nonparametric distribution of those data, we reported medians and IQRs for hematologic, hepatic, and renal function laboratory tests. We used Wilcoxon 2-sample tests to compare laboratory results among patients with and without the most common neurologic symptoms.
Table 1. Neurologic symptoms during hospital admissions for babesiosis in patients admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, January 2011–October 2021*.
| Neurologic symptom | No. (%) patients | Peak parasitemia, no. (%) patients |
p value | ||
|---|---|---|---|---|---|
| <1.0%, n = 45 | 1.0%–10.0%, n = 81 | >10.0%, n = 37 | |||
| Headache | 52 (31.9) | 14 (31.1) | 28 (34.6) | 10 (27.0) | 0.711 |
| Confusion/delirium | 27 (16.6) | 2 (4.4) | 13 (16.1) | 12 (32.4) | 0.003 |
| Impaired consciousness | 24 (14.7) | 4 (8.9) | 9 (11.1) | 11 (29.7) | 0.018 |
| Ataxia/gait disorder | 17 (10.4) | 3 (6.7) | 10 (12.4) | 4 (10.8) | 0.632 |
| Vision impairment | 10 (6.1) | 5 (11.1) | 4 (4.9) | 1 (2.7) | 0.266 |
| Acute syncope | 6 (3.7) | 2 (4.4) | 4 (4.9) | 0 (0.0) | 0.482 |
| Language deficit | 5 (3.1) | 0 (0.0) | 5 (6.2) | 0 (0.0) | 0.106 |
| Nerve pain | 4 (2.5) | 1 (2.2) | 2 (2.5) | 1 (2.7) | 1.000 |
| Focal weakness | 3 (1.8) | 1 (2.2) | 1 (1.2) | 1 (2.7) | 0.794 |
| Tremor | 3 (1.8) | 1 (2.2) | 2 (2.5) | 0 (0.0) | 1.000 |
| Seizure | 2 (1.3) | 1 (2.2) | 0 (0.0) | 1 (2.7) | 0.252 |
*Total sample (n = 163); Column percentages do not sum to 100% due to many patients being affected by multiple neurologic conditions throughout hospitalization. Boldface type indicates statistical significance.
To represent the distribution density of neurologic symptoms by peak parasitemia, we used Prism 9 software (GraphPad Software) to generate violin plots. We analyzed the relationship between peak parasitemia and each reported neurologic symptom by using Mann-Whitney U tests for continuous variables.
We used univariate logistic regression models to test associations between comorbid conditions and the 3 most common neurologic symptoms: headache, confusion/delirium, and impaired consciousness. We noted variables that were significant in unadjusted univariate analysis and entered them into a multivariate model for each neurologic symptom. We set the significance level for univariate and multivariate analysis to p<0.05.
Results
Demographic and Clinical Characteristics
We identified a total of 163 hospitalized patients with laboratory-confirmed babesiosis during the study period (January 2011–October 2021). The median age was 67 years (IQR 45–89; range 30–93). Most patients were male (n = 104 [63.8%]). The study population was predominately White or Caucasian (n = 118 [74.7%]). Most patients (n = 160 [98.2%]) were diagnosed with babesiosis by a blood smear positive for intra-erythrocytic organisms that were consistent with Babesia. Three patients were diagnosed with babesiosis on the basis of a positive PCR blood specimen in the context of an appropriate acute clinical syndrome. Most patients (n = 117 [71.8%]) had >1 medical comorbidity at admission. The most common comorbidities were hypertension (n = 82 [50.3%]), immunodeficiency (n = 40 [24.5%]), and cardiac disorder (n = 39 [23.9%]). Most of the immunodeficient patients (n = 21 [52.5%]) were asplenic. Other comorbid conditions included diabetes (n = 23 [14.1%]), stroke (n = 13 [8.0%]), chronic kidney disease (n = 8 [4.9%]), migraine (n = 4 [2.5%]), dementia (n = 3 [1.84%]), and seizure disorder (n = 2 [1.2%]). Twelve patients (7.4%) had erythema migrans rash, indicating active co-infection with Lyme disease.
Neurologic Symptoms of Babesiosis
We recorded the frequency of neurologic symptoms experienced by patients admitted to YNHH for babesiosis (Table 1). More than half (n = 97 [59.5%]) experienced >1 neurologic symptoms. The most frequent symptoms were headache (n = 52 [31.9%]), confusion/delirium (n = 27 [16.6%]), impaired consciousness (n = 24 [14.7%]), ataxia/gait disorder (n = 17 [10.4%]), and vision impairment (n = 10 [6.1%]). Patients noted to have impaired consciousness were 20 (12.3%) classified as lethargic, 3 (1.8%) as obtunded, and 1 (0.6%) as stuporous.
Confusion/Delirium and Parasitemia
Although we found no association between the degree of parasitemia and the presence of any neurologic symptoms, patients with confusion/delirium and impaired consciousness were significantly more likely to have a higher peak parasitemia than those without these symptoms (Figure). All 163 hospitalized patients were stratified by peak parasitemia into 3 groups: peak parasitemia of <1% (n = 45), peak parasitemia of 1.0%–10% (n = 81), and peak parasitemia of >10% (n = 37). The 27 patients with confusion/delirium were also stratified into the peak parasitemia groups. Among the 45 patients with a peak parasitemia <1%, 2 (4.4%) patients experienced confusion/delirium. Among the 81 patients with a peak parasitemia of 1.0%–10%, 13 (16.1%) experienced confusion/delirium. Among the 37 patients with a peak parasitemia of >10%, 12 (32.4%) experienced confusion/delirium (Table 1). The median peak parasitemia of the patients who experienced confusion/delirium was significantly higher than that of the patients who did not experience confusion/delirium (p = 0.001) (Figure). Those findings indicate that as parasitemia increases, so does the prevalence of confusion/delirium.
Figure.
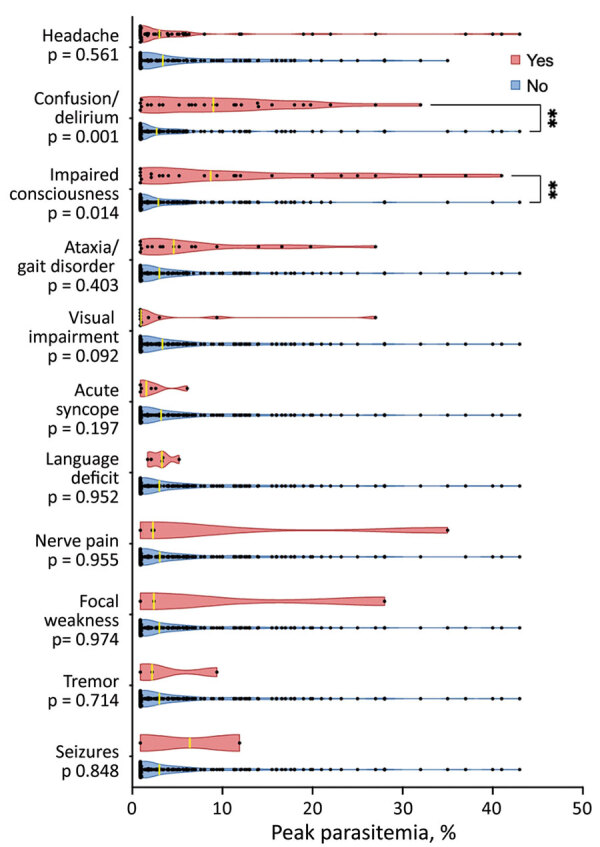
Violin plot depicting the peak parasitemia distribution in patients with and without select neurologic symptoms associated with babesiosis admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, January 2011–October 2021. Asterisks (**) indicate that the median peak parasitemia significantly differed between the patients who did and the patients who did not experience the specific symptom (p<0.05).
Impaired Consciousness and Parasitemia
We found a significant association between peak parasitemia and impaired consciousness (p<0.005). Of the 163 hospitalized patients, 14.7% experienced impaired consciousness at the time of hospital admission. When we stratified by peak parasitemia groups, we observed impaired consciousness in 4 (8.9%) of the group with the lowest (<1%) peak parasitemia, 9 (11.1%) of the group with mid (1.0%–10%) peak parasitemia, and 11 (29.7%) of those in the group with the highest (>10%) peak parasitemia. That distribution was similar to that of patients with confusion/delirium. Patients with impaired consciousness (of any degree) had a higher median peak parasitemia than those without impaired consciousness (p = 0.014) (Figure).
Ophthalmologic Symptoms
Ten (6.1%) patients reported transient vision impairment, one of whom was formally evaluated by the ophthalmology department after reportedly seeing different colored lights and shapes when she closed her eyes. Ophthalmologic examination revealed no evidence of ocular infection or inflammation.
Neuroimaging Findings
Thirty-three (20.2%) of the hospitalized patients underwent neuroimaging with cranial computerized tomography (n = 28), brain magnetic resonance imaging (n = 7), or electroencephalogram (n = 2) (Table 2). Only 2 patients had acute abnormalities seen on neuroimaging, 1 with ischemic stroke and 1 with subarachnoid hemorrhage. The patient who suffered a stroke had fever, malaise, and anemia; an episode of expressive aphasia during an acute babesiosis episode resulting from a small, left-middle cerebral artery stroke, confirmed on imaging to be secondary to high-grade internal carotid artery stenosis. The patient with a subarachnoid hemorrhage had confusion and jaundice and was in critical condition because of multiorgan failure, including respiratory, kidney, and liver failure, and disseminated intravascular coagulation. Computed tomography revealed subarachnoid hemorrhage, most conspicuous within the left frontal lobe. Most other patients who underwent neuroimaging had nonspecific white matter changes and some element of volume loss, which were considered to be related to chronic disease and aging. Electroencephalograms results were unremarkable for both patients who underwent this examination.
Table 2. Imaging indications and findings in patients with neurologic symptoms associated with babesiosis admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, January 2011–October 2021*.
| Imaging | No. (%) patients |
|---|---|
| Neuroimaging modality | |
| Computed tomography | 28 (17.2) |
| Magnetic resonance imaging | 7 (4.3) |
| Electroencephalogram |
2 (1.2) |
| Indication | |
| Altered mental status/confusion | 15 (9.2) |
| Headache | 8 (4.9) |
| Fever | 5 (3.1) |
| Evaluate CNS abnormalities | 3 (1.8) |
| Weakness | 3 (1.8) |
| Dizziness | 1 (0.6) |
| Dysphagia, slurred speech | 1 (0.6) |
| Fall | 1 (0.6) |
| Head injury | 1 (0.6) |
| Visual changes | 1 (0.6) |
| Syncope | 1 (0.6) |
| Numbness | 1 (0.6) |
| Fatigue | 1 (0.6) |
| Seizure |
1 (0.6) |
| Findings | |
| Nonspecific white matter changes | 23 (14.1) |
| Volume loss | 14 (8.6) |
| Acute changes | 2 (1.2) |
| Evidence of previous stroke | 3 (1.8) |
*Total sample consisted of 163 patients. Neuroimaging modalities and indications sum to >33 because some patients received multiple imaging modalities or had multiple indications. CNS, central nervous system.
Laboratory Findings
We assessed laboratory measures of hematologic, hepatic, and renal function among patients with the most frequently reported neurologic symptoms: headache, confusion/delirium, and impaired consciousness. Anemia was common in the overall sample population. Median hematocrit was 26.7% for male patients (reference range 40%–52%) and 25% for female patients (reference range 37%–53%). Patients with impaired consciousness had a lower hematocrit than did those without impaired consciousness (23.6% vs. 26.4%; p = 0.024) (Table 3). Patients with confusion/delirium and those with impaired consciousness had significantly higher median blood urea nitrogen and creatinine values and significantly lower glomerular filtration rate compared with patients without these symptoms (Table 3).
Table 3. Laboratory result comparisons by neurologic symptoms in patients with babesiosis admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, January 2011–October 2021*.
| Laboratory result, median (IQR) | Reference range | Headache |
Confusion/delirium |
Impaired consciousness |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes, n = 52 | No, n = 111 | p value† | Yes, n = 27 | No, n = 136 | p value† | Yes, n = 24 | No, n = 139 | p value† | ||||
| Hematologic function | ||||||||||||
| Lowest hematocrit | M, 40%–52%; F, 37%–53% | 25.9 (16.2–35.6) | 26.0 (19.4–32.6) | 0.581 | 24.0 (16.5–31.5) | 26.4 (18.8–34) | 0.088 | 23.6 (15.7–31.5) | 26.4 (18.9–33.9) | 0.024 | ||
| Lowest platelet count |
150–400 × 109/L |
78.5 (4.0–153.0) |
82.0 (23.0–141.0) |
0.881 |
|
68.0 (1.0–135.0) |
82.0 (−5.0 to 230.0) |
0.339 |
|
59.5 (−5.0 to 124.0) |
84.0 (17.0–151.0) |
0.084 |
| Hepatic function | ||||||||||||
| Highest AST | 3–40 U/L | 66.0 (25.5–106.5) | 76.0 (38.0–114.0) | 0.277 | 76.0 (42.0–110.0) | 75.5 (32.5–118.5) | 0.813 | 81.0 (48.0–114.0) | 75.0 (33.0–117.0) | 0.909 | ||
| Highest ALT |
3–40 U/L |
61.0 (10.0–112.0) |
63.0 (9.0–117.0) |
0.809 |
|
54.0 (8.0–100.0) |
63.5 (12.5–114.5) |
0.568 |
|
49.0 (4.0–102.0) |
63.0 (13.0–113.0) |
0.441 |
| Renal function | ||||||||||||
| Highest BUN | 7–20 mg/dL | 17.0 (4.5–29.5) | 24.0 (5.0–43.0) |
0.002 | 31.0 (−3.0 to 65.0) |
20.0 (5.5–34.5) | 0.025 | 33.0 (0.0–66.0) |
20.0 (5.0–35.0) | 0.005 | ||
| Highest creatinine | 0.5–1.2 mg/dL | 1.00 (0.96–1.40) | 1.1 (0.3–1.9) | 0.091 | 1.5 (0.7–2.3) | 1.0 (0.5–1.5) | <0.001 | 1.5 (0.3–2.7) | 1.0 (0.5–1.5) | 0.002 | ||
| Lowest GFR | ≥60 mL/min | 76.0 (37.5–114.5) | 67.0 (21.0–113.0) | 0.007 | 46.0 (3.0–89.0) | 73.0 (33.5–112.5) | <0.001 | 47.0 (1.0–93.0) | 73.0 (33.0–113.0) | 0.003 | ||
*Boldface indicates significance. ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; GFR, glomerular filtration rate; IQR, interquartile range. †By Wilcoxon 2-sample test.
Six patients underwent lumbar puncture during hospital admission. The indications for lumbar puncture were headache (n = 2), confusion (n = 2), unresponsive state (n = 1), and confusion with bilateral hearing loss (n = 1). None of those patients were found to have a pleocytosis (i.e., all had cerebrospinal fluid [CSF] leukocyte counts <5 cells/µL). One patient had slightly elevated CSF protein (82 g/dL); CSF parameters were otherwise within reference ranges. Five of the 6 patients who underwent lumbar puncture were tested for Lyme antibody in CSF, but results were negative in all cases.
Risk Factors for Neurologic Complications
Univariate logistic regression models tested associations between comorbid conditions and the 3 most common neurologic symptoms: headache, confusion/delirium, and impaired consciousness. In univariate modeling, we found significant associations (p<0.05) between hypertension, diabetes mellitus, and stroke/transient ischemic attack and the presence of confusion/delirium during hospital admission. Those same factors were also significantly associated with impaired consciousness in univariate analysis and were entered into multivariate models of the respective complications (Table 4).
Table 4. Associations between comorbid conditions and neurologic symptoms in patients with babesiosis admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, January 2011–October 2021*.
| Comorbid condition |
Confusion/delirium, no. (%) |
Adjusted odds of confusion/delirium |
Impaired consciousness, no. (%) |
Adjusted odds of impaired consciousness |
||
|---|---|---|---|---|---|---|
| OR (95% CI) |
p value |
OR (95% CI) |
p value |
|||
| Diabetes mellitus | ||||||
| No | 140 (13.6) | 1.00 | 140 (10.7) | |||
| Yes |
23 (34.8) |
3.04 (1.11–8.34) |
0.031
|
23 (39.1) |
5.36 (1.98–14.48) |
<0.001
|
| Stroke/transient ischemic attack | ||||||
| No | 150 (14.7) | |||||
| Yes | 13 (38.5) | 3.06 (0.88–10.66) | 0.079 | |||
*Univariate logistic regression models tested associations between the following comorbid conditions and the 3 most frequent neurologic symptoms (headache, confusion/delirium, impaired consciousness): cardiac disorder, hypertension, diabetes mellitus, immunocompromised status, stroke/transient ischemic attack, obesity, chronic kidney disease, migraine, and malignancy. Variables that were significant in unadjusted univariate analysis were entered into a multivariate model for each neurologic symptom. The significance level for univariate and multivariate analysis was set to p<0.05. Boldface indicates statistical significance.
Multivariate analysis revealed increased adjusted odds of confusion/delirium (OR 3.04 [95% CI 1.11–8.34]; p = 0.031) and impaired consciousness (OR 5.36 [95% CI 1.98–14.48]; p<0.001) among patients with diabetes mellitus. There was a significant association between stroke/transient ischemic attack and confusion/delirium in univariate modeling, but the association was nonsignificant in the multivariate model (p = 0.079). Patients affected by confusion/delirium or impaired consciousness tended to be older (median age 72.5 years [IQR 55.5–89.5]) compared with patients without these symptoms (median age 66.5 years [IQR 46.5–86.5]) (p = 0.012).
Outcomes
The median length of hospital stay was 6.5 (IQR 2.5–10.5) days for patients with confusion/delirium or impaired consciousness compared with 5 (IQR 2–8) days for those without those symptoms (p = 0.019). A greater percentage of patients with those symptoms were admitted to the intensive care unit (55.3% vs. 44.7%; p = 0.002). Four (2.5%) of the 163 patients in the study group died during hospitalization. Among those 4 patients, 2 had neurologic complications. One experienced ataxia/gait disturbance, confusion/delirium, impaired consciousness, tremor, and vision impairment. The other patient experienced confusion/delirium and impaired consciousness.
Discussion
More than half (59.5%) of the 163 patients admitted to YNHH with babesiosis had >1 neurologic complication. Confusion/delirium and impaired consciousness were the 2 most common severe neurologic complications among our study patients. Other severe neurologic symptoms, including seizure or stroke, were seldom reported. Confusion/delirium and impaired consciousness were significantly associated with high peak parasitemia (p<0.005) and with markers of renal injury. We also found that the prevalences of confusion/delirium and of impaired consciousness were greater in patients with diabetes mellitus. Although Lyme disease can cause several neurologic complications, including confusion/delirium and impaired consciousness, less than one tenth of patients hospitalized for babesiosis had an erythema migrans rash. We found no relationship between babesiosis–Lyme disease co-infection and prevalence of neurologic complications (26–28).
The etiology of Babesia-associated neurologic symptoms is unknown. Central nervous system complications observed in B. bovis in cattle and B. canis in dogs are thought to be caused by erythrocyte, platelet, and leukocyte cytoadherence to vascular endothelium with vascular obstruction, excessive proinflammatory cytokine activation associated with high parasitemia, or both (12,29–32). One of the patients in our study had an acute left-middle cerebral artery stroke consistent with vascular obstruction; however, a direct link between stroke and this parasitic infection cannot be established with a single case. Retinopathy has been documented in both cerebral malaria and babesiosis and is thought to be the result of vascular obstruction in the brain and retina (33–35).
We noted an association between renal impairment (defined by elevated blood urea nitrogen and creatinine and low glomerular filtration rate) and patients who experienced confusion/delirium or impaired consciousness. Confusion/delirium and impaired consciousness may be a direct result of impaired renal function (i.e., secondary to uremia). Alternatively, those neurologic symptoms and renal impairment might be a consequence of a common pathologic mechanism, such as microvascular obstruction from cytoadhering infected erythrocytes or excessive proinflammatory cytokine release. Animal studies likewise have found an association between renal complications and cerebral complications of babesiosis (31,36–38).
Limitations of this study include the retrospective nature of the study design, the lack of histopathologic data, and incomplete access to prehospitalization data, including severity of comorbid conditions and medication use. Our sample size of 163 patients is modest because severe cases of babesiosis requiring hospitalization are uncommon. This study cohort reflects the clinical manifestations of babesiosis patients who experienced severe infection that required hospitalization and, as such, the results may not be directly applicable to all patients with babesiosis. Neurologic symptom severity scores were not available, and duration of symptoms after discharge is unknown, although most patients had cleared or improved neurologic symptoms by the time of discharge. The long-term outcomes of neurologic complications of babesiosis warrant further research.
The lack of histopathologic data limits the specificity of our findings. Patients with severe infections other than babesiosis may also experience many of the neurologic complications described in this study. Regarding the patients we studied, it is challenging to delineate whether signs and symptoms were part of the typical course of systemic infection, were worsened by the presence of babesiosis, or were characteristic of the babesiosis agent itself. Despite those limitations, this study provides a baseline description of the prevalence of neurologic complications of babesiosis.
In conclusion, more than half of the patients admitted to YNHH from January 2011–October 2021 for acute babesiosis experienced >1 neurologic complication. Confusion/delirium and impaired consciousness were each significantly associated with peak parasitemia, renal impairment, and preexisting diabetes mellitus. Further research is needed to clarify the pathogenesis of neurologic manifestations of babesiosis and determine possible long-term neurologic sequelae. Clinicians caring for patients in endemic areas should be aware that babesiosis can manifest with a range of symptoms, including neurologic.
Acknowledgments
This work was supported by the National Institute of Mental Health and the National Institute on Aging of the National Institutes of Health (K23 MH118999 and T35 AG049685); the Llura A. Gund Laboratory for Vector-borne Diseases; and the Gordon and Llura Gund Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
P.J.K. is on the board of the American Lyme Disease Foundation, for which he receives no remuneration, and coauthored a chapter on babesiosis in UpToDate. All other authors report no potential conflicts.
Biography
Ms. Locke is a second-year medical student at Yale School of Medicine, New Haven, Connecticut. She is interested in the epidemiology of vector-borne diseases.
Footnotes
Suggested citation for this article: Locke S, O’Bryan JO, Zubair AS, Rethana M, Spichler Moffarah A, Krause PJ, et al. Neurologic complications of babesiosis, United States, 2011–2021. Emerg Infect Dis. 2023 June [date cited]. https://doi.org/10.3201/eid2906.221890
These first authors contributed equally to this article.
These senior authors contributed equally to this article.
References
- 1.Centers for Disease Control and Prevention. Surveillance for babesiosis—United States, 2019 Annual Summary. Atlanta: The Centers; 2021. [cited 2023 Mar 17]. https://www.cdc.gov/parasites/babesiosis/resources/Surveillance_Babesiosis_US_2019.pdf
- 2.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–407. 10.1056/NEJMra1202018 [DOI] [PubMed] [Google Scholar]
- 3.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19. 10.7326/0003-4819-155-8-201110180-00362 [DOI] [PubMed] [Google Scholar]
- 4.Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res. 2015;2015:587131. 10.1155/2015/587131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32:30–42. 10.1016/j.pt.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox LM, Wingerter S, Ahmed A, Arnold A, Chou J, Rhein L, et al. Neonatal babesiosis: case report and review of the literature. Pediatr Infect Dis J. 2006;25:169–73. 10.1097/01.inf.0000195438.09628.b0 [DOI] [PubMed] [Google Scholar]
- 7.Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–6. 10.1086/525852 [DOI] [PubMed] [Google Scholar]
- 8.Akel T, Mobarakai N. Hematologic manifestations of babesiosis. Ann Clin Microbiol Antimicrob. 2017;16:6. 10.1186/s12941-017-0179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117–25. 10.1086/319742 [DOI] [PubMed] [Google Scholar]
- 10.Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA): 2020 guideline on diagnosis and management of babesiosis. Clin Infect Dis. 2021;72:185–9. 10.1093/cid/ciab050 [DOI] [PubMed] [Google Scholar]
- 11.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29:357–70. 10.1016/j.idc.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usmani-Brown S, Halperin JJ, Krause PJ. Neurological manifestations of human babesiosis. Handb Clin Neurol. 2013;114:199–203. 10.1016/B978-0-444-53490-3.00014-5 [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw MJ, Bloch KC. Tick-borne infections of the central nervous system. In: Hasbun R, Bloch KC, Bhimraj MDA, editors. Neurological complications of infectious diseases. Cham: Springer International Publishing; 2021. p. 325–49. [Google Scholar]
- 14.Ortiz JF, Millhouse PW, Morillo Cox Á, Campoverde L, Kaur A, Wirth M, et al. Babesiosis: Appreciating the pathophysiology and diverse sequela of the infection. Cureus. 2020;12:e11085. 10.7759/cureus.11085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun T, Tenenbaum MJ, Greenspan J, Teichberg S, Wang RT, Degnan T, et al. Morphologic and clinical observations in human infection with Babesia microti. J Infect Dis. 1983;148:239–48. 10.1093/infdis/148.2.239 [DOI] [PubMed] [Google Scholar]
- 16.Venigalla T, Adekayode C, Doreswamy S, Al-Sudani H, Sekhar S. Atypical presentation of babesiosis with neurological manifestations as well as hematological manifestations. Cureus. 2022;14:e26811. 10.7759/cureus.26811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reubush TK II, Cassaday PB, Marsh HJ, Lisker SA, Voorhees DB, Mahoney EB, et al. Human babesiosis on Nantucket Island. Clinical features. Ann Intern Med. 1977;86:6–9. 10.7326/0003-4819-86-1-6 [DOI] [PubMed] [Google Scholar]
- 18.Maxwell SP, Brooks C, McNeely CL, Thomas KC. Neurological pain, psychological symptoms, and diagnostic struggles among patients with tick-borne diseases. Healthcare (Basel). 2022;10:1178. 10.3390/healthcare10071178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oleson CV, Sivalingam JJ, O’Neill BJ, Staas WE Jr. Transverse myelitis secondary to coexistent Lyme disease and babesiosis. J Spinal Cord Med. 2003;26:168–71. 10.1080/10790268.2003.11754578 [DOI] [PubMed] [Google Scholar]
- 20.Reese M, Maru D. Unexplained recurrent fevers and the importance of inquiring about occupation: a case report. Med Forum. 2016;17. 10.29046/TMF.017.1.012 [DOI] [Google Scholar]
- 21.Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, et al. ; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184–91. 10.1086/339813 [DOI] [PubMed] [Google Scholar]
- 22.Walker HK. The origins of the history and physical examination. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 23.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 24.Edwards SL. Using the Glasgow Coma Scale: analysis and limitations. Br J Nurs. 2001;10:92–101. 10.12968/bjon.2001.10.2.5391 [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. ; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halperin JJ. Nervous system Lyme disease—facts and fallacies. Infect Dis Clin North Am. 2022;36:579–92. 10.1016/j.idc.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 27.Schwenkenbecher P, Pul R, Wurster U, Conzen J, Pars K, Hartmann H, et al. Common and uncommon neurological manifestations of neuroborreliosis leading to hospitalization. BMC Infect Dis. 2017;17:90. 10.1186/s12879-016-2112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36:495–521. 10.1016/j.idc.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Krause PJ, Daily J, Telford SR, Vannier E, Lantos P, Spielman A. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol. 2007;23:605–10. 10.1016/j.pt.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 30.Puri A, Bajpai S, Meredith S, Aravind L, Krause PJ, Kumar S. Babesia microti: Pathogen genomics, genetic variability, immunodominant antigens, and pathogenesis. Front Microbiol. 2021;12:697669. 10.3389/fmicb.2021.697669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Kabra A, Igarashi I, Krause PJ. Animal models of the immunology and pathogenesis of human babesiosis. Trends Parasitol. 2023;39:38–52. 10.1016/j.pt.2022.11.003 [DOI] [PubMed] [Google Scholar]
- 32.Allred DR, Al-Khedery B. Antigenic variation and cytoadhesion in Babesia bovis and Plasmodium falciparum: different logics achieve the same goal. Mol Biochem Parasitol. 2004;134:27–35. 10.1016/j.molbiopara.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 33.Maude RJ, Beare NA, Abu Sayeed A, Chang CC, Charunwatthana P, Faiz MA, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2009;103:665–71. 10.1016/j.trstmh.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz JM, Eagle RC Jr. Ocular findings in human babesiosis (Nantucket fever). Am J Ophthalmol. 1982;93:307–11. 10.1016/0002-9394(82)90530-X [DOI] [PubMed] [Google Scholar]
- 35.Zweifach PH, Shovlin J. Retinal nerve fiber layer infarct in a patient with babesiosis. Am J Ophthalmol. 1991;112:597–8. 10.1016/S0002-9394(14)76867-9 [DOI] [PubMed] [Google Scholar]
- 36.de Scally MP, Lobetti RG, Reyers F, Humphris D. Are urea and creatinine values reliable indicators of azotaemia in canine babesiosis? J S Afr Vet Assoc. 2004;75:121–4. 10.4102/jsava.v75i3.466 [DOI] [PubMed] [Google Scholar]
- 37.Aikawa M, Pongponratn E, Tegoshi T, Nakamura K, Nagatake T, Cochrane A, et al. A study on the pathogenesis of human cerebral malaria and cerebral babesiosis. Mem Inst Oswaldo Cruz. 1992;87(Suppl 3):297–301. 10.1590/S0074-02761992000700051 [DOI] [PubMed] [Google Scholar]
- 38.Tsuji M, Fujioka H, Arai S, Taniyama H, Ishihara C, Aikawa M. A mouse model for cerebral babesiosis. Parasitol Today. 1996;12:203–5. 10.1016/0169-4758(96)40002-3 [DOI] [PubMed] [Google Scholar]


