Abstract
Introduction
The OneTouch Verio Reflect® (OTVR) Blood Glucose Meter features a color range indicator and provides on-meter guidance, insights, and encouragement. Diabetes management is enhanced by the OneTouch Reveal® (OTR) Mobile App. We sought to provide real-world evidence (RWE) that combining devices improves glycemia.
Methods
Anonymized glucose and app analytics from more than 55,000 people with diabetes (PWDs) were extracted from a server. Data from their first 14 days using OTVR Meter and OTR App was compared with 14 days prior to 90- and 180-day timepoints using paired within-subject differences.
Results
In people with type 1 (PwT1D) or type 2 diabetes (PwT2D), readings in-range (RIR 70–180 mg/dL) improved by 7.8 percentage points (57.9–65.7%) and 12.0 percentage points (72.8–84.8%), respectively, over 180 days and hyperglycemia (> 180 mg/dL) was reduced by − 8.4 percentage points (37.9–29.5%) and − 12.2 percentage points (26.2–14.1%). RIR improved by > 10 percentage points in 38% of PwT1D and 39% of PwT2D. PwT1D spending > 2 to 4 sessions or > 10 to 20 min per week on the app improved RIR by 7.0 and 8.2 percentage points, respectively. PwT2D spending > 2 to 4 sessions or > 10 to 20 min per week on the app improved RIR by 12.6 and 12.1 percentage points, respectively. In PwT1D or T2D, mean blood glucose reduced by − 14.3 and − 19.8 mg/dL, respectively, from baseline to 180 days, with no clinically meaningful changes in percentage of hypoglycemic readings (< 70 mg/dL). PwT1D 65 years and older performed the most app sessions (10 per week) and improved RIR by 7.9 percentage points. PwT2D 65 years and older spent more time on the app (45 min per week) than PwT2D of any other age and improved RIR by 7.6 percentage points. All glycemic changes were statistically significant (p < 0.0005).
Conclusion
Real-world data from more than 55,000 PWDs demonstrates sustained improvements in readings in-range in PWDs using the OneTouch Verio Reflect Blood Glucose Meter and OneTouch Reveal App.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-023-01415-3.
Keywords: Real-world evidence, Digital ecosystem, Diabetes application, Blood glucose meter, Readings in range
Key Summary Points
| Why carry out this study? |
| Traditional blood glucose meters (BGMs), mostly without Bluetooth® connectivity or advanced on-meter features, have been investigated in controlled, small-scale studies. It is important to understand how PWDs might benefit under real-world conditions. |
| This study builds upon a prior analysis but extends device exposure to a more clinically meaningful timeframe, greatly increases the size of the data set to enable a more robust overall analysis, and presents a sub-analysis of the impact of age on various outcomes. |
| What was learned from this study? |
| Improved glycemic outcomes, such as more readings in-range, were sustained over 6 months by PwT1D and PwT2D and were proportionate with increasing app engagement. |
| Despite PWDs under 25 years of age interacting the least with the app compared to other age groups, they still achieved clinically significant improvements in glycemic outcomes. |
| PWDs 65 years or older spent the most time and performed the most app sessions, demonstrating the strongest affinity to this technology, and achieved significant glycemic improvements, although the magnitude of their improvements was smaller than for younger age groups. |
Introduction
A comprehensive analysis of randomized controlled trials (RCTs) found blood glucose monitoring (BGM) reduced glycated hemoglobin (A1c) by − 0.3% compared with usual care, with further A1c improvement of − 0.2% in those performing structured BGM [1]. These benefits are welcome at a time when the percentage of adults with diabetes in whom glycemic control was achieved (A1c < 7%) declined from 57.4% to 50.5% [2]. The advent of continuous glucose monitoring (CGM) has provided a wealth of data for patients and healthcare professionals (HCPs) [3]. The clinical value of both real-time and intermittently scanned CGMs has been reported in people with type 1 diabetes (PwT1D) and type 2 diabetes (PwT2D) in RCTs [4, 5], observational studies [6], retrospective chart review studies [7], and from sensor readings uploaded to servers [8]. However, at least one meta-analysis examining RCTs (from 2014 to 2021) found Freestyle Libre CGM did not significantly reduce A1c compared to BGM [9]. Perhaps this is because realizing the benefits of both BGM and CGM technologies requires people with diabetes (PWDs) and HCPs to interpret and act upon the available insights.
The focus on CGM overshadows the fact that new innovations in BGM continue at a brisk pace, including on-meter features (such as color range indicators and automatic pattern recognition), virtually pain-free lancing devices, and Bluetooth® connectivity to mobile diabetes apps [10, 11]. The value of advanced BGM technologies was exemplified in the recent MOBILE study in PwT2D on basal insulin, which demonstrated that regular monitoring using a connected OneTouch Verio Flex® (OTVF) Meter, which features a color range indicator (CRI), combined with additional insights from the OneTouch Reveal® (OTR) Mobile Diabetes App, elicited a clinically meaningful A1c reduction of − 0.6%, with the Dexcom G6 CGM lowering A1c by a further − 0.4% [12]. It is worth noting that structured monitoring (e.g., seven-point profiles prior to site visits) was not mandated for the BGM group in the MOBILE study, which might have further improved A1c outcomes in the BGM group had it been used. Support for this theory is twofold: a recent RCT found an additional − 0.32% reduction in A1c in PwT2D performing structured monitoring compared with those performing routine BGM [13] and Bergenstal et al. found consistent use of glucose data, regardless of device (structured monitoring using BGM or CGM), led to similar improvements in time in range and A1c over 16 weeks [14]. The latest OneTouch Verio Reflect® (OTVR) Meter builds upon the attributes of the simpler Verio Flex Meter with an enhanced ColorSure Dynamic Range Indicator and Blood Sugar Mentor Feature providing personalized guidance, insight, and encouragement directly on the meter screen [15]. This real-time advice feature of the OTVR Meter was highlighted in the diabetes technology section of the 2023 American Diabetes Association (ADA) Standards of Care [16]. For these reasons, it is unsurprising that the OTVR Meter was strongly preferred over three leading BGMs in a survey of 353 HCPs, who rated the OTVR Meter as the best meter to support PWDs in achieving a variety of self-management goals recommended in ADA/European Association for the Study of Diabetes (EASD) clinical practice guidelines [17]. The concept of time in range (TIR) is commonly applied to clinical outcomes using CGM, but this metric cannot be applied to episodic testing with BGM; therefore we recommend an alternative, and potentially more readily understood, term of “readings in range” (RIR) to describe the clinical utility of a BGM intervention.
Although our prior analysis of real-world data for the OTVR Meter and the OTR app [18] demonstrated improved glycemic outcomes, our findings were limited by relatively short exposure to both devices and we thought it important to demonstrate that clinically relevant outcomes would be sustained. Therefore, the current analysis includes 7107 PwT1D and 32,719 PwT2D who experienced both devices for 6 months. Furthermore, the expanded scale of 55,000 PWDs allowed us to robustly investigate glycemic outcomes across various age ranges beyond simply above or below 65 years of age to determine how age might influence outcomes using these monitoring technologies.
Methods
The OTVR Meter has a ColorSure Dynamic Range Indicator® (see Fig. S1 in the electronic supplementary material for details) and a Blood Sugar Mentor® feature displaying one of 24 different on-meter messages based on a patient’s past and present blood glucose readings. Concordance with monitoring is supported by the OneTouch Delica Plus® Lancing System, the only lancing system cited in the ADA Standards of Care as a less painful system that may make BGM less burdensome to perform [19]. When used in conjunction with the OTR App, meter readings automatically sync with the app via Bluetooth®. PWDs can grant permission to share app data in real time with their HCPs who have the professional (web) version of the OTR App. For this retrospective data analysis, we used de-identified data from our LifeScan data lake. Data were obtained from registered users of LifeScan’s OneTouch Reveal app. Users who download the app are informed about LifeScan’s processing of personal data in accordance with its privacy policy. Users provide their explicit consent to the processing of their personal data as set out in the privacy policy for the app. The app’s privacy policy permits LifeScan to deidentify data and use the data to perform analytics, research, and product development. Additional ethics committee approval was not required, and no clinical sites or external investigators were involved in this analysis study. Our prior publication [18] also describes in detail the method we used to extract uploaded app data from our server while maintaining user data privacy and data protection. The current analysis request automatically fetched data from users who registered with the app between 7 May 2018 and 8 Sept 2022.
Statistical Analysis
All analyses were performed separately for each diabetes type. The number of days from when a subject first started using the OTR App with the OTVR Meter was determined, and time windows created for baseline (first 14 days of app usage) and 90 days and 180 days (last 14 days prior to the 90- and 180-day timepoints, respectively). For inclusion in either data set, subjects were required to have at least 90 readings within the 90-day timeframe or at least 180 readings within the 180-day timeframe. Subjects with data available within the respective 90- or 180-day windows were retained to enable pairwise comparisons between starting and ending values. Readings in range (RIR) were defined as glucose values from 70 to 180 mg/dL, hyperglycemia as > 180 mg/dL, and hypoglycemia as < 70 mg/dL. For each subject, the mean BG and percentage of readings within each of the glycemic indicator categories were calculated for baseline and 90- and 180-day time windows and the within-subject changes from baseline determined. All statistical comparisons between baseline and 90 and 180 days were performed by paired-sample t tests using IBM SPSS Statistics 21 and Minitab 20.
Results
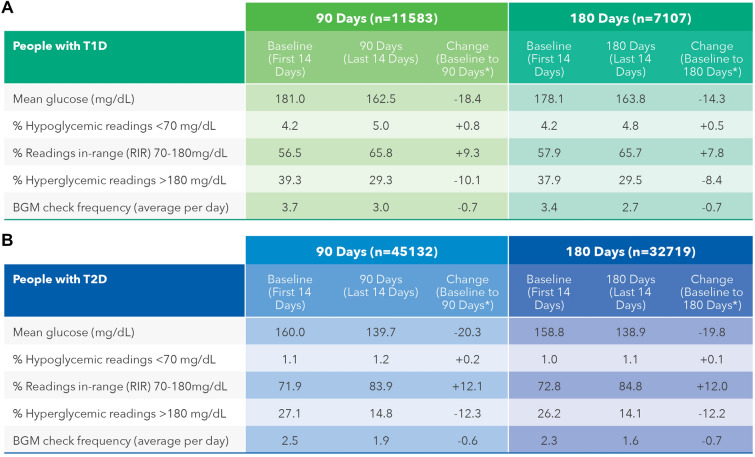
This analysis includes two separate data sets. The first data set covers 11,583 PwT1D and 45,132 PwT2D using the OTVR Meter with the OTR App over a 90-day timeframe and the second data set covers 7107 PwT1D and 32,719 PwT2D over a 180-day timeframe also using the OTVR Meter and OTR App. This section focuses on changes over 180 days given that sustained outcomes for PWDs are more clinically relevant.
Overall Changes in Glycemic Outcomes Over 180 Days
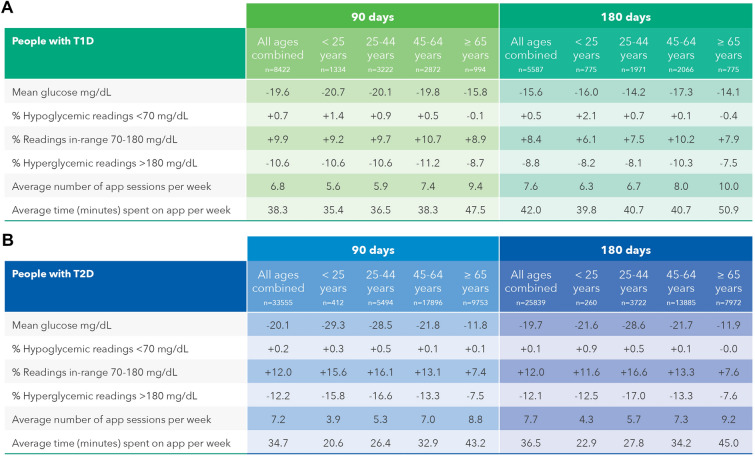
In PwT1D or T2D, mean BG reduced by − 14.3 and − 19.8 mg/dL, respectively, comparing baseline to 180 days. RIR improved significantly by 7.8 percentage points (57.9 to 65.7%) and 12.0 percentage points (72.8 to 84.8%) in PwT1D and PwT2D, respectively, over 180 days. Hyperglycemic readings were reduced by − 8.4 percentage points (37.9 to 29.5%) and − 12.2 percentage points (26.2 to 14.1%) in PwT1D and PwT2D, respectively, mirroring the positive changes in RIR over 180 days. Hypoglycemic readings increased marginally, but not clinically meaningfully, by 0.5 percentage points (4.2 to 4.8%) in PwT1D. The proportion of hypoglycemic readings in PwT2D was far lower and remained essentially unchanged during the study, increasing by only 0.1 percentage point (from 1.0% to 1.1%). Baseline monitoring frequency was higher for PwT1D than PwT2D (3.4 versus 2.3 checks per day). Both groups significantly reduced their monitoring frequency by 0.7 checks per day comparing baseline with 180 days. Similar glycemic changes and trends were observed in the 90-day data set for PWDs (Fig. 1). All above glycemic changes were statistically significant at p < 0.0005. Further analysis of the 180-day data set found 47.5% (3377 of 7107) of PwT1D improved RIR by > 5 percentage points and 37.8% (2686 of 7107) improved > 10 percentage points. Similarly, 48.9% (16,003 of 32,719) of PwT2D improved RIR by > 5 percentage points and 38.6% (12,629 of 32,719) improved > 10 percentage points over 180 days.
Fig. 1.
Summary of aggregated glycemic outcomes over 90 and 180 days. All above glycemic changes were statistically significant at p < 0.0005
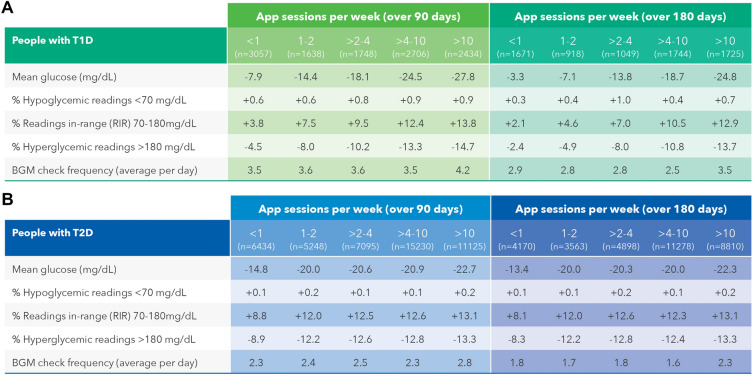
Effect of Number of OTR App Sessions on Glycemic Outcomes
Clinically significant lowering in mean BG was seen in PwT1D who did > 2 to 4 app sessions per week (− 13.8 mg/dL) over 180 days, with the largest reductions seen in those doing > 4 to 10 sessions (− 18.7 mg/dL) and > 10 sessions per week (− 24.8 mg/dL). PwT1D who conducted > 2 to 4 app sessions per week improved RIR by 7.0 percentage points and this increased to 10.5 percentage points when performing > 4 to 10 sessions per week. Clinically beneficial increases in RIR were detected alongside comparable reductions in hyperglycemic readings in PwT1D performing either > 2 to 4 or > 10 app sessions per week, respectively lowering readings in the hyperglycemic range by − 8.0 or − 13.7 percentage points (Fig. 2a). In the larger data set of 32,719 PwT2D, similar improvements in mean BG, RIR, and hyperglycemic readings were seen compared to those measured in PwT1D. However, glycemic improvements in PwT2D doing as few as just 1 or 1–2 app sessions per week were higher than in PwT1D and continued at this enhanced level as app engagement also increased (Fig. 2b). A higher frequency of BG checking was observed in PwT1D and PwT2D who completed the most app sessions per week compared with those performing the fewest. Changes in monitoring frequency could not account for the improvements seen in glycemic measures given that monitoring frequency did not change as a function of the number of app sessions performed at baseline or over 180 days.
Fig. 2.
Effect of number of OTR App sessions on glycemic outcomes in PWDs
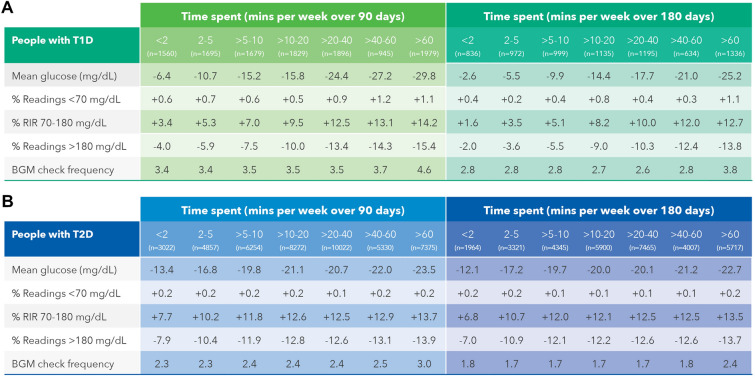
Effect of Time Spent Using the OTR App on Glycemic Outcomes
Lower mean BG values were found in PwT1D spending > 10 to 20 min per week on the OTR App (− 14.4 mg/dL) over 180 days, with the most pronounced changes seen in those spending either > 40 to 60 min (− 21.0 mg/dL) or > 60 min per week (− 25.2 mg/dL). Readings in range improved by 8.2 percentage points in PwT1D who spent > 10 to 20 min on the app, and this rose to 12.7 percentage points in those who spent > 60 min on the app. Improved RIR in PwT1D were consistent with nearly equivalent reductions in hyperglycemia, with hyperglycemic readings reducing by − 9.0 percentage points in those spending > 10 to 20 min on the app, improving to a − 13.8 percentage point reduction in those spending > 60 min per week on the app. (Fig. 3a). Encouragingly, glycemic outcomes in PwT2D were consistent with those seen in PwT1D, although PwT2D had more clinically meaningful improvements in outcomes at lower levels of app usage than PwT1D. For example, PwT2D who spent just > 5 to 10 min on the app lowered mean BG (− 19.7 mg/dL), increased RIR (12.0 percentage points), and reduced hyperglycemic readings by − 12.1 percentage points (Fig. 3b). In terms of app sessions, a higher frequency of checking was seen in both PwT1D (3.8 vs 2.8 checks per day) and PwT2D (2.4 vs 1.8 checks per day) in those spending the most compared to the least time on the app. Nonetheless, monitoring frequency was similar in PWDs within each category of time spent using the app from baseline to 180 days and this indicates that monitoring frequency per se cannot explain the glycemic changes seen.
Fig. 3.
Effect of time spent on the OTR App on glycemic outcomes in PWDs
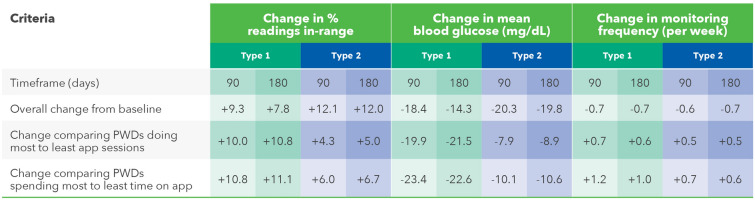
Effect of App Engagement Level on Glycemic Outcomes and Monitoring Behavior in PWDs
Improvements in overall mean BG and RIR were clinically significantly higher for PwT2D than for PwT1D (Fig. 1), although in PwT1D, enhancements in both metrics exceeded those of PwT2D comparing the most to the least app-engaged (Fig. 4). For example, RIR improved by 10.8 percentage points in PwT1D compared with 5.0 percentage points in PwT2D, when comparing PWDs completing the most to the fewest app sessions per week over 180 days. These findings are more apparent graphically (see Figs. S2 and S3 in the electronic supplementary material details), where changes in RIR were plotted in relation to app engagement. Although monitoring levels remained similar compared to baseline, we observed that those engaging the most with the app checked BG levels more often those who were least engaged with the app.
Fig. 4.
Effect of OTR App engagement level on glycemic outcomes and monitoring behavior in PWDs
Effect of Age of PWD on Overall Changes in Glycemic Outcomes
We created four categories of age ranges to enable analysis of the data. Unsurprisingly, there were far fewer PwT1D in the age 65 and over category at 180 days (11% of 7107 PWDs) compared with PwT2D (24% of 32,719 PWDs). Hypoglycemic readings increased more in PwT1D less than 25 years old (2.1 percentage points), which was fourfold higher than the increase in all age groups combined (0.5 percentage points). Hypoglycemic readings actually reduced in the 65 and over group (− 0.4 percentage points) at 180 days, a unique finding compared with all other age categories. PwT1D between 45 and 64 years of age had the largest improvement in RIR of 10.2 percentage points. Interestingly, PwT1D aged 65 or over were the most app-engaged compared with all age groups combined (performing 10 sessions per week vs 7.6) and especially compared with those aged less than 25 years (who performed 6.3 sessions per week). However, despite this engagement, improvement in RIR in PwT1Ds 65 years and older was lower (7.9 percentage points) than the 8.4 percentage points observed in the combined age data (Fig. 5a).
Fig. 5.
Effect of age of PWDs on overall changes in glycemic outcomes
Glycemic control as measured by mean BG, RIR, and exposure to hyperglycemia improved after 180 days in PwT2D 65 years and over, although improvements were lower compared with all ages combined and to each of the three younger age categories. People 65 years and older performed the most app sessions and spent the most time on the mobile app of any age category, with twice as many app sessions as the under 25s (9.2 sessions per week versus 4.3). Hypoglycemic readings increased more in PwT2D under 25 years (0.9 percentage points) and this declined markedly as age increased and, in fact, the 65 and older group experienced no increase in hypoglycemic readings despite an improved RIR of 7.6 percentage points, mimicking the hypoglycemia trends seen in PwT1D 65 years and older. The most pronounced glycemic improvements in mean BG and RIR were detected in PwT2D between 25 and 44 years of age, with mean BG reduced by − 28.6 mg/dL and RIR improved by 16.6 percentage points, outperforming all other age categories. In keeping with PwT1D, app engagement in PwT2D increased with advancing age (Fig. 5b).
Discussion
Our real-world analysis of more than 55,000 PWDs consolidates previous evidence and confirms the benefits gained by PwT1D and T2D who initiated use of the Bluetooth® connected OTVR Meter and the companion OTR Mobile App. This extensive data set enabled us to confirm in sub-analyses that PwT1D and T2D who interacted more frequently with the mobile app achieved better glycemic outcomes, such as reductions in mean BG and achievement of more RIR, than those with fewer interactions. Identifying the specific attributes that drove our positive patient outcomes is more challenging with a retrospective analysis of data; however, prior publications involving PWDs and HCPs illuminated how unique features of the OTVR Meter and OTR Mobile App support insights, decision-making, and potentially behavior change. For example, using color to enable PWDs to rapidly interpret the importance or context of a numerical reading is a prominent feature in both devices. We previously reported that insulin- and non-insulin-using PWDs experiencing a three-bar color range indicator were more inclined to act upon readings shown with color, and that associating readings with color was viewed as particularly beneficial by those with lower numeracy [20]. A follow-up study focusing on the seven-bar ColorSure Dynamic Range Indicator and associated Blood Sugar Mentor screens [21] on the OTVR Meter found PWDs were more willing to act on data displayed on the OTVR Meter compared with their current BGMs, with 71% saying they would check their glucose more often, especially when they could identify below-range glucose readings in real time, and a further 71% responding they were more likely to adjust insulin doses when information was presented with the variety of Blood Sugar Mentor screens available in the OTVR Meter compared with their current BGMs. These experiential studies provide insights regarding how long-term “home use” of the OTVR Meter and OTR Mobile App might support better diabetes management and lead to the improved readings in range we observed in this study.
It is important that PWDs perform “meaningful monitoring,” meaning they check blood glucose values at appropriate times and act upon individual readings (or glucose patterns) with behavioral, lifestyle, or therapeutic adjustments. However, a study in 207 PwT2D demonstrated subjects were more tolerant of high BG levels than is clinically advisable [22] and a further survey of 886 PwT2D found half of insulin- and non-insulin-using patients regularly took no action for out-of-range values with any self-care adjustments [23]. Therefore, there is value in highlighting below-range, in-range, and above-range readings and prompting PWDs to act both in the moment and after retrospectively reviewing trends or patterns on a mobile app.
Our aggregated data clearly show PwT1D have far fewer readings in range (57.9%) compared with PwT2D (72.8%) at baseline. This finding, based on many thousands of individual patients, may reflect that PwT1D experience more glycemic variability than PwT2D, and often check blood glucose values both pre- and post-prandially, such that additional readings falling outside of the in-range zone are more frequently available than for PwT2D. Irrespective of these differing clinical factors, we still observe significantly improving glycemic measures in both PwT1D and T2D, although PwT2D achieved higher magnitudes of reduction in their exposure to hyperglycemia (− 12.2 percentage points vs − 8.4 percentage points in PwT1D).
These overall changes in glycemia are given extra context by comparing OTR Mobile App engagement in PwT1D and T2D. Real-time Google analytics (GA) data recorded every interaction PWDs made with the OTR App, allowing us to categorize by engagement level, which we describe in terms of number of sessions and time spent on the app, noting that all PWDs visit the app home page, which shows a snapshot of recent BGM data and a timeline graphic showing BG data alongside any information PWDs have manually entered (e.g., insulin doses and lifestyle events). Higher app engagement of > 4 app sessions per week was found in 61% of PwT2D compared with 48% of PwT1D. Both of these levels of app interaction are impressive given that they represent sustained average engagement over 26 consecutive weeks. Encouragingly, there were almost twice as many PwT2D who performed more than 10 sessions per week than performed fewer than 1 session per week. The extra investment in reviewing and reflecting upon the data summaries and manually entered data paid dividends in terms of significantly better glycemic outcomes in those who used the OTR Mobile App more often. This benefit was more evident in PwT1D, who experienced a steeper trajectory of improvement, with RIR improvement of 2.1 percentage points in those performing fewer than 1 session per week, improving dramatically by an additional 10 percentage points in those performing more than 10 sessions per week.
A unique aspect to this analysis explored how age affected glycemic outcomes and the degree of engagement PWDs had with OTR. Our age data set comprised people who entered their birthdate during app registration (78.6% of PwT1D and 79.0% of PwT2D in the 180-day data set). We found that 14% of PwT1D and 31% of PwT2D were 65 years of age and older, in keeping with the later age of onset of type 2 diabetes. The outcomes and behaviors of PwT1D and PwT2D who were 65 years and older were remarkably similar, each improving RIR by 7.9 percentage points and 7.6 percentage points, respectively, performing similar numbers of sessions (10.0 vs 9.2 sessions per week), and spending similar amounts of time per week on the app (50.9 vs 45.0 min). It is not surprising that in people aged 65 and older, having the highest app engagement, did not directly translate to the largest improvement in glycemic outcomes compared to the other age categories. ADA Standards of Care state that glycemic goals for some older adults might reasonably be relaxed such that while older adults who are otherwise healthy with few coexisting chronic illnesses and intact cognitive function and functional status should have lower glycemic goals (such as A1c < 7.0–7.5%), those with multiple coexisting chronic illnesses, cognitive impairment, or functional dependence should have less-stringent glycemic goals (such as A1c < 8.0%) [24].
We anticipated more hypoglycemic readings in PwT1D than in PwT2D, but found the number of hypoglycemic readings were low and fairly similar in both data sets in people aged 65 and older. Number of hypoglycemic readings diminished significantly with increasing age in PwT1D and PwT2D, and this trend was mirrored by patients with increasing age spending increasingly more time on the app. It would be difficult to conclude that app usage per se lowered exposure to hypoglycemia but the meter and app complement each other in automatically alerting patients to low readings and low glucose patterns, highlighting times of the day and/or week when patients are at elevated risk for experiencing lows. This information enables patients to consider what triggers such events and make appropriate adjustments to lower future risk. This is important given that a retrospective study of PwT2D found that a higher proportion of those who experienced severe hypoglycemia had a documented prior history of non-severe hypoglycemia than those who did not have severe hypoglycemia [25]. When our real-world evidence (RWE) was stratified by age, we found the highest numbers of hypoglycemic readings in those less than 25 years old, especially in PwT1D. It is clear from published literature that younger adults, especially those with suboptimal glycemic control, appear to be at greater risk for acute (and chronic) complications than older adults with comparable glycemic control [26].
BGM systems offering either cellular or Bluetooth® connectivity to diabetes apps are now commonplace, with emerging evidence to support their clinical value. The REALL study [27] observed that PwT2D using the Contour Next One BGM and Contour App lowered mean BG by − 22.5 mg/dL, which is comparable to mean BG reductions in our study, although over a shorter 4-month timeframe and with a much smaller data set of 461 PwT2D, none of whom were over 65 years of age. However, in contrast to our study, the investigators did not observe significant glycemic differences based on frequency, intensity, or duration of app use. A retrospective study in 2104 highly engaged mySugr app users with T1D (logging app data ≥ 5 days/week for ≥ 6 months) observed that mean BG was lowered on average by − 7.7 mg/dL after 6 months; however, it was unclear which specific connected BGMs were used in tandem with the mySugr app [28]. A further study exploring mySugr in 440 highly app-engaged, high-risk PwT1D found that mean BG decreased by − 17.9% (from 210.8 to 173.1 mg/dL), which is higher than the changes we observed, albeit from a significantly higher baseline [29]. A variety of additional publications from Dario Health, Livongo, One Drop, and Ascensia Diabetes Care attest to the value of combining connected BGMs with diabetes apps [30–33].
With RWE data, there are often limitations in terms of knowledge of the subjects’ medical history, adherence to and/or changes in diabetes medications, the clinical goals set by HCPs, and measured A1c values. Furthermore, we cannot verify the type of HCPs, how they used the meter or app data to adjust therapy, or how often therapy changes were made, and it is unknown if subjects were offered CGM while continuing to use BGM. In order to capture as diverse a population of PwT2D and PwT1D as possible, we set a relatively low BG checking frequency of ≥ 1 check per day when retrieving data from the data lake. Furthermore, the source data did not include information on whether subjects performed structured monitoring; consequently, it was not possible to reliably confirm changes in glycemic variability over time. While it is worth noting that PWDs can upload data using a standard cable from non-connected BGMs and view their data on their home computer using the web version of the OTR app, very few patients are represented by this use case in the data lake. We have observed previously that PWDs using our connected BGMs in the absence of the OTR app achieved clinically meaningful glycemic improvements, suggesting that the on-board insight and guidance features on our latest BGMs also improve outcomes [34, 35].
Conclusion
Sustained improvements in readings in-range over 6 months were observed in PWDs using an advanced Bluetooth® connected blood glucose meter with its companion mobile diabetes management app and these improvements were proportionate with increasing app engagement. Sustained improvements were apparent in all age groups, including PWDs over 65 years of age, who also were more engaged with the diabetes app than younger age groups.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank users of the OneTouch Reveal app for sharing their health data to enable this analysis.
Funding
Funding for analysis and manuscript preparation was provided by LifeScan Inc, Malvern, PA 19355 USA. LifeScan also funded the journals Rapid Service Fee.
Medical Writing and Editorial Assistance
The authors thank Animesh Mahapatra from the LifeScan IT team for assistance sourcing server app data for the manuscript. We also thank INL-agency.com for graphics assistance with all figures and tables, which was funded by LifeScan.
Author Contributions
Mike Grady and Elizabeth Holt wrote the manuscript. Hilary Cameron analyzed the data and reviewed the final manuscript.
Prior Publication
An interim presentation of this analysis, based on fewer study subjects, was presented at ADA 82nd Scientific Session, June, 2022 as Poster 61-LB.
Disclosures
Mike Grady, Hilary Cameron, and Elizabeth Holt are all current employees of LifeScan.
Compliance with Ethics Guidelines
For this retrospective data analysis, we used de-identified data from our LifeScan data lake. Data were obtained from registered users of LifeScan’s OneTouch Reveal app. Users who download the app are informed about LifeScan’s processing of personal data in accordance with its privacy policy. Users provide their explicit consent to the processing of their personal data as set out in the privacy policy for the app. The app’s privacy policy permits LifeScan to deidentify data and use the data to perform analytics, research and product development. Additional ethics committee approval was not required, and no clinical sites or external investigators were involved in this analysis study.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Mike Grady, Email: mgrady@lifescan.com.
Elizabeth Holt, Email: eholt@lifescan.com.
References
- 1.Chircop J, Sheffield D, Kotera Y. Systematic review of self-monitoring of blood glucose in patients with type 2 diabetes. Nurs Res. 2021 doi: 10.1097/NNR.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 2.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S., adults 1999–2018. N Engl J Med. 2021;384:2219–2228. doi: 10.1056/NEJMsa2032271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruger DF, Anderson JE. Continuous glucose monitoring (CGM) is a tool, not a reward: unjustified insurance coverage criteria limit access to CGM. Diabetes Technol Ther. 2021 doi: 10.1089/dia.2021.0193. [DOI] [PubMed] [Google Scholar]
- 4.Beck R, Riddlesworth TD, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 5.Beck R, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. doi: 10.7326/M16-2855. [DOI] [PubMed] [Google Scholar]
- 6.Varzic S, Steiner K, Centner M, et al. Assessment of freestyle libre flash glucose monitoring system implementation in real life clinical setting: a prospective observational study. Diagnostics (Basel) 2021;11(2):305. doi: 10.3390/diagnostics11020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson AL, Daniel TD, DeSantis A, et al. Flash glucose monitoring in type 2 diabetes managed with basal insulin in the USA: a retrospective real-world chart review study and meta-analysis. BMJ Open Diab Res Care. 2022;10:e002590. doi: 10.1136/bmjdrc-2021-002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37–46. doi: 10.1016/j.diabres.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Liang B, Koye DN, Hachem M, Zafari N, Braat S, Ekinci EI. Efficacy of flash glucose monitoring in type 1 and type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Front Clin Diabetes Healthc. 2022;3:849725. doi: 10.3389/fcdhc.2022.849725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz LB, Grady M, Levy BL, Setford SJ. The impact of new technologies on the efficacy of self-monitoring blood glucose using OneTouch blood glucose monitoring systems. US Endocrinol. 2018;14(suppl. 1):2–8. [Google Scholar]
- 11.Grady M, Lamps G, Shemain A, Cameron H, Murray L. Clinical evaluation of a new, lower pain, One Touch lancing device for people with diabetes: virtually pain-free testing and improved comfort compared to current lancing systems. J Diabetes Sci Technol. 2019 doi: 10.1177/1932296819856665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262–2272. doi: 10.1001/jama.2021.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura A, Harashima S, Fujita Y, et al. Effects of structured testing versus routine testing of blood glucose in diabetes self-management: a randomized controlled trial. J Diabet Complicat. 2017 doi: 10.1016/j.jdiacomp.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Bergenstal RM, Mullen DM, Strock E, Johnson ML, Xi MX. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabet Complicat. 2022 doi: 10.1016/j.jdiacomp.2021.108106. [DOI] [PubMed] [Google Scholar]
- 15.Katz LB, Stewart L, Guthrie B, Cameron H. Patient satisfaction with a new, high accuracy blood glucose meter that provides personalized guidance, insight and encouragement. J Diabetes Sci Technol. 2020;14(2):318–323. doi: 10.1177/1932296819867396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Sayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. 7. Diabetes technology: standards of care in diabetes—2023. Diabetes Care. 2023;46(Suppl. 1):S111–S127. doi: 10.2337/dc23-S007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwood DA, Grady M. Healthcare professional perceptions of blood glucose meter features that support achievement of self-management goals recommended by clinical practice guidelines. J Diabetes Sci Technol. 2020 doi: 10.1177/1932296820946112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grady M, Cameron H, Bhatiker A, Holt E, Schnell O. Real-world evidence of improved glycemic control in people with diabetes using a bluetooth-connected blood glucose meter with a mobile diabetes management app. Diabetes Tech Ther. 2022 doi: 10.1089/dia.2022.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. 7. Diabetes technology: standards of care in diabetes—2023. Diabetes Care. 2023;46(Suppl. 1):S111–S127. doi: 10.2337/dc23-SDIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grady M, Katz LB, Strunk CS, Cameron H, Levy BL. Examining the impact of a novel blood glucose monitor with color range indicator on decision-making in patients with type 1 and type 2 diabetes and its association with patient numeracy level. J Med Internet Res Diabetes. 2017;2(2):e24. doi: 10.2196/diabetes.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grady M, Cameron H, Katz LB. Patients with diabetes using a new glucose meter with blood sugar mentor and dynamic color range indicator features show improved interpretation and willingness to act on blood glucose results (ASCEND study) J Diabetes Sci Technol. 2021;15(5):1168–1176. doi: 10.1177/1932296820949873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans JMM, Mackison D, Vivien SV. Self-monitoring of blood glucose in type 2 diabetes: patients’ perceptions of ‘high’ readings. Diabetes Res Clin Pract. 2013;102(1):e5–e7. doi: 10.1016/j.diabres.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Polonsky WH, Fisher L, Hessler D, Edelman SV. A survey of blood glucose monitoring in patients with type 2 diabetes: are recommendations from health care professionals being followed? Curr Med Res Opin. 2011;27(Suppl 3):31–37. doi: 10.1185/03007995.2011.599838. [DOI] [PubMed] [Google Scholar]
- 24.ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. 13. Older adults: standards of care in diabetes—2023. Diabetes Care. 2023;46(1):S216–S229. doi: 10.2337/dc23-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misra-Hebert AD, Pantalone KM, Ji X, Milinovich A, et al. Patient characteristics associated with severe hypoglycemia in a type 2 diabetes cohort in a large, integrated health care system from 2006 to 2015. Diabetes Care. 2018;41(6):1164–1171. doi: 10.2337/dc17-1834. [DOI] [PubMed] [Google Scholar]
- 26.Pettus JH, Zhou FL, Shepherd L, Preblick R, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019;42(12):2220–2227. doi: 10.2337/dc19-0830. [DOI] [PubMed] [Google Scholar]
- 27.Fisher L, Fortmann A, Knaebel J, Stuhr A. Can a basic management app paired with a glucose meter help reduce glucose levels among adults with type 2 diabetes? The REALL study. J Diabetes Sci Technol. 2022;9:19322968221096163. doi: 10.1177/19322968221096163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hompesch M, Hergesheimer L, Kalcher K, et al. Retrospective analysis of Impact on SMBG and glycemic control of mobile health (mHealth)-application for diabetes management. J Diabetes Sci Technol. 2017;11:A31. [Google Scholar]
- 29.Hompesch M, Kalcher K, Debong F, Morrow L. Significant improvement of blood glucose control in a high risk population of type 1 diabetes using a mobile health app: a retrospective observational study. Diabetes Technol Ther. 2017;64(Suppl. 1):2337. [Google Scholar]
- 30.Fundoiano-Hershcovitz Y, Hirsch A, Dar S, et al. Role of digital engagement in diabetes care beyond measurement: retrospective cohort study. JMIR Diabetes. 2021;6(1):e24030. doi: 10.2196/24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amante D, Harlan DM, Lemon SC, et al. Evaluation of a diabetes remote monitoring program facilitated by connected glucose meters for patients with poorly controlled type 2 diabetes: randomized crossover trial. JMIR Diabetes. 2021;6(1):e25574. doi: 10.2196/25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborn CY, Van Ginkel JR, Rodbard D, et al. One drop | mobile: an evaluation of hemoglobin A1c improvement linked to app engagement. JMIR Diabetes. 2017;2(2):e21. doi: 10.2196/diabetes.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandran SR, Tan HC, Bee YM, et al. Telemonitoring with a connected glucose meter improves glycemia among people with insulin-treated type 2 diabetes. J Diabetes Sci Technol. 2023 doi: 10.1177/19322968231157387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grady M, Katz LB, Cameron H, Levy BL. Diabetes app-related text messages from health care professionals in conjunction with a new wireless glucose meter with a color range indicator improves glycemic control in patients with type 1 and type 2 diabetes: randomized controlled trial. J Med Internet Res Diabetes. 2017;2(2):e19. doi: 10.2196/diabetes.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grady M, Katz LB, Levy BL. Blood glucose meters featuring color range indicators improve glycemic control in patients with diabetes in comparison to blood glucose meters without color (ACCENTS Study) J Diabetes Sci Technol. 2018;12(6):1211–1219. doi: 10.1177/19322968187757555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.