Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has shown diverse life-threatening effects, most of which are considered short-term. In addition to its short-term effects, which has claimed many millions of lives since 2019, the long-term complications of this virus are still under investigation. Similar to many oncogenic viruses, it has been hypothesized that SARS-CoV-2 employs various strategies to cause cancer in different organs. These include leveraging the renin angiotensin system, altering tumor suppressing pathways by means of its nonstructural proteins, and triggering inflammatory cascades by enhancing cytokine production in the form of a “cytokine storm” paving the way for the emergence of cancer stem cells in target organs. Since infection with SARS-CoV-2 occurs in several organs either directly or indirectly, it is expected that cancer stem cells may develop in multiple organs. Thus, we have reviewed the impact of coronavirus disease 2019 (COVID-19) on the vulnerability and susceptibility of specific organs to cancer development. It is important to note that the cancer-related effects of SARS-CoV-2 proposed in this article are based on the ability of the virus and its proteins to cause cancer but that the long-term consequences of this infection will only be illustrated in the long run.
Keywords: SARS-CoV-2, COVID-19, Oncogenesis, Cancer
Abbreviations
- PI3K-AKT
Phosphatidylinositol-3-Kinase and Protein Kinase B
- NF-κB
nuclear factor-kappa B
- IGF-1
insulin-like growth factor 1
- HIF-1α
Hypoxia-inducible factor 1-alpha
- NO
nitric oxide;
- ICAM-1
Intercellular Adhesion Molecule 1
- VCAM-1
vascular cell adhesion molecule-1
- 8-oxo-Dg
8-Oxo-2′-deoxyguanosine;
- 8-NG
8-nitroguanine;
- CAF
cancer associated fibroblasts
- CCL2/CXCR2
C–C Motif Chemokine Ligand 2/CXC chemokine receptor 2
- ER
Endoplasmic reticulum
- TNF
Tumor necrosis factor
- PTEN
phosphatase and tensin homolog
- CREB1
cAMP-regulatory element-binding protein-1
- CASP3
caspase 3
- SMAD3
SMAD family member 3
- SNAIL
Zinc finger protein SNAI1
- EMT
epithelial–mesenchymal transition
- SCFA
Short-chain fatty acids
- EMMPRIN
extracellular matrix metalloproteinase inducer
- RAAS
renin-angiotensin-aldosterone system
- Ang
angiotensin
- AT1R
angiotensin 1 receptor
- AT2R
angiotensin 1 receptor
- MAS1R
MAS1 receptor
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- TGF-β
Transforming growth factor beta
- VEGFA
Vascular endothelial growth factor A
- STAT
signal transducer and activator of transcription
- Pim1
Proviral integration site for Moloney murine leukemia virus-1
- Pim2
Proviral integration site for Moloney murine leukemia virus-2
- C-MYC
Cellular Myelocytomatosis Oncogene
- IL-6
Interleukin 6
- ROS
Reactive oxygen species
- HSP-27
heat shock protein 27
- DDX10
DEAD-box helicase 10
- NUP-98
nucleoporin-98
- PRB
retinoblastoma protein
- GNB-1
Guanine nucleotide-binding protein subunit beta-1
- ETC
electron transport chain
- HMOX1
heme oxygenase-1
- SIRT5
Sirtuin 5
- NSD2
Nuclear receptor binding SET domain protein 2
- HDAC2
Histone deacetylase 2
- NUP214
nucleoporin 214
- AKAP9
A Kinase Anchor Protein 9
- BRD2
Bromodomain-containing protein 2
- BRD4
Bromodomain-containing protein 4
- LARP1
La-related Protein 1
1. Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019, turned scientists’ attention from around the globe to the pathogenesis, prevention, and management of this detrimental infectious disease. Depending on the target organ of SARS-CoV-2, manifestations and the severity of symptoms varied from person to person. Fever, cough, headache, sore throat, diarrhea, tiredness, and the loss of taste or smell are a number of commonly reported symptoms [1]. One of the most severe symptoms of coronavirus disease 2019 (COVID-19) is acute respiratory distress symptom (ARDS), which can trigger a series of several inflammatory incidents in the lungs of an affected patient [2,3].
The genome of this virus consists of a positive-sense single strand RNA (ss-RNA) which is 26–32 kilobases and unlike retroviruses, reverse transcriptase (RT) enzyme is not encoded by its genome [4]. One of the structural proteins of corona viruses (including SARS-CoV-2) is the spike protein (S protein) that makes cell invasion possible through the interaction of the receptor-binding domain (RBD) domain of the S1 subunit with angiotensin-converting enzyme 2 (ACE2) on the host cell surface [5]. Mutations in the S protein impair the ability of circulating antibodies to bind to and protect against viral infection which undermines the protection against recurrent infection with this virus [6].
It is proposed that SARS-CoV-2 may have long-term life-threatening complications that will be revealed over time. This issue bears some resemblance to common chronic viral infections including human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), human papillomavirus (HPV), and Epstein-Barr virus (EBV) [7]. In this review paper, we summarize the latest information regarding the predisposition to cancer in humans infected with the SARS-CoV-2 virus with a focus on the mechanism(s) of the viral tumorigenicity which lead to cancer development.
2. Oncogenic viruses
There are at least seven tumor-inducing viruses and their actions have been extensively reviewed elsewhere. These include human papillomavirus (HPVs), hepatitis viruses B and C (HBV and HCV), human gamma herpes viruses (HHV4/Epstein-Barr Virus, EBV), HHV8/Kaposi's sarcoma-Associated Herpesvirus (KSHV), Merkel cell polyomavirus (MCPyV), and human T-cell leukemia virus I (HTLV-1) [8]. There are critical stages and molecules that play a role in their oncogenicity [8] including blocking tumour suppressor molecules such as P53 or by activation of oncogenes [9]. For example, human immunodeficiency virus (HIV) has potential cancer-causing effects [10,11]. This virus leverages multiple mechanisms that eventually result in three cancer types namely, Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), and invasive cervical cancer (ICC) by different mechanisms [10,11].
Other human beta-coronaviruses such as SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV) also evoke various pathways that lead to carcinogenesis such as the repression of the tumour repressor retinoblastoma protein (PRB) by the nonstructural protein (nsp)15 of SARS-CoV-1 [12]. In addition, loss of cell-cell contact inhibition following infection [13] and generation of reactive oxygen species (ROS) are triggers for cancer development [14]. In several cancer-related viruses, virus-encoded circular RNAs (circRNAs) have been identified and characterized [15,16]. MERS-CoV-, SARS-CoV-1- and SARS-CoV-2-derived circRNAs showed competitive interactions with human miRNAs [17]. The analysis of these interactions in MERS-CoV viral infection showed that regulated genes in the late stages of disease were involved in multiple pathways such as cancer, metabolism and autophagy [17]. SARS-CoV-2 circRNAs interaction analysis indicated that the down-regulated genes are involved in cellular metabolism including the metabolism of fatty acids and cholesterol in the early stages of disease and conversely, the late stages of disease were associated with pathways involved in oxidative stress [17]. Despite of the potential role of these three viruses for oncogenicity, follow-up studies on long-term symptoms of SARS-CoV-1 have not reported cancers associated with this virus [[18], [19], [20]].
SARS-CoV-2, due to its characteristics and inflammatory features, which will be discussed in the following parts, seems to act like oncogenic viruses but there is no direct evidence or observation over time to support this hypothesis as yet.
3. SARS-CoV-2 mechanism of action
SARS-CoV-2 has infected millions of people worldwide and resulted in millions of deaths [21]. Although extensive research has been performed on the pathobiology of this virus over the past 2 and a half years, there are no reports of viral-induced tumorigenesis as yet [22,23]. However, there are some reports suggesting that SARS-CoV-2 might be considered as an oncogenic virus [24]. In the following section, we describe the possible mechanisms for the induction of cancers by SARS-CoV-2.
3.1. The renin-angiotensin-aldosterone system (RAAS)
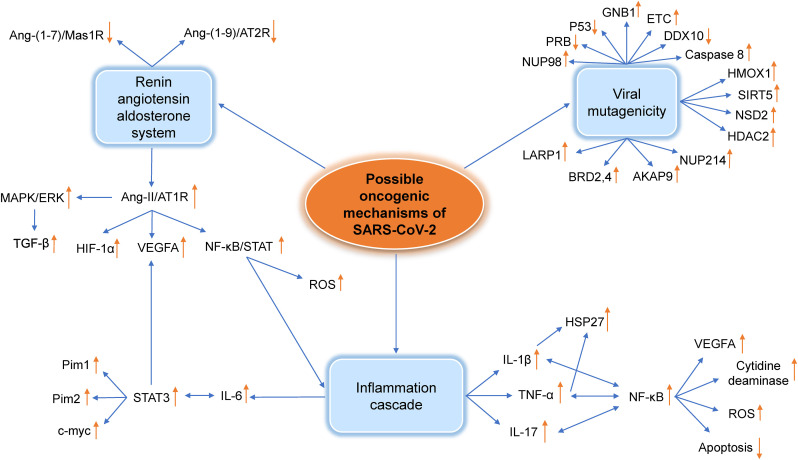
The renin-angiotensin-aldosterone system (RAAS) is responsible for chronic alteration and maintenance of blood pressure and of systemic vascular resistance. The three key factors in this system are renin, angiotensin II (Ang-II), and aldosterone [25]. Decreased renal perfusion causes secretion of renin from the kidney. Ang-II attaches to its receptor called angiotensin 1 receptor (AT1R), which is a member of the G protein-coupled receptor (GPCR) family. In SARS-CoV-2 infection, the virus binds to ACE2 and as a consequence, downregulates AT1R which leads to dysregulation of the RAAS system [[26], [27], [28], [29], [30]]. This dysregulation results in lower Ang-(1–9)/angiotensin 2 receptor (AT2R) and Ang-(1–7)/Mas1 receptor downstream signaling but higher Ang-II/AT1R signaling pathway [31]. Overall, dysregulation of the RAAS leads to inflammation, vasoconstriction, fibrosis, oxidation, and capillary permeability which can all be a factor in triggering cancer progression and development [[26], [27], [28], [29], [30],32,33].
The formation of cancer stem cells may also result from the actions of Ang-II, resulting in its link to carcinogenesis, metastasis and relapse [34]. For example, in non-small cell lung cancer (NSCLC), Ang-II regulates cancer aggressiveness and the number of cancer stem cells [35]. Moreover, the binding of Ang-II to AT1R causes the activation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways which, in turn, results in the production of transforming growth factor-beta (TGF-β) [[36], [37], [38]].
Despite of the role of TGF-β in carcinogenesis where it may play a pivotal role in cell growth and proliferation, TGF-β may also regulate tumor cell migration in favor of cancer [[36], [37], [38]]. In addition, the Ang-II/AT1R pathway contributes to the production of inflammatory growth factors and cytokines, including IL-1α, IL-6, IL-8, MCP1 and macrophage colony stimulating factor (M-CSF) by inflammatory cells [39]. The production of inflammatory cytokines is induced by the activation of transcription factors [34,[40], [41], [42], [43], [44], [45], [46], [47], [48]].
Production of vascular endothelial growth factor (VEGF) is connected with the angiogenesis, a procedure hijacked by cancer cells to increase the blood supply for the growth and metastasis [34,39]. In a number of studies, it is reported that the upregulation of VEGF, due to the dysregulation of RAAS, promotes angiogenesis in solid tumors such as ovarian and breast cancers [28,49,50]. Furthermore, the generation of ROS by neutrophils and macrophages after the Ang-II/AT1R activation in the inflammatory microenvironment causes extra damage by its deleterious effects on the DNA structure [39,51].
3.2. Viral mutagenicity
There are various mechanisms through which a virus can promote oncogenicity. For example, viral infections stimulate the human oncogenes or disrupt tumor suppression mechanisms which is the most likely way for SARS-CoV-2 to cause cancer [9]. Infection with SARS-CoV-2 may induce the expression of nsp15 which acts upon the tumor suppressor PRB through an LXCXE motif [12,52]. The interaction between nsp15 and PRB causes proteasomal digestion of PRB [12]. In addition, cells responsible for expressing nsp15 become overactive and rapidly divide [12]. In addition to PRB, another tumor suppressor gene P53 is degraded by SARS-CoV-2 via its nsp3 protein which results in the stabilization of an RCHY1 (Ring Finger and CHY Zinc Finger Domain Containing 1) ubiquitin E3 ligase that targets P53 for degradation [53,54]. This leads to lower levels of P53 which is a predisposing condition for developing cancer [[55], [56], [57]].
Oncogenic viruses are recognized as inducing mutations and cell transformation pursuant to viral infection due to modulation of the cell cycle. Infection by SARS-CoV-2 is likely to block the cell cycle leading to activation of apoptotic mechanisms including production of caspase 8 [58,59]. Viruses regulate the cell cycle, damage host DNA, use host translation machines, disrupt apoptosis, and suppress the host immune responses [[60], [61], [62], [63]]. SARS-CoV-2 interacts with epigenetic modifiers or chromatin, and epigenetic changes that are known to be important for both viral infection and cancer progression [64]. In addition, cell translation mechanisms and RNA processing could be affected by SARS-CoV-2 proteins [65].
In this line, SARS-CoV-2 possesses a few important proteins which play a role in cell-cycle progression, metabolism, epigenetics and translation and RNA processing [66]. These proteins are involved in viral genome replication and cause damage to cell cycle interactions. Among them, nsp7 is a crucial cofactor of RNA polymerase as well as interacting with GPCR signaling pathway elements, such as GNB1 (Guanine nucleotide-binding protein subunit beta-1), RHOA (rat sarcoma virus homolog family member A) and electron transport chain (ETC)-associated CYB5B [67]. The ETC is one of the sources of ROS in oxidative stress and is proposed to have a major role in the progression of cancer [68]. Other proteins of SARS-CoV-2, such as open reading frame (ORF)3 and nsp14 interacts with HMOX1 and Sirtuin 5 (SIRT5), respectively [69]. SIRT5 regulates the expression of the anti-oxidant NFE2L2 (Nuclear factor erythroid 2-related factor 2) at the transcriptional level, and increased levels of SIRT5 in lung cancer contribute to tumor progression [69].
Alpha-DNA polymerase complexes are essential for the initiation of DNA replication, and the SARS-CoV-2 nsp1 protein interacts with this complex. In addition, the interaction between SARS-CoV-2 with the RBP pathway stops the cell cycle in the G1 phase [70]. NSP8 interacts with histone methyltransferase NSD2 whilst histone deacetylase 2 (HDAC2) interacts with NSP5. Several oncogenic pathways connect NSD2 and HDAC2 [67]. Overexpression of NSD2 up-regulates the RAS-transcriptional program that is present in many cancers [71]. HDAC2 is a p53 tumor suppressor activator that induces DNA damage responses [72]. NSD2 also interacts with the bromodomain and extra-terminal (BET) family protein BRD4 [65]. Protein E is a SARS-CoV-2 envelope protein associated with BRD4 and BRD2 [66]. BRD4 and BRD2 are involved in carcinogenesis by activating oncogenes, such as MYC [65].
The SARS-CoV-2 N protein interacts with several host factors such as La-related Protein (LARP1). LARP1 acts as a phosphorylation-sensitive switch to inhibit or activate the translation of mRNAs with a 5′ terminal oligopyrimidine (Top) motif [73]. Top mRNAs are involved in cell growth and proliferation because they encode factors for translation and ribosome biogenesis. LARP1 expression is dysregulated in cancers, especially those related to viral infections, and appears to cause tumors [73]. LARP4B can stimulate translation and is associated with viral NSP12 and N protein [65].
Chromosomal alterations of the NUP98 gene act as a proto-oncogene. However, repeated chromosomal shifts are probably necessary to make cells malignant [74]. DDX10, a DEAD RNA helicase box and NUP98 fusion partner that is involved in ribosome assembly, interacts with the nsp8 viral protein [65]. DDX10 forms a characteristic combination with NUP98 [65]. DDX10 is a tumor suppressor whose expression is diminished in ovarian cancer leading to cell proliferation through the nuclear factor kappa B (NF-κB) pathway [75]. All of these interactions can lead to cancer.
3.3. Inflammatory cascade
SARS-CoV-2 can induce a plethora of pro-inflammatory cytokines known as a cytokine storm [76,77]. One of the main inflammatory cytokines participating in this process is interleukin-6 (IL-6) [78]. The extent to which IL-6 is important in the pathogenesis of COVID-19 is indicated by a high level of serum IL-6 (>32.1 pg/mL) being a prognostic factor for disease severity and poor prognosis [[78], [79], [80]]. In addition, tocilizumab, a recombinant humanized anti-human IL-6 receptor monoclonal antibody, which is conventionally used for rheumatoid arthritis has shown promising results in treating some, but not all, patients with COVID-19 [51,81].
The association of chronic inflammation with autoimmune diseases and cancer has been recently reviewed [82,83]. The IL-6 amplifier (IL-6 Amp) mechanism involves the activation of signal transducer and activator of transcription 3 (STAT3) by IL-6, and the activation of NF-κB by IL-17 or TNF-α [82,83]. STAT3 activation can cause G1 to S cell cycle transition along with the induction of c-myc, pim1 and pim2 protooncogenes [[84], [85], [86]]. The evidence of cancer progression via the activation of IL-6, STAT3, and subsequently pim1 has been illustrated in pancreatic and breast cancer [[87], [88], [89]]. In addition, the IL-6-stimulated STAT3 signaling pathway can cause metastasis by epithelial–mesenchymal transition (EMT) in head and neck cancer [90]. This signaling pathway can also cause angiogenesis via induction of hypoxia-inducible factor-1α (HIF-1α) and VEGF in cervical cancer [[91], [92], [93], [94]]. Therefore, since IL-6 activates STAT-3, which has a key role in cancer progression and inflammation, there might be a possible relationship between SARS-CoV-2 infection and the susceptibility of these patients to cancer [82].
TNF-α also plays a critical role in initiating an inflammation cascade via NF-κB activation [82]. Inflammation and NF-κB can cause tumors by evoking DNA damage, chromosomal instability and mutations via different processes including the production of ROS, the induction of activation-induced cytidine deaminase and the prevention of apoptosis [95]. In addition, some viruses including EBV stimulate NF-κB to exert their oncogenic effects [96]. As with the IL-6/STAT3 pathway, activation of the TNF-α/NF-κB pathway enhances angiogenesis via VEGF, EMT and metastasis [95,[97], [98], [99]]. The plethora of cytokines released during the cytokine storm, including interleukin-1β (IL-1β) and TNF-α, are responsible for the activation of protein kinases involved in the phosphorylation of heat shock protein 27 (HSP-27) [100,101]. Since HSP-27 can inhibit multiple steps involved in apoptosis it is a sign of poor prognosis in many different cancers [102]. Therefore, TNF-α may indirectly have a key role in cancer progression [103] (Fig. 1 ).
Fig. 1.
Interactions between oncogenic factors induced by SARS-CoV-2 infection. For details of the interactions see text. RAAS, renin-angiotensin-aldosterone system; Ang, angiotensin; AT1R, angiotensin 1 receptor; AT2R, angiotensin 1 receptor; MAS1R, MAS1 receptor; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; TGF-β, Transforming growth factor beta; VEGFA, Vascular endothelial growth factor A; HIF-1α, Hypoxia-inducible factor 1-alpha; STAT, signal transducer and activator of transcription; Pim1, Proviral integration site for Moloney murine leukemia virus-1; Pim2, Proviral integration site for Moloney murine leukemia virus-2; C-MYC, Cellular Myelocytomatosis Oncogene; NF-κB, nuclear factor-kappa B; IL-6, Interleukin 6; ROS, Reactive oxygen species; IL-17, Interleukin 17; IL-1β, Interleukin 1β; TNF, Tumor necrosis factor; HSP-27, heat shock protein 27; DDX10, DEAD-box helicase 10; NUP-98, nucleoporin-98; PRB, retinoblastoma protein; GNB-1, Guanine nucleotide-binding protein subunit beta-1; ETC, electron transport chain; HMOX1, heme oxygenase-1; SIRT5, Sirtuin 5; NSD2, Nuclear receptor binding SET domain protein 2; HDAC2, Histone deacetylase 2; NUP214, nucleoporin 214; AKAP9, A Kinase Anchor Protein 9; BRD2, Bromodomain-containing protein 2; BRD4, Bromodomain-containing protein 4; LARP1, La-related Protein 1.
4. COVID-19 mutagenicity perspectives
As indicated above, the infection of many organs by SARS-CoV-2 suggests that it may lead to the development of cancer at many sites. In the following section, we will discuss the possible involvement of different organs in greater detail (Fig. 2 ).
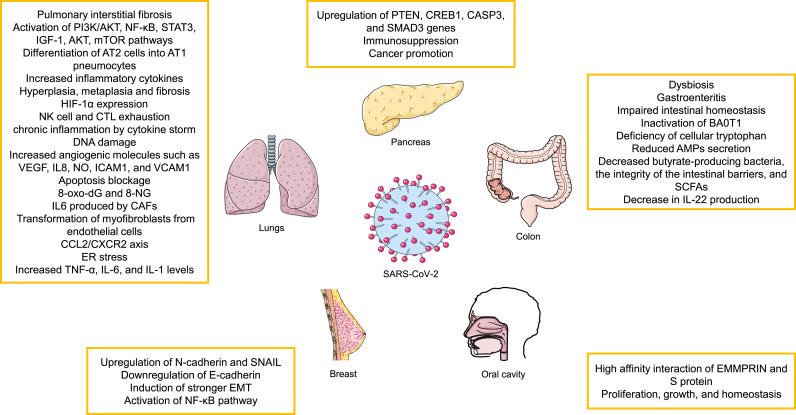
Fig. 2.
Possible oncogenic effects of SARS-CoV-2 infection on different organs. See text for more details. PI3K-AKT, Phosphatidylinositol-3-Kinase and Protein Kinase B; NF-κB, nuclear factor-kappa B; STAT, signal transducer and activator of transcription; IGF-1, insulin-like growth factor 1; AT, angiotensin; HIF-1α, Hypoxia-inducible factor 1-alpha; NK, natural killer cell; CTL, Cytotoxic T-lymphocyte; VEGFA, Vascular endothelial growth factor A; IL, Interleukin; NO, nitric oxide; ICAM-1, Intercellular Adhesion Molecule 1; VCAM-1, vascular cell adhesion molecule-1; 8-oxo-Dg, 8-Oxo-2′-deoxyguanosine; 8-NG, 8-nitroguanine; CAF, cancer associated fibroblasts; CCL2/CXCR2, C–C Motif Chemokine Ligand 2/CXC chemokine receptor 2; ER, Endoplasmic reticulum; TNF, Tumor necrosis factor; PTEN, phosphatase and tensin homolog; CREB1, cAMP-regulatory element-binding protein-1; CASP3, caspase 3; SMAD3, SMAD family member 3; SNAIL, Zinc finger protein SNAI1; EMT, epithelial–mesenchymal transition; SCFA, Short-chain fatty acids; EMMPRIN, extracellular matrix metalloproteinase inducer.
4.1. Lung cancer
SARS-CoV-2 triggers pulmonary interstitial fibrosis and inflammatory changes [104], which are known risk factors for lung cancer. SARS-CoV-2 also activates oncogenic signaling pathways such as PI3K/AKT [105]. Viral infection can differentiate alveolar epithelial type II (AT2) cells into alveolar epithelial type I (AT1) pneumocytes, which is accompanied by the active AT1 cell proliferation, which is also associated with the pathogenesis of lung injury [[106], [107], [108]] as well as pro-fibrotic mechanisms and enhanced production of inflammatory cytokines [109]. Patients exposed to SARS-CoV-2, experience inflammatory changes of trachea, bronchus and alveoli. Continuous damage to alveolar epithelium leads to hyperplasia, metaplasia and fibrosis which stimulates the incidence of lung cancer [[110], [111], [112]]. ACE2 has important role in modulating ang II expression resulting in the heightened expression of NF-κB-regulated inflammatory cytokines and activation of the PI3K/AKT pathway, a known factor in tumor development [113,114]. Furthermore, HIF-1α expression is induced by the SARS-CoV-2-driven hypoxemia [115].
SARS-CoV-2 infection causes NK cell and CTL exhaustion which leads to the suppression of immune system. Patients with NK and CTL exhaustion are more susceptible to autoimmune disorders, chronic obstructive pulmonary disease (COPD) and cancer [116].
GGO (ground glass opacity), a characteristic feature of several lung pathologies with a high-risk of developing cancer is seen in computerized tomography (CT) scans of patients with COVID-19 [117,118]. Two types of GGO nodules are detected by CT: pure ones without any solid particles in them and mixed nodules which contain solid components. The presence of GGO, especially the solid ones, in the lung is indicative of the development of cancer [[119], [120], [121], [122]].
As mentioned above, a cytokine storm occurs due to the viral infection which leads to chronic inflammation and potentially the onset of cancer [123,124]. There are other oncogenic mechanisms in association with pro-inflammatory cytokines including DNA damage, increased amounts of angiogenic molecules such as VEGF, IL8, NO, Intercellular Adhesion Molecule 1 (ICAM1), and vascular cell adhesion molecule-1 (VCAM1), induction of cell proliferation and prevention of apoptosis are of importance [[125], [126], [127]]. Furthermore, ROS and reactive nitrogen species (RNS) can also drive inflammation-induced carcinogenesis by causing DNA damage, inducing post-translational changes in proteins and lipids, activating oncogenes and repressing tumor suppressor genes [128]. For example, 8-Oxo-2′-deoxyguanosine (8-oxo-dG) and 8-nitroguanine (8-NG) are ROS and RNS metabolites respectively which are related to DNA damage and cancer onset [129,130].
IL-6 activates oncogenic pathways and also blocks antitumor defenses [131]. Furthermore, IL6 produced by cancer associated fibroblasts (CAF) promotes metastasis and EMT in lung cancer via STAT3 [132]. Moreover, the transformation of myofibroblasts from endothelial cells is triggered by TGF-β, IL-6, and galectin-3 (Gal-3) during the process of fibrosis in the lungs of COVID-19 patients [133]. When injury occurs in the lung, a large number of growth and fibrogenic factors as well as cytokines are expressed and released by AT2 cells. These factors overstimulate AT2 cells, recruit fibroblasts to fibrotic sites, and induce transactivation of fibroblasts to myofibroblasts, leading to the loss of alveolar function, especially alveolar-capillary gas exchange [134]. The role of myofibroblasts in the tumor microenvironment of NSCL cancer has also been reported [135]. Moreover, TGF-β and IL-6 play a role in lung AT1 cell EMT [133]. IL-1β and IL-17 also have carcinogenic effects by stimulating epithelial cell proliferation and tumor growth [136,137]. The C–C Motif Chemokine Ligand 2/CXC chemokine receptor 2 (CCL2/CXCR2) axis is known to be oncogenic and cause lung damage and fibrosis through driving the expression of TNF-α and iNOS [[138], [139], [140]].
The cytokine storm has other detrimental effects leading to the development of cancer including cachexia and sarcopenia. The presence of cachexia leads to ER stress and increased cytokine levels such as TNF-α, IL-6, and IL-1 that are critical in the activation of different signaling pathways including NF-κB, STAT3, IGF-1, AKT and mTOR involved in lung cancer onset [[141], [142], [143]].
4.2. Colorectal cancer (CRC)
Intestinal dysbiosis means an imbalance of commensal bacteria that puts the gut microbiota in a pathogenic state including an association with cancer in distal organs [144]. There are many ways in which SARS-CoV-2 infection could affect CRC including the ability of ACE2 to regulate nutrient uptake and modulate intestinal inflammation [145]. Intestinal SARS-COV-2 infection and the subsequent ACE2 imbalance observed in SARS-CoV-2 infection may cause symptoms similar to gastroenteritis, impaired intestinal homeostasis and gut microbiota (GM) dysbiosis in COVID-19 patients [146,147]. The susceptibility to infection with SARS-CoV-2 and mortality are higher in elderly patients with less diverse intestinal microbiota [148]. Metagenomic sequencing (MGS) analysis in hospitalized patients with COVID-19 indicates a reduced microbial diversity, loss of beneficial intestinal bacteria as well as an increase in opportunistic pathogens [146,149]. Systemic inflammation may occur due to intestinal dysbiosis, which allows bacterial products and components to enter the circulatory system [150]. Studies have identified the presence of dysbiosis in patients with CRC [151]. GM dysbiosis affects the local immune system and is associated with the onset and progression of CRC [152]. For example, in CRC patients, persistent changes in the GM are observed during tumor growth which are linked to disease progression [153]. As mentioned earlier, decreased ACE2 increases the level of angiotensinogen and activates the renin-angiotensin system, which leads to the inactivation of the amino acid transporter BA0T1, resulting in a deficiency of cellular tryptophan, which ultimately reduces the secretion of antimicrobial peptides (AMPs) and enabling intestinal dysbiosis [154].
In COVID-19 patients, the numbers of butyrate-producing bacteria are significantly decreased [155] whilst the increased presence of short-chain fatty acid (SCFA)-producing bacteria is linked with mild SARS-CoV-2 infection [156]. Butyrate-producing bacteria are very important in preserving the integrity of the intestinal barrier and SCFAs are important for IL-22 signaling which is essential for the integration of gut and lung epithelial barriers [157]. CRC is metabolically dependent on anaerobic glycolysis. SCFA acts as an HDAC inhibitor and sensitizes cancer cells to apoptosis with the accumulation of butyrate in the cytoplasm of cancer cells. The decrease in IL-22 production may be due to a decrease in butyrate-producing bacteria and intestinal dysbiosis [158,159]. Accordingly, SARS-CoV-2 infection may alter the regulation of the GM, inflammation, gut permeability and thereby increase the risk of carcinogenesis and the progression of CRC [160].
4.3. Pancreatic adenocarcinoma
Pancreatic adenocarcinoma is responsible for the majority of pancreatic cancers and due to its poor prognosis and difficult in diagnosis, this type of cancer results in metastasis and advanced stages of disease [161]. The early stages of pancreatic cancer are asymptomatic and patients are not referred to the hospital until the disease has reached a critical stage which is difficult to cure [162]. Symptoms of pancreatic cancer consists of jaundice, pain in the abdomen or back, weight loss, nausea, vomiting, gallbladder or liver enlargement, blood clots in large veins, and diabetes [163].
It has been hypothesized that after an infection with the SARS-CoV-2 virus, the expression of some genes related to the pancreatic adenocarcinoma are increased. An in-silico study predicted that pancreatic adenocarcinoma was the most likely cancer to occur following infection with SARS-CoV-2 [164]. In this analysis, four genes: phosphatase and tensin homolog (PTEN), cAMP-regulatory element-binding protein-1 (CREB1), caspase 3 (CASP3) and SMAD family member 3 (SMAD3) were the major driver genes for SARS-CoV-2 infection-induced carcinogenesis [164].
PTEN is a tumor suppressor gene exerting its function by a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase to negatively regulate the of AKT/PKB signaling pathway [165]. CREB1 is a transcription factor ubiquitously expressed in most tissues and is a member of leucin zipper family [166]. CREB1 is important in cell survival, proliferation and differentiation and in tumor formation [167]. CASP3 is a cysteine-aspartic acid protease that has a fundamental role in apoptosis [168]. Cancer treatment strategies, such as radiotherapy and chemotherapy induce tumor cell apoptosis by activating CASP3 [169]. SMAD3 is a member of a family of intracellular signal transducer proteins that participate in TGF-β signaling [170]. SMAD3 mediates metastasis and TGF-β related immunosuppression. Depending on a cell type, SMAD3 can act as a promoter or suppressor of cancer [171].
4.4. Breast cancer
Breast cancer tops the list of life-threatening cancers for women. Since breast cancer develops insidiously, routine screening can help early diagnosis and prevent metastasis. Breast cancer symptoms include a lump in the breast or underarm, thickening parts of the breast, dimpling of breast skin, nipple discharge (except milk), change in the size or shape of breasts and pain [172,173].
The hyperglycosylated S protein of the SARS-CoV-2 gamma variant can downregulate E-cadherin expression and upregulate the levels of N-cadherin and of the Zinc finger protein SNAI1 (SNAIL), resulting in increased EMT in breast epithelia cells. This, as with NF-κB pathway activation, can cause metastasis of breast cancers [174]. In another study the correlation between inhibiting the SARS-CoV-2 receptor, ACE2, and breast cancer has been illustrated [175]. COVID-19 treatment strategies which target ACE2 contributes to a dysregulated immune system which favors cancer progression. This pro-tumorigenic state is dominated by immunosuppressive T regulatory (Treg) cells and anti-inflammatory cytokines, such as IL-10. This immunosuppressive state in group 1 or luminal A breast cancer may enable tumor cells to proliferate and metastasize to distant organs [175].
4.5. Oral cancer
Oral cancer is one of the most common types of cancer and is a major public health problem especially in developing countries [176]. As we mentioned in previous sections, Ang-2 has a role in carcinogenesis by modulating cell proliferation and angiogenesis following binding to AT1R [177]. ACE2 is overexpressed in the oral cavity [178] and COVID-19 infection decreases ACE2 availability in patients leading to higher levels of Ang-2 that can promote pro-tumoral activity [177].
The immunoglobulin superfamily member extracellular matrix metalloproteinase inducer (EMMPRIN), which is also called BASIGIN/CD147 is another SARS-COV-2 entry target [179]. EMMPRIN has various roles such as proliferation, growth, and homeostasis and is associated with many malignancies and carcinogenic pathways [180]. There is a high affinity interaction between EMMPRIN and the SARS-CoV-2 S protein [181]. Increased EMMPRIN expression is observed in oral premalignant cells and in primary and metastatic cell lines of oral squamous cell carcinoma (OSCC) [182]. In contrast, SARS-CoV-2 infection in OSCC patients may deplete EMMPRIN by binding to the S protein leading to a downregulation of carcinogenesis-related pathways [183].
5. Conclusion
The long-term impact of COVID-19 on morbidity and mortality cannot be neglected. Experimental studies show that SARS-CoV-2 is able to induce re-infection/reactivation and persistent infection in the same manner as seen with other viral infections. One of the most worrying long-term effects of infection is the potential to induce malignant neoplasms, which will be a major health concern over the coming decades. SARS-CoV-2 infection affects many mechanisms that play a crucial role in cancer onset and progression including cell cycle regulation, the RAAS system and inflammation/proliferation signaling pathways.
Author contributions
Kasra Jahankhani and Fatemeh Ahangari contributed to the study conception and design, Writing and Gathering content.
Conceptualization, Reviewing, Editing, and Supervision was performed by Esmail Mortaz.
Ian M. Adcock ∗contributed to the writing of the reported study and editing∗ of the revised version.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Declarations of interest
None.
Declaration of competing interest
The authors declare no conflicts of interest.
Handling Editor: O.A. Dontsova
References
- 1.Coronavirus. https://www.who.int/health-topics/coronavirus#tab=tab_3 Available from:
- 2.Crimi E., Slutsky A.S. Inflammation and the acute respiratory distress syndrome. Best Pract. Res. Clin. Anaesthesiol. 2004;18(3):477–492. doi: 10.1016/j.bpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Notz Q., et al. Pro- and anti-inflammatory responses in severe COVID-19-induced acute respiratory distress syndrome—an observational pilot study. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.581338. 2631-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey W.T., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masrour-Roudsari J., Ebrahimpour S. Causal role of infectious agents in cancer: an overview. Caspian journal of internal medicine. 2017;8(3):153–158. doi: 10.22088/cjim.8.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tempera I., Lieberman P.M. Oncogenic viruses as entropic drivers of cancer evolution. Frontiers in Virology. 2021;1 doi: 10.3389/fviro.2021.753366. 28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesri Enrique A., Feitelson M.A., Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15(3):266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borges A.H., Dubrow R., Silverberg M.J. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr. Opin. HIV AIDS. 2014;9(1):34–40. doi: 10.1097/COH.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dlamini Z., et al. HIV-associated cancer biomarkers: a requirement for early diagnosis. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158127. 8127-8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhardwaj K., et al. The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J. Virol. 2012;86(8):4294–4304. doi: 10.1128/JVI.07012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteil V., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivas U.S., et al. ROS and the DNA damage response in cancer. Redox Biol. 2019;25 doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toptan T., et al. Circular DNA tumor viruses make circular RNAs. Proc. Natl. Acad. Sci. USA. 2018;115(37):E8737–E8745. doi: 10.1073/pnas.1811728115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J.-t., et al. Identification of virus-encoded circular RNA. Virology. 2019;529:144–151. doi: 10.1016/j.virol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Cai Z., et al. Identification and characterization of circRNAs encoded by MERS-CoV, SARS-CoV-1 and SARS-CoV-2. Briefings Bioinf. 2021;22(2):1297–1308. doi: 10.1093/bib/bbaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam M.H.-B., et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch. Intern. Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 19.Ngai J.C., et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P., et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Research. 2020;8(1):8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Home - johns hopkins coronavirus resource center. https://coronavirus.jhu.edu/ Available from:
- 22.Leung T.Y.M., et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg. Microb. Infect. 2020;9(1):2190–2199. doi: 10.1080/22221751.2020.1825914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yelin D., et al. Long-term consequences of COVID-19: research needs. Lancet Infect. Dis. 2020;20(10):1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpalhão M., Ferreira J.A., Filipe P. Persistent SARS-CoV-2 infection and the risk for cancer. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109882. 109882-109882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fountain J.H., Lappin S.L. StatPearls; 2021. Physiology, Renin Angiotensin System. [PubMed] [Google Scholar]
- 26.Gupta A., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. 2020. 26(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gressens S.B., et al. Controversial roles of the renin angiotensin system and its modulators during the COVID-19 pandemic. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.624052. 59-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues-Ferreira S., Nahmias C. G-protein coupled receptors of the renin-angiotensin system: new targets against breast cancer? Front. Pharmacol. 2015;6(FEB) doi: 10.3389/fphar.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Augustine R., et al. Increased complications of COVID-19 in people with cardiovascular disease: role of the renin–angiotensin-aldosterone system (RAAS) dysregulation. Chem. Biol. Interact. 2022;351 doi: 10.1016/j.cbi.2021.109738. 109738-109738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Rayas J.M., et al. COVID-19 and ACE -inhibitors and angiotensin receptor blockers-: the need to differentiate between early infection and acute lung injury. Rev. Colomb. Cardiol. 2020;27(3):129–131. [Google Scholar]
- 31.Mortaz E., et al. Decreased serum levels of angiotensin converting enzyme (ACE)2 and enhanced cytokine levels with severity of COVID-19: normalisation upon disease recovery. Heliyon. 2022;8(2) doi: 10.1016/j.heliyon.2022.e08957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alipoor S.D., et al. COVID-19: molecular and cellular response. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.563085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortaz E., et al. The immune response and immunopathology of COVID-19. Front. Immunol. 2020:11. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshayes F., Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol. Metabol. 2005;16(7):293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Tawinwung S., Ninsontia C., Chanvorachote P. Angiotensin II increases cancer stem cell-like phenotype in lung cancer cells. Anticancer Res. 2015;35(9) 4789-4789. [PubMed] [Google Scholar]
- 36.Elshafei A., et al. RAAS, ACE2 and COVID-19; a mechanistic review. Saudi J. Biol. Sci. 2021;28(11):6465–6470. doi: 10.1016/j.sjbs.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syed V. TGF-Β signaling in cancer. J. Cell. Biochem. 2016;117(6):1279–1287. doi: 10.1002/jcb.25496. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., et al. Long non-coding RNA HIF1A-AS1 is upregulated in intracranial aneurysms and participates in the regulation of proliferation of vascular smooth muscle cells by upregulating TGF-β1. Exp. Ther. Med. 2019;17(3):1797–1801. doi: 10.3892/etm.2018.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George A.J., Thomas W.G., Hannan R.D. The renin–angiotensin system and cancer: old dog, new tricks. Nat. Rev. Cancer. 2010;10(11):745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 40.Wolf G., Schroeder R., Stahl R.A.K. Angiotensin II induces hypoxia-inducible factor-1α in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am. J. Nephrol. 2004;24(4):415–421. doi: 10.1159/000080086. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., et al. STAT proteins mediate angiotensin II–induced production of TIMP-1 in human proximal tubular epithelial cells. Kidney Int. 2003;64(2):459–467. doi: 10.1046/j.1523-1755.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 42.Touyz R.M., Berry C. Recent advances in angiotensin II signaling. Braz. J. Med. Biol. Res. 2002;35(9):1001–1015. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- 43.Qi D., et al. Hypoxia inducible factor 1α in vascular smooth muscle cells promotes angiotensin II-induced vascular remodeling via activation of CCL7-mediated macrophage recruitment. Cell Death Dis. 2019;10(8) doi: 10.1038/s41419-019-1757-0. 544-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan J., et al. Role of angiotensin II in activation of the JAK/STAT pathway induced by acute pressure overload in the rat heart. Circ. Res. 1997;81(4):611–617. doi: 10.1161/01.res.81.4.611. [DOI] [PubMed] [Google Scholar]
- 45.Higuchi S., et al. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin. Sci. 2007;112(8):417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 46.Wolf G., et al. Angiotensin II activates nuclear transcription factor-κB through AT1 and AT2 receptors1. Kidney Int. 2002;61(6):1986–1995. doi: 10.1046/j.1523-1755.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.M., et al. Mechanism of Ang II involvement in activation of NF-κB through phosphorylation of p65 during aging. Age (Dordrecht, Netherlands) 2012;34(1):11–25. doi: 10.1007/s11357-011-9207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satou R., Gonzalez-Villalobos R.A. JAK-STAT and the renin-angiotensin system: the role of the JAK-STAT pathway in blood pressure and intrarenal renin-angiotensin system regulation. JAK-STAT. 2012;1(4):250–256. doi: 10.4161/jkst.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ino K., et al. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br. J. Cancer. 2006;94(4):552–560. doi: 10.1038/sj.bjc.6602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arrieta O., et al. Association between AT1 and AT2 angiotensin II receptor expression with cell proliferation and angiogenesis in operable breast cancer. Tumor Biol. 2015;36(7):5627–5634. doi: 10.1007/s13277-015-3235-3. [DOI] [PubMed] [Google Scholar]
- 51.Forrester S.J., et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98(3):1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stingi A., Cirillo L. SARS-CoV-2 infection and cancer. Bioessays. 2021;43(8) doi: 10.1002/bies.202000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma-Lauer Y., et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLprovia E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. USA. 2016;113(35):E5192 LP–E5201. doi: 10.1073/pnas.1603435113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leng R.P., et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112(6):779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 55.Sheng Y., et al. Molecular basis of Pirh2-mediated p53 ubiquitylation. Nat. Struct. Mol. Biol. 2008;15(12):1334–1342. doi: 10.1038/nsmb.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surget S., Khoury M.P., Bourdon J.-C. Uncovering the role of p53 splice variants in human malignancy: a clinical perspective. OncoTargets Ther. 2013;7:57–68. doi: 10.2147/OTT.S53876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burkhart D.L., Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer. 2008;8(9):671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su M., et al. A mini-review on cell cycle regulation of coronavirus infection. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.586826. 586826-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S., et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Targeted Ther. 2020;5(1) doi: 10.1038/s41392-020-00334-0. 235-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai Y., et al. Regulation of apoptosis by enteroviruses. Front. Microbiol. 2020:11. doi: 10.3389/fmicb.2020.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luftig M.A. Viruses and the DNA damage response: activation and antagonism. Annual Review of Virology. 2014;1(1):605–625. doi: 10.1146/annurev-virology-031413-085548. [DOI] [PubMed] [Google Scholar]
- 62.Christiaansen A., Varga S.M., Spencer J.V. Viral manipulation of the host immune response. Curr. Opin. Immunol. 2015;36:54–60. doi: 10.1016/j.coi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagga S., Bouchard M.J. Cell cycle regulation during viral infection. Methods Mol. Biol. 2014;1170:165–227. doi: 10.1007/978-1-4939-0888-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atlante S., et al. The epigenetic implication in coronavirus infection and therapy. Clin. Epigenet. 2020;12(1):156. doi: 10.1186/s13148-020-00946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tutuncuoglu B., et al. The landscape of human cancer proteins targeted by SARS-CoV-2. Cancer Discov. 2020;10(7):916 LP–921. doi: 10.1158/2159-8290.CD-20-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon D.E., et al. vol. 22. Preprint posted March; 2020. (A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv). [Google Scholar]
- 67.Gordon D.E., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X., Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J. Transl. Med. 2017;15(1):1–13. doi: 10.1186/s12967-017-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu W., et al. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(11):10699–10705. doi: 10.1007/s13277-014-2372-4. [DOI] [PubMed] [Google Scholar]
- 70.Yuan X., et al. G1 phase cell cycle arrest induced by SARS-CoV 3a protein via the cyclin D3/pRb pathway. Am. J. Respir. Cell Mol. Biol. 2007;37(1):9–19. doi: 10.1165/rcmb.2005-0345RC. [DOI] [PubMed] [Google Scholar]
- 71.García-Carpizo V., et al. NSD2 contributes to oncogenic RAS-driven transcription in lung cancer cells through long-range epigenetic activation. Sci. Rep. 2016;6(1):1–17. doi: 10.1038/srep32952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun D., et al. Histone deacetylase 2 is involved in DNA damage-mediated cell death of human osteosarcoma cells through stimulation of the ATM/p53 pathway. FEBS Open Bio. 2019;9(3):478–489. doi: 10.1002/2211-5463.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong S., et al. vol. 6. eLife; 2017. (LARP1 Functions as a Molecular Switch for mTORC1-Mediated Translation of an Essential Class of mRNAs). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frieman M., et al. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007;81(18):9812–9824. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gai M., Bo Q., Qi L. Epigenetic down-regulated DDX10 promotes cell proliferation through Akt/NF-κB pathway in ovarian cancer. Biochem. Biophys. Res. Commun. 2016;469(4):1000–1005. doi: 10.1016/j.bbrc.2015.12.069. [DOI] [PubMed] [Google Scholar]
- 76.Ragab D., et al. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blanco-Melo D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu F., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. : the official publication of the Pan American Society for Clinical Virology. 2020;127 doi: 10.1016/j.jcv.2020.104370. 104370-104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han H., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung M.K., et al. vol. 58. eBioMedicine; 2020. (SARS-CoV-2 and ACE2: the Biology and Clinical Data Settling the ARB and ACEI Controversy). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021;33(3):127–148. doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atsumi T., et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014;74(1):8–14. doi: 10.1158/0008-5472.CAN-13-2322. [DOI] [PubMed] [Google Scholar]
- 84.Shirogane T., et al. Synergistic roles for pim-1 and c-myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999;11(6):709–719. doi: 10.1016/s1074-7613(00)80145-4. [DOI] [PubMed] [Google Scholar]
- 85.Kiuchi N., et al. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J. Exp. Med. 1999;189(1):63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukada T., et al. STAT3 orchestrates contradictory signals in cytokine-induced G1 to S cell-cycle transition. EMBO J. 1998;17(22):6670–6677. doi: 10.1093/emboj/17.22.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Block K.M., et al. IL-6 stimulates STAT3 and Pim-1 kinase in pancreatic cancer cell lines. Pancreas. 2012;41(5):773–781. doi: 10.1097/MPA.0b013e31823cdd10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu J., et al. PIM-1 contributes to the malignancy of pancreatic cancer and displays diagnostic and prognostic value. J. Exp. Clin. Cancer Res. 2016;35(1) doi: 10.1186/s13046-016-0406-z. 133-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao X., et al. PIM1 is responsible for IL-6-induced breast cancer cell EMT and stemness via c-myc activation. Breast Cancer. 2019;26(5):663–671. doi: 10.1007/s12282-019-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yadav A., et al. IL-6 promotes head and neck tumor metastasis by inducing epithelial–mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol. Cancer Res. 2011;9(12):1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Q., et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 92.Niu G., et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 93.Wei L.-H., et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 94.Adachi Y., et al. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int. J. Cancer. 2006;119(6):1303–1311. doi: 10.1002/ijc.22006. [DOI] [PubMed] [Google Scholar]
- 95.Zhang T., et al. NF-κB signaling in inflammation and cancer. MedComm. 2021;2(4):618–653. doi: 10.1002/mco2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charostad J., et al. The interplay between EBV and KSHV viral products and NF-κB pathway in oncogenesis. Infect. Agents Cancer. 2020;15 doi: 10.1186/s13027-020-00317-4. 62-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber M.A., et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 2004;114(4):569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang S., et al. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 99.Wang R., et al. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 2020;11(1) doi: 10.1038/s41419-020-2252-3. 55-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Satoh J.-I., Kim S.U. Cytokines and growth factors induce HSP27 phosphorylation in human astrocytes. J. Neuropathol. Exp. Neurol. 1995;54(4):504–512. doi: 10.1097/00005072-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 101.Mehlen P., et al. Tumor necrosis factor-α induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J. Cell. Biochem. 1995;58(2):248–259. doi: 10.1002/jcb.240580213. [DOI] [PubMed] [Google Scholar]
- 102.Choi S.-K., et al. Targeting heat shock protein 27 in cancer: a druggable target for cancer treatment? Cancers. 2019;11(8) doi: 10.3390/cancers11081195. 1195-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saha A., Anirvan P. Cancer progression in COVID-19: integrating the roles of renin angiotensin aldosterone system, angiopoietin-2, heat shock protein-27 and epithelial mesenchymal transition. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1099. 1099-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.John A.E., et al. COVID-19 and pulmonary fibrosis: a potential role for lung epithelial cells and fibroblasts. Immunol. Rev. 2021;302(1):228–240. doi: 10.1111/imr.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delpino M.V., Quarleri J. SARS-CoV-2 pathogenesis: imbalance in the renin-angiotensin system favors lung fibrosis. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wigén J., et al. Converging pathways in pulmonary fibrosis and Covid-19 - the fibrotic link to disease severity. Respir. Med. X. 2020;2 doi: 10.1016/j.yrmex.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olajuyin A.M., Zhang X., Ji H.-L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discovery. 2019;5(1):63. doi: 10.1038/s41420-019-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahn D.-G., et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez I.E., Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet (London, England) 2012;380(9842):680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 111.Harapan H., et al. Coronavirus disease 2019 (COVID-19): a literature review. Journal of Infection and Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh N., et al. Inflammation and cancer. Ann. Afr. Med. 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Passos-Silva D.G., Verano-Braga T., Santos R.A.S. Angiotensin-(1-7): beyond the cardio-renal actions. Clin. Sci. 2013;124 7:443–456. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- 114.Celec P. Nuclear factor kappa B--molecular biomedicine: the next generation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2004;58(6–7):365–371. doi: 10.1016/j.biopha.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 115.Wu G., et al. Hypoxia exacerbates inflammatory acute lung injury via the toll-like receptor 4 signaling pathway. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01667. 1667-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Osman M.S., van Eeden C., Cohen Tervaert J.W. 2020. Fatal COVID-19 Infections: Is NK Cell Dysfunction a Link with Autoimmune HLH? 102561-102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou S., et al. CT Features of coronavirus disease 2019 (COVID-19) Pneumonia in 62 Patients in wuhan, China. AJR. Am. J. Roentgenol. 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 118.Luo L., et al. CT differential diagnosis of COVID-19 and non-COVID-19 in symptomatic suspects: a practical scoring method. BMC Pulm. Med. 2020;20(1) doi: 10.1186/s12890-020-1170-6. 129-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee H.Y., et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR. American journal of roentgenology. 2014;202(3):W224–W233. doi: 10.2214/AJR.13.11819. [DOI] [PubMed] [Google Scholar]
- 120.Lee L., et al. COVID-19 follow-up planning: what will we be missing? ERJ open research. 2020;6(2) doi: 10.1183/23120541.00198-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Migliore M., et al. Ground glass opacities management in the lung cancer screening era. Ann. Transl. Med. 2018;6(5) doi: 10.21037/atm.2017.07.28. 90-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alipoor S.D., et al. Immunopathogenesis of pneumonia in COVID-19. TANAFFOS (Respiration) 2020;19(2):79–82. [PMC free article] [PubMed] [Google Scholar]
- 123.Gomes M., et al. The role of inflammation in lung cancer. Adv. Exp. Med. Biol. 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 124.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kundu J.K., Surh Y.-J. Inflammation: gearing the journey to cancer. Mutat. Res. 2008;659(1–2):15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 126.Xu-Welliver M., Carbone D.P. Blood-based biomarkers in lung cancer: prognosis and treatment decisions. Transl. Lung Cancer Res. 2017;6(6):708–712. doi: 10.21037/tlcr.2017.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. : MCR. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 128.Costela-Ruiz V.J., et al. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kawanishi S., et al. Nitrative and oxidative DNA damage in infection-related carcinogenesis in relation to cancer stem cells. Gene Environ. : the official journal of the Japanese Environmental Mutagen Society. 2016;38 doi: 10.1186/s41021-016-0055-7. 26-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pinlaor S., et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J. Gastroenterol. 2005;11(30):4644–4649. doi: 10.3748/wjg.v11.i30.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taniguchi K., Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014;26(1):54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 132.Wang L., et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8(44):76116–76128. doi: 10.18632/oncotarget.18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giacomelli C., et al. Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients. Biochem. Pharmacol. 2021;193 doi: 10.1016/j.bcp.2021.114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Razzaque M.S., Taguchi T. Pulmonary fibrosis: cellular and molecular events. Pathol. Int. 2003;53(3):133–145. doi: 10.1046/j.1440-1827.2003.01446.x. [DOI] [PubMed] [Google Scholar]
- 135.Shintani Y., et al. Pulmonary fibroblasts induce epithelial mesenchymal transition and some characteristics of stem cells in non-small cell lung cancer. Ann. Thorac. Surg. 2013;96(2):425–433. doi: 10.1016/j.athoracsur.2013.03.092. [DOI] [PubMed] [Google Scholar]
- 136.Numasaki M., et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J. Immunol. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
- 137.McLoed A.G., et al. Neutrophil-derived IL-1β impairs the efficacy of NF-κB inhibitors against lung cancer. Cell Rep. 2016;16(1):120–132. doi: 10.1016/j.celrep.2016.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liao M., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 139.Sun L., et al. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;300(3):L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu J.Q., Kosten T.R., Zhang X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2013;46:200–206. doi: 10.1016/j.pnpbp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 141.Roy A., Kumar A. ER stress and unfolded protein response in cancer cachexia. Cancers. 2019;11(12) doi: 10.3390/cancers11121929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dunne R.F., et al. Cachexia and sarcopenia in older adults with cancer: a comprehensive review. Cancers. 2019;11(12) doi: 10.3390/cancers11121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morley J.E., Kalantar-Zadeh K., Anker S.D. 2020. COVID-19: a Major Cause of Cachexia and Sarcopenia? pp. 863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernandes R., et al. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865(7):1876–1897. doi: 10.1016/j.bbadis.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 145.Guo Y., et al. ACE2 in the gut: the center of the 2019-nCoV infected pathology. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.708336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gu S., et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trottein F., Sokol H. Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Rep. 2020;32(3) doi: 10.1016/j.celrep.2020.107915. 107915-107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. 198018-198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zuo T., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terruzzi I., Senesi P. Does intestinal dysbiosis contribute to an aberrant inflammatory response to severe acute respiratory syndrome coronavirus 2 in frail patients? Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.110996. 110996-110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sánchez-Alcoholado L., et al. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers. 2020;12(6) doi: 10.3390/cancers12061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scanlan P.D., et al. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ. Microbiol. 2008;10(3):789–798. doi: 10.1111/j.1462-2920.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 153.Sanapareddy N., et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6(10):1858–1868. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mönkemüller K., Fry L.C., Rickes S. 2020. Systemic Inflammatory Response and Thrombosis Due to Alterations in the Gut Microbiota in COVID-19; pp. 584–585. [DOI] [PubMed] [Google Scholar]
- 155.Tang L., et al. Clinical significance of the correlation between changes in the major intestinal bacteria species and COVID-19 severity. Engineering (Beijing, China) 2020;6(10):1178–1184. doi: 10.1016/j.eng.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zuo T., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70(2):276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jayasimhan A., Mariño E. Dietary SCFAs, IL-22, and GFAP: the three musketeers in the gut-neuro-immune network in type 1 diabetes. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02429. 2429-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Encarnação J.C., et al. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34(3):465–478. doi: 10.1007/s10555-015-9578-9. [DOI] [PubMed] [Google Scholar]
- 159.Lo B.C., et al. IL-22 preserves gut epithelial integrity and promotes disease remission during chronic Salmonella infection. J. Immunol. 2019;202(3):956–965. doi: 10.4049/jimmunol.1801308. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
- 160.Howell M.C., et al. SARS-CoV-2-Induced gut microbiome dysbiosis: implications for colorectal cancer. Cancers. 2021;13(11) doi: 10.3390/cancers13112676. 2676-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pancreatic Cancer Types | Johns Hopkins Medicine.
- 162.Vareedayah A.A., Alkaade S., Taylor J.R. Pancreatic adenocarcinoma. Mo. Med. 2018;115(3):230–235. [PMC free article] [PubMed] [Google Scholar]
- 163.Signs and Symptoms of Pancreatic Cancer.
- 164.Ebrahimi Sadrabadi A., et al. The risk of pancreatic adenocarcinoma following SARS-CoV family infection. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-92068-4. 12948-12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.PTEN Phosphatase and Tensin Homolog [Homo sapiens (Human)] - Gene - NCBI.
- 166.CREB1 cAMP Responsive Element Binding Protein 1 [Homo sapiens (Human)] - Gene - NCBI.
- 167.Sakamoto K.M., Frank D.A. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin. Cancer Res. : an official journal of the American Association for Cancer Research. 2009;15(8):2583–2587. doi: 10.1158/1078-0432.CCR-08-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sagini M.N., et al. 1947. The Expression of Genes Contributing to Pancreatic Adenocarcinoma Progression Is Influenced by the Respective Environment. 6019 (Print)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou M., et al. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int. J. Cancer. 2018;143(4):921–930. doi: 10.1002/ijc.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.SMAD3 SMAD Family Member 3 [Homo sapiens (Human)] - Gene - NCBI.
- 171.Millet C., Zhang Y.E. Roles of Smad3 in TGF-beta signaling during carcinogenesis. Crit. Rev. Eukaryot. Gene Expr. 2007;17(4):281–293. doi: 10.1615/critreveukargeneexpr.v17.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Breast Cancer - Symptoms and Causes - Mayo Clinic.
- 173.What Are the Symptoms of Breast Cancer? | CDC.
- 174.Huang H.-C., et al. Hyperglycosylated spike of SARS-CoV-2 gamma variant induces breast cancer metastasis. American journal of cancer research. 2021;11(10):4994–5005. [PMC free article] [PubMed] [Google Scholar]
- 175.Bhari V.K., et al. SARS-CoV-2 cell receptor gene ACE2 -mediated immunomodulation in breast cancer subtypes. Biochemistry and Biophysics Reports. 2020;24 doi: 10.1016/j.bbrep.2020.100844. 100844-100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Karmakar D., et al. Road map to understanding SARS-CoV-2 clinico-immunopathology and COVID-19 disease severity. Pathogens. 2020;10(1) doi: 10.3390/pathogens10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sarode S.C., et al. Biological behavior of oral squamous cell carcinoma in the background of novel corona virus infection. Oral Oncol. 2020;110 doi: 10.1016/j.oraloncology.2020.104781. 104781-104781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu H., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1) doi: 10.1038/s41368-020-0074-x. 8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nabeshima K., et al. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 2006;56(7):359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 180.Xin X., et al. CD147/EMMPRIN overexpression and prognosis in cancer: a systematic review and meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep32804. 32804-32804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang K., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vigneswaran N., et al. Increased EMMPRIN (CD 147) expression during oral carcinogenesis. Exp. Mol. Pathol. 2006;80(2):147–159. doi: 10.1016/j.yexmp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 183.Varadarajan S., et al. EMMPRIN/BASIGIN as a biological modulator of oral cancer and COVID-19 interaction: novel propositions. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110089. 110089-110089. [DOI] [PMC free article] [PubMed] [Google Scholar]