Abstract
Radionuclide perfusion studies have an established ancillary role in determination of death by neurologic criteria (DNC). While critically important, these examinations are not well understood by individuals outside of the imaging specialties. The purpose of this review is to clarify relevant concepts and nomenclature and provide a lexicon of relevant terminology of value to non-nuclear medicine practitioners who wish to better understand these examinations. Radionuclides were first employed to evaluate cerebral blood flow in 1969. Radionuclide DNC examinations that use lipophobic radiopharmaceuticals (RPs) entail a flow phase followed immediately by blood pool images. On flow imaging, presence of intracranial activity within the arterial vasculature is scrutinized following arrival of the RP bolus into the neck. Lipophilic RPs designed for functional brain imaging were introduced to nuclear medicine in the 1980s and were engineered to cross the blood–brain–barrier and be retained in the parenchyma. The lipophilic RP 99mTc-hexamethylpropyleneamine oxime (99mTc-HMPAO) was first used as an ancillary investigation in DNC in 1986. Examinations using lipophilic RPs entail both flow and parenchymal phase images. According to some guidelines, parenchymal phase uptake should be assessed by tomographic imaging, while other investigators consider simple planar imaging sufficient. Findings of perfusion on either the flow or parenchymal phase of the examination effectively precludes DNC. If the flow phase is omitted or somehow compromised, the parenchymal phase remains sufficient for DNC. A priori, parenchymal phase imaging is superior to flow phase imaging for several reasons and lipophilic RPs are favoured over lipophobic RPs in that both flow and parenchymal phase imaging are performed. Disadvantages of lipophilic RPs are increased cost and the need to procure them from a central laboratory, which can prove difficult, especially outside usual working hours. According to most current guidelines, both lipophilic and lipophobic RP categories are acceptable for use in ancillary investigations in DNC, with a growing overt preference for studies using the lipophilic RPs based on their ability to capture the parenchymal phase. The new adult and pediatric Canadian recommendations favour use of lipophilic RPs to variable degrees, specifically 99mTc-HMPAO, the lipophilic moiety which has undergone the greatest validation. Although ancillary use of radiopharmaceuticals is quite settled in multiple DNC guidelines and best practices, several areas of further research remain open to investigation. Examens auxiliaires de perfusion nucléaire pour la détermination du décès selon des critères neurologiques : méthodes, interprétation et lexique—un guide de l’utilisateur à l’intention du clinicien
Keywords: ancillary investigation, brain death, death by neurologic criteria, death determination, radionuclide imaging, scintigraphy
Résumé
Les examens de la perfusion nucléaire jouent un rôle auxiliaire bien établi dans la détermination du décès selon des critères neurologiques (DCN). Bien qu’ils soient d’une importance cruciale, ces examens ne sont pas bien compris par les personnes en dehors des spécialités d’imagerie. Le but de cette revue est de clarifier les concepts et la nomenclature pertinents et de fournir un lexique de terminologie pertinente utile aux praticiens non spécialisés en médecine nucléaire qui souhaitent mieux comprendre ces examens. Les radionucléides ont été utilisés pour la première fois pour évaluer la circulation sanguine cérébrale en 1969. Les examens de DCN par radionucléides qui utilisent des produits radiopharmaceutiques (RP) lipophobes impliquent une phase de circulation suivie immédiatement d’images de pool sanguin. Sur l’imagerie en circulation, la présence d’une activité intracrânienne dans le système vasculaire artériel est examinée après l’arrivée du bolus de RP dans le cou. Les RP lipophiles conçus pour l’imagerie cérébrale fonctionnelle ont été introduits en médecine nucléaire dans les années 1980 et ont été conçus pour franchir la barrière hémato-encéphalique et être retenus dans le parenchyme. Le RP lipophile 99mTc-hexaméthylpropylèneamine-oxime (99mTc-HMPAO) a été utilisé pour la première fois comme examen auxiliaire pour le DCN en 1986. Les examens utilisant des RP lipophiles impliquent à la fois des images de circulation et de phase parenchymateuse. Selon certaines lignes directrices, l’absorption durant la phase parenchymateuse devrait être évaluée par imagerie tomographique, tandis que d’autres chercheurs considèrent qu’une imagerie planaire simple suffit. Les résultats de perfusion sur la phase de circulation ou la phase parenchymateuse de l’examen excluent effectivement un DCN. Si la phase de circulation est omise ou compromise d’une manière ou d’une autre, la phase parenchymateuse reste suffisante pour établir un DCN. A priori, l’imagerie en phase parenchymateuse est supérieure à l’imagerie en phase de circulation pour plusieurs raisons et les RP lipophiles sont privilégiés par rapport aux RP lipophobes parce que l’imagerie en circulation et en phase parenchymateuse sont toutes deux réalisées. Les inconvénients des RP lipophiles sont l’augmentation des coûts et la nécessité de les obtenir auprès d’un laboratoire central, ce qui peut s’avérer difficile, surtout en dehors des heures de travail habituelles. Selon la plupart des lignes directrices actuelles, les catégories de RP lipophiles et lipophobes sont toutes deux acceptables pour une utilisation dans les examens auxiliaires pour un DCN, avec une préférence manifeste croissante pour les études utilisant les RP lipophiles en fonction de leur capacité à capturer la phase parenchymateuse. Les nouvelles recommandations canadiennes pour adultes et enfants privilégient l’utilisation de RP lipophiles à des degrés variables, en particulier le 99mTc-HMPAO, le fragment lipophile qui a subi la plus grande validation. Bien que l’utilisation auxiliaire des produits radiopharmaceutiques soit tout à fait établie dans de multiples lignes directrices et meilleures pratiques de DCN, plusieurs domaines de recherche supplémentaires restent ouverts à l’étude.
Blood flow studies have an important role to play in the determination of death by neurologic criteria (DNC), a condition commonly referred to as “brain death.” As codified in many guidelines and best-practices,1–4 ancillary investigation1 becomes essential to DNC when the clinical exam cannot be completed in its entirety. Examples where ancillary investigation is essential include inability to perform apnea testing in an unstable patient, presence of confounding factors such as hypothermia or sedative medications, or situations where brainstem reflexes cannot be examined because of physical injury. Ancillary investigation may also be useful for social reasons, such as allowing family members to better comprehend DNC.5,6 Radionuclide perfusion2 studies offer a unique combination of ease of performance, accuracy, and a relatively high degree of validation, making them amongst the most recommended and preferred modalities in many guidelines.1–4
While radionuclide studies are therefore well-established, details regarding these examinations, especially differences between the two major categories of studies, are not well understood by individuals outside of the imaging specialties. Not infrequently, procedures that use lipophilic radiopharmaceuticals (RPs) are conflated with those that use lipophobic RPs, in spite of markedly disparate criteria of interpretation,7,8 which can result in misunderstanding or error.
The purpose of the present narrative review is therefore to address this knowledge gap by concisely clarifying the concepts and nomenclature relating to the nuclear medicine studies performed in the context of DNC. Terms used in the following discussion are further elucidated in a lexicon presented in Table 1. Readers unfamiliar with nuclear medicine terminology may find this aid beneficial when reviewing this and other manuscripts.
Table 1.
Lexicon of terms relevant to radionuclide studies of brain perfusion
| Term | Explanation |
|---|---|
| Absorbed dose | The amount of energy deposited by ionizing radiation in materials through which they pass, measured in units of rad (radiation-absorbed dose) or gray. |
| Activity | When used in the context of radionuclides, a measure of amount of a radioactive substance based on its rate of decay, as in millicurie or megabecquerel. |
| Anger camera | A specific design of gamma camera, eponymously named for the inventor Hal Anger. |
| Angiographic | A series of dynamic images designed to portray flow within vessels. |
| Blood pool | Distribution of activity within the vascular system following initial mixing and dilution of RP within the intravascular volume. |
| Blush | Nonspecific visualization of activity within a tissue on flow or blood pool imaging. |
| Bolus | As in "RP bolus." A concentration of material that when administered will introduce a relatively brief perturbation of the system. |
| Cine-loop | A method of displaying dynamic images as a short repeating image loop ("movie"). |
| Diffusible | See lipophilic. |
| Dosage | An amount of a medicine or RP. Radioactive substances administered to patients are measured in units of millicurie or megabecquerel. |
| Dose | Short for “absorbed dose.” See “absorbed dose.” |
| Dynamic | As in "dynamic imaging." Acquisition of a number of images at fixed time intervals to portray the change in distribution of an RP over time. |
| Flow phase | In brain imaging, referring to a dynamic series of images with a 1- or 2-second time base portraying the intravascular flow of blood. |
| Gamma (emission) | Referring to the emission of gamma particles, photons of energy that originate in the nuclei of radioactive atoms upon their radioactive decay. |
| Gamma-camera | A generic term for imaging devices used to spatially map the distribution of gamma-emitting radionuclides. |
| Lipophilic | Category of RPs that can freely diffuse across cell membranes such as the blood brain barrier. |
| Lipophobic | Category of RPs that cannot freely diffuse across cell membranes in the blood brain barrier, also known as "nonlipophilic." |
| Megabecquerel (MBq) | Système International measurement of radioactive material related to the number of disintegrations per second. One becquerel (Bq) represents a rate of radioactive decay equal to 1 disintegration per second. |
| Millicurie (mCi) | A common measurement of radioactive material which predates adoption of the becquerel. One curie is equal to 37 billion (3.7 × 1010) disintegrations per second. |
| Nondiffusible | See lipophobic. |
| Nonlipophilic | See lipophobic. |
| Nuclear medicine | The discipline of medicine involved in use of unsealed sources of radioactivity to diagnose and treat disease. |
| Parenchymal phase | Referring to the delayed phase of brain imaging with lipophilic RPs following diffusion of the RP into cells and trapping within the cytoplasm. |
| Particulate emission | Emission of alpha, beta, or neutron particles in the course of radioactive decay. |
| Perfuse | To force blood or other fluid (1) to flow from the artery through the vascular bed of a tissue or (2) to flow through the lumen of a hollow structure. |
| Planar | As in "planar imaging." Referring to a 2-dimensional projection of activity from the subject being imaged onto an external detector. |
| Projection (as in "anterior projection") | Angular positioning of the radiation detector with respect to the subject being imaged. |
| Radioactivity | The release of energy by unstable nuclei. |
| Radionuclide | A radioactive nuclide. |
| Radionuclide angiography | Series of dynamic scintigraphic images designed to portray flow within vessels. |
| Radiopharmaceutical (RP) | A radioactive compound administered for diagnostic or therapeutic purposes. |
| Radiotracer | Colloquial term for RP. See RP. |
| Scalp tourniquet | Application of a tight band around the supraorbital skull to minimize scalp flow. |
| Scintigraphy | The method of producing images based on the spatial distribution of radioactive materials. |
| SPECT | Acronym for “Single Photon Emission Computer Tomography,” a method of tomography used in radionuclide imaging. |
| Tomography | A method of generating 3-dimensional images from 2-dimensional planar images. |
| Tracer | Short form of “radiotracer.” See “radiotracer.” |
RP = radiopharmaceutical
Radionuclide studies
Historically, the original blood flow examination performed as an ancillary investigation for DNC was the four-vessel angiogram. This radiographic procedure was technically demanding, entailed sequential injection of iodinated contrast into four arteries with potential for nephrotoxicity, and carried risk of vascular and thromboembolic complications. Flow imaging using radionuclides was first employed to evaluate cerebral blood flow in 1969.7 A compact bolus of activity is injected and rapidly flushed into a vein while a sequence of one- to two-second flow images, typically in anterior projection, is recorded immediately thereafter as the activity reaches the carotid circulation, emulating angiographic imaging, but avoiding administration of iodinated contrast. It is desirable to have the bolus enter the venous bloodstream as close to the heart as possible to preserve concentration of the activity,9 though injection via a large-bore peripheral intravenous line can also suffice. Some authors apply a tightly fitting band (“scalp tourniquet”) around the supraorbital skull to minimize confounding activity in the scalp circulation.8 Images are reviewed as a series of serial frames or displayed as a short dynamic movie (“cine-loop”) (Fig. 1). “Blood pool” imaging, a relatively dispensable component of the examination, is acquired immediately following the flow images and shows the distribution of activity within the vascular compartment, after initial mixing and dilution of the RP within the systemic blood volume. This portrays blood primarily within the more capacious venous sinuses.
Fig. 1.
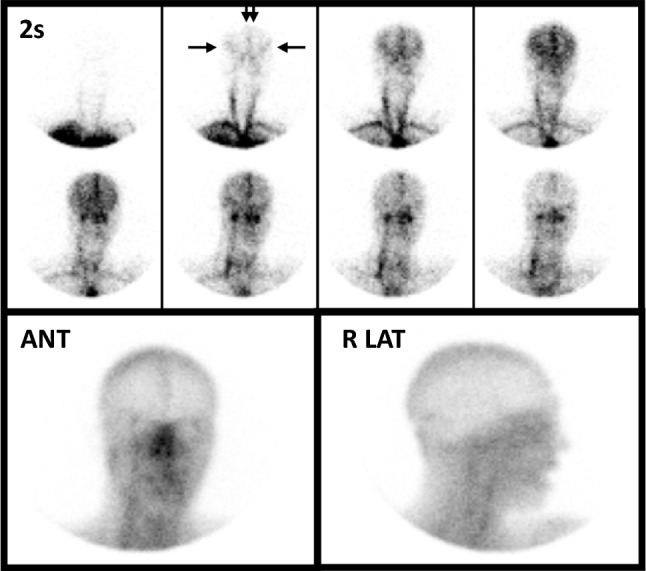
Normal lipophobic perfusion study using 99mTc-DTPA in a young man following a ruptured aneurysm of the left posterior inferior cerebral artery. Eight sequential two-second flow images appear in the upper panel and show excellent visualization of the common carotid arteries as well as the anterior (double vertical arrows) and middle (horizontal arrows) cerebral arteries, forming a “trident” appearance, which indicates presence of intracranial perfusion. The activity progresses to a transient blush of activity within the parenchyma followed by visualization of the intracranial venous sinuses on the final flow images. On immediate static images (lower row) in anterior (ANT) and right lateral (R LAT) projections, activity is noted in the venous sinuses but there is no localization within the brain parenchyma—an expected and normal finding when imaging lipophobic RPs. This Figure was originally published by author L. S. Z. in the Journal of Nuclear Medicine (Zuckier LS. Radionuclide evaluation of brain death in the post-McMath era. J Nucl Med 2016; 57: 1560–8, © SNMMI) and is reproduced in accordance with applicable Authors’s Permission to Reprint (https://jnm.snmjournals.org/page/permissions [accessed October 2022])
The initial lipophobic RPs employed were various 99mTc-labelled imaging agents in common use during the 1970s, which were conveniently co-opted for brain flow imaging (Table 2). These RPs are labelled with 99mTc-technetium, a nearly ideal radionuclide with an easily imaged gamma-emission, a relatively short physical half-life, and absence of injurious particulate emissions. While the six-hour half-life minimizes long-term exposure of radiation within the patient, it does represent a logistic challenge in that RPs have to be constituted close to the time of use, precluding any day-to-day storage. Renal imaging agents were often preferred because of their rapid clearance from the blood into the urine, and lack of localization in the head and neck, making them ideal if repeat imaging and reinjection was required. Imaging was typically performed in the nuclear medicine suite, although some facilities employed portable scintigraphic cameras that allowed planar imaging to take place at the bedside, obviating the need to move a ventilated patient to the imaging area.
Table 2.
99mTc‐based radiopharmaceuticals used for ancillary investigation in the determination of death by neurologic criteria
| Lipophobic radiopharmaceutical | Lipophilic radiopharmaceutical | |
|---|---|---|
| Radiopharmaceutical examples (original imaging purpose) |
99mTc‐DTPA (renal) 99mTc‐GHA (renal) 99mTc‐pertechnetate (thyroid) |
99mTc‐HMPAO (functional brain) 99mTc‐ECD (functional brain) |
| Flow (angiographic) phase# | Primary diagnostic role | Confirmatory, nonmandatory, diagnostic role |
| Immediate (blood pool) phase# | Minimally contributory role* | Noncontributory |
| Parenchymal (delayed uptake) phase~ | Not applicable | Primary diagnostic role |
| Criteria necessary to support DNC | Nonvisualization of intracranial flow | Absent parenchymal uptake in brain and brainstem and absent intracranial flow (if performed) |
| Conceptual and diagnostic limitations |
Posterior fossa/brainstem not well‐visualized on anterior flow images 1‐ or 2‐second images are low count and statistically noisy, obscuring signal Specificity for DNC not adequately characterized |
Brainstem not well‐visualized, especially on planar imaging. Use in premature and young infants limited Specificity for DNC not adequately characterized |
#By planar imaging only
*Nonvisualization of venous sinuses supports diagnosis of DNC while visualization of venous sinuses is nonspecific
~By planar or tomographic (SPECT) imaging
DNC = determination of death by neurologic criteria; DTPA = diethylenetriaminepentaacetic acid (pentetate); ECD = ethyl cysteinate dimer; GHA = glucoheptonate; HMPAO = hexamethylpropyleneamine oxime; SPECT = single photon emission computed tomography
Lipophilic RPs designed for functional brain imaging were introduced to nuclear medicine in the 1980s.10–12 These RPs were engineered to cross the blood brain barrier and be retained in the parenchyma, to a degree proportional to regional blood flow. An early lipophilic RP was N-isopropyl-p-[123I] iodoamphetamine (123I-IMP),13 which was labelled with 123I-iodine and enjoyed a brief period of use prior to being effectively eclipsed by the superior 99mTc-technetium labelled alternatives.14 There are two 99mTc-based lipophilic RPs currently used in functional brain imaging: 99mTc-exametazime (99mTc-hexamethylpropyleneamine oxime [99mTc- HMPAO]) and the subsequently introduced 99mTc-bicisate dihydrochloride (99mTc-ethyl cysteinate dimer [99mTc-ECD]). Although both passively cross the blood brain barrier and become fixed in the brain parenchyma, the biochemical mechanisms of localization differ; retention of 99mTc-ECD is dependent on the presence of intracellular esterases, whereas 99mTc- HMPAO requires only intracellular glutathione.15 For these reasons, minor differences in biodistribution and behaviour between these RPs have been described under given circumstances. For functional evaluation of the brain, such as the differentiation of dementing processes or localization of epileptogenic foci, imaging is best performed using a tomographic technique (single photon emission computer tomography [SPECT]) to portray localization of uptake in specific brain regions (Fig. 2). With current technology, brainstem uptake of lipophilic RPs remains poorly resolved on either planar or tomographic (SPECT) imaging.
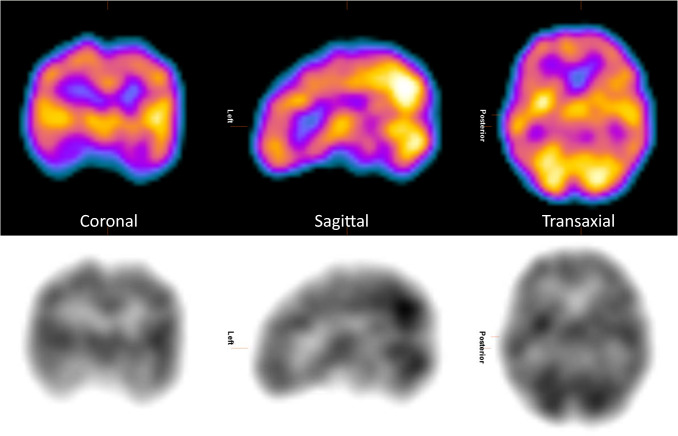
Fig. 2.
Example of functional tomographic brain imaging following intravenous injection of the lipophilic RP 99mTc-ECD. The reconstructed three-dimensional distribution of RP activity has been displayed in coronal, sagittal, and transaxial planes, in both colour (upper row) and black and white (lower role) greyscales. While tomographic imaging is standard for functional imaging of the brain, opinions vary whether it is required for determination of death by neurologic criteria.
The lipophilic RP 99mTc-HMPAO was first used in DNC in 198616 and validation studies have almost exclusively been performed with this agent. Some guidelines recommend performing tomographic SPECT imaging in DNC, while other investigators consider simple planar imaging sufficient to assess presence or absence of intracranial activity. The downside of SPECT imaging is that it requires a more protracted acquisition period in the imaging department, which may subject the patient to potential risks inherent in leaving the intensive care unit for a greater period of time.
In clinical practice, lipophilic RPs are typically injected as a bolus to enable acquisition of angiogram-like flow phase images as an additional, secondary component of the examination (Fig. 3) (Table 2). Following visualization of the vessels, activity will be noted to immediately accumulate in the brain parenchyma. In most published reports evaluating 99mTc-HMPAO as an ancillary blood flow investigation, emphasis is on the parenchymal phase though in some instances, findings on both flow and parenchymal phase imaging have been separately reported.17 An advantage of flow phase over parenchymal imaging is its independence from RP quality control issues. Because the flow phase does not depend on particular binding or metabolism of the injected RP, degradation or substitution of the RP with a compromised moiety would not affect integrity of the angiographic visualization of flow, though it could conceivably affect parenchymal uptake. At one time, 99mTc-HMPAO was chemically unstable and required administration within 30 min of constitution prior to degradation; presence of the flow phase was desirable as it independently confirmed absent perfusion in DNC. Current commercial kits are stabilized with a reducing agent, which has obviated this concern.18 Nonetheless, congruent findings on the flow phase of the examination are a welcome independent confirmation in DNC when no uptake is detected on the parenchymal phase.
Fig. 3.
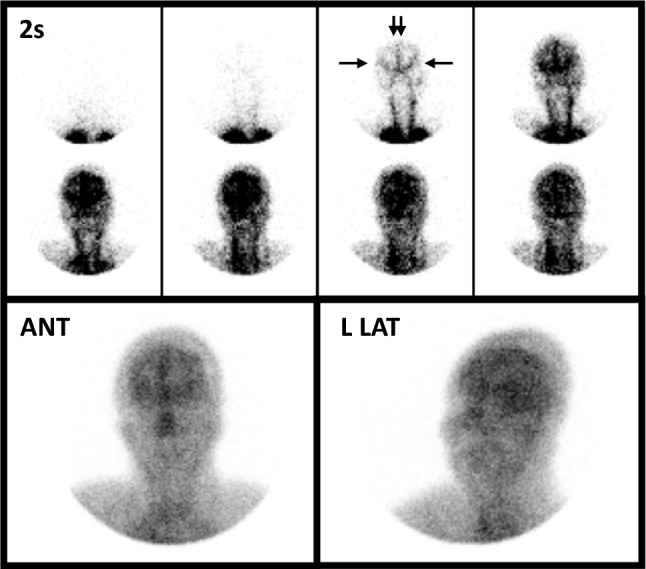
Normal lipophilic perfusion study in a young man status-post motor vehicle accident with severe head trauma, showing abundant intracranial perfusion. The eight sequential two-second flow images show excellent visualization of the common carotid arteries as well as the anterior (double vertical arrows) and middle (horizontal arrows) cerebral arteries, forming a “trident” appearance. Activity immediately localizes in the brain parenchyma. On delayed parenchymal phase imaging in anterior (ANT) and left lateral (L LAT) projections, there is extensive though somewhat inhomogeneous localization within the brain. This Figure was originally published by author L. S. Z. in the Journal of Nuclear Medicine (Zuckier LS. Radionuclide evaluation of brain death in the post-McMath era. J Nucl Med 2016; 57: 1560–8, © SNMMI) and is reproduced in accordance with applicable Authors’s Permission to Reprint (https://jnm.snmjournals.org/page/permissions [accessed October 2022])
Interpretation of radionuclide studies
Overview
Examinations that use lipophobic RPs entail acquisition of the flow phase followed immediately by blood pool imaging, the latter actually having minimal if any bearing on diagnosis. In contrast, examinations based on lipophilic RPs include both flow and parenchymal phase images, both of which are important to the diagnosis (Table 2). When adequately performed, both phases must be compatible with absent perfusion for the ancillary investigation to support DNC. Nonetheless, if the dynamic phase is omitted or somehow compromised, the parenchymal phase remains sufficient for DNC. The parenchymal phase is believed to be more sensitive for detection of intracranial perfusion and is sufficiently robust to stand on its own.
Flow phase criteria
Essence of the blood flow phase is evaluation of intracranial activity within arterial vasculature following arrival of an adequate bolus of activity into the neck, typically achieved by confirming visualization of activity within the common and external carotid arteries (Figs. 1 and 4). In a patient with normal intracranial flow, the paired midline anterior cerebral arteries and laterally situated middle cerebral arteries appear as a three-pronged “trident-”like structure. One may see a transient “blush” of activity in the brain parenchyma as the RP transits from the larger arteries into the capillaries; however, this finding is variable and is not necessary for documenting the presence of perfusion. If arrival of the bolus into neck vessels is not apparent, the study cannot be regarded as adequate for documenting lack of internal carotid flow. Visualization of any of the anterior cerebral, middle cerebral, or even other smaller intracranial arteries is sufficient to indicate presence of intracranial blood flow, thereby excluding DNC.
Fig. 4.
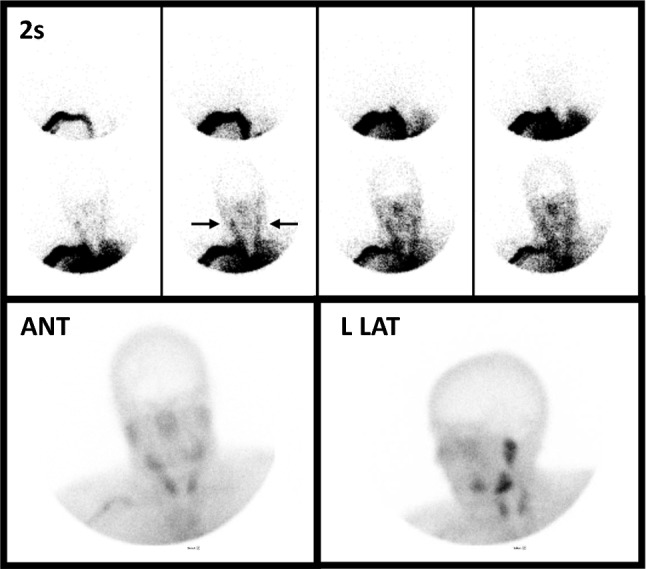
Abnormal lipophobic perfusion study using 99mTc-pertechnetate in a young woman with diffuse cerebral edema due to hypoxic ischemic injury following hanging. The eight sequential two-second flow images show excellent visualization of the common carotid arteries (horizontal arrows) without visualization of flow into the internal carotid circulation. On immediate static images, there is absent visualization of activity within the venous sinuses. Lipophobic RPs do not normally localize within the brain parenchyma, rendering the anterior (ANT) and left lateral (L LAT) delayed images of limited value. Pertechnetate, an RP used for thyroid imaging, localizes in the salivary glands and thyroid, which are visible overlying the lower face and neck. This Figure was originally published by author L. S. Z. in the Journal of Nuclear Medicine (Zuckier LS. Radionuclide evaluation of brain death in the post-McMath era. J Nucl Med 2016; 57: 1560–8, © SNMMI) and is reproduced in accordance with applicable Authors’s Permission to Reprint (https://jnm.snmjournals.org/page/permissions [accessed October 2022])
Parenchymal phase criteria
Lipophilic RPs normally accumulate within the brain parenchyma, which can be assessed several minutes following completion of the flow phase. Evaluation of parenchymal uptake can be performed by either planar or tomographic technique; when tomographic imaging is not obtained, guidelines recommend obtaining planar imaging at several different projections.8,19 Lateral views are considered especially important to evaluate the posterior fossa, which is not clearly discernable on anterior views.20 Detection of any parenchymal uptake precludes DNC as it reflects perfusion of the brain (Figs. 3 and 5).
Fig. 5.
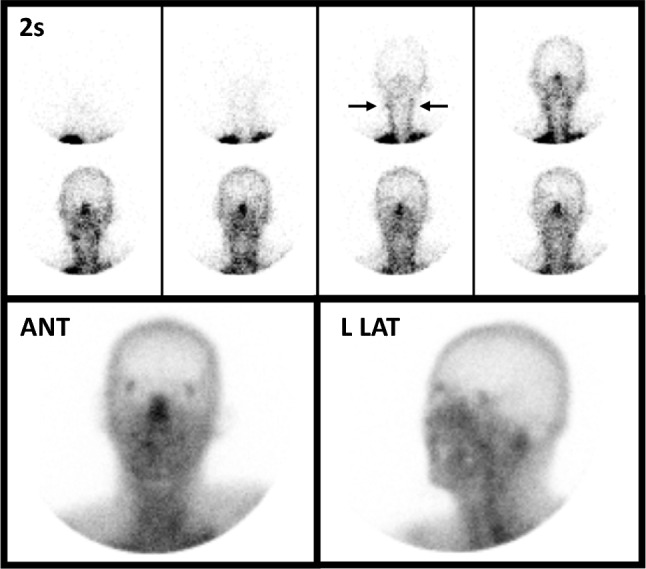
Abnormal lipophilic perfusion study using 99mTc-HMPAO in a young man status post hanging. There is excellent visualization of the common carotid arteries (horizontal arrows); however, no intracranial perfusion in present, as evidenced by absent flow (upper eight images) and lack of parenchymal uptake (ANT and L LAT views). Lack of perfusion within the boney skull has been dubbed the “light-bulb” or “hollow-skull” sign. Prominence of the midface is noted on anterior view (termed the “hot nose” sign); however, this finding is not specific for determination of death by neurologic criteria and therefore is potentially misleading.21 This figure was originally published by author L. S. Z. in the Journal of Nuclear Medicine (Zuckier LS. Radionuclide evaluation of brain death in the post-McMath era. J Nucl Med 2016; 57: 1560–8, © SNMMI) and is reproduced in accordance with applicable Authors’s Permission to Reprint (https://jnm.snmjournals.org/page/permissions [accessed October 2022])
Hot nose sign
A finding in ancillary radionuclide studies that has been reported in the context of DNC is presence of relatively prominent perfusion in the centre of the face, imaginatively coined the “hot nose” sign.21,22 There is discussion as to whether the activity ascribed to the nose is actually located more posterior thereof.23 Of clinical relevance, this sign is neither sensitive nor specific for DNC, and is therefore best ignored.24
Sagittal sinus visualization
While early authors had suggested that activity within the sagittal sinus implies presence of intracranial circulation, the predominant opinion today is that these may fill from scalp perfusion and do not necessarily imply presence of intracranial blood flow.25–28 Absent venous sinus activity is abnormal but specificity of this finding for complete absence of brain perfusion is uncertain.
Relative advantages of the radionuclide imaging agents
A priori, parenchymal phase imaging is superior to flow phase imaging for several reasons. Perfusion of the brain can be assessed in a static manner, allowing more prolonged acquisition and superior counting statistics, which is more sensitive for detecting small amounts of activity than statistically noisy one- or two-second flow images. A fixed distribution activity, as imaged during the parenchymal phase, is also more forgiving of technical faults—if additional views are requested, or if a technical camera blunder occurs, repeat imaging can be performed without administration of additional material, which is not true of dynamic flow imaging.15 The fixed nature of the parenchymal phase also permits imaging in multiple obliquities to enable clarification of ambiguous findings or acquisition of tomographic SPECT images. For these reasons, lipophilic RPs, which capture parenchymal phase uptake in addition to flow, are diagnostically superior to flow-only lipophobic RPs. Disadvantages of lipophilic RPs include increased cost, and the need to procure them at a central laboratory, which can prove difficult especially after usual working hours.
According to most current guidelines, both lipophilic and lipophobic categories of RPs are acceptable for use as ancillary investigations in DNC, with a growing overt preference for studies using the lipophilic RPs based on their ability to capture the parenchymal phase. According to guidelines released in 2012 by the Society of Nuclear Medicine and Molecular Imaging, lipophilic RPs are “increasing in popularity” but there is “no clear evidence that they are more accurate.”8 Joint guidelines issued by the American College of Radiology, the American College of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging and the Society of Pediatric Radiology in 2021 have stated that either 99mTc- ECD or 99mTc-HMPAO are preferred while 99mTc-DTPA or 99mTc-pertechnetate may be used if the former are not available.19 The Belgian Society of Nuclear Medicine guidelines suggest that 99mTc-HMPAO should be favoured over 99mTc-ECD, which reflects the fact that the latter has been less validated in the medical literature.29 In an earlier clinical guideline, the American Academy of Neurology has referred to only 99mTc-HMPAO30 while a more recent update references “nuclear scans” as an ancillary investigation.1 According to the World Brain Death Project, if scintigraphic techniques are used as an alternative to digital subtraction angiography, then lipophilic radiopharmaceuticals should be preferentially used, optimally with tomographic imaging.3
Our current systematic review in pediatric patients indicated a preference for lipophilic RPs, specifically 99mTc-HMPAO, while 99mTc-ECD was believed to be similar by extension, though not validated. Lipophobic RPs, such as 99mTc-DTPA or 99mTc-GHA, were considered acceptable if the “first line” lipophilic RPs were not available.31 The systematic review in adult patients also favoured 99mTc-HMPAO; however, the lipophobic RPs were not recommended because of much greater statistical uncertainty in the specificity estimate for the flow phase studies compared with parenchymal phase evaluation.32
Topics requiring further elucidation
Areas that require further elucidation include accuracy of 99mTc-ECD, which has not been adequately studied in this context. In addition, all the measures of intracranial perfusion do not have well defined specificities for determination of DNC because these patients are simply not studied unless their clinical presentation is completely in concert with DNC. Effectiveness of these tests in premature and young infants with open fontanelles and unfused bones is questionable because of the frequent persistence of minimal blood flow in the unfused skull. Finally, thresholds of minimal detectable perfusion have never been determined for scintigraphic studies using either lipophobic or lipophilic radiopharmaceuticals, though these parameters are basic to their interpretation.33
Acknowledgments
Author contributions
Nicole K. McKinnon and Lionel S. Zucker contributed to the manuscript and the revised manuscript.
Disclosures
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Helen Opdam, Guest Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Footnotes
In this article, adjunct examinations used to complement an incomplete physical examination have been termed ancillary investigations, in concert with the usage and glossary of terms contained within the report Shemie SD, Wilson LC, Hornby L, et al. A brain-based definition of death and criteria for its determination after arrest of circulation or neurologic function in Canada: a 2023 Clinical Practice Guideline. Can J Anesth 2023; 10.1007/s12630-023-02431-4.
According to Stedmans Medical Dictionary (Lippincott Lippincott & Wilkins, Baltimore, MD, 2016), the term perfuse has two meanings—to force blood or other fluid (1) to flow from the artery through the vascular bed of a tissue or (2) to flow through the lumen of a hollow structure. Both senses of the word are reflected in the various radionuclide studies performed in the context of DNC.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wijdicks EF, Varelas PN, Gronseth GS, Greer DM, American Academy of Neurology Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:1911–1918. doi: 10.1212/wnl.0b013e3181e242a8. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa TA, Ashwal S, Mathur M, Mysore M, Committee for Determination of Brain Death in Infants Children Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations-executive summary. Ann Neurol. 2012;71:573–585. doi: 10.1002/ana.23552. [DOI] [PubMed] [Google Scholar]
- 3.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: the World Brain Death Project. JAMA. 2020;324:1078–1097. doi: 10.1001/jama.2020.11586. [DOI] [PubMed] [Google Scholar]
- 4.Shemie SD, Lee D, Sharpe M, Tampieri D, Young B, Canadian Critical Care Society Brain blood flow in the neurological determination of death: Canadian expert report. Can J Neurol Sci. 2008;35:140–145. doi: 10.1017/s0317167100008544. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa TA, Ashwal S, Mathur M, et al. Clinical report—guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Pediatrics. 2011;128:e720–e740. doi: 10.1542/peds.2011-1511. [DOI] [PubMed] [Google Scholar]
- 6.Lewis A, Adams N, Chopra A, Kirschen MP. Use of ancillary tests when determining brain death in pediatric patients in the United States. J Child Neurol. 2017;32:975–980. doi: 10.1177/0883073817724697. [DOI] [PubMed] [Google Scholar]
- 7.Zuckier LS, Kolano J. Radionuclide studies in the determination of brain death: criteria, concepts, and controversies. Semin Nucl Med. 2008;38:262–273. doi: 10.1053/j.semnuclmed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Donohoe KJ, Agrawal G, Frey KA, et al. SNM practice guideline for brain death scintigraphy 2.0. J Nucl Med Technol. 2012;40:198–203. doi: 10.2967/jnmt.112.105130. [DOI] [PubMed] [Google Scholar]
- 9.Pjura GA, Kim EE. Radionuclide evaluation of brain death. In: Wessmann HS, Freeman LM, editors. Nuclear medicine annual. New York: Raven Press; 1987. pp. 269–293. [Google Scholar]
- 10.Holmes RA, Chaplin SB, Royston KG, et al. Cerebral uptake and retention of 99Tcm- hexamethylpropyleneamine oxime (99Tcm-HM-PAO) Nucl Med Commun. 1985;6:443–447. doi: 10.1097/00006231-198508000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Ell PJ, Hocknell JM, Jarritt PH, et al. A 99Tcm-labelled radiotracer for the investigation of cerebral vascular disease. Nucl Med Commun. 1985;6:437–441. doi: 10.1097/00006231-198508000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Neirinckx RD, Canning LR, Piper IM, et al. Technetium-99m d, l-HM-PAO: a new radiopharmaceutical for SPECT imaging of regional cerebral blood perfusion. J Nucl Med. 1987;28:191–202. [PubMed] [Google Scholar]
- 13.Kuhl DE, Barrio JR, Huang SC, et al. Quantifying local cerebral blood flow by N-isopropyl-p- [123I]iodoamphetamine (IMP) tomography. J Nucl Med. 1982;23:196–203. [PubMed] [Google Scholar]
- 14.Kung HF, Ohmomo Y, Kung MP. Current and future radiopharmaceuticals for brain imaging with single photon emission computed tomography. Semin Nucl Med. 1990;20:290–302. doi: 10.1016/s0001-2998(05)80235-1. [DOI] [PubMed] [Google Scholar]
- 15.Zuckier LS, Sogbein OO. Brain perfusion studies in the evaluation of acute neurologic abnormalities. Semin Nucl Med. 2013;43:129–138. doi: 10.1053/j.semnuclmed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Roine RO, Launes J, Lindroth L, Nikkinen P. 99mTc-hexamethylpropyleneamine oxime scans to confirm brain death. Lancet. 1986;2:1223–1224. doi: 10.1016/s0140-6736(86)92237-3. [DOI] [PubMed] [Google Scholar]
- 17.Laurin NR, Driedger AA, Hurwitz GA, et al. Cerebral perfusion imaging with technetium-99m HM- PAO in brain death and severe central nervous system injury. J Nucl Med. 1989;30:1627–1635. [PubMed] [Google Scholar]
- 18.Hung JC, Volkert WA, Holmes RA. Stabilization of technetium-99m—d, l- hexamethylpropyleneamine oxime (99mTc—d, l-HMPAO) using gentisic acid. Int J Rad Appl Instrum B. 1989;16:675–680. doi: 10.1016/0883-2897(89)90137-2. [DOI] [Google Scholar]
- 19.American College of Radiology. ACR–ACNM–SNMMI–SPR practice parameter for the performance of single‐photon emission brain perfusion imaging (including SPECT and SPECT/CT), 2021. Available from URL: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/BrainPerf-SPECT.pdf. Accessed Aug 2022
- 20.Spieth M, Abella E, Sutter C, Vasinrapee P, Wall L, Ortiz M. Importance of the lateral view in the evaluation of suspected brain death. Clin Nucl Med. 1995;20:965–968. doi: 10.1097/00003072-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Zuckier LS. “Hot nose sign” is not so hot. Radiology. 2005;237:749–750. doi: 10.1148/radiol.2372050556. [DOI] [PubMed] [Google Scholar]
- 22.Mishkin FS, Dyken ML. Increased early radionuclide activity in the nasopharyngeal area in patients with internal carotid artery obstruction: “hot nose”. Radiology. 1970;96:77–80. doi: 10.1148/96.1.77. [DOI] [PubMed] [Google Scholar]
- 23.Huang AH. The hot nose sign. Radiology. 2005;235:216–217. doi: 10.1148/radiol.2351030537. [DOI] [PubMed] [Google Scholar]
- 24.Appelt EA, Song WS, Phillips WT, Metter DF, Salman UA, Blumhardt R. The, “hot nose” sign on brain death nuclear scintigraphy: where does the flow really go? Clin Nucl Med. 2008;33:55–57. doi: 10.1097/rlu.0b013e31815c4fbf. [DOI] [PubMed] [Google Scholar]
- 25.Lee VW, Hauck RM, Morrison MC, Peng TT, Fischer E, Carter A. Scintigraphic evaluation of brain death: significance of sagittal sinus visualization. J Nucl Med. 1987;28:1279–1283. [PubMed] [Google Scholar]
- 26.Schwartz JA, Baxter J, Brill D, Burns JR. Radionuclide cerebral imaging confirming brain death. JAMA. 1983;249:246–247. doi: 10.1001/jama.1983.03330260064035. [DOI] [PubMed] [Google Scholar]
- 27.Goodman JM, Heck LL, Moore BD. Confirmation of brain death with portable isotope angiography: a review of 204 consecutive cases. Neurosurgery. 1985;16:492–497. [PubMed] [Google Scholar]
- 28.Coker SB, Dillehay GL. Radionuclide cerebral imaging for confirmation of brain death in children: the significance of dural sinus activity. Pediatr Neurol. 1986;2:43–46. doi: 10.1016/0887-8994(86)90039-1. [DOI] [PubMed] [Google Scholar]
- 29.Vander Borght T, Laloux P, Maes A, et al. Guidelines for brain radionuclide imaging. Perfusion single photon computed tomography (SPECT) using Tc-99m radiopharmaceuticals and brain metabolism positron emission tomography (PET) using F-18 fluorodeoxyglucose. The Belgian Society for Nuclear Medicine. Acta Neurol Belg. 2001;101:196–209. [PubMed] [Google Scholar]
- 30.Quality Standards Subcommittee of the American Academy of Neurology Practice parameters for determining brain death in adults (summary statement) Neurology. 1995;45:1012–1014. doi: 10.1212/wnl.45.5.1012. [DOI] [PubMed] [Google Scholar]
- 31.McKinnon NK, Maratta C, Zuckier LS, et al. Ancillary test for death determination in infants and children: a systematic review and meta-analysis. Can J Anesth. 2023 doi: 10.1007/s12630-023-02418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neves Briard J, Nitulescu R, Lemoine É, et al. Diagnostic accuracy of ancillary tests for death by neurologic criteria: a systematic review and meta-analysis. Can J Anesth. 2023 doi: 10.1007/s12630-023-02426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuckier LS. Radionuclide evaluation of brain death in the post-McMath era, epilogue and enigmata. J Nucl Med. 2022 doi: 10.2967/jnumed.122.263972. [DOI] [PubMed] [Google Scholar]