Because of a paucity of high-quality evidence, the updated Canadian Death Determination Guidelines featured in this month’s Special Issue of the Journal include several recommendations based on low to moderate certainty of evidence or expert opinion.1 In generating its recommendations, the guideline development group identified numerous knowledge gaps.2 Many of these suggest research questions answerable only through nontherapeutic studies involving imminently dying or recently deceased adult and pediatric patients in controlled intensive care unit environments.
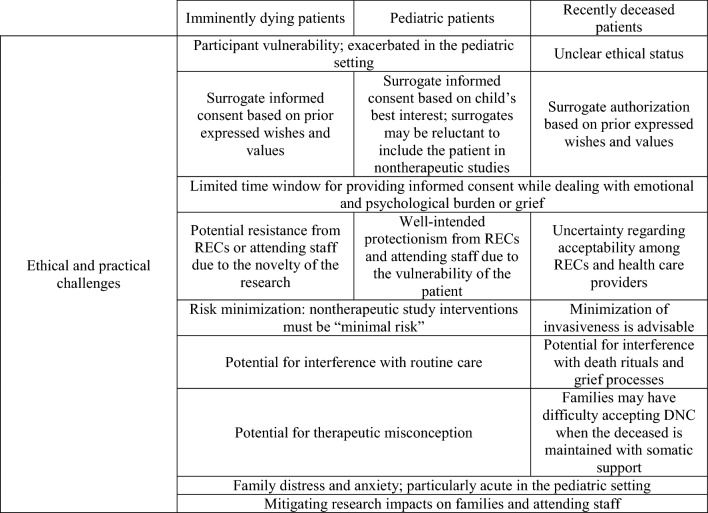
While advancing the science of death determination is in the interest of patients, families, health care providers, health care institutions, and society, there are currently no dedicated Canadian ethical guidelines addressing the substantial challenges of research with imminently dying or recently deceased patients (Table 1). Indeed, to our knowledge, nor are there authoritative international guidelines for research with these populations, perhaps owing to the novelty of these areas of research. Uncertainty regarding the ethics of research with the imminently dying and recently deceased has hindered research into important scientific questions. Accordingly, below we explore the ethical and practical challenges of research with these populations, highlight existing guidance where available, and point to areas where further guidance is needed.
Table 1.
Ethical and practical challenges of research with imminently dying and recently deceased study populations
DNC = determination of death by neurologic criteria; REC = research ethics committee
Ethical lacunae
The principles of respect for persons, justice, and beneficence guide all research with human participants.3 These principles ground moral rules to which researchers must adhere (Table 2).
Table 2.
Ethical principles and normative entailments
| Principle | Definition | Normative guidelines |
|---|---|---|
| Justice | The potential benefits, risks, and burdens of research participation must be distributed equitably | Fair procedures must be in place for the selection of research participants |
| Vulnerable research participants are entitled to additional protection | ||
| Respect for persons | Candidates for research participation must be treated as autonomous agents, and those with diminished autonomy are entitled to protection | Informed consent must be obtained from prospective research participants |
| When prospective participant autonomy is lacking, informed consent must be obtained from an authorized surrogate decision-maker | ||
| Protect the confidentiality of private information | ||
| Beneficence | Research participants must be protected from harm and their welfare must be promoted | Therapeutic procedures must satisfy equipoise |
| Any risks of nontherapeutic procedures must be minimized consistent with sound scientific design, and reasonable in relation to the knowledge to be gained |
Table adapted from Murphy et al.7
Nevertheless, there is no consensus on the application of these moral rules to studies involving imminently dying or recently deceased patients. Nontherapeutic studies do not benefit participants medically, and uncertainties concerning the permissible limits of such research and the protection owed to participants therefore persist.4 Moreover, the ethical status of recently deceased patients has been debated, with international variation regarding whether they ought to be afforded research participant protection.4
Additionally, recent scholarship has illuminated how nonparticipant “bystanders” can be impacted by research activities.5 Studies involving imminently dying or recently deceased patients will impact families facing emotional burdens and health care providers concerned that patient participation will negatively affect patient care or family grief.6 Measures to support families and health care providers are therefore warranted in research of this kind.7,8 Just what these supports should be, however, has not been determined.
Research with imminently dying patients
Research enrolling patients expected to die within hours or minutes is critical to improving practices for death determination in the interest of future patients. For example, a study to document cerebral blood flow and cerebral electrical activity in relation to arterial pulse pressure following withdrawal of life-sustaining measures is needed to determine when cessation of brain function occurs during the dying process.2
While the prospect of research with imminently dying patients provokes unease because of participant vulnerability, there is no compelling reason why this population should be excluded from research, provided they are afforded adequate protection. Indeed, exclusion would unjustly deny patients and families the opportunity to altruistically contribute to science and deprive future patients of the benefits of scientific knowledge.7
Ethical issues
Nontherapeutic studies like that described above—which will involve physiologic monitoring of dying patients purely for research purposes—test the limits of existing ethical guidance.7 While research involving the imminently dying has been successfully undertaken,9 it poses significant ethical challenges.6 These include participant vulnerability, difficulties obtaining informed consent, the possibility that research could interfere with routine care and the dying process, potential effects on families, and the reservations of health care providers and research ethics committees.6,7,10 These ethical challenges—and the practical difficulties attending them—are principal obstacles to achieving important scientific ends.
Available guidance
Imminently dying patients generally lack decision-making capacity and are therefore vulnerable. Vulnerable participants are entitled to protections beyond what is typical for competent participants.11 Below, we outline these protections and their implications. Table 3 describes strategies for ensuring these requirements are met (Table 3).
Table 3.
Additional ethical requirements for nontherapeutic research with imminently dying patients and strategies for ensuring these requirements are met
| Requirement | Suggested strategies |
|---|---|
| Study hypothesis must require participation of the vulnerable population |
Ensure the study question could not be answered using a population less vulnerable than the imminently dying Ensure the study stands to benefit the patient population in future Ensure scientific rigor (e.g., sample size must be sufficient to answer the study question) |
| Study must have a favorable risk-benefit ratio |
Ensure risks are reasonable in relation to the knowledge benefits Ensure the study has social value insofar as it will benefit the study population in future |
| Study interventions must be minimal risk |
Ensure nontherapeutic interventions pose risks no higher than those typically experienced by the study population as part of their routine care (e.g., blood draws, imaging, etc.) Ensure adequate protection is in place for storage of data and biological samples |
| Risks and intrusiveness must be minimized and consistent with sound scientific design |
Follow standard of care so far as is possible, and mitigate interference with routine care Minimize obtrusiveness of study personnel and equipment Make use of clinically indicated monitoring for data collection where feasible Combine nontherapeutic imaging with indicated imaging where feasible Account for risks of patient transport (e.g., for imaging) Account for risks and inconveniences associated with any delays to withdrawal of life-sustaining measures Include plans for dealing with participant distress in study protocol |
| Surrogate informed research consent is required |
Consult attending staff regarding surrogate’s emotional and psychological state before approaching for consent to ensure distressed family members are not overburdened Ensure surrogates understand their role as surrogate decision-makers Ensure surrogates are aware that refusal will not impact patient care Ensure surrogates are aware the withdrawal from the study will not impact patient care Mitigate risk of therapeutic misconception by having a third party who is not part of the care team make the approach when feasible Ensure surrogates have adequate time to consider the patient’s participation and ask questions Repeat information at intervals if necessary Discuss participation in a private setting where possible Consider the use of multimedia educational instruments for detailing study rationale and procedures Ensure consent discussion and documents are in lay terms |
| Impacts on families and surrogates must be minimized and opportunities for benefits must be maximized |
Include patient and family partners in protocol design to ensure responsivity to family perspectives Prepare families for study processes using visual aids and discussion Minimize intrusiveness of study procedures and personnel Ensure supports are available (e.g., spiritual care providers, social workers) Promote meaning-making by communicating the social value of the study Plan to provide summary findings to families |
| Healthcare workers must be supported |
Include health care providers who care for patients at the end of life in protocol development Offer workshops on study processes and aims before participant enrollment Provide informational pamphlets/printouts and ensure health care providers are aware of the study before commencing enrollment Acknowledge concerns and incorporate suggestions for mitigating interference with routine care Communicate the social value of the study Discuss the study regularly with health care providers to assess perspectives and address concerns |
The ethical principle of justice demands that answering a study’s research question necessitates the inclusion of the vulnerable population. This will usually require that the study aims to produce knowledge of benefit to future members of the study population—in this case, imminently dying patients.
As imminently dying patients are typically incapable of consenting to study participation, the ethical principle of respect for persons requires that consent be obtained from surrogate decision-makers. Obtaining surrogate consent to research in the context of an intensive care unit is challenging, particularly because of surrogate distress, confusion, or decisional burden.7 Nonetheless, it is critical to ensuring that research participation aligns with the patient’s prior expressed wishes or values.7,10
The ethical principle of beneficence prescribes that the risks of research participation stand in reasonable relation to knowledge benefits for imminently dying patients. First, risks of nontherapeutic procedures must be minimized consistent with sound scientific design. This involves prioritizing patient care over research goals, minimizing the risks of nontherapeutic interventions, and avoiding alterations to routine care as far as possible.7 Second, the risks of nontherapeutic study interventions must not exceed a “minimal risk” threshold, defined in Canada as the risks of daily life for the study population.11 While this may appear to permit highly risky interventions given the risks facing imminently dying patients, in practice “minimal risk” is considered synonymous with the risks involved in routine clinical care.12
Finally, while not mandated by research ethics guidelines, studies with the imminently dying ought to consider “bystanders” affected by the research.5 Strategies for minimizing impacts on participants’ families and health care providers should be included in study protocols.7
Although it does not amount to authoritative guidance, a recent article coauthored by several of us outlines the major ethical challenges of research with the imminently dying and further identifies strategies for their resolution.7 The user-friendly ethical checklist we advance in this paper may prove useful to researchers when designing study protocols.
Roadmap
While the above requirements for research with the imminently dying are instructive, we caution that they may fall short of what is needed to support stakeholders. Dedicated guidelines for research with this population are therefore urgently needed. Importantly, guideline development must involve those stakeholders who will be most impacted: patients, families, and health care providers who care for patients at the end of life.
Pediatric considerations
Due to a dearth of high-quality research, the updated Canadian Death Determination Guidelines include several pediatric-specific recommendations based on low certainty of evidence.1 Answering important questions regarding the physiology of the dying process in pediatric populations will require studies involving imminently dying children. For example, a study to document the incidence of autoresuscitation following circulatory arrest is needed to inform the appropriate “hands-off” period prior to organ recovery in pediatric donation after the determination of death by circulatory criteria.2
Ethical issues
The practical and ethical challenges to research involving imminently dying children cannot be overstated.13–16 Foremost among these is a “well-intended protectionism” by health care providers and research ethics committees.14 Critically ill children are vulnerable. They are typically unable to voice assent or dissent, and younger children have yet to develop the capacities required to express an autonomous interest in research participation. Parents and guardians are distraught, profoundly concerned for the welfare of the child, and strive to be “the good parent” by focusing on the child’s quality of life.17 Hence, there is an understandable reluctance to impose potential research burdens on the patient.13–15
Moreover, parents of critically ill children frequently seek to make decisions that the rest of the family accepts,17 complicating surrogate consent to research. Further, parents are prone to emotionally driven decision-making,18 and may therefore be susceptible to therapeutic misconception—a mistaken belief that research interventions are administered with therapeutic warrant.19 Finally, given the uniquely tragic circumstances attending a child’s critical illness or injury, parents are themselves vulnerable, meaning that impacts on research bystanders could be more substantial than in research with adult populations.
Available guidance
Researchers, attending staff, and research ethics committees should not assume that research opportunities will be unwelcome to the patient-family unit. Indeed, families may benefit from meaning-making by contributing to the advancement of science.7,13,14 While it is important to acknowledge stakeholder reservations regarding nontherapeutic research with critically ill children, research with this population can also be ethically justifiable provided protocols include adequate protection for patients and families.16 Nonetheless, further discussion and debate are warranted.
Roadmap
The challenges to nontherapeutic research with critically ill children are immense, and it is advisable to proceed with caution while awaiting dedicated ethical guidance. Guideline development should follow consultation with health care providers, youth advisors, and families of children who have suffered critical illness or death to determine under what circumstances—if any—nontherapeutic research with critically ill children is perceived to be acceptable.
Research with recently deceased patients
Filling some knowledge gaps identified by the guideline development group will require studies involving recently deceased patients.2 For example, most recommendations regarding ancillary tests were based on studies that did not include children; the validity of these tests for neonates and pediatric patients is unknown.2 Studies involving nonindicated ancillary tests following clinical determination of death by neurologic criteria may therefore be undertaken to assess the sensitivity of ancillary tests in children.
Ethical issues
Dedicated Canadian guidelines for research with the deceased are lacking. While Canadian research ethics guidelines suggest that deceased patients involved in research are research participants and owed the full range of standard protection,11 there are compelling reasons to dispute this. The dead are not moral persons. They are neither vulnerable nor subject to welfare harms. It is therefore unclear what would underpin requirements such as risk minimization. Indeed, the contention that deceased patients are research participants is out of step with influential guidelines from jurisdictions that stipulate only living persons meet the criteria for participant status.4
Nevertheless, there are independent reasons to afford deceased patients some protection.8,20 For example, doing so reflects the dignity accorded human bodies, shows respect for the deceased and their family, and maintains public trust in research.8 Such reasoning suggests that one credible rationale supporting protection for the deceased is to mitigate effects on the living.4
Available guidance
To our knowledge, there are no authoritative international guidelines for whole-body research with recently deceased study populations. That said, the high-level recommendations issued by the North American Consensus Panel on Research with the Recently Dead are likely to be useful to Canadian researchers.20 Importantly, while the panel rightly emphasizes that “cadavers should be treated in a manner that is consistent with respect for the value and dignity of the once-living person,” it also acknowledges that research procedures “need not be identical to those used with the living.”20 This observation highlights the uniqueness of research in this domain and suggests that what constitutes an ethically permissible intervention may be determined by a calculus different than that used for living participants.
In the absence of dedicated Canadian guidelines for research with the recently deceased, researchers should adhere to stipulations in Canada’s regulatory framework for the time being by affording the deceased participant protections.11 Involving family partners and health care providers in study design will help to ensure that research procedures are sensitive to the needs of recently bereaved families.2
Roadmap
The ethics of research with the recently deceased currently lack a sound foundation, meaning that—here again—dedicated Canadian guidelines are needed. Further thinking is required to establish what protection the deceased and their families are owed in the context of postmortem research. Sensible measures may include scientific oversight, confidentiality protection, clear protocols for storage of biological materials (where appropriate), an approved plan for final disposition of remains, and surrogate authorization to ensure that the use of the body is compatible with the deceased’s values.8,20 Other protection, such as research ethics committee oversight and risk minimization, should be explored, but it is currently unclear what would justify its applicability to this setting.
Conclusion
Strengthening the evidence base supporting guidelines for death determination in Canada requires studies that test existing research ethics frameworks. Although research with imminently dying and recently deceased populations is ethically justifiable, further discussion and debate are warranted. Indeed, given the importance of public trust for the research enterprise, it is advisable to approach research with the imminently dying and recently deceased cautiously. While some relevant guidance is available,7,8,10,20 dedicated Canadian guidelines for research with these populations are urgently needed. In the interim, we encourage researchers undertaking studies with these populations to reflect on the above considerations when designing study protocols, and to consult the references cited in this article for further nuance.
Acknowledgments
Author contributions
Nicholas B. Murphy, Charles Weijer, Saptharishi Lalgudi Ganesan, Marat Slessarev, Sonny Dhanani, and Teneille Gofton conceived the project. Nicholas B. Murphy authored the first and all subsequent drafts of the manuscript. All authors critically reviewed and revised each draft of the manuscript for important intellectual content.
Acknowledgments
The authors thank Sam Shemie and Lindsay Wilson for their support throughout the development of this manuscript. They also thank two anonymous reviewers for their constructive comments and suggestions.
Disclosures
Marat Slessarev reports salary support from Trillium Gift of Life Network. Charles Weijer receives consulting income from Cardialen, Eli Lilly & Company, and Research Triangle Institute (RTI) International. All other authors have nothing to disclose.
Funding statement
This work was conducted as part of the project entitled, “A Brain-Based Definition of Death and Criteria for its Determination After Arrest of Circulation or Neurologic Function in Canada,” made possible through a financial contribution from Health Canada through the Organ Donation and Transplantation Collaborative and developed in collaboration with the Canadian Critical Care Society, Canadian Blood Services, and the Canadian Medical Association. The views expressed herein do not necessarily represent the views of Health Canada, the Canadian Critical Care Society, Canadian Blood Services, or the Canadian Medical Association.
Editorial responsibility
This submission was handled by Dr. Maureen Meade, Guest Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shemie SD, Wilson LC, Hornby L, et al. A brain-based definition of death and criteria for its determination after arrest of circulation or neurologic function: a 2023 Clinical Practice Guideline. Can J Anesth. 2023 doi: 10.1007/s12630-023-02431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maitre G, Shemie SD, Baker A, et al. Knowledge gaps in the definition and determination of death. Can J Anesth. 2023 doi: 10.1007/s12630-023-02422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. The Belmont report: ethical principles and guidelines for the protection of human subjects of research, 1979. Available from URL: https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html (accessed December 2022).
- 4.Martin DE, Cronin AJ, Dalle Ave A, et al. Addressing ethical confusion in deceased donation and transplantation research: the need for dedicated guidance. Transpl Int. 2021;34:2459–2468. doi: 10.1111/tri.14108. [DOI] [PubMed] [Google Scholar]
- 5.Eyal N. Risk to bystanders in clinical trials: a symposium. Clin Trials. 2019;16:447–449. doi: 10.1177/1740774519862758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Beinum A, Hornby L, Dhanani S, Ward R, Chambers-Evans J, Menon K. Feasibility of conducting prospective observational research on critically ill, dying patients in the intensive care unit. J Med Ethics. 2017;43:47–51. doi: 10.1136/medethics-2016-103683. [DOI] [PubMed] [Google Scholar]
- 7.Murphy N, Weijer C, Debicki D, et al. Ethics of non-therapeutic research on imminently dying patients in the intensive care unit. J Med Ethics. 2022 doi: 10.1136/medethics-2021-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicclair MR, DeVita M. Oversight of research involving the dead. Kennedy Inst Ethics J. 2004;14:143–164. doi: 10.1353/ken.2004.0025. [DOI] [PubMed] [Google Scholar]
- 9.Dhanani S, Hornby L, van Beinum A, et al. Resumption of cardiac activity after withdrawal of life-sustaining measures. N Engl J Med. 2021;384:345–352. doi: 10.1056/nejmoa2022713. [DOI] [PubMed] [Google Scholar]
- 10.Luce JM, Cook DJ, Martin TR, et al. The ethical conduct of clinical research involving critically ill patients in the United States and Canada: principles and recommendations. Am J Respir Crit Care Med. 2004;170:1375–1384. doi: 10.1164/rccm.200406-726st. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada. Tri-Council policy statement: ethical conduct for research involving humans, 2018. Available from URL: https://ethics.gc.ca/eng/policy-politique_tcps2-eptc2_2018.html (accessed December 2022).
- 12.Weijer C. The ethical analysis of risk in intensive care unit research. Crit Care. 2004;8:85–86. doi: 10.1186/cc2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapoport A. Addressing ethical concerns regarding pediatric palliative care research. Arch Pediatr Adolesc Med. 2009;163:688–691. doi: 10.1001/archpedi.163.8.688. [DOI] [PubMed] [Google Scholar]
- 14.Weaver MS, Mooney-Doyle K, Kelly KP, et al. The benefits and burdens of pediatric palliative care and end-of-life research: a systematic review. J Palliat Med. 2019;22:915–926. doi: 10.1089/jpm.2018.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinds PS, Burghen EA, Pritchard M. Conducting end-of-life studies in pediatric oncology. West J Nurs Res. 2007;29:448–465. doi: 10.1177/0193945906295533. [DOI] [PubMed] [Google Scholar]
- 16.Fine PG. Maximizing benefits and minimizing risks in palliative care research that involves patients near the end of life. J Pain Symptom Manage. 2003;25:S53–62. doi: 10.1016/s0885-3924(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 17.October TW, Fisher KR, Feudtner C, Hinds PS. The parent perspective: “being a good parent” when making critical decisions in the PICU. Pediatr Crit Care Med. 2014;15:291–298. doi: 10.1097/pcc.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett RA, LeBaron VT. Parental perspectives on roles in end-of-life decision making in the pediatric intensive care unit: an integrative review. J Pediatr Nurs. 2019;46:18–25. doi: 10.1016/j.pedn.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care. 2002;40:V55–63. doi: 10.1097/01.mlr.0000023956.25813.18. [DOI] [PubMed] [Google Scholar]
- 20.Pentz RD, Cohen CB, Wicclair M, et al. Ethics guidelines for research with the recently dead. Nat Med. 2005;11:1145–1149. doi: 10.1038/nm1105-1145. [DOI] [PubMed] [Google Scholar]