Abstract
Purpose
We performed a systematic review and meta-analysis to determine the diagnostic test accuracy of ancillary investigations for declaration of death by neurologic criteria (DNC) in infants and children.
Source
We searched MEDLINE, EMBASE, Web of Science, and Cochrane databases from their inception to June 2021 for relevant randomized controlled trials, observational studies, and abstracts published in the last three years. We identified relevant studies using Preferred Reporting Items for Systematic Reviews and Meta-Analysis methodology and a two-stage review. We assessed the risk of bias using the QUADAS-2 tool, and applied Grading of Recommendations Assessment, Development, and Evaluation methodology to determine the certainty of evidence. A fixed-effects model was used to meta-analyze pooled sensitivity and specificity data for each ancillary investigation with at least two studies.
Principal findings
Thirty-nine eligible manuscripts assessing 18 unique ancillary investigations (n = 866) were identified. The sensitivity and specificity ranged from 0.00 to 1.00 and 0.50 to 1.00, respectively. The quality of evidence was low to very low for all ancillary investigations, with the exception of radionuclide dynamic flow studies for which it was graded as moderate. Radionuclide scintigraphy using the lipophilic radiopharmaceutical 99mTc-hexamethylpropyleneamine oxime (HMPAO) with or without tomographic imaging were the most accurate ancillary investigations with a combined sensitivity of 0.99 (95% highest density interval [HDI], 0.89 to 1.00) and specificity of 0.97 (95% HDI, 0.65 to 1.00).
Conclusion
The ancillary investigation for DNC in infants and children with the greatest accuracy appears to be radionuclide scintigraphy using HMPAO with or without tomographic imaging; however, the certainty of the evidence is low. Nonimaging modalities performed at the bedside require further investigation.
Study registration: PROSPERO (CRD42021278788); registered 16 October 2021.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12630-023-02418-1.
Keywords: ancillary testing, brain death, death determination, neurologic, pediatrics
Résumé
Objectif
Nous avons réalisé une revue systématique et une méta-analyse pour déterminer la précision des tests diagnostiques des examens auxiliaires pour la déclaration du décès selon des critères neurologiques (DCN) chez les nourrissons et les enfants.
Sources
Nous avons effectué des recherches dans les bases de données MEDLINE, EMBASE, Web of Science et Cochrane de leur création jusqu’en juin 2021 pour trouver des études randomisées contrôlées, des études observationnelles et des résumés pertinents publiés au cours des trois dernières années. Nous avons identifié les études pertinentes utilisant la méthodologie PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) et une revue en deux étapes. Nous avons évalué le risque de biais en utilisant l’outil QUADAS-2 et appliqué la méthodologie GRADE (Grading of Recommendations Assessment, Development and Evaluation) afin d’évaluer la certitude des données probantes. Un modèle à effets fixes a été utilisé pour méta-analyser les données de sensibilité et de spécificité regroupées pour chaque examen auxiliaire avec au moins deux études.
Constatations principales
Trente-neuf manuscrits admissibles évaluant 18 examens auxiliaires uniques (n = 866) ont été identifiés. La sensibilité et la spécificité variaient de 0,00 à 1,00 et de 0,50 à 1,00, respectivement. La qualité des données probantes était faible à très faible pour tous les examens auxiliaires, à l’exception des études de circulation nucléaire dynamique, pour lesquelles elle a été classée comme modérée. La scintigraphie nucléaire à l’aide du produit radiopharmaceutique lipophile 99mTc- hexa-méthyl-propylène amine oxime (HMPAO) avec ou sans imagerie tomographique était à la base des examens auxiliaires les plus précis, avec une sensibilité combinée de 0,99 (intervalle de densité le plus élevé [IDE] à 95 %, 0,89 à 1,00) et une spécificité de 0,97 (IDE à 95 %, 0,65 à 1,00).
Conclusion
L’examen auxiliaire pour un DCN chez les nourrissons et les enfants offrant la plus grande précision semble être la scintigraphie nucléaire utilisant le HMPAO avec ou sans imagerie tomographique; cependant, la certitude des données probantes est faible. Les modalités sans imagerie réalisées au chevet du patient nécessitent un examen plus approfondi.
Enregistrement de l’étude: PROSPERO (CRD42021278788); enregistrée le 16 octobre 2021.
Traditionally, death determination was based on cessation of cardiopulmonary function. Nevertheless, the advent of cardiopulmonary resuscitation and positive pressure mechanical ventilation in the mid-20th century challenged conventional definitions of death; an increasing number of patients with catastrophic brain injuries were manifesting with absent brainstem function on clinical examination. Death determination was decoupled from cardiopulmonary arrest in 1968 when the first standards for death by neurologic criteria (DNC) were outlined by a multidisciplinary committee at Harvard Medical School.1 Since then, clinical examination has prevailed as the gold standard for the determination of neurologic death across various jurisdictions.2–4
Clinical criteria for DNC have primacy in adult and pediatric patients in Canada. In 2006, the forum on Severe Brain Injury to Neurological Determination of Death published specific recommendations for ancillary testing in infants and children in situations where physicians are unable to complete the required clinical test.2 The clinical criteria for diagnosis include irreversible coma, absence of brainstem reflexes, and inability to breath spontaneously, which is formally evaluated by the apnea test, in the setting of severe neurologic injury. Prior to testing for DNC, providers must attempt to correct potential confounding conditions including hypothermia, hypotension, metabolic disturbances, and ensure systemic clearance of sedative medications that can interfere with clinical assessment.2, 5
Scenarios frequently arise where the clinical exam is either confounded by metabolic derangements, sedatives, or hypothermia. In other instances, physicians may be unable to complete the clinical examination because of anatomic derangements including perforated tympanic membranes, injury to the pupils, high cervical spine injuries, or severe lung disease preventing apnea testing. In such situations, ancillary investigations are required to augment the clinical examination and minimize the false-positive and false-negative determinations of DNC.2
While there are a multitude of potential ancillary investigations, all of them evaluate one of three surrogate physiologic processes that underscore the clinical examination: brain blood flow, brain perfusion, and/or brain function. These three distinct physiologic processes necessitate different types of ancillary investigations; imaging modalities are better suited to evaluate brain blood flow or brain perfusion, while electrophysiologic modalities evaluate brain function.6
While these physiologic processes remain proximal to the clinical examination, their diagnostic accuracy in determining DNC has not yet been quantified. There is an urgent need to consolidate the evidence base and derive estimates of how accurate ancillary investigations are for determining DNC. In doing so, we will be better positioned to inform the development of pediatric specific clinical practice guidelines on the optimal ancillary testing strategy.
In response to the heterogeneity in the choice and conduct of ancillary investigations in determining DNC,7, 8 we sought to perform a systematic review of the literature to determine the diagnostic accuracy of ancillary investigations for determination of DNC in infants and children.
Methods
This work was conducted as part of the “Brain-Based Definition of Death and Criteria for its Determination After Arrest of Circulation or Neurologic Function in Canada” project. We performed a systematic review following the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy and reported the findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines,9 including the PRISMA checklist (Electronic Supplementary Material [ESM] eAppendix 1), and the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The study protocol was registered with the International Prospective Register of Systematic Reviews registry (PROSPERO) prior to data extraction (CRD42021278788; registered 16 October 2021).
Search question, population, and inclusion and exclusion criteria
The primary objective of our review was to estimate the diagnostic test accuracy of ancillary investigations for DNC. The population of interest included all pediatric patients, defined as full-term infants (≥ 37 weeks gestational age) to children < 18 yr of age, who had either confirmed or suspected death as determined by clinical DNC. Children with confirmed DNC had a clinical assessment consistent with DNC prior to the ancillary investigation being performed. Children with suspected DNC had an exam concerning for DNC; however, formal testing was not undertaken prior to the ancillary investigations being performed. We did not prespecify any ancillary investigations as index tests, as we aimed to report on various potential ancillary investigations used in a pediatric cohort. An index test included any potential ancillary investigation evaluating brain blood flow, perfusion, or function that was performed in conjunction with or in addition to the clinical exam in patients with confirmed or suspected DNC. Furthermore, index tests could be administered either before or after the clinical assessment for DNC. In cases where an index test was administered multiple times to a patient, we used the results of the last index test performed. For the reference standard, we accepted the clinical assessment (either with or without an alternative ancillary investigation if the patient had any confounders) or four-vessel angiography as it has been previously recommended as a gold standard test in Canadian DNC guidelines.2 The clinical assessment included evaluation of brainstem function (absent pupillary, corneal, oculocephalic, and vestibulo–ocular reflexes, and absence of response to painful stimuli) and apnea test or absent respirations (for studies published prior to the implementation of the apnea test). In case series reporting on individual adult and pediatric data, we included pediatric data if there were two or more patients.
We excluded published editorials, letters, reviews, guidelines, scoping reviews, systematic reviews, meta-analyses, nonresearch articles, and abstracts published prior to 2018 but reviewed their bibliographies for additional eligible articles.
Search strategy
An information specialist (R. F.) modified a search that had been updated for a systematic review on ancillary investigations for adult DNC. The modified search was verified by content experts.10 The search in the original ancillary investigations systematic review was peer reviewed according to the PRESS Peer Review of Electronic Search Strategies 2015 Guideline Statement.11 We conducted a search of Ovid MEDLINE, Ovid Embase, the Cochrane Central Register of Controlled Trials (CENTRAL) via EBM Reviews (Ovid), and Web of Science (Science Citation) from inception until June 2021. Medical Subject Headings were used to identify synonyms. The MEDLINE search strategy appears in ESM eAppendix 2. Conference proceedings from the preceding three years (2018–2021) were retrieved by the Embase search and trial registry records from ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform were retrieved by the CENTRAL search. The reference lists of our full-text articles were searched for additional relevant articles. Filters were applied to limit results to references published in English or French.
Study selection
We used Covidence® software (Veritas Health Innovation Ltd., Melbourne, VIC, Australia) to screen articles in two stages. We screened titles and abstracts in the first stage followed by full-text articles in the second stage. For both stages, screening was conducted by two independent reviewers (N. M., J. K., M. S., J. G. B., O. M., L. H., L. W., C. M., J. B.). Disagreements were resolved by discussion or through arbitration by a third reviewer (J. B., N. M.).
Data extraction and summary measures
Groups of two independent reviewers (N. M., C. M./O. M., J. K./A. K., J. P./M. S., J. G. B.) extracted data through a standardized form. We collected author name, year of publication, country of origin, study design, total sample size, total number of pediatric patients, patient characteristics, presence of any confounders, data related to the flow, timing, and administration of the index test, and reference standard. We derived true positive, true-negative, false-positive, and false-negative values and summarized the data as sensitivity and specificity values for each ancillary investigation.
Statistical analysis
We analyzed studies that included patients with confirmed DNC separately from studies that included patients with suspected DNC. Additionally, we pooled and analyzed studies using the lipophilic radiopharmaceutical 99mTc-hexamethylpropyleneamine oxime (HMPAO) whether imaged by planar or single-photon emission computerized tomography (SPECT) techniques. We also pooled and analyzed all nuclear medicine imaging modalities with an initial dynamic flow phase (ESM eTable 1). We could only report pooled sensitivities for the studies that included patients with confirmed DNC because this study population did not contain false-positive cases from which to calculate specificity. For studies with suspected DNC, we reported both sensitivity and specificity. We estimated pooled sensitivities and specificities, when possible, for each ancillary investigation type with at least two studies. Pooled estimates for sensitivity were computed under the assumption that studies are exchangeable and sampled from the same binomial distribution with common sensitivity parameters. Similarly, for ancillary investigations with at least two studies with nonzero total negative cases, pooled estimates were also computed for specificity, under a similar assumption as for sensitivity. Estimates of pooled sensitivity and specificity were reported as posterior modes and 95% highest density intervals (HDI).12 For index tests with only one study, we generated 95% confidence intervals using Review Manager (RevMan, London, UK). All computations were carried out using R (R Foundation for Statistical Computing, Vienna, Austria) and Stan statistical software (Stan Development Team, New York, NY, USA).13, 14
Rating the certainty of evidence
We evaluated the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework.15 In keeping with the GRADE framework, studies started as high-certainty evidence. We downgraded certainty based on limitations in risk of bias, inconsistency, indirectness, and imprecision (ESM eTable 1). The risk of bias was assessed at both the study and outcome levels using the Quality Assessment Tool for Diagnostic Accuracy Studies-2 (QUADAS-2) tool.16 To evaluate imprecision, we used pooled sensitivity and specificity values and their respective confidence intervals to estimate the absolute number of true-positive, false-positive, true-negative, and false-negative cases per 1,000 people at prevalence levels of 50, 90, and 95%.
Results
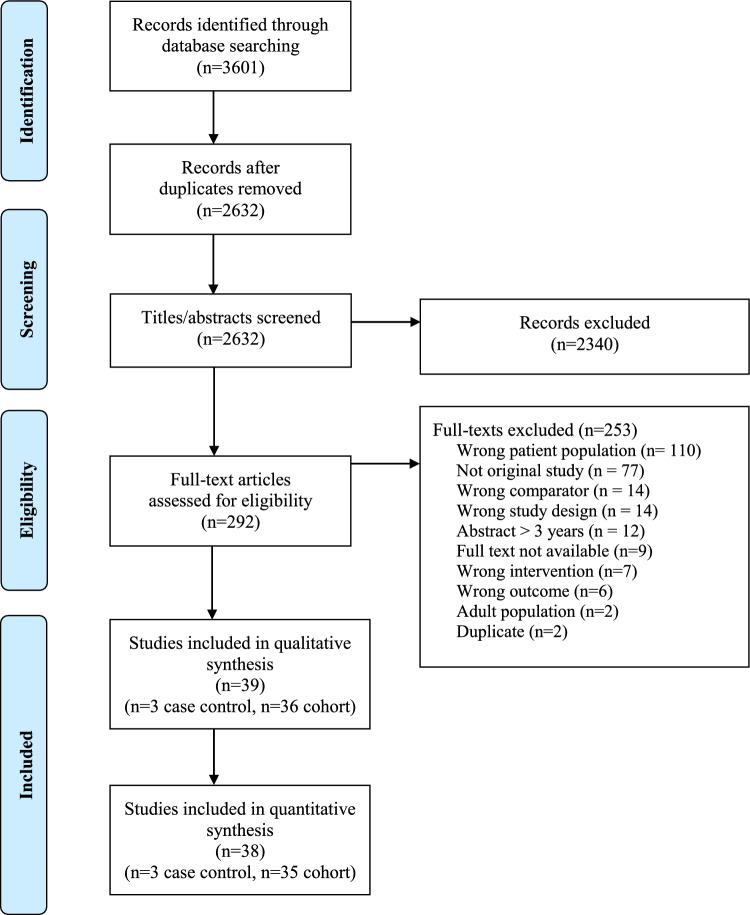
We found 39 studies that met the inclusion criteria, published between 1971 and 2017 (Figure 1; Table 1). Of these, we meta-analyzed the data from 38 studies and narratively reported the outcomes of one study given we were unable to extract patient characteristics for the index test.17 Thirty-six studies were cohort studies and three were case-controlled designs. Eighteen different ancillary investigations for DNC were evaluated across the included studies, with a total of 55 comparative evaluations of the index tests across the 38 manuscripts (Table 1). Of the 18 different ancillary investigations, eight were evaluated in only a single study (Table 1).18–25
Figure 1.
PRISMA flow diagram. Details of the citation search and screening process in this systematic review
Table 1.
Characteristics of included studies
| Author (year) | Country | Number of pediatric patients (n) | DNC suspected or confirmed | Age (yr), mean (SD) | Reference standard | Criteria used for DNC | Ancillary investigation |
|---|---|---|---|---|---|---|---|
| Ashwal 1977 | USA | 13 | Suspected | 3.1 (4.0) | Clinical exam + ancillary test (EEG) | Unresponsive to external stimuli, absent brainstem reflexes, lack of spontaneous respiration† | EEG, lipophobic RP (99mTc-pertechnetate) |
| Ashwal 1979 | USA | 3 | Suspected | 1.0 (1.3) | Clinical exam | Unresponsive to external stimuli, absent brainstem reflexes, lack of spontaneous respiration† | EEG |
| Ashwal 1989 | USA | 21 | Confirmed* | 3.0 (3.9) | Clinical exam | Unresponsive coma, absent brainstem reflexes, apnea test PCO2 > 60 mm Hg | EEG, xenon CT |
| Ashwal 1993 | USA | 52 | Confirmed | 1.2 (2.0) | Clinical exam | Unresponsive coma, fixed dilated pupils, no brainstem reflexes, apnea test | EEG, lipophobic RP (not specified) |
| Blanot 2016 | France | 43 | Confirmed | 6.0 (5.0) | Clinical exam | No confounders, unresponsive coma, absent brainstem reflexes, apnea test | TCD |
| Bode 1988 | Germany | 27 | Confirmed | 5.4 (5.0) | Clinical exam + ancillary test (TCD) | Deep coma, absent brainstem reflexes, no spontaneous respiration† | TCD |
| Coker 1986 | USA | 55 | Suspected | NR (NR) | Clinical exam + ancillary test (EEG) | Unresponsive coma, fixed dilated pupils, absent oculocephalic and oculovestibular reflexes, no spontaneous respirations† | Lipophobic RP (not specified) |
| deTribolet 1977 | Switzerland | 2 | Suspected | 8.5 (2.8) | Clinical exam +4-vessel angiography | Did not define clinical exam criteria. Angiography showing complete cerebral circulatory arrest (contrast interruption at base of cranium) | Lipophobic RP (99mTc-DTPA) |
| Duya 2000 | Turkey | 23 | Confirmed | 5.5 (4.9) | Clinical exam | Turkish guidelines including apnea test | CT angiography |
| Erbengi 1990 | Turkey | 5 | Suspected | 12.4 (2.3) | Clinical exam + ancillary test (BAEP) | Complete unresponsiveness, fixed and dilated pupils, absent brainstem reflexes, absent spinal reflexes, apnea test | Lipophobic RP (99mTc-DTPA), lipophilic RP (99mTc-HMPAO SPECT) |
| Erbengi 1991 | Turkey | 7 | Confirmed | 12.9 (2.3) | Clinical exam | Unresponsive coma, absent brainstem reflexes, fixed, dilated pupils, apnea test | Lipophobic RP (99mTc-DTPA), lipophilic RP (99mTc-HMPAO SPECT), BAEP |
| Fackler 1988 | USA | 45 | Confirmed | 5.3 (4.2) | Clinical exam | Unresponsive coma, no brainstem reflexes, apnea test, referred to “President’s commission” | EEG, 4-vessel cerebral angiogram or radionuclide scan (not specified) |
| Flowers 2000 | USA | 19 | Confirmed | NR (NR) | Clinical exam | GCS = 3, absent brainstem reflexes, apnea test pCO2 > 60 mm Hg | Lipophobic RP (99mTc-pertechnetate) |
| Furgiuele 1984 | USA | 6 | Suspected | 0.8 (0.5) | Clinical exam + ancillary test (EEG) | Absence of spontaneous movements, absence of motor response to light, noise or pain, absent brainstem reflexes, apnea test | EEG, cranial sector ultrasound |
| Gencpinar 2015 | Turkey | 28 | Confirmed | 6.0 (4.2) | Clinical exam + ancillary test (variable) | No confounders, cause for irreversible brain injury, fixed dilated pupils, absent brainstem reflexes, apnea test | TCD |
| Goh 2004 | England | 31 | Confirmed | 4.3 (4.5) | Clinical exam | No confounders, unresponsive coma, cause for irreversible brain injury, fixed dilated pupils, absent brainstem reflexes, apnea test | EEG, BAEP |
| Hindy-Fancois 2009 | France | 14 | Confirmed | 3.5 (NR) | Clinical exam + ancillary test (EEG + TCD) | Clinical exam not described + isoelectric EEG + TCD | 2-Phase CT |
| Holzman 1982 | USA | 18 | Suspected | 4.5 (4.3) | Clinical exam + ancillary test (EEG) | Cortical function absent, spontaneous movement absent, apnea for 3 minutes, absent brainstem reflexes, no pupillary response to light or pain + electrocerebral silence on EEG | EEG, lipophobic RP (99mTc-glucoheptonate) |
| Jalili 1994 | USA | 17 | Suspected | 2.1 (1.7) | Clinical exam + ancillary test (EEG) | Absence of brainstem function, loss of cerebral function, flaccid tone, absence of spontaneous or induced movements, apnea test + isoelectric EEG | Carotid artery Doppler ultrasonography |
| Kahveci 2002 | Turkey | 5 | Confirmed | 10.3 (3.6) | Clinical exam | Coma with cerebral unresponsiveness, absence of corneal light reflexes, light-fixed mydriatic pupils, absence of oculovestibular reflexes, apnea with PaCO2 > 60 Torr | Lipophilic RP (99mTc-HMPAO SPECT) |
| Kraft 2006 | Czech Republic | 6 | Suspected | 9.5 (2.8) | Clinical exam | Two clinical exams minimum 4 hours apart with a focus on absent brainstem reflexes | Lipophilic RP (99mTc-HMPAO) |
| Laurin 1989 | Canada | 9 | Suspected | 8.8 (5.2) | Clinical exam | Deep unresponsive coma, absent brainstem reflexes, apnea test | Lipophilic RP (99mTc-HMPAO) |
| Mohandas 1971 | USA | 8 | Confirmed | 11.2 (6.5) | Clinical exam | No spontaneous movement, no spontaneous respiration for 4 minutes, absence brainstem reflexes (all above criteria must be present for 12 hours) | EEG |
| Newell 1989 | USA | 3 | Suspected | 9.8 (7.2) | Clinical exam + ancillary test (TCD) | Unresponsive coma, absent brainstem reflexes, apnea test | TCD |
| Okuyaz 2004 | Turkey | 8 | Confirmed | 2.6 (2.0) | Clinical exam + ancillary test (EEG) | Unresponsiveness to noxious stimuli, fixed and dilated pupils, absence of brain stem reflexes, apnea test + isoelectric EEG | EEG, lipophilic RP (99mTc-HMPAO SPECT) |
| Okuyaz 2006 | Turkey | 8 | Confirmed | 6.0 (5.7) | Clinical exam + ancillary test (EEG) | Unresponsive coma, absent brainstem reflexes, fixed dilated pupils, apnea test, isoelectric EEG | Bispectral index |
| Parker 1995 | Canada | 59 | Confirmed | NR (NR) | Clinical exam | Unresponsive coma, no posturing, no spontaneous movement, no brainstem reflexes, apnea test | EEG, lipophilic RP (99mTc-HMPAO) |
| Pistoia 1991 | USA | 6 | Confirmed | 6.5 (4.4) | Clinical exam + ancillary test (EEG) | Unresponsive coma, absent brainstem reflexes + isoelectric EEG | Xenon CT |
| Powers 1989 | USA | 3 | Suspected | 9.3 (1.5) | Clinical exam + ancillary test (radionuclide angiography) | No confounders, unresponsive coma, absent brainstem reflexes, flaccid tone, apnea test (pCO2 > 55 mm Hg) | TCD |
| Qian 1998 | China | 58 | Suspected | 2.3 (3.3) | Clinical exam + ancillary test (EEG) | No confounders, deep coma, absent brainstem reflexes, apnea test, fixed heart rate after atropine, 30-min isoelectric EEG | TCD |
| Riggs 2017 | USA | 13 | Suspected | 1.4 (1.7) | Clinical exam | 2 Separate exams consistent with known irreversible cause for coma, absent neurologic function, apnea test | Ophthalmic US of central retinal vessels |
| Rodriguez 2002 | Canada | 15 | Confirmed* | 4.8 (5.0) | Clinical exam + ancillary test (EEG, radionuclide angiography) | GCS 3, lack of cerebral and brainstem reactivity, lack of respiratory effort during apnea testing, pupillary diameter 3 mm, absence of pupillary light and corneal reflexes, unstable systolic blood pressure (variations: 20 mmHg), and inadequate body temperature control (variations: 1 °C) + 30 min isoelectric EEG + radionuclide angiography | TCD |
| Ruiz-Garcia 2000 | Mexico | 125 | Confirmed | 2.0 (2.8) | Clinical exam + ancillary test (EEG) | Unresponsive coma, no brainstem reflexes, apnea test + 2 x EEG 24 hr apart | EEG, lipophobic RP (99mTc-DTPA), BAEP, and SSEP |
| Ruiz-Lopez 1999 | Spain | 51 | Confirmed | 5.3 (4.8) | Clinical exam | No confounders, unresponsive coma, absent brainstem reflexes | BAEP and SSEP |
| Schwartz 1984 | USA | 9 | Suspected | 5.6 (4.2) | Clinical exam + ancillary test (4-vessel angiography) | Nonresponse, unreceptive to external stimulation, absent brainstem reflexes, apnea test with PaCO2 > 60 Torr + 4-vessel angiography | 4-Vessel cerebral angiography, lipophobic RP (99mTc-pertechnetate) |
| Schober 1987 | Germany | 14 | Confirmed | 3.5 (3.4) | Clinical exam + ancillary test (EEG) | 2 Neurologic examinations showing unresponsivity and receptivity, absent brainstem reflexes, no spontaneous respiration + isoelectric EEG | Lipophobic RP (99mTc-DTPA), lipophilic radiopharmaceuticals (99mTc-HMPAO and 123I-IMP) |
| Steinhart 1985 | USA | 23 | Confirmed* | 6.6 (4.5) | Clinical exam | No confounders, unresponsive coma, absent brainstem reflexes, apnea test | BAEP |
| Thompson 1986 | USA | 10 | Suspected | 2.1 (3.3) | Clinical exam + ancillary test (EEG) | Absence of brainstem reflexes, absences of cephalic responses to stimuli, no spontaneous respirations, confirmatory lab studies including EEG | EEG, xenon CT, lipophobic RP (99mTc-Pertechnetate) |
| Wilson 1993 | USA | 12 | Suspected | 6.6 (5.1) | Clinical exam + ancillary test (EEG) | Not clearly described: exam included cold calorics, doll’s eyes, apnea test + isoelectric EEG | Lipophilic RP (99mTc-HMPAO) |
*Denotes case–control studies (n = 3)
†Prior to 1986, formal apnea testing was seldom performed; instead, the criteria included loss of spontaneous respirations
BAEP = brainstem auditory evoked potentials; DNC = death determination by neurologic criteria; DTPA = diethylenetriamine pentaacetic acid; EEG = electroencephalography; GCS = Glasgow coma scale; HMPAO = hexamethylpropyleneamine oxime; 123I-IMP = N-isopropyl-p-[123I]iodoamphetamine; NA = not calculable; NR = not recorded; PaCO2 = arterial partial pressure of carbon dioxide; RP = radiopharmaceutical; SPECT = single-photon emission computed tomography; SSEP = somatosensory evoked potentials; TCD = transcranial Doppler
Eighteen studies included patients who met clinical exam criteria for DNC and 18 studies included patients who were suspected of DNC but had not undergone formal testing. The case–control studies (n = 3, 7.7%) included pediatric patients who were confirmed to have met DNC.26–28 All studies included a clinical exam or a clinical exam and an ancillary investigation as the gold standard. One study did not define the criteria for a clinical exam consistent with DNC; however, the clinical exam was combined with four-vessel cerebral angiography for all pediatric patients and as such was determined to meet criteria to be included as a reference standard.29
Imaging-based ancillary investigations (Tables 2 and 3)
Table 2.
Summary of findings for suspected DNC
| Ancillary investigation | Studies | Test accuracy | Test results | Effect per 1,000 patients tested | Certainty of evidence | ||
|---|---|---|---|---|---|---|---|
| Prevalence 50% typically seen in | Prevalence 90% typically seen in | Prevalence 95% typically seen in | |||||
| Carotid artery ultrasonography | Based on data from 17 patients in one study | Sensitivity: 0.71 (95% CI, 0.29 to 0.96) | True positives (patients meeting DNC) | 355 (145 to 480) | 639 (261 to 864) | 675 (275 to 912) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 145 (20 to 355) | 261 (36 to 639) | 275 (38 to 675) | ||||
| Specificity: 1.00 (95% CI, 0.69 to 1.00) | True negatives (patients without DNC) | 500 (345 to 500) | 100 (69 to 100) | 50 (35 to 50) | Very low (serious risk of bias, indirectness, and imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 0 (0 to 155) | 0 (0 to 31) | 0 (0 to 15) | ||||
| Cranial sector ultrasound | Based on data from 6 patients in one study | Sensitivity: 1.00 (95% CI, 0.52 to 1.00) | True positives (patients meeting DNC) | 500 (260 to 500) | 900 (468 to 900) | 950 (494 to 950) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 240) | 0 (0 to 432) | 0 (0 to 456) | ||||
| Specificity: Not estimable | True negatives (patients without DNC) | Not estimable | Not estimable | Not estimable | |||
| False positives (patients incorrectly classified as meeting DNC) | Not estimable | Not estimable | Not estimable | ||||
| BAEP | Based on data from 23 patients from one study | Sensitivity: 0.90 (95% CI, 0.55 to 1.00) | True positives (patients meeting DNC) | 450 (275 to 500) | 810 (495 to 900) | 855 (523 to 950) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 50 (0 to 225) | 90 (0 to 405) | 95 (0 to 427) | ||||
| Specificity: 1.00 (95% CI, 0.75 to 1.00) | True negatives (patients without DNC) | 500 (375 to 500) | 100 (75 to 100) | 50 (38 to 50) | Very low (serious risk of bias, indirectness, and imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 0 (0 to 125) | 0 (0 to 25) | 0 (0 to 12) | ||||
| EEG | Based on data from 68 patients in six studies | Sensitivity: 0.88 (95% HDI, 0.78 to 0.96) | True positives (patients meeting DNC) | 440 (390 to 480) | 792 (702 to 864) | 836 (741 to 912) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 60 (20 to 110) | 108 (36 to 198) | 114 (38 to 209) | ||||
| Specificity: 0.96 (95% HDI, 0.82 to 1.00) | True negatives (patients without DNC) | 480 (430 to 500) | 96 (86 to 100) | 48 (43 to 50) | Very low (serious risk of bias, indirectness, and imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 20 (0 to 70) | 4 (0 to 14) | 2 (0 to 7) | ||||
| Four-vessel cerebral angiography | Based on data from 9 patients in one study | Sensitivity: 1.00 (95% CI, 0.66 to 1.00) | True positives (patients meeting DNC) | 500 (330 to 500) | 900 (594 to 900) | 950 (627 to 950) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 170) | 0 (0 to 306) | 0 (0 to 323) | ||||
| Specificity: Not estimable | True negatives (patients without DNC) | Not estimable | Not estimable | Not estimable | |||
| False positives (patients incorrectly classified as meeting DNC) | Not estimable | Not estimable | Not estimable | ||||
| Flow-based nuclear medicine imaging | Based on data from 116 patients in eight studies | Sensitivity: 0.95 (95% HDI, 0.89 to 0.98) | True positives (patients meeting DNC) | 475 (445 to 490) | 855 (801 to 882) | 903 (845 to 931) | Moderate (serious risk of bias) |
| False negatives (patients incorrectly classified as not having met DNC) | 25 (10 to 55) | 45 (18 to 99) | 47 (19 to 105) | ||||
| Specificity: 0.88 (95% HDI, 0.67 to 0.98) | True negatives (patients without DNC) | 440 (335 to 490) | 88 (67 to 98) | 44 (34 to 49) | Moderate (serious risk of bias) | ||
| False positives (patients incorrectly classified as meeting DNC) | 60 (10 to 165) | 12 (2 to 33) | 6 (1 to 16) | ||||
| Nuclear medicine ( 99m Tc-DTPA) | Based on data from seven patients in two studies | Sensitivity: 0.87 (95% HDI, 0.53 to 0.99) | True positives (patients meeting DNC) | 435 (265 to 495) | 783 (477 to 891) | 827 (503 to 941) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 65 (5 to 235) | 117 (9 to 423) | 123 (9 to 447) | ||||
| Specificity: Not estimable | True negatives (patients without DNC) | Not estimable | Not estimable | Not estimable | |||
| False positives (patients incorrectly classified as meeting DNC) | Not estimable | Not estimable | Not estimable | ||||
| Nuclear medicine ( 99m Tc-glucoheptonate) | Based on data from data from 15 patients in one study | Sensitivity: 0.89 (95% CI, 0.52 to 1.00) | True positives (patients meeting DNC) | 445 (260 to 500) | 801 (468 to 900) | 845 (494 to 950) | Very low (serious risk of bias, indirectness, and imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 55 (0 to 240) | 99 (0 to 432) | 105 (0 to 456) | ||||
| Specificity: 0.67 (95% CI, 0.22 to 0.96) | True negatives (patients without DNC) | 335 (110 to 480) | 67 (22 to 96) | 34 (11 to 48) | Very low (serious risk of bias, indirectness and imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 165 (20 to 390) | 33 (4 to 78) | 16 (2 to 39) | ||||
| Nuclear medicine ( 99m Tc-HMPAO SPECT and non-SPECT combined) | Based on data from 31 patients in four studies | Specificity: 0.99 (95% HDI, 0.89 to 1.00) | True positives (patients meeting DNC) | 495 (445 to 500) | 891 (801 to 900) | 941 (845 to 950) | Low (serious risk of bias, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 5 (0 to 55) | 9 (0 to 99) | 9 (0 to 105) | ||||
| Sensitivity: 0.97 (95% HDI, 0.65 to 1.00) | True negatives (patients without DNC) | 485 (325 to 500) | 97 (65 to 100) | 49 (33 to 50) | Low (serious risk of bias, imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 15 (0 to 175) | 3 (0 to 35) | 1 (0 to 17) | ||||
| Nuclear medicine ( 99m Tc-HMPAO) | Based on data from 27 patients in three studies | Sensitivity: 0.99 (0.87 to 1.00) | True positives (patients meeting DNC) | 495 (435 to 500) | 891 (783 to 900) | 941 (827 to 950) | Low (serious risk of bias, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 5 (0 to 65) | 9 (0 to 117) | 9 (0 to 123) | ||||
| Specificity: 0.97 (95% HDI, 0.65 to 1.00) | True negatives (patients without DNC) | 485 (325 to 500) | 97 (65 to 100) | 49 (33 to 50) | Low (serious risk of bias, imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 15 (0 to 175) | 3 (0 to 35) | 1 (0 to 17) | ||||
| Nuclear medicine ( 99m Tc-HMPAO SPECT) | Based on data from four patients in one study | Sensitivity: 1.00 (95% CI, 0.40 to 1.00) | True positives (patients meeting DNC) | 500 (200 to 500) | 900 (360 to 900) | 950 (380 to 950) | Very low (serious risk of bias; very serious risk of imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 300) | 0 (0 to 540) | 0 (0 to 570) | ||||
| Specificity: Not estimable | True negatives (patients without DNC) | Not estimable | Not estimable | Not estimable | |||
| False positives (patients incorrectly classified as meeting DNC) | Not estimable | Not estimable | Not estimable | ||||
| Nuclear medicine ( 99m Tc-pertechnetate) | Based on data from 30 patients in three studies | Sensitivity: 0.91 (95% HDI, 0.77 to 0.99) | True positives (patients meeting DNC) | 456 (385 to 495) | 820 (693 to 891) | 865 (731 to 941) | Low (serious risk of bias, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 44 (5 to 115) | 80 (9 to 207) | 85 (9 to 219) | ||||
| Specificity: 0.97 (95% HDI, 0.65 to 1.00) | True negatives (patients without DNC) | 485 (325 to 500) | 97 (65 to 100) | 49 (33 to 50) | Low (serious risk of bias, imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 15 (0 to 175) | 3 (0 to 35) | 1 (0 to 17) | ||||
| Ophthalmic ultrasound of central retinal vessels | Based on data from one study in 13 patients | Sensitivity: 0.92 (95% CI, 0.64 to 1.00) | True positives (patients meeting DNC) | 460 (320 to 500) | 736 (512 to 800) | 874 (609 to 950) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 40 (0 to 180) | 64 (0 to 288) | 76 (0 to 342) | ||||
| Specificity: Not estimable | True negatives (patients without DNC) | Not estimable | Not estimable | Not estimable | |||
| False positives (patients incorrectly classified as meeting DNC) | Not estimable | Not estimable | Not estimable | ||||
| Transcranial Doppler | Based on data from 79 patients in 4 studies | Sensitivity: 0.91 (95% HDI, 0.77 to 0.98) | True positives (patients meeting DNC) | 455 (385 to 490) | 819 (693 to 882) | 864 (731 to 931) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 45 (10 to 115) | 81 (18 to 207) | 86 (19 to 219) | ||||
| Specificity: 0.88 (95% HDI, 0.77 to 0.95) | True negatives (patients without DNC) | 440 (385 to 475) | 88 (77 to 95) | 44 (39 to 48) | Very low (serious risk of bias, indirectness, imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 60 (25 to 115) | 12 (5 to 23) | 6 (2 to 11) | ||||
| Xenon CT | Based on data from 30 patients in 2 studies | Sensitivity: 0.81 (95% HDI, 0.57 to 0.94) | True positives (patients meeting DNC) | 405 (285 to 470) | 729 (513 to 846) | 770 (542 to 893) | Very low (serious risk of bias, indirectness, inconsistency, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 95 (30 to 215) | 171 (54 to 387) | 180 (57 to 408) | ||||
| Specificity: 0.99 (95% HDI, 0.83 to 1.00) | True Negatives (patients without DNC) | 495 (415 to 500) | 99 (83 to 100) | 50 (42 to 50) | Very low (serious risk of bias, indirectness, imprecision) | ||
| False positives (patients incorrectly classified as meeting DNC) | 5 (0 to 85) | 1 (0 to 17) | 0 (0 to 8) | ||||
BAEP = brainstem auditory evoked potentials; DTPA = diethylenetriamine pentaacetic acid; EEG = electroencephalography; HMPAO = hexamethylpropyleneamine oxime; SPECT = single-photon emission computed tomography; SSEP = somatosensory evoked potentials; TCD = transcranial Doppler
Table 3.
Summary of findings for confirmed DNC
| Ancillary investigation | Studies | Test accuracy (sensitivity) | Test results | Effect per 1,000 patients tested | Certainty of evidence | ||
|---|---|---|---|---|---|---|---|
| Prevalence 50% typically seen in | Prevalence 90% typically seen in | Prevalence 95% typically seen in | |||||
| BAEP | Based on data from eight patients in two studies | 0.88 (95% HDI, 0.56 to 0.99) | True positives (patients meeting DNC) | 450 (275 to 500) | 810 (495 to 900) | 855 (523 to 950) | Very low (serious risk of bias, serious indirectness; very serious risk of imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 50 (0 to 225) | 90 (0 to 405) | 95 (0 to 427) | ||||
| BAEP and SSEP | Based on data from 158 patients in two studies | 0.92 (95% HDI, 0.87 to 0.96) | True positives (patients meeting DNC) | 460 (435 to 480) | 828 (783 to 864) | 874 (827 to 912) | Low (serious risk of bias, serious indirectness) |
| False negatives (patients incorrectly classified as not having met DNC) | 40 (20 to 65) | 72 (36 to 117) | 76 (38 to 123) | ||||
| Bispectral index | Based on data from eight patients in one study | 1.00 (95% CI, 0.63 to 1.00) | True positives (patients meeting DNC) | 500 (315 to 500) | 900 (567 to 900) | 950 (598 to 950) | Very low (serious risk of bias, indirectness; very serious risk of imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 185) | 0 (0 to 333) | 0 (0 to 352) | ||||
| EEG | Based on data from 231 patients in seven studies | 0.87 (95% HDI, 0.82 to 0.91) | True positives (patients meeting DNC) | 435 (410 to 455) | 783 (738 to 819) | 827 (779 to 864) | Very low (serious risk of bias, indirectness, inconsistency) |
| False negatives (patients incorrectly classified as not having met DNC) | 65 (45 to 90) | 117 (81 to 162) | 123 (86 to 171) | ||||
| CT angiography | Based on data from 19 patients in one study | 1.00 (95% CI, 0.83 to 1.00) | True positives (patients meeting DNC) | 500 (410 to 500) | 900 (738 to 900) | 950 (779 to 950) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 90) | 0 (0 to 162) | 0 (0 to 171) | ||||
| Nuclear medicine ( 99m Tc-flow-based imaging) | Based on data from 133 patients in five studies | 0.94 (95% HDI, 0.88 to 0.97) | True positives (patients meeting DNC) | 470 (440 to 485) | 846 (792 to 873) | 893 (836 to 922) | Low (serious risk of bias, indirectness) |
| False negatives (patients incorrectly classified as not having met DNC) | 30 (15 to 60) | 54 (27 to 108) | 57 (28 to 114) | ||||
| Nuclear medicine ( 123 I-IMP) | Based on data from four patients in one study | 1.00 (95% CI, 0.40 to 1.00) | True positives (patients meeting DNC) | 500 (200 to 500) | 900 (360 to 900) | 950 (380 to 950) | Very low (serious risk of bias, serious indirectness. Very serious risk of imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 300) | 0 (0 to 540) | 0 (0 to 570) | ||||
| Nuclear medicine ( 99m Tc-DTPA) | Based data from 93 patients in three studies | 0.92 (95% HDI, 0.86 to 0.97) | True positives (patients meeting DNC) | 460 (430 to 485) | 828 (774 to 873) | 874 (817 to 922) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 40 (15 to 70) | 72 (27 to 126) | 76 (28 to 133) | ||||
| Nuclear medicine ( 99m Tc-HMPAO SPECT and non-SPECT) | Based on data from 53 patients in five studies | 0.92 (95% HDI, 0.83 to 0.98) | True positives (patients meeting DNC) | 460 (415 to 490) | 828 (747 to 882) | 874 (789 to 931) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 40 (10 to 85) | 72 (18 to 153) | 76 (19 to 161) | ||||
| Nuclear medicine ( 99m Tc-HMPAO SPECT) | Based on data from 15 patients in three studies | 0.99 (95% HDI, 0.83 to 1.00) | True positives (patients meeting DNC) | 495 (415 to 500) | 891 (747 to 900) | 941 (789 to 950) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 5 (0 to 85) | 9 (0 to 153) | 9 (0 to 161) | ||||
| Nuclear medicine ( 99m Tc-HMPAO) | Based on data from 38 patients in two studies | 0.89 (95% HDI, 0.77 to 0.97) | True positives (patients meeting DNC) | 445 (385 to 485) | 801 (693 to 873) | 845 (731 to 922) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 55 (15 to 115) | 99 (27 to 207) | 105 (28 to 219) | ||||
| Nuclear medicine ( 99m Tc-pertechnetate) | Based on data from 19 patients in one study | 1.00 (95% CI, 0.83 to 1.00) | True positives (patients meeting DNC) | 500 (410 to 500) | 900 (738 to 900) | 950 (779 to 950) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 0 (0 to 90) | 0 (0 to 162) | 0 (0 to 171) | ||||
| Transcranial Doppler | Based on data from 70 patients in three studies | 0.91 (95% HDI, 0.83 to 0.97) | True positives (patients meeting DNC) | 455 (415 to 485) | 819 (747 to 873) | 864 (789 to 922) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 45 (15 to 85) | 81 (27 to 153) | 86 (28 to 161) | ||||
| Xenon CT cerebral blood flow | Based on data from six patients in one study | 0.83 (95% CI, 0.36 to 1.00) | True positives (patients meeting DNC) | 415 (180 to 500) | 747 (324 to 900) | 789 (342 to 950) | Very low (serious risk of bias, indirectness, imprecision) |
| False negatives (patients incorrectly classified as not having met DNC) | 85 (0 to 320) | 153 (0 to 576) | 161 (0 to 608) | ||||
BAEP = brainstem auditory evoked potentials; DTPA = diethylenetriamine pentaacetic acid; EEG = electroencephalography; HMPAO = hexamethylpropyleneamine oxime; SPECT = single-photon emission computed tomography; SSEP = somatosensory evoked potentials; TCD = transcranial Doppler
Accuracy of radionuclide dynamic flow imaging
Thirteen studies (n = 249) used radiopharmaceutical-based flow imaging to determine DNC. Of those, 12 studies used a lipophobic radiopharmaceutical—five with 99mTc-diethylenetriaminepentaacetic acid (DTPA),23, 29–32 four with 99mTc-pertechnetate,22, 33–35 one with 99mTc-glucoheptonate (GHA),23 two with unspecified radiopharmaceuticals36, 37—and one study reported the initial flow phase of a 99mTc-labeled lipophilic radiopharmaceutical in sufficient detail to be included38 (ESM eTable 2). We included one study that used 99mTc-pertechnetate and a bedside triple probe scintillation counting system to assess the presence or absence of cerebral blood flow. This is not a conventional nuclear medicine scan; however, it used a lipophobic radiopharmaceutical and assessed cerebral blood flow, so we included it in our analysis.34 We meta-analyzed these studies together based on the identical functional properties of the radiopharmaceuticals used. In eight studies that enrolled patients suspected of DNC (n = 116),22, 24, 29, 31, 34, 35, 37, 38 radionuclide flow imaging had a sensitivity of 0.95 (95% HDI, 0.89 to 0.98) and a specificity of 0.88 (95% HDI, 0.67 to 0.98). The certainty of evidence was downgraded to moderate for both sensitivity and specificity because of serious risk of bias (ESM eTable 3).
99mTc-diethylenetriaminepentaacetic acid (DTPA)
Five studies (n = 100) assessed cerebral blood flow using 99mTc-DTPA23, 29–32; and two studies (n = 7)29, 31 reported diagnostic accuracy outcomes in patients with suspected DNC. In the first study,29 the two patients with suspected DNC had no detectable flow in their craniums. In the second study, Erbengi et al. (1990)31 included five pediatric patients suspected of DNC. The absence of intracranial arterial flow and absence of sagittal sinus activity on dynamic and static images were the criteria set for a test to be consistent with DNC. Of the five pediatric patients, three had absent flow and no uptake on static blood pool images, consistent with DNC. One patient had flow present, and another had no flow present but sagittal sinus activity on static images not consistent with DNC per the author’s criteria. In patients with suspected DNC, 99mTc-DTPA had a sensitivity of 0.87 (95% HDI, 0.53 to 0.99). The certainty of evidence is very low because of serious risk of bias, indirectness, and imprecision.
Of the three studies involving patients with confirmed DNC, Erbengi et al. (1991)30 included only one pediatric participant in whom both the dynamic and static images showed an absence of cerebral circulation. In Schober et al. (1987),23 two patients showed no flow, consistent with DNC. In the third study, no cerebral blood flow was detected in 83 patients with confirmed DNC, whereas persistent intracranial blood flow was present in seven patients.32 Pooled analysis for these three studies showed a sensitivity of 0.92 (95% HDI, 0.86 to 0.97). The certainty of evidence was very low because of serious risk of bias, indirectness, and imprecision.
99mTc-pertechnetate
Four studies enrolling 49 patients assessed cerebral blood flow using 99mTc-pertechnetate.22, 33–35 Of those, 30 patients in three studies were suspected of DNC while 19 patients22, 34, 35 in one study were confirmed to be DNC.33 In patients with suspected DNC, 99mTc-pertechnetate showed a sensitivity of 0.91 (95% HDI, 0.77 to 0.99) and a specificity of 0.97 (95% HDI, 0.65 to 1.00). The certainty of the evidence for both sensitivity and specificity was downgraded to low because of high risk of bias and imprecision.
Ashwal et al. (1977)34 used 99mTc-pertechnetate and a bedside triple probe scintillation counting system to assess the presence or absence of cerebral blood flow. Nine patients with a clinical exam concerning for DNC had no cranial isotope bolus detected in the presence of a systemic bolus. Three children who did not meet DNC criteria had detectable flow, showed by a positive cranial and systemic isotope bolus.
Schwartz et al. (1984)22 reported on nine children with suspected DNC. None of the nine children had arterial flow detected after systemic isotope injection; however, activity was detected in the sagittal sinus, which they discounted as insignificant (n = 3). In a study by Thompson et al. (1986)35 that used dynamic brain scintigraphy, flow was graded from 0 to +4, with 0 reflecting no cerebral activity and absent or minimal but delayed sinus activity to +4 representing peak cerebral activity to sagittal sinus in less than six seconds. A study with a scintigraphy grade of 0 meets DNC criteria. The study results included three patients without cerebral activity (grade flow of 0) and clinical assessments consistent with DNC, four patients with graded flow of +3 and +4 and a clinical assessment inconsistent with DNC, and two patients with graded flow of +1 and +3 but meeting clinical assessment criteria for DNC; both of these infants survived.
Flowers et al. (2000)33 was the only study to include patients with a confirmed diagnosis of DNC. They defined a study with the absence of arterial flow in the cerebral circulation as consistent with DNC. No arterial flow was detected in the 19 patients studied; however, one patient did have evidence of radiopharmaceutical detected in the superior sagittal sinus, which the authors state was likely to have been due to flow from extradural perforating arteries originating from the external carotid circulation,37 and as such was discounted as not indicative of brain viability or cortical flow. The sensitivity for this study was 1.0.
Accuracy of radionuclide parenchymal uptake studies
Ten studies used lipophilic radiopharmaceuticals, one (n = 14) using N-isopropyl-p-[123I]iodoamphetamine (123I-IMP)23 and nine (n = 84) using 99mTc-HMPAO.23, 30, 31, 38–43 Of the nine 99mTc-radiopharmaceutical studies, five used planar imaging23, 38, 41–43 and four SPECT imaging.23, 38, 41–43 We combined the parenchymal uptake studies using 99mTc-HMPAO. The pooled sensitivity and specificity were 0.99 (95% HDI, 0.89 to 1.00) and 0.97 (95% HDI, 0.65 to 1.00), respectively. The certainty of the evidence for both sensitivity and specificity was downgraded to low because of high risk of bias and imprecision.
99mTc-hexamethylpropyleneamine oxime (HMPAO) (planar imaging)
Five studies (n = 65) reported on the diagnostic accuracy of 99mTc-HMPAO with planar imaging.23, 38, 41–43 Of those, two studies included patients with confirmed DNC by clinical exam23, 41 and three studies included patients with suspected DNC.38, 42, 43 Parker et al. (1995)41 included 13 patients in whom clinical criteria for DNC could not be completed. None of these patients showed parenchymal uptake. Laurin et al. (1989)38 described a mixed cohort of both children and adults suspected of meeting DNC. The authors noted that nine of 25 patients with no arterial flow did have 99mTc-HMPAO uptake into the dural venous sinus, which they discounted as not contradicting the diagnosis of DNC. The authors did not stratify this finding by age; therefore, it is unclear if any of the pediatric patients (n = 9) without arterial flow had blood pool noted in their dural venous sinuses. The sensitivity and specificity of 99mTc-HMPAO was 0.99 (95% HDI, 0.87 to 1.00) and 0.97 (95% HDI, 0.65 to 1.00), respectively. The certainty of evidence for both sensitivity and specificity was deemed to be low because of serious risk of bias, imprecision, and heterogeneity.
99mTc-hexamethylpropyleneamine oxime (HMPAO) (SPECT imaging)
Four studies (n = 19) used 99mTc-HMPAO with SPECT imaging.30, 31, 39, 40 One study investigated patients with suspected DNC31, while three studies reported the diagnostic accuracy in patients with confirmed DNC. All studies defined a scan consistent with DNC as the absence of parenchymal uptake in both the cerebral hemispheres and cerebellum on the tomographic images. None of the 19 patients had parenchymal uptake in either their cerebral hemispheres or cerebellum. In patients with suspected DNC (n = 4), sensitivity of 99mTc-HMPAO SPECT was 1.00 (95% CI, 0.40 to 1.00). Specificity could not be determined because no patients who had absent flow were deemed to be alive. Certainty of evidence for sensitivity is very low because of serious risk of bias and very serious concerns of imprecision.
Accuracy of computed tomography (CT) angiography
Only one study (n = 19) reported on the diagnostic accuracy of CT angiography.21 The estimated sensitivity was 1.00 (95% CI, 0.82 to 1.00). The certainty of this evidence was very low because of high risk of bias, indirectness, and imprecision.
Accuracy of four-vessel cerebral angiography
Only one study (n = 9) investigated four-vessel cerebral angiography as the ancillary investigation in children (n = 9) with clinical exams suggestive of DNC.22 In their study, all children had absent cerebral arterial filling and met criteria for DNC. The sensitivity of four-vessel cerebral angiography was 1.00 (95% CI, 0.66 to 1.00). The certainty of this evidence was low because of serious risk of bias, indirectness, and imprecision. A second study had 28 patients who underwent either four-vessel cerebral angiography or an unspecified radionuclide scan following clinical exam consistent with DNC.44 No cerebral blood flow was present for any patients with suspected DNC (n = 28); however, given the investigations were not stratified per patient, we were unable to use the data in the four-vessel cerebral angiography analysis. Unfortunately, no pediatric or adult studies included patients without DNC, so no data exist from which to calculate specificity.
Accuracy of transcranial Doppler (TCD)
We identified seven studies27, 45–50 (n = 149) that reported on the use of TCD in determination of DNC. Three studies defined a priori the waveforms that were consistent with DNC,47, 49, 50 with two of these studies further clarifying the required waveforms to be present bilaterally for the investigation to be consistent with DNC.47, 50 One study did not define transcranial waveforms consistent with DNC, but stated that “cerebral circulatory arrest” is consistent with DNC.48 The remaining studies described waveforms in patients with exams suspicious for DNC but did not a priori define the criteria for a TCD consistent with DNC.27, 45, 46 In patients with suspected DNC (four studies, n = 79 patients), the sensitivity and specificity were 0.91 (95% HDI, 0.77 to 0.98) and 0.88 (95% HDI, 0.77 to 0.95), respectively. The certainty of the evidence was very low for both sensitivity and specificity because of serious risk of bias, indirectness, and imprecision.
Nonimaging-based ancillary investigations (Tables 2 and 3)
Accuracy of electroencephalography (EEG)
We identified 13 studies (n = 299) that evaluated EEG as an ancillary investigation for DNC.20, 24, 26, 32, 34–36, 40, 41, 44, 51–53 Thirteen patients studied had known confounders including detectable phenobarbital levels (n = 11),34, 41, 44 or hypothermia and a detectable phenobarbital level (n = 2)35 at the time of EEG. Typical studies defined an EEG with electrocerebral silence as consistent with DNC, one stated an isoelectric EEG was consistent and another defined a “flat” EEG as consistent with DNC.40 Seven studies included patients with confirmed DNC.32, 36, 40, 41, 44, 52, 53 In patients with suspected DNC, pooled analysis showed a sensitivity of 0.88 (95% HDI, 0.78 to 0.96) and a specificity of 0.96 (95% HDI, 0.82 to 1.00). The certainty of evidence is very low for both sensitivity and specificity because of serious risk of bias, indirectness, and imprecision (ESM eTable 4).
Accuracy of brain auditory evoked potentials (BAEPs)
A total of three studies (n = 31)28, 30, 52 reported on the diagnostic accuracy of BAEPs as determining DNC. Only one study a priori defined a BAEP consistent with DNC, stating that a recording with no response in the C2 to A1/A2 electrode30 was consistent with DNC, whereas the other studies were descriptive.28, 52 Typical patients did not have confounders.28, 30 In the one study (n = 23) of patients with suspected DNC, the sensitivity and specificity were 0.90 (95% CI, 0.55 to 1.00) and 1.00 (95% CI, 0.75 to 1.00), respectively. The certainty of the evidence was very low for both sensitivity and specificity because of serious risk of bias, indirectness, and imprecision.
Accuracy of brain auditory evoked potentials and somatosensory evoked potentials (SSEPs)
Two studies (n = 158) evaluated the accuracy of BAEPs and SSEPs in patients with confirmed DNC.32, 54 Confounding variables such as barbiturate coma or hypothermia were not described in any patients. Ruiz-García et al. (2000)32 defined an investigation consistent with DNC as no observable waveforms for both brainstem and somatosensory evoked potentials. Ruiz-López et al. (1999)54 did not explicitly define the methodology waveforms consistent with DNC and instead described waveforms in the patient population. Pooled sensitivity for the two studies was 0.92 (95% HDI, 0.87 to 0.96). The certainty of the evidence was very low because of serious risk of bias, indirectness, and imprecision.
Discussion
Ancillary investigations are essential for DNC when the clinical assessment cannot be completed in its entirety. Nevertheless, the wide variety of available ancillary investigations and uncertainty around their diagnostic accuracy creates a challenge for patient families, clinicians, and organ donation organizations when DNC is being considered. This systematic review and meta-analysis consolidates the literature to date on the diagnostic accuracy of ancillary investigations in infants and children with suspected or confirmed DNC. We identified 39 manuscripts reporting on 18 different ancillary investigations in 866 infants and children. Of all ancillary investigations examined, the radionuclide studies, including pooled lipophobic radiopharmaceuticals and pooled lipophilic radiopharmaceuticals, showed the highest sensitivities and specificities.
The World Brain Death Project recommends the use of radionuclide angiography as the preferred ancillary investigation for DNC in infants and children.3 Despite high diagnostic accuracy, the certainty of evidence is moderate for studies based on lipophobic flow and low for studies based on lipophilic parenchymal uptake. Certainty in diagnostic accuracy estimates could be further improved by refining the criteria for DNC. For example, in one study, activity in the superior sagittal sinus was defined as not consistent with DNC.31 Superior sagittal sinus activity is believed to be due to flow from extradural perforating arteries originating from the external carotid circulation and has been shown to occur not infrequently in children meeting DNC criteria.37 A final caveat in the analysis of lipophilic radiopharmaceuticals is that only studies that used 99mTc-HMPAO have been evaluated; a second lipophilic radiopharmaceutical in common use today, 99mTc-bicisate, may be similar but has not been rigorously evaluated.
Estimates of the diagnostic accuracy of all ancillary investigations were limited by low certainty of evidence due to risk of bias, indirectness, imprecision, and heterogeneity. For the other imaging modalities, we could not derive specificity data for CT angiography and four-vessel cerebral angiography. Extrapolation of CT angiography from adult studies is severely limited by the anatomical and physiologic differences of infants and young children for several reasons. First, newer multiphase CT angiography, an increasingly common ancillary investigations in adults, requires a dose of radiation that would be uncharacteristically high for infants and young children. Furthermore, their smaller veins limit the contrast injection rates required to perform these studies. Two-phase spiral CT has been shown in a small international study to be an ancillary investigation for DNC in smaller children, details on the protocol used or vessels evaluated preclude its generalizability.17 Future studies investigating CT angiography would benefit from clearly defining if 4, 7, or 10 vessels were evaluated, and should include vessels in both the supratentorial and infratentorial regions of the brain to evaluate blood flow to the cortex and the brainstem.
Transcranial Doppler is a potentially promising ancillary investigation for determination of DNC since it can be performed at the bedside without the need for patient transport. Nevertheless, the findings of our review suggest that TCDs are more specific than they are sensitive. Few studies reported the diagnostic criteria for TCDs with respect to the vessels that were evaluated, the cut-offs for Doppler waveforms, and whether TCDs were done bilaterally or unilaterally. Standardizing TCD criteria for DNC in pediatric patients as well as the inclusion of both the anterior and posterior cerebral circulation could minimize false negatives and improve sensitivity measures. Future implementation of TCD as an ancillary investigation for pediatric DNC would require additional resources and education of pediatric sonographers, given that currently, TCDs are not performed regularly at all pediatric institutions. Nevertheless, prior to this, we require additional studies to improve the certainty in the diagnostic accuracy of TCDs, and to better delineate the diagnostic thresholds for the determination of DNC.
Electroencephalography is another promising ancillary investigation that has been used for determination of DNC. Although the sensitivity and specificity of EEGs in our review were 88% and 96%, respectively, our findings are limited by low certainty evidence. Very few studies we identified included patients with confounders that could meaningfully alter the diagnostic accuracy of EEGs. As a result, there is uncertainty in how EEGs would perform if used on patients who have been heavily sedated or those with uncorrectable hypothermia. Future studies are needed to validate the diagnostic accuracy of EEGs in the context of clinical confounders.
In terms of strengths, this systematic review and meta-analysis was conducted using established systematic review methodology and is the largest and most inclusive evaluation of ancillary investigations for DNC in infants and children. We registered the systematic review protocol prior to the initiation of the review and employed a search strategy that did not restrict studies by publication type. We employed a two-step review process for both title and abstract screening as well as full-text review to minimize bias due to selective reporting.
Our review also has important limitations. The originally planned analyses were to use (1) multi-level models to estimate partially pooled sensitivities; and (2) hierarchical summary receiver operating characteristic curve models to estimate partially pooled sensitivities and specificities, in both cases per type of ancillary investigation and per study nested within each ancillary investigation type. Unfortunately, these analyses were not feasible because of the limited number of studies per ancillary investigation, the relatively small sample sizes of most of the studies, and missing data from within the studies. Because of all these shortcomings, our analysis used fixed-effect models and pooled sensitivity and specificity independently of one another, and as such our estimates do not account for between-study heterogeneity nor the inherent trade-off between sensitivity and specificity. This is a limitation to keep in mind when interpreting the results. Other limitations include the exclusion of studies published in languages other than English and French, omission of the gray literature, and the exclusion of abstracts published prior to 2018.
Conclusion
Our systematic review suggests that flow-based nuclear medicine imaging modalities are the most accurate ancillary investigations for DNC in infants and children—more specifically, radionuclide scintigraphy using 99mTc-HMPAO, combined with planar or SPECT imaging (low certainty of evidence). This lipophilic radiopharmaceutical incorporates both a parenchymal and a flow phase. Further studies are required to refine our diagnostic accuracy estimates and certainty of the evidence in more readily available and bedside ancillary investigations such as EEGs and TCDs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Author contributions
Nicole K. McKinnon, Lionel S. Zuckier, J. Gordon Boyd, Michaël Chassé, Andreas Kramer, Julie Kromm, Owen Mooney, Marat Slessarev, and John Basmaji designed the study. Nicole K. McKinnon, Julie Kromm, Marat Slessarev, J. Gordon Boyd, Owen Mooney, Laura Hornby, Christina Maratta, and John Basmaji assessed citations for eligibility. Nicole K. McKinnon, Christina Maratta, Owen Mooney, Julie Kromm, Andreas Kramer, Jaewoo Park, Marat Slessarev, J. Gordon Boyd, and John Basmaji abstracted data. Nicole K. McKinnon, Christina Maratta, Owen Mooney, Julie Kromm, Andreas Kramer, Jaewoo Park, Marat Slessarev, J. Gordon Boyd, and John Basmaji assessed risk of bias, and Nicole K. McKinnon, Christina Maratta, Lionel S. Zuckier, Prakash Muthusami, and John Basmaji checked data for accuracy. Nicole K. McKinnon, Lionel S. Zuckier, Prakash Muthusami, Roy Nitulescu, Michaël Chassé, and John Basmaji conducted analyses. All authors contributed to interpret the data. All authors read the manuscript and provided feedback.
Acknowledgements
The authors would like to express their appreciation to Robin Featherstone, MLIS, and Daniela Ziegler, MSI, for modifying our search strategy. Additionally, the authors would like to thank Lindsay C. Wilson, MHA, and Sam D. Shemie, MD, for their leadership, support, and coordination.
Disclosures
Laura Hornby is a paid consultant for Canadian Blood Services.
Funding statement
This work was conducted as part of the project entitled, “A Brain-Based Definition of Death and Criteria for its Determination After Arrest of Circulation or Neurologic Function in Canada,” made possible through a financial contribution from Health Canada through the Organ Donation and Transplantation Collaborative and developed in collaboration with the Canadian Critical Care Society, Canadian Blood Services, and the Canadian Medical Association. The views expressed herein do not necessarily represent the views of Health Canada, the Canadian Critical Care Society, Canadian Blood Services, or the Canadian Medical Association.
Prior conference presentations
These findings have been presented at The Canadian Association of Nuclear Medicine Annual Conference in Montreal, QC on 30 September 2022 and the Critical Care Canada Forum meeting in Toronto, ON on 24 November 2022.
Editorial responsibility
This submission was handled by Dr. Maureen Meade, Guest Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beecher HK. A definition of irreversible coma: report of the ad hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1968;205:337–340. doi: 10.1001/jama.1968.03140320031009. [DOI] [PubMed] [Google Scholar]
- 2.Shemie SD, Doig C, Dickens B, et al. Severe brain injury to neurological determination of death: Canadian forum recommendations. CMAJ. 2006;174:S1–12. doi: 10.1503/cmaj.045142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: the World Brain Death Project. JAMA. 2020;324:1078–1097. doi: 10.1001/jama.2020.11586. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa TA, Ashwal S, Mathur M, et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 Task Force recommendations. Crit Care Med. 2011;39:2139–2155. doi: 10.1097/ccm.0b013e31821f0d4f. [DOI] [PubMed] [Google Scholar]
- 5.Kanji S, Williamson D, Hartwick M. Potential pharmacological confounders in the setting of death determined by neurologic criteria: a narrative review. Can J Anesth. 2023 doi: 10.1007/s12630-023-02415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plourde G, Briard JN, Shemie SD, Shankar JJS, Chassé M. Flow is not perfusion, and perfusion is not function: ancillary testing for the diagnosis of brain death. Can J Anesth. 2021;68:953–961. doi: 10.1007/s12630-021-01988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis A, Liebman J, Kreiger-Benson E, et al. Ancillary testing for determination of death by neurologic criteria around the world. Neurocrit Care. 2021;34:473–484. doi: 10.1007/s12028-020-01039-6. [DOI] [PubMed] [Google Scholar]
- 8.Lewis A, Bakkar A, Kreiger-Benson E, et al. Determination of death by neurologic criteria around the world. Neurology. 2020;95:e299–309. doi: 10.1212/wnl.0000000000009888. [DOI] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chassé M, Glen P, Doyle MA, et al. Ancillary testing for diagnosis of brain death: a protocol for a systematic review and meta-analysis. Syst Rev. 2013;2:100. doi: 10.1186/2046-4053-2-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Kruschke JK. Doing Bayesian Data Analysis: A Tutorial with R, JAGS and Stan. 2. London: Elsevier; 2014. [Google Scholar]
- 13.R Core Team (2021). R: a language and environment for statistical computing. Available from URL: https://www.R-project.org/ (accessed December 2022).
- 14.Stan Development Team. Stan modeling language users guide and reference manual. Available from URL: https://mc-stan.org (accessed December 2022).
- 15.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hindy-François C, Orliaguet G, Meyer P, et al. Pediatric brain death diagnosis in the view of organ donation in France. Transplantation. 2009;87:616–617. doi: 10.1097/tp.0b013e3181963d91. [DOI] [PubMed] [Google Scholar]
- 18.Riggs BJ, Cohen JS, Shivakumar B, et al. Doppler ultrasonography of the central retinal vessels in children with brain death. Pediatr Crit Care Med. 2017;18:258–264. doi: 10.1097/pcc.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 19.Okuyaz Ç, Birbiçer H, Doruk N, Atici A. Bispectral index monitoring in confirmation of brain death in children. J Child Neurol. 2006;21:799–801. doi: 10.1177/08830738060210090101. [DOI] [PubMed] [Google Scholar]
- 20.Furgiele TL, Frank LM, Riegle C, Wirth F, Earley LC. Prediction of cerebral death by cranial sector scan. Crit Care Med. 1984;12:1–3. doi: 10.1097/00003246-198401000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Duyu M, Karakaya Z. Evaluation of patients diagnosed with brain death in paediatric critical care. J Pediatric Res. 2020;7:250–256. doi: 10.4274/jpr.galenos.2020.82474. [DOI] [Google Scholar]
- 22.Schwartz JA, Baxter J, Brill DR. Diagnosis of brain death in children by radionuclide cerebral imaging. Pediatrics. 1984;73:14–18. doi: 10.1542/peds.73.1.14. [DOI] [PubMed] [Google Scholar]
- 23.Schober O, Galaske R, Heyer R. Determination of brain death with 123I-IMP and 99mTc-HM-PAO. Neurosurg Rev. 1987;10:19–22. doi: 10.1007/BF01780588. [DOI] [PubMed] [Google Scholar]
- 24.Holzman BH, Curless RG, Sfakianakis GN, Ajmone-Marsan C, Montes JE. Radionuclide cerebral perfusion scintigraphy in determination of brain death in children. Neurology. 1983;33:1027–1031. doi: 10.1212/wnl.33.8.1027. [DOI] [PubMed] [Google Scholar]
- 25.Jalili M, Crade M, Davis AL. Carotid blood-flow velocity changes detected by Doppler ultrasound in determination of brain death in children. Clin Pediatr. 1994;33:669–674. doi: 10.1177/000992289403301106. [DOI] [PubMed] [Google Scholar]
- 26.Ashwal S, Schneider S, Thompson J. Xenon computed tomography measuring cerebral blood flow in the determination of brain death in children. Ann Neurol. 1989;25:539–546. doi: 10.1002/ana.410250603. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez RA, Cornel G, Alghofaili F, Hutchison J, Nathan HJ. Transcranial Doppler during suspected brain death in children: potential limitation in patients with cardiac “shunt”. Pediatr Crit Care Med. 2002;3:153–157. doi: 10.1097/00130478-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Steinhart CM, Weiss IP. Use of brainstem auditory evoked potentials in pediatric brain death. Crit Care Med. 1985;13:560–562. doi: 10.1097/00003246-198507000-00010. [DOI] [PubMed] [Google Scholar]
- 29.de Tribolet N, Schäfer K, Oberson R, Zander E. Radioisotope diagnosis of brain death [French] Schweiz Med Wochenschr. 1977;107:464–467. [PubMed] [Google Scholar]
- 30.Erbengi A, Erbengi G, Cataltepe O, Topcu M, Erbas B, Aras T. Brain death: determination with brain stem evoked potentials and radionuclide isotope studies. Acta Neurochir. 1991;112:118–125. doi: 10.1007/bf01405139. [DOI] [PubMed] [Google Scholar]
- 31.Erbengi G, Erbengi A, Erbas B, Aras T. Diagnosis of brain death using TC-99m-HMPAO/SPECT, Tc-99m-DTPA scintigraphy and radionuclide angiography. NucCompact. 1990;21:177–179. [Google Scholar]
- 32.Ruiz-García M, Gonzalez-Astiazarán A, Collado-Corona MA, Rueda-Franco F, Sosa-de-Martínez C. Brain death in children: clinical, neurophysiological and radioisotopic angiography findings in 125 patients. Childs Nerv Syst. 2000;16:40–45. doi: 10.1007/s003810050010. [DOI] [PubMed] [Google Scholar]
- 33.Flowers WM, Jr, Patel BR. Accuracy of clinical evaluation in the determination of brain death. South J Med. 2000;93:203–206. doi: 10.1097/00007611-200093020-00010. [DOI] [PubMed] [Google Scholar]
- 34.Ashwal S, Smith AJ, Torres F, Loken M, Chou SN. Radionuclide bolus angiography: A technique for verification of brain death in infants and children. J Pediatr. 1977;91:722–727. doi: 10.1016/s0022-3476(77)81023-8. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JR, Ashwal S, Schneider S, Hasso AN, Hinshaw DB, Kirk G. Comparison of cerebral blood flow measurements by xenon computed tomography and dynamic brain scintigraphy in clinically brain dead children. Acta Radiologica Suppl. 1986;369:675–679. [PubMed] [Google Scholar]
- 36.Ashwal S. Brain death in early infancy. J Heart Lung Transplant. 1993;12:S176–S178. [PubMed] [Google Scholar]
- 37.Coker SB, Dillehay GL. Radionuclide cerebral imaging for confirmation of brain death in children: the significance of dural sinus activity. Pediatr Neurol. 1986;2:43–46. doi: 10.1016/0887-8994(86)90039-1. [DOI] [PubMed] [Google Scholar]
- 38.Laurin NR, Driedger AA, Hurwitz GA, et al. Cerebral perfusion imaging with technetium-99m HM-PAO in brain death and severe central nervous system injury. J Nucl Med. 1989;30:1627–1635. [PubMed] [Google Scholar]
- 39.Kahveci F, Bekar A, Tamgac F. Tc-99 HMPAO cerebral SPECT imaging in brain death patients with complex spinal automatism. Ulus Travma Derg. 2002;8:198–201. [PubMed] [Google Scholar]
- 40.Okuyaz Ç, Gücüyener K, Karabacak Nİ, Aydin K, Serdaroğlu A, Cingi E. Tc-99m-HMPAO SPECT in the diagnosis of brain death in children. Pediatr Int. 2004;46:711–714. doi: 10.1111/j.1442-200x.2004.01976.x. [DOI] [PubMed] [Google Scholar]
- 41.Parker BL, Frewen TC, Levin SD, et al. Declaring pediatric brain death: current practice in a Canadian pediatric critical care unit. CMAJ. 1995;153:909–916. [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft O, Samlík J, Chmelová J. The diagnosis of brain death—own experience. Nucl Med Rev Cent East Eur. 2006;9:132–137. [PubMed] [Google Scholar]
- 43.Wilson K, Gordon L, Selby JB., Sr The diagnosis of brain death with Tc-99m HMPAO. Clin Nucl Med. 1993;18:428–434. doi: 10.1097/00003072-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Fackler JC, Troncoso JC, Gioia FR. Age-specific characteristics of brain death in children. Am J Dis Child. 1988;142:999–1003. doi: 10.1001/archpedi.1988.02150090097034. [DOI] [PubMed] [Google Scholar]
- 45.Powers AD, Graeber MC, Smith RR. Transcranial Doppler ultrasonography in the determination of brain death. Neurosurgery. 1989;24:884–889. doi: 10.1227/00006123-198906000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Qian SY, Fan XM, Yin HH. Transcranial Doppler assessment of brain death in children. Singap Med J. 1998;39:247–250. [PubMed] [Google Scholar]
- 47.Bode H, Sauer M, Pringsheim W. Diagnosis of brain death by transcranial Doppler sonography. Arch Dis Child. 1988;63:1474–1478. doi: 10.1136/adc.63.12.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanot S, Montmayeur J, Salvadori A, Ottonello G, Orliaguet G. Évaluation rétrospective de l’épreuve d’apnée chez l’enfant en mort encéphalique. Méd Intensive Réa. 2016;25:171–178. doi: 10.1007/s13546-016-1222-3. [DOI] [Google Scholar]
- 49.Newell DW, Grady SM, Sirotta P, Winn RH. Evaluation of brain death using transcranial Doppler. Neurosurgery. 1989;24:509–513. doi: 10.1227/00006123-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Gencpinar P, Dursun O, Teküc H, Ünal A, Haspolat Ş, Duman Ö. Pediatric brain death: experience of a single center. Turkiye Klinikleri J Medical Sci. 2015;35:60–66. doi: 10.5336/medsci.2014-41309. [DOI] [Google Scholar]
- 51.Ashwal S, Schneider S. Failure of electroencephalography to diagnose brain death in comatose children. Ann Neurol. 1979;6:512–517. doi: 10.1002/ana.410060609. [DOI] [PubMed] [Google Scholar]
- 52.Goh A, Mok Q. Clinical course and determination of brainstem death in a children’s hospital. Acta Paediatr. 2004;93:47–52. doi: 10.1111/j.1651-2227.2004.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 53.Mohandas A, Chou SN. Brain death: a clinical and pathological study. J Neurosurg. 1971;35:211–218. doi: 10.3171/jns.1971.35.2.0211. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-López MJ, de Azagra AM, Serrano A, Casado-Flores J. Brain death and evoked potentials in pediatric patients. Crit Care Med. 1999;27:412–416. doi: 10.1097/00003246-199902000-00051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.