Abstract
Advances in biomedical research have demonstrated that inflammation and its related diseases are the greatest threat to public health. Inflammatory action is the pathological response of the body towards the external stimuli such as infections, environmental factors, and autoimmune conditions to reduce tissue damage and improve patient comfort. However, when detrimental signal-transduction pathways are activated and inflammatory mediators are released over an extended period of time, the inflammatory process continues and a mild but persistent pro-inflammatory state may develop. Numerous degenerative disorders and chronic health issues including arthritis, diabetes, obesity, cancer, and cardiovascular diseases, among others, are associated with the emergence of a low-grade inflammatory state. Though, anti-inflammatory steroidal, as well as non-steroidal drugs, are extensively used against different inflammatory conditions, they show undesirable side effects upon long-term exposure, at times, leading to life-threatening consequences. Thus, drugs targeting chronic inflammation need to be developed to achieve better therapeutic management without or with a fewer side effects. Plants have been well known for their medicinal use for thousands of years due to their pharmacologically active phytochemicals belonging to diverse chemical classes with a number of these demonstrating potent anti-inflammatory activity. Some typical examples include colchicine (alkaloid), escin (triterpenoid saponin), capsaicin (methoxy phenol), bicyclol (lignan), borneol (monoterpene), and quercetin (flavonoid). These phytochemicals often act via regulating molecular mechanisms that synergize the anti-inflammatory pathways such as increased production of anti-inflammatory cytokines or interfere with the inflammatory pathways such as to reduce the production of pro-inflammatory cytokines and other modulators to improve the underlying pathological condition. This review describes the anti-inflammatory properties of a number of biologically active compounds derived from medicinal plants, and their mechanisms of pharmacological intervention to alleviate inflammation-associated diseases. The emphasis is given to information on anti-inflammatory phytochemicals that have been evaluated at the preclinical and clinical levels. Recent trends and gaps in the development of phytochemical-based anti-inflammatory drugs have also been included.
Keywords: inflammation, phytochemical, medicinal drug, preclinical, clinical
1 Introduction
Chronic inflammation and associated disorders are the biggest public health issues and expected to increase enormously in the United States during the next 30 years (Pahwa et al., 2020). Inflammation is the pathological response of the body towards the external stimuli such as infectious, chemical, mechanical, and autoimmune stressors. Depending on post inflammatory responses, inflammation may be acute or chronic. Acute inflammation concentrates immune cells at the site of infection to combat dangerous foreign material while chronic inflammation is defined by the type of inflammatory cells in tissues when acute inflammation persists for a longer time (Ward, 2010). Advances in molecular studies show that chronic inflammation causes diabetes, heart disease, cancer, stroke, arthritis, and obesity (Pahwa et al., 2020) (Figure 1). It should be noted that inflammation is a self-healing process that proceeds in three crucial steps which are interconnected and occur sequentially such as swelling, redness, immobility, pain, and heat (Yatoo et al., 2018). Firstly, it starts from an increased vascular permeability followed by infiltration of immune cells that finally results in granuloma formation and tissue repair (Eddouks et al., 2012). Activated immunogenic response triggers mitogen-activated protein kinase (MAPK), Janus kinase/signal transducers and activators of transcription (JAK-STAT), and nuclear factor-κB (NF-κB) pathways, as well as the production of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL) 1β (IL-1β), and chemokines (Afonina et al., 2017). Cytokines and chemokines both are critical for attracted activating additional immune cells at infection site, such as circulating neutrophils that boost interferon γ (IFN-γ), proteases, and reactive oxygen species (ROS). Cytokines also increase cyclooxygenase-2 (COX-2) that promotes the synthesis of inflammatory prostaglandins (Gandhi et al., 2017). After removing the immunogenic factor, the immune system reprograms signaling pathways to resolve inflammation in a dynamic process regulated by several biological systems. First, deployed effector cells are killed and reduced to baseline levels following elimination of proinflammatory agents and signals. Non-inflammatory macrophages remove apoptotic neutrophil vesicles and restores tissue equilibrium (Maskrey et al., 2011). However, sometimes the underlying conditions of the body interrupts with this phenomenon and lead to dysregulation of the inflammatory system, resulting in uncontrolled pathways and the production of inflammatory mediators that cause chronic inflammation and other degenerative diseases. One evidence meets here with regards to a link between inflammation and obesity (Stepien et al., 2014). In the present review, we have postulated a basic understanding of inflammation, obesity and other related complications while more emphasized on recent investigations of medicinal phytochemicals for their anti-inflammatory properties using preclinical and clinical studies.
FIGURE 1.
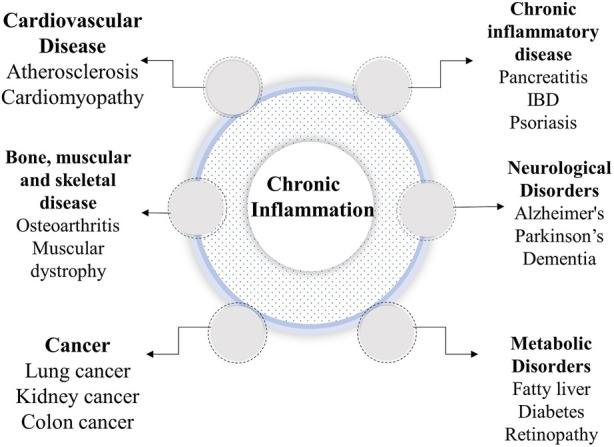
Complications caused by chronic inflammation.
2 Inflammation, obesity and related complications
Chronic inflammation is a condition that typically lasts for a long time and is characterized by the presence of immune cells such as lymphocytes and macrophages along with the proliferation of blood vessels and connective tissues. One remarkable discovery postulated that obesity is the biggest cause of chronic inflammation, following severe disorders (Ellulu et al., 2017). World Health Organization (WHO) estimated that 1.9 billion people are overweight and 600 million are obese (World Health Organization, 2015). Obesity increases pro-inflammatory IL-6 and TNF-α levels and decreases anti-inflammatory hormone adiponectin (Stepien et al., 2014). The overexpressed pro-inflammatory cytokines are considered to be the link between obesity and inflammation and this sustained chronic inflammation is a strong risk factor for developing many metabolic disorders and cancer (Hotamisligil, 2006).
The adipose tissues are the determining factor of the occurrence of obesity. These tissues respond to additional nutrients by hyperplasia and hypertrophy, causing adipocyte expansion and obesity, which reduces blood flow and causes hypoxia (Cinti et al., 2005). Hypoxia is thought to cause necrosis and macrophage infiltration into adipose tissue, which leads to increased pro-inflammatory mediator production, including leptin, adiponectin, IL-6, TNF-α, monocyte chemoattractant protein-1 (MCP-1), and resistin (Lafontan, 2005). IL-6 induces hepatocytes to produce and release inflammatory molecules, c-reactive protein (CRP) that indicates liver-caused systemic inflammation which controls obesity regardless of race and gender (Choi et al., 2013). Klisic et al. (2014) measured CRP and metabolic markers among normal weight and overweight postmenopausal women and reported higher levels of CRP and triglycerides (TG) in overweight women. Adiponectin and leptin have a major role in inflammation; IL-6 also modulates the secretion of these hormones (Matsuda et al., 2002; Matsuzawa, 2006; Klisic et al., 2014). IL-6, adiponectin, leptin, and CRP are significant mediators of localized inflammation in adipose tissues when abnormalities are present. In this situation, obesity-related comorbidities develop, indicating an inflammatory state that contributes to the onset and progression of many diseases (Trayhurn and Wood, 2004; Hansson, 2005; Danesh et al., 2008; Zhang et al., 2009; Sansone and Bromberg, 2012) (Figure 2).
FIGURE 2.
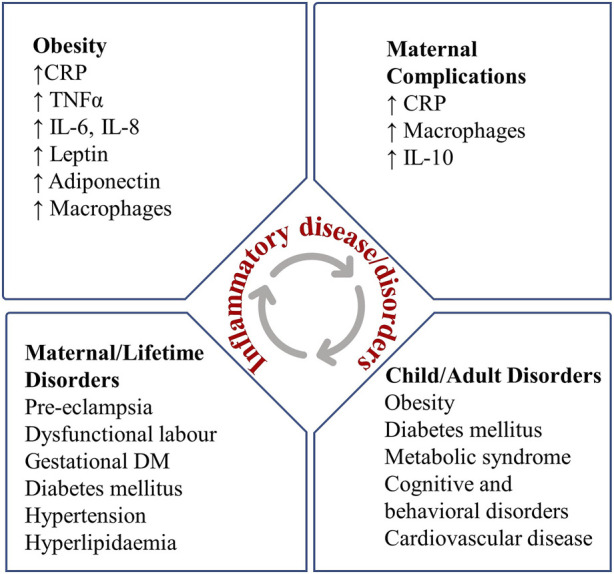
Relation between maternal obesity, inflammation and birth complications.
Obesity and inflammation have interrelated effects on the immune system, body weight, and metabolism (Castanon et al., 2014; McNelis and Olefsky, 2014). A study found a link between inflammation and ω-3 and ω-6 polyunsaturated fatty acids (PUFA) ratio. Larger consumption of ω-3 fatty acids reduces proinflammatory cytokines, IL-2, IL-6, and TNF-α, and increases anti-inflammatory IL-10 and tumor growth factor- β (TGF-β) (Alfano et al., 2012). High ω-6 PUFA diets increase adipokine levels, pro-inflammatory cytokine production, and hyperinsulinemia (Chan and Norat, 2015; Ghose et al., 2015). In animal studies, Polyak et al. (2014) found that chemokine fractalkine receptor knockout animals gained less weight and had less white adipose tissue than controls. These animals also had lower adipose MCP-1, IL-1α, and TNF-α levels (Polyak et al., 2014). IL-18 knockout animals fed a high-fat diet gained weight and burned less energy. Additionally, central IL-18 infusion reduced high-fat meal consumption, demonstrating that IL-18 can influence food intake centrally (Zorrilla and Conti, 2014). In conclusion, chemokine/cytokines, fractalkine, and IL-18 affect weight gain and metabolic diseases, indicating an interdisciplinary approach to inflammation and high-fat diet/obesity. The data also shows a link between obesity, diet, and chronic inflammation, which causes multiple diseases/disorders.
2.1 Birth complications
Preeclampsia (PE) has a global incidence of 2.16% during pregnancy (Abalos et al., 2014) and causes proteinuria, thrombocytopenia, renal insufficiency, and liver disease (Pennington et al., 2012; Abalos et al., 2014). In a healthy pregnancy, the processes that promote uteroplacental vascular remodeling can lead to placental ischemia after placental inflammation, which releases substances into the maternal circulation. These substances stimulate immune cells in the body’s periphery, especially T and B lymphocytes, which cause endothelial cell dysfunction, vascular dysfunction, and high blood pressure (LaMarca et al., 2013; Roberts, 2014). Since pro-inflammatory processes influence placental ischemia-induced hypertension, these mechanisms are likely amplified in obese people. Obesity before pregnancy is linked to high levels of pro-inflammatory cytokines in the placenta and circulating IL-6 throughout pregnancy. Overweight women have thicker placental blood vessel walls than normal-weight women (Roberts et al., 2011). Increased leptin gene expression may also contribute to PE (Lepercq et al., 2003; Iwagaki et al., 2004), decreased uterine natural killer cells (Parker et al., 2014), and increased CD4+ T cells (Wallace et al., 2001).
2.2 Cognitive and behavioral disorders
Obesity-related inflammation also affects the neonatal child and gives birth to neurological complications and brain disorders (Edlow, 2018). Thus, obesity-induced or direct inflammation during pregnancy make autism, schizophrenia, attention-deficit hyperactivity disorder and major depressive disorder more prevalent (Patterson, 2009; Knuesel et al., 2014; Estes and McAllister, 2016). Schizophrenia is characterized by delusions, hallucinations, disordered thinking, and cognitive impairment. Its prevalence rose from 13.1 million in 1990 to 20.9 million in 2016 (Charlson et al., 2018). Severe infections and autoimmune diseases may increase the lifetime risk of schizophrenia and schizophrenia spectrum disorders (Meyer, 2011; Benros et al., 2014). In response to maternal inflammation, placental cytokines (IL-1, IL-6, and interferon-γ) increase fetal oxidative stress (Meyer et al., 2009). This irreversible dysregulation affects brain growth and function and increases schizophrenia risk. Proinflammatory cytokine IL-6 may link maternal inflammation to fetal brain development and later psychopathology (Kohli et al., 2007; Buss et al., 2012). A recent study with 84 newborns used machine learning and resting-state functional magnetic resonance imaging. It showed that variations in maternal IL-6 concentrations across the course of pregnancy are associated with individual differences in functional brain networks in the neonatal period and relate to future working memory performance (Rudolph et al., 2018).
2.3 Cardiovascular diseases
Inflammation plays a key role in atherosclerosis, which raises risk of cardiovascular diseases (CVD) (Steinberg, 2006). Atherosclerosis begins with low-density lipoproteins (LDL) build up in abnormally permeable artery endothelium. Overexpression of IL-6 in atheromatous fatty streaks, endothelium, smooth muscle, and adipose tissue accelerates atherosclerosis (Szekanecz et al., 1994; Hlatky et al., 2009). TNF-α plays a role in endothelial dysfunction, vascular dysregulation, monocyte adherence to endothelial cells, vascular oxidative stress, apoptosis, and the atherogenic response, which lead to thrombosis and coagulation (Ueland et al., 2012; Zhang and Zhang, 2012). Leptin and adipokine influence atherosclerosis after CVD (Chen et al., 2003; Sierra-Johnson et al., 2007). Obesity is a risk factor for endothelial dysfunction-related cardiovascular diseases like arterial hypertension and atherosclerosis. Adipokines affect triglyceride metabolism and adipocyte hypertrophy, which can lead to macrophage expansion in adipose tissue, inflammation, and increased production of proinflammatory cytokines TNF-α and IL-6 (Samad et al., 1997; Fried et al., 1998; Mahabadi et al., 2009). Increased macrophages and local inflammation may cause obesity-related metabolic dysfunctions like systemic inflammation and atherosclerosis.
2.4 Osteoarthritis
Arthritis is another chronic inflammatory condition that causes disability and pain and hinders socioeconomic life. Osteoarthritis (OA) affects 250 million people worldwide, mostly the elderly (Kotti et al., 2014). Cartilage degeneration, subchondral bone remodeling, osteophyte production, and synovium and joint capsule inflammation characterize OA (Goldring and Goldring, 2010). Numerous soluble mediators, like cytokines or prostaglandins, can stimulate chondrocyte matrix metalloproteinases (MMP) synthesis, causing inflammation. OA causes an imbalance between pro-inflammatory and anti-inflammatory cytokines in the synovium (Kulkarni et al., 2021). Osteophytes are pro-inflammatory due to high mast cell activity (Kulkarni et al., 2022). Once thought to be cartilage-driven, OA is characterized by inflammatory synovium (Goldring and Otero, 2011; Kapoor et al., 2011; Loeser et al., 2012). In obese people, obesity may link OA and inflammation where obese people have twice the risk of OA as normal-weight people (Yusuf et al., 2010). Obesity imbalances adipokines and other cytokines, which may cause osteoarthritis (Gomez et al., 2011). White adipose tissue is the most common source of adipokines, but the knee’s infrapatellar fat pad also may produce inflammatory mediators like IL-6, TNF-α, adipsin, adiponectin, and visfatin that reach the synovium and cartilage (Clockaerts et al., 2010; Klein-Wieringa et al., 2011).
2.5 Diabetes
The International Diabetes Federation (IDF) predicts 578 million cases of diabetes by 2030 and 700 million by 2045 (International Diabetes Federation, 2019). Diabetes is characterized by impaired glucose tolerance and hyperglycemia caused by insulin deficiency or resistance (Blair, 2016). Type 1 diabetes is caused by β-cell death due to autoimmune disorder whereas type 2 diabetes (T2DM) is linked to genetics, ethnicity, age, overweight, unhealthy diet, and lack of exercise. Growing evidence suggests these causal variables follow the same inflammatory pathways as a shared pathogenetic mediator in diabetes progression (Shoelson et al., 2006). Diabetes etiology, relationship with obesity, and biological function of adipose tissue are studied extensively. The amount of inflammatory factors produced by adipose tissue macrophages defines obesity (Weisberg et al., 2003; Xu et al., 2003). When macrophages and immune cells move into adipose tissue, they cause chronic low-grade inflammation. The latter produces TNF-α, IL-1, IL-6, IL-10, leptin, adiponectin, MCP, angiotensinogen, resistin, and other cytokines and chemokines (Kanda et al., 2006; Shoelson et al., 2007; Antonopoulos et al., 2015) that serve as the pathologic link between obesity, insulin resistance and diabetes (Nikolajczyk et al., 2011).
2.6 Cancer
Lifestyle and environmental factors, rather than inherited genetic defects, regulate the development of 90%–95% of all cancers (Aggarwal et al., 2009). Chronic inflammation produces reactive oxygen species (ROS) leading to mutations and proliferation of the pro-cancerous cells. Cancer-promoting cytokines like IL-6, IL-11, TNF-α, IL-1β, and IL-23 vary by tumor type and stage. Thus, inflammation is a central component of tumor development and progression. In tumor microenvironments, inflammatory cells and mediators promote proliferative signaling, migration, metastasis, and blood vessel growth (Anand et al., 2008; Hanahan and Coussens, 2012). Inflammation accelerates many phases of metastasis, a key factor in cancer mortality (Hanahan and Weinberg, 2011). One recent study has estimated that 3.6% of all new cancer cases worldwide are attributable to excess adiposity and that uterine, postmenopausal breast, and colon cancer account for 63.6% of cancers attributable to high body mass index (BMI) (Arnold et al., 2015). As obesity-induced chronic inflammation is a cancer precondition, it increases cancer incidence and death. Obesity modifies release of adipokines and cytokines, affecting many systemic processes, including the tumor environment. Adiponectin, leptin, IL-6, TNF-α, YKL-40 (chitinase-3-like-protein-1), osteopontin, and plasminogen activator inhibitor-1 (PAI-1) are all produced by adipocytes and stimulate cancer growth, progression, and metastasis (Quail and Dannenberg, 2019).
In summary, obesity and inflammation are two sides of the same coin; it doesn’t matter which comes first. Both conditions are subjected with one causing the other and give rise to multiple health complications. Moreover, the facts about inflammation-related diseases and disorders, with an emphasis on obesity, show that chronic inflammation is the main cause of these complications. The information on diseases associated with inflammation demonstrates that chronic inflammation is the primary outcome of these complications. Our immune effector cells produce ROS and cytokines that trigger paracrine and autocrine inflammation. Unchecked oxidative stress can cause inflammation and tissue damage (Bennett et al., 2018). Chemically synthesized drugs can treat these inflammatory complications. Two drug classes 1) Steroid-based anti-inflammatory drugs (SAIDs) and 2) Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) were developed to overcome the side effects and limitations of steroidal anti-inflammatory drugs (Celotti and Laufer, 2001; Rainsford, 2007). Even though high-class drugs are available, there are cost, availability and most importantly, side effect restrictions. To address these disadvantages, medicines must target underlying inflammation to make therapeutic advances with no or fewer adverse effects. Since inflammation is complex, it requires multidimensional treatment. In this regard, medicinal herbs are gaining importance to prevent and treat inflammatory disorders. In traditional use, clinical trials, and experimental studies, multiple plants have shown anti-inflammatory effects (Arulselvan et al., 2016; Allegra, 2019).
3 Plant derived drugs: a historical perspective
Historical observation of folklore medicines reveals Ayurveda and herbalism with ancient plant uses (4500 BC) (Karunamoorthi et al., 2013). Herbal medicine is the practice of treating disease with plants, plant extracts, herbal preparations, and finished herbal products called phytomedicines that contain phytochemicals as active ingredients (Pan et al., 2014). Traditional Chinese, Indian, and Arabic herbal medicine are the three main herbal treatment systems today. Archaeological evidence shows that Iraq and China have used herbal medicine for 6,000 and 8,000 years ago, respectively (Leroi-Gourhan, 1975; Pan et al., 2014). The earliest records of natural products are from Mesopotamia (2600 B.C.), where clay tablets documented the use of oils derived from Commiphora species (myrrh) and Cupressus sempervirens L. (Cypress) to treat coughs, colds, and inflammation (Cragg and Newman, 2005). In the past 40 years, both developing and developed countries have used more herbs and herbal products for health. Aspirin, or acetylsalicylic acid (Salix alba L., White willow), is a well-known anti-inflammatory drug. Other important drugs include morphine and codeine (opium poppy), digitoxin (lady’s glove), anti-malarial quinine, and Pilocarpine (Pilocarpus jaborandi Holmes, Pilocarpus) (Tarver, 2014). With advances in technology and chemical sciences, herbal active ingredients are being isolated and studied for pharmacological uses. This revolution in phytopharmacology has led to the development of various phytomedicines. Table 1 lists plant-based chemicals that have been shown to treat illness.
TABLE 1.
Plant derived drugs for commercial use in various diseases.
| Drug | Class of drug | Plant source | Disease | References |
|---|---|---|---|---|
| Paclitaxel | Taxanes | Taxus brevifolia | Breast cancer | Cragg (1998) |
| Ingenol 3-O-angelate | Polyhydroxy diterpenoid | Euphorbia peplus | Skin cancer | Kedei et al. (2004); Ogbourne et al. (2004) |
| PG490-88(14-succinyl triptolide sodium salt) | Diterpene-diepoxide | Tripterygium wilfordii | Autoimmune and inflammatory diseases | Kiviharju et al. (2002); Fidler et al. (2003) |
| Tiotropium | Muscarinic receptor antagonist | Atropa belladonna | Asthma and COPD | Kumar and Reddy (2003) |
| Arteether | Sesquiterpene lactones | Artemisia annua | Antimalarial | Newman and Cragg (2007) |
| Grandisines A and B | Indole alkaloids | Elaeocarpus grandis | Analgesic | Carroll et al. (2005) |
| Galantamine hydrobromide | Amaryllidaceae alkaloid | Galanthus nivalis | Alzheimer’s | Howes et al. (2003) |
| Apomorphine | Dopamine | Papaver somniferum | Parkinson’s | Deleu et al. (2004) |
4 Phytochemicals evaluated in anti-inflammatory properties
Increasing knowledge of folklore medicinal plants as a therapeutic target opened the door for anti-inflammatory plant extracts. Polyherbal formulation of Ashwagandharishta, Balarishta, Dashmoolarishta, and Triphala extract reduces synovial inflammation (Ingale et al., 2018). Pawar et al. (2011) tested Withania somnifera L. root extracts in an inflammatory bowel disease (IBD) rat model (Pawar et al., 2011). Piper ovatum Vahl leaves have been examined for their anti-inflammatory properties by Rodrigues Silva et al. (2008). Ayurveda describes fermented Asava and Arishta formulations. These formulations are plant extracts fermented with microbes, allowing biological transformation and potentially generating novel fermentative products of phytochemicals with superior bioavailability and anti-inflammatory activity (Bhondave et al., 2014). Carrageenan-injected rats showed anti-inflammatory effects from Eulophia ochreata L. tubers extract (Jagtap et al., 2009). An animal model of carrageenan-induced inflammation was used to test the anti-inflammatory properties of the ethanolic root extract of Swertia chirata Buch.-Ham. ex Wall (Das et al., 2012). To understand the plant’s anti-inflammatory role and mechanism, researchers are isolating and characterizing phytochemicals and organizing them by structure and chemical properties. Understanding phytochemical mechanisms of action could lead to new anti-inflammatory drugs.
5 Preclinical trials
First, phytochemicals are tested in vitro, then in vivo using animal models, and finally in humans. Selecting the right experimental model prevents bias and errors. This study examined in vitro and in vivo anti-inflammatory phytochemicals and plant-based anti-inflammatory drug possibilities. In this section, potential phytochemicals (Figure 3) studied for anti-inflammatory diseases/complications in preclinical experiments are discussed (Tables 2, 3).
FIGURE 3.
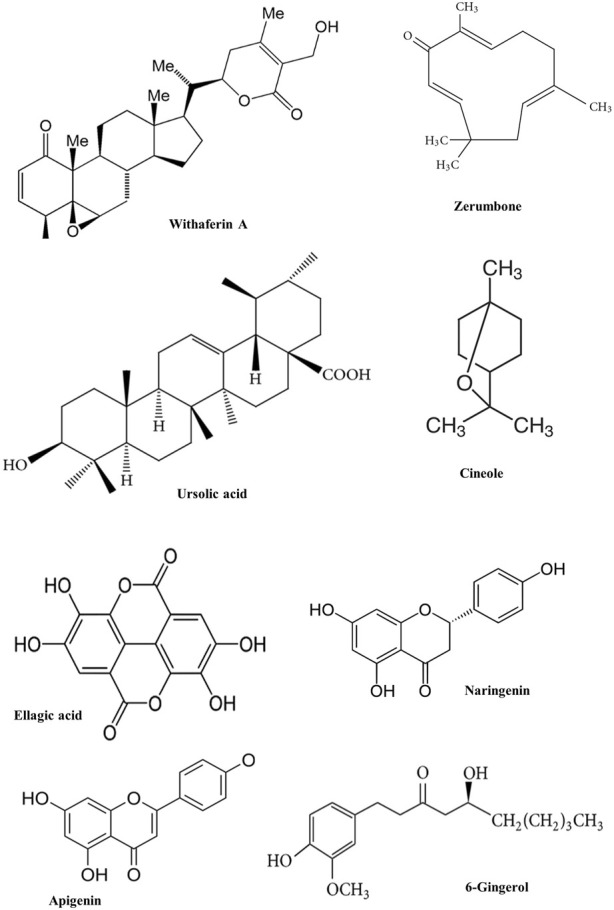
Chemical structure of some phytochemicals used in pre-clinical trials.
TABLE 2.
List of anti-inflammatory phytochemicals used in pre-clinical trials.
| Class | Phytochemicals | Botanical name (family) | Molecular targets | References |
|---|---|---|---|---|
| Flavones | Apigenin | Chamaemelum nobile (Asteraceae) | NF-κB and STAT 1 Signalling pathways, expression of miR-155, activation of PPAR-γ | Nicholas et al. (2007), Arango et al. (2015), Feng et al. (2016) |
| Luteolin | Rosmarinus officinalis (Lamiaceae) | JNK, NF-κB p65 Signalling pathways | Ando et al. (2009), Xiong et al. (2017) | |
| Baicalin and Baicalein | Scutellaria baicalensis (Lamiaceae) | Fizz1 expression, CHOP/STAT pathway | Kim et al. (2018), Zhu et al. (2016) | |
| Flavonols | Quercetin | Malus domestica (Rosaceae) | NF-κB p65, ERK, JNK and STAT pathways, AKT Signalling modulation | Bian et al. (2018), Maurya and Vinayak, (2017) |
| Kaempferol | Camellia sinensis (Theaceae) | NF-κB, STAT, and JNK Signalling pathways | Bian et al. (2019), Park et al. (2015) | |
| Flavanone | Naringenin | Citrus paradise (Rutaceae) | NF-κB activation, NF-kB/IL-6/STAT-3 pathways, NO-cGMP-PKG KATP channel Signalling | Pinho-Ribeiro et al. (2016), Zhang et al. (2018b), Manchope et al., (2016) |
| Hesperidin | Citrus limon (Rutaceae) | expression of p65, Foxo1, Foxo3, and Nrf2 Signalling pathways | Xiao et al. (2018), Tsai et al. (2019) | |
| Isoflavones | Genistein | Genista tinctorial (Fabaceae) | NF-κB/Akt Signalling pathway, AMPK activation, expression of p65 | Howes and Simmonds (2014), Lee et al. (2019), Yuan et al. (2019) |
| Puerarin | Pueraria lobate (Fabaceae) | NF-κB activation, Fizz1 expression, Nrf2 regulation | Liu et al. (2014a), Nguyen Ngo Le et al. (2019), Jeon et al. (2020) | |
| Catechins | Epigallocatechin gallate | Camellia sinensis (Theaceae) | Suppression of neuronal apoptosis, NF-κB/p65/IκB-α Signalling pathway | Cai et al. (2014) |
| Anthocyanidins | Cyanidin-3-O-glycoside | Lonicera caerulea (Caprifoliaceae) | MAPK and NF-κB Signalling pathway, regulation of iNOS and COX-2 expression | Wu et al. (2017), Pereira, Almeida, and Dinis (2018) |
| Monoterpenes | Cineole | Eucalyptus globulus (Myrtaceae) | PPAR-γ dependent modulation of NF-κB, PRR pathways, NF-κB/MAPKs/MKP-1 Signalling pathways | Linghu et al. (2019), Yadav and Chandra (2017) |
| Paeoniflorin | Paeonia lactiflora (Paeoniaceae) | Nrf2/HO-1 Signalling pathways, MAPK pathway, ERK1/2 and Akt regulation, NF-κB/p65/IκBα signalling pathways | Wu et al. (2019), Yu et al., (2017), Gong et al. (2015), Yu et al. (2019) | |
| Sesquiterpenes | Parthenolide | Tanacetum parthenium (Asteraceae) | NF-κB and MAPKs signalling pathways Nrf2/Keap1 signalling pathway | Kim et al. (2019), Li et al. (2015) |
| Zerumbone | Zingiber zerumbet (Zingiberaceae) | NF-κB/HO-1 signalling pathway, NF-κB/MAPK/PI3K-Akt signalling pathways | Kim et al. (2009), Haque et al., (2018) | |
| Diterpenoids | Ginkgolides | Ginkgo biloba (Ginkgoaceae) | Regulation of Caspase-1/NF-κB P65 expression, CD40-NF-κB signal pathway | Chen et al. 2018b), Zhang et al. (2018) |
| Triterpenoids | Ursolic acid | Glechoma hederacea (Lamiaceae) | NF-κB/p65 signalling pathway | Zhao et al. (2018) |
| Escin | Aesculus hippocastanum, (Sapindaceae) | mRNA expression of NF-κB/reduction of TNF-α, P-selectin, and VCAM-1 | Wang et al., (2014), Zhao et al. (2018) | |
| Withaferin A | Withania somnifera (Solanaceae) | IKKβ/NF-κβ pathway, regulation of LPS/TLR4 pathway | Martorana et al., (2015), Batumalaie et al., (2016) | |
| β –sitosterol | Glycine max (Fabaceae) | SHP-1/NF-κB regulation, NLRP3/caspase-1 signalling pathway | Valerio and Awad (2011), Liao et al., (2018) | |
| Curcuminoids | Curcumin | Curcuma longa, (Zingiberaceae) | TLR4/MyD88/NF-κB signalling pathway, PI3K/Akt/NF-κB signalling pathway, NF-κB/PPAR-γ signalling, MAPK/ERK/p38/Akt/NF-κB pathway, HO-1, and Nrf-2 pathway | Zhu et al. (2014), Song et al., (2013), Liu et al., (2016b), Yu et al., (2018) |
| Stilbenes | Resveratrol | Vitis vinifera (Vitaceae) | Modulation of AP-1/NF-κB/COX-2, ICAM-1, iNOS, and IL-1β mRNA expression, VEGF/p38-MAPK/NF-κB pathway | Latruffe et al., (2015), Huang et al., (2017), Yan et al., (2018) |
| Phenolic acids | Rosmarinic acid | Rosmarinus officinalis (Lamiaceae) | NF-κB and p65 expression, NF-κB/p65/pSTAT3 pathway | Cao et al., (2016), Jin et al., (2017) |
| Ellagic acid | Punica granatum (Lythraceae) | expression of RANTES protein, IRAK4/TRAF-6/IKK-β/NF-κB/p65 expressions | (Promsong et al., (2015), Zhou et al., 2019) | |
| Gallic acid | Camellia sinensis (Theaceae) | TLR-4/NF-κB/PPARγ signalling pathway | Fan et al. (2018) | |
| Protocatechuic acid | Allium cepa (Amaryllidaceae) | SIRT1/NF-κB signalling pathway PI3K/Akt-mediated nuclear-factor-κB activation, STAT-6/PPAR-γ pathway | Kaewmool et al. (2020) | |
| Vanillic acid | Vanilla planifolia (Orchidaceae) | Nrf2/HO-1 expression | Calixto-Campos et al. (2015) | |
| 6-gingerol | Zingiber officinale Rosc. (Zingiberaceae) | PI3K and p-Akt expression, RANKL/PGE2 expressions | Xu et al. (2018b), Hwang et al. (2018) | |
| Caffeic acid phenethyl ester | Populus nigra L. (Salicaceae) | NF-κB/p65 signalling pathway, Nrf2/HO-1 signalling pathway | Takakura et al. (2018) |
TABLE 3.
List of some animal models with the target pathologies in pre-clinical trials.
| Phytochemicals | Animal models | Target pathologies | References |
|---|---|---|---|
| Apigenin | Male C57BL/6J | Colonic inflammatory and motor abnormalities | Gentile et al. (2018) |
| Luteolin | C57BL/6J Obese mice model | Insulin resistance (IR) and type 2 diabetes pathophysiology | Liu et al. (2014b) |
| Baicalin and Baicalein | LPS-induced mastitis in BALB/c mice | Mastitis | He et al. (2015) |
| Quercetin | Male C57BL/6N mice | Angiogenesis in lymphoma-bearing mice | Khan et al. (2018) |
| Kaempferol | BALB/c mice models | Allergic asthma | Park et al. (2015) |
| Naringenin | Male Swiss mice | Superoxide anion-driven inflammatory pain | Manchope et al. (2016) |
| Hesperidin | Sprague-Dawley rats | Diabetic neuropathy | Visnagri et al. (2014) |
| Genistein | Diethyl nitrosamine induced C57BL/6 N mice | Hepatocellular carcinoma | Lee et al. (2019) |
| Puerarin | Male Sprague-Dawley rats | Streptozotocin (STZ)-induced diabetes | Liu et al. (2014a) |
| Epigallocatechin gallate | Male adult Sprague–Dawley (SD) rats | Chronic constriction injury | Cai et al. (2014) |
| Cyanidin-3-O-glycoside | TNBS-challenged mice | Inflammation in colitis | Gan et al. (2019) |
| Cineole | Male Kunming mice | LPS-induced acute inflammatory injury | Linghu et al. (2019) |
| Paeoniflorin | Adult male Sprague-Dawley rats | Chronic constriction injury | Zhou et al. (2019b) |
| Parthenolide | Collagen antibody-induced arthritis (CAIA) BALB/c mouse model | Rheumatoid arthritis | Williams et al. (2020) |
| Zerumbone | Mono-iodoacetate (MIA)-induced male SD rat OA model | Osteoarthritis | Chien et al. (2016) |
| Ginkgolides | Male Sprague-Dawley rats | Myocardial ischemia/reperfusion | Zhang et al. (2018a) |
| Ursolic acid | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated Parkinson mouse model | Neuroinflammation | Rai et al. (2019) |
| Escin | Swiss albino mice | Indomethacin-induced gastric ulcers | Wang et al. (2014) |
| Withaferin A | BALB/c mice | Spinal cord tissues in traumatized mice | Yan et al. (2017) |
| β –sitosterol | CIA mice model | Arthritis | Liu et al. (2019a) |
| Curcumin | BALB/c mice | Allergic asthma | Shahid et al. (2019) |
| Resveratrol | Cigarette smoke COPD mouse models | Chronic obstructive pulmonary disease | Chen et al. (2016) |
| Rosmarinic acid | DSS-induced colitis mouse model | Splenomegaly | Jin et al. (2017) |
| Ellagic acid | streptozotocin-induced diabetic nephropathy mouse model | Diabetic Nephropathy | Zhou et al. (2019a) |
| Gallic acid | sulfonic acid (TNBS)-induced ulcerative colitis (UC) mouse model | Ulcerative colitis | Zhu et al. (2019) |
| Protocatechuic acid | castrated rats | Benign prostatic hyperplasia | Akanni et al. (2020) |
| Vanillic acid | carrageenan-induced inflammatory pain mouse model | Analgesic and anti-inflammatory effects | Calixto-Campos et al. (2015) |
| 6-gingerol | Sprague-Dawley rats | myocardial infarction | Xu et al. (2018b) |
| Caffeic acid phenethyl ester | rat model of optic nerve crush (ONC) injury | Glaucoma | Takakura et al. (2018) |
5.1 Flavones
5.1.1 Apigenin (APG)
Apigenin (APG) is found in Chamaemelum nobile (L.) All. (Asteraceae) ligulate flowers, celery, parsley, coriander, and peppermint. Anti-inflammatory activity of APG involves inhibiting of NF-κB translocation by suppressing p65 phosphorylation (Nicholas et al., 2007). In an IFN-γ activated murine microglia cell model, APG’s effect on STAT1 phosphorylation reduced IL-6 and TNF-α levels (Rezai-Zadeh et al., 2008). APG and APG-rich diets may have anti-inflammatory effects in vivo by lowering lipopolysaccharide (LPS)-induced microRNA-155 (Arango et al., 2015). Diet-induced obesity in male C57BL/6J mice was used to study APG’s effects on inflammatory and motor abnormalities in the colon. APG (10 mg/kg) stopped the increase in body fat, epididymal fat, and metabolic indexes. There was also a reduction in malondialdehyde (MDA), IL-1β, IL-6, eosinophil infiltration, substance P, and inducible nitric oxide synthase (iNOS expression) (Gentile et al., 2018). Alzheimer’s, Parkinson’s, and Huntington’s are neurodegenerative diseases caused by neuroinflammation. APG showed strong anti-inflammatory properties in a human-induced pluripotent stem cell (iPSC) model of familial and sporadic Alzheimer by protecting neurites and cell viability by downregulating cytokine and nitric oxide (NO) release in inflammatory cells (Balez et al., 2016). Non-alcoholic steatohepatitis (NASH) causes a fatty, inflamed liver. APG (0.005%, w/w) reduced inflammation by lowering plasma MCP-1, IFN-γ, TNF-α, and IL-6 levels in mice with NASH and a high-fat diet (Jung et al., 2016). In diabetic rats, APG (10, 30, 50 mg/kg) reduced metabolic inflammation by successfully polarizing infiltrating macrophages to an anti-inflammatory M2 phenotype. The mechanism involved binding and activating peroxisome proliferator-activated receptor gamma (PPAR-γ) and the subsequent suppression of the NF-κB pathway (Feng et al., 2016).
5.1.2 Luteolin
This is a common flavone found in rosemary (Rosmarinus officinalis L., Lamiaceae), pomegranate (Punica granatum L., Lythraceae) and artichoke (Cynara scolymus L., Asteraceae). Luteolin suppresses chronic inflammation in adipocytes and macrophages coculture, as well as c-Jun N-terminal Kinase (JNK) phosphorylation in macrophages (Ando et al., 2009). In the C57BL/6J obese mice model, luteolin (10 mg/kg) reduces MCP-1 and resistin in blood, while elevated adiponectin level that improved insulin resistance (IR) and T2DM (Liu et al., 2014b). Multiple sclerosis (MS), a neurodegenerative and immune-inflammatory disorder, causes problems throughout the body. Immunomodulatory effects on peripheral blood mononuclear cells (PBMC) derived from MS patients were observed in the presence of luteolin where it suppressed pro-inflammatory cytokines, including IL-1β, MMP-9, and TNF-α (Sternberg et al., 2009). The effects of luteolin were also examined on irinotecan-induced mice model of intestinal mucositis. It reduced ROS levels and inflammation by lowering TNF-α, IL-1β, and IL-6 whereas increased the levels of IL-4 and IL-10 (Boeing et al., 2020). Severe acute pancreatitis (SAP) is pancreatic inflammation and the outcome may be life-threatening. Xiong et al. (2017) studied the effects of luteolin in an ICR mouse model induced by cerulein/LPS where luteolin (100 mg/kg) reduced SAP symptoms by lowering TNF-α and IL-6 levels while raising IL-10 via NF-κB p65 and IκBα expressions (Xiong et al., 2017). In a study, skin from BALB/c mice donors was grafted in C57BL/6 mice recipients and allografts were treated with luteolin (25 and 50 mg/kg). The recipient mice survived longer showing decreased cellular infiltration and proinflammatory cytokine gene expression (Ye et al., 2019).
5.1.3 Baicalin and baicalein
Scutellaria baicalensis Georgi (Lamiaceae) is a traditional Chinese herb that contains the compounds baicalin and baicalein. IBD is a long-term, idiopathic inflammation which causes small and large intestine complications. Zhu et al. (2016) studied the baicalin (100 mg/kg) effects on macrophage polarization and IBD therapy. He found that LPS-stimulated mouse peritoneal macrophages had a lower ratio of M1 to M2 macrophages, indicating a shift from M1 to M2 polarization, especially Fizz1 expression in M2a subtypes. Baicalin has also been found effective in colitis, an auto-immune or infectious colon inflammation. A report suggested that baicalin upregulated both interferon regulatory factor 4 and 5 in lamina propria mononuclear cells isolated from dextran sulfate sodium (DSS)-induced colitis mice model (Zhu et al., 2016).
Multiple studies are also present that emphasize the anti-inflammatory properties of baicalein. Kim et al. (2018) showed that baicalein blocks NO, cytokines, chemokines and growth factors through the endoplasmic reticulum stress CHOP/STAT pathway in RAW 264.7 murine macrophages induced by dsRNA (Kim et al., 2018). Tubular-interstitial nephritis is characterized by kidney inflammation and cell damage. A report suggested that baicalein alleviated LPS induced cell viability and apoptosis of renal tubular epithelial cells, while decreased the activation of NF-κB and MAPKs (Chen et al., 2018a). Hepatic ischemia/reperfusion (I/R) is an inflammatory liver pathology. It was found that baicalein (300 mg/kg) preconditioning reduced NF-κB expression and pro-inflammatory cytokine production whereas TNF-α/IL-10 ratio and leukocyte infiltration were reduced (Mahmoud et al., 2019). Furthermore, in a report, baicalein (20 mg/kg) consistently suppressed T-cell proliferation in collagen-induced C57BL/6J male mice of arthritis (CIA) (Xu et al., 2018a). Mastitis is a breast inflammation which is usually caused by a bacterial infection. In BALB/c mice with LPS-induced mastitis, baicalein (20 mg/kg) reduced mammary gland damage, myeloperoxidase activity, TNF-α and IL-1β levels, while blocked the TLR4 expression. Baicalein suppressed TLR4-mediated NF-κB and MAPK signaling, reducing inflammation (He et al., 2015).
5.2 Flavonol
5.2.1 Quercetin
Quercetin is a common flavonol found in fruits and vegetables (Malus domestica Borkh., Rosaceae). Activated endothelial cells control leukocyte trafficking to inflammation sites in early atherosclerosis. One report found that quercetin reduced COX, 5-LOX 9 (arachidonate 5-lipoxygenase), MPO, NOS, CRP, and IL-6 mRNA expression in Sprague-Dawley (SD) rats on a hypercholesterolemic diet (Bhaskar et al., 2016). Interstitial inflammation is the primary pathogen following a kidney insult, as inflammatory macrophages become polarized. Quercetin (20 mg/kg) reduced tubulointerstitial damage and inflammatory factor production in ICR/JCL mice with obstructed kidneys while CD68+ macrophages infiltrated the renal interstitium less often. Reduced iNOS and IL-12 levels and increased F4/80+/CD11b+/CD86+ macrophages in kidneys of renal injury patients suggested quercetin prevented M1 macrophage polarization (Lu et al., 2018). Inflammation in IBD requires activated microvascular endothelial cells and cell adhesion. In LPS-stimulated rat intestinal microvascular endothelial cells, quercetin reduced intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1) protein levels. This phytochemical reduced TLR4, NF-κB p65, extracellular signal-regulated kinase (ERK), JNK, STAT phosphorylation and IκB-α degradation (Bian et al., 2018). AKT (protein kinase B) signaling is often activated in cancer, which keeps the tumor microenvironment oxidized for adaptability. A report found that quercetin reduced cell survival, inflammation, and angiogenesis in lymphoma-bearing mice (Maurya and Vinayak, 2017). Khan et al. (2018) explained that quercetin (30 mg/kg/day) reduced activated gliosis and inflammatory markers and stopped neuroinflammation in adult male of C57BL/6N brain and hippocampal regions (Khan et al., 2018).
5.2.2 Kaempferol
It is a flavonoid found in tea [Camellia sinensis (L.) Kuntze, Theaceae] and many fruits and vegetables (also known as kaempferol-3 or kaempferide). Intervertebral disc degeneration has been considered an irreversible process when cell viability decreases, type II collagen is synthesized and the nucleus pulposus is dehydrated. Research proved that in the presence of Kaempferol, proinflammatory cytokines decreases while IL-10 increases (Zhu et al., 2017). Wang et al. (2018) reported that kaempferol suppressed concanavalin A-induced T-cell proliferation and NO/ROS generation in LPS-infected RAW 264.7 macrophage cells (Wang et al., 2018). It is known that endothelial expression of cytokines and adhesion molecules triggers IBD. One report emphasized the role of kaempferol where it stopped rat intestinal microvascular endothelial cells from making too much TNF-α, IL-1β, IL-6, ICAM-1, and VCAM-1 via NF-κB and STAT signaling pathways (Bian et al., 2019). The NF-κB pathway, is critical in inflammation, proliferation, and carcinogenesis. Kaempferol reduced NF-κB activity in secreted embryonic alkaline phosphatase (SEAP)-driven NF-κB reporter cells with varying TNF-α concentrations (Kadioglu et al., 2015). Allergic asthma is a respiratory condition which causes airway inflammation. kaempferol (20 mg/kg) reduced allergic asthmatic mucus production in BALB/c mice by disrupting TGF-β-triggered ER stress signaling of inositol-requiring enzyme 1α/TNF receptor-associated factor 2/c-Jun N-terminal kinase (Park et al., 2015).
5.3 Flavanones
5.3.1 Naringenin
Grapefruits contain bitter, colorless flavonoid naringenin (Citrus paradisi Macfad., Rutaceae) which is known to reduce inflammatory and nerve pain. It was reported that oxidative stress, hyperalgesic cytokines (IL-33, TNF-α and IL-1β), and NF-κB activation were inhibited in mice paw skin treated with naringenin (16.7–150 mg/kg) (Pinho-Ribeiro et al., 2016). Naringenin also reduced colitis by inhibiting myeloid-derived suppressor cells, pro-inflammatory mediators, and the NF-κB/IL-6/STAT-3 cascade in colonic tissues (Zhang et al., 2018). Naringenin’s anti-inflammatory and anti-allergy properties were tested on mice models of ear edema caused by arachidonic acid and tetradecanoylphorbol-13-acetate (TPA). Naringenin showed anti-inflammatory effects against otitis media in female CD-1 mice at 1% in arachidonic acid and 50% in TPA (Escribano-Ferrer et al., 2019). Narringenin (50 mg/kg) reduces nociceptive effects and inflammation in male Swiss mice by activating the NO-cGMP-PKG-KATP channel signaling pathway involving nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) (Manchope et al., 2016).
5.3.2 Hesperidin
Flavonoid hesperidin is found in citrus fruits, especially oranges and lemons (Citrus limon (L.) Osbeck, Rutaceae). Diabetic neuropathy (DN) is one of the most common long-term complications of diabetes mellitus. In the diabetic neuropathy model of SD rats, hesperidin (50 and 100 mg/kg) reduced IL-1β and TNF-α (Visnagri et al., 2014). Moreover, hesperidin effectively enhanced chondrogenesis of human mesenchymal stem cells (MSCs) by inhibiting pro-inflammatory cytokines IFN-γ, IL-2, IL-4 and IL-10, and suppressing the expression of p65 to facilitate cartilage tissue repair (Xiao et al., 2018). Oxidative stress can cause chondrocytes to secrete inflammatory mediators, causing a senescence-associated secretory phenotype. Hesperidin showed chondroprotective properties, increased cellular antioxidant capacity, decreased COX-2, IL-1β, TNF-α, MMP-3, MMP-9 mRNA levels, and increased IL-10, tissue inhibitors of metalloproteinases-1, SRY-box transcription factor 9, and altered forkhead box O 1 (Foxo1), Foxo3, and Nrf2 signaling pathways in H2O2 stimulated primary human chondrocytes (Tsai et al., 2019). OA is one of the degenerative and chronic diseases of articular joints with chondrocytes degeneration. Hesperidin reduces IL-1β-induced MMP-3 and MMP-13 expression in OA chondrocytes and NF-κB (Fu et al., 2018). Hesperidin (100 mg/kg) inhibited inflammation in an Alzheimer’s disease (AD) APP/PS1 mouse model, restored APP synthesis and Aβ peptide deposition, and improved nesting and social interactions (Li et al., 2015a).
5.4 Isoflavones
5.4.1 Genistein
Genistein is an isoflavone polyphenol extracted from Genista tinctorial L., the dyer’s broom (Fabaceae). Genistein suppresses NF-κB activation, reduces TNF-α and IL-6 production, and reactivates insulin-mediated Akt and endothelial NO synthase phosphorylation to improve insulin resistance-related endothelial dysfunction. Endothelin-1, a cytokine that plays a role in insulin’s mitogenic effects, was also downregulated by the treatment and VCAM-1 overexpression (Howes and Simmonds, 2014). Genistein also inhibited NO, Prostaglandin E2 (PGE2), IL-1, TNF-α, TLR4 and MyD88 in LPS-induced BV2 microglia (Jeong et al., 2014). It has been evidenced that chronic inflammation develops hepatocellular carcinoma (HCC) and other malignancies. When C57BL/6N mice were given 80 mg/kg of Genistein, it slows down HCC development while AMP-activated protein kinase activation killed hepatocytes through caspase pathways and reduced liver macrophage inflammation (Lee et al., 2019). Breast cancer is the most common malignancy in women of developed countries. The effects of the phytoestrogen genistein on the inflammatory profile in breast cancer cell lines were studied. Genistein-dependent expression of inflammatory-related genes was seen through its interaction with alpha and beta estrogen receptors (ER), and its effects depend on the ERα/ERβ ratio (Pons et al., 2019). In experimentally induced condylar cartilage degradation in male rats, genistein (180 mg/kg) treatment had significantly reduced the expression of p65 and inflammatory cytokines (IL-1β and TNFα) showing therapeutic effects on condyle cartilage damages of OA rats (Yuan et al., 2019).
5.4.2 Puerarin
Puerarin is a key component of Pueraria lobata (Willd.) Ohwi (Pueraria lacei Craib) (Fabaceae). Xue et al. (2016) reported that puerarin inhibited MDA, NO, NF-κB, TNF-α, IL-1β, and IL-6 production in an animal I/R model (Xue et al., 2016). In streptozotocin induced diabetic male SD rats, Puerarin reduced spinal cord inflammation and neuropathic pain by inhibiting NF-κB activation and cytokine upregulation (Liu et al., 2014a). A rat model (rAION) of anterior ischemic optic neuropathy was used to test puerarin’s antiapoptotic and anti-inflammatory effects. Anti-apoptotic factors were increased by reducing iNOS, IL-1β, TNF-α, and IL-10 and inducing IL-10, arginase-1, and Fizz1 (found in inflammatory zone protein) (Le et al., 2019). In vitro and in vivo OA models were used to study the therapeutic effects of puerarin. It increases OA chondrocyte proliferation and suppresses IL-1β induced inflammatory cytokines and monocytes/macrophages. In a mono-iodoacetate-induced OA mouse model, puerarin (50 mg/kg) reduced inflammatory monocyte recruitment and cartilage destruction (Peng et al., 2019). Ulcerative colitis is an IBD accompanied by abdominal pain, diarrhea, and rectal bleeding. Puerarin was given to male BALB/c mice with DSS-induced colitis at 10 and 50 mg/kg, where it showed antioxidant mechanism by controlling the Nrf2 pathway and antioxidant enzymes. It also inhibited NF-κB and pro-inflammatory mediators of inflammation (Jeon et al., 2020).
5.5 Catechins
5.5.1 Epigallocatechin gallate (EGCG)
Green tea leaves (Camellia sinensis (L.) Kuntze, Theaceae) have the most EGCG catechins. Chronic constriction injury (CCI)-induced neuropathic pain in male adult SD rats are improved by intrathecal injection of EGCG (1 mg/kg), which reduces TLR4, NF-κB, High mobility group box 1, TNF-α, and IL-1β and increases spinal cord IL-10 (Kuang et al., 2012). Infrasound, a common source of vibroacoustic illness, can harm the central nervous system (CNS). EGCG inhibited infrasound-induced microglial activation in rat hippocampi, as evidenced by reduced expression of IL-1, IL-6, IL-18, and TNF-α cytokines and decreased neuronal apoptosis. EGCG reduced microglia IκBα and infrasound-induced nuclear NF-κB, p65, and phosphorylated IκBα (Cai et al., 2014). Sun et al. (2017) reported that EGCG improved renal pathology and reduced inflammatory markers in diabetic mice, including ICAM1 and VCAM-1 (Sun et al., 2017). EGCG (50 mg/kg) reduced macrophage and T-cell infiltration in Dahl salt-sensitive rats (Luo et al., 2020). In Balb/c mouse models with bronchial asthma, EGCG (20 mg/kg) reduces airway inflammation via the TGF-1β pathway and eventually reduced Th17 cells and increased Treg cells (Shan et al., 2018).
5.6 Anthocyanidins
5.6.1 Cyanidin-3-O-glycoside (C3G)
C3G is a pigment in red and blue fruits and vegetables. Lonicera caerulea L contains anti-inflammatory anthocyanins (Caprifoliaceae). C3G inhibits the NF-κB pathway in epithelial cells, protecting against chronic gut inflammatory diseases (Ferrari et al., 2017). C3G may reduce LPS-induced inflammation through TAK1 (transforming growth factor-β-activated kinase 1) mediated MAPK and NF-κB pathways, according to a mouse paw edema and macrophage cell model (Wu et al., 2017). Researchers used an LPS-activated macrophage cell line (RAW264.7) to test C3G and 5-aminosalicylic acid’s anti-inflammatory properties. iNOS and COX-2 expression inhibition were more effective than 5-aminosalicylic acid at countering LPS-induced NO and prostaglandin release (Pereira et al., 2018). 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice and LPS-stimulated C3G and cyanidin were used to examine Caco-2 cell monolayer inflammation. Chronic exposure to TNBS reduced the animal’s clinical symptoms and histological brain damage. Activation of myeloperoxidase and release of inflammatory cytokines TNF-α, IL-1β, IL-6, and IFN-γ were dramatically reduced. Caco-2 cells treated with LPS produced less nitric oxide and inflammatory cytokines when C3G or Cy was added (Gan et al., 2019). Microglia are resident macrophages involved in many neurodegenerative diseasescause brain inflammation. Pre-treatment with C3G reduced microglial activation and the production of neurotoxic mediators like NO, PGE2, and pro-inflammatory cytokines (IL-1β and IL-6). C3G suppressed NF-κB and p38 MAPK signaling pathways, reducing iNOS, COX-2, and proinflammatory cytokines (Kaewmool et al., 2019).
5.7 Monoterpenes (terpenoids)
5.7.1 Cineole
Cineole is also called eucalyptol or 1,8-cineole and the main volatile oil in Eucalyptus spp. (Myrtaceae). In vitro studies of normal and non-smoking monocytes showed IL-6 was inhibited more than IL-1β, IL-8, and TNF-α at 0.15–1.5 µM of 1,8-cineole (Juergens et al., 2017). 1,8-cineole protects vascular endothelium in LPS-induced mice, and human umbilical vein endothelial cells (HUVECs), inhibits IL-6 and IL-8 and boosts serum IL-10. Male Kunming mice given LPS had less inflammation and VCAM-1 expression in the thoracic aorta. In vitro and in vivo results showed that 1,8-cineole reduced LPS damage to endothelial cells through PPAR-dependent NF-κB modulation (Linghu et al., 2019). Eucalyptus oil, long used in traditional medicine, is helpful in aromatherapy for respiratory problems. Yadav et al. (2017) studied 1,8-cineole and eucalyptol regulate anti-inflammatory pathways by downregulating pattern recognition receptors (PRR) receptors (TREM-1 and NLRP3) and downstream signaling cascade partners (NF-κB, MAPKs, MKP-1) (Yadav and Chandra, 2017).
5.7.2 Paeoniflorin (PF)
The main ingredient in Paeonia lactiflora Pall is paeoniflorin (PF) (Paeoniaceae). When LPS was added to Caco-2 cells, PF blocked COX-2, iNOS, TNF-α, IL-6, and MMP-9 and inhibited NF-κB signaling by activating Nrf2/HO-1 (Wu et al., 2019). It was shown that PF-treated psoriasis animal models had thinner epidermis, less parakeratosis, and less lymphocyte infiltration. PF suppressed IL-6, IL-17A, and IL-22 mRNA. It also stopped HaCat cells from making IL-22, possibly by blocking the MAPK pathway (Yu et al., 2017). PF inhibited astrocytes and microglia from activating chronic constriction-injured rats. It reduced inflammation-promoting cytokines in the spinal cord, such as TNF-α, IL-1β, IL-6, and chemokine (C-X-C motif) ligand (Zhou et al., 2019b). PF also inhibited IL-8 mRNA expression and secretion by lowering ERK1/2 and Akt phosphorylation in human hepatic sinusoidal endothelial cells (Gong et al., 2015). When LPS was added to human oral keratinocytes, PF inhibited the production of pro-inflammatory cytokines such as TNF-α and IL-6. It also suppressed the phosphorylation of NF-κB p65 and IκBα proteins, which hampered NF-κB and p65 from moving into the nucleus (Yu et al., 2019).
5.8 Sesquiterpenes
5.8.1 Parthenolide (PAR)
Feverfew [Tanacetum parthenium (L.) Sch. Bip.], an Asteraceae medicinal herb, contains PAR. PAR inhibited the inflammatory response in 3T3-CM-cultured macrophages co-cultured with adipose tissue by downregulating IL-6 and MCP-1. PAR reduced adiponectin and resistin dysregulations in macrophage-conditioned medium-cultured adipocytes. In the same study, PL-administered to high-fat diet (HFD)-fed mice, showed an anti-obese effect, connected to anti-inflammatory responses with the regulation of inflammatory cytokines, and the downregulation of NF-κB and MAPKs and inhibited obesity and obesity-induced inflammatory responses via activation of Nrf2/Keap1 signalling pathway (Kim et al., 2019). To understand anti-inflammatory and anti-cancer effects of PAR, researchers used LPS-induced human leukemia monocytic THP-1 cells and human primary monocytes. IL-12p40, IL-6, IL-1β, IL-8, TNF-α, IL-18, and NO were all reduced by PAR in THP-1 cells, with IC50 values ranging from 1.091–2.620 µM TLR4-mediated MAPK and NF-κB signaling contributed to PAR’s anti-inflammatory effects (Li et al., 2015c). Studies focuses that chronic inflammation causes joint destruction and excruciating pain in rheumatoid arthritis. PAR (4 mg/kg) reduced paw inflammation, bone degradation, and pain-like behavior in moderate collagen antibody-induced arthritis (CAIA) BALB/c mice (Williams et al., 2020).
5.8.2 Zerumbone (ZER)
This phytochemical is mainly found in Zingiber zerumbet (L.) Roscoe ex Sm. Oral treatment (100, 250, and 500 ppm) in mice repressed NF-κB and HO-1, causing apoptosis and inhibiting colon cancer growth (Kim et al., 2009). A ZER-rich diet (250 and 500 ppm) reduced lung cancer multiplication by reducing growth, inflammation, and NF-κB and HO-1 expression, killing cancer cells in animals (Kim et al., 2009). ZER reduced iNOS and COX-2 in LPS-stimulated RAW 264.7 cells by inducing the HO-1 pathway, which impacted OA dose-dependently. Chien et al. (2016) showed that ZER (1–5 mg/kg) reduced paw edema and pain in a male SD rat OA model (Chien et al., 2016). It also reduces neuroinflammation, β-amyloid deposition, and behavioral deficits in APP/PS1 mice. MAPK signaling pathway inhibition promoted a phenotypic switch from pro-inflammatory to anti-inflammatory in microglia (Li et al., 2020). Using human U937 macrophages generated by LPS, another study found that ZER decreased the up-regulation of pro-inflammatory mediators such as TNF-α, IL-1β, PGE2, the COX-2 protein, and NF-κB (p65), IκBα, and IKKα/β. ZER suppression of inflammatory markers in macrophages required MyD88, demonstrating its potential as a powerful treatment for inflammatory-mediated immunological diseases (Haque et al., 2018).
5.9 Diterpenoids
5.9.1 Ginkgolides (GB)
Maidenhair tree extract is a common and old herbal remedy (Ginkgo biloba L., Ginkgoaceae) where ginkgo flavonol glycosides (GFGs) and ginkgolides are active ingredients (GGs). GGs include ginkgolide A (GA), ginkgolide B (GB), ginkgolide C (GC), ginkgolide J (GJ), ginkgolide M (GM), ginkgolide K (GK), ginkgolide L (GL), ginkgolide P (GP), ginkgolide Q (GQ), and bilobalide. Hypoxic-ischemic injury to the brain is a significant cause of mortality and severe neurologic disability. One report showed that GB reduced NLRP3 expression in microglia in a rat pup model of hypoxic-ischemic brain injury and stopped Caspase-1 and NF-κB P65 from entering the nucleus. NLRP3 inflammasome activation was less likely (Chen et al., 2018b). Clinical therapy can alleviate myocardial ischemia/reperfusion (MI/R) illnesses by reducing inflammation. Male SD rats with left anterior descending coronary (LAD) artery blockage mimicked MI/R damage. GC may provide an alternative therapy for MI/R disorders by suppressing the CD40-NF-κB signal pathway and downstream inflammatory cytokine production (Zhang et al., 2018a). GB inhibited inflammation and protected LPS-induced chondrocytes by upregulating synthesis-related genes and downregulating matrix-degrading genes to increase chondrocyte collagen II and aggrecan expression and reduced LPS-induced MAPK activation (Hejia et al., 2018).
5.10 Triterpenoids
5.10.1 Ursolic acid (UA)
Basil, rosemary, sage, apples and pears may contain this phytochemical in Glechoma hederacea L. (Lamiaceae). It was reported that UA decreased TNF-α production in RAW 267.4 macrophages, A549 alveolar epithelial infected with Mycobacterium tuberculosis H37Rv, and mouse splenocytes stimulated with Con A. UA activity reduces the levels of COX-2 and NO synthase in stimulated cells. Finally, UA may be future tuberculosis and antibiotic therapy due to its anti-inflammatory properties (Zerin et al., 2016). Inflammation in the brain may play a role in Parkinson’s. The UA therapy reversed neuroinflammation and neurodegeneration and improved biochemical and behavioral indicators. In Parkinson’s mice models, researchers used UA (25 mg/kg) to reduce MPTP-induced neuroinflammation and inflammatory markers (Iba1 and TNF-α) and transcription factor NF-κB (Rai et al., 2019). DSS caused ulcerative colitis in male BALB/c mice, causing colon damage. DSS increased IL-1β and TNF-α, MDA, and SOD in colon homogenate. UA restored DSS’s effects and reduced NF-κB levels in colon tissue (Liu et al., 2016a).
5.10.2 Escin
Horse chestnut extract (Aesculus hippocastanum L., Sapindaceae). The glucocorticoid receptor in escin gel may be anti-inflammatory. Both paw edema and capillary permeability rat models treated with escin gel had elevated glucocorticoid receptor levels and reduced NF-κB mRNA (Zhao et al., 2018). Intragastric escin (0.45, 0.9, or 1.8 mg/kg) reduced Indomethacin-induced gastric ulceration in Swiss albino mice, reducing MDA, TNF-α, and VCAM-1. In the same assay, intragastric escin inhibited myeloperoxidase, superoxide dismutase, catalase, and glutathione peroxidase (Wang et al., 2014). In cecal ligation and puncture (CLP) induced intestinal mucosal injury in a mouse model, a low dose of escin ameliorated endotoxin-induced liver injury and intestinal mucosal injury and increased the expression of tight junction protein claudin-5. They add to evidence that escin is a potent anti-inflammatory agent that reduces intestinal mucosa damage in animal models (Li et al., 2015b).
5.11 Steroidal compounds
5.11.1 Withaferin A (WA)
WA is a steroidal lactone in Ashwagandha [Withania somnifera (L.) Dunal, Solanaceae] with many biological effects. Obesity gives rise to insulin resistance and endothelial dysfunction by the activation of inflammatory pathways. Endothelial cells treated with WA reduced TNF-α and IL-6 production in palmitic acid (PA)-induced insulin-resistant human umbilical vein endothelial cells. When used to treat PA, WA decreased endothelin-1 and plasminogen activator inhibitor type-1 levels and restored endothelium-mediated vasodilation. In the presence of acetylcholine-stress relief (Batumalaie et al., 2016). CNS affects the immune response to infections, traumas, or diseases. WA may treat neuroinflammatory and stress-related diseases. WA reduces astrocyte NF-κB activity and TNF-α, COX-2, and iNOS production in response to LPS/TLR4 pathway activation (Martorana et al., 2015). BALB/c mice given WA (10 mg/kg) improved neurobehavioral function and reduced spinal cord histological changes. WA increased TGF-1β and IL-10 while decreasing IL-1β and TNF-.α (Yan et al., 2017). WA reduced ovalbumin-induced lung damage and fibrosis in mice. WA reduced inflammation-inducing cell infiltration into bronchoalveolar lavage fluid, pro-inflammatory cytokine production, and inflammasome activation via the NLRP3 pathway in human lungs (Zhao et al., 2019). Pulmonary fibrosis is an interstitial lung disease evidenced by chronic inflammation. WA (2 and 4 mg/kg) decreased connective tissue growth factor, collagen 1A2, collagen 3A1, and fibronectin in a bleomycin-induced lung fibrosis mouse model where it reduced NF-κB p65, IL-1β, and TNF-α expression (Bale et al., 2018). People often take too much acetaminophen, which causes liver damage. Our team looked at the hepatoprotective effects of a withanolide-rich fraction (WRF) from Withania somnifera (L.) Dunal contains WA (12.9 mg/gm). Male Wistar rats given acetaminophen were given 50, 100, or 200 mg/kg of WRF, which stopped the TNF-α, IL-1β, COX-2, and iNOS proteins from causing inflammation and oxidative stress (Devkar et al., 2016).
5.11.2 β -sitosterol (BSS)
It’s found in wheat germ, rice bran, flax seeds, peanuts, and soybeans (Glycine max (L.) Merr., Fabaceae). In murine J774A.1 macrophage, BSS reduced pro-inflammatory cytokines and chemokines and increased anti-inflammatory IL-10. NF-κB translocation to the nucleus was inhibited by protein tyrosine phosphatase (SHP-1) (Valerio and Awad, 2011). BSS nanoparticles (7.5–30 µM) prevented keratinocytes and macrophages from releasing TNF-α, IL-1β, IL-6, IL-8, and ROS when triggered by peptidoglycan, TNF-α, or LPS. Also, BSS decreased NLRP3, a key part of NLRP3 inflammasomes, and stopped caspase-1 (Liao et al., 2018). In CIA mice, intraperitoneal BSS (20 or 50 mg/kg) or adoptive transfer of BSS-BMDMs reduced ankle swelling, collagen-specific antibodies (IgG and IgG1), and pro-inflammatory cytokines (Liu et al., 2019a).
5.12 Curcuminoids
5.12.1 Curcumin
Turmeric’s roots contain curcumin (Curcuma longa L., Zingiberaceae) which adds flavor to food and has medical uses. Curcumin protects neurons and slows microglia and macrophage activation and death. In male C57BL/6 mice with traumatic brain injury, TLR4/MyD88/NF-κB signaling was involved (Zhu et al., 2014). Curcumin’s effects on myocarditis were studied in rodents where it inhibited phosphoinositide 3-kinase (PI3K)/Akt/NF-κB signaling in coxsackievirus B3-induced myocarditis mice. It also inhibited inflammatory cytokines like TNF-α, IL-6, and IL-1β, reducing inflammation (Song et al., 2013). Neuroinflammation contributes to AD. Curcumin’s anti-inflammatory effects may aid AD patients. Liu et al. (2016b) found curcumin improved mice’s spatial memory and cholinergic neurons. This improvement was related to NF-κB signaling pathways and PPARγ mediated transcription (Liu et al., 2016b). Curcumin and curcumol were also tested on macrophage cells exposed to cigarette smoke extract. It was found that curcumol and curcumin inhibited the NF-κB signaling pathway and downregulated proinflammatory factors (Li et al., 2019). BALB/c mice given ovalbumin developed asthma. Curcumin (20 mg/kg and 100 mg/kg) reduced inflammatory cell infiltration, goblet cell hyperplasia, alveolar thickening, edema, and vascular congestion in BALB/c with ovalbumin-induced allergic asthma; and decreased mRNA expression levels of cytokines IL-4, IL-5, TNF-α, TGF-β (Shahid et al., 2019). Lipoteichoic acid (LTA) stimulates neuroinflammatory molecules, contributing to neurodegeneration. In LTA-stimulated BV-2 microglial cells, curcumin’s anti-inflammatory effects decreased TNF-α, PGE2, NO, iNOS, and COX-2. Another study found that curcumin reduced LTA-induced phosphorylation of MAPK, ERK, p38, Akt and NF-κB translocation. Curcumin stimulated HO-1 and Nrf-2 expression in microglial cells (Yu et al., 2018).
5.13 Stilbenes
5.13.1 Resveratrol (RSV)
Red grapes (Vitis vinifera L., Vitaceae) and wine have one of the anti-inflammatory polyphenols known as resveratrol (RSV). A review concluded the multifaceted approach of RSV such as activation of protein-1 (AP-1), NF-κB, Cox-2 and regulation of proinflammatory cytokines like IL-6, IL-8, IL-10 and TNF-α as well as ICAM-1 and MCP expression (Latruffe et al., 2015). RSV inhibited ICAM-1, iNOS, and IL-1β mRNA expression in TNF-α-treated human coronary endothelial cells, demonstrating anti-inflammatory properties (Huang et al., 2017). RSV also improved lung histological damage and decreased pro-inflammatory cytokines (IL-6, IL-17, TNF-α, and TGF-β) in cigarette smoke chronic obstructive pulmonary disease (COPD) animals (Chen et al., 2016). RSV improves circulation in streptozotocin-treated rats, a pancreatic cell toxin. The improvement was associated with lower blood levels of TNF-α, IL-1β, and IL-6 and suppression of vascular endothelial growth factor (VEGF) via the p38-MAPK and NF-κB pathways (Yan et al., 2018). Yanez et al. (2019) examined the effects of RSV and nicotinamide on the downregulation of high levels of TNF-α, IL-6, and VEGF in LPS-induced macrophages. Nicotinamide increased RSV-induced PARP1 activation and its related anti-inflammatory effects, which were mediated through B-cell lymphoma 6 upregulation and COX-2 downregulation (Yanez et al., 2019).
5.14 Phenolic acids
5.14.1 Rosmarinic acid (RosA)
RosA is an ester of caffeic acid and 3, 4-dihydroxyphenyl lactic acid found in rosemary herb (Rosmarinus officinalis L., Lamiaceae). Rahbardar et al. (2017) found that RosA (40 mg/kg) decreased spinal inflammatory markers, including matrix MMP-2, PGE-2, IL-1β, and COX-2, in rats with sciatic nerve CCI-induced neuropathic pain (Rahbardar et al., 2017). Cao et al. (2016) reported that RosA (75, 150, and 300 mg/kg) reduced TNF-α, IL-6, IL-1β, TGF-β, and VEGF in HCC while NF-κB and p65 was also decreased in the xenograft microenvironment (Cao et al., 2016). RosA from pomegranate peel reduced TNF-α in Freund’s complete adjuvant-induced arthritis by increasing GSH and SOD while reducing MDA levels (Gautam et al., 2019). Jin et al. (2017) found that RosA reduced DSS-induced colon shortening and splenomegaly in mice. RosA prevented COX-2 and iNOS expression and IL-1β, IL-6, and IL-22 production in inflamed mucosa by inhibiting NF-κB, p65, and pSTAT3 expression and nuclear transport (Jin et al., 2017). One of the research also described RosA anti-inflammatory effect on LPS-induced mouse mastitis and mouse mammary epithelial cells. It reduced myeloperoxidase activity, TNF-α, IL-1β, and IL-6 levels (Jiang et al., 2018).
5.14.2 Ellagic acid (EA)
Ellagic acid (EA) is present in fruits, such as pomegranates (Punica granatum, Lythraceae), seeds, and vegetables. Innate immunity plays an important role in managing oral cavity homeostasis, infections, and cancers. Promsong et al. (2015) measured the effects of EA (12.5–100 μM) on innate immune mediators in primary human gingival epithelial cells (HGEs). EA increased the expression of RANTES (regulated on activation of normal T-cell expressed and secreted), IL-1β, and IL-2, while decreased TNF-α, C-C Motif Chemokine Ligand 20 (CCL20), IL-6, IL-8, and C-X-C Motif Chemokine Ligand 5 (CXCL5) (Promsong et al., 2015). In a different study, EA (50, 100, and 150 mg/kg) decreased the levels of blood glucose, TNF-α in serum, and the expression levels of TLR-4, IL-1 receptor-associated kinase 4 (IRAK4), TNF-receptor associated factor 6 (TRAF-6), IKK-β-, and NF-κB p65 in the kidney tissue of mice with streptozotocin-induced diabetic nephropathy (Zhou et al., 2019a). Guan et al. (2017) studied EA’s effects on LPS-induced lung damage in mice. He found that EA (5 mg/kg) reduced LPS-induced protein dispersion in bronchoalveolar lavage fluid and inflammatory cell infiltration into lung tissue while reduced TNF-α, IL-6, and IL-1β and increased IL-10 (Guan et al., 2017). One more research evidenced that treatment with EA (50 mg/kg) reduced paw swelling, inflammation, NF-κB, IL-1β, MMP-9, VEGF, caspase-3 expression, blood oxidative stress, and NO levels in a rat model of adjuvant-induced arthritis (Fikry et al., 2019). In addition, pomegranate peel extract high in EA inhibited the generation of IL-17 by activated T cells isolated from mice with experimental autoimmune encephalomyelitis (Stojanović et al., 2017). Furthermore, wistar rat hippocampi were exposed to arsenic, which caused neuroinflammation and mitochondrial dysfunction. EA reduced arsenic-induced neurotoxicity in rats by reducing ROS, Bax, Bcl2, and inflammatory biomarkers (IL-1β, TNF-α, IFN-γ) (Firdaus et al., 2018).
5.14.3 Gallic acid (GA)
Gallic acid (GA) is abundant in tea leaves (Camellia sinensis (L.) Kuntze, Theaceae), along with gall nuts, apple peels, sumac, green tea, and grapes. Recent research examined the effects of GA on IL-1-induced human intestinal epithelial cell line and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced ulcerative colitis (UC) in mice. GA raised the expressions of IL-4 and IL-10, whereas blocking the NF-κB pathway decreased the expressions of IL-1, IL-6, IL-12, IL-17, IL-23, TGF-β, and TNF-α. These modifications alleviated inflammation, reversed the loss in body weight and the rise in colon weight, and mitigated the histological alterations caused by UC (Zhu et al., 2019). Generally, hypertrophic scars are the result of prolonged intense inflammation. Fan et al. (2018) studied GA’s effect on LPS-induced inflammation in hypertrophic scar fibroblasts and reported reduced TNF-α, IL-6, IL-1β, and IL-8 levels. This indicated an inflammatory response via TLR-4/NF-κB/PPARγ pathway (Fan et al., 2018). Endometriosis is a gynecologic disease in women that can cause infertility and chronic pelvic pain with a relatively high recurrence rate. GA (102.4 μg/ml) and its derivatives showed ameliorating effects on endometriosis primary cultures by regulating NF-κB mRNA expression and IL-6 secretions (Bustami et al., 2018).
5.14.4 Protocatechuic acid (PCA)
Protocatechuic acid (PCA) is a phenolic chemical extracted from onion (Allium cepa L., Amaryllidaceae) and found in many plants and fruits. Recent research shows PCA’s anti-inflammatory mechanism via sirtuin1(SIRT1)/NF-κB in LPS-activated BV2 microglia (Kaewmool et al., 2020). Inflamed visceral adipose tissue (VAT) causes insulin resistance and T2DM in obese patients. By increasing insulin receptor substrate-1 and Akt phosphorylation, PCA can modulate insulin sensitivity and inflammation in obese-VAT and normal-weight T2DM patients. This may be due to reduced protein tyrosine phosphatase 1B activity in obese-VAT treated with PCA. Thus, PCA is a powerful phytochemical against obesity-related inflammation and IR (Ormazabal et al., 2018). The polarization of macrophages affects atherosclerosis. PCA blocked PI3K-Akt-mediated NF-κB activation and M1 polarization. In J774 cells and mouse bone marrow macrophages, it phosphorylated STAT-6 and activated PPAR-γ, increasing M2 activation. These findings showed PCA relieved atherosclerosis by modulating M1-M2 conversion (Liu et al., 2019b). Benign prostatic hyperplasia (BPH) causes an enlarged prostate. Akanni et al. (2020) reported that BPH castrated rats treated with PA showed reduction in inflammation and oxidative stress and caused histological changes (Akanni et al., 2020).
5.14.5 Vanillic acid (VA)
Vanillic acid (VA) is the major component of the extracts of the vanilla (Vanilla planifolia Jacks. ex Andrews, Orchidaceae) bean and pod, commonly utilized in food flavoring agents, cosmetics and drugs. In a mouse model of inflammation produced by carrageenan, VA reduced hyperalgesia, leukocyte recruitment, oxidative stress, IL-33, TNF-α, and IL-1β production, as well as NF-κB activation. This study proves analgesic and anti-inflammatory actions of VA, associated with Nrf2 activation (Calixto-Campos et al., 2015). In another study, VA reduced Aβ1-42-induced oxidative stress, neuroinflammation, and cognitive impairment in mice by activating Nrf2 and increasing HO-1 expression (Amin et al., 2017). The anti-inflammatory potential of VA was evaluated in LPS-induced macrophages and in in vivo animal models. VA reduced LPS-induced gene expression and pro-inflammatory mediators, including iNOS/COX-2 and cytokines. The mechanism involved was suppression of NF-κB activation in macrophages and improve acetic acid-induced vascular permeability and zymosan-induced leukocyte migration in mice (Lee et al., 2018).
5.14.6 6-Gingerol (6-G)
This phytochemical is found in ginger (Zingiber officinale, Rosc., Zingiberaceae), spice and herbal medicine. 6-G (6 mg/kg) pre-treatment alleviated MI/R in SD rats by improving the cardiac functions. The later involved reduced myocardial infarction area and cardiac pathological injury, lowered myocardial enzyme level and inhibited inflammatory response by upregulating PI3K and p-Akt expression (Xu et al., 2018b). Additionally, 6-G rich fraction inhibited the inflammatory markers such as myeloperoxidase, NO, and TNF-α in brains, ovaries, and uterus of chlorpyrifos-treated rats (Abolaji et al., 2017). A report assessed 6-G inhibition on IL-1 induced osteoclast differentiation in co-cultures of osteoblasts and osteoclast precursor cells and found that 6-G suppressed NF-κB ligand and reduced PGE2, indicating its potential use in the treatment of inflammatory bone destruction associated with excessive PGE2 production (Hwang et al., 2018). The AD model of whiskers rats produced by streptozotocin was investigated to examine whether 6-G therapy might reduce inflammation and ameliorate cognitive impairment. The researchers observed that pre-treatment with 10 and 20 mg/kg 6-G decreased levels of neuroinflammatory and α, β-secretases, APH1a (Aph-1 Homolog A, Gamma-Secretase Subunit), and COX-2, resulting in an improvement in cognitive behaviors (Halawany et al., 2017). 6-G (25 mg/kg) antioxidant and anti-inflammatory properties protected rat kidneys from septic acute damage by reducing ROS, RNS, MDA and increasing GSH activity (Rodrigues et al., 2018). Additionally, orally administered 6-G rich extract reduced the levels of the proinflammatory marker TNF-α and expression of NF-κB and vascular endothelial growth factor in the retinal tissue of the streptozotocin-induced diabetic Wistar albino rats (Dongare et al., 2016).
5.14.7 Caffeic acid phenethyl ester (CAPE)
It’s a polyphenolic chemical mostly found in black poplar (Populus nigra L., Salicaceae) and beehive propolis. Glaucoma is characterized by the death of retinal ganglion cells (RGCs) and is a leading cause of blindness worldwide. Jia et al. (2019) reported that CAPE inhibits NF-κB activation, reduces the production of inflammatory cytokines like IL-8, IL-6, iNOS, COX-2, TNF-α, and C-C ligand-2 in a glaucoma rat model of optic nerve crush (Jia et al., 2019). One more study found that CAPE inhibits NF-κB activation via thiol group modification and p65 phosphorylation in RAW 264.7 cells (Takakura et al., 2018). In the host’s defense against dental caries, odontoblasts produce growth factors and develop reparative dentin. CAPE increased VEGF mRNA expression and production in rat odontoblast-like KN-3 cells and enhanced NF-κB transcription factor. Thus, CAPE is predicted as a unique biological material for dental pulp treatment (Kuramoto et al., 2019). Salles et al. (2019) showed that treatment with CAPE (10 µM) improved wound inflammatory and oxidative profile with decreased TNF-α, phosphorylated NF-kB p65 protein, NOS2 and COX-2 expression in male Swiss diabetic rats (Salles et al., 2019). Periodontal disease is linked to chronic oxidative stress and inflammation. It was reported that in primary murine macrophages, CAPE showed antioxidative effects via the Nrf2-mediated HO-1 pathway and anti-inflammatory effects via NF-κB suppression (Stähli et al., 2019).
6 Phytochemicals evaluated in clinical trials
The effectiveness of phytoconstituents for different health complications is known since ancient days. Recent advances in research has provided a larger platform to find out the efficacy and mechanism of these plant-derived components. Animal models are, of course, invaluable to study the pharmacological capacity of a drug. However, these models do not satisfactorily represent the human conditions and have limitations. In this context, we have summarized some major phytochemicals that are being studied for their role in inflammation in different complications and are undergoing clinical trials as well (Figure 4; Table 4).
FIGURE 4.
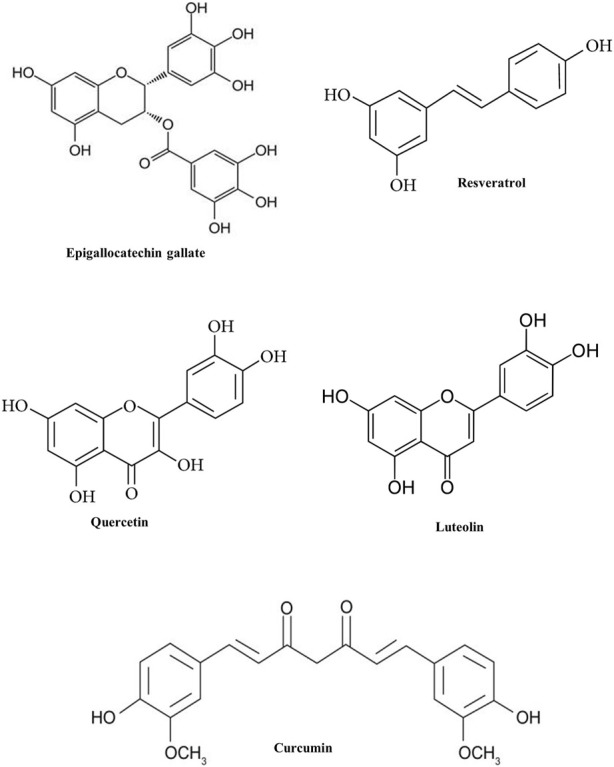
Chemical structure of some phytochemicals used in clinical trials.
TABLE 4.
List of anti-inflammatory phytochemicals used in clinical trials.
| Phytochemicals | Class of compound | Disease/disorder | Assessment | References |
|---|---|---|---|---|
| Resveratrol | Vitis vinifera (Vitaceae) | Gulf War Illness | Improvements in cognitive functioning, functional status, mood, hippocampal neurogenesis, and functional connectivity as well as anti-inflammatory and antioxidant effects | NCT03665740 a |
| Curcumin | Curcuma longa, (Zingiberaceae) | Bladder Spasm | Reducing inflammation for ureteral stent-induced symptoms | NCT02598726 a |
| Malignant Neoplasm | ||||
| Pain | ||||
| Urinary Urgency | ||||
| Epigallocatechin gallate | Camellia sinensis (Theaceae) | Obesity | To assess endotoxin and inflammatory biomarkers | NCT03413735 a |
| Endotoxemia | ||||
| Inflammation | ||||
| Quercetin | Malus domestica, (Rosaceae) | COVID-19 | Prophylaxis and treatment of COVID-19 | NCT04377789 a |
| Luteolin | Rosmarinus officinalis (Lamiaceae) | Frontotemporal Dementia | To assess the brain correlates related to the clinical improvement associated with PEA-LUT treatment | NCT04489017 a |
Indicates reference found at www.clinicaltrials.gov with corresponding identifier code (NCT).
6.1 Resveratrol
Resveratrol clinical research focuses on T2DM/metabolic syndrome, polycystic ovary syndrome, and non-alcoholic fatty liver disease. This phytochemical activates SIRT1 and may help with metabolic, inflammatory, and cell cycle disorders. A low grade of systemic inflammation and oxidative damage can be seen in smokers. Bo et al. (2013) reported that resveratrol (500 mg/day) reduced CRP and TGs and improved antioxidant status in 50 healthy adult smokers during a 90-day cross-over, randomized and double-blind study. These effects were depending on anti-inflammatory and anti-oxidant properties of resveratrol that ultimately subsided cardiovascular risk in participants (Bo et al., 2013). In another study, healthy Japanese participants were given resveratrol (1 g/day for 28 days) to examine its effects on immune cells. Here, increased γδ-T cells and regulatory T cells reduced plasma TNF-α and MCP-1 levels (Espinoza et al., 2017). In a 24-week randomized controlled trial, 93 veterans participated to evaluate cognitive functioning, functional status, mood, hippocampal neurogenesis, and functional connectivity, as well as the anti-inflammatory and antioxidant effects of resveratrol (500 mg, 1,000 mg, 1,500 mg, and 2,000 mg) (NCT03665740). Study results have not been posted yet.
6.2 Curcumin
Curcumin, also known as diferuloylmethane is turmeric’s main component (Curcuma longa L., Zingiberaceae) and used to treat inflammatory illnesses in Ayurvedic medicine. A randomized controlled trial evaluated curcumin’s anti-inflammatory effects in people with metabolic syndrome. Here, 117 participants received curcumin (1 g/day) or a placebo for 8 weeks and showed reduction in TNF-α, IL-6, TGF-β, and MCP-1 in blood (Panahi et al., 2016). A report found that peptic ulcers treated with 600 mg of curcumin per day for 12 weeks improved the condition from 48% to 76%, depending on treatment length (Prucksunand et al., 2001). The function of inflammation in the development of pancreatitis and subsequent tissue damage is crucial (Vaquero et al., 2001). A 6-week pilot study of tropical pancreatitis with 15 patients was performed where administration of curcumin (5 mg/day) with piperine (5 mg) reduced MDA levels, but there was no significant differences in GSH or pain scores as compared to placebo group (Durgaprasad et al., 2005). In another study, forty cancer patients are being examined in a phase I pilot study to examine the adverse effects and optimal dose of curcumin when combined with piperine extract to reduce ureteral stent-induced symptoms (NCT02598726).
6.3 Epigallocatechin gallate (EGCG)
EGCG, also known as epigallocatechin-3-gallate, is a component of green tea, Camellia sinensis (L.) Kuntze (Theaceae). The inflammatory nature of MS increases IL-6 levels in the blood that elevates and often exacerbates pain associated with a physical disability. In a pilot trial, the effects of coconut oil and EGCG on IL-6, anxiety, and functional impairment in MS patients were evaluated. 51 patients with MS were given EGCG (800 mg) and coconut oil (60 ml) for 4 weeks following the Mediterranean diet. The results showed improvement in anxiety and functional capacity along with a decrease in IL-6 (Platero et al., 2020). One more study uses catechin-rich green tea and is being tested on 40 humans to improve gut barrier function and prevent endotoxin translocation and inflammation (NCT03413735).
6.4 Quercetin
Quercetin, a flavonol and plant secondary metabolite found in apples, grapevines, berries, broccoli, onions, and capers. Quercetin targets prominent pro-inflammatory signaling pathways such as STAT1, NF-κB, MAPK and scavenges reactive oxygen and nitrogen species (Hämäläinen et al., 2007). It has been postulated that oxidative stress and low antioxidant levels cause inflammatory sarcoidosis. It was reported that quercetin (15 mg per day) treatment reduced inflammation and boosts antioxidant defense by increasing total plasma antioxidant capacity in sarcoidosis patients participated in double-blind study (Boots et al., 2011). In a randomized, double-blind 8 weeks study, subjects with systematic and regular exercise showed reduction in oxidative stress and inflammatory markers CRP and IL-6 upon treatment with quercetin alone (500 mg) and/or with vitamin C (250 mg) (Askari et al., 2012). COPD is a chronic pulmonary condition that affects millions of people worldwide and reduction of oxidative stress and inflammation are essential part of COPD management (King, 2015). Quercetin (2000 mg/day) efficacy is being evaluated using IL-β, IL-8, bronchoalveolar lavage, CRP, and surfactant protein-D involving 15 COPD patients in a double-blind, placebo-controlled study (NCT03989271). The coronavirus emerged in late 2019, caused multiple deaths via a disease called COVID-19 with challenging health burden around the globe (Zhou et al., 2020). Based on quercetin’s strong scavenger and anti-inflammatory activity, some researchers hypothesized it could prevent and treat COVID-19. The randomized clinical trial included 50 participants with COVID-19 infection and a 1,000 mg/day quercetin dose (NCT04377789).
6.5 Luteolin
Luteolin is a flavone found in carrots, cabbage, artichoke, tea, and celery while it is used majorly for cancer and inflammation due to its antitumor and anti-inflammatory properties. A correlation has been found between autism spectrum disorders (ASD) and cognitive function-related brain inflammation (Pardo et al., 2005; El-Ansary and Al-Ayadhi, 2012). Taliou et al. (2013) reported that treatment with luteolin (100 mg/10 kg) effectively reduced ASD symptoms in children in a 6 week pilot research using an open-label design (Taliou et al., 2013). Frontotemporal dementia (FTD) is a disease where neuroinflammation may play a role and that neuroinflammation-targeting medications may be effective in treating this condition (Cordaro et al., 2020). A clinical trial is being conducted with 50 FTD patients to evaluate palmitoylethanolamide mixed with luteolin (PEA-LUT) at 700 mg × 2/day for 24 weeks (NCT04489017).
7 Phytochemicals used currently in inflammatory diseases/disorders
Natural products are a vital resource for global pharmaceutical firms developing new medicines. About 25% of this natural resource comes from pharmaceuticals i) A direct supply of therapeutic substances (both pure medications and phytomedicines); ii) raw materials for manufacturing complex, semi-synthetic therapeutics; iii) models for developing lead compounds; and iv) taxonomic markers for discovering novel drugs (Calixto, 2019). In in vitro and in vivo studies, many phytochemicals have shown anti-inflammatory activity, and most have been tested in clinical trials. Not all are approved as medicines/drugs; but are used as supplements. Using phytochemicals as drugs or medicines depends on country norms. In this review, we list some effective anti-inflammatory drugs used around the world (Figure 5; Table 5).
FIGURE 5.
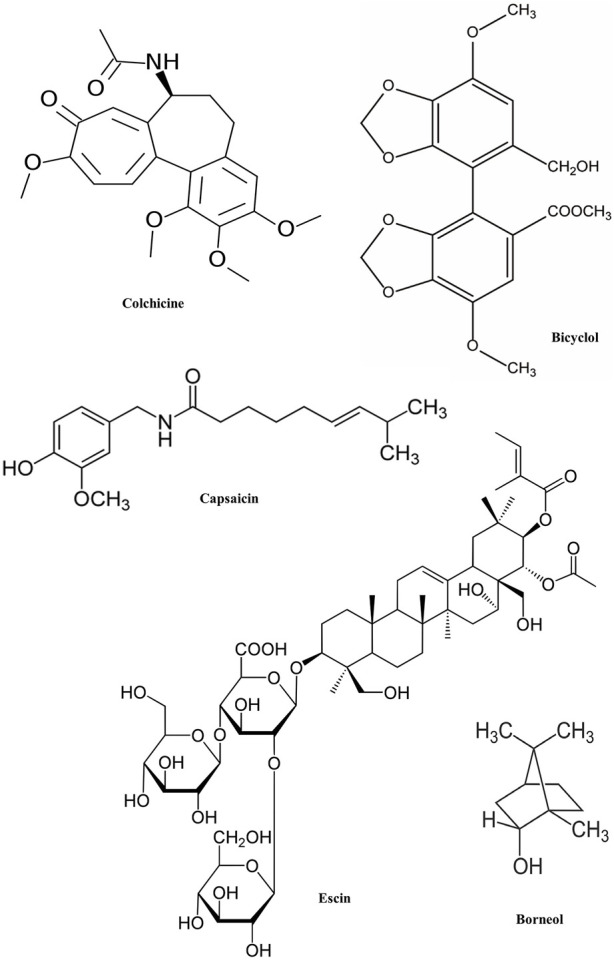
Chemical structure of phytochemicals used as current drugs/medicine.
TABLE 5.
List of anti-inflammatory phytochemicals used as current drugs/medicines.
| Drug/medicine (class/group of compounds) | Pharmacological action | Disease/disorder | Molecular targets | References |
|---|---|---|---|---|
| Colchicine (alkaloid) | Microtubule polymerization by binding to tubulin | Gout attacks Joint Pain | Tubulin | Leung et al. (2015) |
| Escin (triterpenoid saponin) | Anti-inflammatory, reduces vascular permeability by inducing endothelial nitric oxide synthesis | Cerebral edema and chronic venous insufficiency | NO synthesis | Gallelli (2019) |
| Capsaicin (methoxy phenol) | Defunctionalisation of nociceptor fibers by inducing a topical hypersensitivity reaction on the skin | Neuropathic pain associated with postherpetic neuralgia | Nociceptor fibers | Fattori et al. (2016) |
| Bicyclol (lignan) | cytochrome P-450 enzyme system stimulants | Liver complications | cytochrome P-450 enzyme | Liu et al. (2005); Bao and Liu (2008) |
| Borneol (monoterpene) | Induces anesthesia and analgesia | Anxiety, fatigue, and insomnia | — | Xiong et al. (2013); Ji et al. (2020) |
| Bromelain | Reduces inflammation by interfering with the enzymatic synthesis involved in the arachidonic acid metabolic pathway | Osteoarthritis, hay fever, ulcerative colitis, and debridement | Arachidonic acid | Hale et al. (2005); Rathnavelu et al. (2016) |
7.1 Colchicine
Colchicine is an alkaloid of Colchicum autumnale L. (Colchicaceae), also called autumn crocus or meadow saffron. This phytochemical is an alternative medication for those who are unable to tolerate NSAIDs in gout. Colchicine prevents microtubule polymerization by binding to tubulin and suppressing leukocyte and other inflammatory cell proliferation and reduces urate crystal inflammation (Leung et al., 2015).
7.2 Escin
Escin is a horse chestnut triterpenoid saponin (Aesculus hippocastanum L.), which is known for its vasoprotective, anti-inflammatory, anti-edematous, and anti-nociceptive properties. Traditional Chinese medicine uses escin to treat cerebral edema and chronic venous insufficiency. Recent research shows that escin can reduce vascular permeability in inflamed tissues, preventing swelling (Gallelli, 2019).
7.3 Capsaicin
Various non-steroidal drugs and phytochemicals are analgesics and anti-inflammatory agents (Kim et al., 2003). Capsaicin is a topical analgesic approved by the FDA for alleviating the neuropathic pain associated with postherpetic neuralgia. It's available in cream, powder, and patch forms, but also present in some nutritional supplements. The exact mechanism of action is not known, however it is attributed to the defunctionalisation of nociceptor fibres by inducing a topical hypersensitivity reaction on the skin (Fattori et al., 2016).
7.4 Bicyclol
A synthetic compound derived from Schisandra C, a lignan extracted from the Chinese medicinal herb Schisandra chinensis Fructus (Turcz.) Baill. Chinese Medical Association approved this anti-inflammatory drug for liver complications. Mechanisms of action include cytochrome P-450 stimulants, free radical-scavenging HSP70 stimulants, and protein kinase C inhibitors (Liu et al., 2005; Bao and Liu, 2008).
7.5 Borneol
Borneol is present in many essential oils and it’s a bicyclic monoterpene with a strong, bitter aroma and flavor. Research shows borneol’s effectiveness in inflammation and related complications (Ji et al., 2020). For instance, in Chinese medicine, borneol treats anxiety, fatigue, and insomnia. Borneol not only causes anesthesia, pain relief for abdominal pain, wounds, and burns but also treats rheumatism, hemorrhoids, skin diseases, and ulcers. More precisely, it is well known to relieves pain, inflammation, digestive issues, stress, and anxiety (Xiong et al., 2013).
7.6 Bromelain
Bromelain is a group of protein-digesting enzymes found in pineapple juice and the pineapple stem. In the US, it's a dietary supplement, but elsewhere it is a medicine. Bromelain stimulates inflammatory pathways to produce pain and inflammation-fighting substances. Hence it is generally prescribed for osteoarthritis, hay fever, ulcerative colitis, and debridement (Rathnavelu et al., 2016). It stops the release of IL-1β, IL-6, and TNF-α by activated immune cells when inflammation causes them to make too many cytokines (Hale et al., 2005).
8 Discussion and conclusion
This review briefly describes recent investigations on the anti-inflammatory properties of medicinal phytochemicals using preclinical and clinical studies. The preclinical studies of these phytochemicals have led to a better understanding of their mode of action for the therapeutic management of a variety of chronic inflammatory diseases and disorders and steered the way to the development of many anti-inflammatory drugs which are being used clinically. It is evidenced that phytochemicals may suppress the expression of proinflammatory genes and stimulate the expression of anti-inflammatory genes; this differential gene expression is governed by epigenetic changes. In this study, we demonstrate that phytochemicals exert their anti-inflammatory impact by modulating the expression of proinflammatory miRNAs, particularly those that are increased after NF-κB activation. These phytochemicals also modulate key inflammatory signaling pathways, such as MAPKs, STAT, and Nrf-2.
Additionally, the present review gave insights towards the relation of inflammation and obesity, with one causing the other. Some of the studies suggested in preclinical studies gives evidence of the linkage between inflammation and obesity. For example, obesity mice model treated with apigenin showed reduction in body weight along with improvement in inflammatory parameters (Gentile et al., 2018). One more study proved that PL administration showed anti-obese effect and inhibited obesity-induced inflammatory responses (Kim et al., 2019). We have also discussed how inflammatory conditions are linked with birth complications that decide future disease/disorders in neonatal stage. In fact, during pregnancy, mother provides a variety of food and conventional nutrients that contain a variety of phytochemicals in various concentrations to the foetus. It also indicates that concentration-dependent effects of phytochemicals must be present to control the repercussions of mother’s health and food habits.
It is of utmost interest to understand the specific role of phytochemicals in different inflammatory diseases rather than depending upon the crude extracts or partially purified mixture of phytochemicals. It is also important to understand the right time for the intervention by phytochemicals in different diseases. It’s very likely that the same phytochemicals may not be effective at different ages for a similar inflammatory disorder. The clinical studies are not addressing in detail the above facts regarding phytochemicals intervention, specifying the needs for controlled treatment with conventional allopathic drugs. This kind of study may trigger the competitive use of phytochemicals against allopathic drugs also. Finally, it is important to discuss and study the above fundamentals to better understand the mechanism of action of phytochemicals in inflammation associated diseases and disorders. Another treatment modality is combination therapy that combines two or more therapeutic agents such as certain specific phytochemicals with known therapeutic effects. Combination therapy is the cornerstone of cancer treatment where a combination of anticancer drugs is used to enhance treatment efficacy compared to the monotherapy because a combination has the potential to target key signaling pathways that control tumor growth where synthetic drugs are used with one or mixture of the phytochemicals. The application of complementary and alternative medicine, which includes phytochemicals and herbal extracts that leads to chances of herb-drug interactions (HemaIswarya and Doble, 2007). In another study, anticancer activities of each of the three phytochemicals baicalein, curcumin, and resveratrol in combination with a chemotherapy drug paclitaxel indicated that combination of paclitaxel with curcumin showed synergistic growth inhibition and significant apoptosis in human breast cancer MCF-7 cell lines (Zhan et al., 2014).
The use of phytochemicals as therapeutic agents has certain limitations that deserve some attention, such as the larger dose requirement for some compounds, poor solubility, isolation, and procurement, etc. In fact, most of the clinical trials do not take into consideration the inflammatory parameters in the assessment. A small number of phytochemicals which have been approved for clinical trials, are essentially those that have already been tested in preclinical studies as anti-inflammatory molecules. On the contrary, a large number of phytochemicals are being used as supplements and are available over the counter, are also found to be effective but these are not approved as medicines/drug due to their lack of proper clinical evaluation. Finally, it seems that there is a broad difference in basic preclinical studies of these anti-inflammatory phytochemicals and their availability as drugs/medicines. For future perspectives, it looks like that the design of the study should be more specific at the molecular level and more clinical trials should be introduced through targeted treatments with therapeutic phytochemicals. Furthermore, in silico studies can be initiated to increase the spectrum of the study as well as to find more pronounced details regarding the feasibility and therapeutic usefulness of these phytochemicals. Finally, this review has focused on those phytochemicals which are at the preclinical and clinical level and summarized the mechanism of action of these phytochemicals at the molecular level. It is expected that present study will provide the necessary understanding to define specific phytochemicals with anti-inflammatory properties that can be used as therapeutics in complex diseases such as obesity, diabetes, and cancer.
Acknowledgments
The authors are thankful to Prof. Sahebrao Mahadik for valuable suggestions in revising, editing, and formatting the manuscript and authorities of Bharati Vidyapeeth (Deemed to be University) for their overall support.
Author contributions
AN wrote and edited original draft. SJ conceptualized and supervised the study. SV, MD, and AH reviewed and edited the draft. PR and OP reviewed, revised and reformatted the scientific content, and critical feedback in the preparation of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author PR was employed by Innovation Biologicals Pvt., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abalos E., Cuesta C., Carroli G., Qureshi Z., Widmer M., Vogel J. P., et al. (2014). Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: A secondary analysis of the world health organization multicountry survey on maternal and newborn health. BJOG 121, 14–24. 10.1111/1471-0528.12629 [DOI] [PubMed] [Google Scholar]
- Abolaji A. O., Ojo M., Afolabi T. T., Arowoogun M. D., Nwawolor D., Farombi E. O. (2017). Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 270, 15–23. 10.1016/j.cbi.2017.03.017 [DOI] [PubMed] [Google Scholar]
- Afonina I. S., Zhong Z., Karin M., Beyaert R. (2017). Limiting inflammation—The negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 18, 861–869. 10.1038/ni.3772 [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Vijayalekshmi R. V., Sung B. (2009). Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 15, 425–430. 10.1158/1078-0432.CCR-08-0149 [DOI] [PubMed] [Google Scholar]
- Akanni O. O., Owumi S. E., Olowofela O. G., Adeyanju A. A., Abiola O. J., Adaramoye O. A. (2020). Protocatechuic acid ameliorates testosterone-induced benign prostatic hyperplasia through the regulation of inflammation and oxidative stress in castrated rats. J. Biochem. Mol. Toxicol. 34 (8), e22502. 10.1002/jbt.22502 [DOI] [PubMed] [Google Scholar]
- Alfano C. M., Imayama I., Neuhouser M. L., Kiecolt-Glaser J. K., Smith A. W., Meeske K., et al. (2012). Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 30, 1280–1287. 10.1200/JCO.2011.36.4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra M. (2019). Antioxidant and anti-inflammatory properties of plants extract. Antioxidants 8, 549. 10.3390/antiox8110549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin F. U., Shah S. A., Kim M. O. (2017). Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 7, 40753. 10.1038/srep40753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A. B., Sundaram C., Harikumar K. B., Tharakan S. T., Lai O. S., et al. (2008). Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 25, 2097–2116. 10.1007/s11095-008-9661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando C., Takahashi N., Hirai S., Nishimura K., Lin S., Uemura T., et al. (2009). Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 583, 3649–3654. 10.1016/j.febslet.2009.10.045 [DOI] [PubMed] [Google Scholar]
- Antonopoulos A. S., Margaritis M., Coutinho P., Shirodaria C., Psarros C., Herdman L., et al. (2015). Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: The regulatory role of perivascular adipose tissue. Diabetes 64, 2207–2219. 10.2337/db14-1011 [DOI] [PubMed] [Google Scholar]
- Arango D., Diosa-Toro M., Rojas-Hernandez L. S., Cooperstone J. L., Schwartz S. J., Mo X., et al. (2015). Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol. Nutr. Food Res. 59, 763–772. 10.1002/mnfr.201400705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M., Pandeya N., Byrnes G., Renehan P. A. G., Stevens G. A., Ezzati P. M., et al. (2015). Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 16, 36–46. 10.1016/S1470-2045(14)71123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulselvan P., Fard M. T., Tan W. S., Gothai S., Fakurazi S., Norhaizan M. E., et al. (2016). Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 5276130. 10.1155/2016/5276130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari G., Ghiasvand R., Feizi A., Ghanadian S. M., Karimian J. (2012). The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J. Res. Med. Sci. 17, 637–641. [PMC free article] [PubMed] [Google Scholar]
- Bale S., Venkatesh P., Sunkoju M., Godugu C. (2018). An adaptogen: Withaferin A ameliorates in vitro and in vivo pulmonary fibrosis by modulating the interplay of fibrotic, matricelluar proteins, and cytokines. Front. Pharmacol. 9, 248. 10.3389/fphar.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balez R., Steiner N., Engel M., Muñoz S. S., Lum J. S., Wu Y., et al. (2016). Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 6, 31450. 10.1038/srep31450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X. Q., Liu G. T. (2008). Bicyclol: A novel antihepatitis drug with hepatic heat shock protein 27/70-inducing activity and cytoprotective effects in mice. Cell. Stress Chaperones 13, 347–355. 10.1007/s12192-008-0034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batumalaie K., Amin M. A., Murugan D. D., Sattar M. Z. A., Abdullah N. A. (2016). Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci. Rep. 6, 27236. 10.1038/srep27236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. M., Reeves G., Billman G. E., Sturmberg J. P. (2018). Inflammation–Nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 5, 316. 10.3389/fmed.2018.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros M. E., Pedersen M. G., Rasmussen H., Eaton W. W., Nordentoft M., Mortensen P. B. (2014). A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am. J. Psychiatry 171, 218–226. 10.1176/appi.ajp.2013.13010086 [DOI] [PubMed] [Google Scholar]
- Bhaskar S., Sudhakaran P. R., Helen A. (2016). Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell. Immunol. 310, 131–140. 10.1016/j.cellimm.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Bhondave P. D., Devarshi P. P., Mahadik K. R., Harsulkar A. M. (2014). Ashvagandharishta” prepared using yeast consortium from Woodfordia fruticosa flowers exhibit hepatoprotective effect on CCl4 induced liver damage in Wistar rats. J. Ethnopharmacol. 151, 183–190. 10.1016/j.jep.2013.10.025 [DOI] [PubMed] [Google Scholar]
- Bian Y., Liu P., Zhong J., Hu Y., Fan Y., Zhuang S., et al. (2019). Kaempferol inhibits multiple pathways involved in the secretion of inflammatory mediators from LPSinduced rat intestinal microvascular endothelial cells. Mol. Med. Rep. 19, 1958–1964. 10.3892/mmr.2018.9777 [DOI] [PubMed] [Google Scholar]
- Bian Y., Liu P., Zhong J., Hu Y., Zhuang S., Fan K., et al. (2018). Quercetin attenuates adhesion molecule expression in intestinal microvascular endothelial cells by modulating multiple pathways. Dig. Dis. Sci. 63, 3297–3304. 10.1007/s10620-018-5221-2 [DOI] [PubMed] [Google Scholar]
- Blair M. (2016). Diabetes mellitus review. Urol. Nurs. 36, 27–36. 10.7257/1053-816x.2016.36.1.27 [DOI] [PubMed] [Google Scholar]
- Bo S., Ciccone G., Castiglione A., Gambino R., De Michieli F., Villois P., et al. (2013). Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 20, 1323–1331. 10.2174/0929867311320100009 [DOI] [PubMed] [Google Scholar]
- Boeing T., de Souza P., Speca S., Somensi L. B., Mariano L. N. B., Cury B. J., et al. (2020). Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 177 (10), 2393–2408. 10.1111/bph.14987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots A. W., Drent M., de Boer V. C. J., Bast A., Haenen G. R. M. M. (2011). Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 30, 506–512. 10.1016/j.clnu.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Buss C., Entringer S., Wadhwa P. D. (2012). Fetal programming of brain development: Intrauterine stress and susceptibility to psychopathology. Sci. Signal. 5, pt7. 10.1126/scisignal.2003406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustami A., Sopiah P., Muharam R., Wibowo H. (2018). Effects of gallic acid and its derivates on inflammatory regulation of endometriotic primary cultures: Study on NF-kB mRNA expression and IL-6 secretion. Biomed. Pharmacol. J. 11, 1479–1484. 10.13005/bpj/1514 [DOI] [Google Scholar]
- Cai J., Jing D., Shi M., Liu Y., Lin T., Xie Z., et al. (2014). Epigallocatechin gallate (EGCG) attenuates infrasound-induced neuronal impairment by inhibiting microglia-mediated inflammation. J. Nutr. Biochem. 25, 716–725. 10.1016/j.jnutbio.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Calixto J. (2019). The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 91, e20190105. 10.1590/0001-3765201920190105 [DOI] [PubMed] [Google Scholar]
- Calixto-Campos C., Carvalho T. T., Hohmann M. S. N., Pinho-Ribeiro F. A., Fattori V., Manchope M. F., et al. (2015). Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 78, 1799–1808. 10.1021/acs.jnatprod.5b00246 [DOI] [PubMed] [Google Scholar]
- Cao W., Hu C., Wu L., Xu L., Jiang W. (2016). Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 132, 131–137. 10.1016/j.jphs.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Carroll A., Arumugan G., Quinn R., Redburn J., Guymer G., Grimshaw P. (2005). Grandisine A and B, novel indolizidine alkaloids with human delta-opioid receptor binding affinity from the leaves of the Australian rainforest tree Elaeocarpus grandis . J. Org. Chem. 70, 1889–1892. 10.1021/jo048525n [DOI] [PubMed] [Google Scholar]
- Castanon N., Lasselin J., Capuron L. (2014). Neuropsychiatric comorbidity in obesity: Role of inflammatory processes. Front. Endocrinol. (Lausanne). 5, 74. 10.3389/fendo.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotti F., Laufer S. (2001). Anti-inflammatory drugs: New multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol. Res. 43, 429–436. 10.1006/phrs.2000.0784 [DOI] [PubMed] [Google Scholar]
- Chan D. S., Norat T. (2015). Obesity and breast cancer: Not only a risk factor of the disease. Curr. Treat. Options Oncol. 16, 22. 10.1007/s11864-015-0341-9 [DOI] [PubMed] [Google Scholar]
- Charlson F. J., Ferrari A. J., Santomauro D. F., Diminic S., Stockings E., Scott J. G., et al. (2018). Global epidemiology and burden of schizophrenia: Findings from the global burden of disease study 2016. Schizophr. Bull. 44, 1195–1203. 10.1093/schbul/sby058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Montagnani M., Funahashi T., Shimomura I., Quon M. J. (2003). Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 278, 45021–45026. 10.1074/jbc.M307878200 [DOI] [PubMed] [Google Scholar]
- Chen J., Yang X., Zhang W., Peng D., Xia Y., Lu Y., et al. (2016). Therapeutic effects of resveratrol in a mouse model of LPS and cigarette smoke-induced COPD. Inflammation 39, 1949–1959. 10.1007/s10753-016-0430-3 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zheng Y., Zhou Z., Wang J. (2018a). Baicalein alleviates tubular-interstitial nephritis in vivo and in vitro by down-regulating NF-kB and MAPK pathways. Braz. J. Med. Biol. Res. 51, e7476–e7479. 10.1590/1414-431X20187476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Bai S., Hu Q., Shen P., Wang T., Liang Z., et al. (2018b). Ginkgo biloba extract and its diterpene ginkgolide constituents ameliorate the metabolic disturbances caused by recombinant tissue plasminogen activator in rat prefrontal cortex. Neuropsychiatr. Dis. Treat. 14, 1755–1772. 10.2147/NDT.S167448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien T., Huang S. K., Lee C., Tsai P., Wang C. C. (2016). Antinociceptive and anti-inflammatory effects of zerumbone against mono-iodoacetate-induced arthritis. Int. J. Mol. Sci. 17, 249. 10.3390/ijms17020249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Joseph L., Pilote L. (2013). Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 14, 232–244. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., et al. (2005). Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355. 10.1194/jlr.M500294-JLR200 [DOI] [PubMed] [Google Scholar]
- Clockaerts S., Bastiaansen-Jenniskens Y. M., Runhaar J., Van Osch G. J. V. M., Van Offel J. F., Verhaar J. A. N., et al. (2010). The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: A narrative review. Osteoarthr. Cartil. 18, 876–882. 10.1016/j.joca.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Cordaro M., Cuzzocrea S., Crupi R. (2020). An Update of palmitoylethanolamide and luteolin effects in preclinical and clinical studies of neuroinflammatory events. Antioxidants 9, 216. 10.3390/antiox9030216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G. M., Newman D. J. (2005). Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 77, 7–24. 10.1351/pac200577010007 [DOI] [Google Scholar]
- Cragg G. M. (1998). Paclitaxel (taxol): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 18, 315–331. [DOI] [PubMed] [Google Scholar]
- Danesh J., Kaptoge S., Mann A. G., Sarwar N., Wood A., Angleman S. B., et al. (2008). Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 5, e78. 10.1371/journal.pmed.0050078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. C., Bhadra S., Roy S., Saha S. K., Islam S., Bachar S. C. (2012). Analgesic and anti-inflammatory activities of ethanolic root. Jordan J. Biol. Sci. 5, 31–36. [Google Scholar]
- Deleu D., Hanssens Y., Northway M. G. (2004). Subcutaneous apomorphine: An evidence-based review of its use in Parkinson’s disease. Drugs Aging 21, 687–709. 10.2165/00002512-200421110-00001 [DOI] [PubMed] [Google Scholar]
- Devkar S. T., Kandhare A. D., Zanwar A. A., Jagtap S. D., Katyare S. S., Bodhankar S. L., et al. (2016). Hepatoprotective effect of withanolide-rich fraction in acetaminophen-intoxicated rat: Decisive role of TNF-α, IL-1β, COX-II and iNOS. Pharm. Biol. 54, 2394–2403. 10.3109/13880209.2016.1157193 [DOI] [PubMed] [Google Scholar]
- Dongare S., Gupta S. K., Mathur R., Saxena R., Mathur S., Agarwal R., et al. (2016). Zingiber officinale attenuates retinal microvascular changes in diabetic rats via anti-inflammatory and antiangiogenic mechanisms. Mol. Vis. 22, 599–609. [PMC free article] [PubMed] [Google Scholar]
- Durgaprasad S., Pai C. G., VasanthkumarAlvres J. F., Namitha S. (2005). A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 122, 315–318. [PubMed] [Google Scholar]
- Eddouks M., Chattopadhyay D., Zeggwagh N. A. (2012). Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evidence-Based Complement. Altern. Med. 2012, 142087. 10.1155/2012/142087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow A. G. (2018). Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 37, 95–110. 10.1002/pd.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary A., Al-Ayadhi L. (2012). Neuroinflammation in autism spectrum disorders. J. Neuroinflammation 9, 265. 10.1186/1742-2094-9-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellulu M. S., Patimah I., Khaza H., Rahmat A., Abed Y. (2017). Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 13, 851–863. 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano-Ferrer E., Regué J., Garcia-Sala X., Boix-montañes A., Lamuela-Raventós R. M. (2019). In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice. J. Nat. Prod. 82, 177–182. 10.1021/acs.jnatprod.8b00366 [DOI] [PubMed] [Google Scholar]
- Espinoza J. L., Trung L. Q., Inaoka P. T., Yamada K., An D. T., Mizuno S., et al. (2017). The repeated administration of resveratrol has measurable effects on circulating T-cell subsets in humans. Oxid. Med. Cell. Longev. 2017, 6781872. 10.1155/2017/6781872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. L., McAllister A. K. (2016). Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777. 10.1126/science.aag3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P., Chen S., Li Z., Yang X., Lei S., Tan W. (2018). Gallic acid inhibits LPS induced hypertrophic scar inflammation via toll-like receptor 4/nuclear factor-κB/peroxisome proliferator-activated receptor γ signaling. Int. J. Clin. Exp. Med. 11, 12124–12132. [Google Scholar]
- Fattori V., Hohmann M. S. N., Rossaneis A. C., Pinho-Ribeiro F. A., Verri W. A. (2016). Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 21, 844. 10.3390/molecules21070844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Weng D., Zhou F., Owen Y. D., Qin H., Zhao J., et al. (2016). Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 9, 61–76. 10.1016/j.ebiom.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D., Cimino F., Fratantonio D., Molonia M. S., Bashllari R., Busa R., et al. (2017). Cyanidin-3-O-glucoside modulates the in vitro inflammatory crosstalk between intestinal epithelial and endothelial cells. Mediat. Inflamm. 2017, 3454023. 10.1155/2017/3454023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler J. M., Li K., Chung C., Wei K., Ross J. A., Gao M., et al. (2003). PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol. Cancer Ther. 2, 855–862. [PubMed] [Google Scholar]
- Fikry E. M., Gad A. M., Eid A. H., Arab H. H. (2019). Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharmacother. 110, 878–886. 10.1016/j.biopha.2018.12.041 [DOI] [PubMed] [Google Scholar]
- Firdaus F., Zafeer M. F., Anis E., Ahmad M., Afzal M. (2018). Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol. Rep. 5, 411–417. 10.1016/j.toxrep.2018.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried S. K., Bunkin D. A., Greenberg A. S. (1998). Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83, 847–850. 10.1210/jcem.83.3.4660 [DOI] [PubMed] [Google Scholar]
- Fu Z., Chen Z., Xie Q., Lei H., Xiang S. (2018). Hesperidin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Exp. Ther. Med. 16, 3721–3727. 10.3892/etm.2018.6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallelli L. (2019). Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des. devel. Ther. 13, 3425–3437. 10.2147/DDDT.S207720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., Fu Y., Yang L., Chen J., Lei H., Liu Q. (2019). Cyanidin-3-O-glucoside and cyanidin protect against intestinal barrier damage and 2,4,6-trinitrobenzenesulfonic acid-induced colitis. J. Med. Food 23, 90–99. 10.1089/jmf.2019.4524 [DOI] [PubMed] [Google Scholar]
- Gandhi J., Khera L., Gaur N., Paul C., Kaul R. (2017). Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front. Microbiol. 8, 538. 10.3389/fmicb.2017.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R. K., Gupta G., Sharma S., Hatware K., Patil K., Sharma K., et al. (2019). Rosmarinic acid attenuates inflammation in experimentally induced arthritis in Wistar rats, using Freund’s complete adjuvant. Int. J. Rheum. Dis. 22, 1247–1254. 10.1111/1756-185X.13602 [DOI] [PubMed] [Google Scholar]
- Gentile D., Fornai M., Colucci R., Pellegrini C., Tirotta E., Benvenuti L., et al. (2018). The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS One 13, e0195502–e0195519. 10.1371/journal.pone.0195502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh Rahbardar M., Amin B., Mehri S., Mirnajafi-Zadeh S. J., Hosseinzadeh H. (2017). Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 86, 441–449. 10.1016/j.biopha.2016.12.049 [DOI] [PubMed] [Google Scholar]
- Ghose A., Kundu R., Toumeh A., Hornbeck C., Mohamed I. (2015). A review of obesity, insulin resistance, and the role of exercise in breast cancer patients. Nutr. Cancer 67, 197–202. 10.1080/01635581.2015.990569 [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Goldring S. R. (2010). Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 1192, 230–237. 10.1111/j.1749-6632.2009.05240.x [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Otero M. (2011). Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 23, 471–478. 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R., Conde J., Scotece M., Gomez-Reino J. J., Lago F., Gualillo O. (2011). What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 7, 528–536. 10.1038/nrrheum.2011.107 [DOI] [PubMed] [Google Scholar]
- Gong W. G., Lin J. L., Niu Q. X., Wang H. M., Zhou Y. C., Chen S. Y., et al. (2015). Paeoniflorin diminishes ConA-induced IL-8 production in primary human hepatic sinusoidal endothelial cells in the involvement of ERK1/2 and Akt phosphorylation. Int. J. Biochem. Cell. Biol. 62, 93–100. 10.1016/j.biocel.2015.02.017 [DOI] [PubMed] [Google Scholar]
- Guan S., Zheng Y., Yu X., Li W., Han B., Lu J. (2017). Ellagic acid protects against LPS-induced acute lung injury through inhibition of nuclear factor kappa B, proinflammatory cytokines and enhancement of interleukin-10. Food Agric. Immunol. 28, 1347–1361. 10.1080/09540105.2017.1339670 [DOI] [Google Scholar]
- Halawany A. M. El, Sayed N. S. E. L., Abdallah H. M., Dine R. S. El. (2017). Protective effects of gingerol on streptozotocin-induced sporadic Alzheimer’s disease: Emphasis on inhibition of β-amyloid, COX-2, alpha-beta - secretases and APH1a. Sci. Rep. 7, 2902. 10.1038/s41598-017-02961-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hale L. P., Greer P. K., Trinh C. T., Gottfried M. R. (2005). Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin. Immunol. 116, 135–142. 10.1016/j.clim.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Hamalainen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. (2007). Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 45673. 10.1155/2007/45673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Coussens L. M. (2012). Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 21, 309–322. 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: The next generation. Cell. 144, 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hansson G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Haque M. A., Jantan I., Harikrishnan H. (2018). Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-κB/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 55, 312–322. 10.1016/j.intimp.2018.01.001 [DOI] [PubMed] [Google Scholar]
- He X., Wei Z., Zhou E., Chen L., Kou J., Wang J., et al. (2015). Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int. Immunopharmacol. 28, 470–476. 10.1016/j.intimp.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Hejia H., Yan L., Zengfeng X., Xiangfeng Z. (2018). Ginkgolide B exerts anti-inflammatory and chondroprotective activity in LPS-induced chondrocytes. Adv. Clin. Exp. Med. 27, 913–920. 10.17219/acem/70414 [DOI] [PubMed] [Google Scholar]
- Hemaiswarya S., Doble M. (2007). “Mechanistic Studies on combination of phytochemicals and synthetic drugs as anti-cancer agents.” in Alternative treatment for cancer, World Scientific, Singapore, 233–253. 10.1142/9789812709301_0010 [DOI] [Google Scholar]
- Hlatky M. A., Greenland P., Arnett D. K., Ballantyne C. M., Criqui M. H., Elkind M. S. V., et al. (2009). Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American heart association. Circulation 119, 2408–2416. 10.1161/CIRCULATIONAHA.109.192278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Howes M. J. R., Perry N. S. L., Houghton P. J. (2003). Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 17, 1–18. 10.1002/ptr.1280 [DOI] [PubMed] [Google Scholar]
- Howes M. J. R., Simmonds M. S. J. (2014). The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 17, 558–566. 10.1097/MCO.0000000000000115 [DOI] [PubMed] [Google Scholar]
- Huang F. C., Kuo H. C., Huang Y. H., Yu H. R., Li S. C., Kuo H. C. (2017). Anti-inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: Implication for the treatment of kawasaki disease. BMC Pharmacol. Toxicol. 18, 3. 10.1186/s40360-016-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. H., Kim T., Kim R., Ha H. (2018). The natural product 6-gingerol inhibits inflammation-associated osteoclast differentiation via reduction of prostaglandin E₂ levels. Int. J. Mol. Sci. 19, 2068. 10.3390/ijms19072068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingale D., Kulkarni P., Koppikar S., Harsulkar A., Moghe A., Jagtap S. (2018). Reduced synovial inflammation and inhibition of matrix metalloproteinases explicates anti-osteoarthritis activity of polyherbal formulations. Indian J. Pharmacol. 50, 22–29. 10.4103/ijp.IJP_29_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation (2019). IDF diabetes atlas 9th edn. Brussels, Belgium. [Google Scholar]
- Iwagaki S., Yokoyama Y., Tang L., Takahashi Y., Nakagawa Y., Tamaya T. (2004). Augmentation of leptin and hypoxia-inducible factor 1alpha mRNAs in the pre-eclamptic placenta. Gynecol. Endocrinol. 18, 263–268. 10.1080/0951359042000196277 [DOI] [PubMed] [Google Scholar]
- Jagtap S., Suhit G., Prashant B., Anant P., Pankaj P., Abhay H., et al. (2009). Validation of the potential of Eulophia ochreata L. tubers for its anti-inflammatory and antioxidant activity. Pharmacologyonline 2, 307–316. [Google Scholar]
- Jeon Y. D., Lee J. H., Lee Y. M., Kim D. K. (2020). Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 124, 109847. 10.1016/j.biopha.2020.109847 [DOI] [PubMed] [Google Scholar]
- Jeong J. W., Lee H. H., Han M. H., Kim G. Y., Kim W. J., Choi Y. H. (2014). Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem. Biol. Interact. 212, 30–39. 10.1016/j.cbi.2014.01.012 [DOI] [PubMed] [Google Scholar]
- Ji J., Zhang R., Li H., Zhu J., Pan Y., Guo Q. (2020). Analgesic and anti-inflammatory effects and mechanism of action of borneol on photodynamic therapy of acne. Environ. Toxicol. Pharmacol. 75, 103329. 10.1016/j.etap.2020.103329 [DOI] [PubMed] [Google Scholar]
- Jia Y., Jiang S., Chen C., Lu G., Xie Y., Sun X., et al. (2019). Caffeic acid phenethyl ester attenuates nuclear factor‑κB‑mediated inflammatory responses in Müller cells and protects against retinal ganglion cell death. Mol. Med. Rep. 19, 4863–4871. 10.3892/mmr.2019.10151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K., Ma X., Guo S., Zhang T., Zhao G., Wu H., et al. (2018). Anti-inflammatory effects of rosmarinic acid in lipopolysaccharide-induced mastitis in mice. Inflammation 41, 437–448. 10.1007/s10753-017-0700-8 [DOI] [PubMed] [Google Scholar]
- Jin B. R., Chung K. S., Cheon S. Y., Lee M., Hwang S., Noh Hwang S., et al. (2017). Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 7, 46252. 10.1038/srep46252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens L. J., Racké K., Tuleta I., Stoeber M., Juergens U. R. (2017). Anti-inflammatory effects of 1,8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma? Synergy 5, 1–8. 10.1016/j.synres.2017.08.001 [DOI] [Google Scholar]
- Jung U. J., Cho Y. Y., Choi M. S. (2016). Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients 8, 305. 10.3390/nu8050305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu O., Nass J., Saeed M. E. M., Schuler B., Efferth T. (2015). Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 35, 2645–2650. [PubMed] [Google Scholar]
- Kaewmool C., Kongtawelert P., Phitak T., Pothacharoen P., Udomruk S. (2020). Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-κB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J. Neuroimmunol. 341, 577164. 10.1016/j.jneuroim.2020.577164 [DOI] [PubMed] [Google Scholar]
- Kaewmool C., Udomruk S., Phitak T., Pothacharoen P., Kongtawelert P. (2019). Cyanidin-3- O-glucoside protects PC12 cells against neuronal apoptosis mediated by LPS-stimulated BV2 microglial activation. Neurotox. Res. 37, 111–125. 10.1007/s12640-019-00102-1 [DOI] [PubMed] [Google Scholar]
- Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., et al. (2006). MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505. 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.-P., Fahmi H. (2011). Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42. 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- Karunamoorthi K., Jegajeevanram K., Vijayalakshmi J., Mengistie E. (2013). Traditional medicinal plants: A source of phytotherapeutic modality in resource-constrained health care settings. J. Evid. Based. Complement. Altern. Med. 18, 67–74. 10.1177/2156587212460241 [DOI] [Google Scholar]
- Kedei N., Lundberg D. J., Toth A., Welburn P., Garfield S. H., Blumberg P. M. (2004). Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 64, 3243–3255. 10.1158/0008-5472.can-03-3403 [DOI] [PubMed] [Google Scholar]
- Khan A., Ali T., Rehman S. U., Khan M. S., Alam S. I., Ikram M., et al. (2018). Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol. 9, 1383. 10.3389/fphar.2018.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. S., Kawada T., Kim B. S., Han I. S., Choe S. Y., Kurata T., et al. (2003). Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 15, 299–306. 10.1016/S0898-6568(02)00086-4 [DOI] [PubMed] [Google Scholar]
- Kim C. Y., Kang B., Suh H. J., Choi H. S. (2019). Parthenolide, a feverfew-derived phytochemical, ameliorates obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 pathway. Pharmacol. Res. 145, 104259. 10.1016/j.phrs.2019.104259 [DOI] [PubMed] [Google Scholar]
- Kim M., Miyamoto S., Yasui Y., Oyama T., Murakami A., Tanaka T. (2009). Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int. J. cancer 124, 264–271. 10.1002/ijc.23923 [DOI] [PubMed] [Google Scholar]
- Kim Y., Kim H., Lee J. Y., Kim D., Kang M. S., Park W. (2018). Anti-inflammatory effect of baicalein on polyinosinic–polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses 10, e224. 10.3390/v10050224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. T. (2015). Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 4, 68. 10.1186/s40169-015-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviharju T. M., Lecane P. S., Sellers R. G., Peehl D. M. (2002). Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer Res. 8, 2666–2674. [PubMed] [Google Scholar]
- Klein-Wieringa I. R., Kloppenburg M., Bastiaansen-Jenniskens Y. M., Yusuf E., Kwekkeboom J. C., El-Bannoudi H., et al. (2011). The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann. Rheum. Dis. 70, 851–857. 10.1136/ard.2010.140046 [DOI] [PubMed] [Google Scholar]
- Klisic A. N., Vasiljevic N. D., Simic T. P., Djukic T. I., Maksimovic M. Z., Matic M. G. (2014). Association between C-reactive protein, anthropometric and lipid parameters among healthy normal weight and overweight postmenopausal women in Montenegro. Lab. Med. 45, 12–16. 10.1309/lmi6i2rn7ampeuul [DOI] [PubMed] [Google Scholar]
- Knuesel I., Chicha L., Britschgi M., Schobel S. A., Bodmer M., Hellings J. A., et al. (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10, 643–660. 10.1038/nrneurol.2014.187 [DOI] [PubMed] [Google Scholar]
- Kohli S., Griggs J. J., Roscoe J. A., Jean-Pierre P., Bole C., Mustian K. M., et al. (2007). Self-reported cognitive impairment in patients with cancer. J. Oncol. Pract. 3, 54–59. 10.1200/JOP.0722001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotti M., Duffell L. D., Faisal A. A., McGregor A. H. (2014). The complexity of human walking: A knee osteoarthritis study. PLoS One 9, e107325. 10.1371/journal.pone.0107325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X., Huang Y., Gu H. F., Zu X. Y., Zou W. Y., Song Z. B., et al. (2012). Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur. J. Pharmacol. 676, 51–56. 10.1016/j.ejphar.2011.11.037 [DOI] [PubMed] [Google Scholar]
- Kulkarni P., Harsulkar A., Märtson A. G., Suutre S., Märtson A., Koks S. (2022). Mast cells differentiated in synovial fluid and resident in osteophytes exalt the inflammatory pathology of osteoarthritis. Int. J. Mol. Sci. 23, 541. 10.3390/ijms23010541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni P., Martson A., Vidya R., Chitnavis S., Harsulkar A. (2021). Pathophysiological landscape of osteoarthritis. Adv. Clin. Chem. 100, 37–90. 10.1016/bs.acc.2020.04.002 [DOI] [PubMed] [Google Scholar]
- Kumar V., Reddy B. M. (2003). Status of Austro-Asiatic groups in the peopling of India: An exploratory study based on the available prehistoric, linguistic and biological evidences. J. Biosci. 28, 507–522. 10.1007/BF02705125 [DOI] [PubMed] [Google Scholar]
- Kuramoto H., Hirao K., Yumoto H., Hosokawa Y., Nakanishi T., Takegawa D., et al. (2019). Caffeic acid phenethyl ester (CAPE) induces VEGF expression and production in rat odontoblastic cells. Biomed. Res. Int. 2019, 5390720. 10.1155/2019/5390720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M. (2005). Fat cells: Afferent and efferent messages define new approaches to treat obesity. Annu. Rev. Pharmacol. Toxicol. 45, 119–146. 10.1146/annurev.pharmtox.45.120403.095843 [DOI] [PubMed] [Google Scholar]
- LaMarca B., Cornelius D., Wallace K. (2013). Elucidating immune mechanisms causing hypertension during pregnancy. Physiology 28, 225–233. 10.1152/physiol.00006.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latruffe N., Lançon A., Frazzi R., Aires V., Delmas D., Michaille J. J., et al. (2015). Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation. Ann. N. Y. Acad. Sci. 1348, 97–106. 10.1111/nyas.12819 [DOI] [PubMed] [Google Scholar]
- Lee J. K., Lee S., Shin T., Khang D., Kim S., Heo E. Y. (2018). Effect of perioperative systemic steroid treatment on patients with obstructive lung disease undergoing elective abdominal surgery. J. Food Nutr. Res. 6, 227–233. 10.1111/crj.12520 [DOI] [PubMed] [Google Scholar]
- Lee S. R., Kwon S. W., Lee Y. H., Kaya P., Kim J. M., Ahn C., et al. (2019). Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK- mediated apoptosis and anti-inflammation. BMC Cancer 19, 6–12. 10.1186/s12885-018-5222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepercq J., Guerre-Millo M., André J., Caüzac M., Hauguel-de Mouzon S. (2003). Leptin: A potential marker of placental insufficiency. Gynecol. Obstet. Invest. 55, 151–155. 10.1159/000071529 [DOI] [PubMed] [Google Scholar]
- Leroi-Gourhan A. (1975). The Flowers found with shanidar IV, a neanderthal burial in Iraq. Science 190, 562–564. 10.1126/science.190.4214.562 [DOI] [Google Scholar]
- Leung Y. Y., Yao Hui L. L., Kraus V. B. (2015). Colchicine--Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 45, 341–350. 10.1016/j.semarthrit.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zug C., Qu H., Schluesener H., Zhang Z. (2015a). Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 281, 32–42. 10.1016/j.bbr.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Li L., Wu X. H., Zhao X. J., Xu L., Pan C. L., Zhang Z. Y. (2020). Zerumbone ameliorates behavioral impairments and neuropathology in transgenic APP/PS1 mice by suppressing MAPK signaling. J. Neuroinflammation 17, 61. 10.1186/s12974-020-01744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Lu C., Zhang L., Zhang J., Du Y., Duan S., et al. (2015b). Oral administration of escin inhibits acute inflammation and reduces intestinal mucosal injury in animal models. Evidence-Based Complement. Altern. Med. 2015, 503617. 10.1155/2015/503617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu T. H., Yu J. Z., Li C. X., Liu Y., Wu Y. Y., et al. (2019). Curcumin and curcumol inhibit NF-κB and TGF-β 1/Smads signaling pathways in CSE-treated RAW246.7 cells. Evidence-Based Complement. Altern. Med. 2019, 3035125. 10.1155/2019/3035125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Gao X., Wu X., Wu Z., Cheng L., Zhu L., et al. (2015c). Parthenolide inhibits LPS-induced inflammatory cytokines through the toll-like receptor 4 signal pathway in THP-1 cells. Acta Biochim. Biophys. Sin. 47, 368–375. 10.1093/abbs/gmv019 [DOI] [PubMed] [Google Scholar]
- Liao P. C., Lai M. H., Hsu K. P., Kuo Y. H., Chen J., Tsai M. C., et al. (2018). Identification of beta-sitosterol as in vitro anti-inflammatory constituent in Moringa oleifera . J. Agric. Food Chem. 66, 10748–10759. 10.1021/acs.jafc.8b04555 [DOI] [PubMed] [Google Scholar]
- Linghu K., Wu G., Fu L., Yang H., Li H., Chen Y., et al. (2019). 1,8-Cineole ameliorates LPS-induced vascular endothelium dysfunction in mice via PPAR-γ dependent regulation of NF-κB. Front. Pharmacol. 10, 178. 10.3389/fphar.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Piao X., Guo L., Liu S., Chai F., Gao L. (2016a). Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 13, 4779–4785. 10.3892/mmr.2016.5094 [DOI] [PubMed] [Google Scholar]
- Liu G. T., Li Y., Wei H. L., Zhang H., Xu J. Y., Yu L. H. (2005). Mechanism of protective action of bicyclol against CCl-induced liver injury in mice. Liver Int. Off. J. Int. Assoc. Study Liver 25, 872–879. 10.1111/j.1478-3231.2005.01103.x [DOI] [PubMed] [Google Scholar]
- Liu M., Liao K., Yu C., Li X., Liu S., Yang S. (2014a). Puerarin alleviates neuropathic pain by inhibiting neuroinflammation in spinal cord. Mediat. Inflamm. 2014, 485927. 10.1155/2014/485927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Hao D., Xu W., Li J., Li X., Shen D., et al. (2019a). β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 57, 161–168. 10.1080/13880209.2019.1577461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Fu X., Lan N., Li S., Zhang J., Wang S., et al. (2014b). Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav. Brain Res. 267, 178–188. 10.1016/j.bbr.2014.02.040 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Pang J., Zhang H., Luo J., Qian X., et al. (2019b). Attenuation of atherosclerosis by protocatechuic acid via inhibition of M1 and promotion of M2 macrophage polarization. J. Agric. Food Chem. 67, 807–818. 10.1021/acs.jafc.8b05719 [DOI] [PubMed] [Google Scholar]
- Liu Z. J., Li Z. H., Liu L., Tang W. X., Wang Y., Dong M. R., et al. (2016b). Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease. Front. Pharmacol. 7, 261. 10.3389/fphar.2016.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. (2012). Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707. 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Wu L., Liu L., Ruan Q., Zhang X., Hong W., et al. (2018). Quercetin ameliorates kidney injury and fi brosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 154, 203–212. 10.1016/j.bcp.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Luo D., Xu J., Chen X., Zhu X., Liu S., Li J., et al. (2020). (−)-Epigallocatechin-3-gallate (EGCG) attenuates salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Sci. Rep. 10, 4783. 10.1038/s41598-020-61794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabadi A. A., Massaro J. M., Rosito G. A., Levy D., Murabito J. M., Wolf P. A., et al. (2009). Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The framingham heart study. Eur. Heart J. 30, 850–856. 10.1093/eurheartj/ehn573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M. F., Gamal S., El-Fayoumi H. M. (2019). Baicalein preconditioning modulates hepatocellular injury following liver ischemia and reperfusion in rats via anti-inflammatory and antioxidant signaling. Dubai Med. J. 2, 73–80. 10.1159/000501449 [DOI] [Google Scholar]
- Manchope M. F., Calixto-campos C., Coelho-silva L., Zarpelon A. C., Pinho-ribeiro F. A., Georgetti S. R., et al. (2016). Naringenin inhibits superoxide anion-induced inflammatory pain: Role of oxidative stress, cytokines, Nrf-2 and the NO − cGMP − PKG − K ATP channel Signaling Pathway. PLoS One 11, e0153015–e0153020. 10.1371/journal.pone.0153015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorana F., Guidotti G., Brambilla L., Rossi D. (2015). Withaferin a inhibits nuclear factor-κB-dependent pro-inflammatory and stress response pathways in the astrocytes. Neural Plast. 2015, 381964. 10.1155/2015/381964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskrey B. H., Megson I. L., Whitfield P. D., Rossi A. G. (2011). Mechanisms of resolution of inflammation: A focus on cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 31, 1001–1006. 10.1161/ATVBAHA.110.213850 [DOI] [PubMed] [Google Scholar]
- Matsuda M., Shimomura I., Sata M., Arita Y., Nishida M., Maeda N., et al. (2002). Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J. Biol. Chem. 277, 37487–37491. 10.1074/jbc.M206083200 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. (2006). The metabolic syndrome and adipocytokines. FEBS Lett. 580, 2917–2921. 10.1016/j.febslet.2006.04.028 [DOI] [PubMed] [Google Scholar]
- Maurya A. K., Vinayak M. (2017). Quercetin attenuates cell survival, inflammation, and angiogenesis via modulation of AKT signaling in murine T-cell lymphoma. Nutr. Cancer 69, 470–480. 10.1080/01635581.2017.1267775 [DOI] [PubMed] [Google Scholar]
- McNelis J. C., Olefsky J. M. (2014). Macrophages, immunity, and metabolic disease. Immunity 41, 36–48. 10.1016/j.immuni.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Meyer U. (2011). Anti-inflammatory signaling in schizophrenia. Brain. Behav. Immun. 25, 1507–1518. 10.1016/j.bbi.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Yee B. K. (2009). A Review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr. Bull. 35, 959–972. 10.1093/schbul/sbn022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2007). Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 70, 461–477. 10.1021/np068054v [DOI] [PubMed] [Google Scholar]
- Nguyen Ngo Le M. A., Wen Y. T., Ho Y. C., Kapupara K., Tsai R. K. (2019). Therapeutic effects of puerarin against anterior ischemic optic neuropathy through antiapoptotic and anti-inflammatory actions. Invest. Ophthalmol. Vis. Sci. 60, 3481–3491. 10.1167/iovs.19-27129 [DOI] [PubMed] [Google Scholar]
- Nicholas C., Batra S., Vargo M. A., Voss O. H., Gavrilin M. A., Wewers M. D., et al. (2007). Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 179, 7121–7127. 10.4049/jimmunol.179.10.7121 [DOI] [PubMed] [Google Scholar]
- Nikolajczyk B. S., Jagannathan-Bogdan M., Shin H., Gyurko R. (2011). State of the union between metabolism and the immune system in type 2 diabetes. Genes. Immun. 12, 239–250. 10.1038/gene.2011.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbourne S. M., Suhrbier A., Jones B., Cozzi S. J., Boyle G. M., Morris M., et al. (2004). Antitumor activity of 3-ingenyl angelate: Plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 64, 2833–2839. 10.1158/0008-5472.can-03-2837 [DOI] [PubMed] [Google Scholar]
- Ormazabal P., Scazzocchio B., Varì R., Santangelo C., D’Archivio M., Silecchia G., et al. (2018). Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 42, 2012–2021. 10.1038/s41366-018-0075-4 [DOI] [PubMed] [Google Scholar]
- Pahwa R., Goyal A., Bansal P., Jialal I. (2020). Chronic inflammation. Treasure island (FL). Florida, USA: StatPearls Publishing. [PubMed] [Google Scholar]
- Pan S. Y., Litscher G., Gao S.-H., Zhou S. F., Yu Z. L., Chen H. Q., et al. (2014). Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evidence-Based Complement. Altern. Med. 2014, 525340. 10.1155/2014/525340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahi Y., Hosseini M. S., Khalili N., Naimi E., Simental-Mendía L. E., Majeed M., et al. (2016). Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 82, 578–582. 10.1016/j.biopha.2016.05.037 [DOI] [PubMed] [Google Scholar]
- Pardo C. A., Vargas D. L., Zimmerman A. W. (2005). Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 17, 485–495. 10.1080/02646830500381930 [DOI] [PubMed] [Google Scholar]
- Park S. H., Gong J. H., Choi Y. J., Kang M. K., Kim Y. H., Kang Y. H. (2015). Kaempferol inhibits endoplasmic reticulum stress-associated mucus hypersecretion in airway epithelial cells and ovalbumin-sensitized mice. PLoS One 10, e0143526. 10.1371/journal.pone.0143526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker V. J., Solano M. E., Arck P. C., Douglas A. J. (2014). Diet-induced obesity may affect the uterine immune environment in early-mid pregnancy, reducing NK-cell activity and potentially compromising uterine vascularization. Int. J. Obes. 38, 766–774. 10.1038/ijo.2013.164 [DOI] [PubMed] [Google Scholar]
- Patterson P. H. (2009). Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav. Brain Res. 204, 313–321. 10.1016/j.bbr.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Pawar P., Gilda S., Sharma S., Jagtap S., Paradkar A., Mahadik K., et al. (2011). Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and muco-restorative activity in TNBS-induced inflammatory bowel disease. BMC Complement. Altern. Med. 11, 34. 10.1186/1472-6882-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Xie Z., Pei J. I. E., Wang B., Gao Y. I., Qu Y. (2019). Puerarin alters the function of monocytes/macrophages and exhibits chondroprotection in mice. Mol. Med. Rep. 8, 2876–2882. 10.3892/mmr.2019.9936 [DOI] [PubMed] [Google Scholar]
- Pennington K. A., Schlitt J. M., Jackson D. L., Schulz L. C., Schust D. J. (2012). Preeclampsia: Multiple approaches for a multifactorial disease. Dis. Model. Mech. 18, 9–18. 10.1242/dmm.008516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S. R., Almeida L. M., Dinis T. C. P. (2018). Cyanidin-3-glucoside potentiates the anti-inflammatory and antioxidant activity of 5-aminosalicylic acid, in an in vitro model of inflammation. Free Radic. Biol. Med. 120, S124–S125. 10.1016/j.freeradbiomed.2018.04.410 [DOI] [Google Scholar]
- Pinho-Ribeiro F. A., Zarpelon A. C., Fattori V., Manchope M. F., Mizokami S. S., Casagrande R., et al. (2016). Naringenin reduces inflammatory pain in mice. Neuropharmacology 105, 508–519. 10.1016/j.neuropharm.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Platero J. L., Cuerda-Ballester M., Ibanez V., Sancho D., Lopez-Rodriguez M. M., Drehmer E., et al. (2020). The impact of coconut oil and epigallocatechin gallate on the levels of IL-6, anxiety and disability in multiple sclerosis patients. Nutrients 12, 305. 10.3390/nu12020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak A., Ferenczi S., Denes A., Winkler Z., Kriszt R., Pinter-Kubler B., et al. (2014). The fractalkine/Cx3CR1 system is implicated in the development of metabolic visceral adipose tissue inflammation in obesity. Brain. Behav. Immun. 38, 25–35. 10.1016/j.bbi.2014.01.010 [DOI] [PubMed] [Google Scholar]
- Pons D. G., Vilanova-Llompart J., Gaya-Bover A., Alorda-Clara M., Oliver J., Roca P., et al. (2019). The phytoestrogen genistein affects inflammatory-related genes expression depending on the ERα/ERβ ratio in breast cancer cells. Int. J. Food Sci. Nutr. 70, 941–949. 10.1080/09637486.2019.1597025 [DOI] [PubMed] [Google Scholar]
- Promsong A., Chung W. O., Satthakarn S., Nittayananta W. (2015). Ellagic acid modulates the expression of oral innate immune mediators: Potential role in mucosal protection. J. Oral Pathol. Med. 44, 214–221. 10.1111/jop.12223 [DOI] [PubMed] [Google Scholar]
- Prucksunand C., Indrasukhsri B., Leethochawalit M., Hungspreugs K. (2001). Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J. Trop. Med. Public Health 32, 208–215. [PubMed] [Google Scholar]
- Quail D. F., Dannenberg A. J. (2019). The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154. 10.1038/s41574-018-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S. N., Zahra W., Singh S., Birla H., Keswani C., Dilnashin H., et al. (2019). Anti-inflammatory activity of ursolic acid in MPTP-induced Parkinsonian mouse model. Neurotox. Res. 36, 452–462. 10.1007/s12640-019-00038-6 [DOI] [PubMed] [Google Scholar]
- Rainsford K. D. (2007). Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 42, 3–27. 10.1007/1-4020-5688-5_1 [DOI] [PubMed] [Google Scholar]
- Rathnavelu V., Alitheen N. B., Sohila S., Kanagesan S., Ramesh R. (2016). Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 5, 283–288. 10.3892/br.2016.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K., Ehrhart J., Bai Y., Sanberg P. R., Bickford P., Tan J., et al. (2008). Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflammation 5, 41. 10.1186/1742-2094-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M. (2014). Pathophysiology of ischemic placental disease. Semin. Perinatol. 38, 139–145. 10.1053/j.semperi.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. A., Riley S. C., Reynolds R. M., Barr S., Evans M., Statham A., et al. (2011). Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–254. 10.1016/j.placenta.2010.12.023 [DOI] [PubMed] [Google Scholar]
- Rodrigues F. A. de P., Santos A. D. da C., de Medeiros P. H. Q. S., Prata M. de M. G., Santos T. C. de S., da Silva J. A., et al. (2018). Gingerol suppresses sepsis-induced acute kidney injury by modulating methylsulfonylmethane and dimethylamine production. Sci. Rep. 8, 12154. 10.1038/s41598-018-30522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues Silva D., Baroni S., Svidzinski A. E., Bersani-Amado C. A., Cortez D. A. G. (2008). Anti-inflammatory activity of the extract, fractions and amides from the leaves of Piper ovatum Vahl (Piperaceae). J. Ethnopharmacol. 116, 569–573. 10.1016/j.jep.2007.12.018 [DOI] [PubMed] [Google Scholar]
- Rudolph M. D., Graham A. M., Feczko E., Miranda-dominguez O., Rasmussen J. M., Nardos R., et al. (2018). Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosience 21, 765–772. 10.1038/s41593-018-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles J., de Souza B. R., Costa A. M. A. (2019). Caffeic acid phenethyl ester improves diabetic mice cutaneous wound healing. FASEB J. 33, 812. 10.1096/fasebj.2019.33.1_supplement.812.3 [DOI] [Google Scholar]
- Samad F., Yamamoto K., Pandey M., Loskutoff D. J. (1997). Elevated expression of transforming growth factor-β in adipose tissue from obese mice. Mol. Med. 3, 37–48. 10.1007/BF03401666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P., Bromberg J. (2012). Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 30, 1005–1014. 10.1200/JCO.2010.31.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid H., Shahzad M., Shabbir A., Saghir G. (2019). Immunomodulatory and anti-inflammatory potential of curcumin for the treatment of allergic asthma: Effects on expression levels of pro-inflammatory cytokines and aquaporins. Inflammation 42, 2037–2047. 10.1007/s10753-019-01066-2 [DOI] [PubMed] [Google Scholar]
- Shan L., Kang X., Liu F. E. N., Cai X., Han X., Shang Y. (2018). Epigallocatechin gallate improves airway inflammation through TGF-β1 signaling pathway in asthmatic mice. Mol. Med. Rep. 18, 2088–2096. 10.3892/mmr.2018.9183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., Herrero L., Naaz A. (2007). Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180. 10.1053/j.gastro.2007.03.059 [DOI] [PubMed] [Google Scholar]
- Shoelson S. E., Lee J., Goldfine A. B. (2006). Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801. 10.1172/JCI29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Johnson J., Romero-Corral A., Lopez-Jimenez F., Gami A. S., Sert Kuniyoshi F. H., Wolk R., et al. (2007). Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am. J. Cardiol. 100, 234–239. 10.1016/j.amjcard.2007.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ge W., Cai H., Zhang H. (2013). Curcumin protects mice from coxsackievirus B3-induced myocarditis by inhibiting the phosphatidylinositol 3 kinase/Akt/nuclear factor-κB pathway. J. Cardiovasc. Pharmacol. Ther. 18, 560–569. 10.1177/1074248413503044 [DOI] [PubMed] [Google Scholar]
- Stähli A., Maheen C. U., Strauss F. J., Eick S., Sculean A., Gruber R. (2019). Caffeic acid phenethyl ester protects against oxidative stress and dampens inflammation via heme oxygenase 1. Int. J. Oral Sci. 11, 6. 10.1038/s41368-018-0039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. (2006). Thematic review series: The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: The discovery of the statins and the end of the controversy. J. Lipid Res. 47, 1339–1351. 10.1194/jlr.R600009-JLR200 [DOI] [PubMed] [Google Scholar]
- Stepien M., Stepien A., Wlazel R. N., Paradowski M., Banach M., Rysz J. (2014). Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: A comparative pilot study. Lipids Health Dis. 13, 29. 10.1186/1476-511X-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg Z., Chadha K., Lieberman A., Drake A., Hojnacki D., Weinstock-Guttman B., et al. (2009). Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: Additive effects of IFN-beta. J. Neuroinflammation 6, 28. 10.1186/1742-2094-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanović I., Šavikin K., Đedović N., Živković J., Saksida T., Momčilović M., et al. (2017). Pomegranate peel extract ameliorates autoimmunity in animal models of multiple sclerosis and type 1 diabetes. J. Funct. Foods 35, 522–530. 10.1016/j.jff.2017.06.021 [DOI] [Google Scholar]
- Sun W., Liu X., Zhang H., Song Y., Li T., Liu X., et al. (2017). Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 108, 840–857. 10.1016/j.freeradbiomed.2017.04.365 [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Shah M. R., Pearce W. H., Koch A. E. (1994). Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: The possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions 42, 159–162. 10.1007/bf01983484 [DOI] [PubMed] [Google Scholar]
- Takakura K., Takatou S., Tomiyama R., Le T. M., Nguyen D. T., Nakamura Y., et al. (2018). Inhibition of nuclear factor-κB p65 phosphorylation by 3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester. J. Pharmacol. Sci. 137, 248–255. 10.1016/j.jphs.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Taliou A., Zintzaras E., Lykouras L., Francis K. (2013). An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 35, 592–602. 10.1016/j.clinthera.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Tarver T. (2014). “The review of natural products,” in Journal of consumer health on the internet. Editors Beutler J. A. (England, UK: Routledge; ), 291–292. 10.1080/15398285.2014.932189 [DOI] [Google Scholar]
- Trayhurn P., Wood I. S. (2004). Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 92, 347–355. 10.1079/bjn20041213 [DOI] [PubMed] [Google Scholar]
- Tsai Y. F., Chen Y. R., Chen J. P., Tang Y., Yang K. C. (2019). Effect of hesperidin on anti-inflammation and cellular antioxidant capacity in hydrogen peroxide-stimulated human articular chondrocytes. Process Biochem. 85, 175–184. 10.1016/j.procbio.2019.07.014 [DOI] [Google Scholar]
- Ueland T., Yndestad A., Dahl C. P., Gullestad L., Aukrust P. (2012). TNF revisited: Osteoprotegerin and TNF-related molecules in heart failure. Curr. Heart Fail. Rep. 9, 92–100. 10.1007/s11897-012-0088-6 [DOI] [PubMed] [Google Scholar]
- Valerio M., Awad A. B. (2011). β-Sitosterol down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in J774A.1 murine macrophages. Int. Immunopharmacol. 11, 1012–1017. 10.1016/j.intimp.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Vaquero E., Gukovsky I., Zaninovic V., Gukovskaya A. S., Pandol S. J. (2001). Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G1197–G1208. 10.1152/ajpgi.2001.280.6.G1197 [DOI] [PubMed] [Google Scholar]
- Visnagri A., Kandhare A. D., Chakravarty S., Ghosh P., Bodhankar S. L. (2014). Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 52, 814–828. 10.3109/13880209.2013.870584 [DOI] [PubMed] [Google Scholar]
- Wallace F. A., Miles E. A., Evans C., Stock T. E., Yaqoob P., Calder P. C. (2001). Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J. Leukoc. Biol. 69, 449–457. 10.1189/jlb.69.3.449 [DOI] [PubMed] [Google Scholar]
- Wang J., Fang X., Ge L., Cao F., Zhao L., Wang Z., et al. (2018). Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One 13, e0197563. 10.1371/journal.pone.0197563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhao S., Wang Y., Yang Y., Yao L., Chu L., et al. (2014). Protective effects of escin against indomethacin-induced gastric ulcer in mice. Toxicol. Mech. Methods 24, 560–566. 10.3109/15376516.2014.951815 [DOI] [PubMed] [Google Scholar]
- Ward P. A. (2010). “Acute and chronic inflammation,” in Fundamentals of inflammation, eds. Serhan C. N., Gilroy D. W., Ward P. A. (Cambridge: Cambridge University Press; ), 1–16. 10.1017/CBO9781139195737.002 [DOI] [Google Scholar]
- Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W. J. (2003). Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808. 10.1172/JCI19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Lees F., Tsangari H., Hutchinson M. R., Perilli E., Crotti T. N. (2020). Assessing the effects of parthenolide on inflammation, bone loss, and glial cells within a collagen antibody-induced arthritis mouse model. Mediat. Inflamm. 2020, 6245798. 10.1155/2020/6245798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015). Obesity and overweight . http://who.int/mediacentre/factsheets/fs311/en/ (Accessed October 10, 2022). [Google Scholar]
- Wu S., Yano S., Chen J., Hisanaga A., Sakao K., He X., et al. (2017). Polyphenols from Lonicera caerulea L. berry inhibit LPS-Induced Inflammation through dual modulation of inflammatory and antioxidant mediators. J. Agric. Food Chem. 65, 5133–5141. 10.1021/acs.jafc.7b01599 [DOI] [PubMed] [Google Scholar]
- Wu X. X., Huang X. L., Chen R. R., Li T., Ye H. J., Xie W., et al. (2019). Paeoniflorin prevents intestinal barrier disruption and inhibits lipopolysaccharide (LPS)-induced inflammation in caco-2 cell monolayers. Inflammation 42, 2215–2225. 10.1007/s10753-019-01085-z [DOI] [PubMed] [Google Scholar]
- Xiao S., Liu W., Bi J., Liu S., Zhao H., Gong N., et al. (2018). Anti-inflammatory effect of hesperidin enhances chondrogenesis of human mesenchymal stem cells for cartilage tissue repair. J. Inflamm. 15, 14. 10.1186/s12950-018-0190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Wang K., Yuan C., Xing R., Ni J., Hu G., et al. (2017). Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 39, 113–125. 10.3892/ijmm.2016.2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z. Y., Xiao F. M., Xu X., Wu Y. F., Jiang X. M. (2013). Studies on pharmacological activity of borneol. Zhongguo Zhong Yao Za Zhi 38, 786–790. 10.4268/cjcmm20130602 [DOI] [PubMed] [Google Scholar]
- Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830. 10.1172/JCI19451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Liu J., Yue G., Sun M., Li J., Xiu X. I. A., et al. (2018a). Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol. Med. Rep. 18, 1149–1154. 10.3892/mmr.2018.9054 [DOI] [PubMed] [Google Scholar]
- Xu T., Qin G., Jiang W., Zhao Y., Xu Y., Lv X. (2018b). 6-Gingerol Protects Heart by suppressing myocardial ischemia/reperfusion induced inflammation via the PI3K/Akt-dependent mechanism in rats. Evidence-Based Complement. Altern. Med. 2018, 6209679. 10.1155/2018/6209679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q., Liu Y., He R., Yang S., Tong J., Li X., et al. (2016). Lyophilized powder of catalpol and puerarin protects neurovascular unit from stroke. Int. J. Biol. Sci. 12, 367–380. 10.7150/ijbs.14059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav N., Chandra H. (2017). Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS One 12, e0188232. 10.1371/journal.pone.0188232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Sun X., Xu C. (2018). Protective effects of resveratrol improve cardiovascular function in rats with diabetes. Exp. Ther. Med. 15, 1728–1734. 10.3892/etm.2017.5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Huang G., Liu Q., Zheng J., Chen H., Huang Q., et al. (2017). Withaferin A protects against spinal cord injury by inhibiting apoptosis and inflammation in mice. Pharm. Biol. 55, 1171–1176. 10.1080/13880209.2017.1288262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez M., Jhanji M., Murphy K., Gower R. M., Sajish M., Jabbarzadeh E. (2019). Nicotinamide augments the anti-inflammatory properties of resveratrol through PARP1 activation. Sci. Rep. 9, 10219. 10.1038/s41598-019-46678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatoo M. I., Gopalakrishnan A., Saxena A., Parray O. R., Tufani N. A., Chakraborty S., et al. (2018). Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders - a review. Recent Pat. Inflamm. Allergy Drug Discov. 12, 39–58. 10.2174/1872213x12666180115153635 [DOI] [PubMed] [Google Scholar]
- Ye S., Liu H., Chen Y., Qiu F., Liang C., Zhang Q., et al. (2019). A novel immunosuppressant, luteolin, modulates alloimmunity and suppresses murine allograft rejection. J. Immunol. 203, 3436–3446. 10.4049/jimmunol.1900612 [DOI] [PubMed] [Google Scholar]
- Yu F., Xu N., Zhou Y., Li B., Li M., Wang Q., et al. (2019). Anti-inflammatory effect of paeoniflorin combined with baicalin in oral inflammatory diseases. Oral Dis. 25, 1945–1953. 10.1111/odi.13171 [DOI] [PubMed] [Google Scholar]
- Yu J., Xiao Z., Zhao R., Lu C., Zhang Y. (2017). Paeoniflorin suppressed IL-22 via p38 MAPK pathway and exerts anti-psoriatic effect. Life Sci. 180, 17–22. 10.1016/j.lfs.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Yu Y., Shen Q., Lai Y., Park S. Y., Ou X., Lin D., et al. (2018). Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 9, 386. 10.3389/fphar.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Ding W., Wu N., Jiang S., Li W. (2019). Protective effect of genistein on condylar cartilage through downregulating NF-κB expression in experimentally created osteoarthritis rats. Biomed. Res. Int. 2019, 2629791. 10.1155/2019/2629791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf E., Nelissen R. G., Ioan-Facsinay A., Stojanovic-Susulic V., DeGroot J., van Osch G., et al. (2010). Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann. Rheum. Dis. 69, 761–765. 10.1136/ard.2008.106930 [DOI] [PubMed] [Google Scholar]
- Zerin T., Lee M., Jang W. S., Nam K. W., Song H. Y. (2016). Anti-inflammatory potential of ursolic acid in Mycobacterium tuberculosis -sensitized and Concanavalin A-stimulated cells. Mol. Med. Rep. 13, 2736–2744. 10.3892/mmr.2016.4840 [DOI] [PubMed] [Google Scholar]
- Zhan Y., Chen Y., Liu R., Zhang H., Zhang Y. (2014). Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch. Pharm. Res. 37, 1086–1095. 10.1007/s12272-013-0311-3 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang C. (2012). Vasoprotection by dietary supplements and exercise: Role of TNFα signaling. Exp. Diabetes Res. 2012, 972679. 10.1155/2012/972679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Han D., Li Z., Shen C., Zhang Y., Li J., et al. (2018a). Ginkgolide C alleviates myocardial ischemia/reperfusion-induced inflammatory injury via inhibition of CD40-NF-κB pathway. Front. Pharmacol. 9, 109–115. 10.3389/fphar.2018.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu Q., Wang J., Harnish D. C. (2009). Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem. Biophys. Res. Commun. 379, 476–479. 10.1016/j.bbrc.2008.12.117 [DOI] [PubMed] [Google Scholar]
- Zhang Y. S., Wang F., Cui S. X., Qu X. J. (2018b). Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol. Ther. 19, 735–744. 10.1080/15384047.2018.1453971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. M., Gao Z. W., Xie S. X., Han X., Sun Q. S. (2019). Withaferin A attenuates ovalbumin induced airway inflammation. Front. Biosci. 24, 576–596. 10.2741/4737 [DOI] [PubMed] [Google Scholar]
- Zhao S. Q., Xu S. Q., Cheng J., Cao X. L., Zhang Y., Zhou W. P., et al. (2018). Anti-inflammatory effect of external use of escin on cutaneous inflammation: Possible involvement of glucocorticoids receptor. Chin. J. Nat. Med. 16, 105–112. 10.1016/S1875-5364(18)30036-0 [DOI] [PubMed] [Google Scholar]
- Zhou B., Li Q., Wang J., Chen P., Jiang S. (2019a). Ellagic acid attenuates streptozocin induced diabetic nephropathy via the regulation of oxidative stress and inflammatory signaling. Food Chem. Toxicol. 123, 16–27. 10.1016/j.fct.2018.10.036 [DOI] [PubMed] [Google Scholar]
- Zhou D., Zhang S., Hu L., Gu Y.-F., Cai Y., Wu D., et al. (2019b). Inhibition of apoptosis signal-regulating kinase by paeoniflorin attenuates neuroinflammation and ameliorates neuropathic pain. J. Neuroinflammation 16, 83. 10.1186/s12974-019-1476-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Bian C., Yuan J., Chu W., Xiang X., Chen F., et al. (2014). Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J. Neuroinflammation 11, 59. 10.1186/1742-2094-11-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Tang H., Zhang Z., Zhang Y., Qiu C., Zhang L., et al. (2017). Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int. Immunopharmacol. 43, 236–242. 10.1016/j.intimp.2016.12.020 [DOI] [PubMed] [Google Scholar]
- Zhu L., Gu P., Shen H. (2019). Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 67, 129–137. 10.1016/j.intimp.2018.11.049 [DOI] [PubMed] [Google Scholar]
- Zhu W., Jin Z., Yu J., Liang J., Yang Q., Li F., et al. (2016). Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 35, 119–126. 10.1016/j.intimp.2016.03.030 [DOI] [PubMed] [Google Scholar]
- Zorrilla E. P., Conti B. (2014). Interleukin-18 null mutation increases weight and food intake and reduces energy expenditure and lipid substrate utilization in high-fat diet fed mice. Brain. Behav. Immun. 37, 45–53. 10.1016/j.bbi.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


