Abstract
Retinoic-acid-receptor-related orphan receptor γ (RORγ) is a major transcription factor for proinflammatory IL-17A production. Here, we revealed that the RORγ deficiency protects mice from STZ-induced Type 1 diabetes (T1D) through inhibiting IL-17A production, leading to improved pancreatic islet β cell function, thereby uncovering a potential novel therapeutic target for treating T1D. We further identified a novel RORγ inverse agonist, ginseng-derived panaxadiol, which selectively inhibits RORγ transcriptional activity with a distinct cofactor recruitment profile from known RORγ ligands. Structural and functional studies of receptor-ligand interactions reveal the molecular basis for a unique binding mode for panaxadiol in the RORγ ligand-binding pocket. Despite its inverse agonist activity, panaxadiol induced the C-terminal AF-2 helix of RORγ to adopt a canonical active conformation. Interestingly, panaxadiol ameliorates mice from STZ-induced T1D through inhibiting IL-17A production in a RORγ-dependent manner. This study demonstrates a novel regulatory function of RORγ with linkage of the IL-17A pathway in pancreatic β cells, and provides a valuable molecule for further investigating RORγ functions in treating T1D.
Keywords: RORγ, crystal structure, drug discovery, inverse agonist, panaxadiol
Introduction
Type 1 diabetes (T1D) occurs when the autoimmune destruction of insulin-producing pancreatic β cells caused insulin deficiency, and eventually resultant hyperglycaemia [1, 2]. Nowadays, there had been an increased incidence of T1D around the world, and the effective treatment is limited, such as the lifelong insulin therapy that can delay the progression of the disease [3]. However, insulin therapy is often accompanied by T1D-associated complications, including retinopathy, neuropathy, cardiovascular disease and hypoglycaemia [4]. Thus, it is important to study the T1D-associated regulating mechanism in pancreatic β cells, which may provide new therapeutic targets and strategies. The pathogenic role of T helper 17 (Th17) cells has been demonstrated as a contributor to T1D development through stimulating the production of proinflammatory cytokine interleukin 17 A (IL-17A) [5, 6], since the production of IL-17A results in inflammatory responses and autoimmune destruction of insulin-producing β cells in the pancreas [7, 8]. Therefore, therapies that target the IL-17A/Th17 cells might be a promising strategy for the prevention and treatment of T1D [9].
The retinoic-acid-receptor-related orphan receptor γ (RORγ) is a member of the nuclear receptor (NR) superfamily whose activity has been implicated in immune responses and autoimmune diseases [10–12]. Like other nuclear receptors, RORγ regulates the gene transcription by binding to specific sequences of DNA of its target genes [13, 14]. RORγ contains an activation function (AF-2) located at the C terminus of its ligand-binding domain (LBD) that is key to the transcriptional regulation. The ligand binding to RORγ LBD induces the conformational changes of the AF-2 helix that can recruit or expel coactivators/corepressors in regulating the transcription of its target genes [15, 16]. Notably, RORγ has been reported as a major transcription factor for Th17 cell differentiation and IL-17A production [17–19]. So far, RORγ inverse agonists have been developed as one of the promising strategies for treating IL17A/Th17 cell-mediated autoimmune diseases [20, 21]. For example, both RORγ natural antagonist digoxin and ursolic acid (UA) suppress IL-17A production [22–24]. However, the toxic side effects and cross-activity with other targets of these compounds have limited their therapeutic uses for autoimmune diseases [25, 26]. As such, the identification of alternative RORγ ligands is still an utmost need to yield more efficacious RORγ-targeted drugs.
Given the critical roles of IL-17A involved in RORγ signaling and T1D progression, we hypothesized that RORγ is involved in the development of T1D. We established STZ-induced T1D mice models and found that RORγ deficiency protects mice from STZ-induced T1D with improved pancreatic islet β cell function through inhibiting IL-17A production [27, 28], implying that RORγ inverse agonists can be considered as a new attractive strategy for T1D treatment. Herein, we performed a high-throughput AlphaScreen™ assay to search for novel RORγ inverse agonists with distinct binding modes and improved safety from the Traditional Chinese Medicine Monomer Library. Surprisingly, ginseng-derived panaxadiol was uncovered to selectively inhibit RORγ transcriptional activity with a unique cofactor recruiting profiles from known RORγ inverse agonists. Unlike RORγ-UA complex structures, the C-terminal AF-2 helix of RORγ-panaxadiol complex is positioned in a canonical active conformation. Interestingly, panaxadiol also alleviates STZ-induced T1D symptoms by inhibiting IL-17A production. Consequently, RORγ may be a crucial regulator and new therapeutic target in treating T1D.
Materials and methods
Protein preparation
The human RORγ LBD (residues 262–507) was expressed as N-terminal 6×His fusion protein from the expression vector pET24a (Novagen). BL21 (DE3) cells transformed with expression plasmids were grown in LB broth at 25 °C to an OD600 of ~1.0 and induced with 0.1 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG) at 16 °C for 16–18 h. Cells were harvested and sonicated in 200 mL extraction buffer (25 mM Tris pH 7.5, 500 mM NaCl, 10% glycerol and 25 mM imidazole) per 6 liters of cells. The lysate was centrifuged at 20,000 rpm for 30 min, and the supernatant was loaded on a 5 mL Ni-loaded HiTrap HP column (GE Healthcare). The column was washed with extraction buffer and the protein was eluted with a gradient of 25–500 mM imidazole. The RORγ LBD was further purified by gel filtration (elution buffer, 25 mM Tris-HCl (pH 7.5), 100 mM NaCl, 2 mM DTT) using a HiLoad 26/600 Superdex 200 column (GE Healthcare) with a 5-fold excess of panaxadiol and a 2-fold excess of the SRC2-2 peptide (KHKILHRLLQDSS) to the purified protein, followed by filter concentration to 10 mg/ml.
Coactivator binding assays
The binding of the various cofactor peptide motifs to the RORγ LBD in response to ligands was determined by AlphaScreenTM (Amplified Luminescent Proximity Homogeneous Assay Screen) assays, using a hexahistidine detection kit from Perkins-Elmer as described before [29]. Compounds were added to the mixture comprised of approximately 20–40 nM RORγ LBD and 20 nM biotinylated cofactor peptides in the presence of 5 µg/mL streptavidin donor and nickel chelate acceptor beads in a buffer containing 50 mM MOPS, 50 mM NaF, 0.05 mM CHAPS, and 0.1 mg/mL bovine serum albumin, all adjusted to a pH of 7.4. Luminescence signal was detected by a Perkins-Elmer multimode microplate reader. The peptides with an N-terminal biotinylation are listed below:
SRC1-2, SPSSHSSLTERHKILHRLLQEGSP;
SRC2-3, QEPVSPKKKENALLRYLLDKDDTKD;
SRC3-3, PDAASKHKQLSELLRGGSG;
NCOR-2, GHSFADPASNLGLEDIIRKALMGSF;
SMRT-2, ASTNMGLEAIIRKALMGKYDQ.
Luciferase reporter assays
HEK-293T cells were maintained in DMEM containing 10% fetal bovine serum and were transiently transfected using Lipofectamine 2000 (Invitrogen). All mutant RORγ plasmids were created using the Quick-Change site-directed mutagenesis kit (Stratagene). The resulting plasmids were confirmed by DNA sequencing. Before 24 h of transfection, 24-well plates were plated (5 × 104 cells per well). For nuclear receptor luciferase reporter assay, the cells were transfected with 200 ng Gal4-LBDs of various nuclear receptors and 200 ng of pG5Luc reporter (Promega). For native promoter reporter assays, the cells were co-transfected with plasmids encoding full length RORγ and ROR response element (RORE) or Il17a promoter luciferase reporter plasmid [18, 30]. Ligands were added 5 h after transfection. Cells were harvested 24 h later for the luciferase assays with the Dual-Luciferase Reporter assay system (Promega). The luciferase activities were normalized to renilla activity co-transfected as an internal control. The dose curves were fitted by GraphPad Prism 8.
Thermal shift assay
Thermal shift assay (TSA) was performed using 20 μL experimental unit containing 10 μM RORγ LBD, five-fold molar compounds and 2.5× SYPRO Orange (Sigma) in buffer containing 25 mM Tris, 150 mM NaCl and 5 mM dithiothreitol (DTT), pH 7.5. The samples were heated from 35 to 75 °C at a rate of 0.5 °C per 5 s and the fluorescence data were obtained on a CFX96 Real-Time PCR System (Bio-Rad). The 50% of maximum temperature (Tm) values of proteins were calculated by GraphPad Prism 8 and fitted using Boltzmann sigmoid curves.
Animals
6–8-week-old male C57BL/6 wild-type (WT) mice and RORγ−/− mice were used. RORγ−/− mice (Stock No: 007571) were purchased from The Jackson Laboratory. Mice were housed and bred under specific pathogen-free conditions. All of the animal experiments were approved by the Animal Ethics Committee of Xiamen University (acceptance no. XMULAC20170313). All animal experiments were performed in compliance with the guidelines from the Institutional Animal Care and Use Committee at Experimental Animal Centre at Xiamen University.
Type 1 diabetes induction and treatment
6-8-week-old male wild-type C57BL/6 J (WT) and homozygous RORγ-deficient (RORγ KO) mice were daily injected intraperitoneally (i.p.) with 50 mg/kg of STZ (MCE) for 5 consecutive days. STZ was diluted in sodium citrate buffer (pH = 4.5), and immediately injected within 20 min of preparation. After blood glucose monitoring on 4 and 7 days post the first injection, mice with fasting blood glucose higher than 13.3 mM were regarded as diabetic. UA (50 mg/kg every day, TargetMol) and panaxadiol (50 mg/kg every day, TargetMol) were administered from first STZ injection, and continued until the end of the experiments.
IL-17A production assay in mouse Th17 cells
Mouse CD4+ T cells were prepared from mouse splenocytes by negative selection using magnetic beads coated with antibodies that capture unwanted cells using MojoSort™ Mouse CD4+ Naïve T Cell Isolation Kit (BioLegend) following the manufacturer’s protocol. Isolated naive CD4+ T cells were cultured in Th17-skewing conditions for 5 days. Th17 skewed using CellXVivo Mouse Th17 Cell Differentiation Kit (R&D Systems). The concentrations of IL-17A in the culture media were measured by ELISA kit (R&D Systems).
Quantitative real-time PCR (qPCR)
Total RNA was extracted from jurkat cells with Trizol reagent (Sigma). RNA was reverse transcribed using the TAKARA reverse transcription kit. Real-time quantitative PCR was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad) using Hieff qPCR SYBR Green Master Mix (Yeasen Biotech). The primer sequence of IL-17A was reported before [31]. The mRNA expression was normalized to GAPDH.
ELISA
The production of IL-17A in the pancreatic tissue of mice was quantified by ELISA kit following the manufacturer’s instruction (R&D Systems).
Histology and immunohistochemistry
Pancreas samples were fixed overnight in 4% paraformaldehyde, embedded in paraffin and the sections (5 μm) stained with hematoxylin-eosin (H&E). Immunohistochemistry reactions were performed on sections (5 μm) of paraformaldehyde-fixed tissue. The sections were dewaxed, rehydrated, and incubated with 3% H2O2 to block endogenous peroxidase. Then, the slides were incubated with 5% BSA (Boster) to block unspecific staining. Next, the sections were stained with anti-insulin (1:64000, Abcam 181547) or anti-glucagon (1:8000, Abcam ab92517) antibodies, and the staining was visualized with anti-rabbit DAB-HRP (Boser) secondary antibodies. Finally, the sections were counterstained with hematoxylin and analyzed.
Crystallization and structure determination
The crystals of RORγ/panaxadiol complex were grown at room temperature in hanging drops containing 1.0 μl of the ligand-protein solutions and 1.0 μl of well buffer containing 0.1 M BIS-TRIS pH 5.5, 3.0 M sodium chloride. The crystals were directly flash frozen in liquid nitrogen for data collection. Diffraction data were collected at beamline BL17U1 of the Shanghai Synchrotron Radiation Source. The observed reflections were reduced, merged and scaled with DENZO and SCALEPACK in the HKL2000 package [32]. The search model was 3L0L in the Protein Data Bank. The structures were determined by molecular replacement in the CCP4 suite [33]. Manual model building was carried out with Coot [34], followed by Refmac5 refinement in the CCP4 suite [35]. The structure figures were made with PyMOL (version 2.3.3, Schrödinger). The structure has been deposited in the PDB with accession code 7W3P.
Statistical analysis
All values are expressed as mean ± SEM Differences were analyzed using Student’s t-test and P < 0.05 was considered statically significant.
Results
RORγ deficiency protects mice from STZ-induced type 1 diabetes
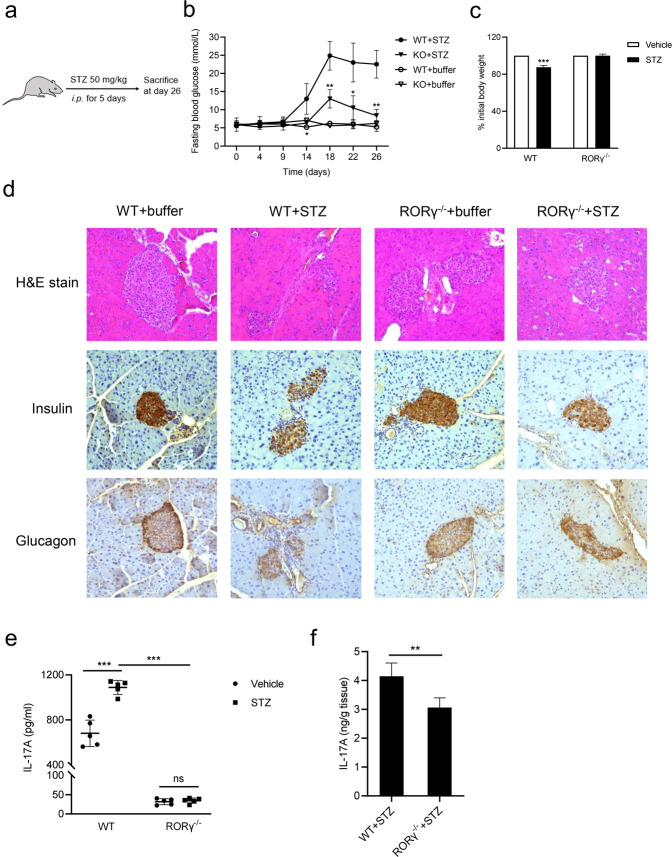
To investigate the possible role of RORγ in T1D, we established multiple low-dose STZ models of wild-type (WT) mice or RORγ deficiency (RORγ−/−) mice, by continuous blood glucose monitoring (Fig. 1a). As expected, STZ-treated WT mice showed a significantly increased blood glucose level. Interestingly, STZ-treated RORγ−/− mice presented remarkably lower blood glucose level compared with STZ-treated WT mice (Fig. 1b). The body weight of STZ-treated WT mice lost significantly compared with WT mice, whereas STZ-treated RORγ−/− mice remained unchanged (Fig. 1c). In addition, STZ-treated RORγ−/− mice showed a decreased inflammatory infiltration around the pancreatic islets and better preservation of insulin-producing β-cells and glucagon-producing α-cells compared with STZ-treated WT mice (Fig. 1d). Recent evidence suggested that IL-17A has been involved in the development of T1D [36–38], while RORγ is a major transcription factor associated with IL-17A production. As such, we detected the abundance of IL-17A in STZ-treated RORγ−/− mice. As expected, IL-17A production in WT mice increased after STZ treatment, whereas IL-17A levels in STZ-treated RORγ−/− mice were significantly lower compared with that in STZ-treated WT mice (Fig. 1e). The level of IL-17A was also reduced in the pancreas of STZ-treated RORγ−/− mice (Fig. 1f), in agreement with the positive regulatory roles of IL-17A by RORγ activation. Together, our results demonstrate that RORγ deficiency protects mice from STZ-induced T1D through improving pancreatic islet β cell function by inhibiting IL-17A production, thereby uncovering a potential novel therapeutic target for the treatment of T1D.
Fig. 1. RORγ deficiency protects mice from STZ-induced type 1 diabetes.
a Schematic diagram for the WT and RORγ−/− mice experiment. b Fasting blood glucose levels were measured from day 1 (first injection) to day 26. c Percentage of initial body weight during STZ treatment. d Pancreata were collected for hematoxylin and eosin (H&E) staining, and histology and immunohistochemistry analysis of insulin and glucagon. e IL-17A expression levels of splenocytes in STZ-treated WT and RORγ−/− mice. Isolated naive CD4+ T cells were cultured in Th17-skewing conditions for 5 days, and IL-17A in the supernatants were measured by ELISA. f Pancreata were collected at day 26 for the measurement of IL-17A production by ELISA. The results are representative of three independent experiments and are expressed as the means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
We further explored the potential therapeutic possibility of RORγ modulation in T1D by treating STZ-treated WT mice with RORγ modulators. For now, RORγ inverse agonist UA has been shown to bind to the ligand-binding domain of RORγ, inhibiting its transcriptional activity and thereby suppressing Th17 cells differentiation and dramatically reducing IL-17A levels [24, 39]. Indeed, UA treatment markedly ameliorated blood glucose change during STZ induction of T1D (Supplementary Fig. S1a). Haematoxylin and eosin (H&E) and histochemical staining results showed that UA treatment prevented inflammatory infiltration into the pancreatic islets with better preservation of insulin-producing β-cells and glucagon-producing α-cells (Supplementary Fig. S1b). It also decreased IL-17A levels in the splenocytes and pancreas of STZ-treated mice (Supplementary Fig. S1c, d). These results indicate that RORγ antagonism alleviates STZ-induced T1D symptoms.
Identification of Ginseng-derived panaxadiol as a RORγ inverse agonist
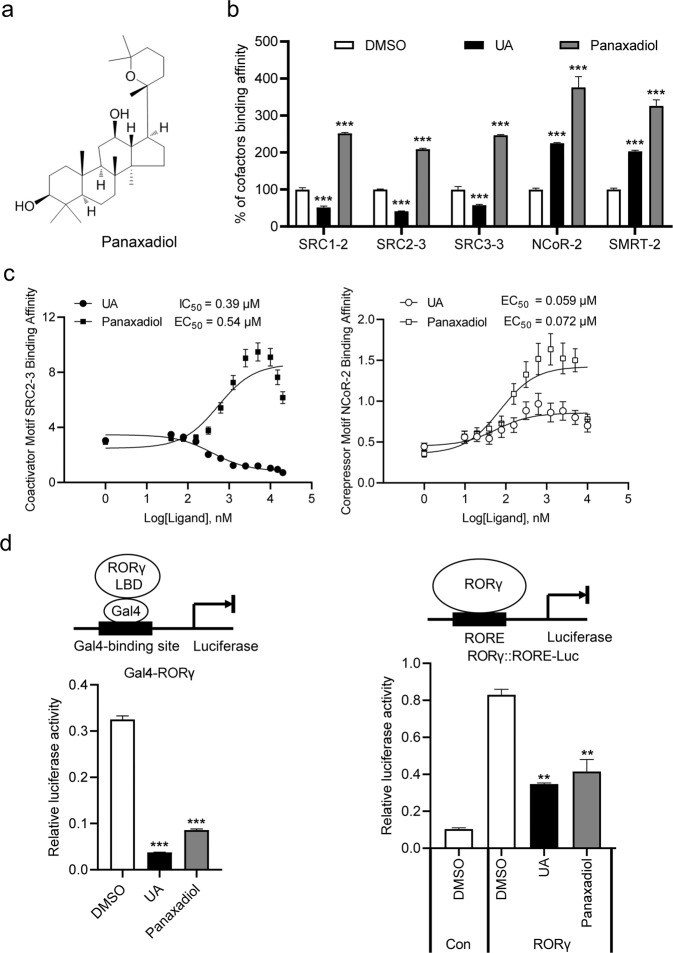
Since that the severe toxicity and side effects of current RORγ ligands, like UA, limit their therapeutic uses, we need to search for novel modulators of RORγ with distinct binding modes and improved safety. We used RORγ LBD as a bait to screen Traditional Chinese Medicine Monomer Library based on AlphaScreen biochemical assay, which is widely used for detecting ligand-dependent interactions between nuclear receptors and their cofactors [40], and the ginseng-derived panaxadiol was discovered as an interesting RORγ modulator (Fig. 2a). Surprisingly, panaxadiol shows a different cofactor recruitment profile from RORγ antagonist UA. Unlike the selective recruitment of corepressor motifs by UA-bound RORγ, panaxadiol enhanced the interaction of RORγ with both coactivator motifs (SRC1-2, SRC2-3 and SRC3-3) and corepressor motifs (NCoR-2 and SMRT-2) (Fig. 2b). Furthermore, full-dose curves revealed that panaxadiol promoted the interaction of RORγ with coactivator and corepressor motifs in a concentration-dependent manner (Fig. 2c), reaffirming panaxadiol as a highly potent RORγ ligand.
Fig. 2. Identification of Ginseng-derived panaxadiol as a unique RORγ inverse agonist.
a Chemical structure of panaxadiol. b Panaxadiol promotes the interaction of co-activator and co-repressor motifs with RORγ, shown by AlphaScreen assay. This displays the interaction of RORγ with various co-factor motifs in response to 1 μM panaxadiol and UA. c Concentration-response curves for UA and panaxadiol in inducing RORγ to recruit the coactivator and corepressor motifs, respectively, by AlphaScreen assay. d Transactivation of Gal4-RORγ and full-length RORγ reporter assay for UA and panaxadiol at a 1 μM concentration. The results are the average of experiments performed in triplicate, with error bars indicating SDs; **P < 0.01, ***P < 0.001, compared with vehicle.
To further explore the physiological roles of panaxadiol in RORγ signaling, cell-based reporter assays were employed to characterize the transcriptional properties of RORγ in response to panaxadiol. Interestingly, panaxadiol significantly inhibited RORγ transcriptional activity in cell-based Gal4-RORγ and full length RORγ-dependent reporter assays (Fig. 2d). Furthermore, full-dose curves revealed that panaxadiol inhibited RORγ transcriptional activity in a concentration-dependent manner with an IC50 similar to that of UA (Supplementary Fig. S2a), without impacts on RORα and RORβ tested (Supplementary Fig. S2b). Additionally, the thermal shift assay (TSA) [41] showed that panaxadiol increased the thermal stability of RORγ (Supplementary Fig. S3). Taken together, our results suggest that panaxadiol is a highly potent RORγ selective inverse agonist.
Structure of the RORγ LBD in complex with panaxadiol
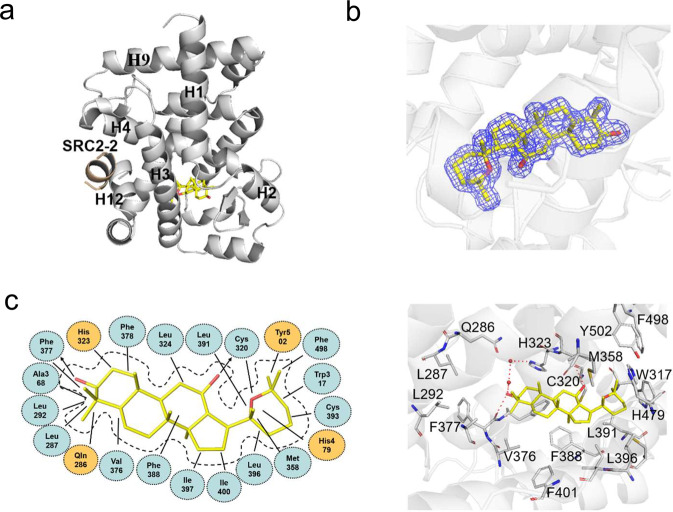
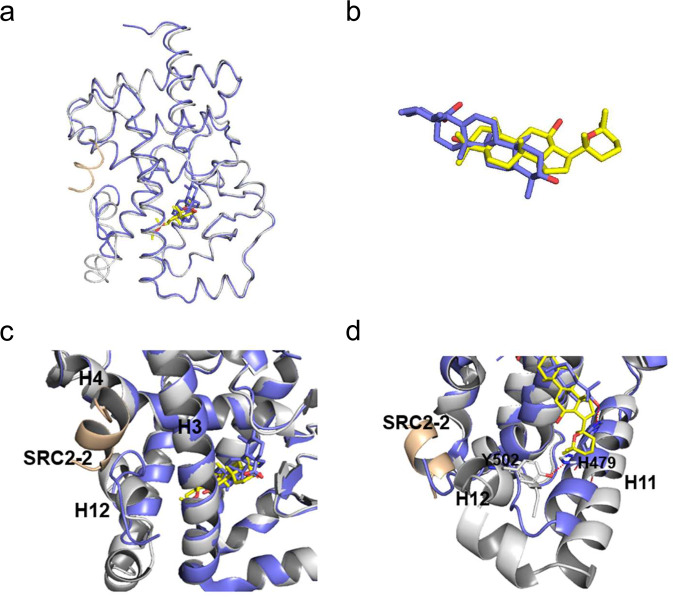
To determine the molecular basis of the specific interaction with RORγ, we solved the crystal structure of RORγ complexed with panaxadiol (Supplementary Table S1), which reveals a classical structure of a three-layer helical sandwich that resembles most nuclear receptor structures (Fig. 3a) [42, 43]. The presence of panaxadiol was apparent in the highly revealing electron density map shown in Fig. 3b, whose interaction with RORγ was stabilized by a combination of Van der Waals interactions and hydrogen bonds (Fig. 3c). Alignment of structures of RORγ/panaxadiol with RORγ/UA revealed that both ligand-bound RORγ LBDs aligned well with ligands occupying the similar binding sites in the RORγ pocket (Fig. 4a, b). Specifically, in contrast to the destabilized AF-2 helix shown in RORγ-UA complex [39], the C-terminal AF-2 helix positions in a canonical active conformation of RORγ-panaxadiol complex (Fig. 4c), in agreement with its dual activity in recruiting both coactivators and corepressors. Structural analysis reveals that UA forms a hydrogen bond with H479, resulting in breaking the hydrogen bond between H479 and Y502, which is critical to stabilize the C-terminal AF-2 helix and induce coactivator recruitment by RORγ (Fig. 4d) [44, 45].
Fig. 3. Molecular recognition of panaxadiol by RORγ.
a Structural determination of RORγ LBD bound with ligand panaxadiol. The structure is in cartoon representation with RORγ LBD in gray and the SRC2-2 motif is in wheat, respectively. Bound panaxadiol is shown as a stick representation with carbon and oxygen atoms depicted in yellow and red, respectively. b The 2Fo-Fc electron density map (1.0σ) show the bound panaxadiol to the RORγ. c Key interactions of RORγ with panaxadiol. Red dashes represent hydrogen bond interactions.
Fig. 4. Structural comparison of the RORγ-panaxadiol complex with RORγ-UA.
a Overlays of the structures of panaxadiol (yellow)-bound RORγ (gray) with UA-bound RORγ (slate blue) (PDB ID 5x8s). b Superposition of panaxadiol (yellow) with UA (slate blue). c, d Overlays of RORγ-panaxadiol (gray) and RORγ-UA (slate blue), showing the Y502 − H479 lock and the SRC2-2 binding site.
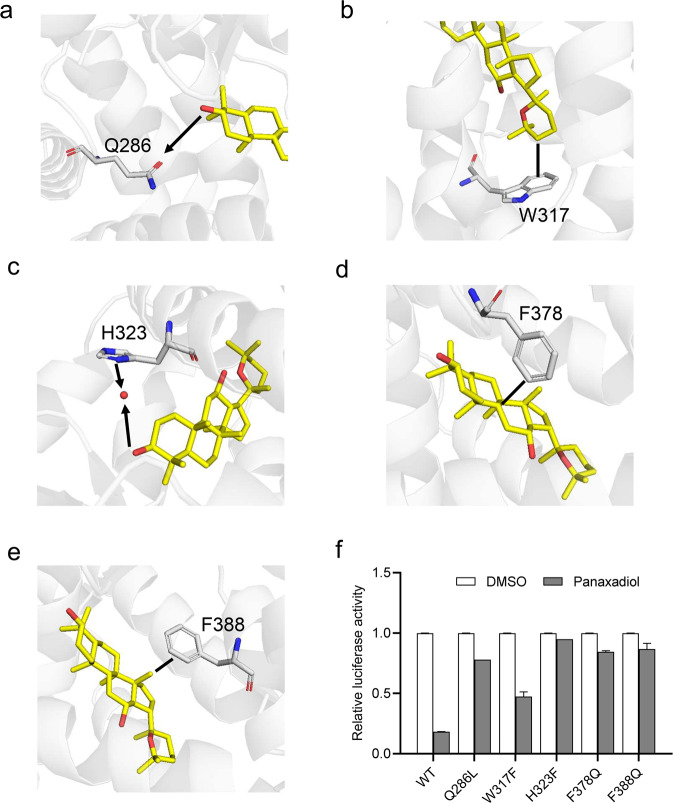
To further verify the roles of pocket residues in panaxadiol binding and RORγ suppression, we mutated several key RORγ residues in contact with panaxadiol and then tested the transcriptional activity of these mutated RORγ in response to panaxadiol in cell-based reporter assays using a GAL4 driven RORγ response reporter. Both Gln286 and His323 of RORγ pocket residues form hydrogen bonds with the OH group of panaxadiol (Fig. 5a, c). The Q286L and H323F mutations decrease the suppression of RORγ by panaxadiol in cell-based reporter assays using a GAL4-driven RORγ response reporter (Fig. 5f). The CH-π interaction between panaxadiol and Trp317 is critical for its binding to RORγ (Fig. 5b). Accordingly, the W317F mutation decreases the suppression of RORγ by panaxadiol (Fig. 5f). In addition, both F378Q and F388Q mutations decrease the suppression activity of RORγ by panaxadiol (Fig. 5d–f), which emphasizes the importance of hydrophobic interactions for panaxadiol in binding to RORγ. Together, these data affirm that panaxadiol interacts directly with RORγ.
Fig. 5. The structural determinants of the interactions of RORγ with panaxadiol.
a–e Molecular determinants of the interactions between panaxadiol and RORγ. The bound panaxadiol is shown in stick representation, with carbon and oxygen atoms depicted in yellow and red, respectively. The hydrophobic interactions and hydrogen bonds are shown with lines and arrows, respectively. f Effects of mutations of key RORγ residues on their transcriptional activity in response to panaxadiol treatment in cell-based reporter gene assays. 293 T cells were co-transfected with plasmids encoding RORγ–LBD WT or mutants as indicated in the figures, fused with the Gal4DNA-binding domain together with the pG5Luc reporter. The cells were treated with 1 μM panaxadiol. The results equate to the average of experiments performed in triplicate, with error bars indicating SDs.
Panaxadiol suppresses IL-17A expression and production in vitro
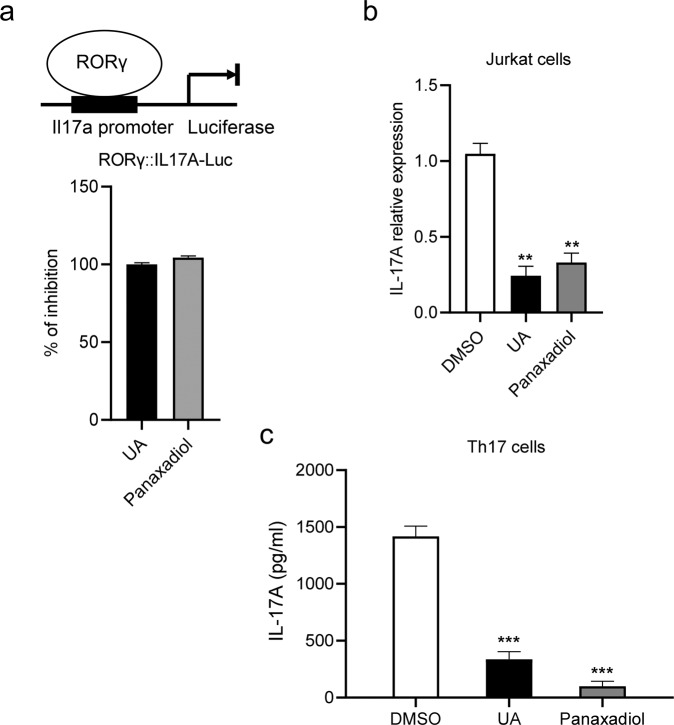
Next, we investigated the effect of panaxadiol in IL-17A expression and production in vitro. In order to determine if ginseng-derived panaxadiol modulates the IL-17A transcriptional activity, we first cloned the promoter of Il17a gene in a luciferase reporter. 293 T cells were co-transfected with plasmids encoding full-length RORγ together with an Il17a promoter luciferase reporter. As expected, the transcriptional activity of Il17a promoter was repressed by panaxadiol (Fig. 6a). To confirm that panaxadiol also affects IL-17A expression, jurkat cells were treated with panaxadiol and IL-17A mRNA levels were measured by qPCR. The results showed a significantly reduced mRNA expression level of the IL-17A after treatment with panaxadiol (Fig. 6b). Then we further assessed whether panaxadiol can inhibit the production of IL-17A in mature Th17 cells. Isolated splenocytes from WT mice were cultured under Th17 differentiation medium in the presence of panaxadiol, and IL-17A protein levels were measured by ELISA (Fig. 6c). The results demonstrate that panaxadiol indeed inhibited the secretion of IL-17A from differentiated Th17 cells.
Fig. 6. Panaxadiol decreases IL-17A expression and production.
a Panaxadiol represses the transcription of IL-17A. IL-17A transactivation by panaxadiol at a 1 μM concentration compared with UA. 293 T cells were transfected with the Il17a-promoter-driven luciferase plasmids and plasmid encoding full length RORγ. b IL-17A mRNA expression in stimulated Jurkat cells activated with PMA/ionomycin treatment for 5 h. c Inhibition of IL-17A production by UA and panaxadiol. Naive CD4+ T cells were isolated from spleen of wild-type mice and subjected to TH17 differentiation in the presence of 1 μM UA and panaxadiol. The results are the average of experiments performed in triplicate, with error bars indicating SDs; **P < 0.01, ***P < 0.001 compared with vehicle.
Panaxadiol alleviates STZ-induced T1D through inhibiting IL-17A production
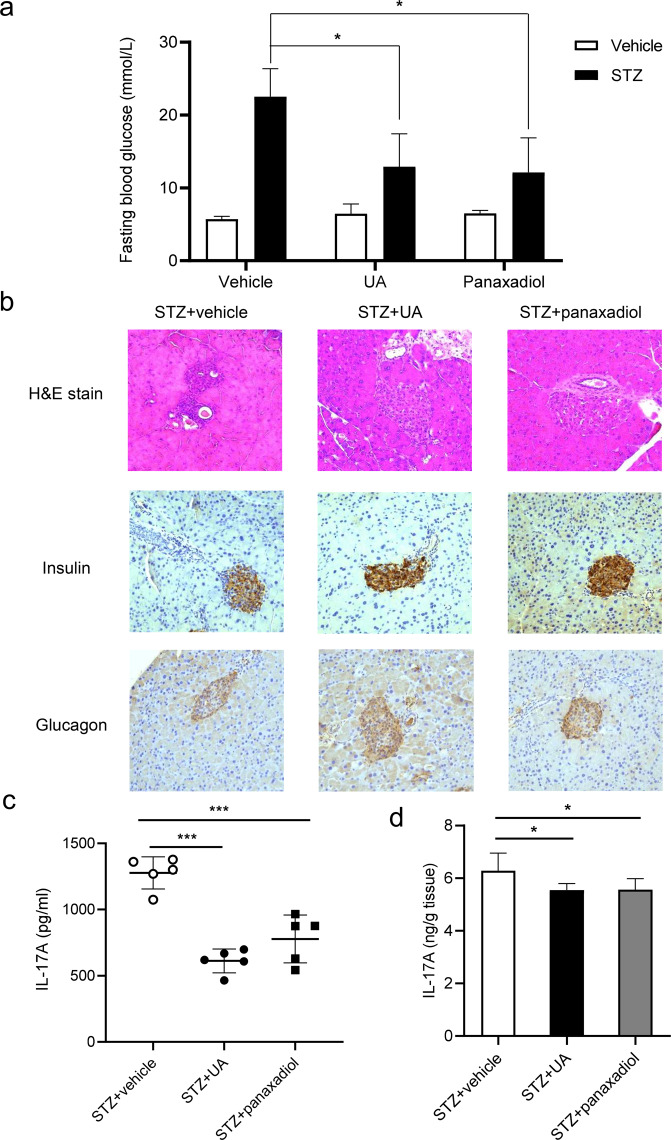
To further examine the therapeutic potential of panaxadiol in vivo, we tested its effects in STZ-induced T1D mice. Like UA, panaxadiol treatment group lowered blood glucose level than vehicle-treated group (Fig. 7a). Haematoxylin and eosin (H&E) and histochemical staining analysis showed that panaxadiol prevented inflammatory infiltration in the pancreatic islets and better preserved the insulin-producing β-cells and glucagon-producing α-cells (Fig. 7b). Importantly, panaxadiol also decreased IL-17A level in the splenocytes and pancreas of STZ-treated mice (Fig. 7c, d). Therefore, these results indicate that panaxadiol can suppress STZ-induced T1D through inhibiting IL-17A production to improve pancreatic islet β cell function.
Fig. 7. Panaxadiol alleviates STZ-induced type 1 diabetes in mice.
a Fasting blood glucose levels of STZ-induced mice at day 26 treated with UA and panaxadiol. b H&E, insulin and glucagon staining of pancreatic sections of three group mice. c IL-17A expression levels of splenocytes in UA and panaxadiol-treated STZ-induced mice. Isolated naive CD4+ T cells were cultured in Th17-skewing conditions for 5 days, and IL-17A in the supernatants was measured by ELISA. d The levels of IL-17A in pancreata were measured by ELISA. The results are representative of three independent experiments and are expressed as the means ± SEM. *P < 0.05, ***P < 0.001.
Discussion
The pathogenic role of IL-17A/Th17 cells in T1D has been well reported [9, 38]. Targeting the IL-17A/Th17 cells is a potential new clinical therapy for T1D. However, monotherapies of anti-IL-17A showed no sustained anti-diabetic effects in the IDDM rat model of T1D [46, 47]. As such, targeting the upstream of IL-17A/Th17 cells regulating pathway is a potential alternative therapy strategy for T1D. Among the regulators of IL-17A signaling, RORγ is reported as a key transcription factor for IL-17A production. To date, the role of RORγ in T1D is still unclear. It has been reported that RORα/γ inverse agonist SR1001 suppresses insulitis and prevents hyperglycemia in nonobese diabetic (NOD) mice [48]. In our study, we established multiple low-dose STZ-induced T1D mice models and found that RORγ deficiency protects mice from STZ-induced T1D through inhibiting IL-17A production to improve pancreatic islet β cell function. Interestingly, ginseng-derived panaxadiol was revealed to selectively inhibit RORγ transcriptional activity which alleviates STZ-induced T1D through inhibiting IL-17A production.
Recently, RORγ inverse agonists have attracted great attention in the research community worldwide as a possible therapy for IL-17A-mediated autoimmune diseases [49]. The distinctive functional profile of RORγ in response to various ligand binding is largely determined by the selective usage of transcriptional cofactors. Thus, ligand-bound RORγ may show diverse pharmacological functions depending on the specific binding of cofactors induced by different ligands. Unlike a typical antagonist, such as UA, panaxadiol-bound RORγ has a marked binding preference for both the coactivator and corepressor motifs. The selective usage of cofactors may contribute to the unique characteristics of panaxadiol in modulating RORγ activity in metabolic and autoimmune diseases. Thus, discrimination of the subtle differences between the coactivator and corepressor interaction helices by the RORγ AF2 core may provide the molecular basis for specific molecular basis for pharmacological potentials of panaxadiol.
Accumulating evidence suggests a role for traditional Chinese medicine in the modulation of Th17/IL-17A axis-mediated diseases [50, 51]. As an important herbal supplement, ginseng has been widely used for many health-related purposes in traditional Chinese medicine for thousands of years, however, of which the targeting mechanisms still remain unclear. The physiological function of ginseng-derived panaxadiol has been linked to hypoxia-inducible factor (HIF)-1α and STAT3 signaling pathways [52]. Our results indicate that at least part of the panaxadiol effects are in fact through targeting the nuclear receptor RORγ and RORγ-target genes, thus uncovering a novel signaling route for this important herb medicine. Similarly, the distinct properties of panaxadiol may provide a novel means for the regulation of RORγ or the treatment of RORγ-associated diseases. The structural mechanism may provide a basis for designing panaxadiol-based compounds that can be used more specifically either for RORγ- or HIF-1α/STAT3-regulated diseases, or for a combinatorial therapy. The beneficial and side effects arising from the cross interaction with each target can be optimized by designing new panaxadiol-based compounds with more selectivity.
In conclusion, we demonstrate that RORγ deficiency protects mice from STZ-induced T1D, thereby uncovering a potential novel therapeutic target for T1D. Moreover, we identified a unique RORγ inverse agonist, ginseng-derived panaxadiol, which selectively inhibits RORγ transcriptional activity with a selective cofactor recruitment profile distinct from known RORγ ligands. Our study thereby demonstrates a novel regulatory function of RORγ with linkage of the IL-17A pathway in pancreatic β cells, which may lead to a new drug-design strategy targeting RORγ functions in treating T1D.
Supplementary information
Acknowledgements
We thank the staff at BL19U1 of the Shanghai Synchrotron Radiation Source for assistance in data collection. This work was supported by grants from the National Natural Science Foundation of China (31770814).
Author contributions
SYT, SMC, YYF and JLH conducted the experiments. SYT contributed to the experiment design, performed structural analysis and wrote the manuscript. YYF contributed in editing the manuscript. YL designed the experiment and revised the manuscript.
Data availability
The structure of RORγ/panaxadiol/SRC2 ternary complex was deposited to the Protein Data Bank with PDB ID of 7W3P.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Si-yu Tian, Shu-ming Chen.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-022-01042-x.
References
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349–62. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 3.Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020;8:226–38. doi: 10.1016/S2213-8587(19)30412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, et al. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185:1959–67. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran S, Quesada-Masachs E, Zilberman S, Graef M, Kiosses WB, Chu T, et al. IL-17 is expressed on beta and alpha cells of donors with type 1 and type 2 diabetes. J Autoimmun. 2021;123:102708. doi: 10.1016/j.jaut.2021.102708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu F, Guo F, Zhu Y, Zhou Q, Li T, Xiang H, et al. IL-17 in pancreatic disease: pathogenesis and pharmacotherapy. Am J Cancer Res. 2020;10:3551–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Z, Zheng F. A complex auxiliary: IL-17/Th17 signaling during type 1 diabetes progression. Mol Immunol. 2019;105:16–31. doi: 10.1016/j.molimm.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol Metab. 2012;23:619–27. doi: 10.1016/j.tem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang MR, Rosen H, Griffin PR. RORs in autoimmune disease. Curr Top Microbiol Immunol. 2014;378:171–82. doi: 10.1007/978-3-319-05879-5_8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Luo XY, Wu DH, Xu Y. ROR nuclear receptors: structures, related diseases, and drug discovery. Acta Pharmacol Sin. 2015;36:71–87. doi: 10.1038/aps.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu HE. Family reunion of nuclear hormone receptors: structures, diseases, and drug discovery. Acta Pharmacol Sin. 2015;36:1–2. doi: 10.1038/aps.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol Endocrinol. 2010;24:923–9. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strutzenberg TS, Zhu Y, Novick SJ, Garcia-Ordonez RD, Doebelin C, He Y, et al. Conformational changes of RORγ during response element recognition and coregulator engagement. J Mol Biol. 2021;433:167258. doi: 10.1016/j.jmb.2021.167258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 20.Huh JR, Littman DR. Small molecule inhibitors of RORγt: targeting Th17 cells and other applications. Eur J Immunol. 2012;42:2232–7. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jetten AM, Cook DN. (Inverse) Agonists of retinoic acid-related orphan receptor γ: regulation of immune responses, inflammation, and autoimmune disease. Annu Rev Pharmacol Toxicol. 2020;60:371–90. doi: 10.1146/annurev-pharmtox-010919-023711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–90. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, et al. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286:31409–17. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem. 2011;286:22707–10. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patocka J, Nepovimova E, Wu W, Kuca K. Digoxin: pharmacology and toxicology-A review. Environ Toxicol Pharmacol. 2020;79:103400. doi: 10.1016/j.etap.2020.103400. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q, He M, Zhang M, Zeng S, Chen L, Zhou L, et al. Ursolic acid: a systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia. 2020;147:104735. doi: 10.1016/j.fitote.2020.104735. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Prigozin H, Polak N, Rapoport M, Lohse AW, Cohen IR. Autoimmune diabetes induced by the beta-cell toxin STZ. Immunity to the 60-kDa heat shock protein and to insulin. Diabetes. 1994;43:992–8. doi: 10.2337/diab.43.8.992. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, He X, Cai P, Li T, Peng R, Dang J, et al. Induced regulatory T cells suppress Tc1 cells through TGF-β signaling to ameliorate STZ-induced type 1 diabetes mellitus. Cell Mol Immunol. 2021;18:698–710. doi: 10.1038/s41423-020-00623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L, Feng X, Rong H, Pan Z, Inaba Y, Qiu L, et al. The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat Commun. 2013;4:1937. doi: 10.1038/ncomms2924. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Zhang J, Fang L, Zhou L, Wang S, Xiang Z, et al. Increasing human Th17 differentiation through activation of orphan nuclear receptor retinoid acid-related orphan receptor γ (RORγ) by a class of aryl amide compounds. Mol Pharmacol. 2012;82:583–90. doi: 10.1124/mol.112.078667. [DOI] [PubMed] [Google Scholar]
- 31.Chung BH, Kim BM, Doh KC, Min JW, Cho ML, Kim KW, et al. Suppressive effect of 1α,25-Dihydroxyvitamin D3 on Th17-immune responses in kidney transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2017;101:1711–9. doi: 10.1097/TP.0000000000001516. [DOI] [PubMed] [Google Scholar]
- 32.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–26. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 33.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–42. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–67. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaochite JN, Caliari-Oliveira C, Davanso MR, Carlos D, Malmegrim KC, Cardoso CR, et al. Dynamic changes of the Th17/Tc17 and regulatory T cell populations interfere in the experimental autoimmune diabetes pathogenesis. Immunobiology. 2013;218:338–52. doi: 10.1016/j.imbio.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Tong Z, Liu W, Yan H, Dong C. Interleukin-17A deficiency ameliorates streptozotocin-induced diabetes. Immunology. 2015;146:339–46. doi: 10.1111/imm.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother. 2018;101:287–92. doi: 10.1016/j.biopha.2018.02.103. [DOI] [PubMed] [Google Scholar]
- 39.Noguchi M, Nomura A, Murase K, Doi S, Yamaguchi K, Hirata K, et al. Ternary complex of human RORγ ligand-binding domain, inverse agonist and SMRT peptide shows a unique mechanism of corepressor recruitment. Genes Cells. 2017;22:535–51. doi: 10.1111/gtc.12494. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Suino K, Daugherty J, Xu HE. Structural and biochemical mechanisms for the specificity of hormone binding and coactivator assembly by mineralocorticoid receptor. Mol Cell. 2005;19:367–80. doi: 10.1016/j.molcel.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 41.de Vries R, Meijer FA, Doveston RG, Leijten-van de Gevel IA, Brunsveld L. Cooperativity between the orthosteric and allosteric ligand binding sites of RORγt. Proc Natl Acad Sci USA. 2005;118:e2021287118. doi: 10.1073/pnas.2021287118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gampe RT, Jr., Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000;5:545–55. doi: 10.1016/S1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 43.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–6. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.René O, Fauber BP, Boenig Gde L, Burton B, Eidenschenk C, Everett C, et al. Minor structural change to tertiary sulfonamide RORc ligands led to opposite mechanisms of action. ACS Med Chem Lett. 2015;6:276–81. doi: 10.1021/ml500420y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–72. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marwaha AK, Tan S, Dutz JP. Targeting the IL-17/IFN-γ axis as a potential new clinical therapy for type 1 diabetes. Clin Immunol. 2014;154:84–9. doi: 10.1016/j.clim.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Jörns A, Ishikawa D, Teraoku H, Yoshimoto T, Wedekind D, Lenzen S. Remission of autoimmune diabetes by anti-TCR combination therapies with anti-IL-17A or/and anti-IL-6 in the IDDM rat model of type 1 diabetes. BMC Med. 2020;18:33. doi: 10.1186/s12916-020-1503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solt LA, Banerjee S, Campbell S, Kamenecka TM, Burris TP. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology. 2015;156:869–81. doi: 10.1210/en.2014-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandya VB, Kumar S, Sachchidanand, Sharma R, Desai RC. Combating autoimmune diseases with retinoic acid receptor-related orphan receptor-γ (RORγ or RORc) inhibitors: hits and misses. J Med Chem. 2018;61:10976–95. doi: 10.1021/acs.jmedchem.8b00588. [DOI] [PubMed] [Google Scholar]
- 50.Asadi-Samani M, Bagheri N, Rafieian-Kopaei M, Shirzad H. Inhibition of Th1 and Th17 cells by medicinal plants and their derivatives: a systematic review. Phytother Res. 2017;31:1128–39. doi: 10.1002/ptr.5837. [DOI] [PubMed] [Google Scholar]
- 51.Xu YY, Wang DM, Liang HS, Liu ZH, Li JX, Wang MJ, et al. The role of Th17/Treg Axis in the traditional Chinese medicine intervention on immune-mediated inflammatory diseases: a systematic review. Am J Chin Med. 2020;48:535–58. doi: 10.1142/S0192415X20500275. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Li MY, Zhang ZH, Zuo HX, Wang JY, Xing Y, et al. Panaxadiol inhibits programmed cell death-ligand 1 expression and tumour proliferation via hypoxia-inducible factor (HIF)-1α and STAT3 in human colon cancer cells. Pharmacol Res. 2020;155:104727. doi: 10.1016/j.phrs.2020.104727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The structure of RORγ/panaxadiol/SRC2 ternary complex was deposited to the Protein Data Bank with PDB ID of 7W3P.