Abstract
Streptococcus mutans has been implicated as the primary pathogen in childhood caries (tooth decay). While the role of polymicrobial communities is appreciated, it remains unclear whether other microorganisms are active contributors or interact with pathogens. Here, we integrate multi-omics of supragingival biofilm (dental plaque) from 416 preschool-age children (208 males and 208 females) in a discovery-validation pipeline to identify disease-relevant inter-species interactions. Sixteen taxa associate with childhood caries in metagenomics-metatranscriptomics analyses. Using multiscale/computational imaging and virulence assays, we examine biofilm formation dynamics, spatial arrangement, and metabolic activity of Selenomonas sputigena, Prevotella salivae and Leptotrichia wadei, either individually or with S. mutans. We show that S. sputigena, a flagellated anaerobe with previously unknown role in supragingival biofilm, becomes trapped in streptococcal exoglucans, loses motility but actively proliferates to build a honeycomb-like multicellular-superstructure encapsulating S. mutans, enhancing acidogenesis. Rodent model experiments reveal an unrecognized ability of S. sputigena to colonize supragingival tooth surfaces. While incapable of causing caries on its own, when co-infected with S. mutans, S. sputigena causes extensive tooth enamel lesions and exacerbates disease severity in vivo. In summary, we discover a pathobiont cooperating with a known pathogen to build a unique spatial structure and heighten biofilm virulence in a prevalent human disease.
Subject terms: Biofilms, Bacterial pathogenesis, Oral diseases
Streptococcus mutans (S. mutans) has been implicated as a major pathogen in childhood caries. Here, the authors show that Selenomonas sputigena, a flagellated anaerobe, interacts with S. mutans in the supragingival biofilm, builds a honeycomb-like multicellular-superstructure that encapsulates it, and promotes the development of childhood dental caries.
Introduction
Biofilm-forming pathogens have been implicated in a myriad of human infectious diseases and contamination of biomedical devices and implants1–3. Although established pathogens display emergent properties that facilitate biofilm lifestyle and virulence expression, they often reside in polymicrobial communities with increasing evidence of interspecies interactions4,5. Among various microbiomes, the human plaque biofilm communities harbor diverse microbiota at the oral mucosal barrier and mineralized dental surfaces that play key roles in modulating health and disease yet remain underappreciated6. Although microbiome-wide association studies have revealed novel microbial species implicated in oral diseases, most evidence has been generated by small samples of participants recruited from clinics or undergoing dental treatment, whereas the identified species’ causal roles remain largely undefined. Comprehensive investigations of the taxonomic and functional aspects of microbial interactions in oral biofilms among community-based samples are warranted. Such studies may not only reveal additional pathogens with population relevance but can also shed light on novel pathogenetic mechanisms and interspecies interactions.
Dental caries (tooth decay) is a widespread, biofilm-mediated, and diet-modulated disease that affects approximately 600 million children worldwide and remains a major unresolved public health problem7. The disease has a complex etiology, but it is generally understood as a host-diet-dependent process of dental tissue demineralization that relies on dysbiotic community and polymicrobial acidogenesis4,8. Streptococcus mutans is a gram-positive, biofilm-forming, acidogenic and aciduric bacterium that has been clinically associated with childhood caries9, has been shown to cause caries in animal models10, and has been established as a keystone oral pathogen11. Other bacterial taxa, e.g., Scardovia wiggsiae and mitis group streptococci such as Streptococcus gordonii and Streptococcus oralis, have been reported as collaborating with or antagonizing S. mutans12,13. Although other microbial species on tooth surfaces have been associated with dental caries14–17, it remains unclear whether they are active contributors, inactive cohabitants, or they interact with S. mutans as pathobionts18 to promote disease development.
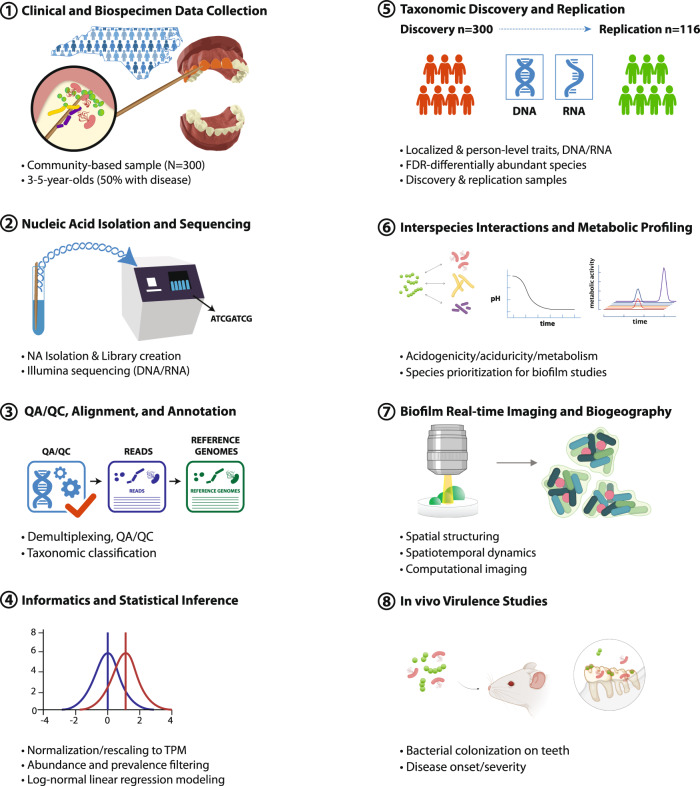
Most evidence to date on the microbial basis of dental caries has been generated from targeted, culture-based, or 16S ribosomal gene-based methods. While providing foundational information, this knowledge base can be substantially augmented using DNAseq-based metagenomics (MTG), as well as RNAseq-based metatranscriptomics (MTX), to gain deeper insights into microbial taxonomy and functional activity. The few studies employing next-generation sequencing have been done in small clinical samples19,20, and with little or no mechanistic validation. Meanwhile, advances in imaging technology have revealed spatially structured communities in multispecies biofilms, warranting better understanding of the spatial arrangement and positioning (i.e., the biogeography) of the interacting microbes21,22. To overcome these limitations and gain fundamental knowledge, we have developed a multi-modality, discovery-validation pipeline integrating clinical data from community-based studies and informatics discovery with in vitro and in vivo experimental models to study disease-associated microbial taxa and their interactions (Fig. 1).
Fig. 1. Overview of methods employed in the study.
Overview of the design of the multi-method, discovery-validation experimental approach employed in the present investigation.
Our data highlight Selenomonas sputigena, Prevotella salivae and Leptotrichia wadei as species with previously unrecognized roles in supragingival biofilms, with functional repertoires and interactions relevant to the pathogenesis of a prevalent childhood dysbiotic disease. We unexpectedly find that the flagellated, gram-negative anaerobic bacterium S. sputigena, found in saliva and commonly reported as member of the periodontal niche microbiome23, has a significant role in the supragingival biofilm microbiome and is strongly associated with childhood dental caries. Here, we show that the motile S. sputigena becomes trapped into extracellular glucan-matrix produced by S. mutans, loses its motility, but rapidly proliferates to form a multicellular honeycomb-like scaffold that wraps the streptococcal cell clusters and results in enhanced acid production—a key virulence factor in dental caries. We verify experimentally that, although S. sputigena alone does not cause caries, it significantly increases disease severity when co-infected with S. mutans causing extensive cavitation of tooth enamel in a rodent model. Taken together, our findings reveal a novel pathobiont capable of unique spatial structuring and interspecies cooperation that enhances biofilm virulence—a phenomenon that substantially improves our understanding of childhood dental disease pathogenesis and may have implications for established pathogen and pathobiont interactions in other polymicrobial infections.
Results
Established and novel taxa are associated with childhood caries
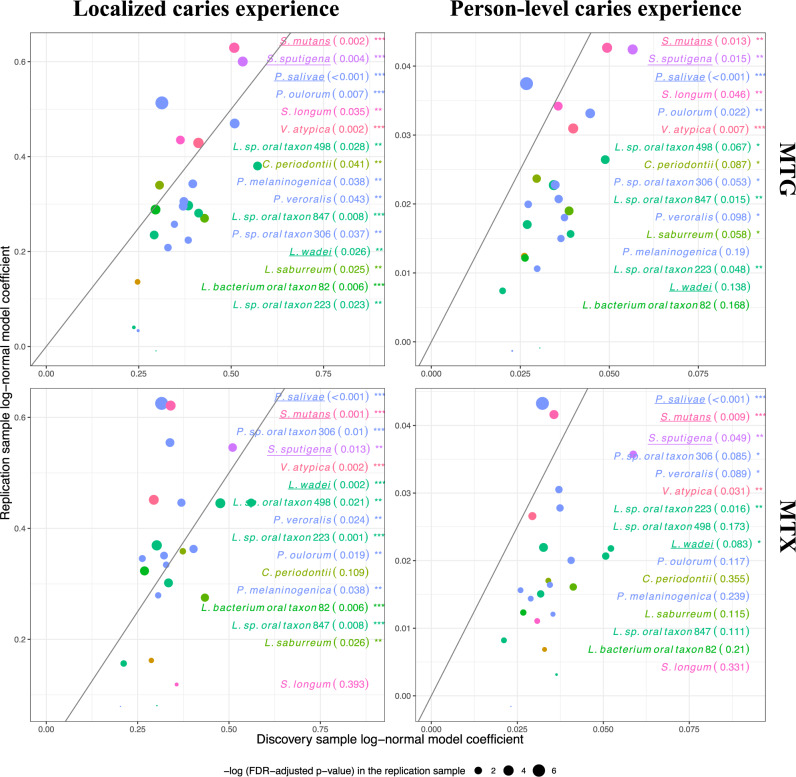
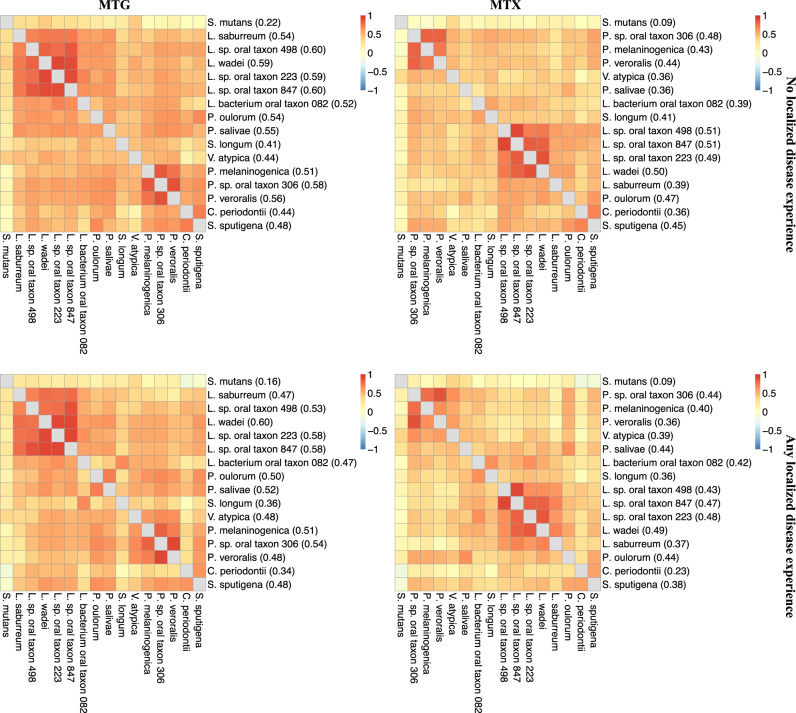
We carried out comprehensive taxonomic association analyses in MTG (i.e., whole genome sequencing shotgun) and MTX (i.e., RNAseq) data and identified 16 bacterial species significantly associated with clinically measured childhood caries experience. We used strict, multiple testing (i.e., false discovery rate, FDR)-controlled, across-trait criteria to identify this set of 16 disease-associated species. Specifically, we required taxa to demonstrate statistically significant associations with quantitative disease experience measured both locally (i.e., number of tooth surfaces with caries experience considering tooth surfaces where plaque was harvested from) and at the person-level (i.e., number of tooth surfaces with caries experience considering the entire dentition), in DNA and RNA data, in the discovery sample set (n = 300 children), and with evidence of replication in an independent sample of similarly-aged children (n = 116, Fig. 2 and Supplementary Table 1). S. mutans (beta = 0.51 ± 0.21, p = 2.6 × 10−6) emerged strongly associated with caries experience as the known and expected suspect. Other significantly associated taxa included P. salivae (beta = 0.31 ± 0.14, p = 8.5 × 10−6 in the association of DNA-based species taxonomic abundance with localized caries experience), S. sputigena (beta = 0.53 ± 0.22, p = 3.1 × 10−6), L. wadei (beta = 0.41 ± 0.15, p = 2.2 × 10−7), Veillonella atypica (beta = 0.41 ± 0.18, p = 1.1 × 10−5), Lachnospiraceae bacterium oral taxon 082 (beta = 0.30 ± 0.15, p = 1.1 × 10−4), Stomatobaculum longum (beta = 0.36 ± 0.22, p = 1.6 × 10−3), Lachnoanaerobaculum saburreum (beta = 0.43 ± 0.17, p = 9.6 × 10−7), and Centipeda periodontii (beta = 0.31 ± 0.21, p = 3.8 × 10−3). These associations were consistent in MTG and MTX data (Supplementary Fig. 1), and, as expected, there was a high degree of correlation between the abundance of taxonomically neighboring significant taxa in MTG and MTX (e.g., Leptotrichia spp. and Prevotella spp.) (Fig. 3). S. mutans, being the only Streptococcus among the 16 significant species, showed the smallest correlations with the other 15 taxa. For example, S. mutans’ mean correlations in MTX were 0.09 both in health and disease, whereas L. wadei’s were 0.50 and 0.49, and S. sputigena’s were 0.45 and 0.38, respectively.
Fig. 2. Association of oral microbial species with dental caries.
Estimates of association between dental caries experience and the abundance of 23 species that were significantly associated with caries experience in MTG and MTX analyses in the discovery (n = 300 children) sample. The magnitude of association was measured by log-normal model coefficients (the difference of the log-abundance level of each species between the binary caries statuses), and two-sided Wald tests were performed. 16 of those species showed evidence of association in the replication sample (n = 116 children) and are annotated by name in the figure. Nominal p-values are presented in parentheses, and *, **, and *** denote nominal p-values of less than 0.1, 0.05, 0.01, respectively in the replication sample. The effect sizes and their 95% confidence intervals are available in the source data file in the Supplementary Information. Diagonal lines represent y = x. Similarly colored taxa are members of the same genus. The 4 underlined taxa are the “top species” that were prioritized for in vitro virulence assays, biofilm studies, and were candidates for in vivo experiments.
Fig. 3. Pairwise correlations between oral microbial species.
Pairwise correlations (i.e., Pearson correlations of residuals generated from a log-normal model for quantitative localized caries experience including terms for batch effects and age) between the 16 significant species’ abundance in MTG and MTX, in dental health (i.e., no localized caries experience) and disease (i.e., any localized caries experience). Parenthesized values are individual species’ mean correlations with the other 15.
Glycolysis is upregulated in biofilm transcriptomes where novel taxa are implicated
Six pathways emerged as significantly associated with caries experience in MTX: glycolysis IV, pyruvate fermentation to acetate and lactate II, chorismate biosynthesis from 3-dehydroquinate, UDP-N-acetyl-D-glucosamine biosynthesis I, inosine-5’-phosphate biosynthesis III, and superpathway of menaquinol-8 biosynthesis II (Supplementary Table 2). Of note, the first 4 of these pathways involved one or more of the 16 significant species. Glycolysis IV, one of the most abundant pathways in expression abundance (ranked #3 overall) and an important pathway for acidogenesis implicated in disease, involves S. sputigena, L. wadei and 4 more significant species. Pyruvate fermentation to acetate and lactate II involves L. saburreum and its significant downregulation is consistent with a decrease in the availability of pyruvate in an acidogenic caries-promoting biofilm, wherein it may be preferentially consumed by S. mutans. Additionally, the upregulated UDP-N-acetyl-D-glucosamine biosynthesis I, involving L. wadei, C. periodontii, S. sputigena, and V. atypica, is important for peptidoglycan synthesis and cell growth. In addition, lactose and galactose degradation I, the pathway with the highest proportional representation of significant species (i.e., L. wadei and S. mutans) was also upregulated in disease although not statistically significant (Supplementary Fig. 2).
Three new candidate pathogens nominated for virulence and biofilm studies
Next, we sought to prioritize a subset of species that was practically feasible to carry forward into the multi-modal validation pipeline (Fig. 1 steps 6–8). To select this shortlist of candidates that could be fully characterized in virulence, biofilm, and in vivo experiments in this study, we considered statistical evidence of association in the discovery sample (i.e., p-value), evidence independent replication, representation of different genera (i.e., one species per genus), and availability of clinical isolates. Based on these criteria, from the list of 16 significant species we prioritized S. mutans (the known, well-established pathogen), and S. sputigena, P. salivae, and L. wadei (as new candidates). These four taxa, hereafter referred to as “top species”, were carried forward to in vitro virulence assessments and biofilm characterization and served as candidates for further in vivo colonization and pathogenicity studies.
Interspecies metabolic interactions and acidogenesis
To form the acidic microenvironment that causes demineralization of tooth surfaces, bacterial pathogens within the biofilm microbiota must efficiently metabolize dietary sugars such as sucrose (the primary sugar associated with dental decay) and produce acids (acidogenicity) while surviving in an acidified milieu (aciduricity)24. We first sought to determine whether the top species fit the pathogenic profile of being both acidogenic and aciduric.
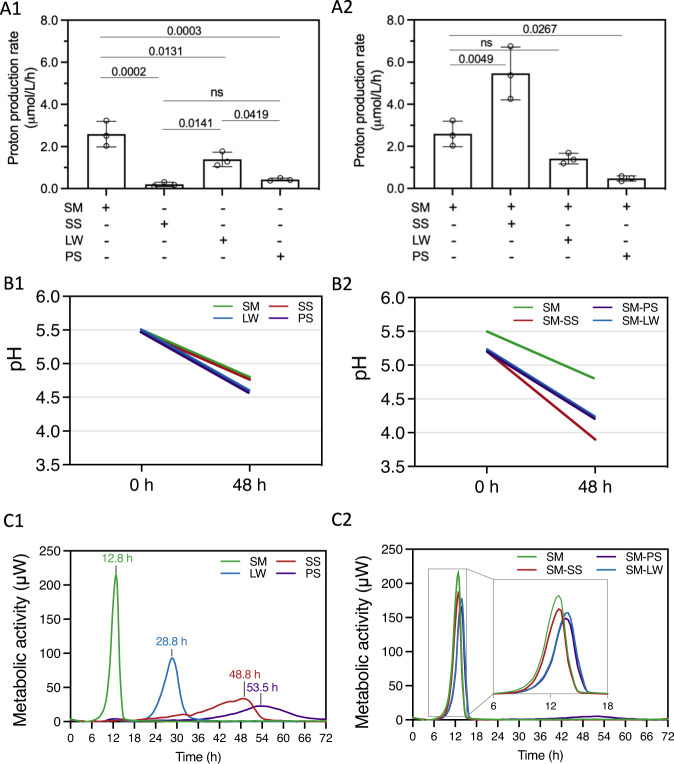
Glycolytic pH-drop assays for each species individually (Fig. 4A1) and the new candidates combined with the established S. mutans (Fig. 4A2) showed that all were active acid producers in the presence of sucrose, lowered pH to highly acidic values, i.e., pH 4.3 to 5.5 which can cause enamel demineralization25, consistent with up-regulation of glycolysis in the disease-associated plaque biofilm transcriptome. S. mutans was the most acidogenic, followed by L. wadei, P. salivae, and S. sputigena (Fig. 4A1). Intriguingly, the combination of S. mutans and S. sputigena resulted in the highest average acid production rate, significantly higher than that of S. mutans alone (Fig. 4A2) and the most rapid pH drop. These findings are suggestive of a possible co-metabolic relationship between S. mutans and S. sputigena.
Fig. 4. Metabolic and acidogenic profile of the top species.
Top species include S. mutans, S. sputigena, L. wadei, and P. salivae. Each species was grown alone or in combination with S. mutans. A1 Average proton production rate of mono-species culture over a 12-h glycolytic assay. A2 Average proton production rate of mixed-species cultures (S. mutans + new species) and S. mutans alone over a 12-h glycolytic assay. B1 Acid tolerance presented as the lowest starting pH that each of the species could survive and the final pH (after 48 h) B2 lowest starting pH that each of the mixed-species could survive, and the final pH (after 48 h). C1 Curves of the metabolic activity (μW) of mono-species cultures. Data above individual peaks indicate the peak metabolic activity time of each species. C2 Curves of the metabolic activity (μW) of mixed-species cultures (S. mutans + new species) and that of S. mutans alone. Inset, metabolic curves between 6–18 h. SM, S. mutans; SS, S. sputigena; LW, L. wadei; PS, P. salivae. For A1 and A2, data are plotted as mean ± standard deviation from three independent experiments and significant p-values are noted above the bars (p < 0.05 derived by one-way ANOVA with post hoc Tukey HSD test). ns denotes differences not statistically significant (p > 0.05).
All 4 species could grow in an acidic culture medium (i.e., pH pre-adjusted to 5.5), demonstrative of their ability to tolerate acidic conditions required for the development of caries lesions. Acid tolerance challenges of monocultures showed that all species could grow at an initial pH of 5.5, and further decreased pH to 4.8–4.6 (Fig. 4B1). Notably, acid tolerance was higher when S. sputigena, L. wadei, and P. salivae were in co-cultures with S. mutans, demonstrating ability to grow at a starting pH of 5.2 and further decrease the pH to a range between 3.9 to 4.2 after 48 h (Fig. 4B2). Of note, the mixed culture of S. sputigena and S. mutans achieved the lowest final pH of 3.9 after 48 h.
S. mutans dominates metabolic activity in top species’ mixed cultures
Next, we used real-time isothermal microcalorimetry to characterize the energy (total heat) released by top species’ sucrose metabolic activity, individually and when the three new candidates were co-cultured with S. mutans. In monocultures, S. mutans produced the highest peak and rate of metabolic activity, the fastest times to activity and peak, but also the shortest decay time, reflecting a rapid drop in its metabolic activity (Fig. 4C1). In contrast, S. sputigena’s metabolic activity peaked much later (i.e., at 49 h) and decayed slowly, findings aligned with being a slow growing anaerobic bacterium26, whereas L. wadei presented the second highest peak. Co-cultures of S. sputigena, L. wadei and P. salivae with S. mutans had metabolic peaks close to the S. mutans’ monoculture peak (Fig. 4C2) indicating the metabolic dominance by the pathogen within co-cultures.
In summary, we found that S. sputigena, L. wadei and P. salivae, alongside the known S. mutans, display aciduric and acidogenic properties. All species can metabolize sucrose, the primary disease-associated dietary sugar, produce acids in the glycolytic assay, and survive in the resulting acidic pH. While the metabolic activity is driven by S. mutans, we unexpectedly found that the addition of S. sputigena results in faster acid production and increased aciduricity, significantly higher than S. mutans alone. This suggests a possible cooperative relationship between S. mutans and S. sputigena that favors the formation of pathogenic biofilms.
S. mutans enhances S. sputigena colonization and biofilm formation
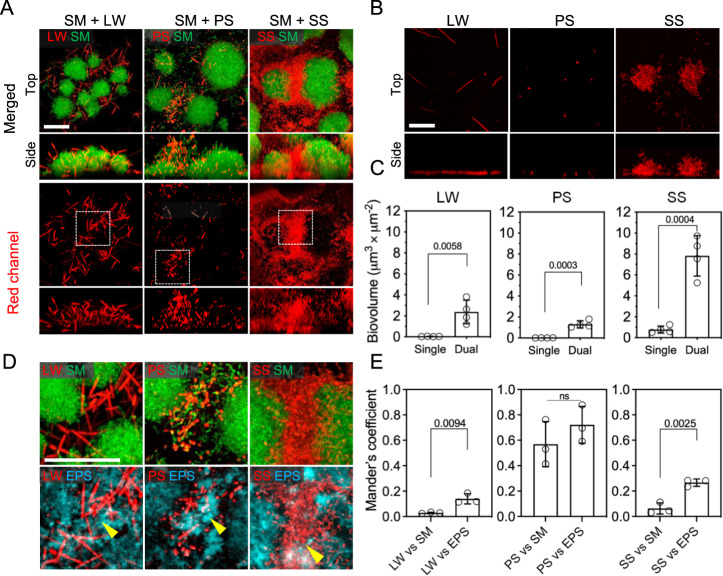
Dental caries is a prime example of a biofilm-mediated disease that is initiated by colonization of microbes on tooth surfaces and development of structured biofilm communities27. We developed an experimental model using saliva-coated hydroxyapatite discs (sHA, a tooth enamel surrogate) and confocal live-cell imaging to investigate the four top species’ biofilm-forming abilities. Because S. mutans is a well-characterized pathogen known for its exceptional ability to assemble biofilms on teeth28, we focused on investigating biofilm forming properties of L. wadei, P. salivae, and S. sputigena alone and in combination with S. mutans. We found that the new candidates could co-colonize the sHA surface, with S. mutans forming structured but morphologically distinct mixed-species biofilms after 24 h (Fig. 5A upper panel). In contrast, none of the new species alone could efficiently colonize the surface and develop biofilms, with only single cells (L. wadei and P. salivae) or small bacterial clusters (S. sputigena) on the surface (Fig. 5B). Higher abundances of each of the new species were noted when grown as mixed biofilms with S. mutans (Fig. 5A lower panel). These findings were further corroborated by quantifying the surface-bound cell volume (Fig. 5A lower panel and Fig. 5B) using a computational image analysis toolbox optimized for biofilms29. Indeed, we confirmed higher amounts of L. wadei, P. salivae, and S. sputigena on the hydroxyapatite surface after 24 h when co-cultured with S. mutans compared to the same species alone (Fig. 5C), indicating that the presence of S. mutans can facilitate their surface colonization. Notably, we found that surface-colonized S. sputigena displayed the highest (>10-fold) increase when co-cultured with S. mutans, which was higher than that of L. wadei or P. salivae, suggesting that interspecies interactions may significantly enhance the colonization and biofilm formation by S. sputigena.
Fig. 5. Biofilm formation and spatial structuring on tooth-mimetic surface.
A Confocal images (top and side views) of 24-h mixed-species biofilms on saliva-coated hydroxyapatite surfaces formed by each of the new species together with S. mutans. The upper panel is a merged image demonstrating the spatial structuring of S. mutans (in green) and the new species (in red) within mixed biofilms. Lower panel, red channel only. Dotted box, areas that are shown magnified in panel D. B 24-h mono-species biofilms formed by the top species (top and side views) C Biovolume of the new species within single and mixed biofilms, based on computational image analysis of panels A and B. D Top, magnified confocal images of mixed species biofilms; bottom, the new species physically interacting with extracellular α-glucan matrix (EPS, in cyan). E Computational colocalization analysis of the new species versus S. mutans cells or EPS. SM, S. mutans; SS, S. sputigena; LW, L. wadei; PS, P. salivae. Scale bars, 20 μm. For C and E, data are plotted as mean ± standard deviation from three independent experiments, and significant p-values are noted above the bars (p < 0.05 derived from two-sided Student’s t-test). ns denotes differences not statistically significant (p > 0.05).
To further understand the growth dynamics of the single and mixed biofilms, we assessed their attachment at an initial stage (i.e., at 4 h) and at an intermediate stage (i.e., at 10 h). The data indicate that S. sputigena, L. wadei, and P. salivae all bind similarly to the surface either alone or co-cultured with S. mutans at 4 h (Supplementary Fig. 3, upper). Interestingly, when co-cultured, S. sputigena appears to form clusters near S. mutans aggregates in the mixed biofilm at 10 h (Supplementary Fig. 3, middle, white arrowheads), suggesting early physical interactions between the two species during biofilm initiation. These observations suggest that the post-binding events, i.e., close interactions and co-metabolism with S. mutans, may mediate the biofilm spatial structure and community development at later stages, i.e., at 24 h (Supplementary Fig. 3, lower).
Interspecies spatial structuring and co-localization within biofilms
Given that S. mutans produces extracellular polysaccharides (EPS) that enhance bacterial cell co-adhesion and biofilm accumulation30, we investigated the spatial localization of the three new candidate species, S. mutans, and EPS. High-resolution images of magnified areas showed that most of L. wadei, P. salivae, and S. sputigena cells were spatially located in between streptococcal clusters (known as microcolonies), rather than intermixing with the S. mutans cells within (Fig. 5D, upper panel). Interestingly, we found a densely packed accumulation of S. sputigena, whereas L. wadei or P. salivae sparsely colonized these areas. Using a fluorescent marker that specifically labels S. mutans-derived EPS α-glucans28, we found that most of L. wadei, P. salivae, or S. sputigena cells were co-localized with the extracellular polymeric matrix (Fig. 5D, lower panel).
We performed three-dimensional analyses to characterize the co-localization of two fluorescent signals in relation to each other using Mander’s overlap coefficient, a quantitative measure of spatial proximity31. For each mixed-species biofilm, we calculated two Manders’ coefficients: 1) between each new species and S. mutans and 2) between each new species and EPS. We found that L. wadei and S. sputigena were more proximal to EPS than S. mutans (Fig. 5E, left and right panels), indicating that these species may be primarily associated with EPS than to S. mutans cells. In contrast, P. salivae showed similar proximity to S. mutans cells and EPS (Fig. 5E, middle panel). In summary, our findings show that the new candidates form mixed biofilms with S. mutans, and this coexistence enhances their surface colonization and co-development of structured biofilms. S. sputigena forms a densely populated community interspersed between S. mutans clusters, leading to highly cohesive, co-assembled biofilms.
The motile bacterium S. sputigena becomes trapped in streptococcal exoglucans
Motivated by the enhanced surface colonization of S. sputigena in the presence of S. mutans and the enhanced acidogenicity of this species combination, we sought to characterize the spatial structuring of S. sputigena within the mixed-species biofilm. As a backdrop, S. sputigena was originally found in the aerodigestive system (including saliva), the subgingival microbiota of patients affected with periodontitis32–34, and endodontic infections35. Selenomonas multispecies communities were common (i.e., among the top 10 abundant taxa) in the biofilms obtained in our recent in vivo experimental study of caries progression and arrest36. Its role in supragingival biofilms and how it interacts with other microbes remain unknown.
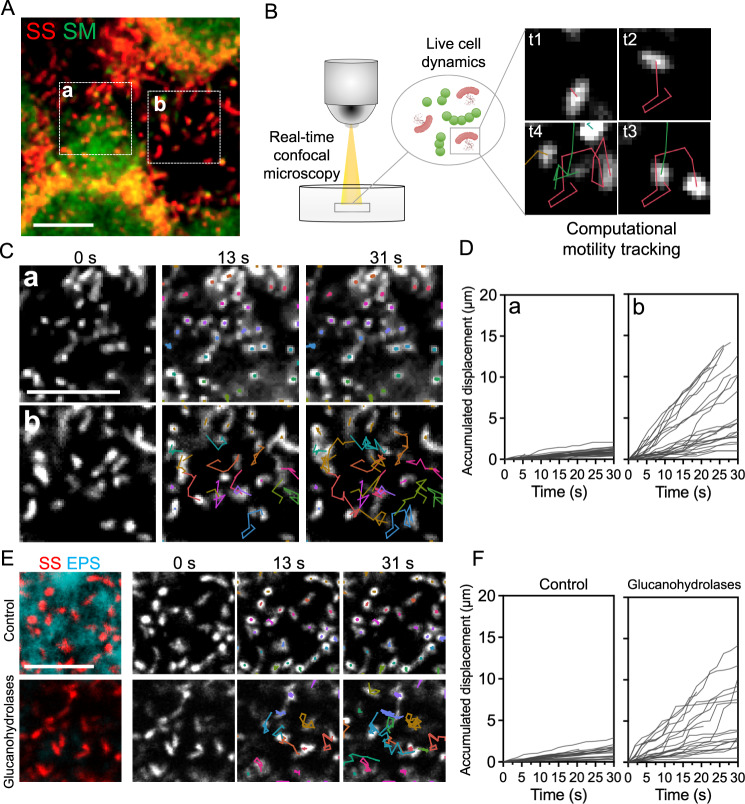
Unlike most oral microbes, S. sputigena has flagella, which are surface-attached appendages that allow motility in liquid environments. Unexpectedly, we found that S. sputigena cells were motile even after their colonization on the sHA surface, displaying a tumbling, multi-directional motion (Supplementary Movie 1) while remaining attached to the hydroxyapatite surface. We used real-time live imaging and computational motion tracking to study the surface motility of S. sputigena cells when co-cultured with S. mutans (Fig. 6A, B and Supplementary Movie 2). The spatial coordinates of individual S. sputigena cells were followed at each time frame and a time-resolved trajectory was generated (representative frames shown in Fig. 6B). Our results revealed distinctive motility behaviors of S. sputigena cells depending on their spatial location relative to that of S. mutans. The upper panels of Fig. 6C (same areas as “Box a”, in Fig. 6A) show that S. sputigena cells near S. mutans cells display no motility (Fig. 6C top). In contrast, S. sputigena cells located far away from S. mutans remained motile (lower panels of Fig. 6C; same areas as “Box b” in Fig. 6A). We also calculated accumulated displacements (total path length) of individual S. sputigena cells relative to their original position. The displacement curves revealed that most S. sputigena cells adjacent to S. mutans did not move or only moved for short distances (Fig. 6D, left panel), whereas those with no S. mutans nearby moved actively for longer distances (Fig. 6D, right panel).
Fig. 6. S. sputigena, a motile bacterium, becomes trapped in extracellular α-glucans.
A Confocal image of S. sputigena-S. mutans mixed biofilm formed on the surface. Red, S. sputigena; green, S. mutans. Dotted boxes, biofilm subareas with S. sputigena physically associated with S. mutans clusters (a) or surface-attached S. sputigena alone (b). B Surface-attached microbes were visualized by fluorescence staining and real-time confocal microscopy. Individual S. sputigena cells (red channel in panel A) were tracked computationally over time to generate spatiotemporal trajectories. C Trajectories of surface-attached S. sputigena cells in Areas (a) and (b), as shown in panel A. Colors indicate trajectories that originated from individual cells. D Accumulated S. sputigena cell displacement (total path length) relative to the initial position. Left, accumulated displacement of S. sputigena cells in Area (a); right, in Area (b). E Immobilized S. sputigena cells trapped by S. mutans-derived α-glucan matrix. Red, S. sputigena; cyan, α-glucan matrix. Top panel, S. sputigena cells trapped by α-glucans showed no mobility; bottom panel, upon EPS degradation using glucanohydrolases (dextranase and mutanase), surface mobility of S. sputigena cells is recovered. F Accumulated S. sputigena cell displacement (total path length) relative to the initial position. Left, accumulated displacement of S. sputigena cells in the control group; right, after EPS enzymatic degradation. SM, S. mutans; SS, S. sputigena. Representative images from three independent experiments are shown. Scale bars, 10 μm.
Given the contrasting motility between S. sputigena cells at different spatial locations, we hypothesized that S. sputigena cells that lost their surface motility might be physically trapped in S. mutans-derived EPS. We first tracked S. sputigena cells that were co-localized with EPS α-glucans and found that the cells were devoid of mobility (Fig. 6E, upper panels). Then, we performed EPS degradation using glucanohydrolases (mutanase and dextranase) that specifically hydrolyze α-glucans without antibacterial activity37 and examined the motility behavior of S. sputigena in real-time. We found that the surface motility of S. sputigena was recovered following α-glucans degradation (trajectories shown in Fig. 6E, lower panels). These observations were confirmed by computational motility tracking before and after glucanohydrolase treatment (Fig. 6F), indicating that EPS degradation released trapped S. sputigena cells restoring their surface motility. Additionally, S. sputigena single-species biofilms were formed in the presence of exogenously supplemented purified glucosyltransferase B (GtfB), an S. mutans-derived exoenzyme that produces α-glucans30. S. sputigena cells grown with GtfB (without S. mutans) were trapped by α-glucans produced by the enzyme, showing no surface motility whereas most S. sputigena cells growing without Gtf were motile (trajectories in Supplementary Fig. 4A; displacement curves in Supplementary Fig. 4B), further supporting EPS-mediated entrapment and loss of motility.
By co-colonizing the surface with S. mutans, S. sputigena cells appear to lose motility and become immobilized through interspecies cell-glucan matrix interactions yet remain viable and active, which may enhance surface binding and accumulation of these motile bacteria that could influence the scaffolding of the mixed biofilm community. Our biofilm MTX data corroborate these observations. First, we found that S. mutans’ gtfC gene (encoding glucosyltransferase GtfC, involved in glucan synthesis) and the glucose-1-phosphate adenylyltransferase gene were significantly upregulated in disease (Supplementary Table 3). gtfC encodes an enzyme that synthesizes both soluble and insoluble α-glucans30 that are highly susceptible to degradation by dextranase (breaks down soluble glucans) and mutanase (digests insoluble glucans) which were used in this study’s motility experiments. Second, we found several negative S. mutans and S. sputigena gene-gene expression interactions. Intriguingly, 11 of 13 gene-gene interactions were negative, and S. mutans' glucose-1-phosphate adenylyltransferase gene demonstrated a strong negative interaction (p = 3.7 × 10−6) with S. sputigena’s motility-related flagellin gene (Supplementary Table 4).
Spatial arrangement of S. sputigena and S. mutans within intact biofilm structure
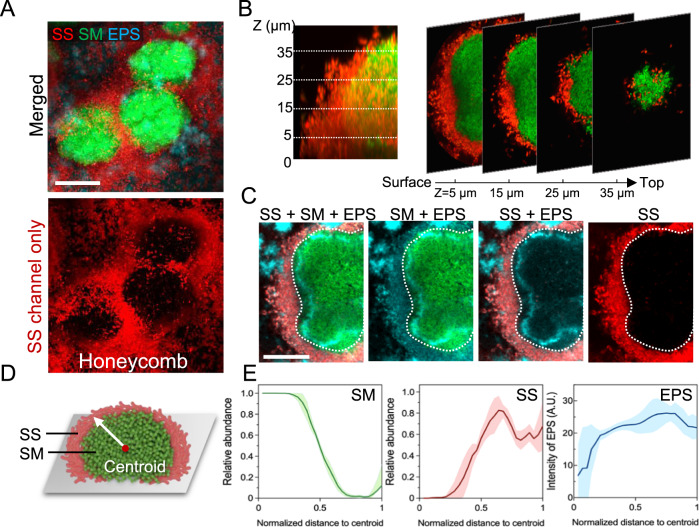
Considering that S. mutans can significantly enhance S. sputigena colonization to the tooth-mimetic surface and modulate its surface motility, we investigated how this co-colonizing community mediates biofilm spatial structure (biogeography) at multi-length scales. To this end, we used super-resolution confocal microscopy coupled with computational analyses for mixed biofilm communities38. We found a unique 3D multicellular organization composed of densely packed S. sputigena cells encasing S. mutans microcolonies (Fig. 7A). Using layer-by-layer imaging sectioning, we found an orderly arrangement of S. sputigena cells across the entire height of the biostructure (Fig. 7B). A representative sectional rendering (z = 15 μm from the surface) of the 3D biostructure revealed a spatially segregated inner core formed primarily by S. mutans, and a dense ring-like outer layer of S. sputigena, both associated with EPS α-glucans (Fig. 7C). We investigated the composition and the spatial structuring of the microbial and EPS components in relation to the center of mass (referred to as centroid) of the biostructure (see diagram in Fig. 7D). We found that the core near the centroid consisted predominantly of S. mutans, whereas the periphery harbored predominantly S. sputigena (Fig. 7E, left and middle panels). Notably, S. mutans-derived EPS α-glucans were detected throughout the entire biostructure, including the peripheral areas where the abundance of S. mutans cells was low (Fig. 7E, right panel), suggesting that the secreted EPS could mediate co-adhesion without direct cellular contact providing scaffolding for the interspecies assembly.
Fig. 7. Biogeography of S. sputigena-S. mutans biofilm.
A The mixed biofilm featured by a honeycomb-like structure formed by S. sputigena (in red) encapsulating cell clusters of S. mutans. Both species are embedded by EPS α-glucan matrix (in cyan) B Optical sectioning of the mixed-species community reveals physical segregation between S. mutans (green) and S. sputigena (red) within the biofilm. C Cross-sectional confocal image of the spatial arrangement of S. sputigena, S. mutans and EPS. S. mutans-derived EPS (cyan) is present both inside and outside of the S. mutans inner core cluster (green); S. sputigena cells (red) are predominantly outside, embedded in EPS while encapsulating S. mutans cluster D A diagram of the biogeography and the centroid of mass used as reference for spatial analysis. E Spatial distribution of S. sputigena, S. mutans and EPS relative to the distance to the centroid. Lines correspond to means and shaded regions to standard deviations from three independent experiments. Representative images from three independent experiments are shown. SM, S. mutans; SS, S. sputigena. Scale bars, 20 μm.
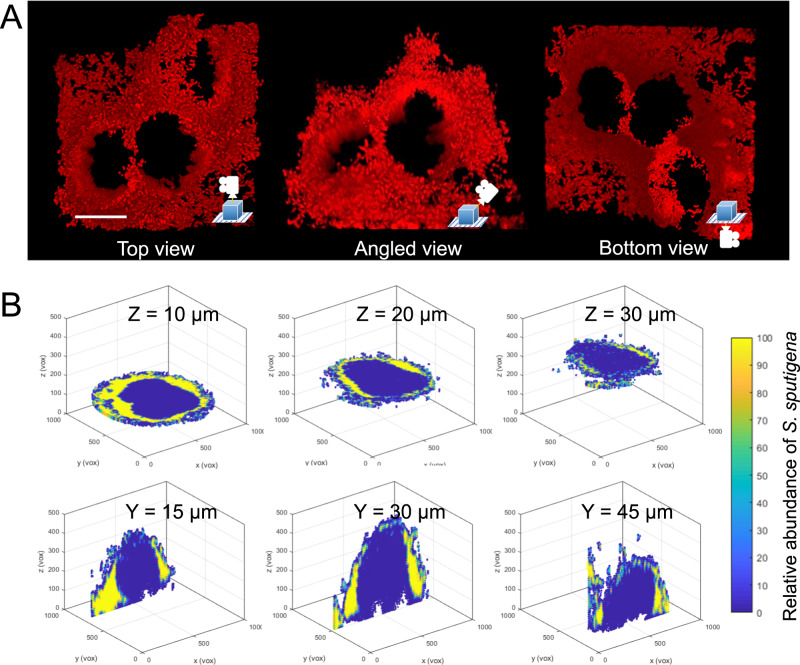
To further assess the structural organization of the biofilm, we performed 3D reconstruction of the confocal images and quantitative analysis of the spatial distribution of the bacterial cells. This analysis revealed a unique honeycomb-like architecture consisting of S. sputigena cells forming a multicellular superstructure (Fig. 8A and Supplementary Movie 3). To determine the spatial distribution of bacterial cells, we computationally dissected the large mixed-species biostructure into small cubic volumes in micron scale, and calculated the relative abundance of (i.e., the proportion of the total cellular volume occupied by) S. sputigena cells within each cube in correspondence to their 3D spatial coordinates29. Using this method which provides local distribution with 3D spatial resolution, we found high S. sputigena abundance near the entire outer surface of the biostructure (selected horizontal or vertical planes shown in Fig. 8B). These results indicate a non-random pattern with densely packed yet spatially segregated biogeography between S. sputigena and S. mutans cells. Taken together, we find evidence for a motile bacterium, S. sputigena, becoming immobilized by a foreign EPS produced by a co-colonizing species (S. mutans), promoting localized growth and accumulation, and forming a distinctive multicellular honeycomb superstructure guided by the matrix scaffolding.
Fig. 8. Spatial distribution of S. sputigena within the biofilm superstructure.
A Three-dimensional rendering of the honeycomb structure formed by S. sputigena in the S. sputigena-S. mutans mixed biofilm. B Spatial distribution of S. sputigena (relative abundance, defined as the proportion of the total cellular volume occupied by S. sputigena) within the community structure at different horizontal (top) and vertical planes (bottom). Scale bar, 20 μm.
S. sputigena-mediated interspecies spatial structuring is required and specific for enhanced biofilm virulence
To investigate whether spatial structuring is required for the biofilm to create acidic microenvironments that are critical for dental caries development, we measured the biofilm-apatite interface pH in situ. We used a pH-responsive fluorescent probe39 and high-resolution confocal live imaging to measure pH changes in real-time. We found rapid acidification at the biofilm-apatite interface of the superstructure formed by S. sputigena and S. mutans after glucose exposure, which resulted in highly acidic (pH < 5.5) regions (Supplementary Fig. 5A, upper). Given that S. mutans-derived EPS α-glucans were detected throughout the entire biostructure mediating co-adhesion for the interspecies assembly (Fig. 7C, E), we used a S. mutans double-knockout of gtfB and gtfC (i.e., defective in α-glucans production30) to disrupt the biofilm spatial structuring with S. sputigena. We found that S. sputigena and S. mutans ΔgtfBC mutants were unable to form structured biofilms and did not result in acidic pH regions (Supplementary Fig. 5A, middle). This finding was further corroborated by co-culturing S. sputigena and wild type S. mutans in the presence of glucanohydrolases (dextranase and mutanase) that specifically break down α-glucans and dismantle the biofilm structure without affecting bacterial viability, impairing the ability to create localized acidic-pH regions (Supplementary Fig. 5A, lower). The data suggest that the interspecies spatial structuring between S. sputigena and S. mutans is required to create a virulent acidic microenvironment.
We further investigated the specificity of S. sputigena-S. mutans interactions to promote biofilm virulence using an ex vivo human tooth-enamel biofilm model. This method allows direct assessment of the acid-induced damage (i.e., demineralization) on the enamel surface inflicted by the biofilm40. Given that P. salivae does not increase the proton production rate when co-cultured with S. mutans (Fig. 4, A2), we used a P. salivae-S. mutans co-culture as control and compared to the acid-induced enamel demineralization caused by S. sputigena-S. mutans biofilms, as well as by S. mutans alone. Macroscopically, we found large areas of enamel demineralization associated with the S. mutans-S. sputigena biofilm, which was characterized by chalky-like opaque surfaces under the stereoscope (Supplementary Fig. 6A, lower left), similar to caries lesions found clinically. In contrast, only small areas of opaque demineralized areas were found on the enamel surface from P. salivae-S. mutans (Supplementary Fig. 6A, middle left) and S. mutans alone (Supplementary Fig. 6A; upper left) biofilms. These differences were confirmed using confocal topography imaging and transverse microradiography analysis.
The enamel surfaces under S. mutans-S. sputigena biofilms showed higher roughness compared to those from S. mutans biofilms and P. salivae-S. mutans biofilms (Supplementary Fig. 6C, “Roughness of enamel surface”; p < 0.05), with widespread regions of surface damage (Supplementary Fig. 6A, lower right). In contrast, the surface roughness from P. salivae-S. mutans biofilms was similar to that from S. mutans alone (Supplementary Fig. 6C; p > 0.05), both showing milder enamel surface demineralization (Supplementary Fig. 6A right). Transverse microradiography analysis validated the extent of demineralized enamel lesion, showing significantly higher mineral loss and deeper acid-damage caused by S. mutans-S. sputigena biofilms (p < 0.05) versus P. salivae-S. mutans or S. mutans biofilms (Supplementary Fig. 6B, C). Taken together, the data suggest specificity of S. sputigena-S. mutans interactions and spatial structuring to promote biofilm virulence.
S. sputigena can colonize tooth surfaces and increases caries experience in vivo
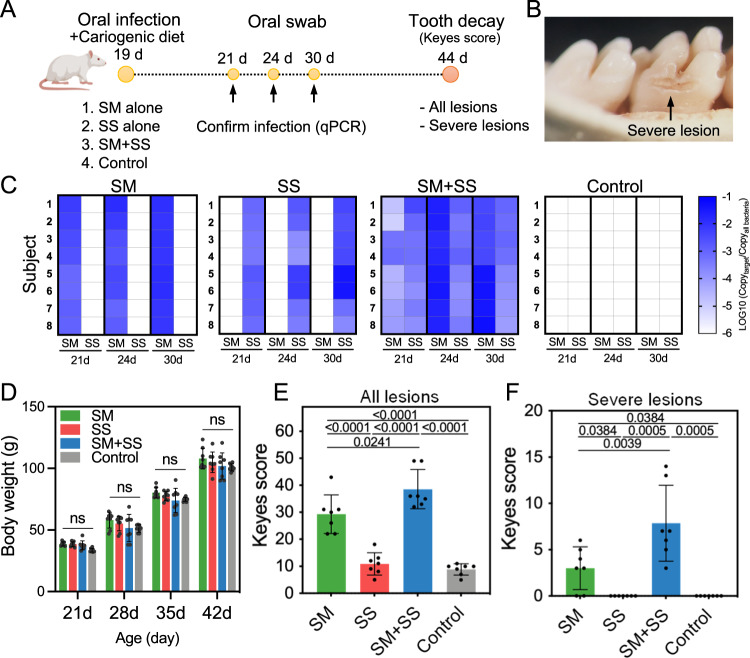
The in vitro and ex vivo experimental data presented thus far demonstrate that S. sputigena can bind and form highly structured, densely packed biofilms with S. mutans on apatitic surface—their combination produces more acid and more enamel demineralization than either species alone. It is thus logical to expect that they can co-colonize tooth surfaces and exhibit enhanced biofilm virulence in vivo. To experimentally test this hypothesis, we sought to determine whether S. sputigena alone or when co-infected with S. mutans (Fig. 9A) can colonize tooth surfaces and cause tooth decay based on an established rodent model that mimics the characteristics of early childhood caries, including exposure to a sugar-rich diet and rampant dental cavitation41 (Fig. 9B). We found that the animals were infected by S. mutans, S. sputigena, or both and remained persistently infected (Fig. 9C) as determined by real-time PCR using species-specific probes. The uninfected (control) animals remained free of infection by S. mutans or S. sputigena (Fig. 9C). All animals maintained good health showing a steady body weight gain with no significant difference between groups (Fig. 9D). These data confirm the ability of S. sputigena to co-colonize tooth enamel surfaces with S. mutans in vivo.
Fig. 9. In vivo study of the role of S. sputigena and S. mutans in dental caries.
A A diagram of the experimental design. B Cavitated (severe) carious lesions developed on the animal’s teeth, similar to those found clinically in severe childhood tooth decay. C Confirmation of infection by qPCR. Oral swabs were taken on Day 21, Day 24, and Day 30 and were subject to qPCR analysis using species-specific probes. D Body weight of the animals was measured weekly to monitor the systematic impact of the bacterial infection on animal health during the experiment period. No significant differences were observed between groups. E Keyes scoring of total caries (tooth decay) developed on the smooth surfaces. F Keyes scoring of cavitated (severe) lesions developed on smooth surfaces. Caries scores were recorded as stages and extent of carious lesion severity according to Larson’s modification of Keyes’ scoring system. Data are presented as mean ± standard deviation. (n = 8 animals) and significant p-values are provided above the bars (p < 0.05, derived from one-way ANOVA with post hoc Tukey HSD test). ns denotes differences not statistically significant (p > 0.05).
The impact on caries development and severity was then assessed for each experimental condition. In this model, tooth enamel progressively develops caries lesions (analogous to those observed in humans), proceeding from initial areas of demineralization to moderate lesions and on to extensive (i.e., severe) lesions characterized by enamel structure damage and cavitation. Consistent with previous studies42,43, S. mutans infection led to the onset of dental caries whereas control uninfected animals harboring their natural oral microbiota developed minor tooth demineralization with no severe lesions (Fig. 9E). Infection with S. sputigena did not induce development of caries lesions on the tooth surface compared to the uninfected control (Fig. 9E) despite confirmed bacterial colonization, suggesting that, alone, S. sputigena has limited cariogenic potential. However, S. sputigena co-infected with S. mutans resulted in a significant increase of both total caries experience and the severity of caries lesions compared to those infected with either species alone (Fig. 9E), indicating enhanced virulence within resident microbiota. Notably, we found that co-infection produced more severe lesions (Fig. 9F), characterized by enamel destruction and frank cavities (Fig. 9B). In sum, we show that S. sputigena interacts with S. mutans for enhanced co-colonization and the presence of S. sputigena leads to the assembly of uniquely structured biofilms with enhanced virulence that cause the onset of severe dental disease in vivo, suggesting a new pathobiont and interspecies biogeography to promote disease-causing conditions.
Discussion
In this study, we integrated multi-omics of human plaque samples from two community-based clinical cohorts in a discovery-validation pipeline combining informatics, laboratory, and in vivo experimental models to identify pathogen candidates and obtain insights into how these previously uncharacterized species interact and form virulent oral biofilms (Fig. 10). Comprehensive taxonomic association analyses and multiple testing-controlled, across-clinical trait criteria identified 16 species significantly associated with early childhood caries, including several new candidates. Among them, S. mutans (an established pathogen), S. sputigena, P. salivae, and L. wadei (as new candidates) were strongly associated with disease and were carried forward into comprehensive biofilm studies. We found that the flagellated and motile S. sputigena develops a cooperative interaction with S. mutans to exacerbate biofilm virulence. Specifically, S. sputigena and S. mutans can co-colonize apatitic surfaces, metabolize sugars, and produce and tolerate acids. Together, the two species contribute to significantly enhanced acidogenesis and aciduricity. This interaction is underlaid by S. sputigena’s entrapment in streptococcal exoglucans, wherein it loses motility, and builds a densely packed honeycomb-like multicellular structure. Negative interactions between S. mutans’ glucose-1-phosphate adenylyltransferase gene and several S. sputigena’s genes including the flagellin gene44 in MTX, corroborate the inter-species interactions manifested in the experimental studies. Crucially, while incapable of causing disease on its own, S. sputigena significantly increases the biofilm virulence in vivo when co-infected with S. mutans and augments the severity of dental caries. S. sputigena has so far been implicated in periodontal disease23,45, endodontic infections35 , and is a member of aerodigestive tract microbiome with emerging prognostic significance in cancer46,47. Our findings demonstrate that S. sputigena acts as a pathobiont outside its typical habitat to modulate biogeography (i.e., spatial structure), metabolic activity, and pathogenicity of supragingival biofilms in the context of a prevalent childhood disease.
Fig. 10. Application of the multi-method, discovery-validation pipeline in the present study.
Outline of the study’s experimental approach that can serve as a guide for future oral microbiome discovery-validation investigations. We began by analyzing and integrating multi-omics data generated from human plaque samples obtained in two community-based clinical samples of preschool-age children. Then, we carried forward initial discovery results into a validation pipeline combining informatics, laboratory, and in vivo experimental models seeking to identify pathogen candidates and obtain insights into how these previously uncharacterized species interact and form virulent oral biofilms.
We leveraged robust clinical and multi-omics data generated among sizeable community-based samples of young children and used conservative selection criteria to identify bacterial species associated with disease experience and guide the prioritization of candidates for characterization and experimental validation. The large sample size for a multi-omics study of this kind and the application of strict discovery and validation criteria led to a set of high-confidence species associated with caries experience, including known and novel taxa. While we were able to comprehensively test four of these 16 species, we anticipate that this set of high-confidence candidates will be further studied in the near future by us and others. Biofilm transcriptomics analyses affirmed significantly upregulated metabolic pathways (e.g., glycolysis, pyruvate fermentation, and UDP-N-acetylglucosamine) and bacterial genes (e.g., glucose-1-phosphate adenylyltransferase) in disease. These findings are aligned with metabolic interactions between microbiota and dietary sugars expected to be found in dental caries24. Sugars fuel the emergence of dysbiosis by favoring proliferation of species that can adapt to ecological changes and promote acidification of the biofilm microenvironment that underlies destruction of mineralized dental tissues. Enhanced acidogenesis, up-regulation of UDP-N-acetyl-D-glucosamine biosynthesis I, and gene-gene interactions in MTX between the two species strengthen the case for S. sputigena being an important species in the caries-associated dental plaque biofilm36.
A key finding of the present report is that the cooperative interaction between S. sputigena and S. mutans hinges upon an unexpected phenomenon wherein a motile bacterium that is typically reported as resident of the subgingival (i.e., below gum line) niche is entrapped in an exopolymeric matrix produced by another species residing in the supragingival (i.e., above gum line) area and forms a unique spatial biofilm structure that increases virulence. We show that the dense growth of immobilized S. sputigena cells colonizing the apatite surface and surrounding S. mutans cell clusters create a cohesively packed community superstructure that cannot be achieved by either species alone. Importantly, we demonstrate that the immobilization of S. sputigena and the spatial structuring of the mixed community are associated with the creation of localized regions of highly acidic pH, a hallmark for biofilm virulence in dental caries. The exact mechanisms by which S. sputigena loses motility and forms the densely packed honeycomb structures remain unknown although S. mutans-derived EPS α-glucans appear to play a key role. S. sputigena expresses a heavily glycosylated flagellin, building blocks of flagella44. It is possible that the glycans on the surface of S. sputigena display glucan-binding properties, interacting with streptococcal exoglucans, and thereby immobilize the cell. Additionally, fucose is a major monosaccharide constituent of the O-linked glycans in S. sputigena flagellin44, and our recent metabolomics work among the same discovery sample has demonstrated elevated fucose abundance in early childhood caries48. Glucosyltransferases (Gtfs) secreted by S. mutans can bind to sugar moieties of different polysaccharides on the surface of other microbes and produce EPS α-glucans in situ49. It is possible that Gtf can bind to fucose moiety allowing glucan synthesis in situ that could contribute to immobilization. Future studies need to investigate the interactions of Gtf with S. sputigena, glucan-fucose interactions, and determine whether these interactions are strain-dependent.
S. sputigena has been recalcitrant to genetic manipulation which hinders in-depth molecular studies to further understand its pathogenic roles, such as the functions of flagellin on inter-species biofilm interactions. However, new genetic engineering methodologies involving restriction modification-silent tools50 may circumvent this limitation. Aside from S. sputigena, several new species emerged as prime targets for additional studies. For example, L. wadei and P. salivae, both strongly associated with caries experience in our discovery and replication cohorts, may play pathogenic roles in the context of polymicrobial biofilms that are not necessarily associated with S. mutans and could be investigated in the future. Another candidate is Lachnoanaerobaculum saburreum, which is capable of producing acid from glucose, lactose, and sucrose among other sugars51 and is involved in pyruvate fermentation to acetate and lactate II—one of the four pathways that we found as significantly differentially expressed in disease. Examination of multi-species combinations of these candidates may provide additional insights about the biofilm virulence mechanisms.
A limitation of our current multi-omics pipeline is that it is not optimized for studying inter-kingdom interactions (i.e., viruses and fungi, besides bacteria). For example, the nucleic extraction protocols were not optimal for detecting and analyzing fungi, which are important in the context of childhood caries52. This can be overcome with the incorporation of nucleic acid extraction protocols for downstream fungal analyses. At the same time, the detection of phages in MTG and MTX data is a rapidly growing area that is likely to provide high yields in the near future53. Another limitation is the cross-sectional nature of our human observational data, which limits the samples’ inferential potential regarding temporality and causality—it is likely that some taxonomic and functional changes found in the initial discovery of candidate species in human plaque samples are downstream (i.e., consequences) of established disease. This is where the multi-modality aspect of our study is of the essence—the experimental studies of the pipeline are well-positioned to help clarify the actual pathogenic potential of candidate species. Nevertheless, community-based longitudinal human studies are warranted to enhance both discovery and validation of new species, not only in the presence of dental caries but also in its onset and progression.
In summary, we employed a multimodal pipeline to gain new knowledge about taxonomic and functional features of the childhood oral microbiome in health and disease from two sizeable discovery and replication community-based samples of human plaque biofilms. We discover a novel inter-species interaction and unique biogeography at microscale wherein a motile flagellated species becomes immobilized in the EPS matrix produced by a disease-causing species and proliferates to build a 3D multicellular superstructure with enhanced acidogenesis. We show that the interaction between S. sputigena and S. mutans augments the severity of dental caries in the presence of resident microbiota in vivo, suggesting a new pathobiont exacerbating biofilm virulence for a common yet unresolved disease. Further understanding of the spatial structuring function and pathobiont-mediated virulence may reveal new mechanisms of biofilm assembly and therapeutic targets, which may be relevant to other polymicrobial infections where other species are interacting with known pathogens in complex biofilms.
Methods
Human microbiome studies: context and sampling
Human microbiome studies were done in the context of ZOE 2.0, a genetic epidemiologic study of early childhood oral health among a community-based sample of preschool-age children in North Carolina (NC)54. In brief, between 2016 and 2019, 8059 children ages 36–71 months who attended public preschools in NC were enrolled in the study and 6404 of them underwent comprehensive clinical examinations by trained and calibrated dental examiners. Data on childhood dental caries experience were collected using modified International Caries Detection and Classification System (ICDAS) criteria55. Two supragingival (i.e., “dental plaque”) biofilm samples were collected during the clinical encounters immediately prior to dental examinations, which took place before or at least 30 min after snack or breakfast. The plaque sample that was carried forward to microbiome analyses was obtained using sterile toothpicks from the facial/buccal surfaces of primary teeth in the upper-right quadrant, i.e., Universal tooth numbering system: #A, #B, #C, #D, and #E; FDI tooth numbering system: #55, #54, #53, #52, and #51. Upon collection, plaque samples were placed in RNAlater TissueProtect 1.5 mL tubes and were frozen at −20 °C on-site until transferred to the university core biospecimen processing facility for further processing or long-term storage at −80 °C. Families were provided with a $20 gift card as compensation for their time. Detailed information regarding sample collection, storage, processing, nucleic acid extraction, and sequencing has been reported in a recent protocol publication56.
Participants and phenotyping
We carried forward to whole genome shotgun sequencing (WGS, metagenomics/MTG) and RNA sequencing (RNA-seq, metatranscriptomics/MTX) supragingival samples of the first 300 ZOE 2.0 participants, 50% with and 50% without person-level dental caries experience defined at the “established” caries lesion detection threshold, ICDAS ≥ 3)48. This lesion threshold corresponds to macroscopic tooth structure loss, i.e., a “cavity”. The ZOE 2.0 participants (mean age = 52 months) formed the ‘discovery’ sample whereas the ‘replication’ sample comprised 116 similar-aged children (mean age = 55 months), members of the same study population (i.e., enrolled in NC public preschools) examined under virtually identical conditions (i.e., by one clinical examiner) during the parent study’s pilot phase. The distribution of male/female participants was balanced in the study, with 208 children in each stratum (Supplementary Table 5). This information was obtained from questionnaires completed by parents or legal guardians which was then cross-checked with biological sex data available via genotyping. For the purposes of the present study, we quantified caries experience using the most sensitive clinical criteria including the enumeration of early-stage caries lesions (i.e., at the ICDAS ≥ 1 threshold) according to the recent international consensus definition of early childhood caries (ECC)8. This was done both locally (i.e., within the five tooth surfaces where plaque biofilm was harvested from) and at the person-level (i.e., in the entire dentition, comprising all 88 primary tooth surfaces). We considered the surfaces where plaque was collected from as the most informative in terms of microbiome taxonomy and thus localized caries experience was the primary clinical trait in all analyses. Nevertheless, we posited that the taxonomy of microbiome biofilm in those areas is likely to also be informative for the condition of one’s entire dentition—therefore, we considered a secondary ‘person-level’ caries experience trait as a secondary clinical trait. Estimates of these caries experience traits, in the discovery and replication samples, as well as participants’ demographic information are presented in Supplementary Table 5.
Sequencing and alignment
Total nucleic acid was quantified using QuantIT® PicoGreen®. 5 ng of genomic DNA was processed using the Nextera XT DNA Sample Preparation Kit (Illumina). Target DNA was simultaneously fragmented and tagged using the Nextera Enzyme Mix-containing transposome that fragments the input DNA and adds the bridge PCR (bPCR)-compatible adaptors required for binding and clustering in the flow cell. Next, fragmented and tagged DNA was amplified using a limited-cycle PCR program. In this step index 1(i7) and index 2(i5) were added between the downstream bPCR adapter and the core sequencing library adapter, as well as primer sequences required for cluster formation. The thermal profile for the amplification had an initial extension step at 72 °C for 3 min and initial denaturing step at 95 °C for 30 s, followed by 15 cycles of denaturing of 95 °C for 10 s, annealing at 55 °C for 30 seconds, a 30 second extension at 72 °C, and final extension for 5 min at 72 °C. The DNA library pool was loaded on the Illumina platform reagent cartridge (Illumina) and on the Illumina instrument. Sequencing output from the Illumina HiSeq 4000 2 × 150 was converted to fastq format and demultiplexed using Illumina Bcl2Fastq 2.20.0 (Illumina, Inc. San Diego, CA, USA.). Quality control of the demultiplexed sequencing reads was verified by FastQC (Babraham Institute. Cambridge, UK). Adapters were trimmed using Trim Galore (Babraham Institute. Cambridge, UK). The resulting paired-end reads were classified with Kraken257 and Bracken 2.558 using a custom database including human, fungal, bacterial, and the expanded Human Oral Microbiome Database (eHOMD) genomes59 to produce an initial taxonomic composition profile. All reads identified as ‘host’ were eliminated. Paired-end reads were joined with vsearch 1.10.260. Any remaining adapter reads were trimmed again using Trim Galore. Estimates of gene family, path abundance, and path coverage were produced from the remaining reads using HUMAnN361 based on taxonomic estimates from MetaPhlAn 362,63.
RNA isolation was performed using Qiagen RNeasy Mini Kit (Cat. No. / ID: 74104) and RNA was quantified using NanoDrop1000 (ThermoFisher, Waltham, MA). To generate MTX data via RNA-seq, sequencing output from the Illumina HiSeq 4000 2 × 150 platform was converted to fastq format and demultiplexed using Illumina Bcl2Fastq 2.20.0 (Illumina, Inc. San Diego, CA, USA). Quality control of the demultiplexed sequencing reads was verified by FastQC (Babraham Institute. Cambridge, UK). Adapters were trimmed using Trim Galore (Babraham Institute. Cambridge, UK). The resulting paired-end reads were classified with Kraken257 and Bracken 2.558 using a custom database including human, fungal, bacterial, and the expanded Human Oral Microbiome Database (eHOMD) genomes59 to produce an initial taxonomic composition profile. All reads identified as host were eliminated. Paired-end reads were joined with vsearch 1.10.260. The resulting single-end reads were again trimmed of any remaining adapters using Trim Galore. HUMAnN 3.061 was used to generate gene family and pathway-level data based on taxonomic estimates from the MetaPhlAn 362,63 metagenomic analysis. Additionally, MTX gene expression analyses were performed for the four “top species” that were tested experimentally. Reads classified by Kraken2 as belonging to each of the four top species and any strain thereof were extracted by species, individually aligned to each species' relevant reference transcriptome with STAR64 (v.2.7.10b), and subsequently quantified via Salmon65 (v.1.10.1).
Quality control procedures
An overview of quality control, scaling, and filtering of taxa is presented in Supplementary Fig. 7. After the removal of viral sequences, the remaining data were first arranged in a reads-per-kilobase (RPK) format, then rescaled into transcripts-per-million (TPM), and finally rescaled close to the averaged-over-subject RPK level. The average RPK in MTG in the discovery sample was 8,004,958, and the rescaled TPM was set as 8,000,000 for each subject. To facilitate discovery and inference, species with relative abundance less than 10−5 or with a prevalence rate less than 10% were excluded from all taxonomic discovery analyses. Out of 6411 non-viral species, 5990 low-abundance and 2935 low-prevalence (including 2932 both low-abundant and low-prevalence) taxa were filtered, retaining 418 taxa for all downstream MTG analyses. MTX data in the discovery sample, as well as MTG and MTX data in the replication sample were all processed in a similar fashion. Total RPKs per subject were 11,014,832, 5,077,759, and 2,739,606 on average, and the total rescaled TPMs per subject were on average 11 million, 5 million, and 3 million, respectively. The numbers of retained taxa after applying abundance and prevalence filters were 385 for the MTX in the discovery sample, 422 and 397 for the MTG and MTX data in the replication sample, respectively. We refer to these sets of retained taxa as “core.” TPM normalization was not done for the targeted MTX of the 4 top species, due to limited diversity of the sample. Average RPK per sample was 72,474. We retained genes with average >20 reads for downstream differential expression and gene-gene interaction analyses. Using this filter, out of 9103 total genes available for these 4 top species, 542 genes were retained: 47 for S. mutans, 39 for S. sputigena, 8 for P. salivae, and 448 for L. wadei.
Identification of species significantly associated with caries experience
We used log-normal linear models to test the association between species’ differential abundance in MTG and MTX data and quantitative measures of caries experience. The models included terms for phenotypes of interest (i.e., either localized or person-level caries quantitative experience), batch effects (i.e., the first sequencing batch included 52 samples and the second batch the remaining 248 samples), age at enrollment (measured in months), and race/ethnicity (reported by legal guardians and categorized as white-non Hispanic, African American-non-Hispanic, and others including Hispanics), and unity was added to the rescaled TPM abundance data before log-transformation with base 2. The presence of each species in MTG data was controlled for in models examining differential expression (i.e., abundance in MTX data). A false discovery rate (FDR) correction for multiple testing was applied using the Benjamini-Hochberg procedure66 for each of the four models (caries traits and abundance/expression).
We used strict criteria for the identification of bacterial species associated with caries experience and required taxa to be FDR-significantly differentially abundant for quantitative disease experience defined both locally and at the person level, in MTG and MTX data (i.e., 4 models), in the discovery sample (n = 300). To reduce the potential for false discovery, we sought for additional evidence of association in an independent sample, i.e., we examined the replication of the previously identified associations in a sample of 116 similarly aged participants from the same population as the parent study (i.e., public preschools in NC), similar clinical and microbiome data. Thus 4 more models were created, for localized and person-level disease, in MTG and MTX data. As evidence of replication, we considered, in order of ascending importance, directional consistency of the estimate of association, nominal significance, or FDR-level significance in the replication sample. Species that were FDR-significant in all 4 models in the discovery sample and were at least nominally significant for localized disease experience in MTG data were termed “significant species”. This set of species with high-confidence evidence of association from multiple traits, MTG, and MTX data, and from all 416 study participants were prioritized for reporting and were candidates for consideration in the experimental validation pipeline. The raw data emanating from microbiome analyses that were used to create Figs. 2 and 3, Supplemental Figs. 1 and 2, and Supplemental Tables 1, 2, 3, and 4, as well model coefficients’ 95% confidence intervals (upper and lower bounds, where applicable) and variable legends, are included in the Supplemental Dataset.
Inter-species correlations and pathways involving species significantly associated with caries experience
To provide initial insights into the inter-relationships of the 16 significant species, we examined their pairwise correlation patterns in health and disease. For this purpose, we used Pearson correlation coefficients between model residuals that were generated for each of the 16 significant species. These log-normal models had the same specifications as models used in the main analyses (i.e., adjusted for batch effects and age) with the addition of an adjustment for disease experience. Species’ mean correlations with each of the other 15 significant species were examined between health (i.e., no localized disease experience) and disease (i.e., any localized disease experience), and in MTG and MTX.
To identify pathways significantly associated with caries experience in the biofilm transcriptome, we examined pathway and pathway-species MTX data that were prepared in RPK format as described above. Total RPKs per sample were on average 383,119, and the RPK data were transformed to TPM format with a scale of 400,000, as previously described. Each pathway, summed over all species including those unclassified, was tested using the log-normal model with the same set of covariates as in the main discovery analysis and the addition of a unity before log2 transformation. An FDR correction for testing 297 pathways was applied using the Benjamini-Hochberg procedure. Using this procedure, we identified pathways whose expression in MTX was significantly associated with caries experience. Additionally, we examined the representation of the 16 significant species in these pathways as percent of significant over all species involved in each pathway.
Shortlisting of species for virulence assays and experimental testing
We departed from a list of 16 species that showed strong and replicable evidence of association with dental caries experience (Supplementary Table 1). To select a shortlist of candidates that could be fully characterized in virulence, biofilm, and possibly in vivo studies in this study, we considered species’ statistical evidence of association in the discovery sample (i.e., p-value), evidence replication in the independent sample, representation of different genera (i.e., selected one species per genus), and availability of clinical isolates. Based on these criteria, from the list of 16 significant species we prioritized Streptococcus mutans (the known, well-established pathogen), and Selenomonas sputigena, Prevotella salivae, and Leptotrichia wadei (as 3 new candidates). These 4 taxa, referred to as “top species”, were carried forward to in vitro virulence assessments and biofilm characterization and served as candidates for further in vivo colonization and cariogenicity studies.
Microorganisms and growth conditions used in laboratory validation
Streptococcus mutans UA159 (ATCC 700610), Selenomonas sputigena (ATCC 35185), Leptotrichia wadei (JCM 16777), Prevotella salivae (JCM 12084) were used in in vitro and in vivo studies. Bacteria were grown in Brain Heart Infusion Supplemented (BHIS) medium (Brain Heart Infusion broth supplemented with 5 g/L yeast extract, 5 mg/L Hemin, 1 mg/L Vitamin K1, and 0.5 g/L L-cysteine) at 37 °C to exponential phase in an anaerobic chamber (Anaerobe Systems). For in vitro biofilm studies, a saliva-coated hydroxyapatite disc (2.7 ± 0.2 cm2; Clarkson Chromatography Products) was placed in a vertical position to mimic the tooth-enamel surfaces of human teeth. Single or mixed biofilms were grown on the apatitic surfaces in BHIS medium (107 CFU/mL for the new species and/or 105 CFU/mL S. mutans) supplemented with 1% sucrose, the primary dietary sugar associated with tooth decay, to simulate cariogenic conditions relevant to children with ECC. Biofilms were grown on saliva-coated hydroxyapatite surfaces for up to 24 h to allow the establishment of single or mixed-species communities. For growth dynamics experiments, biofilms were examined at 4 h (i.e., initial attachment stage) and at 10 h (i.e., intermediate stage). For real-time live imaging, a green fluorescent protein (GFP)-tagged S. mutans UA159 strain (S. mutans UA159 Ef-Tu-gfp)67 was used. Biofilm EPS glucan matrices were labeled via supplementing the culture medium with 1 μM Alexa Fluor 647 dextran conjugate (Molecular Probes) during biofilm growth. This labeling method is highly specific for S. mutans-derived α-glucans since the fluorescently labeled dextrans serve as primers for streptococcal glycosyltransferases and are directly incorporated into glucans during biofilm EPS synthesis. S. sputigena single-species biofilms used in the motility tracking assays were grown for 24 h in BHIS (1% sucrose) supplemented with cell-free purified GtfB enzymes (20 U).
Top species’ virulence and metabolic profile assessment
Acid tolerance and acidogenicity
Acid tolerance tests were performed using Brain Heart Infusion (BHI) broth (Anaerobe Systems) with pre-adjusted pH. Lactic acid (13.42 M) was used to adjust the pH of the medium (ranging between 2.9 and 6.5). Each of the top species was serially diluted to 107 CFU/mL. One hundred microliters (100 µL) of bacterial suspension were transferred to 900 µL pH adjusted medium to study the growth of single species under acidic conditions. In parallel, each of the new species (S. sputigena, L. wadei, or P. salivae) was mixed with S. mutans (equal proportion of single species) to examine the growth of mixed species combinations. The cultures were incubated in the anaerobic chamber at 37 °C for 48 h. Bacterial growth was quantified using a microplate reader (SpectraMax M2e) by measuring optical density values at 610 nm, whereas the pH values were measured using a pH meter (FiveEasy Benchtop F20 pH/mV Meter, Mettler Toledo). Both the lowest pH values at which each bacterial species survived (detectable growth) and the final pH values of the culture (after 48 h) were recorded to assess acid tolerance. For acidogenicity (acid production), we used standard glycolytic pH-drop assay. Bacterial cells (a final number of viable cells of 107 CFU/mL, single or mixed-species, with equal numbers, as described above) were incubated in salt solution (50 mM KCl, 1 mM MgCl2.6H2O) with 1% glucose and allowed to produce acids over time. The decrease in pH was measured using a pH meter every 1 h over a 12 h period during which each species actively produced acids. The average proton production rate over the 12 h period was calculated to compare acidogenicity of different species either alone or in combination with S. mutans.
Metabolic profiling
Top species’ metabolic profiles were measured using real-time isothermal microcalorimetry68. S. mutans, S. sputigena, L. wadei, P. salivae, and P. oulorum were transferred to BHI broth and incubated in the anaerobic chamber at 37 °C for 48 h. Each culture was serially diluted to 107 CFU/mL, and 60 µl of each bacterial suspension was transferred, in triplicate, in titanium vials (calWell; SymCel) containing 540 µl of pre-reduced BHI. Real-time heat production, proxying metabolic activity, was measured using a calScreener™ microcalorimeter (SymCel Sverige AB, Stockholm, Sweden) in a 48-well plate (calPlate™) continuously for up to 48 h, as previously described69. The full processing of the samples and plate preparation were performed inside the anaerobic chamber, to maintain the anaerobic condition for optimal bacterial growth. Heat and corresponding energy data were quantified with calView™ software (Version 1.0.33.0, © 2015 SymCel, Sverige AB). The instrument was set and calibrated at 37 °C, with all handling and set-up done according to the manufacturer’s recommendations.
Biofilm live imaging
Biofilm live imaging was performed based on our established fluorescence labeling and confocal imaging protocols optimized for oral biofilms with some modifications70. Biofilms were dip-washed three times in phosphate buffered saline (PBS, pH 7.1) to remove any loosely bound microbes from the surface. To enhance the GFP fluorophore development in GFP-tagged S. mutans cells, we performed aerobic fluorescence recovery immediately before imaging, following the protocol previously reported71. Biofilms were counterstained with SYTO82 (Molecular Probes), which labeled all bacterial cells. Super-resolution live imaging was conducted at 37 °C using a 40× (numerical aperture = 1.2) water immersion objective on a Zeiss LSM800 upright confocal microscope with Airyscan. For real-time imaging of bacterial motility, multi-channel confocal images were taken at a 2.6-second interval for 30 s.
Computational biofilm image analysis
We then used a fluorescence subtraction method to analyze the biofilm spatial structuring (positioning of different species and EPS across the 3D biofilm structure) as detailed previously38. In brief, we applied channel subtraction using the Image Calculator in ImageJ Fiji v.2.11.0 (https://imagej.net/Fiji) to calculate the fluorescence signal from the new species in the mixed-species biofilm, using the following equation: ChNew species = ChAll bacteria- ChS. mutans, where ChAll bacteria is the channel of all bacteria (SYTO82), and ChS. mutans is the channel of S. mutans (GFP). Computational image processing and quantitative analysis were performed using BiofilmQ software v.0.2.2 (https://drescherlab.org/data/biofilmQ), an image analysis toolbox optimized for biofilms29. After image segmentation using Otsu algorithm, we conducted a cube-based object declumping in BiofilmQ that dissected the entire biofilm into small cubic volumes (cube size = 2 μm). This function allows analyses of structural properties within biofilm subdomains with spatial resolution since each cube has a unique spatial coordinate in 3D. Parameters including local shape volume, relative abundance, and intensity were calculated for each channel within the cubes. For co-localization analysis, we used Mander’s overlap coefficient in BiofilmQ to quantify the degree of spatial proximity of two different fluorescence signals in relation to each other. Two Manders’ coefficients were calculated: 1) the new species with S. mutans and 2) the new species with EPS. To track single-cell bacterial motility in real time, we performed computational single-particle tracking and generated time-resolved trajectories using the TrackMate plugin in ImageJ Fiji72. Biofilm visualization was performed using maximum intensity projection and 3D surface rendering in ImageJ Fiji.
In situ biofilm pH measurement and visualization
We used a pH-responsive fluorescent dye (C-SNARF-4) combined with confocal live imaging to determine the in situ pH at the biofilm-apatite interface as described previously39. The C-SNARF-4 exhibits a pH-dependent emission shift, allowing measurement of localized biofilm pH using the ratio of the fluorescence intensities at two emission wavelengths. Briefly, 24 h biofilms were dip-washed and equilibrated in HEPES buffer (50 μM; pH = 7.4) for 60 min. Then, biofilms were stained with 30 μM C-SNARF-4 followed by continuous ratiometric imaging at two detection wavelength ranges (576–608 nm and 629–650 nm). The first image (t = 0 min) was taken before glucose exposure and image acquisition was performed every 5 min at 37 °C after adding glucose (1% final concentration), using a 20x (numerical aperture = 1.0) water dipping objective on the Zeiss LSM800 confocal microscope. Calibration of the ratiometric probe was conducted using HEPES buffer (50 μM; pH = 4.5 to 7.5 in steps of 0.5 pH unit). Computational image analysis and visualization were performed using ImageJ Fiji.
Ex vivo human tooth-enamel biofilm model and enamel surface analyses
We employed an ex vivo human tooth-enamel model which allows analysis of the extent of enamel decay underneath the biofilm40. Briefly, single or mixed-species biofilms (67 h) were formed on sterilized enamel specimens (4 × 4 mm) prepared from de-identified extracted human teeth. The biofilm was removed using enzymatic treatment (dextranase and mutanase) followed by water bath sonication, which was optimized for biofilm removal without causing artificial surface damage40. The macroscopic demineralized areas on the enamel surface (similar to those found clinically manifest early childhood caries) were visualized using stereomicroscopy (ZEISS Axio Zoom V16). Then, the surface topography and roughness of the tooth enamel surface were assessed by non-destructive confocal microscopy using a 50x (numerical aperture = 0.95) objective on the Zeiss LSM800 microscope and ConfoMap software (version 7.3.7984). Next, the human enamel specimens were mounted on acrylic rods and sectioned (100 ± 20 µm thickness) with a hard tissue microtome (Silverstone-Taylor Hard Tissue Microtome, Series 1000 Deluxe) for transverse microradiography. The sections were placed in the TMR-D system and x-rayed (45 kV; 45 mA) at a fixed distance for 12 s. An aluminum step wedge was x-rayed under identical conditions. The digital images were analyzed using TMR software v.3.0.0.18, with sound enamel defined at 87% mineral volume73.
Targeted bacterial gene expression and gene-gene interaction analyses
We used a targeted MTX approach to test S. mutans, S. sputigena, L. wadei, and P. salivae gene expression. Reads were aligned to these species reference genomes using STAR-Salmon64,65. Where multiple strains were available, results were merged to the species level. The association of gene expression with caries experience was then tested for each gene using log-normal models adjusting for batch effects, age, and race/ethnicity, and applying an FDR multiple testing correction for each species. Additionally, we examined gene-gene interactions between S. mutans and S. sputigena—the two species that demonstrated enhanced acidogenesis and unique biofilm structure when co-cultured. To this end, the same log-normal models including gene-gene interactions were used including pairwise combinations of all filtered genes from these two species, applying an FDR multiple testing correction.
In vivo rodent model of childhood caries
Bacterial colonization on teeth and their impacts on disease onset were assessed on an established rodent caries model as detailed elsewhere with some modifications43,74. In brief, 15-d old female Sprague-Dawley rat pups (specific-pathogen-free grade) were purchased with dams (8 pups per dam) from Harlan Laboratories (Indianapolis, IN, USA). Upon arrival, animals were pre-screened for oral infection of S. mutans and S. sputigena by oral swabs and real-time polymerase chain reaction (qPCR) and were determined not to be infected with either organism. Oral swabs (FLOQSwab, COPAN Diagnostics, Murrieta, CA, USA) were taken from each of the animals and bacterial DNA were extracted using DNeasy PowerLyzer Microbial Kit (Qiagen, Valencia, CA, USA). Species-specific qPCR primer sets were used for microorganism detection as follows: S. mutans: Forward 5’ ACCAGAAAGGGACGGCTAAC 3’, Reverse 5’ TAGCCTTTTACTCCAGACTTTCCTG 3’; S. sputigena: Forward 5’ GGTCAGCCTTATCAGTTCCGTT 3’, Reverse 5’ GGCGAGCTTTCAGCAATCTTAG 3’; all bacteria: Forward 5’ TCCTACGGGAGGCAGCAGT 3’, Reverse 5’ GGACTACCAGGGTATCTAATCCTGTT 3’.
The pups were randomly assigned into four groups that received different bacterial infections and were housed separately: (1) S. mutans alone; (2) S. sputigena alone; (3) S. mutans plus S. sputigena; (4) control without S. mutans or S. sputigena infection. Animals were inoculated daily using cotton oral swabs with actively growing cultures of S. mutans and/or S. sputigena (~108 CFU/mL in BHIS) at the age of 19–23 d (five doses in total). Each of the infected groups were confirmed for its designated microbial infection at 21 d, 24 d, and 30 d by oral swabs and qPCR, while the control group remained free of either S. mutans or S. sputigena. No cross-contamination was detected throughout the experiment. All animals were provided with the National Institutes of Health cariogenic diet #2000 and 5% sucrose water ad libitum to mimic the cavity-promoting diet relevant to childhood caries. The experiment proceeded for three weeks, at the end of which the animals were euthanized using carbon dioxide. The jaws were dissected and processed for caries scoring of teeth according to Larson’s modification of Keyes’ system75. Caries scores were determined by a calibrated examiner. Investigators were masked to experimental group (i.e., infection) allocations during the infection, sampling, and assessment stages, by using color-coded samples. In vitro and in vivo experimental data were presented as mean ± standard deviation. Data were subjected to Student’s pairwise t-test or analysis of variance (ANOVA) with post-hoc Tukey HSD test to account for multiple comparisons. Differences between groups were considered statistically significant when p < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by research grants from the National Institutes of Health: National Center for Advancing Translational Sciences National UL1TR001111 (K.D.); National Institute for Dental and Craniofacial Research U01DE025046 (K.D.), R01DE025220 (H.K.), and R03DE028983 (D.W.); Z. R. is supported by the NIDCR Postdoctoral Training Program under award number R90DE031532. Z. R. was a recipient of the Colgate–Palmolive Fellowship. The UNC Microbiome Core is funded in part by the Center for Gastrointestinal Biology and Disease (CGIBD P30 DK034987) and the UNC Nutrition Obesity Research Center (NORC P30 DK056350). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The authors thank Dr. Patricia V. Basta and her team at the UNC-Chapel Hill Biospecimen Processing facility for the accessioning, storage, and disbursement of the supragingival biofilm microbiome specimens in the ZOE studies; Dr. Anderson Hara at Indiana University for technical assistance in transverse microradiography; Dr. Justin Merritt at Oregon Health & Science University for kindly providing the S. mutans UA159 Ef-Tu-gfp strain; and Dr. Lamprini Karygianni and Dr. Thomas Thurnheer at University of Zurich for the gift of S. sputigena ATCC 35185 strain. Some schematic diagrams in the figures are created with BioRender.com (Publication License #RS251UWR8N).
Source data
Author contributions
K.D., K.E.N., A.A.R., D.W., and H.K. designed the study. K.D., M.A.S.P, P.S., J.G., and A.G.F.G participated in data collection and sampling. J.R. and M.A.A.P. carried out sample processing and sequencing. H.C., J.R., B.M.L, C.L., A.O., and D.W. conducted bioinformatics analyses. Z.R., A.A.R., and H.K. carried out in vitro experiments. Z.R. and H.K. carried out in vivo experiments. H.C., Z.R., K.D., A.A.R., D.W., and H.K. wrote the paper with contributions from all the authors.
Peer review
Peer review information
Nature Communications thanks Rodrigo Alex Arthur, and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Raw DNA and RNA sequence data for ZOE 2.0 have been deposited and are publicly available as part of dbGaP accession phs002232.v1.p1 “TOPDECC-Trans-omics for Precision Dentistry and Early Childhood Caries: Genome-Wide Genotyping (CIDR) and Microbiome in the ZOE 2.0 Study” and the Sequence Read Archive (SRA) as BioProject 671299 (PRJNA671299; dbGaP: phs002232; https://www.ncbi.nlm.nih.gov/bioproject/671299). Raw DNA and RNA sequence data for ZOE pilot (the replication sample) have been deposited and are publicly available as part of BioProject 843091 “ZOE 2.0 pilot study” (PRJNA843091). Microbiome taxonomy (MTG and MTX) and pathway information for the entire biofilm microbial community, as well as targeted MTX data for the four top species used in this study have been deposited and are publicly available alongside metadata (i.e., demographic and clinical phenotype information) via the Carolina Digital Repository and accession number 5d86p890x. Reference genomes were obtained from the expanded Human Oral Microbiome Database (https://www.homd.org/). The source data file used to generate figures and the tables is available in the Supplementary Information. Source data are provided with this paper.
Code availability
The scripts used to perform this analysis can be found at 10.5281/zenodo.770729776.
Competing interests
The authors declare no competing interests.
Ethical approval
Human observational data and analyses received approval (#14-1992) from the University of North Carolina-Chapel Hill Office of Human Research Ethics Institutional Review Board on September 18, 2014. Legal guardians of all children provided written informed consent for participation in the study. The in vivo experimental study was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (IACUC#805735). All research was performed in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hunyong Cho, Zhi Ren.
Contributor Information
Kimon Divaris, Email: Kimon_Divaris@unc.edu.
Di Wu, did@email.unc.edu.
Hyun Koo, Email: koohy@upenn.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-38346-3.
References
- 1.Rossi E, et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021;19:331–342. doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 2.Ch’ng JH, Chong K, Lam LN, Wong JJ, Kline KA. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019;17:82–94. doi: 10.1038/s41579-018-0107-z. [DOI] [PubMed] [Google Scholar]
- 3.Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev.: MMBR. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadiq FA, et al. Community-wide changes reflecting bacterial interspecific interactions in multispecies biofilms. Crit. Rev. Microbiol. 2021;47:338–358. doi: 10.1080/1040841X.2021.1887079. [DOI] [PubMed] [Google Scholar]
- 6.Marsh PD. In sickness and in health-what does the oral microbiome mean to us? an ecological perspective. Adv. Dent. Res. 2018;29:60–65. doi: 10.1177/0022034517735295. [DOI] [PubMed] [Google Scholar]
- 7.Uribe SE, Innes N, Maldupa I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Paediatr. Dent. 2021;31:817–830. doi: 10.1111/ipd.12783. [DOI] [PubMed] [Google Scholar]
- 8.Pitts NB, et al. Early childhood caries: IAPD bangkok declaration. J. Dent. Child. 2019;86:72. [PubMed] [Google Scholar]
- 9.Alaluusua S, Renkonen OV. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand. J. Dent. Res. 1983;91:453–457. doi: 10.1111/j.1600-0722.1983.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamada S, et al. Dental caries induction in experimental animals by clinical strains of Streptococcus mutans isolated from Japanese children. Microbiol. Immunol. 1978;22:301–314. doi: 10.1111/j.1348-0421.1978.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 11.Clarke JK. On the bacterial factor in the ætiology of dental caries. Br. J. Exp. Pathol. 1924;5:141–147. [Google Scholar]
- 12.Eriksson L, Lif Holgerson P, Esberg A, Johansson I. Microbial complexes and caries in 17-year-olds with and without Streptococcus mutans. J. Dent. Res. 2018;97:275–282. doi: 10.1177/0022034517731758. [DOI] [PubMed] [Google Scholar]
- 13.Thurnheer T, Belibasakis GN. Streptococcus oralis maintains homeostasis in oral biofilms by antagonizing the cariogenic pathogen Streptococcus mutans. Mol. Oral. Microbiol. 2018;33:234–239. doi: 10.1111/omi.12216. [DOI] [PubMed] [Google Scholar]
- 14.Nyvad B, Crielaard W, Mira A, Takahashi N, Beighton D. Dental caries from a molecular microbiological perspective. Caries Res. 2013;47:89–102. doi: 10.1159/000345367. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 2017;7:41332. doi: 10.1038/srep41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J. Clin. Periodontol. 2017;44:S23–S38. doi: 10.1111/jcpe.12671. [DOI] [PubMed] [Google Scholar]
- 17.Philip N, Suneja B, Walsh L. Beyond Streptococcus mutans: clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br. Dent. J. 2018;224:219–225. doi: 10.1038/sj.bdj.2018.81. [DOI] [PubMed] [Google Scholar]
- 18.Hajishengallis G, Lamont RJ. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24:477–489. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson SN, et al. Functional expression of dental plaque microbiota. Front. Cell. Infect. Microbiol. 2014;4:108. doi: 10.3389/fcimb.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kressirer CA, et al. Functional profiles of coronal and dentin caries in children. J. oral. Microbiol. 2018;10:1495976. doi: 10.1080/20002297.2018.1495976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark Welch JL, Ramírez-Puebla ST, Borisy GG. Oral microbiome geography: micron-scale habitat and niche. Cell host microbe. 2020;28:160–168. doi: 10.1016/j.chom.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azimi, S., Lewin, G. R. & Whiteley, M. The biogeography of infection revisited. Nat. Rev. Microbiol.20, 579–592 (2022). [DOI] [PMC free article] [PubMed]
- 23.Oliveira RR, et al. Levels of candidate periodontal pathogens in subgingival biofilm. J. Dent. Res. 2016;95:711–718. doi: 10.1177/0022034516634619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephan RM. Intra-oral hydrogen-ion concentrations associated with dental caries activity. J. Dent. Res. 1944;23:257–266. doi: 10.1177/00220345440230040401. [DOI] [Google Scholar]
- 26.Kingsley VV, Hoeniger JF. Growth, structure, and classification of Selenomonas. Bacteriological Rev. 1973;37:479–521. doi: 10.1128/br.37.4.479-521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J. Clin. Periodontol. 2017;44:S12–S22. doi: 10.1111/jcpe.12679. [DOI] [PubMed] [Google Scholar]
- 28.Xiao J, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann R, et al. Quantitative image analysis of microbial communities with BiofilmQ. Nat. Microbiol. 2021;6:151–156. doi: 10.1038/s41564-020-00817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, et al. Three-dimensional quantitative imaging of native microbiota distribution in the gut. Angew. Chem. 2021;60:3055–3061. doi: 10.1002/anie.202010921. [DOI] [PubMed] [Google Scholar]
- 32.Drescher J, et al. Molecular epidemiology and spatial distribution of Selenomonas spp. in subgingival biofilms. Eur. J. Oral. Sci. 2010;118:466–474. doi: 10.1111/j.1600-0722.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- 33.Paster BJ, et al. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faveri M, et al. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral. Microbiol. Immunol. 2008;23:112–118. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 35.Rôças IN, Siqueira JF, Jr, Debelian GJ. Analysis of symptomatic and asymptomatic primary root canal infections in adult Norwegian patients. J. Endod. 2011;37:1206–1212. doi: 10.1016/j.joen.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 36.da Costa Rosa T, et al. The bacterial microbiome and metabolome in caries progression and arrest. J. oral. Microbiol. 2021;13:1886748. doi: 10.1080/20002297.2021.1886748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren Z, et al. Dual-targeting approach degrades biofilm matrix and enhances bacterial killing. J. Dent. Res. 2019;98:322–330. doi: 10.1177/0022034518818480. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, et al. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl Acad. Sci. USA. 2020;117:12375–12386. doi: 10.1073/pnas.1919099117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlafer S, et al. Ratiometric imaging of extracellular pH in bacterial biofilms with C-SNARF-4. Appl. Environ. Microbiol. 2015;81:1267–1273. doi: 10.1128/AEM.02831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, et al. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 2018;9:2920. doi: 10.1038/s41467-018-05342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowen WH. Rodent model in caries research. Odontology. 2013;101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- 42.Koo H, et al. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005;84:1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falsetta ML, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rath CB, et al. Flagellin glycoproteomics of the periodontitis associated pathogen selenomonas sputigena reveals previously not described o-glycans and rhamnose fragment rearrangement occurring on the glycopeptides. Mol. Cell. Proteom.: MCP. 2018;17:721–736. doi: 10.1074/mcp.RA117.000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng ZL, Szafrański SP, Jarek M, Bhuju S, Wagner-Döbler I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci. Rep. 2017;7:3703. doi: 10.1038/s41598-017-03804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chao X, et al. Faeces from malnourished colorectal cancer patients accelerate cancer progression. Clin. Nutr. 2022;41:632–644. doi: 10.1016/j.clnu.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, et al. Microbial biomarkers of common tongue coatings in patients with gastric cancer. Microb. pathogenesis. 2019;127:97–105. doi: 10.1016/j.micpath.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 48.Heimisdottir LH, et al. Metabolomics insights in early childhood caries. J. Dent. Res. 2021;100:615–622. doi: 10.1177/0022034520982963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vacca-Smith AM, Bowen WH. Binding properties of streptococcal glucosyltransferases for hydroxyapatite, saliva-coated hydroxyapatite, and bacterial surfaces. Arch. oral. Biol. 1998;43:103–110. doi: 10.1016/S0003-9969(97)00111-8. [DOI] [PubMed] [Google Scholar]
- 50.Johnston CD, et al. Systematic evasion of the restriction-modification barrier in bacteria. Proc. Natl Acad. Sci. USA. 2019;116:11454–11459. doi: 10.1073/pnas.1820256116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedberg ME, et al. Lachnoanaerobaculum gen. nov., a new genus in the Lachnospiraceae: characterization of Lachnoanaerobaculum umeaense gen. nov., sp. nov., isolated from the human small intestine, and Lachnoanaerobaculum orale sp. nov., isolated from saliva, and reclassification of Eubacterium saburreum (Prevot 1966) Holdeman and Moore 1970 as Lachnoanaerobaculum saburreum comb. nov. Int. J. Syst. Evolut. Microbiol. 2012;62:2685–2690. doi: 10.1099/ijs.0.033613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao J, et al. Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res. 2018;52:102–112. doi: 10.1159/000481833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benler S, Koonin EV. Fishing for phages in metagenomes: what do we catch, what do we miss? Curr. Opin. Virol. 2021;49:142–150. doi: 10.1016/j.coviro.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Divaris K, et al. Cohort profile: ZOE 2.0-a community-based genetic epidemiologic study of early childhood oral health. Int. J. Environ. Res. public health. 2020;17:8056. doi: 10.3390/ijerph17218056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ginnis J, et al. Measurement of early childhood oral health for research purposes: dental caries experience and developmental defects of the enamel in the primary dentition. Methods Mol. Biol. 2019;2019:511–523. doi: 10.1007/978-1-4939-9012-2_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Divaris K, et al. The supragingival biofilm in early childhood caries: clinical and laboratory protocols and bioinformatics pipelines supporting metagenomics, metatranscriptomics, and metabolomics studies of the oral microbiome. Methods Mol. Biol. 2019;2019:525–548. doi: 10.1007/978-1-4939-9012-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J, Breitwieser FP, Thielen P, Salzberg SL. Bracken: estimating species abundance in metagenomics data. PeerJ Computer Sci. 2017;3:e104. doi: 10.7717/peerj-cs.104. [DOI] [Google Scholar]
- 59.Dewhirst FE, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abubucker S, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Computational Biol. 2012;8:e1002358. doi: 10.1371/journal.pcbi.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beghini F, et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife. 2021;10:e65088. doi: 10.7554/eLife.65088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Truong DT, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods. 2015;12:902–903. doi: 10.1038/nmeth.3589. [DOI] [PubMed] [Google Scholar]
- 64.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinforma. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- 67.Mu R, Shinde P, Zou Z, Kreth J, Merritt J. Examining the protein interactome and subcellular localization of rnase j2 complexes in Streptococcus mutans. Front. Microbiol. 2019;10:2150. doi: 10.3389/fmicb.2019.02150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braissant O, Bonkat G, Wirz D, Bachmann A. Microbial growth and isothermal microcalorimetry: growth models and their application to microcalorimetric data. Thermochim. Acta. 2013;555:64–71. doi: 10.1016/j.tca.2012.12.005. [DOI] [Google Scholar]
- 69.Braissant O, et al. Isothermal microcalorimetry accurately detects bacteria, tumorous microtissues, and parasitic worms in a label-free well-plate assay. Biotechnol. J. 2015;10:460–468. doi: 10.1002/biot.201400494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paula AJ, Hwang G, Koo H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 2020;11:1354. doi: 10.1038/s41467-020-15165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang C, Xing XH, Lou K. Rapid detection of a gfp-marked Enterobacter aerogenes under anaerobic conditions by aerobic fluorescence recovery. FEMS Microbiol. Lett. 2005;249:211–218. doi: 10.1016/j.femsle.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 72.Tinevez JY, et al. TrackMate: An open and extensible platform for single-particle tracking. Methods. 2017;115:80–90. doi: 10.1016/j.ymeth.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 73.Xiao J, et al. Biofilm three-dimensional architecture influences in situ pH distribution pattern on the human enamel surface. Int. J. oral. Sci. 2017;9:74–79. doi: 10.1038/ijos.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein MI, Scott-Anne KM, Gregoire S, Rosalen PL, Koo H. Molecular approaches for viable bacterial population and transcriptional analyses in a rodent model of dental caries. Mol. oral. Microbiol. 2012;27:350–361. doi: 10.1111/j.2041-1014.2012.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larson RH. Merits and modifications of scoring rat dental caries by Keyes’ method. In Proceedings of” Symposium on animal models in cariology”. Spl. Supp. Microbiol. Abstr. 1981;1981:195–203. [Google Scholar]
- 76.Cho, H. R scripts for “Selenomonas sputigena as a pathobiont mediating spatial structure and biofilm virulence in early childhood caries”, Zenodo, 10.5281/zenodo.7707297 (2023). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Raw DNA and RNA sequence data for ZOE 2.0 have been deposited and are publicly available as part of dbGaP accession phs002232.v1.p1 “TOPDECC-Trans-omics for Precision Dentistry and Early Childhood Caries: Genome-Wide Genotyping (CIDR) and Microbiome in the ZOE 2.0 Study” and the Sequence Read Archive (SRA) as BioProject 671299 (PRJNA671299; dbGaP: phs002232; https://www.ncbi.nlm.nih.gov/bioproject/671299). Raw DNA and RNA sequence data for ZOE pilot (the replication sample) have been deposited and are publicly available as part of BioProject 843091 “ZOE 2.0 pilot study” (PRJNA843091). Microbiome taxonomy (MTG and MTX) and pathway information for the entire biofilm microbial community, as well as targeted MTX data for the four top species used in this study have been deposited and are publicly available alongside metadata (i.e., demographic and clinical phenotype information) via the Carolina Digital Repository and accession number 5d86p890x. Reference genomes were obtained from the expanded Human Oral Microbiome Database (https://www.homd.org/). The source data file used to generate figures and the tables is available in the Supplementary Information. Source data are provided with this paper.
The scripts used to perform this analysis can be found at 10.5281/zenodo.770729776.