Abstract
OBJECTIVES
Right heart failure (RHF) is a major complication following left ventricular assist device (LVAD) implantation. Pulmonary artery pulsatility index (PAPi) has been evaluated as a haemodynamic marker for RHF, but PAPi is dependent on pulmonary vascular resistance (PVR). We conducted a systematic review to assess the relationship between PAPi and RHF and death in patients undergoing LVAD implantation and examined the relationship between PAPi cut-off and PVR.
METHODS
We searched PubMed, EMBASE, CENTRAL and manually screened retrieved references to identify all clinical studies reporting PAPi in adult patients with a durable LVAD. Eligibility criteria were prespecified and 2 reviewers independently screened and extracted data; the Newcastle–Ottawa Scale was used to assess quality of non-randomized studies. This study was prospectively registered on PROSPERO (CRD42021259009).
RESULTS
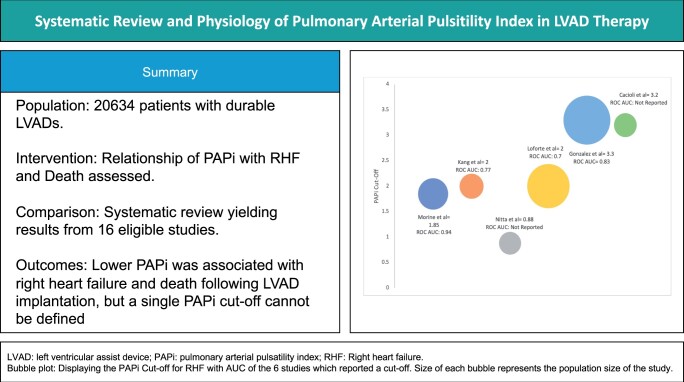
From 283 unique records, we identified 16 studies reporting haemodynamic assessment in 20 634 adult patients with an implanted durable LVAD. Only 2 studies reported on mortality and in both, a lower PAPi was significantly associated with death. Fifteen studies reported RHF data and, in 10 studies, a lower PAPi was significantly associated with RHF. Six studies reported on PAPi cut-offs ranging from 0.88 to 3.3; and the cut-offs were directly related to PVR (r = 0.6613, P = 0.019).
CONCLUSIONS
Lower PAPi was associated with RHF and death following LVAD implantation, but a single PAPi cut-off cannot be defined, as it is dependent on PVR.
Keywords: Left ventricular assist device, Heart failure, Right heart failure, Pulmonary artery pulsatility index
Durable left ventricular assist devices (LVAD) have become an established therapy in patients with end-stage heart failure.
INTRODUCTION
Durable left ventricular assist devices (LVAD) have become an established therapy in patients with end-stage heart failure. Outcomes of LVAD therapy have improved with the introduction of the magnetically levitated centrifugal continuous-flow device [1], but early right heart failure (RHF) remains a major cause of morbidity and mortality following LVAD implant [2]. Therefore, preoperative assessment of the risk of RHF is central to the selection of patients for LVAD therapy.
A number of haemodynamic parameters derived from pulmonary artery catheterization have been used to assess the risk of RHF. Of these, pulmonary artery pulsatility index (PAPi) has been shown to be an independent predictor of mortality due to RHF in acute myocardial infarction, pulmonary arterial hypertension, heart failure, heart transplantation and cardiogenic shock [3–7]. In general, lower PAPi is associated with higher the risk of RHF. PAPi is defined as the ratio of pulmonary artery pulse pressure (PP) to right atrial (or central venous) pressure. Pulmonary artery PP is a function of stroke volume (SV) and pulmonary artery compliance (PAC), and the latter has a hyperbolic relationship with pulmonary vascular resistance (PVR) [8]. On this basis, we hypothesized that the PAPI cut-off associated with RHF would be dependent on the PVR (increase with PVR).
This systematic review first assessed the relationship between PAPi, RHF and death following LVAD implantation. Second, we evaluated the relationship between the reported PAPi cut-off and PVR from the studies identified in this systematic review.
METHODS
Ethics statement
As this is a systematic review, ethics committee review was not applicable. However, this study was formally reviewed and was prospectively registered on PROSPERO (CRD42021259009). The search results are reported in accordance with the PRISMA statement [9]. All eligibility criteria and search strategies were prespecified.
Eligibility
All studies reporting measurement of PAPi in adult patients with a durable LVAD, defined as an intra- or extracorporeal device implanted in the left ventricle for the treatment of advanced heart failure, irrespective of treatment intention (destination therapy or bridge to or transplantation), and published in the English literature were included. Studies were excluded if PAPi was not reported, or data on mortality or RHF were not reported. Studies reported only as a conference abstract were excluded due to insufficient data for analysis.
Search strategy
We searched international primary research databases (PubMed, EMBASE and CENTRAL) from inception to 14 September 2021 and reference lists of relevant articles to identify all eligible studies. The following search strategy was used for all 3 databases:
‘Pulmonary artery pulsatility index’ OR ‘PAPI’.
‘Heart failure’ OR ‘ventricular failure’ OR ‘right ventricular failure’ OR ‘right heart failure’ OR ‘left ventricular failure’ OR ‘ventricular assist device’ OR ‘VAD’ OR ‘LVAD’ OR ‘Heartmate’ OR ‘Heartware’.
1 AND 2.
An updated search was performed on 18 August 2022 using the same search strategy.
Study selection and data extraction
Abstracts and then full-text articles of all identified were screened independently by 2 reviewers (Ivan H.W. Yim and Ayisha M. Khan-Kheil). All studies in patients with a durable LVAD, which contained PAPi data analysed against RHF or death, were included. RHF was author defined, including the Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) definition of RHF [10]. Data were extracted independently by 2 reviewers (Ivan H.W. Yim and Ayisha M. Khan-Kheil) from the full-text publication and any disagreements were resolved by consensus. A full list of the data items and descriptors is available in the Supplementary Material. For all studies included, the Newcastle–Ottawa Scale [11] for assessing the quality of non-randomized studies was employed and based on the number of stars each study gained in each domain; this was then converted to the Agency for Healthcare Research and Quality standards of good, fair and poor.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, Version 27.0 (Armonk, NY). All continuous data are expressed as medians with interquartile ranges and all categorical data are expressed as counts and percentages where applicable.
RESULTS
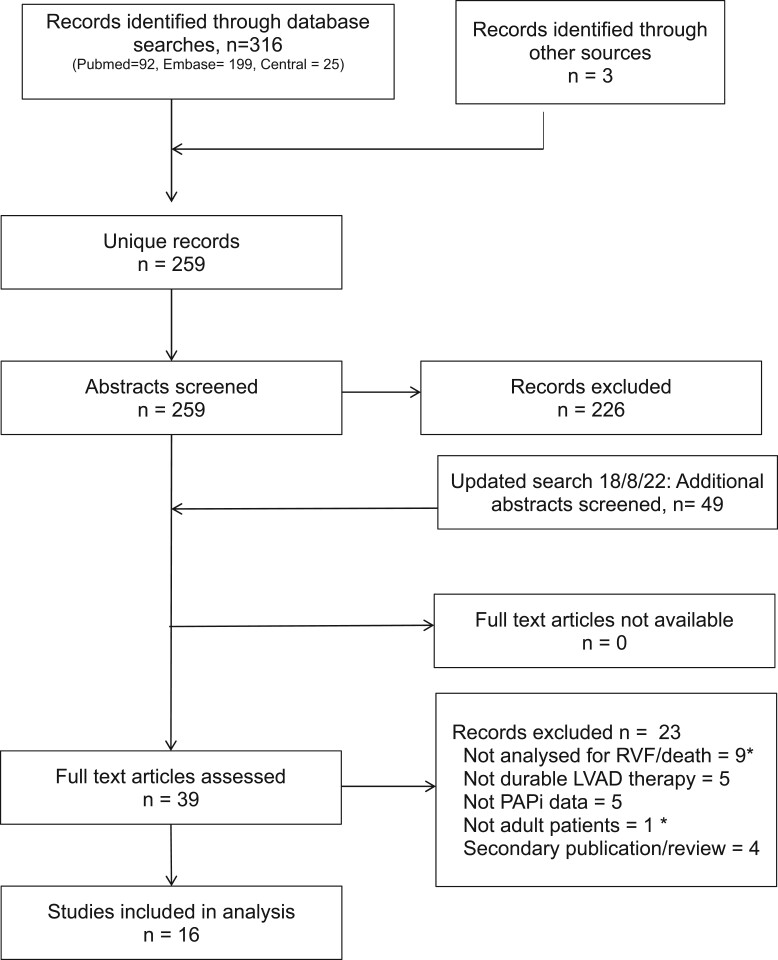
The original search produced 259 unique records and we identified 14 studies reporting haemodynamic assessment in 20 634 adult patients with an implanted durable LVAD (Fig. 1). The updated search performed in August 2022 produced another 49 unique records and all full-text articles were sourced online or via national libraries; a total of 16 studies were included for analysis. Characteristics of the included studies are shown in Table 1. All studies were retrospective cohort studies originating from 4 countries, with 9 (56%) from the USA, 3 from Italy and 1 each from Japan, Germany, the Netherlands and Turkey. Articles were published in specialist heart failure, cardiothoracic surgery, transplantation or anaesthetic journals, and all were published in English.
Figure 1:
PRISMA flow diagram of study selection. *Multiple counting. LVAD: left ventricular assist device;; RHF: right heart failure.
Table 1:
Summary of the 16 studies included in the full-text review
| Authors | N | LVAD | PAPi associated with RHF? | PAPi cut-off for RHF or death | Summary |
|---|---|---|---|---|---|
| Grandin et al. [12] | 151 | Not reported | No | Not reported | Single-centre study. Low PAC combined with a high CVP: PCWP ratio was the strongest predictor for death at 6 months (HR 8.68, P < 0.001) and RHF (OR 4.74, P = 0.02). PAPI was not significantly associated with RHF (P = 0.10) |
| Morine et al. [13] | 132 | HM2, HVAD | Yes | <1.85 (RHF) | A lower PAPi was significantly associated with RHF P < 0.01. A PAPi < 1.85 provided 94% sensitivity and 81% specificity for predicting RHF and was superior to RAP: PCWP ratio, RVSWI and RAP alone. |
| Kang et al. [14] | 83 | HM2, HVAD | Yes | <2 (RHF) | PAPi was an independent predictor of RHF and RVAD implantation following LVAD therapy. A higher PAPi was associated with reduced risk for RVAD placement (OR 0.31, P < 0.0001). PAPi was more predictive of RVAD placement if inotropes were present at the time of catheterization (OR 0.21 vs 0.49). ROC analysis showed optimal sensitivity and specificity achieved using a PAPi threshold of 2. |
| Nitta et al. [15] | 70 | Nipro-VAD | Yes | <0.88 (RHF) | This study aimed to devise a scoring system for predicting RVAD placement following implantation of the Nipro-VAD paracoporeal device. Patients who required RVAD implantation postoperatively had a significantly lower PAPi (P = 0.001). The authors proposed a combination score using PVR > 4.5WU and RAP: PCWP > 0.8 as a scoring system for predicting RVAD requirement following paracoporeal LVAD therapy. |
| Loforte et al. [16] | 258 | HM2, HM3, HVAD, Jarvik 2000, Berlin Heart | Yes | <2 (RHF) | This study aimed to devise the ALMA risk score for predicting RHF following LVAD implantation. Within the haemodynamic data of this study, a PAPi of <2 was found to be associated with unplanned RVAD support (P = 0.001) and on multivariable logistic regression analysis PAPi < 2 had an OR of 3.3 (CI 1.7–6.1, P = 0.001). The ALMA score employs the following 5 variables: destination therapy intention, PAPI < 2, RVSWi <300 mmHg/ml/m2, RV:LV ratio > 0.75 and MELD-XI score >17. The authors proposed a score of 0–1 implies low risk for RVAD requirement and a score above 4 implies very high risk for requiring RVAD following LVAD implantation. |
| Raymer et al. [17] | 216 | HM2, HVAD | Yes | Not reported | This study reported the combination of TAPSE and HeartMate risk score (HMRS) as a scoring system to predict RHF following LVAD implantation. The RHF group had a lower PAPi (P = 0.001). ROC analysis showed PAPi had an AUC of 0.63 (P < 0.001). When the haemodynamic parameters were analysed combination of TAPSE with the HMRS was the best for predicting RHF compared to HMRS + PAPi and HMRS + sRVCPI. |
| Gudejko et al. [18] | 85 | HM2, HVAD | Yes | Not reported | The data used in this study were intraoperative haemodynamic parameters and also echocardiographic data. Higher CVP, lower pre-CPB and post-chest closure PAPi, post-CPB larger right atrial diameter, larger RVES area, lower FAC and lower TAPSE were all associated with severe RHF. |
| Muslem et al. [19] | 375 | HM2, HM3, HVAD | Yes | Not Reported | PAE was found to be the most robust haemodynamic parameter to predict RHF. PAPi was significantly lower in the Severe RHF group compared to no RHF (1.8 vs 2.2, P = 0.017). |
| Alfirevic et al. [20] | 86 | HM2, HM3, HVAD | No | Not reported | Intraoperative TAPSE measurement was the variable of interest in this study. As a secondary comparison, PAPi and the Michigan risk score were not significantly associated with severe RHF. Intraoperative TAPSE, Michigan risk score and PAPi were poor discriminators of RHF following LVAD therapy. |
| Sert et al. [21] | 71 | HM2, HM3, HVAD | No | Not reported | This study compared TAPSE, CVP: PCWP ratio, RVSWI, PAPi, Pennsylvania Score, Michigan score, CRITT score, ALMA score and the EUROMACS score. PAPi was not significantly lower in the group with postop RHF (P = 0.304). They concluded that only the EUROMACS and CRITT score had an ROC AUC above 0.7 and that the combination of TAPSE and the Pennsylvania score was found to be the most sensitive (85%) whereas TAPSE + Michigan score + CVP:PCWP ratio was the most specific (97%). |
| Benjamin et al. [22] | 104 | HM2, HVAD | No | Not reported | Primary end point was duration of inotropic support and the association with RVF. They found patients who were on long-term milrinone had a significantly increased risk of developing RHF post-LVAD insertion. PAPi was not significantly different between the RHF and the group without RVF post-LVAD implant (4 ± 3.9 vs 3.2 ± 2.3, P = 0.255) |
| Ruiz-Cano et al. [23] | 80 | HM3, HVAD | No | Not reported | PAPi was not significantly different between the early RHF group versus no early RHF (2.5 vs 3, P = 0.283). This study found that blood urea nitrogen >44.5 mg/dl and CVP/PCWP > 0.55 were the parameters with the strongest association with early RHF. |
| Guglin and Omar [24] | 18608 | Not reported | No data | Not reported | This was a retrospective cohort study looking at data from the INTERMACS database and primarily assessing RAP and its ability to predict death. RAP was the main predictor of mortality in LVAD recipients. PAPi was lower in non-survivors (P < 0.001), but RAP had superior discriminatory value with a difference in AUC of 0.0105 (P = 0.0052). |
| Gonzalez et al. [25] | 315 | HM2, HVAD | Yes | Optimal PAPi <3.3 (RHF); Delta PAPi <2.08 (Death at 6 months) | This study assessed the change in PAPi during preoperative haemodynamic optimization prior to LVAD implantation. The mean optimal PAPi was lower (P < 0.001) in the group that developed early RHF. A delta PAPI of <2.08 during optimization was associated with higher mortality at 180 days (P = 0.003). |
| Cacioli et al. [26] | 75 | HM2, HM3 | Yes | Not reported | A lower PAPi was strongly associated with RHF following LVAD implant. This study also demonstrated in those who did not develop RHF post-LVAD had a significantly higher PAPi following vasodilator challenge at preop RHC (5.3 ± 3.9 vs 2.7 ± 1.3, P = 0.003). Furthermore, PAPi when combined with established risk scores provided incremental risk stratification for post-LVAD RHF. |
| Stricagnoli et al. [27] | 38 | HM3, Jarvik 2000 | Yes | Not reported | PAPi was the most robust haemodynamic parameter which predicted post LVAD RHF (1.52 ± 0.26 vs 3.95 ± 3.39, P = 0.003) with an ROC AUC of 0.85. |
ALMA: Antonio Loforte and Motalto Andrea; CVP: central venous pressure; CRITT: central venous pressure>15, severe right ventricular dysfunction, intubation, tricuspid regurgitation, tachycardia; EUROMACS: European Registry for Patients with Mechanical Circulatory Support; FAC: fractional area change; HR: heart rate; HVAD: HeartWare; INTERMACS: Interagency Registry of Mechanically Assisted Circulatory Support; LVAD: left ventricular assist device; MELD: Model for End-stage Liver Disease; PAC: pulmonary arterial compliance; PAPi: Pulmonary artery pulsatility index; PCWP: pulmonary capillary wedge pressure; RAP: right atrial pressure; RHF: right heart failure; ROC: receiver operating characteristic; RVAD: right ventricular assist device; RVES: right ventricular end systolic; RVSWI: right ventricular stroke work index; sRVCPI: simplified right ventricular contraction pressure index; TAPSE: tricuspid annular plane systolic excursion.
The study periods ranged from 2004 to 2021. The median number of patients in each study was 95 (interquartile range 78.8–226.5). The types of LVAD implanted were primarily intracorporeal continuous-flow devices, HeartMate (Abbott Laboratories, USA) II or III and HeartWare (Medtronic, USA), with 1 study evaluating the NIPRO-VAD (Nipro, Osaka, Japan) extracorporeal pulsatile pump [28]. Only 6 out of the 16 studies stated the goal of LVAD therapy (bridge to transplantation/candidacy or destination therapy).
Of the 16 studies, the primary outcome was (i) RHF or the event of right ventricular assist device implantation (n = 15) and (ii) mortality associated with right atrial pressure (RAP) (n = 1). All 16 studies reported on the timing of PAPI measurement in relation to LVAD implant. Fourteen studies performed right heart catheterization prior to LVAD implantation to record routine haemodynamic parameters including PAPI (although only 6 studies gave the exact timing in hours, days or months of RHC from LVAD implantation) and 2 studies measured PAPI intraoperatively at the time of LVAD implantation. Twelve studies did not specify medical therapy at the time of haemodynamic assessment (e.g. use of inotropes or intra-aortic balloon pump).
Guglin and Omar [24] analysed the INTERMACS database including 18 733 patients and found that RAP was a haemodynamic predictor of death with an receiver operating characteristic (ROC) curve area under the curve (AUC) of 0.55 (CI 0.539–0.562, P < 0.0001) and an RAP of 13 mmHg or higher had the highest combined sensitivity and specificity in predicting mortality. PAPI and other haemodynamic parameters were also analysed and compared against RAP. Survivors were found to have a significantly higher PAPI (3 ± 3.1 vs 2.6 ± 2.7; P < 0.001) but when compared to RAP, it was found to have a lower ROC curve AUC with a difference in areas of 0.0105, P = 0.005 and therefore RAP was found to be superior at predicting death.
Gonzalez et al. [25] studied PAPI at serial time points before LVAD implantation and during the period of medical optimization, hypothesizing that the magnitude of change of PAPI and other invasive haemodynamic measurements would provide an incremental risk stratification for RHF following LVAD therapy with a secondary end-point of death at 180 days. After optimizing their patients with a combination of diuretic, intravenous sodium nitroprusside, inotropes and non-durable mechanical circulatory support where appropriate, they found that an optimized preoperative PAPi of >3.33 was associated with a significant reduction in early RHF (P < 0.001). In patients with a change in PAPi (delta PAPi) of >2.08 during the optimization period (time period not specified), there was a significant reduction in 6-month mortality following LVAD implantation presumably from reduced early RHF. In a recently published study, Cacioli et al. [26] showed that PAPi following vasodilator challenge with sodium nitroprusside provided incremental risk stratification when combined with established risk scores (EUROMACS-RHF and CRITT). In this study, PAPi alone was significantly lower in patients who developed RHF post-LVAD implantation compared with those who did not have RVF (2.2 ± 1.3 vs 3.3 ± 1.5, P = 0.008). This was even more striking following vasodilator therapy (5.3 ± 3.9 vs 2.7 ± 3, P = 0.003) suggesting a higher PAPi following vasodilator therapy indicates more right ventricular reserve. Similarly to the study performed by Gonzalez et al., this study reported a post-vasodilator challenge PAPi cut-off of 3.2 (with a sensitivity of 66% and a specificity of 68%) for right ventricular failure. Furthermore, the authors combined RV fractional area change and systolic pulmonary artery pressure with post-vasodilator challenge PAPi and found that it had an AUC of 0.949.
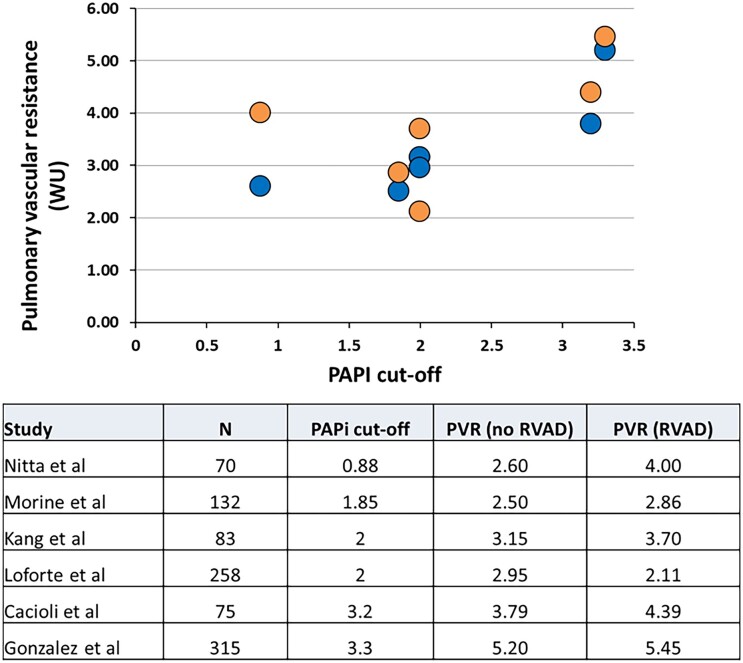
The majority of studies used the INTERMACS definition to describe the end-point of RHF (Table 2). Fifteen studies reported RHF data relating to PAPi: in 10 [13–19, 25–27] of these studies, a lower PAPi was associated with RHF, but there was no significant association between PAPi and RHF in the other 5 studies [12, 20–23]. Six studies performed ROC analyses to determine the optimal PAPi cut-off for RHF following durable LVAD implantation, ranging from 0.88 to 3.3, with a mean of 2.2 and the ROC AUC values ranged from 0.70 to 0.94 with a mean of 0.80. All 6 studies provided data on PVR in patients with and without severe RHF. There was a direct relationship between the PAPi cut-offs and PVR for patients with/without severe RHF (r = 0.6613, P = 0.019) (Fig. 2).
Table 2:
Definition of right heart failure used in the studies
| Authors | RHF definition |
|---|---|
| Kang et al. [14] | INTERMACS definition |
| Nitta et al. [15] | RVAD implantation within 2 weeks of LVAD implantation |
| Raymer et al. [17] | Post-implant inotropic support >14 days, RVAD implantation or death within 14 days due to RHF |
| Loforte et al. [16] | Short or long term right sided MCS despite maximal dosage of continuous inotropic support and NO ventilation within 30 days of LVAD implantation. |
| Gonzalez et al. [25] | INTERMACS definition |
| Gudejko et al. [18] | INTERMACS definition |
| Alfirevic et al. [20] | INTERMACS definition |
| Morine et al. [13] | INTERMACS definition |
| Sert et al. [21] | INTERMACS definition |
| Grandin et al. [12] | RVAD or inotropic support >14 days or death from RHF within 14 days of LVAD implantation |
| Benjamin et al. [22] | RVAD or inotropic support >14 days within 30 days of LVAD implantation. |
| Muslem et al. [19] | INTERMACS definition |
| Ruiz-Cano et al. [23] | INTERMACS definition |
| Cacioli et al. [26] | INTERMACS definition |
| Stricagnoli et al. [27] | INTERMACS definition |
INTERMACS definition of RHF: elevated central venous pressure >18 mmHg in the absence of elevated pulmonary capillary wedge pressure; RVAD implantation; or requirement of prolonged nitric oxide or inotropic therapy [39].
INTERMACS: Interagency Registry of Mechanically Assisted Circulatory Support; LVAD: left ventricular assist device; RHF: right heart failure; NO: nitric oxide; RVAD: right ventricular assist device.
Figure 2:
The relationship between PAPi cut-offs and PVR in the studies that reported on PAPi cut-offs. There is a direct correlation between PAPi and PVR (r = 0.6613, P = 0.019). PAPi: pulmonary artery pulsatility index; PVR: pulmonary vascular resistance.
In assessing the quality of the included studies using the Newcastle–Ottawa Scale, all achieved an Agency for Healthcare Research and Quality grading of good, with at least 1 star in each domain.
DISCUSSION
PAPi has been evaluated as a parameter to predict the risk of RHF and death following LVAD implantation. Notable findings from this systematic review were: (i) lower PAPi measurements before LVAD implantation were associated with higher risk of RHF and/or death; (ii) the reported PAPi cut-offs for discriminating patients at risk of RHF and/or death varied by more than three-fold from 0.80 to 3.3; and (iii) the reported PAPi cut-offs were directly related to PVR.
PAPi is the ratio of PP over RAP (equation 1: PAPI = PP/RAP). Analogous to the charging of capacitors, a significant proportion of the right ventricular SV ‘charges’ the reservoir volume and increases pressure in the compliant pulmonary arteries in systole, which discharges during diastole. Pulmonary arterial compliance defines this relationship between an increase in blood volume (ΔV) and an increase in pressure in the pulmonary arterial system. In practice, PAC is difficult to measure because direct measurement of ΔV is not possible due to the continuous outflow from the arterial system. Therefore, in clinical practice, the ratio of SV/PP is used to determine PAC, accepting that this equation would overestimate the true PAC. Rearranging this equation, it can be appreciated that PAC and SV are the main determinants of PP (equation 2: PP = SV/PAC).
Pulmonary arterial compliance is determined by the prevailing distending pressure [i.e. mean pulmonary artery pressure (MPAP)] and by the elastic properties of the pulmonary arterial wall. The latter is mainly determined by the composition of elastin and collagen in the wall. Pulmonary arterial compliance decreases when MPAP increases in non-linear relationship [8] due to the nature of the stress–strain relationship; and MPAP itself is a function of PVR and left atrial pressure [or pulmonary artery wedge pressure (PAWP)], PVR, heart rate (HR) and SV {equation 3: MPAP = [PVR × (HR × SV)] + PAWP}.
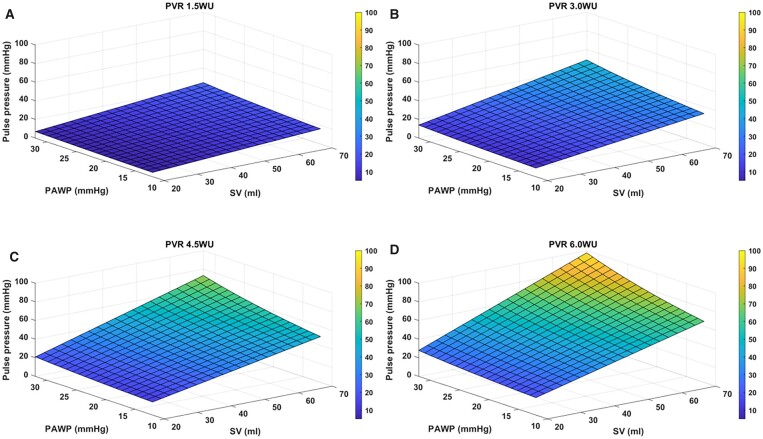
Thus, PAC decreases as a result of: (i) an increasing distending pressure (MPAP) from a combination of increasing PVR, HR and SV; (ii) intrinsic changes in pulmonary arterial wall stiffness (vascular remodelling); and (iii) increase in left atrial pressure (or PAWP). Indeed, the hyperbolic relationship between PAC and PVR [29] and inverse relationship with PAWP are well characterized [30]. By extension, the pulmonary artery PP would be similarly dependent on SV, PAWP and PVR (Fig. 3). This would explain the observed relationship between the PAPi cut-off and PVR levels. We were unable to evaluate the relationship between HR, SV and PAPi, as the data were not reported in the studies.
Figure 3:
Pulmonary artery pulse pressure modelled over a range of pulmonary arterial wedge pressures, stroke volumes and pulmonary vascular resistances. Graphs A–D were modelled on a PVR of 1.5WU, 3WU, 4.5WU and 6WU, respectively, indicating increasing pulse pressure with increasing PVR. PVR: pulmonary vascular resistance.
The implications of these physiological considerations are three-fold. First, in the face of increasing PVR, the right ventricle mal-adapts to maintain relatively low RAP and normal SV; the latter results in disproportionately elevated PP, and by extension an increase in PAPi. The increase in PAPi is a result of progressive adverse remodelling and should not be misconstrued as an improvement in right heart function. Second, a significant drop in PAPi would only occur when the right ventricle uncouples from the pulmonary arterial system, with resultant drop in SV and rise in RAP. Third, because of the higher PVR and lower PAC, the PAPi level would remain higher in the setting of RHF related to pulmonary vascular disease compared to RHF due to primary right ventricular cardiomyopathy with low PVR and high PAC. In this context, Essandoh et al.’s recent description of a mean weighted PAPi of 2.17 as a cut-off to predict RHF post-LVAD implantation should be interpreted and applied with caution, and extrapolation to patients with other pulmonary haemodynamic profiles should be resisted.
Pulmonary artery PP is inversely related to PAC, which is inextricably related to PVR. An increase in PVR increases pulmonary artery PP at any given SV. In this regard, PVR is a determinant of pulmonary artery PP and PAPi and cannot be avoided in interpreting PAPi. The interpretation of PAPi must take into consideration the underlying disease pathophysiology.
In primary right ventricular dysfunction such as right ventricular infarction with low PVR, the compliant pulmonary arterial system would result in low pulmonary artery PP for a given SV and RAP. In contrast, PAPi must be higher at the same SV and RAP in high conditions, such as primary arterial hypertension.
As an illustration, we describe 2 clinical scenarios:
Patient A, assuming a RAP of 10mmHg, has a normal PVR and normal PAC of 5ml/mmHg. Even with supranormal stroke volume of 100ml (pulse pressure=20mmHg), his PAPi would only be 2. Conversely, patient B has a high RAP of 20mmHg and a low PAC of 1ml/mmHg due to pulmonary vascular disease. Even with a RAP that is two times higher and half the stroke volume (50ml, pulse pressure=50mmHg), patient B has a higher PAPi of 2.5. Despite the lower PAPi, it is physiologically implausible to suggest that patient A has more severe right heart failure compared to patient B.
These 2 clinical scenarios highlight the key message in our systematic review—the same PAPi cut-off cannot be applied in different clinical conditions, and a single cut-off averaged from a number of heterogenous studies is likely to be misleading. Interpretation of PAPi requires an appreciation of the underlying disease and pathophysiology. We have developed a calculator available in the Supplementary Material, which shows the mechanisms for the observed PAPi depending on the SV and PVR.
It should be noted that other factors may also contribute to the variable cut-offs and discriminatory value in patients undergoing LVAD implantation. First, there are multiple causes of RHF following LVAD implantation, including technical/surgical factors. Second, the definition of severe RHF is related to the postoperative management strategy. The clinical threshold for the use of right ventricular assist device may explain the variable incidence of severe RHF reported in the literature. Thirdly, the timing of assessment relative to LVAD implantation was not uniformly described in the studies. PAPi would change depending on the effects of medical therapy, including diuretics, vasodilators and inotropes, as reported by Gonzalez et al. [25]. The assessment of PAPi closer to the time of LVAD implantation may have greater discriminatory value.
Limitations
With only 2 publications reporting on death in relation to PAPi this precludes meta-analysis and hence the descriptive nature of this systematic review. However, we believe that our description of the relationship between PAPi and PVR (and therefore a single PAPi cut-off value cannot be defined for heterogenous conditions) is not widely appreciated, yet highly relevant to clinicians, especially with greater adoption of PAPi into clinical practice.
CONCLUSION
In conclusion, lower PAPi is associated with a higher risk of RHF and mortality in patients with durable LVAD therapy. However, PAPi is inherently related to PVR and the different PVR levels may have resulted in the variable PAPi cut-offs described in the studies. The PVR level should be taken into consideration in the interpretation of PAPi.
Supplementary Material
ACKNOWLEDGEMENTS
We are most grateful to the library staff at Birmingham Children’s Hospital for sourcing several of the full-text articles.
Glossary
ABBREVIATIONS
- HR
Heart rate
- INTERMACS
Interagency Registry of Mechanically Assisted Circulatory Support
- LVAD
Left ventricular assist device
- MPAP
Mean pulmonary arterial pressure
- PAC
Pulmonary artery compliance
- PAPi
Pulmonary artery pulsatility index
- PAWP
Pulmonary artery wedge pressure
- PP
Pulse pressure
- PVR
Pulmonary vascular resistance
- RAP
Right atrial pressure
- RHF
Right heart failure
- SV
Stroke volume
Contributor Information
Ivan H W Yim, Department of Cardiac Surgery, Queen Elizabeth Hospital Birmingham, Birmingham, UK.
Ayisha M Khan-Kheil, Department of Cardiology, Queen Elizabeth Hospital Birmingham, Birmingham, UK.
Nigel E Drury, Department of Cardiac Surgery, Queen Elizabeth Hospital Birmingham, Birmingham, UK.
Hoong Sern Lim, Department of Cardiology, Queen Elizabeth Hospital Birmingham, Birmingham, UK; Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston B15 2TT, UK.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Funding
This study was funded by a Novel and Emerging Technologies grant from Heart Research UK (NET31/19). Nigel E. Drury was funded by an Intermediate Clinical Research Fellowship from the British Heart Foundation (FS/15/49/31612).
Conflict of interest: Hoong Sern Lim receives honoraria from Abbott. The other authors report no conflict of interest.
DATA AVAILABILITY
All relevant data are within the article and its supporting information files.
Author contributions
Ivan H.W. Yim: Data curation; Formal analysis; Writing—original draft. Ayisha M. Khan-Kheil: Data curation; Methodology. Nigel E. Drury: Methodology; Supervision; Validation. Hoong Sern Lim: Conceptualization; Methodology; Supervision; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Christoph Knosalla, Leonardo Paim and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Pagani FD, Mehra MR, Cowger JA, Horstmanshof DA, Silvestry SC, Atluri P. et al. Clinical outcomes and healthcare expenditures in the real world with left ventricular assist devices – the CLEAR-LVAD study. J Heart Lung Transplant 2021;40:323–33. [DOI] [PubMed] [Google Scholar]
- 2. Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC, Colombo PC. et al. ; MOMENTUM 3 Investigators. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440–50. [DOI] [PubMed] [Google Scholar]
- 3. Lim Y, Low T-T, Chan SP, Lin W, Teo TW, Jang J-HJ. et al. Does pulmonary artery pulsatility index predict mortality in pulmonary arterial hypertension? ESC Heart Fail 2021;8:3835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kochav SM, Flores RJ, Truby LK, Topkara VK.. Prognostic impact of pulmonary artery pulsatility index (PAPi) in patients with advanced heart failure: insights from the ESCAPE trial. J Card Fail 2018;24:453–9. [DOI] [PubMed] [Google Scholar]
- 5. Hochman JS, Sleeper LA, Godfrey E, McKinlay SM, Sanborn T, Col J. et al. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK: an international randomized trial of emergency PTCA/CABG-trial design. The SHOCK trial study group. Am Heart J 1999;137:313–21. [DOI] [PubMed] [Google Scholar]
- 6. Rong LQ, Rahouma M, Neuburger PJ, Arguelles G, Emerson J, Mauer E. et al. Use of pulmonary artery pulsatility index in cardiac surgery. J Cardiothorac Vasc Anesth 2020;34:1220–5. [DOI] [PubMed] [Google Scholar]
- 7. Aslam MI, Jani V, Lin BL, Dunkerly-Eyring B, Livingston CE, Ramachandran A. et al. Pulmonary artery pulsatility index predicts right ventricular myofilament dysfunction in advanced human heart failure. Eur J Heart Fail 2021;23:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reuben SR. Compliance of the human pulmonary arterial system in disease. Circ Res 1971;29:40–50. [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stewart GC, Kittleson MM, Patel PC, Cowger JA, Patel CB, Mountis MM. et al. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) profiling identifies ambulatory patients at high risk on medical therapy after hospitalizations for heart failure. Circ: Heart Failure 2016;9:e003032. 10.1161/CIRCHEARTFAILURE.116.003032. [DOI] [PubMed] [Google Scholar]
- 11. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2022. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (9 January 2022, date last accessed).
- 12. Grandin EW, Zamani P, Mazurek JA, Troutman GS, Birati EY, Vorovich E. et al. Right ventricular response to pulsatile load is associated with early right heart failure and mortality after left ventricular assist device. J Heart Lung Transplant 2017;36:97–105. [DOI] [PubMed] [Google Scholar]
- 13. Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK.. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail 2016;22:110–6. [DOI] [PubMed] [Google Scholar]
- 14. Kang G, Ha R, Banerjee D.. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 2016;35:67–73. [DOI] [PubMed] [Google Scholar]
- 15. Nitta D, Kinugawa K, Imamura T, Amiya E, Hatano M, Kinoshita O. et al. A useful scoring system for predicting right ventricular assist device requirement among patients with a paracorporeal left ventricular assist device. Int Heart J 2018;59:983–90. [DOI] [PubMed] [Google Scholar]
- 16. Loforte A, Montalto A, Musumeci F, Amarelli C, Mariani C, Polizzi V. et al. Calculation of the ALMA risk of right ventricular failure after left ventricular assist device implantation. ASAIO J 2018;64:e140–7–e147. [DOI] [PubMed] [Google Scholar]
- 17. Raymer DS, Moreno JD, Sintek MA, Nassif ME, Sparrow CT, Adamo L. et al. The combination of tricuspid annular plane systolic excursion and heartmate risk score predicts right ventricular failure after left ventricular assist device implantation. ASAIO J 2019;65:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gudejko MD, Gebhardt BR, Zahedi F, Jain A, Breeze JL, Lawrence MR. et al. Intraoperative hemodynamic and echocardiographic measurements associated with severe right ventricular failure after left ventricular assist device implantation. Anesth Analg 2019;128:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muslem R, Ong CS, Tomashitis B, Schultz J, Ramu B, Craig ML. et al. Pulmonary arterial elastance and INTERMACS-defined right heart failure following left ventricular assist device. Circ Heart Fail 2019;12:e005923. [DOI] [PubMed] [Google Scholar]
- 20. Alfirevic A, Makarova N, Kelava M, Sale S, Soltesz E, Duncan AE.. Predicting right ventricular failure after LVAD implantation: role of tricuspid valve annulus displacement. J Cardiothorac Vasc Anesth 2020;34:1204–10. [DOI] [PubMed] [Google Scholar]
- 21. Sert DE, Karahan M, Aygun E, Kocabeyoglu SS, Akdi M, Kervan U.. Prediction of right ventricular failure after continuous flow left ventricular assist device implantation. J Card Surg 2020;35:2965–73. [DOI] [PubMed] [Google Scholar]
- 22. Benjamin MM, Sundararajan S, Sulaiman S, Miles B, Walker RJ, Durham L. et al. Association of preoperative duration of inotropy on prevalence of right ventricular failure following LVAD implantation. ESC Heart Fail 2020;7:1949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruiz-Cano MJ, Morshuis M, Koster A, Lauenroth V, Prashovikj E, Gummert J. et al. Risk factors of early right ventricular failure in patients undergoing LVAD implantation with intermediate Intermacs profile for advanced heart failure. J Card Surg 2020;35:1832–9. [DOI] [PubMed] [Google Scholar]
- 24. Guglin M, Omar HR.. Right atrial pressure predicts mortality among LVAD recipients: analysis of the INTERMACS Database. Heart Lung Circ 2021;30:592–9. [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez MH, Wang Q, Yaranov DM, Albert C, Wolski K, Wagener J. et al. Dynamic assessment of pulmonary artery pulsatility index provides incremental risk assessment for early right ventricular failure after left ventricular assist device. J Card Fail 2021;27:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cacioli G, Polizzi V, Ciabatti M, Cristiano E, Pergolini A, Distefano G. et al. Prediction of right ventricular failure after left ventricular assist device implantation: role of vasodilator challenge. Eur Heart J Acute Cardiovasc Care 2022;11:629–39. [DOI] [PubMed] [Google Scholar]
- 27. Stricagnoli M, Sciaccaluga C, Mandoli GE, Rizzo L, Sisti N, Aboumarie HS. et al. Clinical, echocardiographic and hemodynamic predictors of right heart failure after LVAD placement. Int J Cardiovasc Imaging 2022;38:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura M, Nishimura T, Kinoshita O, Kashiwa K, Kyo S, Ono M.. Hemodynamic influence of tilting disc valve type on pump performance with the NIPRO-ventricular assist device. J Artif Organs 2012;15:134–9. [DOI] [PubMed] [Google Scholar]
- 29. Chemla D, Lau EMT, Papelier Y, Attal P, Hervé P.. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur Respir J 2015;46:1178–89. [DOI] [PubMed] [Google Scholar]
- 30. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR. et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012;125:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the article and its supporting information files.