Abstract
Introduction
Alzheimer's disease (AD) occurs in aging adults with Down syndrome (DS) at a higher prevalence and an earlier age than in typical aging adults. As with the general aging adult population, there is an urgent need to understand the preclinical and early phases of AD progression in the adult population with DS. The aim of this scoping review was to synthesize the current state of the evidence and identify gaps in the literature regarding functional activity performance and falls and their significance to disease staging (i.e., mild, moderate, and severe defined staging criteria) in relation to Alzheimer's disease and related dementias (ADRD) in adults with DS.
Methods
This scoping review included six electronic databases (e.g., PsycInfo, Academic Search Complete, CINAHL, COCHRANE Library, MEDLINE, and PubMed). Eligible studies included participants with DS ≥25 years of age, studies with functional measures and/or outcomes (e.g., activities of daily living, balance, gait, motor control, speech, behavior, and cognition; falls; and fall risks), and studies that investigated AD pathology and implications.
Results
Fourteen eligible studies were included and categorized through a thematic analysis into the following themes: (1) physical activity and motor coordination (PAMC), (2) cognition, (3) behavior, and (4) sleep. The studies indicated how functional activity performance and engagement may contribute to early identification of those at risk of cognitive decline and AD development and/or progression.
Discussion
There is a need to expand the research regarding ADRD pathology relative to functional outcomes in adults with DS. Functional measures related to disease staging and cognitive impairment are essential to understanding how AD progression is characterized within real‐world settings. This scoping review identified the need for additional mixed‐methods research to examine the use of assessment and intervention related to function and its detection of cognitive decline and AD progression.
Keywords: aging, cognition, Down syndrome, falls, function
1. BACKGROUND
Adults with Down syndrome (DS) are at high risk for the development of Alzheimer's disease and related dementias (ADRD) due to age‐related conditions that include neurological disorders. 1 , 2 , 3 , 4 , 5 Nearly all adults with DS will develop neuropathological AD changes (pathology) in their 30s and 40s, partially due to an overexpression of amyloid beta (Aβ), which accumulates in the brain across the lifespan of adults with DS. 5 , 6 , 7 AD/ADRD pathology refers to the brain neuropathology and changes associated with dementia and AD. 5 , 6 , 7 As with the general older adult population without DS, 8 , 9 there is an urgent need to understand the phases of AD progression in the adult population aging with DS. The estimated number of individuals with DS living in the United States has grown from 49,923 in 1950 to 206,366 since 2010. 10 Given advances in medical care and improvements in the overall health of individuals with DS, life expectancy has increased to 55–60 years of age, and ≈70% will develop AD, a number that will continue to grow with increased life expectancy. 11 , 12 Studies have shown that the average age at onset of dementia is about 3 years prior to the clinical manifestations of AD (e.g., mood and personality changes, memory loss, and difficulty with completing tasks). 13
There is limited evidence on the lived experiences and daily functional performance of adults aging with DS, when changes in functional activity and performance occur, and the significance of those changes to disease staging in relation to ADRD. For example, falls and functional mobility are associated with clinical measures of cognition and may be a non‐cognitive behavioral marker of AD. 14 , 15 , 16 , 17 , 18 A fall is an unexpected event in which a person comes to rest on the ground, floor, or a lower level. 9 Although individuals with DS have a higher rate of falls, it remains unknown when falls occur in the progression of AD in adults with DS. 19 This scoping review aims to synthesize the current state of evidence and identify the gaps in the literature focused on functional performance and engagement (e.g., self‐care, language, sleep, behavior, and physical mobility; fall risks; and falls) in adults with DS, and the relationship of functional performance and engagement with ADRD pathology. 20 We utilized a scoping review methodology to address age‐related functional changes and their relationship to ADRD adults with DS and ADRD, 1 and 2 the current evidence investigating the risk of falling and falls among adults aging with DS and ADRD. 2
RESEARCH IN CONTEXT
Scoping Review: We reviewed the literature regarding age‐related functional changes and fall risk and/or fall incidents in adults with Down syndrome (DS) in relation to Alzheimer's disease (AD) using PsycInfo, Academic Search Complete, CINAHL, COCHRANE library, MEDLINE, and PubMed.
Interpretation: Results showed a wide range of functional outcomes in the areas of physical activity and motor coordination (PAMC), cognition, behavior, and sleep in relation to AD pathology; however, fall risks and/or incidents were not indicated within the literature.
Future directions: Our findings highlight the need for future studies to further examine how functional activity performance and engagement— for example, self‐care, language, sleep, behavior, physical mobility, and falls and fall risks—contribute to early identification of individuals at risk of cognitive decline and AD development, facilitating understanding among adults with DS, their caregivers and family members, and clinicians.
2. METHODS
We conducted this scoping review using the Arksey and O'Malley framework and the Preferred Items for Systematic Reviews and Meta‐Analyses for scoping reviews (PRISMA‐ScR) reporting guidelines to expand the scope of this process. 21 , 22 We elected to use a scoping review design due to the exploratory and broad nature of our aims. The framework process included 21 : (1) identifying the research questions, (2) identifying relevant studies, (3) selecting the studies, (4) charting the data, and (5) summarizing and reporting the results. A study protocol was developed based on our research questions and registered on the OSF.io electronic platform. 23
2.1. Stage 1: Identifying the research questions
We applied the PCC (population, concept, and context) Framework to guide the development of our research questions and scoping review title. 24 The protocol operationalized the PCC as follows: population consisted of adults with full trisomy, partial trisomy, or mosaic DS who were ≥25 years of age; concept was the collection and reporting of functional performance variables in trial and cohort preclinical AD/ADRD studies related to risk, assessment, prevention, prevalence, and incidence; and the context consisted of adults ≥25 years with DS living in residential and/or institutional environmental settings. The research questions guiding this review were: What are the age‐related functional changes in adults with DS in relation to ADRD, and what is the current evidence investigating the risk of falling and falls among adults aging with DS and ADRD.
2.2. Stage 2: Identifying relevant studies
A medical librarian (D.T.) and (S.W.) conducted a search of the literature using the following six databases: PsycInfo, Academic Search Complete, CINAHL, COCHRANE Library, MEDLINE, and PubMed with full‐text available. Three authors (S.W., E.C., and C.L.) screened titles, abstracts, and keywords for possible inclusion and manually screened the reference lists of eligible articles to be considered for inclusion. 21 The Medical Subject Headings (MeSH) search and general terms were divided into two search groups and included (1) Down syndrome, Alzheimer's disease and related dementias, and (2) Down syndrome, Alzheimer's disease and related dementias, Falls.
2.3. Stage 3: Selecting the studies
The inclusion criteria for the studies were: (1) published in English; (2) included participants with DS (full trisomy, partial trisomy, or mosaic DS) ≥25 years of age; (3) included functional measures and/or outcomes (e.g., activities of daily living, balance, gait, motor control, speech, behavior, and cognition; fall risks; and falls); (4) investigated ADRD pathology and/or implications; and (5) was published between January 2012 and April 2022. The exclusion criteria were studies that: (1) included only children with DS, (2) investigated caregivers only, (3) investigated therapists/health professionals only, (4) did not measure and/or report functional outcomes, (5) did not investigate ADRD pathology and/or implications, and (6) were existing meta‐analyses, scoping reviews, or systematic reviews.
2.4. Stage 4: Screening and data charting
The studies were screened in two phases per PRISMA‐ScR 22 : first, a title and abstract review for inclusion and exclusion criteria, and then a full‐text review. In the first phase, a team of three reviewers (S.W., E.C., and C.L.) reviewed articles based on the inclusion and exclusion criteria. The second phase involved an in‐depth, full‐text review of articles that advanced from the first phase. This phase was performed by two authors (S.W., C.L.) who individually completed a full‐text review of articles and then reconciled any disagreements between the independent reviewers.
A descriptive method was used for extraction and analysis of the full‐text review (S.W., E.H., and C.L.) including participant demographics, study design, assessments/evaluations used, evidence‐based intervention, and study outcomes. The extracted data were placed in a shared spreadsheet through an encrypted electronic data drive and reviewed by the study team members. 25
2.5. Stage 5: Summarizing and reporting the results
The team created a summary table of the characteristics that were assessed to identify similarities among the studies regarding population demographics, study design, diagnosis, additional support, research question/line of inquiry, area of function or occupation, evaluations and assessments used, interventions and outcomes, and whether fall incidents and/or functional mobility were discussed in the literature. The team completed a comprehensive analysis and reviewed each study's content to identify its contribution to the research questions. This analysis process enabled a thematic clustering of the articles based on the aforementioned factors and summarized through descriptive themes. 25 , 26
3. RESULTS
A total of 432 abstracts were identified, and duplicate files (n = 232) were removed. The study team reviewed titles, keywords, and abstracts of 200 records; 183 records did not meet the inclusion criteria. The title/abstract‐level review resulted in 17 articles that advanced to full‐text‐level review. The team reviewed the remaining 17 articles in full, mapped the data, and excluded two articles due to lack of functional outcomes reported or assessed, as well as a systematic review. Seven of the studies were published in ADRD‐specific journals 20 , 27 , 28 , 29 , 30 , 31 , 32 ; four articles were in neurology‐specific journals 33 , 34 , 35 , 36 ; and the remaining three articles were in disability, 37 psychiatry, 38 and open research journals. 39 Fourteen articles met the inclusion criteria (Figure 1). 40 The articles were categorized into four themes: physical activity and motor coordination (PAMC), 27 , 29 , 34 , 37 cognition, 20 , 28 , 30 , 35 , 38 , 39 behavior, 31 , 32 and sleep 33 , 36 (Table 1); the themes are described as follows.
FIGURE 1.
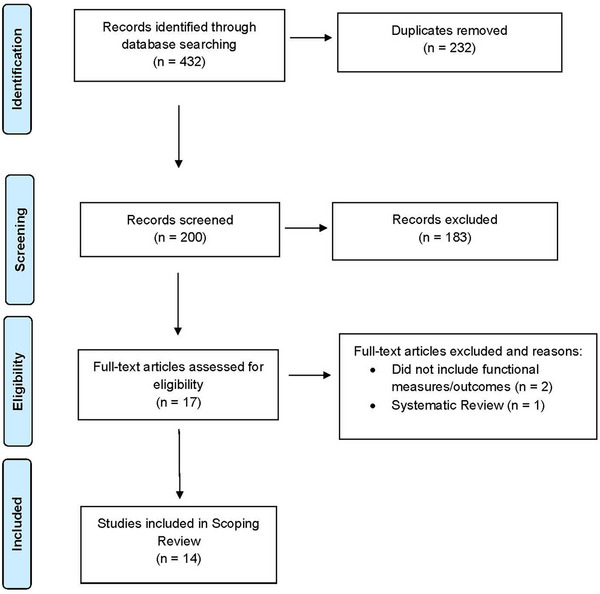
PRISMA 2009 Flow diagram of information through the phases of the scoping review PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. Adapted from Moher et al. 40
TABLE 1.
Study designs of the selected records
| PAMC | Cognition | Behavior | Sleep | |
|---|---|---|---|---|
| RCT | 1 | 0 | 0 | 0 |
| Case control | 0 | 0 | 1 | 0 |
| Cross‐sectional | 2 | 6 | 1 | 1 |
| Other | 1 | 0 | 0 | 1 |
| Total studies | 4 | 6 | 2 | 2 |
Abbreviations: PAMC, Physical Activity & Motor Coordination; RCT, randomized control trial.
3.1. Physical activity and motor coordination
Understanding the association between physical activity and AD in DS has implications for informing interventions with this genetically high‐risk population. The four PAMC studies formulated research questions to investigate: (1) associations between physical activity and cognitive performance and AD biomarkers, 34 (2) physical activity as a protective measure against cognitive decline and dementia in DS, 27 (3) the optimal dosage of physical activity needed to see changes in cognitive function, 37 and (4) whether gait is more impaired in older people with DS and AD pathology than younger people with DS and AD pathology. 29 Assessments for these studies covered domains of motor planning and coordination, gait, falls, memory, executive and/or cognitive functioning, and visuospatial processing, intellectual ability, and dementia symptoms (Table 2).
TABLE 2.
Summary of the studies
| Author(s) | Participant characteristics: number of participants, age, gender, diagnosis, additional support | Research question/line of inquiry | Evaluations/assessments | Intervention | Outcomes | Review of falls | Study type |
|---|---|---|---|---|---|---|---|
| Physical activity | |||||||
| Fleming et al. (2021) 34 |
N = 61 25–55 years Female n = 33 Male n = 28 No dementia |
What is the association between physical activity and cognitive performance and AD biomarkers? |
1. Intelligence scale 2. Motor processing 3. Mental status examination 4. Cued recall test 5. Executive function |
GT9X Actigraph accelerometer to measure physical activity |
1. Sedentary behavior and activity are related to cognitive functioning and white matter. 2. Physical activity intervention may help promote healthy aging in DS. |
No | Cross‐sectional |
| Pape et al. (2021) 27 |
N = 214 Cohort T1: n = 214 17–74 years Female n = 92, Male n = 122 Dementia _______________ Cohort T2: n = 91 Female n = 37, Male n = 54 Dementia at follow‐up post 2 years. |
Is PA a protective measure against cognitive decline? | Comprehensive assessment tool for diagnosing dementia in people with DS |
Current level of PA collected during a semi‐structed interview: High intensity: ≥3 h of PA Moderate: <3 h of PA Low: no PA |
1. Moderate‐to‐high‐intensity exercise could reduce the risk of clinically detectable cognitive decline in a DS population, with possible long‐term benefits. 2. High levels of PA are associated with an 87% reduced risk of declines in personality and behavior. |
No | Cross‐sectional |
| Ptomey et al. (2018) 37 |
N = 27 18–35 years Female n = 11 Male n = 16 No dementia |
What is the optimal dosage of PA needed to affect cognitive function? |
1. Battery of cognitive tests 2. Pre/post intervention to examine cognitive function change |
Group exercise program via video conferencing: 30‐min group activity sessions, 1–2 times/week |
Participants improved their performance on the 2 memory variables: (1) attention switching task and (2) reaction time | No | Randomized control trial |
| Van Pelt et al. (2020) 29 |
N = 28 25–59 years Female n = 13 Male n = 15 No dementia n = 22 Other possible dementia n = 6 |
What is the relationship between gait measure and cognitive performance? Feasibility of dual‐task measure within DS populations? |
1. Intelligence test 2. Picture vocabulary test 3. Dual‐task gait assessment |
None |
1. Borderline/mild ID had a 22% greater dual‐task effect than those with moderate ID due to level of existing physical impairment. 2. Gait speed was slower in older individuals with DS. |
No | Feasibility study |
| Cognition | |||||||
| Aschenbrenner et al. (2021) 20 |
N = 312 ≥35 years of age Female n = 151 Male n = 161 No dementia |
Identification of cognitive tests or test items sensitive to early cognitive change |
1. Cognition examination 2. Executive functioning 3. Paired associate learning 4. Developmental neuropsychological assessment 5. Severe impairment battery |
None |
1. Early markers of cognitive change of AD in DS included prominent declines in memory, language, attention, and praxis. 2. Early markers appear to be comparable to declines in other forms of AD, including sporadic AD and autosomal‐dominant AD. |
No | Cross‐sectional |
| Firth et al. (2018) 35 |
N = 283 Cohort 1 younger adults: 16–35 years, control group n = 119 Female n = 62 Male n = 57 No dementia n = 119 _______________ Cohort 2 older adults: ≥ 35 years, pathological group n = 164 Female n = 77 Male n = 87 No Dementia n = 121 Dementia n = 43 |
The feasibility of an event‐based model to characterize cognitive deterioration associated with AD in DS |
Cognitive battery assessment: 1. Intelligence test 2. Memory test 3. Object‐memory test 4. Informant‐rated questionnaire of cognition |
None |
1. Early occurrence of decline in tests of memory, verbal fluency, and sustained attention/motor coordination (demonstrates AD in DS follows similar pattern of change to other forms of AD). 2. Data‐driven, event‐based model of markers of cognitive decline and informant‐rated ability of individuals with DS to estimate the order of cognitive decline and assign participants to a disease stage |
No | Cross‐sectional |
| Oliver et al. (2021) 38 |
N = 412 26–74 years Female n = 185 Male n = 227 Diagnosis: Dementia or no dementia |
Identify age‐specific prevalence data and functional performance deficits for AMCI in adults with DS |
1. NAID 2. Single point assessment of AMCI 3. Evaluation of early (working memory) and late (aphasia, apraxia, agnosia) stages of dementia within 7 subscales |
None |
1. Evaluation showed NAID scores with split‐half reliability, internal consistency, and concurrent validity with diagnostic/neurophysiological assessment to be robust. 2. NAID scores at subscale show a decline with age. 3. Abnormally low NAID score is not based on ID alone and is consistent with either AMCI or dementia. |
No | Cross‐sectional |
| Startin et al. (2016) 39 |
N = 305 Cohort C1: n = 181 ≥36 years Female n = 85 Male n = 96 Dementia n = 51 No dementia n = 130 ________________ Cohort C2: n = 124 16–35 years Female n = 65 Male n = 59 No dementia |
To establish the pre‐dementia cognitive profile of adults with DS, and to identify factors relating to cognitive abilities. |
1. Cognitive battery assessment 2. Vision and hearing 3. Informant questionnaires 4. Cohort C1: Longitudinal assessments to assess cognitive decline. 5. Cohort C2: Explore cognitive profiles before onset of dementia |
None |
1. Results from participants without dementia suggest high completion rates within tasks. 2. Computer‐based tasks reported lower completion rates; completion rates from those with dementia were lower. 3. Female participants in C2 performed better than male participants on general verbal abilities and showed better cognitive abilities assessed by cognitive assessment. |
No | Cross‐sectional |
| Startin et al. (2019) 28 |
N = 297 Cohort 1: n = 124 16–35 years Female n = 65 Male n = 59 No dementia ________________ Cohort 2: n = 173 ≥36 years Female n = 83 Male n = 90 Dementia: Preclinical n = 66 Prodromal n = 54 Clinical dementia n = 45 |
To understand neuropsycho‐logical changes across the time course of AD development at a population level in adults with DS |
1. Cognitive battery 2. Intelligence test 3. Memory 4. Executive 5. Function 6. Dementia assessment |
None |
1. Memory and attention measures were the most sensitive to aging. 2. Performance for memory/attention outcomes was most sensitive to progression from preclinical to prodromal dementia. |
No | Cross‐ sectional |
| Krinsky‐McHale et al. (2020) 30 |
N = 269 ≥30 years Categorized by: 1. Cognitively stable (CS) 2. Mild cognitive impairment (MCI‐DS) 3. Possible dementia 4. Definite dementia |
The sensitivity of objective measures of performance to the onset of MCI‐DS for individuals ranging in severity of ID |
Comprehensive evaluation: 1. Review of clinical/medical records 2. Informant interviews (day‐to‐day function and concerns) 3. Selective reminding test 4. Cognitive status examination 5. Assessment for severe impairments |
None |
1. Objective methods predicted to be sensitive to the onset of MCI‐DS have utility to recognize cognitive decline associated with prodromal AD/tracking and further disease progression for adults with mild/moderate ID. 2. Category fluency test, visuospatial test, and dementia assessment measures showed changes with dementia. |
No | Cross‐sectional |
| Behavior | |||||||
| Dekker et al. (2021) 31 |
N = 524 ≥30 years Cohorts: 1. No dementia (DS) n = 292 2. Questionable dementia (DS + Q) n = 119 3. Diagnosed dementia (DS + AD) n = 113 |
Assessment of behavioral changes | Behavioral and psychological symptoms of dementia in DS | None |
1. Identified prominent changes in severity/frequency for the following behaviors when comparing item change scores: irritable, anxious, sleep‐related, restless/stereotypic, apathetic, depressive, and eating/drinking. 2. The frequency of listed behaviors exemplified the highest increased frequency in DS + AD. |
No | Case control |
| Dekker et al. (2018) 32 |
N = 281 ≥30 years Mild–severe ID Cohorts: 1. Questionable dementia (DS + Q) n = 65 2. DS without dementia n = 149 3. Diagnosed dementia (DS + AD) n = 67 |
Assessment of behavior in a large, multicenter, cross‐sectional DS cohort |
Comprehensive analysis of behavioral outcomes: 1. Structured interviews 2. Assessment of items in existing scales for DS 3. Analysis of existing literature/frequently observed symptoms in clinical practice |
None |
1. A substantial proportion of DS + Q individuals presented increased anxiety, sleep disturbances, apathy, and depressive symptoms, suggesting that these changes occur early in the course of AD. 2. Prominent changes when comparing the three diagnostic groups for the categories of sleep disturbances, anxiety, stereotypic behavior, agitation, aggression, apathy, and depressive symptoms. |
No | Cross‐sectional |
| Sleep | |||||||
| Cody et al. (2020) 33 |
N = 47 26–56 years Female n = 24 Male n = 23 No dementia N = 40 Mild cognitive impairment n = 7 |
Association between sleep and AD‐related domains of cognitive functioning |
1. Accelerometer MRI scanning cued recall test (learning, episodic, verbal memory) 2. Dementia questionnaire 3. Picture recognition 4. Memory scale 5. Executive functioning 6. Fine motor planning |
None |
1. Identified likelihood of disrupted sleep being associated with Amyloid‐β accumulation/cognitive features of preclinical AD in DS. 2. Sleep disruption, specifically length of awakening at night, was associated with poor memory, executive functioning, and motor planning and coordination cross‐sectionally in adults with DS in the preclinical stages of AD. |
No | Cohort study |
| Fleming et al. (2021) 34 |
N = 29 Age 33–54 Female = 14 Male n = 15 No dementia |
What is the association between disrupted sleep and WM integrity in adults with DS and without dementia? |
1. Accelerometer 2. Caregiver‐reported obstructive sleep apnea |
1. Use of accelerometer for 7 days 2. Daily sleep log by participant/caregiver. 3. Caregiver report regarding sleep apnea |
Disrupted sleep is associated with lower WM integrity in the major association tracts in middle‐aged adults with DS. | No | Exploratory |
Abbreviations: AD, Alzheimer's disease; AMCI, acquired mild cognitive impairment; DS, Down syndrome; ID, intellectual disability; NAID, Neuropsychological Assessment of Dementia; PA, physical activity; WM, white matter.
Fleming et al. 34 used a GT9X Actigraph accelerometer 41 to monitor time of sedentary and moderate‐to‐vigorous physical activity; they noted significant associations between physical activity and brain white matter integrity. The study concluded that adults with DS who routinely engaged in moderate‐to‐vigorous activity and/or less time in sedentary behavior demonstrated higher executive functioning, episodic memory, and visuospatial construction ability and fewer symptoms of dementia than did adults with DS who were less physically active. Pape et al. 27 had a similar study aim, monitoring high‐, moderate‐, and low‐intensity exercise weekly, with an additional measure of semi‐structured interviews to capture the activity data. Study participants who reported moderate levels of exercise at baseline had a 62% reduced risk of declines in memory and orientation. Both studies indicated moderate‐to‐high/vigorous intensity exercise could reduce the risk of clinically detectable cognitive decline in a DS population, with possible long‐term benefits. 27 , 34
Ptomey et al. 37 and Van Pelt et al. 29 used exploratory methods to ascertain the level of exercise needed to affect cognition and/or influence ADRD pathology. 28 , 29 , 37 Ptomey et al. 37 investigated the optimal dosage of physical activity through the use of a Fitbit over the course of 12 weekly virtual group‐exercise sessions. This study noted that individuals who attended two group sessions per week improved their visual memory and new learning performance tasks post intervention. Van Pelt et al. 29 used the GAITRite system 40 to evaluate whether dual‐task and gait‐performance assessments were feasible to conduct with adults with DS, and whether they were associated with age and cognitive impairment, specifically determining the optimal dose of weekly sessions needed to see changes in cognitive function. This study noted, when controlling for intellectual disability and sex, that gait‐performance and dual‐task effects were associated with clinical measures of cognition; and measured gait velocity had stronger associations with clinical dementia than age or level of intellectual disability.
Overall, recommendations were: longitudinal studies are needed to determine whether physical activity promotes healthy aging in DS, and randomized controlled trials are needed to examine changes in cognitive function in individuals receiving physical activity interventions. 37 Assessments that incorporate dual‐task gait, 29 physical activity, 29 , 37 cognitive functioning, 27 , 29 , 34 , 37 and behavioral patterns 27 , 34 are warranted in future studies. The PAMC studies noted that the intensity, performance, and/or frequency of physical activity has modifiable factors to detect and/or affect cognition and/or AD pathology in adults with DS.
3.2. Cognition
The application of cognition in real‐life contexts can be both in constructs of function and cognition relevant to the diagnosis and staging of the progression of AD. 3 , 7 , 42 , 43 Cognition is often assessed using self‐reported or clinician‐administered questionnaires focused on neuropsychological assessments, level of intellectual disability (and/or IQ), visuospatial memory, recall memory, orientation, working memory, hand‐eye coordination, speech, and adaptive abilities (activities of daily living). 3 , 5 , 19 , 30 Six studies provided a comprehensive battery of how cognition is assessed and defined in ADRD pathology. 20 , 27 , 32 , 35 , 37 , 39 Aschenbrenner et al., 20 Firth et al., 35 Oliver et al., 38 and Startin et al. 39 specifically focused on preclinical AD assessment and profile development through a cross‐sectional study design. The research questions addressed by these studies included: What tests/factors are most sensitive for detecting early cognitive change in adults with DS? 20 What factors characterize the cognitive deterioration associated with the development of AD in DS? 35 What is the prevalence of age‐specific data for acquired mild cognitive impairment in adults with DS? 38 What are the risks and protective factors for the development of the clinical signs of dementia in DS? 39
First, Aschenbrenner et al. 20 and Startin et al. 39 investigated functional cognitive measures, which are the most sensitive to detect early cognitive change in adults with DS. Aschenbrenner et al. 20 noted early markers of cognitive change of AD in DS including prominent declines in memory, language, attention, and praxis; they appeared to be comparable to declines seen in other forms of AD, including sporadic AD and autosomal‐dominant AD. Startin et al. 39 demonstrated how a battery of general cognitive ability, memory, executive function, motor coordination, and vision and hearing was suitable for a range of assessments for adults with DS when investigating cognitive abilities and changes in cognitive abilities associated with aging and dementia. 39
Second, Firth et al., 35 Oliver et al., 38 and Startin et al. 28 specifically grouped their participants into categories based on age to investigate the level of cognitive deterioration associated with the development of AD in DS. Firth et al. 35 noted that declines in performance of memory and attention measures were most sensitive to aging, as determined by an event‐based model design developed through biomarker data from cognitive tests and informant questionnaires. Oliver et al. 38 concluded that ≈40% to 45% of adults with DS over age 40 will demonstrate mild memory impairment beyond that expected given the degree of intellectual disability, with a significant increased risk in those 46 years of age and over. Startin et al. 28 had a similar outcome, where memory and attention demonstrated the earliest cognitive decline by ≥40 years of age.
Finally, Firth et al., 35 Krinsky‐McHale et al., 30 and Startin et al. 28 investigated the prevalence of cognitive and functional decline during preclinical (asymptomatic) to prodromal dementia stages and from prodromal to clinical dementia. Startin et al. 28 noted that negative changes in memory and attention outcomes were most sensitive to progression from preclinical to prodromal dementia, which affirmed the findings of Krinsky‐McHale et al., 30 who also noted a negative change in the areas of visual motor performance and semantic (general) memory in adults with DS and mild cognitive impairment. Firth et al. 35 also noted negative changes in memory, attention, and motor coordination in adults with DS and cognitive decline during progression to early AD.
Recommendations to address risks and enact protective factors during the development of the clinical stages of AD in DS were: the use of cognitive batteries, comprehensive assessment, and AD staging based on an event‐based model could help clinicians track decline during the early stages of cognitive decline in DS. 20 , 35 , 39 In addition, intervention studies that consider age, memory, motor coordination, and attention are useful measures to track progression in the preclinical to prodromal stages of AD in DS and may provide the most accurate outcome measures that are the most sensitive to cognitive decline 28 , 35 , 38 ; and clinician insight will continue to be vital during assessment and intervention, and the use of framework models that include other sources of information regarding the lived experiences of adults with DS are vital to identifying early signs and progression of AD. 20 , 30 , 35
3.3. Behavior
Behavioral and psychological symptoms of dementia are common among adults aging with DS and may also serve as early detection of ADRD. 31 , 32 , 38 , 44 Dekker and colleagues 35 , 36 investigated the frequency and severity of behavioral changes through the use of a comprehensive scaled analysis of behavioral items—and a structured interview, as well as a magnetic resonance imaging (MRI) scan. They focused exclusively on behavior, specifically behavior possibly related to AD. The outcomes noted were increased frequency and severity of the following areas of behavior: anxiety, sleep disturbances, agitation and stereotypic behavior, aggression, apathy, depressive symptoms, and eating/drinking behavior. The studies noted that behavioral changes occur and are reported in both early and prodromal stages of dementia, serving as an early alert for additional assessment. 31 Further evaluation of behavioral changes in DS using comprehensive behavioral assessments may contribute to early identification of dementia and could be a useful addition to already existing cognitive questionnaires for dementia in DS. 31 , 32
3.4. Sleep
Sleep disturbances are seen in the early phase of AD, along with irritability and apathy. 32 , 33 The research aim of Cody et al. 33 was to investigate associations among disrupted sleep, Aβ accumulation, and cognitive features of preclinical AD in DS. A comprehensive battery of assessments was used to evaluate motor skills, memory, recall, level of activity, executive functioning, and MRI. This cross‐sectional study concluded that sleep disruption, specifically length of awakening at night, was associated with poor memory, executive functioning, and motor planning and coordination in adults with DS in the preclinical stage of AD.
Fleming et al. 36 used a GT9X Actigraph accelerometer 41 in a manner similar to that of their physical activity study to monitor sleeping patterns in a cohort of middle‐aged adults with DS adults without dementia over seven nights, along with a caregiver report of obstructive sleep apnea. This study hypothesized that adults with DS who demonstrated a higher rate of disrupted sleep (e.g., lower sleep time and sleep efficiency) and/or reported a medical diagnosis of obstructive sleep apnea would evidence greater white matter degeneration than those without disrupted sleep. Partial correlations indicated that more disrupted sleep was associated with lower white matter integrity in the cohort. Ultimately, this study noted sleep as a modifiable factor and highlighted both disrupted sleep and white matter impairment in ADRD in DS. 36
4. DISCUSSION
This scoping review examined the evidence of a range of functional abilities and outcomes related to DS and ADRD pathology. The significance of the themes of PAMC, cognition, behavior, and sleep were discussed with specific outcomes and future directions. Based on our findings and synthesis of the information, we have addressed each of our research questions.
4.1. Age‐related functional changes
Research Question 1: What are the age‐related functional changes in adults with DS in relation to ADRD? Findings regarding age‐related functional changes involved physical activity, memory and attention, behavioral changes, and disrupted sleep. Studies that investigated physical activity found that group exercise had positive effects on cognitive function 29 , 37 ; high levels of physical activity were associated with a reduced risk of behavioral decline 27 ; and sedentary behavior and activity were related to cognitive functioning and white matter development. 34 In addition, physical activity has been shown to activate glial cells (a type of cell that provides physical and chemical support to neurons and maintains their environment), which are important in cleaning up Aβ plaques within the brain. 45 , 46 It is believed that these glial cells become more dormant/inactive as one ages, and exercise can reactivate these glial cells in aging adults. 46 Memory and attention measures were most sensitive to aging, and early markers of cognitive change in AD in DS included memory, language, and attention. 20 , 28 , 30 , 35 , 38 Behavioral changes occurring in early AD were irritability, sleep disturbances, and apathy. 31 Finally, disrupted sleep correlated with both Aβ accumulation of preclinical AD in DS 33 and with lower white matter integrity in adults with DS without dementia. 36
4.2. Current evidence of the risk of falling and falls
Research Question 2: What is the current evidence investigating the risk of falling and falls among adults aging with DS and ADRD? Van Pelt et al. 29 was the only study to our knowledge that investigated dual‐task gait and functional mobility in aging adults with DS. Our study team did not find any additional studies within the search period that addressed fall risk and/or falls incidents. There is limited evidence on the changes in physical function that happen to adults aging with DS prior to any marked AD symptoms. 5 , 47 Growing evidence suggests that functional mobility abnormalities and an increase in falls may precede cognitive impairment and marked AD symptoms. 9 , 48 The relationship between falls and cognition in adults with DS has not been investigated thoroughly. 9 These measures could potentially serve as a direct and less‐invasive method to assess adults aging with DS for impaired cognition. 48 , 49 , 50 The National Institute on Aging (NIA) has called for basic studies to improve the understanding of the genetic and biological causes that lead to AD, including observational research to measure cognitive and functional changes over time. There is a need to explore functional measures such as fall risk, fall incidents, and functional mobility to gain a better understanding of the role they play in the progression of AD in DS. 1 , 8 , 9 Falls and functional mobility are associated with clinical measures of cognition and are a non‐cognitive behavioral biomarker of preclinical AD and AD. 9 , 16 , 18 , 29 A better understanding of falls and functional mobility could inform the non‐cognitive biomarker profile in adults with DS through a direct and less‐invasive method of assessment, thus reducing the burden for both the participant and caregivers. 5 , 9 , 51
4.3. Limitations
The data for this scoping review consist of a small range of studies and methods to create a summary of the current evidence; a potential future scoping review on the broader category of falls in individuals with intellectual and/or developmental disability (IDD), could include a broader scope of individuals with DS. The research team decided to limit the studies from the past 10 years to provide a recent scope of the literature; previous comprehensive and systematic reviews provide a synopsis relevant studies in prior years. 11 , 20 , 52 , 53 This indicates a need for additional studies to confirm and strengthen confidence in these findings. There are specific challenges to research investigating functional activity performance and engagement. These challenges include heterogeneity within the population; variability of responses among participants, caregivers, and/or family members; and accuracy of informant‐based questionnaires, along with the complexity and interpretation of findings. 54 Current research most commonly involves those who have a mild or moderate cognitive impairment status, and findings may not be generalizable to those with more severe cognitive impairment, or those from underrepresented racial or lower socioeconomic status. 54 , 55
5. CONCLUSION
This scoping review describes the extent and themes of the research evidence on AD pathology in adults with DS relative to functional outcomes. The emergent themes from this scoping review highlight functional measures and interventions used to detect and monitor the rate of cognitive decline and AD progression in the aging DS population. 27 , 29 , 31 , 34 , 37 , 56 This scoping review was also designed to identify the literature describing the relationship between falls and functional mobility and ADRD in adults aging with DS; however, there was no study that focused on this area of function. Our intent was to enhance our understanding of real‐world functional measures of preclinical AD, specifically falls, as a potential unknown risk factor for developing preclinical AD in adults with DS. This line of inquiry is similar to the prevalence of falls in older adults and preclinical AD. 9 , 17 , 18 In addition, this line of research could inform the timing of interventions in prevention trials in AD as well as the development of effective intervention for individuals with DS at risk for progression to symptomatic AD. Currently it is recommended AD screening should be initiated at age 40 years in adults with DS, with the assessment of risk factors for cardiovascular disease, stroke, screening for obesity, and secondary causes of osteoporosis (e.g., low bone mineral density). 57 However, further research incorporating functional, clinical, and adaptive behaviors related to cognitive decline is essential to understanding how AD progression is characterized within real‐world settings. 1 , 20 , 28 The scientific evidence that will emerge from these continued research initiatives will help advance efforts toward effective AD intervention and facilitate understanding among adults with DS, their caregivers and family members, and clinicians. 1 , 3 , 13 , 58
CONFLICTS OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. All authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
CONSENT STATEMENT
Human subject consent was not necessary for this scoping review.
ACKNOWLEDGMENTS
This manuscript is funded by the U.S. National Institute on Aging, Falls: A marker of preclinical Alzheimer's disease, 1R01AG057680‐01A1; and Research Supplement to Promote Diversity in Health‐Related Research, R01AG057680‐05S1, Evaluation of Falls as a Behavioral Biomarker for Preclinical Alzheimer's Disease in Adults Aging with Down syndrome (DS).
Washington SE, Cler E, Lowery C, Stark SL. Down syndrome and Alzheimer's disease: A scoping review of functional performance and fall risk. Alzheimer's Dement. 2023;9:e12393. 10.1002/trc2.12393
REFERENCES
- 1. Alldred MJ, Martini AC, Patterson D, et al. Aging with Down syndrome—where are we now and where are we going? J Clin Med. 2021;10(20):4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartley SL, Handen BL, Devenny DA, et al. Cognitive functioning in relation to brain amyloid‐β in healthy adults with Down syndrome. Brain. 2014;137(Pt 9):2556‐2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartley D, Blumenthal T, Carrillo M, et al. Down syndrome and Alzheimer's disease: common pathways, common goals. Alzheimers Dement. 2015;11(6):700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer's Association 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391‐460. [Google Scholar]
- 5. Handen BL, Lott IT, Christian BT, et al. The Alzheimer's biomarker consortium—Down syndrome: rationale and methodology. Alzheimer Dement. 2020;12(1):e12065‐e12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lott IT. Neurological phenotypes for Down syndrome across the life span. Prog Brain Res. 2012;197:101‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartley SL, Handen BL. Devenny D, et al. Cognitive decline and brain amyloid‐β accumulation across 3 years in adults with Down syndrome. Neurobiol Aging. 2017;58:68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stark S, Roe C, Grant E, et al. P1‐201: risk of falls among older adults with preclinical Alzheimer's disease. Alzheimers Dement 2011;7:S176‐S176. [Google Scholar]
- 9. Stark SL, Roe CM, Grant EA, et al. Preclinical Alzheimer disease and risk of falls. Neurology. 2013;81(5):437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Graaf G, Buckleyand F. Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017;19(4):439‐447. [DOI] [PubMed] [Google Scholar]
- 11. Hartley D, Blumenthal T, Carrillo M, et al. Down syndrome and Alzheimer's disease: common pathways, common goals. Alzheimer Dement. 2015;11(6):700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snyder HM, Bain LJ, Brickman AM, et al. Further understanding the connection between Alzheimer's disease and Down syndrome. Alzheimers Dement. 2020;16(7):1065‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCarron M, McCallion P, Reilly E, et al. A prospective 20‐year longitudinal follow‐up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61(9):843‐852. [DOI] [PubMed] [Google Scholar]
- 14. Van Pelt KL, Koehl L, Caban‐Holt A, et al. Feasibility of dual‐task gait to estimate Alzheimer's related cognitive decline in Down syndrome. Alzheimers Dement. 2020;12(1):e12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hocking DR, Menant JC, Kirk HE, et al. Gait profiles as indicators of domain‐specific impairments in executive control across neurodevelopmental disorders. Res Dev Disabil. 2014;35(1):203‐214. [DOI] [PubMed] [Google Scholar]
- 16. Gonçalves AS, Carvalho CL, Ramos AS, et al. Role of gait and speed performance in predicting cognitive decline and dementia in people with Down syndrome: preliminary findings. Alzheimer Dement. 2020;16(S6):e047466. [Google Scholar]
- 17. Bollinger RM, Keleman A, Thompson R, et al. Falls: a marker of preclinical Alzheimer disease: a cohort study protocol. BMJ Open. 2021;11(9):e050820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keleman A, Wisch JK, Bollinger RM, et al. Falls associate with neurodegenerative changes in ATN framework of Alzheimer's disease. J Alzheimers Dis. 2020;77(2):745‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carfì A, Antocicco M, Brandi V, et al. Characteristics of adults with down syndrome: prevalence of age‐related conditions. Front Med. 2014;77(2):745‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aschenbrenner AJ, Baksh RA, Benejam B, et al. Markers of early changes in cognition across cohorts of adults with Down syndrome at risk of Alzheimer's disease. Alzheimers Dement (Amst). 2021;13(1):e12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arksey H, Malley LO. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. [Google Scholar]
- 22. Tricco, AC , Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 23. Washington S. Down syndrome and Alzheimer's disease: a scoping review of functional performance and fall risk. osf.io/kjgw2. Published April 26, 2023. [DOI] [PMC free article] [PubMed]
- 24. Aromataris E, Munn Z. JBI Manual for Evidence Synthesis [Internet]. Adelaide: JBI; 2020. [Google Scholar]
- 25. Levac D, Colquhounand KKH. Brien O. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77‐101. [Google Scholar]
- 27. Pape SE, Baksh RA, Startin C, et al. The association between physical activity and CAMDEX‐DS changes prior to the onset of Alzheimer's disease in Down syndrome. J Clin Med. 2021;10(9):1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer's disease in Down syndrome. Alzheimers Dement. 2019;15(2):245‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Pelt KL, Koehl L, Caban‐Holt A, et al. Feasibility of dual‐task gait to estimate Alzheimer's related cognitive decline in Down syndrome. Alzheimers Dement (Amst). 2020;12(1):e12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krinsky‐McHale SJ, Zigman WB, Lee JH, et al. Promising outcome measures of early Alzheimer's dementia in adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(1):e12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dekker AD, Ulgiati AM, Groen H, et al. The behavioral and psychological symptoms of dementia in down syndrome scale (BPSD‐DS II): optimization and further validation. J Alzheimers Dis. 2021;81(4):1505‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dekker A.D, Sacco S, Carfi A, et al. The behavioral and psychological symptoms of dementia in down syndrome (BPSD‐DS) scale: comprehensive assessment of psychopathology in Down syndrome. J Alzheimers Dis. 2018;63(2):797‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cody KA, Piro‐Gambetti B, Zammit MD, et al. Association of sleep with cognition and beta amyloid accumulation in adults with Down syndrome. Neurobiol Aging. 2020;93:44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fleming V, Piro‐Gambetti B, Patrick A, et al. Physical activity and cognitive and imaging biomarkers of Alzheimer's disease in down syndrome. Neurobiol Aging. 2021;107:118‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Firth NC, Startin CM, Hithersay R, et al. Aging related cognitive changes associated with Alzheimer's disease in Down syndrome. Ann Clin Transl Neurol. 2018;5(6):741‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fleming V, Piro‐Gambetti B, Bazydlo A, et al. Sleep and white matter in adults with Down SYNDROME. Brain Sciences. 2021;11(10):1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ptomey L.T, Szabo A.N, Willis E.A, et al. Changes in cognitive function after a 12‐week exercise intervention in adults with Down syndrome. Disabil Health J. 2018;11(3):486‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliver C, Adams D, Holland AJ, et al. Acquired mild cognitive impairment in adults with Down syndrome. Int J Geriatr Psychiatry. 2021;37(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Startin CM, Hamburg S, Hithersay R, et al. The LonDownS adult cognitive assessment to study cognitive abilities and decline in Down syndrome. Wellcome Open Res. 2016;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. Physical therapy. 2009;89(9):873‐880. [PubMed] [Google Scholar]
- 41. ActiGraph User guide: ActiGraph GT9X Link+ ActiLife. 2019.
- 42. Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(S1):173‐176. [DOI] [PubMed] [Google Scholar]
- 43. Keleman AA., Bollinger RM, Wisch JK, et al. Assessment of instrumental activities of daily living in preclinical Alzheimer disease. OTJR. 2022;42(4):277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prasher V, Farooqand RA. Holder The Adaptive Behaviour Dementia Questionnaire (ABDQ): screening questionnaire for dementia in Alzheimer's disease in adults with Down syndrome. Res Dev Disabil. 2004;25(4):385‐397. [DOI] [PubMed] [Google Scholar]
- 45. García O, Flores‐Aguilar L. Astroglial and microglial pathology in Down syndrome: focus on Alzheimer's disease. Front Cell Neurosci. 2022;16:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valles S.L., Iradi A, Aldasoro M, et al. Function of glia in aging and the brain diseases. Int J Med Sci. 2019;16(11):1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hartley D, Blumenthal T, Carrillo M, et al. Down syndrome and Alzheimer's disease: common pathways, common goals. Alzheimers Dement. 2015;11(6):700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keleman AA., Bollinger RM, Ances BM, et al. Amyloid accumulation associated with worse performance of complex task. In 2021 Alzheimer's Association International Conference. 2021; ALZ.
- 49. Keleman A, Wisch JK, Bollinger RM, et al. Falls associate with neurodegenerative changes in ATN framework of Alzheimer's disease. J Alzheimers Dis. 2020;(Preprint):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mank A, van Maurik IS, Bakker ED, et al. Identifying relevant outcomes in the progression of Alzheimer's disease; what do patients and care partners want to know about prognosis? Alzheimers Dement. 2021;7(1):e12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Snyder HM, Bain LJ, Brickman AM, et al. Further understanding the connection between Alzheimer's disease and Down syndrome. Alzheimers Dement. 2020;16(7):1065‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lautarescu BA, Hollandand AJ, Zaman SH. The early presentation of dementia in people with Down syndrome: a systematic review of longitudinal studies. Neuropsychol Rev. 2017;27(1):31‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carfì A, Antocicco M, Brandi V, et al. Characteristics of adults with Down syndrome: prevalence of age‐related conditions. Front Med. 2014;1(51). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baumer NT, Becker ML, Capone GT, et al. Conducting clinical trials in persons with Down syndrome: summary from the NIH INCLUDE Down syndrome clinical trials readiness working group. J Neurodev Disord. 2022;14(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lennon JC, Aita SL, Bene VAD, et al. Black and White individuals differ in dementia prevalence, risk factors, and symptomatic presentation. Alzheimers Dement. 2022;18(8):1461‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silverman W, Krinsky‐McHale SJ, Lai F, et al. Evaluation of the national task group‐early detection screen for dementia: sensitivity to ‘mild cognitive impairment'in adults with down syndrome. J Appl Res Intellect Disabil. 2021;34(3):905‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsou AY, Bulova P, Capone G, et al. Medical care of adults with Down syndrome: a clinical guideline. Jama. 2020;324(15):1543‐1556. [DOI] [PubMed] [Google Scholar]
- 58. Dekker AD, Coppus AM, Vermeiren Y, et al. Serum MHPG strongly predicts conversion to Alzheimer's disease in behaviorally characterized subjects with Down syndrome. J Alzheimers Dis. 2015;43(3):871‐891. [DOI] [PubMed] [Google Scholar]


