Abstract
Guidelines for the determination of death by neurologic criteria (DNC) require an absence of confounding factors if clinical examination alone is to be used. Drugs that depress the central nervous system suppress neurologic responses and spontaneous breathing and must be excluded or reversed prior to proceeding. If these confounding factors cannot be eliminated, ancillary testing is required. These drugs may be present after being administered as part of the treatment of critically ill patients. While measurement of serum drug concentrations can help guide the timing of assessments for DNC, they are not always available or feasible. In this article, we review sedative and opioid drugs that may confound DNC, along with pharmacokinetic factors that govern the duration of drug action. Pharmacokinetic parameters including a context-sensitive half-life of sedatives and opioids are highly variable in critically ill patients because of the multitude of clinical variables and conditions that can affect drug distribution and clearance. Patient-, disease-, and treatment-related factors that influence the distribution and clearance of these drugs are discussed including end organ function, age, obesity, hyperdynamic states, augmented renal clearance, fluid balance, hypothermia, and the role of prolonged drug infusions in critically ill patients. In these contexts, it is often difficult to predict how long after drug discontinuation the confounding effects will take to dissipate. We propose a conservative framework for evaluating when or if DNC can be determined by clinical criteria alone. When pharmacologic confounders cannot be reversed, or doing so is not feasible, ancillary testing to confirm the absence of brain blood flow should be obtained.
Keywords: drug confounders, death determination by neurologic criteria, opioids, pharmacokinetics sedatives
Résumé
Les lignes directrices pour la détermination du décès selon des critères neurologiques (DCN) exigent une absence de facteurs confondants si l’examen clinique seul doit être utilisé. Les médicaments qui dépriment le système nerveux central suppriment les réponses neurologiques et la respiration spontanée et doivent être exclus ou neutralisés avant de procéder. Si ces facteurs confondants ne peuvent être éliminés, un examen auxiliaire est nécessaire. Ces médicaments peuvent être présents après avoir été administrés dans le cadre du traitement de patients en état critique. Bien que la mesure des concentrations sériques de médicaments puisse guider l’horaire des évaluations pour un DCN, ces mesures ne sont pas toujours disponibles ou réalisables. Dans cet article, nous passons en revue les médicaments sédatifs et opioïdes qui peuvent confondre un DCN, ainsi que les facteurs pharmacocinétiques qui régissent la durée d’action de ces médicaments. Les paramètres pharmacocinétiques, y compris une demi-vie des sédatifs et des opioïdes sensible au contexte, sont très variables chez les patients gravement malades en raison de la multitude de variables cliniques et de conditions qui peuvent affecter la diffusion et l’élimination des médicaments. Les facteurs liés au patient, à la maladie et au traitement qui influencent la diffusion et l’élimination de ces médicaments sont discutés, notamment la fonction des organes cibles, l’âge, l’obésité, les états hyperdynamiques, l’augmentation de la clairance rénale, l’équilibre liquidien, l’hypothermie et le rôle des perfusions prolongées de médicaments chez les patients gravement malades. Dans ces contextes, il est souvent difficile de prédire combien de temps après l’arrêt du médicament les effets confusionnels prendront pour se dissiper. Nous proposons un cadre conservateur pour évaluer quand ou si un DCN peut être déterminé selon des critères cliniques uniquement. Lorsque les facteurs confondants pharmacologiques ne peuvent pas être neutralisés, ou que cela n’est pas possible, un examen auxiliaire pour confirmer l’absence de circulation sanguine cérébrale doit être réalisé.
Determination of death by neurologic criteria (DNC) requires an established neurologic injury leading to the permanent loss of all brain function. Determination of death by neurologic criteria can be undertaken with clinical examination alone if there are no confounding conditions that render the clinical examination unreliable for confirming loss of brain function.1, 2 In cases where the clinical criteria for DNC appear to be met but the neurologic exam is confounded by the potentially reversible effects of drugs, it is often difficult to predict how long after drug discontinuation the confounding effects will take to dissipate. Sedatives and opioids are commonly administered to brain-injured patients as part of their resuscitation and clinical care prior to DNC. In the absence of ancillary testing, waiting until the effect of the drug(s) have worn off is considered a prerequisite to conducting a reliable neurologic exam but determining how long to wait is a common clinical dilemma fraught with uncertainty and limited guidance.
In this article, we review sedative and opioid drugs that may confound DNC and summarize important pharmacokinetic factors that govern the duration of drug action. We propose a conservative framework for evaluating when or if DNC can be determined by clinical criteria alone. When pharmacologic confounders cannot be reversed, or doing so is not feasible, ancillary testing to confirm an absence of brain blood flow should be obtained. Lastly, we emphasize that guidance is needed regarding the identification, evaluation, and management of confounding pharmacological circumstances that might reduce the reliability of the clinical examination for DNC.
A fictional case scenario
Consider the following fictional case scenario. A 61-yr-old, 142-kg woman who is otherwise healthy falls in her bathroom after losing consciousness and hits her head. She is found by a family member who calls for help and the patient is transferred to the local emergency department via ambulance. Upon presentation she is found to be obtunded (Glasgow Coma Scale score, 4/15) and in shock requiring fluid resuscitation and vasopressors. A computed tomography scan of her head reveals subarachnoid and intraventricular hemorrhage. An intraparenchymal intracranial pressure monitoring device is inserted, and she is cared for in the intensive care unit (ICU) where she receives mechanical ventilation and sedation with high-dose infusions of propofol, midazolam, and fentanyl to manage elevated intracranial pressure. Her care is complicated by kidney injury and persistent shock. After 72 hr, the patient’s sedative medications are discontinued when unfortunately, her bedside examination appears consistent with loss of all brain function. The effect of sedatives and opioids are correctly identified as potential reversible confounding factors. The care team would like to understand when the drugs will have dissipated enough that their effects no longer confound the clinical evaluation of DNC.
This case describes a commonly encountered scenario where the effect of drugs may mimic the clinical signs and confound the examination necessary for DNC. It is important that critical care clinicians can recognize these scenarios and have a process for their evaluation and management.
Guideline recommendations for pharmacologic confounders in DNC
Virtually all published guidelines on DNC including the World Brain Death Project and the new Canadian guidelines published in this Special Issue of the Journal address the impact of reversible clinical, pathological, and environmental conditions that have the potential to confound DNC.1, 3, 4 Similar to other clinical conditions that interfere with the ability to assess neurologic function, the effect of certain drugs can also mimic the clinical criteria required for DNC.5
There are many drugs that can confound a neurologic assessment and guidelines commonly identify central nervous system (CNS) depressants. In critical care settings (i.e., ICUs, trauma units, emergency departments, etc.) these include CNS depressant drugs used for sedation, anxiolysis, and analgesia. These comprise (but are not limited to) opioids, benzodiazepines, barbiturates, propofol, and ketamine. Nevertheless, drugs like baclofen, antidepressants, alcohols, and antiepileptics can also blunt brainstem reflexes.5 Drug exposure in toxicologic settings can also confound the neurologic exam by mimicking signs and symptoms consistent with loss of brain function. A recent systematic review of toxicologic case reports identified snake venom, baclofen, tricyclic antidepressants, bupropion, alcohols, antiepileptic drugs, and barbiturates as the most common drugs taken in overdose to mimic brain death.6 In these cases, patients present with coma and loss of brainstem reflexes. These drugs can alter nerve conduction, cause apnea and CNS depression, and mask intact brain function, but most often this effect is transient and reversible. It must be reiterated that permanent loss of brain function must not be considered in drug overdose settings without an established proximate neurologic injury that could lead to the permanent loss of all brain function.1 Even if a concurrent neurologic diagnosis exists, DNC should be delayed until the confounding effects of drugs have dissipated or the clinical examination can be supported by the lack of brain blood flow on ancillary testing.
Most guidelines recommend that ancillary testing for the absence of brain blood flow be added to the examination of clinical criteria when pharmacologic confounding is suspected, and when it is not possible or feasible for death determination to be delayed until the confounding effect of drug therapy has dissipated.1, 3, 4 In practical terms, “waiting” means five elimination half-lives of each potentially offending drug after discontinuation. By waiting five half-lives we are predicting that almost 97% of the administered drug has been cleared from the body.7 The major limitation of guidance based on drug half-life is that most drug half-lives found in electronic drug databases and product monographs are determined in healthy volunteers or noncritically ill patients (Table). Drug half-life is highly context-sensitive and the variability in pharmacokinetic parameters is greatest in critically ill patients because of patient-, disease-, and treatment-related factors that change from day to day and hour to hour in some cases.8–10 Even when estimations of half-life are available for drugs in critical care settings, interpatient variability is significant, warranting an individualized assessment of patient-specific factors that are known to affect drug disposition. Despite the consideration of all pertinent factors that might extend or shorten a drug’s half-life, one is still only left with a prediction. The reality is that the only way to be truly confident that a neurologic exam is not confounded by a residual drug effect would be to measure serum concentrations of each drug which, for the most part, are not rapidly available or practical. Point-of-care testing for relevant drugs would solve this problem but cost-effective and reliable tools are needed. As testing methods are well described for most of these drugs, setting up time-sensitive regional testing capacity for the main drug confounders could also be an optimal solution.11–13
Table 1.
Characteristics of commonly used sedatives and opioids in critical care
| Drug/(major active metabolite) | Lipophilicity (logP*, partition coefficient) | Primary organ-dependent metabolism | Primary organ-dependent elimination | Protein binding | Elimination half-life in noncritically ill adults |
|---|---|---|---|---|---|
| Morphine | 0.9–1.0 |
Liver (90% metabolized by glucuronidation) |
Kidney | 35% bound to albumin | 2–4 hr |
| (Morphine-6-glucuronide) | -2.9 | N/A | Kidney | 15% bound to albumin | 1–3 hr |
| (Morphine-3-glucuronide) | -3 | N/A | Kidney | 10% bound to albumin | 2–4 hr |
| Hydromorphone | 1.47–1.69 |
Liver (95% metabolized by glucuronidation) |
Kidney | 8–19% bound to plasma proteins | 2–3 hr |
| (Hydromorphone-3-glucuronide) | -2 | N/A | Kidney | N/A | N/A |
| Fentanyl | 3.82–4.12 |
Liver (primarily via CYP3A4 to inactive metabolites) |
Kidney | 79–87% bound to apha-1 acid glycoprotein (primarily) | 2–4 hr |
| Propofol | 3.81–4.16 |
Liver (primarily via glucuronidation to inactive metabolites) |
Kidney | 98% bound to albumin and erythrocytes | The half-life is biphasic (initially 40 min then as long as 4–7 hr in the terminal phase) |
| Midazolam | 3.89–3.97 |
Liver (extensive metabolism via CYP3A4) |
Kidney | 97% bound to albumin | 1.8–6.4 hr |
| (α-hydroxymidazolam) | 2.7 | N/A | Kidney | N/A | Up to 12 hr |
*The higher the logP, the more lipophilic the drug; the lower the logP, the more hydrophilic the drug
For some drugs, determining the duration of their effect or clearance is not as challenging. For example, one can use peripheral nerve stimulation with “train-of-four” monitoring to assess the effect of neuromuscular blocking agents.14 Qualitative screening is available for some drugs, typically used in toxicologic settings. Although these urine and blood tests may not be able to quantify the amount of drug, the presence or absence of evaluable compounds can help in multidrug overdoses or overdose settings with unknown substances consumed. Finally, quantitative assays are clinically available for drugs like some barbiturates, antiepileptics, and alcohols. When available, serial measurements will more accurately guide how long to delay neurologic assessments.
Clinical factors that influence the context-sensitive half-life of drugs in critically ill patients
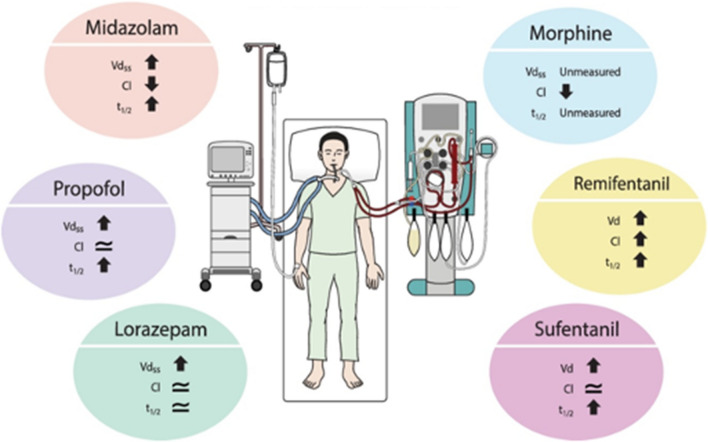
The pharmacokinetic parameters that have the greatest effect on drug half-life are volume of distribution and clearance. Volume of distribution is the theoretical volume within which a drug distributes. For example, lipophilic drugs like midazolam typically have a larger volume of distribution than hydrophilic drugs like morphine. The larger the volume of distribution, the longer the half-life. Drug clearance refers to the rate at which the drug is eliminated from the body. In this case, the greater the clearance, the shorter the half-life (Table). There are many clinical variables in critically ill patients that can influence the volume of distribution or clearance of drugs (Fig. 1).
Fig. 1.
Variables that affect drug clearance and volume of distribution in the ICU. Artwork credit: Viktoriia Panchenko, www.dreamstime.com
Cl = clearance; ICU = intensive care unit; Vd = volume of distribution
Patient-related factors that influence drug elimination half-life: end organ dysfunction, advanced age, obesity, and augmented renal clearance
End organ dysfunction is often the most obvious and sometimes most influential variable to consider when predicting the clearance of drugs.10 Most often, both liver and renal function are relevant. For example, hydromorphone undergoes biotransformation in the liver into both active and inactive metabolites, and all compounds including the parent drug are eliminated by the kidneys.15 The impact of renal dysfunction can be quite significant and prolongs the elimination half-life of renally eliminated drugs. The elimination half-life of midazolam in healthy patients ranges from two to six hours, approximately. The elimination half-life of midazolam when administered as a prolonged continuous infusion to critically ill patients without end organ dysfunction increases to approximately 7–15 hr and as high as 13–34 hr in similar critically ill patients with renal dysfunction despite renal replacement therapy.16, 17 In addition, midazolam also has renally eliminated active metabolites such as α-hydroxymidazolam that may accumulate in renal failure and may prolong sedation.18 The impact of liver dysfunction or cirrhosis on the pharmacokinetics of sedatives and opioids has been less well studied in critically ill patients. Hepatically metabolized drugs can be grouped into those with high and low hepatic extraction ratios, which refer to the fraction of drug metabolized during a single pass through the hepatic circulation. The clearance of drugs with a high hepatic extraction ratio (i.e., morphine, dexmedetomidine, fentanyl, propofol) are typically more sensitive to hepatic blood flow while the clearance of drugs with a low hepatic extraction ratio (i.e., lorazepam, phenytoin) are typically more sensitive to alterations in enzymatic metabolic capacity.19 Opioids are all metabolized by the liver either through phase I or phase II metabolic reactions. While fentanyl has been described as an acceptable opioid choice in renal failure and liver failure because of its lack of active metabolites and common perception of being an agent with a short duration of action, this is based on single-dose studies in noncritically ill patients. The elimination half-life of fentanyl when administered as a continuous infusion has been described to be as long as 25 hr in some critically ill populations compared with two to four hours when administered as a short infusion in noncritically ill patients.20, 21 Fentanyl is a classic example of a drug that has a context-sensitive half-life. The context-sensitive half-life refers to the time for plasma concentrations of a drug to reduce by half after the end of an infusion that is designed to achieve steady state concentrations in the blood (i.e., constant plasma concentration). The “context” refers to the duration of the infusion. Fentanyl is a lipophilic drug that has a multicompartmental pharmacokinetic model meaning that after it distributes throughout the blood volume (the central compartment) it then, at a slower rate, will distribute into peripheral compartment(s) (i.e., adipose tissue) until an equilibrium between compartments is reached. When the infusion is discontinued, the concentration of the drug in the central compartment will reduce due to metabolism and elimination and the concentration gradient between compartments will reverse and the drug in the peripheral compartments will move back into the blood maintaining plasma concentrations, prolonging the pharmacologic effect of the drug and the context-sensitive half-life. In contrast, after a single dose of fentanyl, there is less distribution from the central to peripheral compartments as steady state is never reached. For this reason, context-sensitive half-lives for fentanyl are longer after an infusion than after a single dose
Age is associated with both reduced metabolic capacity within the liver and reduced renal clearance, both of which increase the elimination half-life of relevant drugs.22 Opioids are particularly sensitive as they undergo both hepatic metabolism and renal clearance. Age as a predictor of reduced sedative and opioid clearance has not been well studied in critically ill patients but likely contributes to the overall trajectory of drug disposition. The elimination half-life of oxycodone in noncritically ill adults increases from 3.7 hours in 20- to 40-yr-old individuals to 5.7 hours in 80- to 90-year-old individuals.23, 24 The impact of frailty has not been well studied but the combination of frailty and advanced age is associated with reduced hepatic metabolism.25
Obesity is one of the more common and significant factors complicating the prediction of sedative and opioid clearance because many drugs that interfere with neurologic processes are very lipophilic.26 Midazolam, lorazepam, fentanyl and its analogs, barbiturates, propofol, and most antiepileptics are very lipophilic while morphine and hydromorphone are relatively more hydrophilic. Lipophilic drugs often have a biphasic distribution when administered via continuous infusion. In the initial distributive phase, the drug distributes through the bloodstream but then over time with continued exposure the drug penetrates and saturates adipose tissue. Drug stores in adipose tissue act as reservoirs that translocate slowly back into the bloodstream even after infusions are stopped. The greater the amount of adipose tissue, the larger the volume of distribution of lipophilic drugs and the longer it takes for these stores to clear after discontinuation. Midazolam is particularly lipophilic with a partition coefficient (logP) of almost 4 (higher logP values denote greater lipophilicity; see the Table). In healthy volunteers, the half-life of midazolam after a single dose increases from 2.3 to 6.0 hr with obesity.27, 28 Obesity is also associated with larger volumes of distribution of hydrophilic drugs due to increases in blood volume and total body water and increased renal blood flow, which has been shown to increase drug clearance.29
Augmented renal clearance, defined as a creatinine clearance of > 130 mL·min-1·1.73 m-2, is more commonly encountered in younger patients and in patients with trauma and burns.30 Patients with augmented renal clearance are expected to have greater glomerular filtration rates and thus will clear drugs faster, ultimately shortening the half-life of renally cleared drugs. Studies evaluating the impact of augmented renal clearance are not available for sedatives and opioids but in antimicrobial pharmacokinetic studies it is predictive of subtherapeutic plasma concentrations.30 Augmented renal clearance likely shortens the half-life of renally cleared drugs, but the magnitude and relevance of this factor is unclear.
Disease-related factors that influence context-sensitive half-life: reduced protein binding, systemic inflammation, and hyperdynamic states
Most drugs undergo some degree of protein binding within the blood stream to proteins such as albumin, lipoproteins and proteins expressed on the surface circulating cells such as erythrocytes. It is important to understand that protein binding is typically reversible and only the free fraction (unbound drug) is pharmacologically active and available for excretion. Hypoproteinemia is a common finding in critically ill patients with systemic inflammation as many circulating proteins, including albumin, are sequestered outside the central compartment.10 This reduction in available protein for binding to drugs increases the circulating unbound drug available for distribution, activity, and excretion. This increase in circulating unbound drug is most relevant with highly protein-bound drugs. For example, propofol is 98% protein bound, meaning that 2% of the drug is circulating free or unbound. A reduction in protein binding from 98% to 96% may not seem significant, but represents a 100% increase in the free fraction available for distribution, activity, and clearance.31 Since propofol is titrated to effect, toxicity is rarely encountered. But from a pharmacokinetic perspective, reduced protein binding has conflicting effects on half-life. The increase in the free fraction of propofol should theoretically increase the volume of distribution, which would increase the half-life. But it would also be cleared faster, which would reduce the half-life. The net effect is not clear nor is the relevance of this interaction relative to other clinical factors affecting drug pharmacokinetics in critical illness.32
Aggressive fluid resuscitation in the context of systemic inflammation is a common occurrence in the ICU and usually leads to third-spacing of fluids as inflammation-induced endothelial dysfunction results in “leaky capillaries.” This diffusion across the endothelial membrane results in both fluid and solute accumulation in the extravascular space, effectively increasing the volume of distribution of hydrophilic drugs.9, 10 Drugs that accumulate in the tissue need to return to the central compartment for metabolism and clearance, effectively prolonging the half-life.
Conversely, hyperdynamic states induced by brain injuries, burns, polytrauma, and likely vasopressor therapy can accelerate drug clearance via augmented renal clearance, effectively reducing a drug’s context-sensitive half-life.33 The volume of distribution and clearance of fentanyl in burn patients appears to be almost double that of nonburn matched controls.34
Treatment-related factors that influence context-sensitive drug half-life: prolonged continuous infusions, drug interactions, extracorporeal treatments, fluid balance, and hypothermia
Sedatives and opioids are often administered as continuous infusions in ICU patients to facilitate mechanical ventilation and to provide anxiolysis and analgesia. Typically, shorter-acting agents are used in this setting to allow for frequent titration throughout the day and, hypothetically, faster onset and offset of their clinical effects. Nevertheless, as the duration of these infusions increases, the presumption that these benefits still exist becomes more and more incorrect. After the initial distribution of these drugs within the intravascular space, these drugs begin to translocate across dysfunctional endothelium with extravascular fluid and begin to saturate adipose tissue (for lipophilic drugs), changing the pharmacokinetic profile by increasing the volume of distribution. A recent systematic review compared the pharmacokinetics of prolonged continuous infusions (> 24 hr) of sedatives and opioids in critically ill patients and compared them with those in noncritically ill patients receiving short infusions.35 Effectively, both critical illness and prolonged infusions influenced the pharmacokinetics of these drugs (Fig. 2). For example, the review reported that, for prolonged infusions of midazolam, the volume of distribution doubles and the clearance halves, resulting in a context-sensitive half-life of nine hours compared with 1.7 to 3.5 hours in noncritically ill patients. For prolonged infusions of propofol, the volume of distribution at least triples, and the clearance is similar resulting in a context-sensitive half-life of 33 hr compared with four to seven hours in noncritically ill patients. For prolonged infusions of lorazepam, the volume of distribution triples and the clearance is similar when compared with noncritically ill patients. The authors hypothesized that, since lorazepam has a low hepatic extraction ratio, clearance depends on hepatic function rather than hepatic blood flow. From one study of 20 patients receiving morphine infusions, clearance was reduced by 90% when compared with noncritically ill patients but the volume of distribution and half-life were not measured. No trials evaluating infusions of fentanyl were found but infusions of remifentanil in critically ill patients with renal impairment were associated with a six-fold increase in volume of distribution and a small increase in clearance. The net effect was a prolongation of elimination half-life from 0.08 to 0.13 hr in noncritically ill patients to 0.4 hr in critically ill patients. Similarly, sufentanil infusions in critically ill patients were associated with at least a seven-fold increase in volume of distribution with similar clearance when compared with noncritically ill patients. The net effect was a ten-fold increase in elimination half-life from 0.25–0.33 hr in noncritically ill patients to 26 hr in critically ill patients without organ dysfunction. The key message from this systematic review was that between-patient variability in pharmacokinetic parameters was very large making it difficult to make generalized statements about all critically ill patients. Furthermore, these studies were conducted at single timepoints and the effect of varying durations of continuous infusions can only be hypothesized.
Fig. 2.
Altered pharmacokinetics in ICU patients receiving prolonged continuous infusions of sedatives and analgesics. Figure is reproduced with permission from Tse AH, Ling L, Lee A, Joynt GM. Altered pharmacokinetics in prolonged infusions of sedatives and analgesics among adult critically ill patients: a systematic review. Clin Ther 2018; 40: 1598–615 (Elsevier; license number 5330910863857).
Cl = clearance; T1/2 = half-life; Vdss = volume of distribution at steady state
Drug interactions are also particularly important to consider when predicting the duration of effect of drugs potentially confounding the neurologic exam. Drugs like fentanyl and midazolam are extensively metabolized by the cytochrome P450 (CYP) family of enzymes, specifically the CYP3A4 isoform. Drugs that are strong inhibitors or inducers of this enzyme can have a significant impact on the metabolism of these drugs.36 Strong inducers of CYP3A4 (rifampin, carbamazepine, phenobarbital, and phenytoin) will all accelerate the metabolism of midazolam and fentanyl. In healthy volunteers, the combination of a single dose of midazolam and phenytoin or carbamazepine reduced the elimination half-life almost three-fold from 3.1 hr to 1.3 hr.37 Strong CYP3A4 inhibitors like clarithromycin, voriconazole, itraconazole, ketoconazole, and posaconazole will significantly inhibit the metabolism of midazolam and fentanyl. In a single-dose study of healthy volunteers, the addition of voriconazole to fentanyl reduced fentanyl clearance by 23%.38 It would be reasonable to hypothesize that fentanyl clearance would be further reduced with repeated dosing or continuous infusions at steady state.
Extracorporeal treatments such as continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO) affect the pharmacokinetics of drugs in critical illness. Unfortunately, the independent effects of these treatments are difficult to determine as patients who require ECMO or CRRT are usually the sickest patients in the ICU with multiorgan failure, shock, fluid overload, and low-flow states, all of which contribute to altered drug pharmacokinetics. The volume of distribution of drugs is typically increased in ECMO resulting in prolonged half-lives of drugs due to adsorption and sequestration within the circuit and an increase in circulating blood volume.39 Patients requiring forms of CRRT in the ICU usually already have renal dysfunction and reduced drug clearance and the clearance by CRRT depends on the size of the drug, protein binding, and the efficiency of the mode of CRRT.40 Highly protein-bound drugs are typically poorly removed. But reduced protein binding is associated with critical illness, so there is a larger unbound free fraction of drug available for removal by CRRT. The slower flow rates and longer duration of CRRT typically result in greater clearance of drugs compared with intermittent hemodialysis.
Fluid balance in the critically ill patient is dynamic. Patients upon presentation often require aggressive fluid resuscitation within the first days of admission. Patients with systemic inflammatory syndromes then typically have third-spacing of this fluid because of endothelial dysfunction and those that recover then undergo efforts to mobilize and eliminate this excess fluid with diuresis, CRRT, and drainage.41 During the patient illness course, a steady state of fluid balance is rarely achieved until recovery. This makes drug dosing challenging, particularly for hydrophilic drugs whose volumes of distribution fluctuate widely over the course of the patient’s ICU stay.9 Understanding where on this spectrum the patient is at the time of receiving their potentially confounding drugs is important for predicting the trajectory of drug disposition with respect to when DNC is pursued. Patients who are fluid overloaded will have larger volumes of distribution that will take longer to clear while patients who are volume-contracted may not need as much time.
Hypothermia can be encountered in toxicologic presentations and induced intentionally under controlled settings in the ICU. Hypothermic states are associated with blood flow shunting towards vital organs resulting in low flow states in the gut and compromised drug absorption. Temperature can have a significant impact on drug metabolism as many hepatic enzyme systems are temperature dependent. In most cases, this reduces the metabolic capacity for which drugs with a high hepatic extraction ratio (e.g., fentanyl, propofol, morphine) are more susceptible than those with low hepatic extraction ratios (e.g., lorazepam). Plasma concentrations of both propofol and fentanyl increase by approximately 25% during hypothermic states presumably because of reduced metabolic capacity.42 The magnitude of metabolic impairment appears to be related to the degree of hypothermia as the reduction in midazolam clearance may be as much as 11% per degree below 36.5°C.43
Evaluating how long to wait in pharmacological confounding of DNC
Guideline recommendations are to delay the clinical DNC until the effect of all pharmacological confounders (such as CNS depressant drugs) have dissipated.1 While this is logical, without the ability to measure serum concentrations of offending drugs, it difficult to determine how long to wait. Until such a time that validated point of care serum drug measurement is routinely accessible, we must rely on developing conservative estimates with the clinical information available. As mentioned earlier, serum drug concentrations can be measured for a few drugs, so the first step is to determine if this is available. If possible, serial measurements can be performed to objectively observe the clearance of offending drugs. One controversial issue is whether you should wait until serum levels of offending drugs are reduced to zero or are at least below therapeutic levels. There is limited evidence to support an answer to this question, which is further complicated by the fact that the therapeutic range of most drugs is rarely well determined. When the therapeutic range is known, it is the opinion of these authors that DNC should be delayed at least until the serum concentrations of offending drugs drop below the lower threshold of the therapeutic window. If there are concerns that drugs may confound the clinical examination, then ancillary testing must be undertaken for DNC.
The reality is that measuring serum drug concentrations is not available for most drugs identified as CNS depressants. In these cases, we suggest that determining how long to delay clinical evaluation for DNC begins with determining the reported half-lives of offending drugs (Table). Remember that some drugs will have active metabolites with different half-lives so identifying those with active metabolites (and their respective half-lives) is also important. Acknowledging that these estimates, if drawn from electronic databases and product monographs, are typically not determined in critically ill patients, it at least provides a starting point. It is imperative to recognize that the pharmacokinetics of a drug is not the same as the toxicokinetics of a drug. In an overdose setting, the clearance of drugs can be greatly prolonged and toxicokinetic data should be sought, if available, to determine the starting point.44 The next three questions identify the most important predictors of drug disposition in the critically ill patient: 1) is/are the drug(s) in question lipophilic or hydrophilic and is the patient obese?, 2) does the patient have end organ dysfunction?, and 3) what is the extent of exposure (dose, route of administration, duration)? The answers to these three questions are most likely to influence the estimation of whether the starting half-life should be lengthened or shortened (and by how much). We recommend using multiples of the starting half-life when estimating longer context-sensitive half-lives. Consider a patient who is obese, is receiving a high-dose infusion of fentanyl for more than 72 hr, and has renal injury requiring CRRT. Starting with a three-hour elimination half-life for fentanyl, this estimate could be increased to 12 hr. Of course, if pharmacokinetic data are available from a population of study participants that approximates your patient, then that should also be considered.
The next question should be whether there are any patient-, disease-, and treatment-related factors that are known to affect clearance or volume of distribution of the drugs in question. List them and consider the direction of effect each has on the drug’s half-life (Fig. 1). Estimating the net effect is the most challenging aspect of this process. It is impossible to have confidence in your precision and thus it would be wise to be conservative. Adjustments to the estimate of context-sensitive half-life should be considered in multiples of the reported half-life. It cannot be taken lightly that this process is informing the determination of death. When it is available, ancillary testing should be performed in all cases where pharmacological confounding cannot be feasibly or reliably excluded.45
The question of antidotal therapy with naloxone to reverse opioid exposure and flumazenil to reverse benzodiazepine exposure is a common one. In the toxicology setting, flumazenil is not routinely recommended given the possibility that, in multidrug overdoses, benzodiazepines may be protecting the patient from toxicities of other coingestants (i.e., seizures). Similarly, naloxone used to reverse opioid overdoses has been associated with seizure activity and withdrawal syndromes in chronic opioid users. In the nontoxicologic clinical setting there is less guidance. At this time, this practice cannot be recommended for routine use but if it is to be tried, we suggest using small, intermittent, and sequential doses with constant monitoring by experienced clinicians.
Returning to the original case scenario
Reconsidering the case of the 61-yr-old female who fell in the bathroom; this is a rather complicated scenario. She has received continuous infusions of fentanyl, propofol, and midazolam. Therapeutic drug monitoring is not routinely available for any of these highly lipophilic drugs. Of the three, midazolam has the longest reported half-life at approximately three hours. Assuming an average height, she is obese, has acute kidney injury and has received these drugs at high infusion rates for up to 72 hr. Use of lipophilic drugs in an obese patient at high doses for extended durations would suggest that her volume of distribution would be increased and her renal injury likely is slowing the clearance of these drugs too. It would be reasonable at this point to consider a three-fold increase in our starting midazolam half-life of three hours to 12 hr. There are other factors that might also influence the duration of effect of the midazolam such as shock (reduced hepatic clearance), vasopressor use, and brain injury (increased renal clearance is she has residual renal function). Further questions should be asked about hypoproteinemia (all three drugs are highly protein bound), possible drug interactions, and her state of fluid balance. Based on the available data from the case, the impact of shock and being in a hyperdynamic state have opposite effects on half-life and have less impact than end organ failure, use of lipophilic drugs in obesity, and magnitude of drug exposure; therefore, it would be reasonable not to further adjust our predicted half-life of 12 hr. Five half-lives, therefore, would be a total of 60 hr and would predict that almost 97% of the drug would be removed after a 60-hr delay.
Conclusions
Clinical examination for DNC requires an established proximate neurologic injury that can lead to the permanent loss of all brain function, as well as the exclusion of conditions that might confound the examination. Drugs used in clinical settings can interfere with neurologic clinical assessments and are commonly encountered confounders. Current guidelines recommend delaying neurologic assessments for the purpose of DNC until the effects of these drugs have dissipated. In practice, this length of time is difficult to predict, particularly in the critically ill patient. Patient-, disease-, and treatment-related factors are temporally dynamic and contribute to altered pharmacokinetics of these drugs compounding estimate uncertainty. Most often, measuring serum levels of these drugs is not feasible. In these cases, estimating the half-life of potentially offending drugs is still necessary to predict the duration of effect, and a framework for assessment and decision-making such as that presented in this paper is necessary. Due to the uncertainty of this approach, conservative estimates are recommended and further research is required to evaluate the feasibility of point-of-care and real-time therapeutic drug monitoring. For DNC, ancillary testing should be performed in addition to clinical assessment in all cases where pharmacological confounding cannot feasibly or reliably be excluded.
Acknowledgments
Author contributions
All authors contributed to all aspects of this manuscript including conception and design, acquisition and interpretation of data from published literature, and drafting the article.
Disclosures
No author has conflicts of interest to disclose.
Funding statement
No funding was obtained for the preparation of this review.
Prior conference presentations
Parts of this review are derived from a presentation on this topic by Dr. Kanji at the 19th Annual Meeting of the Neurocritical Care Society in October of 2021 in Chicago, IL, USA.
Editorial responsibility
This submission was handled by Dr. Helen Opdam, Guest Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: the World Brain Death Project. JAMA. 2020;324:1078–1097. doi: 10.1001/jama.2020.11586. [DOI] [PubMed] [Google Scholar]
- 2.Truog RD, Krishnamurthy K, Tasker RC. Brain Death-moving beyond consistency in the diagnostic criteria. JAMA. 2020;324:1045–1047. doi: 10.1001/jama.2020.11665. [DOI] [PubMed] [Google Scholar]
- 3.Shemie SD, Hornby L, Baker A, et al. International guideline development for the determination of death. Intensive Care Med. 2014;40:788–797. doi: 10.1007/s00134-014-3242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijdicks EF, Varelas PN, Gronseth GS, Greer DM, American Academy of Neurology. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010; 74: 1911–8. 10.1212/wnl.0b013e3181e242a8 [DOI] [PubMed]
- 5.Grzonka P, Tisljar K, Rüegg S, Marsch S, Sutter R. What to exclude when brain death is suspected. J Crit Care. 2019;53:212–217. doi: 10.1016/j.jcrc.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Murphy L, Wolfer H, Hendrickson RG. Toxicologic confounders of brain death determination: a narrative review. Neurocrit Care. 2021;34:1072–1089. doi: 10.1007/s12028-020-01114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014;87:93–120. doi: 10.1016/j.bcp.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Boucher BA, Hanes SD. Pharmacokinetic alterations after severe head injury. Clinical relevance. Clin Pharmacokinet. 1998;35:209–221. doi: 10.2165/00003088-199835030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255–271. doi: 10.1016/j.ccc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically ill patient. Chest. 2012;141:1327–1336. doi: 10.1378/chest.11-1396. [DOI] [PubMed] [Google Scholar]
- 11.Ghassabian S, Moosavi SM, Valero YG, Shekar K, Fraser JF, Smith MT. High-throughput assay for simultaneous quantification of the plasma concentrations of morphine, fentanyl, midazolam and their major metabolites using automated SPE coupled to LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;903:126–133. doi: 10.1016/j.jchromb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Jutras M, Williamson D, Chassé M, Leclair G. Development and validation of a liquid chromatography coupled to tandem mass spectrometry method for the simultaneous quantification of five analgesics and sedatives, and six of their active metabolites in human plasma: application to a clinical study on the determination of neurological death in the intensive care unit. J Pharm Biomed Anal 2020; 190: 113521. 10.1016/j.jpba.2020.113521 [DOI] [PubMed]
- 13.Meinitzer A, März W, Mangge H, Halwachs-Baumann G. More reliable brain death diagnosis with chromatographic analysis of midazolam, diazepam, thiopentone, and active metabolites. J Anal Toxicol. 2006;30:196–201. doi: 10.1093/jat/30.3.196. [DOI] [PubMed] [Google Scholar]
- 14.Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44:2079–2103. doi: 10.1097/ccm.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 15.Murray A, Hagen NA. Hydromorphone. J Pain Symptom Manage. 2005;29:S57–66. doi: 10.1016/j.jpainsymman.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Driessen JJ, Vree TB, Guelen PJ. The effects of acute changes in renal function on the pharmacokinetics of midazolam during long-term infusion in ICU patients. Acta Anaesthesiol Belg. 1991;42:149–155. [PubMed] [Google Scholar]
- 17.Swart EL, de Jongh J, Zuideveld KP, Danhof M, Thijs LG, Strack van Schijndel RJ. Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration. Am J Kidney Dis 2005; 45: 360–71. 10.1053/j.ajkd.2004.09.004 [DOI] [PubMed]
- 18.Meinitzer A, Zink M, März W, Baumgartner A, Halwachs-Baumann G. Midazolam and its metabolites in brain death diagnosis. Int J Clin Pharmacol Ther. 2005;43:517–526. doi: 10.5414/cpp43517. [DOI] [PubMed] [Google Scholar]
- 19.Benet LZ, Bowman CM, Koleske ML, Rinaldi CL, Sodhi JK. Understanding drug-drug interaction and pharmacogenomic changes in pharmacokinetics for metabolized drugs. J Pharmacokinet Pharmacodyn. 2019;46:155–163. doi: 10.1007/s10928-019-09626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy EJ. Acute pain management pharmacology for the patient with concurrent renal or hepatic disease. Anaesth Intensive Care. 2005;33:311–322. doi: 10.1177/0310057x0503300306. [DOI] [PubMed] [Google Scholar]
- 21.Soleimanpour H, Safari S, Shahsavari Nia K, Sanaie S, Alavian SM. Opioid drugs in patients with liver disease: a systematic review. Hepat Mon 2016; 16: e32636. 10.5812/hepatmon.32636 [DOI] [PMC free article] [PubMed]
- 22.Cusack BJ. Pharmacokinetics in older persons. Am J Geriatr Pharmacother. 2004;2:274–302. doi: 10.1016/j.amjopharm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Liukas A, Kuusniemi K, Aantaa R, et al. Plasma concentrations of oral oxycodone are greatly increased in the elderly. Clin Pharmacol Ther. 2008;84:462–467. doi: 10.1038/clpt.2008.64. [DOI] [PubMed] [Google Scholar]
- 24.Naples JG, Gellad WF, Hanlon JT. The role of opioid analgesics in geriatric pain management. Clin Geriatr Med. 2016;32:725–735. doi: 10.1016/j.cger.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilmer SN, Kirkpatrick CM. New horizons in the impact of frailty on pharmacokinetics: latest developments. Age Ageing. 2021;50:1054–1063. doi: 10.1093/ageing/afab003. [DOI] [PubMed] [Google Scholar]
- 26.Erstad BL, Barletta JF. Drug dosing in the critically ill obese patient-a focus on sedation, analgesia, and delirium. Crit Care. 2020;24:315. doi: 10.1186/s13054-020-03040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brill MJ, van Rongen A, Houwink AP, et al. Midazolam pharmacokinetics in morbidly obese patients following semi-simultaneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet. 2014;53:931–941. doi: 10.1007/s40262-014-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61:27–35. doi: 10.1097/00000542-198461010-00006. [DOI] [PubMed] [Google Scholar]
- 29.Barletta JF, Erstad BL. Drug dosing in hospitalized obese patients with COVID-19. Crit Care. 2022;26:60. doi: 10.1186/s13054-022-03941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís MÁ. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet. 2018;57:1107–1121. doi: 10.1007/s40262-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 31.Mazoit JX, Samii K. Binding of propofol to blood components: implications for pharmacokinetics and for pharmacodynamics. Br J Clin Pharmacol. 1999;47:35–42. doi: 10.1046/j.1365-2125.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saari TI, Ihmsen H, Mell J, et al. Influence of intensive care treatment on the protein binding of sufentanil and hydromorphone during pain therapy in postoperative cardiac surgery patients. Br J Anaesth. 2014;113:677–687. doi: 10.1093/bja/aeu160. [DOI] [PubMed] [Google Scholar]
- 33.Hefny F, Stuart A, Kung JY, Mahmoud SH. Prevalence and risk factors of augmented renal clearance: a systematic review and meta-analysis. Pharmaceutics. 2022;14:445. doi: 10.3390/pharmaceutics14020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchet B, Jullien V, Vinsonneau C, Tod M. Influence of burns on pharmacokinetics and pharmacodynamics of drugs used in the care of burn patients. Clin Pharmacokinet. 2008;47:635–654. doi: 10.2165/00003088-200847100-00002. [DOI] [PubMed] [Google Scholar]
- 35.Tse AH, Ling L, Lee A, Joynt GM. Altered pharmacokinetics in prolonged infusions of sedatives and analgesics among adult critically ill patients: a systematic review. Clin Ther. 2018;40:1598–1615. doi: 10.1016/j.clinthera.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Roberts DJ, Haroon B, Hall RI. Sedation for critically ill or injured adults in the intensive care unit: a shifting paradigm. Drugs. 2012;72:1881–1916. doi: 10.2165/11636220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Backman JT, Olkkola KT, Ojala M, Laaksovirta H, Neuvonen PJ. Concentrations and effects of oral midazolam are greatly reduced in patients treated with carbamazepine or phenytoin. Epilepsia. 1996;37:253–257. doi: 10.1111/j.1528-1157.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 38.Saari TI, Laine K, Neuvonen M, Neuvonen PJ, Olkkola KT. Effect of voriconazole and fluconazole on the pharmacokinetics of intravenous fentanyl. Eur J Clin Pharmacol. 2008;64:25–30. doi: 10.1007/s00228-007-0398-x. [DOI] [PubMed] [Google Scholar]
- 39.Dreucean D, Harris JE, Voore P, Donahue KR. Approach to Sedation and analgesia in COVID-19 patients on venovenous extracorporeal membrane oxygenation. Ann Pharmacother. 2022;56:73–82. doi: 10.1177/10600280211010751. [DOI] [PubMed] [Google Scholar]
- 40.Schetz M. Drug dosing in continuous renal replacement therapy: general rules. Curr Opin Crit Care. 2007;13:645–651. doi: 10.1097/mcc.0b013e3282f0a3d3. [DOI] [PubMed] [Google Scholar]
- 41.Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17:109. doi: 10.1186/s12882-016-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Poloyac SM. The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function. Expert Opin Drug Metab Toxicol. 2011;7:803–816. doi: 10.1517/17425255.2011.574127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hostler D, Zhou J, Tortorici MA, et al. Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug Metab Dispos. 2010;38:781–788. doi: 10.1124/dmd.109.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neavyn MJ, Stolbach A, Greer DM, et al. ACMT position statement: determining brain death in adults after drug overdose. J Med Toxicol. 2017;13:271–273. doi: 10.1007/s13181-017-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shemie SD, Lee D, Sharpe M, Tampieri D, Young B, Canadian Critical Care Society. Brain blood flow in the neurological determination of death: Canadian expert report. Can J Neurol Sci 2008; 35: 140–5. 10.1017/s0317167100008544 [DOI] [PubMed]