Abstract
Plants program their meristem-associated developmental switches for timely adaptation to a changing environment. Potato (Solanum tuberosum L.) tubers differentiate from specialized belowground branches or stolons through radial expansion of their terminal ends. During this process, the stolon apex and closest axillary buds enter a dormancy state that leads to tuber eyes, which are reactivated the following spring and generate a clonally identical plant. The potato FLOWERING LOCUS T homolog SELF-PRUNING 6A (StSP6A) was previously identified as the major tuber-inducing signal that integrates day-length cues to control the storage switch. However, whether some other long-range signals also act as tuber organogenesis stimuli remains unknown. Here, we show that the florigen SELF PRUNING 3D (StSP3D) and FLOWERING LOCUS T-like 1 (StFTL1) genes are activated by short days, analogously to StSP6A. Overexpression of StSP3D or StFTL1 promotes tuber formation under non-inductive long days, and the tuber-inducing activity of these proteins is graft transmissible. Using the non-tuber-bearing wild species Solanum etuberosum, a natural SP6A null mutant, we show that leaf-expressed SP6A is dispensable for StSP3D long-range activity. StSP3D and StFTL1 mediate secondary activation of StSP6A in stolon tips, leading to amplification of this tuberigen signal. StSP3D and StFTL1 were observed to bind the same protein partners as StSP6A, suggesting that they can also form transcriptionally active complexes. Together, our findings show that additional mobile tuber-inducing signals are regulated by the photoperiodic pathway.
Key words: potato, Solanum tuberosum L.; FLOWERING LOCUS T; StSP3D; tuberization; tuber-inducing signal; long-distance signaling
Tuber induction shares common regulatory elements with flowering regulatory pathways. This work reports that StSP3D and StFTL1, like StSP6A, are mobile proteins that are transported from leaves to stolon tips for tuber induction, and SP3D can induce tuber formation even in the absence of leaf-expressed SeSP6A.
Introduction
Plant organogenesis shapes the morphology of adult plants and is central to vegetative propagation and flowering transition. It is finely controlled and exquisitely sensitive to environmental changes. Potato is a model system for belowground storage organ formation. Potato plants usually form tubers from specialized underground branches or stolons, which initiate from axillary buds at the base of the main stem. Shoot axillary meristems of potato have the potential to remain in a dormant state or to give rise directly to leafy shoots, stolons, and even sessile tubers. Upon inductive conditions such as short days, potato tubers develop from underground stolons by radial expansion of their terminal ends. Newly formed tubers are the economically important parts of the plant. Thus, factors that cause the tip of a stolon to change its developmental program and turn into the very specialized stem/tuber have been widely studied (Zierer et al., 2021).
Potato adjusts tuber formation to day-length conditions. Plants grown in short days (SDs) tuberize earlier than those kept in long-day (LD) conditions. However, in S. tuberosum ssp. andigena, short photoperiods are strictly required for tuber formation, making this species an attractive model for studying photoperiodic tuberization signals (Navarro et al., 2011; Kloosterman et al., 2013; Abelenda et al., 2016). Inductive SD conditions, specifically long nights, are perceived in the leaves, where a tuberization stimulus is produced and then transported to underground stolons to initiate tuber formation (Gregory, 1956; Kumar and Wareing, 1973; Chailakhyan et al., 1981). Elegant grafting studies have shown that flowering tobacco shoots induced tuberization when grafted onto potato stocks, indicating that the floral stimulus may act as a tuber-inducing signal (Chailakhyan et al., 1981). Currently, research in tomato, Arabidopsis, and rice has documented that the molecular nature of this florigenic signal corresponds to the FLOWERING LOCUS T (FT) protein (Weigel et al., 2000; Lifschitz et al., 2006; Corbesier et al., 2007; Tamaki et al., 2007), which belongs to the family of PHOSPHATIDYLETHANOLAMINE BINDING PROTEINS (PEBPs). In potato, overexpression of the FT-like gene StSP6A promotes tuber formation in non-inductive LDs, showing that StSP6A acts as a major component of the tuberigen (Navarro et al., 2011), and StBEL5 mRNA, miR156, and miRNA172 mobile molecules also function as tuber-promoting or tuber-inducing signals (Banerjee et al., 2006; Martin et al., 2009; Bhogale et al., 2014).
Accumulated evidence has shown that the expression of StSP6A is under the control of a conserved CDF/PHYTOCHROME–CONSTANS–FT flowering molecular module. Diurnal oscillation of the StCOL1 gene is partly regulated by the StCDF1–FKFI–GI complex (Kloosterman et al., 2013). Unlike in Arabidopsis, the light receptors PHYTOCHROME B (PHYB) and PHYTOCHROME F (PHYF) are essential for StCOL1 protein stabilization under LD conditions in potato (Abelenda et al., 2016; Zhou et al., 2019). Accumulation of StCOL1 activates the expression of another FT-like gene, SELF PRUNING 5G (StSP5G), which negatively regulates StSP6A expression in leaves to suppress tuber formation (Abelenda et al., 2016). In SDs, the StCOL1 protein is not stabilized, enabling the activation of StSP6A in leaves and its movement to stolons. There, StSP6A forms an autoregulatory loop to amplify the systemic signal and reprograms the expression of genes such as StGA2ox1, StMADS1 (AGL8), and StMADS13 (AGL8-like) (Navarro et al., 2011). Furthermore, the mRNA level of StSP6A is regulated by the small RNA suppressing expression of SP6A (SES) in leaves in response to high temperature (Lehretz et al., 2019; Park et al., 2022), by the StBEL5/POTH1 transcriptional complex in both leaves and stolons (Sharma et al., 2016), and by the interaction of StSP6A with the StTOC1 (TIMING OF CAB EXPRESSION 1) transcription factor in a feedback mechanism (Morris et al., 2019). Studies of StSP6A signaling mechanisms have revealed the functional conservation of storage organ and flowering transitions. StSP6A has been shown to form a tuberigen activation complex (TAC) comprised of StFDL1 and 14-3-3 proteins to promote tuberization (Teo et al., 2017). In addition, StSP6A binds and inactivates SWEET proteins to control source-sink balance (Abelenda et al., 2019). We have previously shown that StABI5 like 1 (StABL1) also interacts with StFT-like proteins to form alternative TACs (aTACs) that promote flowering and tuberization (Jing et al., 2022b). Conversely, TFL1/CEN competes with StSP6A in the TAC to antagonize its tuber-inducing activity (Zhang et al., 2020). Recently, it was reported that the tuber-inducing activity of StSP6A in aerial nodes is spatially blocked by a conserved FT–BRANCHED1b (StBRC1b) genetic module that helps to restrict tuberization belowground (Nicolas et al., 2022). To date, most studies have focused on StSP6A. However, it remains to be determined whether other long-range signals derived from leaves also function as tuber-inducing stimuli to trigger the tuber organogenesis developmental switch.
In potato, the transition to flowering is also dependent on mobile FT-like florigen proteins, and it has been established that SELF PRUNING 3D (StSP3D) is the major player (Navarro et al., 2011; Teo et al., 2017). The FT-like gene family has undergone preferential duplication and sub-functionalization in Solanaceae (Abelenda et al., 2014), and earlier evidence has shown that potato floral and tuber transitions are controlled by two different FT-like paralogs, florigen StSP3D/SFT and tuberigen StSP6A (Navarro et al., 2011; Teo et al., 2017), which respond to different environmental cues. Flowering transition in S. tuberosum can occur independently of photoperiod (Seibert et al., 2020), although LDs combined with high light irradiance accelerate flower development, whereas SDs or LDs and low irradiance significantly decrease flowering. Notably, genetic knockdown of PHYF, StCOL1, or StSP5G led to rapid flowering and tuber formation under LD conditions (Abelenda et al., 2016; Zhou et al., 2019), suggesting that these tuberization pathway repressors also repress flowering. However, further studies are needed to determine whether potato flowering and tuber formation are controlled by independent FT-like proteins.
Here, we provide evidence showing that expression of florigen StSP3D and FLOWERING LOCUS T-like 1 (StFTL1) is activated by SDs analogously to StSP6A. Overexpression of StSP3D or StFTL1 is shown to promote tuber formation in non-inductive LDs, and we observe that these proteins, along with their tuber-inducing effects, are graft transmissible. Leaf-expressed SP6A is dispensable for the promotion of tuberization by SP3D, whereas both FT-like StSP3D and StFTL1 proteins are involved in the regulation of StSP3D, StSP6A, StSP5G, and other tuberization-related genes in stolon tips, possibly via interaction with the same StSP6A-binding proteins. Thus, our study describes a previously unidentified tuber-inducing activity of the StSP3D and StFTL1 proteins, in addition to their activity as florigens, and provides new insights into potato photoperiodic tuberization.
Results
Identification and expression analysis of potato FT-like genes
Previously, Navarro et al. (2011) identified four FT-like genes in potato, StSP3D, StSP5G (StSP5G-A), StSP5G-like, and StSP6A. Using tomato FT-like amino acid sequences as protein BLAST queries of the potato genomics resource (Spud DB, http://spuddb.uga.edu/), we identified two additional FT-like family members in the potato genome (Figure 1A), StSP5G-B, which was named after StSP5G-A (Abelenda et al., 2016), and FLOWERING LOCUS T-Like 1 (StFTL1), which was named after its ortholog in tomato (Song et al., 2020). StSP5G-A and StSP5G-B are organized in tandem on chromosome 5, and StSP5G-like and StFTL1 are arranged in tandem on chromosome 11. The phylogeny of these genes grouped StSP3D, StSP6A, StSP5G-A, StSP5G-B, StFTL1, and StSP5G-like into three sub-clades. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) data showed that the expression of StSP3D, StSP6A, and StFTL1 was induced in leaves in response to SDs, although StSP3D activation was lower than that of StSP6A or StFTL1 (Figure 1B). Expression of StSP5G and StSP5G-like, on the other hand, gradually decreased under SDs. Because StSP5G-A and StSP5G-B share almost identical coding regions, we amplified and quantified both StSP5G-A and StSP5G-B transcripts by qRT-PCR. In stolons, StSP6A and StSP5G transcripts accumulated as the tubers initiated bulking, whereas StSP5G-like and StFTL1 exhibited the opposite expression pattern. StSP3D expression was also slightly induced upon tuber initiation. Previously, we showed that silencing of PHYF (PHYFi) promotes day-neutral tuberization by activating StSP6A (Zhou et al., 2019). Interestingly, the expression of StSP3D was also upregulated in leaves and swelling stolons of PHYFi lines (Supplemental Figure 1A and 1B), in contrast to StFTL1, which was downregulated in these plants. Collectively, these data showed that StSP3D and StFTL1 are SD activated similarly to StSP6A, suggesting a possible role of these proteins as long-distance tuber-inducing signals.
Figure 1.
Identification and expression analysis of potato FT-like genes.
(A) Phylogeny of the StFT-like genes and homologs from Solanum lycopersicum, Oryza sativa, Arabidopsis thaliana, and Nicotiana benthamiana. Potato FT-like genes are indicated by red circles. In the neighbor-joining tree (MEGA X, Poisson model), numbers above the branches indicate the percent support for nodes defining the families in distance bootstrap analyses (1000 replicates).
(B) Heatmap showing the normalized expression levels of StFT-like genes in E109 leaves and stolons. LD, long day. SD2, SD4, SD6, and SD8 indicate leaves sampled at 2, 4, 6, and 8 days after transfer to short-day conditions. S1–S5 indicate stolon tips at different developmental stages. Samples were collected at 8 days after transfer to short days. S1, stolon with apical hook; S2, stolon with straight apical hook; S3, tuber initiation stage; S4, tuber setting stage; S5, tuber bulking stage.
Graft-transmissible induction of potato tuberization by StSP3D
To test whether StSP3D could induce tuber formation, we generated stable transgenic plants expressing a CaMV 35S promoter-driven StSP3D overexpression construct in the SD potato E109 background (Supplemental Figure 2A and 2B). We found that StSP3D-overexpressing (StSP3Dox) plants started to tuberize under non-inductive LD conditions 1 month after planting, whereas no tubers were formed in control E109 plants (Figure 2A). After 10 weeks of cultivation under LDs, one to four mature tubers could be harvested from these transgenic plants, whereas no tubers were formed in control E109 plants (Figure 2B). Likewise, StSP3Dox plants tuberized under natural LD conditions (Supplemental Figure 3). These results suggest that StSP3D also exhibits tuber-inducing activity in potato. We observed that the stolon apical meristem of StSP3Dox plants initiated floral buds (Figure 2C), similar to the phenotype observed in StSP6A-overexpressing plants (Navarro et al., 2011; Lehretz et al., 2019), and that expression of StSP3D led to early flowering (Supplemental Figure 4), indicating the conserved florigenic activity of StSP3D. Moreover, unlike E109 tubers, which formed etiolated shoots from apical buds after tuber dormancy release, most sprouts of the StSP3Dox tubers generated secondary tubers directly (Figure 2D). Normal sprouts of StSP3Dox tubers formed new tubers directly when buried in soil (Figure 2E), unlike sprouts of E109, which developed as upward-growing shoots, suggesting that sprout meristem identity is reprogrammed in StSP3Dox tubers.
Figure 2.
Overexpression of StSP3D induces tuber formation under non-inducing LD conditions.
(A) Tuberization phenotype of StSP3D-overexpressing plants (StSP3Dox) under LD conditions. E109 plants do not tuberize. Thirty-five days after planting. NT, no tuber. Scale bars, 5 cm.
(B) Tuber number and tuber yield of StSP3Dox plants. Twelve weeks under long-day conditions. NT, no tubers. Data are shown as mean ± SD, n ≥ 11. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
(C) Flowering induction in StSP3Dox stolons under LD conditions. Scale bars, 100 μm.
(D) Phenotypes of E109 and StSP3Dox1 tubers after dormancy release. Most StSP3D-overexpressing tuber sprouts formed secondary tubers. Scale bars, 1 cm (upper panels), 5 cm (lower panels).
(E) Phenotypes of tuber-derived E109 and StSP3Dox plants at three weeks after planting of sprouting E109 and StSP3Dox transgenic tubers. Scale bar, 5 cm.
(F) qRT-PCR analysis of StSP5G, StSP6A, and StMADS1 gene expression in leaves of E109 and StSP3Dox plants grown under LD conditions (5 weeks). Light (70 μmol m−2 s−1) and dark conditions are indicated by white and gray backgrounds, respectively. Data are shown as mean ± SD of three independent biological replicates.
(G) Expression analysis of StSP5G, StSP6A, and StFTL1 in non-swelling stolons (NS) or swelling stolons (SS) of E109 and StSP3Dox plants after 35 days of growth under LD conditions. Data are shown as mean ± SD of three independent biological replicates. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
To examine the potential role of StSP3D in regulation of the remaining FT-like genes under LDs, the diurnal oscillation of StSP5G and StSP6A in leaves was analyzed in E109 and StSP3Dox plants. Expression of these genes exhibited no significant differences between E109 and StSP3Dox leaves, although StMADS1, which acts downstream of StSP6A (Navarro et al., 2011), was significantly upregulated in StSP3Dox plants (Figure 2F), suggesting that StSP3D may directly travel to stolons for tuber induction. Indeed, when we analyzed FT-like gene expression in stolons of E109 and StSP3Dox plants, we observed that StSP5G and StSP6A mRNA levels were significantly induced in swelling stolons of StSP3Dox plants (Figure 2G), which suggests that StSP3D may be involved in the secondary activation of StSP5G and StSP6A in swelling stolons.
To further investigate whether StSP3D is a graft-transmissible tuber-inducing signal, we used LD-grown StSP3Dox plants as scions or stocks in grafting studies with E109 plants (scion/stock, E109/StSP3Dox1 and StSP3Dox1/E109) to analyze the tuberization response of these graft combinations. Self-grafted combinations (E109/E109 and StSP3Dox1/StSP3Dox1) served as controls. When StSP3Dox1 plants were used as either the scion or stock, the grafted plants tuberized under LDs, whereas the E109/E109 combination produced almost no tubers (Figure 3A and 3B), suggesting that expression of StSP3D in aerial parts or stolons is sufficient for the stolon-to-tuber transition under LD conditions. We next analyzed the levels of StSP3D-HA transcript and StSP3D-HA protein in stolons and leaves of StSP3Dox1/E109 and E109/StSP3Dox1 heterografts. StSP3D-HA was detectable in stolons of StSP3Dox1/E109 plants but not in leaves of E109/StSP3Dox1 plants (Figure 3C). StSP3D-HA transcripts were not detected in E109 stocks (Figure 3D), showing that the StSP3D-HA protein travels from scion to stock. Long-distance control by StSP3D was also confirmed using LD-grown StSP3Dox plants as scions or stocks in grafting studies with S. etuberosum plants (StSP3Dox1/S. etuberosum and S. etuberosum/StSP3Dox1), followed by harvest of stems above the grafts and belowground stems/rhizomes for StSP3D-HA detection. These studies also showed that StSP3D is graft transmissible, although the StSP3Dox1/S. etuberosum heterografts did not form any tubers (Supplemental Figure 5), both here and in a previous study (Plantenga et al., 2018). Taken together, these results support the identification of StSP3D as a graft-transmissible tuber-inducing signal.
Figure 3.
Graft-transmissible induction of potato tuber formation by StSP3D.
(A) Tuber formation of grafted plants under long day (LD) conditions 12 weeks after grafting. Scale bars, 5 cm.
(B) Tuber number and tuber yield of grafts between StSP3Dox and E109 (wild-type) plants grown for 12 weeks under long-day conditions. Data are shown as mean ± SD, n ≥ 12. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
(C) StSP3D-HA protein was detected using an anti-HA antibody in leaves and stolons of the grafted plants, 1 month after grafting. Coomassie brilliant blue (CBB) staining was used as a loading control.
(D)StSP3D-HA transcript levels were analyzed by RT-PCR in leaves and stolons, 1 month after grafting. Actin was used as a control.
Graft-transmissible induction of potato tuber formation by StFTL1
The fact that the expression of StFTL1 is also activated under SD conditions prompted us to determine whether StFTL1 could also promote potato tuber formation. To this end, we created stable 35S promoter-driven StFTL1 overexpression (StFTL1ox) lines in the E109 background (Supplemental Figure 2C and 2D). Notably, StFTL1ox plants also tuberized under non-inductive LDs (Figures 4A and 4B). As observed for StSP3D overexpression, overexpression of StFTL1 did not direct the diurnal oscillation of StSP3D and StSP6A in leaves, and the expression of StMADS1 was strongly upregulated in leaves of StFTL1ox plants (Figure 4C). Expression of StSP5G and StSP6A was also significantly upregulated in swelling stolons (Figure 4D), suggesting that StFTL1 moves to the stolons for tuber induction. Additional grafting experiments were therefore performed to assess the mobility of this protein. The grafted plants tuberized under LD conditions independently of the use of StFTL1ox plants as scions or rootstocks (Figures 4E and 4F). We next detected the StFTL1-HA protein in stolons and leaves of StFTL1ox26/E109 and E109/StFTL1ox26 heterografts. StFTL1-HA was detectable in stolons of StFTL1ox26/E109 plants but not in leaves of E109/StFTL1ox26 plants (Figure 4G), indicating that StFTL1-HA can travel at least from scion to stock and can also function as a long-range tuberization inducer.
Figure 4.
Expression of StFTL1 promotes tuber formation under non-inducing LD conditions.
(A) LD tuberization phenotype of StFTL1-overexpressing plants (StFTL1ox). E109 plants do not tuberize. Forty days under long-day (LD) conditions. Scale bar, 5 cm.
(B) Tuber number and tuber yield of StFTL1ox plants. NT, no tubers. Ten weeks under long-day conditions. Data are shown as mean ± SD, n = 7 each. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
(C) Diurnal oscillations of StSP6A, StSP3D, and StMADS1 in E109 (wild-type) and StFTL1ox plants under LD conditions (4 weeks). Light (250 μmol m−2 s−1) and dark conditions are indicated by white and gray backgrounds, respectively. Data are shown as mean ± SD of three independent biological replicates.
(D) Expression analysis of StSP5G and StSP6A in non-swelling stolons (NS) or swelling stolons (SS) of E109 and StFTL1ox plants grown under LD conditions (5 weeks). Data are shown as mean ± SD, n = 8 each. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
(E) Tuber formation of grafts under LD conditions (12 weeks). Scale bars, 5 cm.
(F) Tuber number and tuber yield of grafts between StFTL1ox and E109 (wild-type) plants grown under LD conditions for 12 weeks. Data are shown as mean ± SD, n ≥ 12. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
(G) StFTL1-HA protein was detected using an anti-HA antibody in leaves and stolons, 1 month after grafting. Anti-actin was used as a loading control.
SP3D induces tuber formation independently of leaf-expressed SP6A
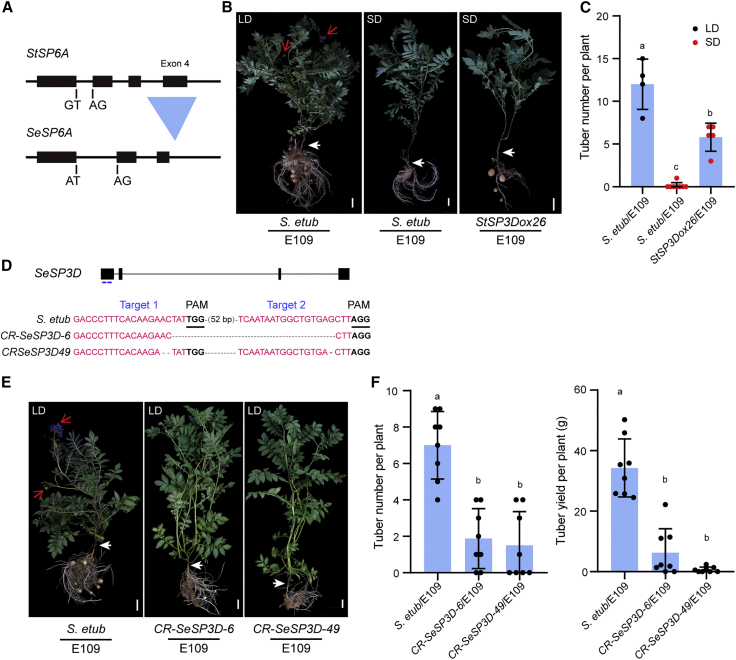
Our expression analyses showed that overexpression of StSP3D or StFTL1 induced tuber formation but did not stimulate StSP6A expression in leaves. To verify that tuber induction by SP3D was independent of leaf-expressed SP6A, we used the non-tuber-bearing wild species S. etuberosum for grafting experiments. We amplified the SeSP6A gene by homology-based cloning and found that it is naturally inactivated by a deletion of the whole fourth exon (Figure 5A and Supplemental Figure 6), leading to the loss of nearly half of the full-length protein sequence. Thus, S. etuberosum is a natural SP6A null mutant. Notably, S. etuberosum strictly requires LD and high light for flowering, and it is more closely related to potato than tobacco. As observed in earlier tobacco-grafting experiments, when LD-grown S. etuberosum scions were grafted onto potato E109 rootstocks, the grafted plants flowered and tuberized. However, none of the grafted plants transitioned to flowering or tuberization when S. etuberosum scions were grown under SD conditions (Figures 5B and 5C). We next created stable transgenic S. etuberosum lines that expressed a CaMV 35S promoter-driven StSP3D overexpression construct (Supplemental Figure 2E and 2F). The StSP3Dox S. etuberosum lines flowered much earlier than the wild type (Supplemental Figure 7A and 7B), but they did not produce any tubers, owing to a lack of tuberization competence (Supplemental Figure 8). When these StSP3Dox26 plants were used as donor scions onto E109, the rootstocks tuberized even under SD conditions (Figures 5B and 5C), indicating that SP3D induces tuber formation independently of leaf-expressed SP6A. In addition, the fact that S. etuberosum/E109 grafts tuberized under LD conditions but not under SD conditions indicates that an SP6A-independent mobile signal accounts for day length control of tuberization in these plants.
Figure 5.
SP3D induces tuber formation independently of leaf-expressed SP6A.
(A) Gene architecture of SP6A in potato and S. etuberosum. The “G” to “A” mutation in the GT-AG sequence in the first exon–intron junction of SeSP6A and the deletion of the whole fourth exon are shown.
(B) Tuberization phenotypes of E109 stocks grafted with StSP3D-overexpressing (StSP3Dox26) and wild-type S. etuberosum scions under LD and SD conditions (8 weeks after grafting). Red arrows indicate fruit or open flowers; white arrows indicate graft unions. Scale bars, 5 cm.
(C) Tuber numbers of 12-week-old E109 stocks grafted with StSP3D-overexpressing S. etuberosum scions (StSP3Dox26) shown in (B) under LD and SD conditions. Black dot, LD; red dot, SD. Data are shown as mean ± SD, n ≥ 4. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
(D) The first exon of SeSP3D was targeted by CRISPR/Cas9 using two single-guide RNAs (sgRNA). Blue lines, sgRNAs. Sequences of wild-type and CR-SeSP3D alleles (CR-SeSP3D-6 and CR-SeSP3D-49) are presented. sgRNA targets and protospacer-adjacent motif (PAM) sequences are indicated by blue and dark lines, respectively. Deletions are indicated by dark dashes.
(E) Tuberization phenotypes of E109 stocks grafted with CR-SeSP3D S. etuberosum scions (CR-SeSP3D-6 and CR-SeSP3D-49) under LD conditions (8 weeks). Red arrows indicate fruit or open flowers; white arrows indicate graft unions. Scale bars, 5 cm.
(F) Tuber number and tuber yield of grafts between CR-SeSP3D/S. etuberosum scions and E109 (wild-type) plants after 8 weeks of growth under LD conditions. Data are shown as mean ± SD, n = 8 each. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
We next tested whether endogenous SeSP3D was involved in tuber induction of S. etuberosum/E109 heterografts. To this end, we knocked out the SeSP3D gene using CRISPR/Cas9 technology. We generated several knockout homozygous mutants (Figure 5D and Supplemental Figure 2G and 2H) that failed to show the flowering transition under normal growth conditions (Supplemental Figure 7C). When shoots of CR-SeSP3D lines or wild-type S. etuberosum were grafted onto E109 rootstocks, the CR-SeSP3D/E109 grafts exhibited significantly delayed tuber formation under LD conditions compared with S. etuberosum/E109 grafted plants (Figures 5E and 5F), suggesting that endogenous SeSP3D is indeed necessary for tuber induction independent of leaf-expressed SP6A. Taken together, these results suggest that leaf-expressed SP6A is dispensable for SP3D induction of tuber formation. In addition, we used the sft mutant in the cultivated tomato (S. lycopersicum) Ailsa Craig (AC57) background (SP6A inactivated, Sato et al., 2012) as a scion for grafting onto E109 rootstocks (Supplemental Figure 9) and observed that both AC57/E109 and sft/E109 grafts tuberized under LD conditions. Thus, it will be interesting in future studies to test whether SD-activated SlFTL1 is responsible for tuber induction of the potato rootstock plants.
Transcriptomic regulatory patterns of SP3Dox and StFTL1ox in non-swelling and swelling stolons
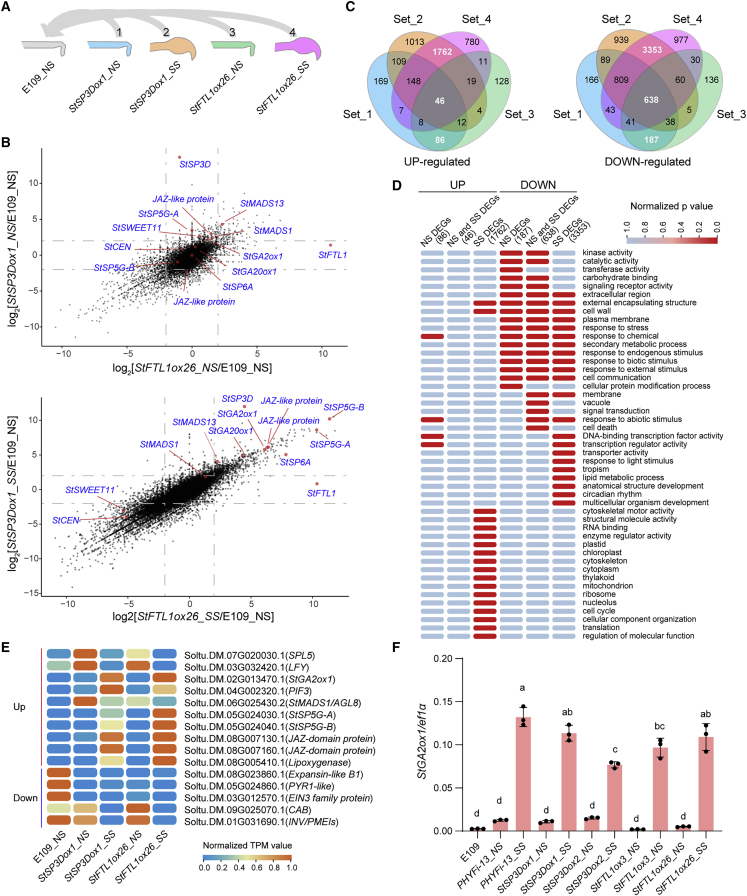
To gain a better understanding of the molecular mechanisms of tuber formation under non-inductive LD conditions, we analyzed the transcriptional profiles of StSP3Dox1 and StFTL1ox26 lines during tuberization by RNA sequencing. To this end, we compared non-swelling stolon tips (NS) and swelling stolon tips (SS) of the transgenic lines to non-swelling stolon tips of E109 (Figure 6A). A total of 27 880 expressed genes (genes with low expression were removed) were identified among the 52 953 annotated protein-coding genes (Supplemental Data 1). Principal component analyses (PCA) of global relative relationships among the various samples showed a clear separation among the three organs (Supplemental Figure 10), consistent with them being quite distinct. However, a strong correlation was observed for StSP3Dox-induced and StFTL1ox-induced transcriptomes (Figure 6B). Indeed, common differentially expressed genes (DEGs) obtained from the four comparisons (Figure 6A) showed that most StSP3D-responsive genes were also coregulated by StFTL1 (Figure 6C).
Figure 6.
Transcriptome analysis at different tuber developmental stages.
(A) Diagram showing the non-swelling and swelling stolon tips used for RNA-seq. The comparisons performed are numbered. E109_NS, E109 non-swelling stolons; StFTL1ox26_NS, StFTL1ox26 non-swelling stolons; StFTL1ox26_SS, StFTL1ox26 swelling stolons; StSP3Dox1_NS, StSP3Dox1 non-swelling stolons; StSP3Dox1_SS, StSP3Dox1 swelling stolons.
(B) Identification of genes with StSP3Dox- and StFTL1ox-dependent expression changes. x axis, log2 [ratio of gene expression in StFTL1ox26_NS/SS and E109_NS]; y axis, log2 [ratio of gene expression in StSP3Dox1_NS/SS and E109_NS]. Genes related to tuberization are indicated in color.
(C) Venn diagram of the numbers of differentially expressed genes (DEGs) in the comparisons shown in (A). Differentially expressed genes were identified using DESeq2 (adjusted p ≤ 0.05, Log2Fold change ≤ −1 or ≥1).
(D) Gene Ontology (GO) enrichment analyses for the upregulated and downregulated DEGs shown in (C). DEGs specific to non-swelling stages are defined as NS DEGs, DEGs shared by non-swelling and swelling stages are defined as NS and SS DEGs, and DEGs specific to swelling stages are defined as SS DEGs. Light blue, not significantly enriched (assigned p = 1). Red, significantly enriched (adjusted p < 0.05). UP and DOWN represent genes significantly upregulated or downregulated, respectively. The number represents the number of DEGs shown in (C).
(E) Expression patterns of genes with high fold changes.
(F) qRT-PCR analysis of StGA2ox1 expression in E109, PHYFi-13_NS, PHYFi-13_SS, StSP3Dox_NS, StSP3Dox_SS, StFTL1ox_NS, and StFTL1ox_SS lines. Data are shown as mean ± SD, n = 3 each. The lowercase letters above the bars indicate significant differences among means (one-way ANOVA, followed by Tukey’s multiple comparison test, p < 0.05).
To identify coregulated genes of interest, we defined DEGs (Supplemental Data 2) specific to the non-swelling stage (NS DEGs), DEGs shared by non-swelling and swelling stages (NS and SS DEGs), and DEGs specific to the swelling stage (SS DEGs). Gene ontology (GO) enrichment analysis of up- and downregulated DEGs revealed that the GO terms response to abiotic stimulus and transcription regulator activity were significantly enriched among the DEGs upregulated in NS (Figure 6D), whereas structural molecule activity, cytoskeleton, and cell cycle were enriched among the upregulated SS DEGs. By contrast, the GO terms cell communication, response to light stimulus, and circadian rhythm were enriched among the downregulated NS or SS DEGs. Notably, the StSP6A-regulated AGAMOUS like genes StMADS1 and StMADS13 were induced before stolon swelling, whereas StSP6A, StSP5G-B, and StGA2ox1 were significantly upregulated only in swelling stolons (Figure 6B, 6E, and 6F), showing that induction of StMADS1 and StMADS13 by StSP3D and StFTL1 occurs prior to that of StSP6A and StSP5G-B.
We next studied the expression patterns of genes with high fold changes in more detail (Figure 6E). Genes encoding the flowering-related LEAFY (LFY) and SQUAMOSA promoter-binding protein-like 5 (SPL5) transcription factors (Lee et al., 2008; Wang et al., 2009) were upregulated only in the non-swelling stage, whereas phytochrome interacting factor 3 (PIF3) and jasmonate-zim-domain protein (JAZ-domain protein) were strongly upregulated in the swelling stage. In addition, ETHYLENE INSENSITIVE3 (EIN3) was downregulated at the non-swelling and swelling stages. This protein is a key regulator of apical hook development, and ein3 eil1 double mutants are defective in apical hook development (An et al., 2012; Zhang et al., 2014). Opening of the stolon apical hook is one of the earliest phenotypic indications of the stolon-to-tuber transition, and StSP6A RNAi plants show an increased apical hook angle, which is reduced in SUC2:StSP6A lines (Abelenda et al., 2019). Thus, downregulation of the E1N3 family protein by StSP3D and StFTL1 is also likely to have a role in inducing the opening of the stolon apical hook.
StSP3D and StFTL1 interact with StSP6A-binding proteins
In Arabidopsis and tomato, FT has been shown to be transported via the phloem to the shoot apical meristem, where it interacts via 14-3-3 proteins with the bZIP transcription factor FD to form a floral activator complex (FAC) that activates the downstream MADS-box genes to trigger floral development (Abe et al., 2005; Wigge et al., 2005; Jung et al., 2012; Lifschitz et al., 2006). Since StSP6A was established as a main component of tuberigen in potato, several StSP6A-binding proteins that regulate tuber development have been identified (Abelenda et al., 2019; Jing et al., 2022b; Nicolas et al., 2022; Teo et al., 2017). We selected the components of the TAC (Teo et al., 2017) and alternative aTAC (Jing et al., 2022b) to test whether StSP3D and StFTL1 interact with these StSP6A partners. First, we investigated the interactions of StSP3D/StFTL1 with StFDL1, StABL1, and a 14-3-3 isoform (St14f) in yeast (Supplemental Figure 11A). Like StSP6A, both StSP3D and StFTL1 interacted with St14f, but only StSP6A showed a strong interaction with StFDL1 in yeast. Both StSP3D and StSP6A interacted with StABL1, but StFTL1 did not. Bimolecular fluorescence complementation (BiFC) assays in tobacco leaf cells showed results similar to those reported for StSP6A: StSP3D and StFTL1 were able to interact with St14f, StFDL1, and StABL1. The BiFC signal for the StSP3D/StFTL1–St14f interaction was predominantly observed in the cytoplasm (Supplemental Figure 11B), whereas the interaction of StSP6A/StSP3D/StFTL1 and StFDL1/StABL1 was observed in the nucleus. Thus, mobile StSP3D and StFTL1 proteins probably promote tuberization in stolon tips in a manner similar to that of long-distance transported StSP6A.
Discussion
Potato, a model for geophytic species (Khosa et al., 2021), has evolved an additional reproductive strategy to survive and propagate under extreme climate conditions through the formation of vegetative storage organs called tubers. Potato is cultivated worldwide for these starch-rich organs, which are used as a staple food in many countries. The adaptation to LD tuberization from the SD-dependent progenitor S. tuberosum Group Andigena greatly contributed to the geographic expansion of potato (Gutaker et al., 2019). Day length is detected by the leaves, which produce a mobile stimulus that is transported to the shoot apex or underground stolons to induce flowering or tuber formation. Given the importance of tubers as a food source, identification of StSP6A in the past decade has promoted extensive research aimed at uncovering the mechanisms that underlie storage organ formation.
In the present study, we have shown that overexpression of SD-activated StSP3D and StFTL1 genes promotes photoperiod-independent tuberization, and this effect is graft transmissible, suggesting that StSP3D and StFTL1 also function as long-range tuber-inducing signals. Previously, characterization of StSP3D showed that StSP3D-silenced plants tuberized at the same time as untransformed wild-type plants under SD conditions, but they exhibited a clear delay in floral transition under LD conditions (Navarro et al., 2011; Teo et al., 2017). In fact, StSP3D, StFTL1, and StSP6A transcripts are all SD-activated in leaves, although the activation of StSP3D expression is weaker than that of StFTL1 and StSP6A. Thus, a partially overlapping tuber-inducing role of StSP3D, StFTL1, and StSP6A in leaves under SD conditions cannot be fully ruled out. Consistent with this notion, we found that SeSP6A of the wild Solanum species S. etuberosum is naturally inactivated, as also reported recently (Tang et al., 2022). However, when S. etuberosum plants were used as scion donors, they were still able to induce tuber formation in potato rootstock under LD conditions. Modern potato cultivars are facultative SD plants, but tuberization is still accelerated under SD conditions (Gutaker et al., 2019). Furthermore, our finding that overexpression of StSP3D in S. etuberosum promoted tuberization of E109 stock plants under SDs suggests that leaf-expressed SP6A is dispensable for SP3D induction of tuber formation. In addition, our use of CRISPR/Cas9 to engineer SP3D mutations in the S. etuberosum background (SeSP6A inactivated) showed that SP3D actually acts as a mobile tuber-inducing signal independently of leaf SP6A expression . However, it is be possible that StSP6A is the normal "real" tuberigen in potato but that other FT homologs (StSP3D and StFTL1) have, for evolutionary reasons, high similarity to StSP6A and thus share some of its tuber-inducing abilities, perhaps serving as a safety net or fallback system to ensure proper tuberization. We thus hypothesize that all SD-activated FT-like genes may contribute to the robustness of SD-responsive tuberization, thus enabling potato to adapt this developmental response to different latitudes. Similarly, GmFT2a and GmFT5a together regulate flowering time in soybean, and the effect of GmFT2a is more prominent than that of GmFT5a under SD conditions, whereas GmFT5a, not GmFT2a, has been found to be essential for soybean adaption to high latitude regions (Cai et al., 2020).
Overexpression of either StSP3D, StSP6A, or StFTL1 promotes tuber formation under non-inductive LD conditions. However, we found that mutation of SeSP3D in S. etuberosum (SP6A inactivated) did not fully block tuberization of S. etuberosum/E109 grafts under LD conditions. Likewise, the tomato sft mutant (SP6A inactivated (Sato et al., 2012)) scion can induce tuber formation in E109 rootstocks (Supplemental Figure 9). Thus, another study using intact potato CRISPR mutants will be required in the future to assess whether these tuber-inducing FT homologs genes are redundant and essential for potato tuberization. In addition, the expression either of StSP3D, StSP6A, or StFTL1 can induce StSP6A expression in stolons, and thus, are involved in the positive feedback loop enhancing StSP6A expression in stolons (Navarro et al., 2011). Tuberization onset was previously reported to be delayed in Hd3a/StSP6A RNAi grafts relative to Hd3a/wild-type grafted controls (Navarro et al., 2011), and thus, the lack of StSP6A may interfere with the tuber-inducing activity of the phloem-transported StSP3D and StFTL1 proteins, leading to developmental epistasis (Hendelman et al., 2021). However, what exactly makes StSP6A unique leaves room for future research. Interestingly, we noticed that StSP3D expression was also weakly induced in wild-type stolon tips at tuber initiation (Figure 1B) and in PHYF-silenced (Figure 1D) and StFTL1ox lines (Figure 6B). This secondary SP3D activation has been also described in the tomato shoot apical meristem (SAM) (Meir et al., 2021), and thus, it is possible that activation of this gene in the stolon tip by phloem-transported tuber-forming FT-like signals acts in part redundantly with the activation of StSP6A.
Members of the PEBP gene family have undergone preferential duplication and subfunctionalization in Solanaceae (Abelenda et al., 2014; Navarro et al., 2015). However, it seems that they still act in concert to modulate the induction of day length-dependent flowering/tuberization. In tomato, disruption of SlSP5G and SlFTL1 in cultivated tomato results in day-neutral flowering and leads to upregulated SlSP3D/SFT expression under LD or SD conditions (Song et al., 2020). Here, expression of both StSP3D and StSP6A was upregulated in PHYFi lines, whereas that of StFTL1 was suppressed, possibly due to the feedback inhibition of increased StSP6A and StSP3D expression. In this regard, the mRNA level of the endogenous StSP6A transcript is decreased in StSP6Acop-overexpressing transgenic plants (Lehretz et al., 2019). On the contrary, from the StFTL1 expression pattern, it is evident that this gene has a different function in stolons than StSP3D or StSP6A. Furthermore, StFTL1 seemed to respond specifically to SD conditions, at least in the genotype used in this study. Consistent with this observation, SlFTL1, the ortholog of StFTL1 in tomato, has been reported to respond specifically to SD conditions, with its loss-of-function leading to impaired SD sensitivity (Song et al., 2020). Furthermore, we observed that the expression of potato FT-like genes is regulated in an organ-specific manner. Similar to the StSP5G-StSP6A module, the tandemly arranged StSP5G-like and StFTL1 paralogs show inverse expression patterns in leaves, but are coregulated in stolon tips (Figure 1B), suggesting that these potato FT-like proteins form different interaction/transcriptional regulatory complexes in leaves and stolons. Furthermore, by RNA-seq analysis, we showed that StSP3D and StFTL1 suppress the expression of the newly identified EIN3 family protein factor in non-swelling and swelling stolons, which possibly contributes to the opening of the stolon apical hook (Abelenda et al., 2019).These identified NS DEGs and SS-specific DEGs are possible targets for FT-mediated signaling and the initiation of tuber development.
Finally, SlSP3D/SFT, the tomato ortholog of StSP3D, drives enhanced heterosis yield via the dosage-dependent inhibition of sp-mediated meristem termination (Krieger et al., 2010; Jiang et al., 2013). Thus, it will be important to examine in future studies whether different allelic combinations of these tuber-inducing FT-like genes drive similar heterosis in potato. In summary, our results uncover the function of SD-induced StSP3D and StFTL1 as long-range tuber-inducing signals in potato. Furthermore, StSP3D and StFTL1-induced tuber formation may partly interact with StFDL1 and StABL1 in stolon tips (Figure 7), which are phloem-transported from leaves to stolons, where they activate the expression of StSP6A to initiate tuber formation. Knockdown of StSP3D delays floral transition under LD conditions, relative transport of this protein to the apical meristem and stolons, as well as the dose-dependent effects on flowering and tuberization, presumably mediating the pleiotropic effects of this FT ortholog. In this way, the control of flowering, tuber induction, and meristem termination by these FT-like genes makes them the prime targets for potato genetic improvement.
Figure 7.
Proposed model for photoperiodic induction of tuberization by FT-like mobile proteins.
Under short-day conditions, StSP6A, StSP3D, and StFTL1 are induced and their proteins synthesized in leaves. These mobile signals are transported to the stolons, where they participate in secondary activation of StSP6A and expression regulation of other tuberization-related genes to induce tuber formation.
Methods
Plant material, growth conditions, and phenotyping
The tetraploid potato cultivar E-potato-109 (E109), which does not form tubers under LD conditions as described previously (Zhou et al., 2019), and the non-tuber-bearing wild species Solanum etuberosum were used in the present study. Plants were propagated in vitro on MS medium supplemented with 3% sucrose (m/v) using a single stem node under LD conditions (16 h light/8 h dark) at 20°C. For phenotyping of transformed lines and wild-type plants in the growth room, 3-week-old in vitro potato plants were transplanted into pots with a diameter of 10 cm (one plant per pot) and grown under LD conditions (16 h light/8 h dark) with light/dark temperatures of 23°C/20°C. Each experiment was performed with three replicates of eight pot-grown plants. For time-course gene expression analyses, plants were grown in the growth room until they reached the 10-leaf stage (5-week-old plants). The fifth leaf from the shoot apex was sampled. Three plants were sampled at 3-h intervals for 24 h, and samples were directly frozen in liquid nitrogen and stored at −80°C until use. For gene expression analysis of stolons, 0.5–1-cm non-swelling stolon (NS) tips and swelling stolon (SS) tips were sampled, directly frozen in liquid nitrogen, and stored at −80°C until use. All plants were grown under a normal light intensity (250 μmol m−2 s−1), except for StSP3Dox plants used for time-course gene expression analysis (70 μmol m−2 s−1), as these transgenic plants exhibited much slower shoot growth. For phenotyping in the net house, 4-week-old plants grown in the growth room or sprouting tubers were planted in pots with a diameter of 25 cm (one plant per pot). The final harvest was performed at 12 weeks after planting.
Cloning and vector construction
All genes in this study were amplified by polymerase chain reaction (PCR) using Phanta Super-Fidelity DNA Polymerase (Vazyme) according to the manufacturer’s instructions. Primers used for amplification are shown in Supplemental Table 1. For TA cloning, the PCR products were cloned using the ClonExpress Ultra One Step Cloning Kit (https://www.vazyme.com/, C115-01). To create 35S-promoter-driven StSP3D-overexpression (StSP3Dox) and StFTL1-overexpression (StFTL1ox) transgenic plants, the coding sequences of StSP3D and StFTL1 were amplified from complementary DNA (cDNA) of E109 plants, inserted into pH7LIC8.0-ccdB-C-HA, and digested with StuI to generate 35S:StSP3D-3×HA and 35S:FTL1-3×HA using Exnase II (Vazyme) according to the manufacturer’s recommendations. The vectors were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into E109, Arabidopsis Col-0, or Solanum etuberosum.
To create knockout mutants, SeSP3D mutagenesis by CRISPR-Cas9 was performed by transforming plants with the PJCV55 vector (Jing et al., 2022a), which contains two reported guide RNAs (Song et al., 2020). For CRISPR/Cas9 vector construction, mutagenesis was confirmed by TA cloning, sequencing, and restriction enzyme digestion.
Transgenic plant generation
For potato genetic transformation, plasmids were introduced into Agrobacterium tumefaciens strain GV3101, which was then used for transformation of the E109 line as described previously (Jing et al., 2022b). For Solanum etuberosum transformation, healthy fully expanded leaves were collected from 4-week-old in vitro plantlets, and 2 or 3 cuts were made along the middle rib with a razor blade. Leaves were placed upside down in co-cultivation medium (MS medium with 2.3 g/L Phytagel supplemented with 30 g/L sucrose, 2 mg/L 6-BA, 0.2 mg/L NAA, and 0.05 mg/L GA3) for two days under dark conditions at 25°C. A. tumefaciens GV3101 with the recombinant T-DNA vector was cultured at 28°C in 10 mL of LB medium with antibiotics overnight, and a 1-mL culture was further cultured at 28°C in 50 mL of LB medium with antibiotics until reaching an OD600nm of 0.6–0.8. Bacterial cells were then collected by centrifugation at 4000 g for 8 min and resuspended in 15 mL of liquid MS medium with 30 g/L sucrose. The A. tumefaciens suspension was incubated with pre-treated leaf explants for 10 min. After inoculation, explants were dried on sterile filter paper and then transferred to co-cultivation medium for two days under dark conditions at 25°C. After 48 h of incubation in the dark at 25°C, the explants were transferred to callus and shoot induction medium (MS medium with 2.3 g/L Phytagel supplemented with 30 g/L sucrose, 2 mg/L 6-BA, 0.2 mg/L NAA, 0.05 mg/L GA3, 50 mg/L kanamycin, and 200 mg/L Timentin) and grown under LD conditions at 22°C for 4 weeks, during which time explants were transferred to fresh callus and shoot induction medium every two weeks. When the regenerated shoots reached 2–3 cm in length, they were transferred to rooting medium (MS medium with 2.3 g/L Phytagel supplemented with 50 mg/L kanamycin and 200 mg/L Timentin).
RNA extraction and qRT-PCR
Total RNA was extracted from frozen samples using the Total RNApure Kit (ZOMANBIO, http://zomanbio.com). Reverse transcription into complementary DNA was performed using the 5× All-in-One RT MasterMix reverse transcription kit (ABM, http://www.abmgood.com). The quality of the cDNA was evaluated using actin primers within 2 adjacent exons. Quantitative RT-PCR was performed on the LightCycler 480 II system (Roche, Switzerland) with EvaGreen 2× qPCR MasterMix (ABM). The potato Ef1α gene was used as the control gene for expression normalization (Nicot et al., 2005). Gene expression levels were calculated by the 2−ΔCq method. All primer sequences for qRT-PCR analysis are described in Supplemental Table 1.
Grafting
For grafting experiments, 3-week-old plants grown under LD conditions were used, and scions were prepared by cutting each stem below the second or third leaf from the apex using a new razor blade. Samples were immediately placed in water to prevent formation of air bubbles in the xylem and subsequent dehydration. Stocks were prepared similarly by cutting the stem at about 5 cm above the soil and removing the lower leaves. A drop of water was applied to the cut stem to prevent water loss. The scion to be grafted was then placed on top of the stock, and the two sections were held in place by binding them together with a grafting clip. Grafts were covered for at least one week with a plastic cup humidified by spraying the inside with water. Cups were sprayed with water for the first 2–3 days and removed one week later. Grafts were grown for another week in the growth room under LD conditions and then transferred to larger pots with a diameter of 20 cm (one plant per pot) for tuberization under LD or SD conditions. Tuber number and tuber yield were quantified 8–12 weeks after grafting.
Western blot analysis
Protein extraction from potato was performed as described previously (Abelenda et al., 2016). The potato protein samples and immunoprecipitates were separated on a 10% SDS-PAGE gel and transferred to a PVDF membrane, and the membrane was blocked in 5% milk in 1× TBS (150 mM NaCl, 10 mM Tris–HCl [pH 7.4]) with 0.05% (v/v) Tween-20 before hybridization with the primary antibodies anti-HA-tag mAb (MBL, M180-3) and anti-actin (Abmart, 26F7) at 1:3000 dilutions. The membrane was washed with 1× TBST before addition of the secondary antibody at a 1:3000 dilution (anti-IgG (H + L chain) (mouse) pAb-HRP, MBL). ECL detection was performed according to the manufacturer’s recommendations.
RNA sequencing
Stolons at the non-swelling stage of E109 (wild-type) plants and the non-swelling and swelling stages (stage 3) of StSP3Dox1 and StFTL1ox26 plants were sampled for RNA sequencing. Stolon samples were collected after 35 days of growth under LD conditions. Total RNA was extracted from three independent replicates for each genotype. RNA quality and concentration were determined using a NanoDrop OneC spectrophotometer (Thermo Fisher Scientific). Two micrograms of total RNA was used for stranded RNA sequencing library preparation using the KC Stranded mRNA Library Prep Kit for Illumina (DR08402, Wuhan Seqhealth, China) according to the manufacturer’s instructions. PCR products corresponding to 200–500 bp were enriched, quantified, and sequenced on the HiSeq X 10 system (Illumina). The raw data were cleaned (with adaptor and low-quality reads filtered out) using Trimmomatic (version 0.36) (Bolger et al., 2014). On average, more than 43.3 million reads with a mean Q30 of 92.6% were generated per sample. The clean reads were mapped to the Solanum tuberosum genome (version 6.1, http://spuddb.uga.edu/) using the transcript quantification tool Salmon (version 1.4.0) with validateMappings (Patro et al., 2017). Reads and transcripts per kilobase of exon model per million mapped reads (TPMs) were calculated. Genes with low expression (total counts in fifteen samples ≤10) were removed from further analysis. DEGs were identified using DESeq2 (Love et al., 2014) (adjusted p < 0.05, fold change >2). Gene Ontology (GO) enrichment, Venn diagram construction, and heatmap analysis of the DEGs were performed using TBtools (version 1.0.98) (Chen et al., 2020).
Yeast two-hybrid assay
The full-length coding sequences of StFTL1 and StFDL1 were cloned into the EcoRI and SalI sites of pGBKT7 using the primers listed in (Supplemental Table 1). The full-length coding sequence of StFTL1 was amplified from E109 and cloned into the EcoRI and BamHI sites of pGADT7. Other vectors were constructed previously (Jing et al., 2022b). Their pairwise combinations or the corresponding empty vectors were co-transformed into yeast strain AH109 using the BD Matchmaker Screening Kit according to the manufacturer’s instructions.
BiFC
The full-length coding sequences of StFTL1 and StFDL1 were amplified using specific primers (Supplemental Table 1) and then cloned into the NYFP and CYFP vectors via restriction digestion with BamHI and SalI. Other vectors were constructed previously (Jing et al., 2022b). These constructs were transformed into A. tumefaciens strain GV3101 by electroporation, and the transformed strain was subsequently infiltrated into leaves of 1-month-old N. benthamiana plants. YFP fluorescence was observed 48 h after infiltration using a confocal laser scanning microscope (CLSM; TCS-SPE; Leica, http://www.leica.com) according to the manufacturer’s instructions.
Funding
This research was supported by the China Agricultural Research System (Potato, CARS-09) and the National Natural Science Foundation of China (3161101332 and 31971988).
Author contributions
S.J., P.J., X.S., L.Y., E.W., and J.Q. conducted the experiments. S.J. and B.S. designed the experiments. S.J., B.S., S.P., and F.Z. wrote the paper.
Acknowledgments
The authors thank Professor Jihua Ding (Huazhong Agricultural University), Professor Chunying Kang (Huazhong Agricultural University), and Dr. Jay Prakash Maurya (Centre for Research in Agricultural Genomics, Spain) for fruitful discussion, helpful comments, and critical reading of this manuscript. We thank Professor Junhong Zhang (Huazhong Agricultural University) and Professor Xia Cui (Chinese Academy of Agricultural Sciences) for tomato material assistance. No conflict of interest declared.
Published: January 11, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Supplemental information
References
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Abelenda J.A., Navarro C., Prat S. Flowering and tuberization: a tale of two nightshades. Trends Plant Sci. 2014;19:115–122. doi: 10.1016/j.tplants.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Abelenda J.A., Cruz-Oró E., Franco-Zorrilla J.M., Prat S. Potato StCONSTANS-like1 suppresses storage organ formation by directly activating the FT-like StSP5G repressor. Curr. Biol. 2016;26:872–881. doi: 10.1016/j.cub.2016.01.066. [DOI] [PubMed] [Google Scholar]
- Abelenda J.A., Bergonzi S., Oortwijn M., Sonnewald S., Du M., Visser R.G.F., Sonnewald U., Bachem C.W.B. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 2019;29:1178–1186.e6. doi: 10.1016/j.cub.2019.02.018. [DOI] [PubMed] [Google Scholar]
- An F., Zhang X., Zhu Z., Ji Y., He W., Jiang Z., Li M., Guo H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012;22:915–927. doi: 10.1038/cr.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A.K., Chatterjee M., Yu Y., Suh S.G., Miller W.A., Hannapel D.J. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell. 2006;18:3443–3457. doi: 10.1105/tpc.106.042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogale S., Mahajan A.S., Natarajan B., Rajabhoj M., Thulasiram H.V., Banerjee A.K. MicroRNA156: a potential graft-transmissible MicroRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp andigena. Plant Physiol. 2014;164:1011–1027. doi: 10.1104/pp.113.230714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Wang L., Chen L., Wu T., Liu L., Sun S., Wu C., Yao W., Jiang B., Yuan S., et al. Mutagenesis of GmFT2a and GmFT5a mediated by CRISPR/Cas9 contributes for expanding the regional adaptability of soybean. Plant Biotechnol. J. 2020;18:298–309. doi: 10.1111/pbi.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chailakhyan M.K., Yanina L.I., Devedzhyan A.G., Lotova G.N. Photoperiodism and tuber formation in graftings of tobacco onto potato. Dokl. Akad. Nauk SSSR. 1981;257:1276–1280. [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Corbesier L., Vincent C., Jang S., Fornara F., Fan Q., Searle I., Giakountis A., Farrona S., Gissot L., Turnbull C., et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Gregory L.E. Some factors for tuberization in the potato plant. Am. J. Bot. 1956;43:281–288. doi: 10.1002/j.1537-2197.1956.tb10492.x. [DOI] [Google Scholar]
- Gutaker R.M., Weiß C.L., Ellis D., Anglin N.L., Knapp S., Luis Fernández-Alonso J., Prat S., Burbano H.A. The origins and adaptation of European potatoes reconstructed from historical genomes. Nat. Ecol. Evol. 2019;3:1093–1101. doi: 10.1038/s41559-019-0921-3. [DOI] [PubMed] [Google Scholar]
- Hendelman A., Zebell S., Rodriguez-Leal D., Dukler N., Robitaille G., Wu X., Kostyun J., Tal L., Wang P., Bartlett M.E., et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell. 2021;184:1724–1739.e16. doi: 10.1016/j.cell.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Jiang K., Liberatore K.L., Park S.J., Alvarez J.P., Lippman Z.B. Tomato yield heterosis is triggered by a dosage sensitivity of the florigen pathway that fine-tunes shoot architecture. PLoS Genet. 2013;9:e1004043. doi: 10.1371/journal.pgen.1004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S., Xiong W., Liu H., Dong F., Chen K., Du J., Song B. Efficient targeted mutagenesis by endogenous promoter controlled tRNA-gRNA array of CRISPR/Cas9 in potato and application in Solanum etuberosum. Research Square. 2022 doi: 10.21203/rs.3.rs-1665829/v1. Preprint at. [DOI] [Google Scholar]
- Jing S., Sun X., Yu L., Wang E., Cheng Z., Liu H., Jiang P., Qin J., Begum S., Song B. Transcription factor StABI5-like 1 binding to the FLOWERING LOCUS T homologs promotes early maturity in potato. Plant Physiol. 2022;189:1677–1693. doi: 10.1093/plphys/kiac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.H., Ju Y., Seo P.J., Lee J.H., Park C.M. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. 2012;69:577–588. doi: 10.1111/j.1365-313X.2011.04813.x. [DOI] [PubMed] [Google Scholar]
- Khosa J., Bellinazzo F., Kamenetsky Goldstein R., Macknight R., Immink R.G.H. PHOSPHATIDYLETHANOLAMINE-BINDING proteins: the conductors of dual reproduction in plants with vegetative storage organs. J. Exp. Bot. 2021;72:2845–2856. doi: 10.1093/jxb/erab064. [DOI] [PubMed] [Google Scholar]
- Kloosterman B., Abelenda J.A., Gomez M.d.M.C., Oortwijn M., de Boer J.M., Kowitwanich K., Horvath B.M., van Eck H.J., Smaczniak C., Prat S., et al. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature. 2013;495:246–250. doi: 10.1038/nature11912. [DOI] [PubMed] [Google Scholar]
- Krieger U., Lippman Z.B., Zamir D. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 2010;42:459–463. doi: 10.1038/ng.550. [DOI] [PubMed] [Google Scholar]
- Kumar D., Wareing P.F. Studies on tuberization in solanum andigena: i. evidence for the existence and movement of a specific tuberization stimulus. New Phytol. 1973;72:283–287. doi: 10.1111/j.1469-8137.1973.tb02034.x. [DOI] [Google Scholar]
- Lee J., Oh M., Park H., Lee I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J. 2008;55:832–843. doi: 10.1111/j.1365-313X.2008.03552.x. [DOI] [PubMed] [Google Scholar]
- Lehretz G.G., Sonnewald S., Hornyik C., Corral J.M., Sonnewald U. Post-transcriptional regulation of FLOWERING LOCUS T modulates heat-dependent source-sink development in potato. Curr. Biol. 2019;29:1614–1624.e3. doi: 10.1016/j.cub.2019.04.027. [DOI] [PubMed] [Google Scholar]
- Lifschitz E., Eviatar T., Rozman A., Shalit A., Goldshmidt A., Amsellem Z., Alvarez J.P., Eshed Y. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA. 2006;103:6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Adam H., Díaz-Mendoza M., Zurczak M., González-Schain N.D., Suárez-López P. Graft-transmissible induction of potato tuberization by the microRNA miR172. Development. 2009;136:2873–2881. doi: 10.1242/dev.031658. [DOI] [PubMed] [Google Scholar]
- Meir Z., Aviezer I., Chongloi G.L., Ben-Kiki O., Bronstein R., Mukamel Z., Keren-Shaul H., Jaitin D., Tal L., Shalev-Schlosser G., et al. Dissection of floral transition by single-meristem transcriptomes at high temporal resolution. Nat. Plants. 2021;7:800–813. doi: 10.1038/s41477-021-00936-8. [DOI] [PubMed] [Google Scholar]
- Morris W.L., Ducreux L.J.M., Morris J., Campbell R., Usman M., Hedley P.E., Prat S., Taylor M.A. Identification of TIMING OF CAB EXPRESSION 1 as a temperature-sensitive negative regulator of tuberization in potato. J. Exp. Bot. 2019;70:5703–5714. doi: 10.1093/jxb/erz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C., Cruz-Oró E., Prat S. Conserved function of FLOWERING LOCUS T (FT) homologues as signals for storage organ differentiation. Curr. Opin. Plant Biol. 2015;23:45–53. doi: 10.1016/j.pbi.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Navarro C., Abelenda J.A., Cruz-Oró E., Cuéllar C.A., Tamaki S., Silva J., Shimamoto K., Prat S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature. 2011;478:119–122. doi: 10.1038/nature10431. [DOI] [PubMed] [Google Scholar]
- Nicolas M., Torres-Pérez R., Wahl V., Cruz-Oró E., Rodríguez-Buey M.L., Zamarreño A.M., Martín-Jouve B., García-Mina J.M., Oliveros J.C., Prat S., Cubas P. Spatial control of potato tuberization by the TCP transcription factor BRANCHED1b. Nat. Plants. 2022;8:281–294. doi: 10.1038/s41477-022-01112-2. [DOI] [PubMed] [Google Scholar]
- Nicot N., Hausman J.F., Hoffmann L., Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- Park J.S., Park S.J., Kwon S.Y., Shin A.Y., Moon K.B., Park J.M., Cho H.S., Park S.U., Jeon J.H., Kim H.S., Lee H.J. Temporally distinct regulatory pathways coordinate thermo-responsive storage organ formation in potato. Cell Rep. 2022;38:110579. doi: 10.1016/j.celrep.2022.110579. [DOI] [PubMed] [Google Scholar]
- Patro R., Duggal G., Love M.I., Irizarry R.A., Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantenga F.D.M., Bergonzi S., Abelenda J.A., Bachem C.W.B., Visser R.G.F., Heuvelink E., Marcelis L.F.M. The tuberization signal StSP6A represses flower bud development in potato. J. Exp. Bot. 2018;570:937–948. doi: 10.1093/jxb/ery420. [DOI] [PubMed] [Google Scholar]
- Tabata S., Tomato Genome Consortium. Hirakawa H., Asamizu E., Shirasawa K., Isobe S., Kaneko T., Nakamura Y., Shibata D., Aoki K., et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485:635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert T., Abel C., Wahl V. Flowering time and the identification of floral marker genes in Solanum tuberosum ssp. andigena. J. Exp. Bot. 2020;71:986–996. doi: 10.1093/jxb/erz484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Lin T., Hannapel D.J. Targets of the StBEL5 transcription factor include the FT ortholog StSP6A. Plant Physiol. 2016;170:310–324. doi: 10.1104/pp.15.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhang S., Wang X., Sun S., Liu Z., Wang K., Wan H., Zhou G., Li R., Yu H., Cui X. Variations in both FTL1 and SP5G, two tomato FT paralogs, control day-neutral flowering. Mol. Plant. 2020;13:939–942. doi: 10.1016/j.molp.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuo S., Wong H.L., Yokoi S., Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Tang D., Jia Y., Zhang J., Li H., Cheng L., Wang P., Bao Z., Liu Z., Feng S., Zhu X., et al. Genome evolution and diversity of wild and cultivated potatoes. Nature. 2022;606:535–541. doi: 10.1038/s41586-022-04822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo C.J., Takahashi K., Shimizu K., Shimamoto K., Taoka K.I. Potato tuber induction is regulated by interactions between components of a tuberigen complex. Plant Cell Physiol. 2017;58:365–374. doi: 10.1093/pcp/pcw197. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Czech B., Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138:738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Weigel D., Ahn J.H., Blázquez M.A., Borevitz J.O., Christensen S.K., Fankhauser C., Ferrándiz C., Kardailsky I., Malancharuvil E.J., Neff M.M., et al. Activation tagging in Arabidopsis. Plant Physiol. 2000;122:1003–1013. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P.A., Kim M.C., Jaeger K.E., Busch W., Schmid M., Lohmann J.U., Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhu Z., An F., Hao D., Li P., Song J., Yi C., Guo H. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell. 2014;26:1105–1117. doi: 10.1105/tpc.113.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Campbell R., Ducreux L.J.M., Morris J., Hedley P.E., Mellado-Ortega E., Roberts A.G., Stephens J., Bryan G.J., Torrance L., et al. TERMINAL FLOWER-1/CENTRORADIALIS inhibits tuberisation via protein interaction with the tuberigen activation complex. Plant J. 2020;103:2263–2278. doi: 10.1111/tpj.14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Song B., Liu T., Shen Y., Dong L., Jing S., Xie C., Liu J. Phytochrome F plays critical roles in potato photoperiodic tuberization. Plant J. 2019;98:42–54. doi: 10.1111/tpj.14198. [DOI] [PubMed] [Google Scholar]
- Zierer W., Rüscher D., Sonnewald U., Sonnewald S. Tuber and tuberous root development. Annu. Rev. Plant Biol. 2021;72:551–580. doi: 10.1146/annurev-arplant-080720-084456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.