Hair cortisol concentration (HCC) can be a valuable indicator of stress in wildlife, but how to best implement this biomarker into existing caribou monitoring programs is unclear. Our results show that HCC is useful in understanding inter-annual and inter-population differences in caribou cortisol, which can inform future caribou management.
Keywords: wildlife monitoring, wildlife health, stress, oestrid, mosquito, Arctic
Abstract
Migratory caribou (Rangifer tarandus sspp.) is an ecotype of conservation concern that is experiencing increased cumulative stressors associated with rapid climate change and development in Arctic Canada. Increasingly, hair cortisol concentrations (HCCs) are being used to monitor seasonal hypothalamic–pituitary–adrenal axis activity of ungulate populations; yet, the effect of key covariates for caribou (sex, season, sampling source, body location) are largely unknown. The objectives of this research were 4-fold: first, we assessed the impact of body location (neck, rump) sampling sites on HCC; second, we assessed key covariates (sex, sampling method, season) impacting HCCs of caribou; third, we investigated inter-population (Dolphin and Union (DU), Bluenose-East (BNE)) and inter-annual differences in HCC and fourth, we examined the association between HCCs and indices of biting insect activity on the summer range (oestrid index, mosquito index). We examined hair from 407 DU and BNE caribou sampled by harvesters or during capture-collaring operations from 2012 to 2020. Linear mixed-effect models were used to assess the effect of body location on HCC and generalized least squares regression (GLS) models were used to examine the impacts of key covariates, year and herd and indices of biting insect harassment. HCC varied significantly by body location, year, herd and source of samples (harvester vs capture). HCC was higher in samples taken from the neck and in the DU herd compared with the BNE, decreased linearly over time and was higher in captured versus hunted animals (P < 0.05). There was no difference in HCC between sexes, and indices of biting insect harassment in the previous year were not significantly associated with HCC. This study identifies essential covariates impacting the HCC of caribou that must be accounted for in sampling, monitoring and data interpretation.
Introduction
Migratory caribou (Rangifer tarandus sspp.) is a keystone ecotype in the Arctic that provides significant ecological, economic and cultural benefits to northern communities and ecosystems (Lyver, 2005; Côté et al., 2010). Many migratory caribou populations have enigmatically and severely declined (by 70–90%) in the last decade (COSEWIC, 2016; COSEWIC, 2017; Campbell et al., 2021). These declines may be associated with an increase in cumulative stressors, such as rising temperatures, increased frequency of extreme weather events and anthropogenic development in the Arctic environment (Vors and Boyce, 2009; Festa-Bianchet et al., 2011; Gunn et al., 2014; Fauchald et al., 2017), among other stressors. Biting insect harassment is also a documented stressor of caribou that is anticipated to increase under current Arctic climate change scenarios of warmer temperatures, longer growing seasons and increasing precipitation (Witter et al., 2012).
Stress responses in wildlife can be reflected in certain biomarkers of hypothalamic–pituitary–adrenal (HPA) axis activity, such as circulating glucocorticoids (GCs) (Baker et al., 2013). GCs are involved in coordinating multiple physiological pathways in response to acute and chronic stress, including the mobilization of energy stores in mammals (Busch and Hayward, 2009). Prolonged and chronic elevation of GC concentrations can result in deleterious impacts such as reduced immunocompetence as well as decreased reproduction in mammals (Bonier et al., 2009; Busch and Hayward, 2009). The dominant GC circulating in Rangifer is cortisol (Koren et al., 2012), and this has previously been measured in the serum, feces and hair of Rangifer sspp. (Omsjoe et al., 2009; Johnson et al., 2010; Ashley et al., 2011; Bondo et al., 2019).
Hair cortisol concentration (HCC) is a promising biomarker that reflects chronic or cumulative stress experienced over weeks to months (Gormally et al., 2020). Cortisol is believed to be incorporated into the hair shaft passively from the bloodstream during the anagen (active) hair growth phase, reflecting the HPA axis activity during that period (Russell et al., 2012). Hair is a practical sample type to collect from caribou because it is a highly stable medium that can be easily transported and stored at room temperature (Felicetti et al., 2003; Jaspers et al., 2010), and it is already collected through community-based monitoring programs and during capture and collaring of caribou (Kutz et al., 2013; Jutha et al., 2022). Hair growth in Rangifer, although not precisely documented, occurs between June and October (Cuyler and Oritsland, 2002; Macbeth, 2013), when migratory caribou herds occupy their summer and fall ranges.
HCC as a biomarker of seasonally circulating cortisol has not been validated in Rangifer due to methodological constraints (Ashley et al., 2011). However, studies in other free-ranging ungulates (Oreamnos americanus, Ovibos moschatus) that used sequential Adrenocorticotropic hormone (ACTH) injections during the hair growth period to simulate chronic HPA axis stimulation demonstrated higher HCC in the hair of experimentally treated animals compared with controls (Dulude-de Broin et al., 2019; Di Francesco et al., 2021). HCCs were previously used in Rangifer to investigate associations with body condition (Macbeth, 2013) and to monitor the effects of environmental disturbance (Ewacha et al., 2017). However, there is a paucity of information examining key covariates (sex, season, sampling method, body location) of HCC that need to be considered to interpret HCC results in free-ranging Rangifer.
In this study, we sought to assess the use of hair cortisol as a biomonitoring tool in caribou and to examine the association between HCCs and indices of biting insect activity in the summer ranges of two migratory caribou herds: the Bluenose-East (BNE) (Rangifer tarandus groenlandicus) and the Dolphin and Union (DU) (R.t. groenlandicus x pearyi) herds. We asked four questions: (1) Is there a significant difference between HCCs of neck and rump hair sampled from the same individual in caribou? (2) What factors associated with the opportunistic sampling of caribou (sex, season, body location, sampling method) are significant covariates of HCC? (3) Does HCC exhibit annual trends and inter-population differences? And (4) is hair cortisol positively associated with the intensity of biting insect harassment?
Methods
Study area and sample collection
The study populations were the BNE herd, which ranges east of Great Bear Lake and west of Kugluktuk, Northwest Territories (NWT), and Nunavut (NU), and the DU herd, which ranges on Victoria Island and mainland NU and NWT (Committee on the Status of Endangered Wildlife in Canada (COSEWIC, 2016) (Fig. 1). The BNE and DU herds have been federally assessed as threatened and endangered, respectively (COSEWIC, 2016, 2017). Hair samples from both herds were collected and archived by two opportunistic sampling methods. The first method was by subsistence hunters through community-based monitoring programs from 2012 to 2021 from the communities of Délįnę, Norman Wells and Ulukhaktok of the NWT and from Kugluktuk and Iqaluktuuttiaq, NU. The second method was by convenience sampling during live capture and collaring activities performed by the Governments of the NWT and NU. The duration of caribou capture is ~15 min, including hair sampling (Cattet, 2018). Paired neck (lateral side) and rump samples from 52 individual caribou were collected in 2019 during a DU caribou harvest (n = 18) and in 2021 during a capture/collaring project (n = 34).
Figure 1.
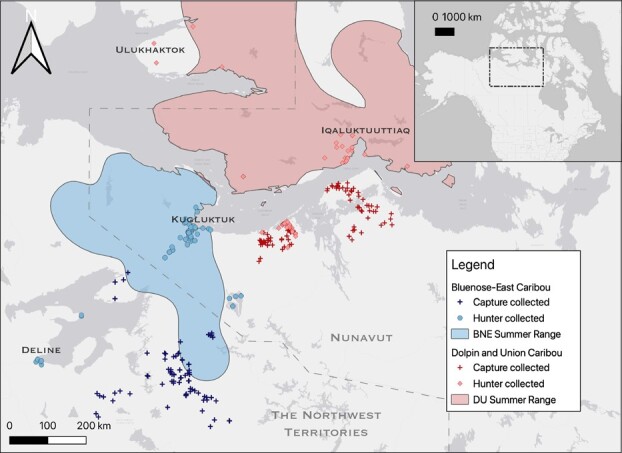
Map of BNE (blue) and DU (red) sampling locations, as collected by hunters (circles) and on captures (crosses) between 2012 and 2020. Summer ranges were delineated from CircumArctic Rangifer Monitoring and Assessment Network database (Russell et al., 2013).
Hunted samples were collected year-round, but predominantly during common harvesting periods: the spring/summer for the BNE and fall/spring for the DU. Hair samples from hunted animals were collected almost exclusively from the neck and/or rump as requested in the collection protocol. Samples from captured caribou were collected during March for the BNE herd and April for the DU herd. Hair samples collected during capture from the BNE were taken from various body locations, including the neck, rump and shoulder, whereas DU capture samples were taken consistently from the neck and rump. Samples collected from capture were generally of plucked hair that contained a hair bulb and follicle. Hair samples collected by hunters were predominantly submitted on the hide, and hair was shaved as close to the skin as possible using a stainless steel razor. A sufficient mass of hair for analysis was collected and submitted from 201 BNE caribou (84 hunted, 117 captured) and 206 DU caribou (116 hunted and 90 captured). Samples from the BNE herd were approximately equal between sexes (F = 106, M = 97), however, for the DU herd, predominantly female caribou were sampled (F = 171, M = 35).
Sample processing and laboratory analysis
All hair cortisol quantification was performed by the Toronto Zoo’s Endocrinology Laboratory (Scarborough, Canada). Hair decontamination, preparation and steroid extraction were completed as described by Mastromonaco et al. (2014) and used by Di Francesco et al. (2021). Briefly, hair samples were examined under a dissecting microscope at 10× power, and hair follicles, bulbs and undercoat, if present, were manually removed such that solely the guard hair remained. Samples were washed by immersing them in 700-ml plastic containers filled with distilled water, rubbed by a gloved hand for 2 min and then dried in a paper towel. Hair was then placed in 20-ml glass vials, vortexed with 15 ml of distilled water for 10 s, then soaked for 5 min. The liquid was removed, and another 15 ml of distilled water was added, vortexed for 10 s and then immediately removed. Finally, 15 ml of 100% methanol was added, vortexed for 10 s and removed immediately. Completely washed hair was dried in a paper towel and then stored in paper envelopes at room temperature.
Washed and dried hair was cut into 5-mm pieces and weighed into 7-ml scintillation vials. Fifty milligrams of hair were extracted with 100% methanol, for a ratio of 0.01 g of hair/ml of methanol, on a rotator plate (MBI Lab Equipment orbital shaker, 100 rpm) at room temperature for 24 h. Samples were centrifuged for 5 min (at 2400g), and the supernatant was pipetted into new glass 7-ml vials. Supernatants (hair extracts) were then stored at −20°C. For cortisol quantification, stored hair extracts were brought to room temperature, and 1500 μl of hair extract was evaporated in a fume hood; the dried extract was then reconstituted using 150 μl of enzyme immune-buffer forming a 10× concentration. Cortisol was quantified using EIAs previously described by Majchrzak et al. (2015) and Kummrow et al. (2011) and used by Di Francesco et al. (2021), and all samples and standards were run in duplicate.
Data processing
Depending on the date of hair collection, hair was either representative of the current or previous year of guard hair growth (Fig. 2), requiring hair growth year to be individually classified. Active guard hair growth is understood to occur between June and September in Rangifer (Cuyler and Oritsland, 2002; Macbeth, 2013). Thus, hair collected between October and December was classified as current year hair growth and hair collected between January and June was classified as previous year growth. Hair collected between July and the end of August (n = 33), during the period of active growth, was classified based on hair length and colour (i.e. long, white, damaged hair signified previous growth, and short, brown hair: current year growth).
Figure 2.
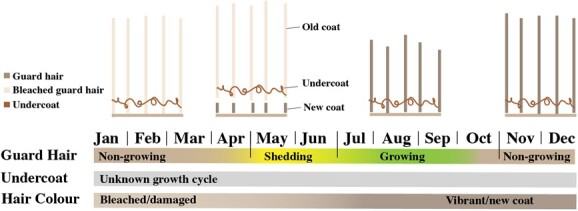
Summary of annual caribou guard hair growth cycle (Cuyler and Oritsland, 2002; Macbeth, 2013). Guard hair is primarily shed (May/June) and grown (June–September) once annually. A fine woollen undercoat is also present in caribou pelage (Ewacha et al., 2017); however, the growth cycle is unknown. Caribou guard hair tends to become bleached and damaged as the hair ages over winter (Macbeth, 2013, Rakic personal obs.).
Sample and demographic information such as date of sample collection, location and sex of animal were obtained from hunter forms and datasheets completed by government wildlife handling staff. Ordered month categories, beginning in September when hair was considered new growth, were created for data analyses. These categories were: 1 = September/October, 2 = November/December, 3 = January/February, 4 = March/April, 5 = May/June and 6 = July/August. Month categories were treated as continuous to test the effect of seasonal changes in hair on HCC. Year was assigned based on hair growth year, not the year of sample collection or analysis. Age was determined based on tooth eruption patterns (calf, sub-adult, or adult) (Miller, 1972) or as reported on hunter and/or capture forms. Only adults (>3 years old) and sub-adults (1–3 years old) were included in statistical analyses; 8 calves (<1 year old) were removed (6 DU, 2 BNE). Furthermore, solely animals of known sex were included, removing a further 10 animals (all BNE caribou), resulting in a sample size of 407 animals for data analysis.
Oestrid and mosquito activity indices for the BNE and DU summer ranges were determined following the guide provided by Witter et al. (2014) and were obtained from the CircumArctic Rangifer Monitoring and Assessment Network MERRA database (Russell et al., 2013). Indices were restricted to the period of biting insect activity from June 15 to September 1 (Witter et al., 2014), and the average activity for that period was calculated. If a caribou was harvested between June and September, then the mean insect harassment index was calculated up to the date of harvest for that individual animal (i.e. an animal harvested on August 18 would have average harassment from June 15 to August 18). HCCs were log-transformed to satisfy assumptions of linear regression. Samples below the limit of hair cortisol quantification (n = 19), reported as <1.00, were treated as low values and assigned a value of 0.5 pg/mg (Vikøren et al., 2011).
Statistical analysis
All analyses were completed in R version 3.6.0 (R Core Team, 2020). To determine the effects of body location (rump or neck), we used a paired hair dataset from 34 captured adult female DU caribou and 18 hunted (5 male, 13 female) DU caribou, totaling 52 pairs (n = 104 total). We compared HCCs in hair from the rump and neck by first using a Wilcoxon signed-rank test to test for differences in rank distribution. Second, an F-test was used to compare possible differences in variance between body locations. Third, correlations between neck and rump hair concentrations were measured using Pearson correlations. Last, the differences by body location (rump or neck) were assessed using a linear mixed-effects model (lme4 R package (Bates et al., 2015). Animal ID was fit as a random effect to account for repeated sampling of the same individual (Paterson and Lello, 2003), and body location (neck/rump) was a fixed effect explaining the log HCC in 52 animals. In a sensitivity analysis, outlier values were detected (n = 2) using a Rosner test using the EnvStats package (Millard, 2013), and all statistical analyses were repeated in the absence of outlier values.
Using the full dataset of all archived DU and BNE hair (n = 407 individuals), we assessed the association of multiple covariates with HCC. Models were fit with seven fixed effects: sex (male/female), herd (BNE/DU), month category (1–6), Oestrid index (OI), mosquito index (MI), body location (neck, rump, shoulder or unknown), sampling method (hunted/captured), and year (2012–2020). Ordered month categories (1–6) were treated as continuous to evaluate the effects of hair aging and seasonal change. Rump values were used for the DU caribou (n = 54), in which both neck and rump values were available. Mixed-effect linear regression was fit using the lme() function. However, after log-transformation, box-cox transformation and weighted regression, non-normality of residuals and heteroskedasticity of best fit models were detected. To overcome these barriers to analysis, generalized least squares regression (GLS), which is more robust to violations of standard regression assumptions (Beale et al., 2010), was done using the gls() function from “nmle” package. Based on multiple top models including different covariates and being within 2 Akaike Information Criterion (AICc) of one another, model averaging was used to generate covariate estimates, confidence intervals and statistical significance. The global model was fit, and all possible combinations of variables were fitted in separate models using ‘MuMIN’ package (Bartoń, 2020). The top models that were within 2 AICc of one another were averaged using the model.avg() function, and averaged effect sizes of included fixed effects were extracted. Lastly, collinearity was assessed by examining variance inflation factors and using a cut-off value of 5 (James et al., 2013).
Results
Eleven immune-assay plates were run between July 2019 and June 2021. Inter-assay coefficients of variability (CVs) were 17.1% (25% binding) and 20.1% (60% binding), and intra-assay CV was 8.5%. Hair cortisol ranged from <1.00 pg/mg to 51.89 pg/mg (Table 1).
Table 1.
Sample size (n), median and range (min, max) of guard HCC (pg/mg) from migratory caribou from the BNE and DU herds from 2012 to 2020
| 2012 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | ||
|---|---|---|---|---|---|---|---|---|---|
| BNE | n | 3 | 21 | 17 | 65 | 27 | 23 | 29 | — |
| med | 2.53 | 2.21 | 3.15 | 3.50 | 2.29 | 2.28 | 2.16 | — | |
| min | 1.52 | 1.06 | 1.17 | 0.50 | 0.50 | 0.50 | 0.50 | — | |
| max | 3.28 | 6.36 | 8.91 | 51.89 | 15.21 | 6.12 | 8.06 | — | |
| DU | n | — | — | — | — | 90 | 40 | 22 | 54 |
| med | — | — | — | — | 8.84 | 5.63 | 2.96 | 4.32 | |
| min | — | — | — | — | 2.91 | 1.54 | 0.50 | 1.41 | |
| max | — | — | — | — | 24.76 | 12.01 | 6.82 | 13.29 |
Body location and hair cortisol concentration
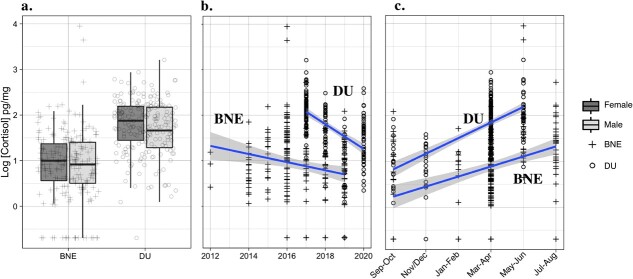
Cortisol concentrations were not significantly influenced by body locations using a Pearson (r = 0.24, P = 0.083) correlation (Fig. 3). However, on removal of two outlier values, cortisol concentrations between body locations were weakly correlated with one another (r = 0.29, P = 0.0043). Neck hair sample variance (4.88 pg/mg) tended to be greater than that of the rump hair (3.23 pg/mg), but these variances were not statistically different (F = 0.824, P = 0.492). Median rank values significantly differed and were higher in neck samples in a paired comparison (Wilcoxon P = 0.004). Results from linear model analyses (Supplementary Materials, Table S1, Table S2) demonstrate that body location was a significant covariate predicting HCC, and concentrations were higher in the neck hair (P < 0.001). All linear models adhered to the assumptions of linear regression analysis (Supplementary Materials, Figure S1).
Figure 3.
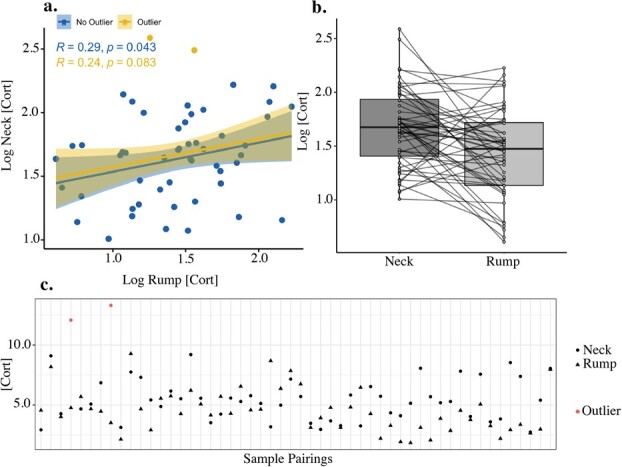
Comparison of log cortisol concentration (pg/mg) of caribou hair between paired neck and rump samples of 52 unique DU caribou from 2021. (a) Scatter plot comparing log neck and rump concentrations, with Pearson correlation (line), associated 95% confidence interval (shading), Pearson correlation (R) and associated P-value of outlier (yellow) and outlier-removed (blue) data. (b) Boxplot comparing log median neck and rump concentrations, lines connect data points from the same individual. (c) Scatter plot showing untransformed hair cortisol (pg/mg) between neck (circle) and rump (triangle) pairings and outliers (red).
HCC in migratory caribou
Of 256 total possible combinations of models to predict caribou HCC, the four top models were within 2 AICc of one another (Supplementary Materials, Table S3). Five parameters—year, herd, month, body location and sampling method—were significant predictors of HCC. Non-significant covariates included the indices of oestrid and mosquito activity and sex. Interaction terms, although included in the set of models, were not present within top fit models from which averages were derived.
Five covariates whose 95% confidence intervals did not include zero were significant: year (2012–2020), herd (DU/BNE), month category (1–6), body location (neck) and sampling method. Sex (P = 0.052) was borderline significant, and males were lower in HCC compared with females; however, when outliers were removed, sex was non-significant (P = 0.17) (Supplementary Materials, Table S5). The herd was a significant covariate in the four averaged models (P < 0.001), with the DU herd HCC being consistently higher compared with the BNE. Month category followed a positive linear trend in both herds such that HCC concentrations were increasing from September to October with a peak in May to June (Fig. 4) and was included in all top models. Year was a significant predictor of HCC (P = 0.04) and cortisol concentrations decreased linearly for both herds—2012–2019 for the BNE and 2017–2020 for the DU—and was included in three of the top models. The sampling method was included in all top models, and HCC was higher in captured animals (P = 0.04). Lastly, the mosquito and oestrid indices were included in all top models, but both estimates include zero in the 95% confidence interval and were therefore non-significant (Supplementary Materials, Table S4, Table S5).
Figure 4.
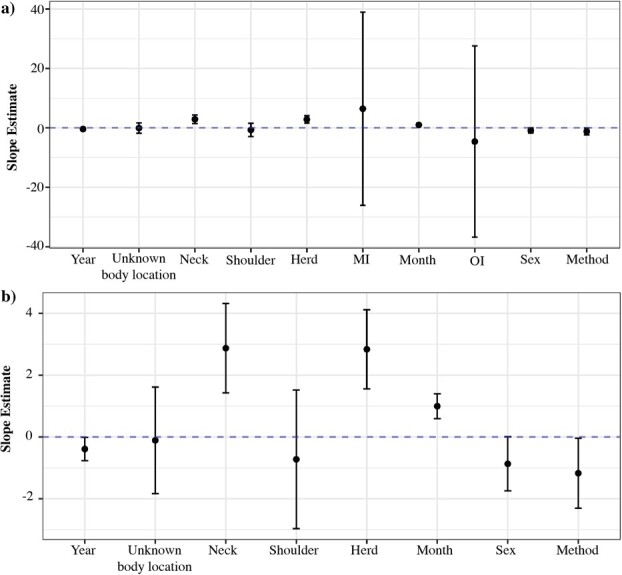
Forest plot of covariates (x axis) predicting HCC in caribou with the associated GLS regression slope estimate (y axis). The 95% confidence intervals are denoted. Estimates were averaged from four top models (ΔAICc < 2). Covariates included are year (2012–2020), unknown, neck and shoulder body locations (rump reference), herd (BNE reference), MI (mosquito index), OI (oestrid index), month categories (1–6), sex (female reference) and sampling method (capture reference). a) with all covariates, b) MI and OI are removed to visualize other covariates.
Discussion
For HCC to be a useful biomarker of stress, the influence of covariates must be considered in the study design and results interpretation. We demonstrated that, for caribou, the key covariates of body location, season and sampling method (captures vs harvested) influenced HCC, whereas sex seemed not to, but should still be considered in future analyses. Accounting for the covariates, we detected significant inter-herd differences, as well as decreasing annual values in HCC that are of wildlife management relevance. We did not, however, detect an influence of oestrid flies or mosquitoes. Values of HCC ranged within and above wild (Ewacha et al., 2017) and captive (Ashley et al., 2011; Carlsson et al., 2016) values previously reported in Rangifer sspp. Wild muskoxen (Ovibos moschatus), sympatric with the DU caribou herd, have a similarly wide range of HCC values (Di Francesco et al., 2017).
Differences between body locations
Health monitoring programs for caribou have collected hair from multiple different body locations (neck, rump and shoulder); however, HCC may vary among body locations, depending on species and study (Macbeth et al., 2010; Ashley et al., 2011; Macbeth et al., 2012; Carlitz et al., 2013; Carlsson et al., 2016; Schell et al., 2017; Acker et al., 2018). We detected significant differences in HCC between neck and rump locations in caribou, and body location was a predictor in paired HCC models.
Differences in HCC between body locations are attributed to various factors, such as hair colour (Ashley et al., 2011; Acker et al., 2018), grooming behaviour (Acker et al., 2018), local cortisol production (Macbeth et al., 2010) and heterogenous molting (Macbeth et al., 2010; Heimbürge et al., 2020). Differences in molting (hair growth covering different periods) and hair length (differences in growth speed or time frame) are the most likely cause for the differences in HCC between body locations (Macbeth, 2013). Although not specifically described for caribou, using moose growth patterns as a proxy, coat replacement begins in the ventral and cranial areas and proceeds dorsally and caudally, and the margins of the rump are generally the last area to be shed (Samuel et al., 1986). In caribou, neck hair is highly variable, with males growing a long mane or beard. We observed a lower variance and lack of outlier values for the rump hair compared with the neck. A similar pattern of increased variability of neck hair was observed in muskoxen (Di Francesco et al., 2021). It follows that standardized sampling in Rangifer should target rump hair, with the caveat that local inflammation induced by oestrid larvae, which occur below the skin on the back and rump, may be a source of local cortisol production and hair contamination.
Sampling method differences
Caribou hair samples are derived from two primary opportunistic sampling methods (hunts and captures), and we found that hair from captured animals had significantly higher HCC values. The mechanistic explanation for these differences in biomarker outcome depending on sampling methodology is unclear. A possible conclusion is that all captured samples were plucked and included the hair follicle, which can increase HCC if not removed (Sergiel et al., 2020), and hunted samples were mostly shaved (lacking a follicle). These follicles are manually removed by hand, and it is likely that not all are removed before hormone extraction, possibly biasing results. Furthermore, there may be a potential sub-population sampling biased between helicopter-based capture and land-based hunting. Acknowledging these differences between sampling methods, the sampling method must be accounted for in future HCC analyses and interpretations when combining samples from multiple sampling methods.
Sex differences
Varied results are reported concerning sex differences of HCCs in ungulates because sex-unique physiology or behaviours during active hair growth may be species-specific. Studies have reported no difference in HCC between sexes in red deer (Cervus elaphus) (Caslini et al., 2016) and white-tailed deer (Odocoileus virginianus) (Potratz et al., 2019), higher HCCs in male moose (Alces alces) (Madslien et al., 2020) and muskoxen (O. moschatus) (Di Francesco et al., 2017) and higher HCCs in female Rocky Mountain goats (Oreamnos americanus) (Dulude-de Broin et al., 2019) and alpine ibex (Capra ibex) (Prandi et al., 2018). In the present study, female caribou tended to have higher HCC compared with males, and these results were not statistically significant when outlier values were removed. Physiologically, differences in body condition, energy mobilization and body size can alter HCCs (Heimbürge et al., 2019), which may explain a potential sex difference; however, this study did not have access to complete body condition nor body mass information to explore these relationships. Nevertheless, sex may still present a significant confounding factor in HCC analyses and interpretations that should be accounted for.
Seasonal variation
The month of hair collection was a significant predictor of HCC in caribou, and these values tended to increase from September to October and peak in May to June (Fig. 5). Hair cortisol values derived in this study are believed to reflect what is present in the hair shaft after a wash procedure. This ‘internal’ cortisol would reflect the circulating free cortisol as well as local cortisol synthesis taking place during the active hair shaft growth period (Ito et al., 2005; Keckeis et al., 2012; Russell et al., 2012), which is June–September for caribou (Cuyler and Oritsland, 2002). We found that HCC levels in the herds increased in the hair shaft during the non-growing quiescent phase (October–June), suggesting cortisol is variably incorporated into the hair beyond the period of active growth. Similar findings in grizzly bears (Ursus arctos) showed a significant increase in HCC beyond the hair growth period (Cattet et al., 2014), and seasonal changes in HCC occur in boreal snowshoe hares (Lepus americanus) (Lavergne et al., 2020), grey wolves (Canis lupus) (Pereira et al., 2022) and Vancouver Island marmots (Marmota vancouverensis) (Acker et al., 2018). Continuous hair growth and local cortisol production and incorporation into the hair shaft are possible explanations for this phenomenon.
Figure 5.
Combined log hair cortisol values (pg/mg) for all animals included in statistical modelling by (a) herd and sex, (b) year (circles = DU herd, crosses = BNE herd) and (c) season. Blue lines correspond to regression lines and grey shading 95% confidence intervals of model fit. Regression lines separated by herd (BNE and DU). Thick horizontal lines of boxplots correspond to medians (dark grey = female, light grey = male).
The chronology of caribou hair growth is not well described; however, it is thought that most guard hairs grow through the summer and that the density of pelage is stable year-round (Cuyler and Oritsland, 2002). Although guard hairs seem to be stable in density, there is a possibility that a select percentage of hair is shed and replaced continuously. In muskoxen, 20% of guard hairs are continuously replaced on the rump year-round (Flood et al., 1989). Our finding of peak HCC in spring is consistent with seasonal-specific stressors in caribou; a peak of HCC in May/June corresponds to spring migration to calving grounds after a long winter (Taillon et al., 2012). Caribou also have a fine wool undercoat that is present throughout the year (Cuyler and Oritsland, 2002). Although this may have a different HCC than guard hairs, it is manually removed before cortisol extraction.
Incorporation of locally produced cortisol into the hair shaft may also be a reason for the increased HCC beyond the hair growth period. Sweat from apocrine glands contains cortisol (Russell et al., 2014), and any cortisol-containing fluid can diffuse into the hair shaft (Otten et al., 2020). The capacity of external substances to diffuse into the hair shaft may also be related to hair integrity and age. Indeed, damaged or distal hair tends to be more susceptible to external cortisol penetration from sweat or sebum (Heimbürge et al., 2020). Conversely, it has also been hypothesized that hair cortisol may be capable of washout in response to grooming or environmental exposure (Acker et al., 2018). Caribou pelage undergoes a marked change during the winter from grey/brown in the fall to white in winter, alluding to a change in hair integrity. This may facilitate the incorporation of locally produced cortisol into the hair shaft. Significant variation of HCC beyond the hair growth period demonstrates that season is a key covariate to consider when interpreting health monitoring data derived from multiple months.
Herd differences
The DU caribou population had significantly higher HCCs compared with the BNE barren-ground caribou herd. The DU herd is a distinct ecotype and subspecies of barren-ground caribou, as defined by morphological, behavioural and genetic characteristics (COSEWIC, 2011). Ecologically, DU caribou range on Victoria Island and seasonally migrate to the mainland across sea ice (GNWT, 2018). On the other hand, the BNE herd ranges on the mainland and seasonally migrates between the tree line and the tundra (COSEWIC, 2016). Prominent ecological, behavioural and anthropogenic pressure differences between these herds may be reflected by differences in baseline HCC. Moreover, ungulate populations inhabiting differing local environments exhibit differing average cortisol concentrations (Caslini et al., 2016). Morphologically, DU caribou are considerably smaller in size and have a lighter white or grey pelage compared with the BNE. Numerous studies have reported hair colour as a significant predictor of HCC (Burnett et al., 2014; Heimbürge et al., 2019), and in some cases, elevated values in lighter coloured hair (Bennett and Hayssen, 2010; Bowland et al., 2020). Coat colour differences between caribou ecotypes may present a confounding factor in comparing HCCs between populations and may be contributing to the differences reported by this study.
Biting insect harassment
We did not detect a significant association between indices of biting harassment and HCC in the two study groups of caribou. This was unexpected given the behavioural impacts of biting insect harassment on Rangifer sspp. (Toupin et al., 1996; Weladji et al., 2003) and the suggested capacity of HCC to reflect impacts of disturbances in caribou (Ewacha et al., 2017). We were limited by solely having access to and considering mosquito or oestrid presence, when black flies (Simuliidae spp.) and horse flies (Tabanidae spp.) may also be significant stressors (Toupin et al., 1996). Statistically, these indices are proxies derived from weather data and only document the total hours of moderate to high insect activity (Witter et al., 2012). Furthermore, these indices are herd-level measurements, and high inter-individual variability in HCC may have prevented the detection of a population-level association. Biologically, biting insect harassment as a potential stressor is endemic to these populations, and migratory caribou may have an adaptive tolerance strategy resulting in a limited HPA axis response (Cizauskas et al., 2015). A tolerance strategy hypothesis is proposed to explain the lack of association between HCC and helminth parasite infection (Carlsson et al., 2016; Trevisan et al., 2017; Di Francesco et al., 2022) and may be extended to insect harassment.
Annual trends
We detected significant differences of annual HCC in migratory caribou. HCC differed between years (model results) and linearly declined in both herds (Fig. 5). During this same time frame, both populations greatly declined in size; the BNE herd declined by 84% from 2012 to 2021 (Adamczewski et al., 2017; Boulanger et al., 2019), and the DU herd declined by 90% from 1997 to 2020 (COSEWIC, 2017; Campbell et al., 2021). However, HCCs decreased in both herds from 2012 to 2020. Density-dependent effects are documented in Rangifer populations (Solberg et al., 2001), and density, alongside environmental factors, influences the dynamics of these populations (Tews et al., 2007). Thus, caribou decline and subsequent relaxation of density-dependent constraints may be associated with a decrease in biomarkers of HPA axis activity. This hypothesis is partially corroborated by findings in woodland caribou (R.t. caribou) (Ewacha et al., 2017), and red deer (C. elaphus) (Caslini et al., 2016). Conversely, there may be a temporal delay between stressor impact and population response, such that stressors that initially caused decline were no longer present or were declining during biomarker sampling. These findings point to HCCs’ capacity to be used as a tool to monitor annual changes in HPA axis activity; the temporal associations with population change must be further investigated.
Hair growth
A major complicating factor for interpreting results in this study was the lack of documentation and understanding of caribou hair phenology. The precise timing of hair growth is rarely well described for wild ungulate species (Nowak et al., 2020), including Rangifer (Ashley et al., 2011), whose coat is not part of the commercial textile industry. The general season of guard hair shedding and growth for Rangifer is described; however, precise timing and pattern of growth by ecotype are unknown, and the undercoat growth pattern is unknown beyond annual persistence (Cuyler and Oritsland, 2002; Laaksonen and Paulsen, 2015). Guard hair and undercoat HCC of other species are weakly correlated and may have significantly different cortisol concentrations (Macbeth et al., 2010; Dulude-de Broin et al., 2019). Furthermore, the season of guard hair and undercoat growth may not be synchronous in ungulates (Nixon et al., 1991). Although undercoat contamination of our samples is possible, every effort was made to remove the undercoat during the initial sorting process. Nevertheless, improved description of the Rangifer hair growth cycle and a comparison of guard hair versus undercoat HCC would improve our understanding of HCC dynamics in Rangifer spp.
Conclusion
Migratory caribou are a keystone species in Canada (Festa-Bianchet et al., 2011; Polfus et al., 2016), with major declines in many herds and populations over the last decade. These declines are variably attributed to cumulative stressors, and the use of HCC as a biomarker of stress holds promise as a tool to monitor chronic stress. Our study used ‘convenience’ samples from harvester-based sampling and capture/collaring operations, and our results highlight the importance of accounting for sample source and location as well as seasonality in study design data interpretation. Accounting for this, we detected HCC variability between herds and trends over time, suggesting this is a useful tool for tracking long-term annual trends of stressors in migratory caribou.
Funding
This work was supported financially by the W. Garfield Weston Scholarship Foundation/Association of Canadian Universities for Northern Studies, the Northern Scientific Training Program, the Alberta Graduate Excellence Scholarship and the University of Calgary Graduate Scholarship, which were awarded to F.R. The research was supported by grants to S.K. from Polar Knowledge Canada [Grant NST-1718-0015], Environment and Climate Change Canada, Irving Maritime Ship Building—Nunavut Arctic College and NSERC (Natural Sciences and Engineering Council of Canada). Canada North Outfitting and the Governments of Nunavut and the Northwest Territories provided invaluable in-kind support.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics Statement
This research was approved under Animal Care and Use Permit No. AC18–0093, Wildlife Research Permit No. WL2021–053 for Nunavut and Wildlife Research Permit No. WL500877, Wildlife Care Committee Permit No. 2020–008 for the Northwest Territories. Statistical analyses demonstrate that at least 30–40 collars per herd are needed to define seasonal range use necessary for conservation and management (Adamczewski and Boulanger, 2016). Because the animal is being handled for these purposes, the opportunity is presented to gain as much biological information as possible, including the collection of non-invasive health data and samples. Animal handling protocols approved by the Wildlife Care Committee include the collection by trained personnel of hair, blood, feces and mucosal swabs, in addition to demographic and morphometric data.
Author Contributions
F.R.: Conceptualization, methodology, formal analysis, writing—original draft. X.F-A.: Conceptualization, reviewing, editing. M.P.: Analysis, reviewing, editing. D.W.: Reviewing, editing. G.M.: Methodology, investigation, reviewing, editing. L-M.L.: Resources, reviewing, editing. N.J.: Resources, reviewing, editing. S.K.: Conceptualization, methodology, reviewing, editing.
Supplementary Material
Acknowledgements
We would first like to thank the hunters from the communities of Kugluktuk (NU) and Ulukhaktok (NWT) for completing and submitting the samples necessary for this research to happen. Our thanks further extend to our government research partners, Don Russell, Kirstyn Falck, Brett Elkin, Jan Adamczewski and Judy Williams of the Government of the Northwest Territories. Our laboratory managers and technicians, Paula Mackie and Christine Gilman of the Toronto Zoo, and Angie Schneider and James Wang from the University of Calgary. Juliette Di Francesco is thanked for her input and expertise.
Contributor Information
F Rakic, Department of Ecosystem and Public Health – Faculty of Veterinary Medicine, University of Calgary; 3280 Hospital Drive NW, Calgary, Alberta, Canada, T2N 4Z6.
X Fernandez-Aguilar, Department of Ecosystem and Public Health – Faculty of Veterinary Medicine, University of Calgary; 3280 Hospital Drive NW, Calgary, Alberta, Canada, T2N 4Z6.
M Pruvot, Department of Ecosystem and Public Health – Faculty of Veterinary Medicine, University of Calgary; 3280 Hospital Drive NW, Calgary, Alberta, Canada, T2N 4Z6.
D P Whiteside, Department of Ecosystem and Public Health – Faculty of Veterinary Medicine, University of Calgary; 3280 Hospital Drive NW, Calgary, Alberta, Canada, T2N 4Z6.
G F Mastromonaco, Reproductive Sciences Unit, Toronto Zoo, 361A Old Finch Avenue, Scarborough, Ontario, Canada, M1B 5K7.
L M Leclerc, Department of Environment, Government of Nunavut, P.O. Box 377, Kugluktuk, Nunavut, Canada, X0B 0E0.
N Jutha, Department of Environment and Natural Resources, Government of the Northwest Territories, 5112 52 st, Yellowknife, The Northwest Territories, Canada, X1A 2L9.
S J Kutz, Department of Ecosystem and Public Health – Faculty of Veterinary Medicine, University of Calgary; 3280 Hospital Drive NW, Calgary, Alberta, Canada, T2N 4Z6.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Acker M, Mastromonaco G, Schulte-Hostedde AI (2018) The effects of body region, season, and external arsenic application on hair cortisol concentration. Conserv Physiol 6: coy037. 10.1093/conphys/coy037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczewski J, Boulanger J (2016) Technical rationale to increase the number of satellite collars on the Bathurst caribou herd. GNWT Manuscript Report 254 Government of the Northwest Territories, Department of Environment and Natural Resources 22. https://www.arlis.org/docs/vol1/K/954094486.pdf. [accessed October 4, 2022].
- Adamczewski J, Boulanger J, Croft B, Davison T, Sayine-Crawford H, Tracz B (2017) Comparing of calving and post-calving photo-surveys of the Bluenose East herd of barren-ground caribou in northern Canada in 2010. Can Wildlife Biol Manag 6: 4–30. ISSN: 1929-3100. [Google Scholar]
- Ashley NT, Barboza PS, Macbeth BJ, Janz DM, Cattet MRL, Booth RK, Wasser SK (2011) Glucocorticosteroid concentrations in feces and hair of captive caribou and reindeer following adrenocorticotropic hormone challenge. Gen Comp Endocr 172: 382–391. 10.1016/j.ygcen.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Baker MR, Gobush KS, Vynne CH (2013) Review of factors influencing stress hormones in fish and wildlife. J Nat Conserv 21: 309–318. 10.1016/j.jnc.2013.03.003. [DOI] [Google Scholar]
- Bartoń K (2020) Package “MuMIn”: multi-model inference. R package version 1.43.17. 1–75. https://CRAN.R-project.org/package=MuMIn.
- Bates D, Maechler M, Bolker B, Walker S (2015) lme4: linear mixed-effects models using Eigen and S4. R package version1. 1–8. https://cran.r-project.org/web/packages/lme4/lme4.pdf.
- Beale CM, Lennon JJ, Yearsley JM, Brewer MJ, Elston DA (2010) Regression analysis of spatial data. Ecol Lett 13: 246–264. 10.1111/j.1461-0248.2009.01422.x. [DOI] [PubMed] [Google Scholar]
- Bennett A, Hayssen V (2010) Measuring cortisol in hair and saliva from dogs: coat color and pigment differences. Domest Anim Endocrin 39: 171–180. 10.1016/j.domaniend.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Bondo KJ, Macbeth B, Schwantje H, Orsel K, Culling D, Culling B, Tryland M, Nymo IH, Kutz S (2019) Health survey of boreal caribou (Rangifer tarandus caribou) in northeastern British Columbia, Canada. J Wild Dis 55: 544–562. 10.7589/2018-01-018. [DOI] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC (2009) Do baseline glucocorticoids predict fitness? Trends Ecol Evol 24: 634–642. 10.1016/j.tree.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Boulanger J, Adamczewski J, Nishi J, Cluff D, Williams J, Sayine-Crawford H, Marie-Leclerc L (2019) Estimates of breeding females & adult herd size and analysis of demographics for the bluenose-east herd of barren-ground caribou: 2018 calving ground photographic survey. GNWT Manuscript No. 278, Government of the Northwest Territories, Department of Environment and Natural Resources, p. 278. https://www.wrrb.ca/sites/default/files/Undertaking 1B Boulanger BNE 2018 calving survey final MR278.pdf. [accessed October 4, 2022].
- Bowland GB, Bernstein RM, Koster J, Fiorello C, Brenn-White M, Liu J, Schwartz L, Campbell A, Stade D, Beagley Jet al. (2020) Fur color and nutritional status predict hair cortisol concentrations of dogs in Nicaragua. Front Vet Sci 7: 565346. 10.3389/fvets.2020.565346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett TA, Madureira AML, Silper BF, Nadalin A, Tahmasbi A, Veira DM, Cerri RLA (2014) Factors affecting hair cortisol concentrations in lactating dairy cows. J Dairy Sci 97: 7685–7690. 10.3168/jds.2014-8444. [DOI] [PubMed] [Google Scholar]
- Busch DS, Hayward LS (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142: 2844–2853. 10.1016/j.biocon.2009.08.013. [DOI] [Google Scholar]
- Campbell M, Ringrose J, Boulanger J, Robert-Charron A, Methuen K, Mutch C, Davison T, Wray C (2021) An aerial abundance estimate of the Dolphin and Union Caribou (Rangifer tarandus groenlandicus x pearyi) herd, Kitikmeot region, Nunavut-fall 2020. Government of Nunavut, Department of Environment No 01-2021-70. https://gov.nu.ca/sites/default/files/wildlife_-_20210301_du_fall_2020_caribou_survey_file_report_feb_1_2021_final.pdf. [accessed August 6, 2022]. [Google Scholar]
- Carlitz EHD, Kirschbaum C, Stalder T, Van Schaik CP (2013) Hair as a long-term retrospective cortisol calendar in orangutans (Pongo spp.): new perspectives for stress monitoring in captive management and conservation. Gen Comp Endocr 195: 151–156. 10.1016/j.ygcen.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Carlsson AM, Mastromonaco G, Vandervalk E, Kutz S (2016) Parasites, stress, and reindeer: infection with abomasal nematodes is not associated with elevated glucocorticoid levels in hair or faeces. Conserv Physiol 4: cow058. 10.1093/conphys/cow058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caslini C, Comin A, Peric T, Prandi A, Pedrotti L, Mattiello S (2016) Use of hair cortisol analysis for comparing population status in wild red deer (Cervus elaphus) living in areas with different characteristics. Eur J Wildl Res 62: 713–723. 10.1007/s10344-016-1049-2. [DOI] [Google Scholar]
- Cattet M (2018) Capture, handling and release of caribou standard operating procedure. In Wildlife Care Committee Government of the Northwest Territories, p. 39. https://www.ecc.gov.nt.ca/sites/ecc/files/resources/caribou_care_sop.pdf. [accessed October 4, 2022].
- Cattet M, Macbeth BJ, Janz DM, Zedrosser A, Swenson J, Dumond M, Stenhouse GB (2014) Quantifying long-term stress in brown bears with the hair cortisol concentration: a biomarker that may be confounded by rapid changes in response to capture and handling. Conserv Physiol 2: cou026. 10.1093/conphys/cou026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas CA, Turner WC, Pitts N, Getz WM (2015) Seasonal patterns of hormones, macroparasites, and microparasites in wild African ungulates: the interplay among stress, reproduction, and disease. PLoS One 10: e0120800. 10.1371/journal.pone.0120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSEWIC (2011) Designatable units for caribou (Rangifer tarandus) in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, p. 88. https://www.canada.ca/content/dam/eccc/migration/cosewic-cosepac/4e5136bf-f3ef-4b7a-9a79-6d70ba15440f/cosewic_caribou_du_report_23dec2011.pdf. [accessed August 6, 2022]. [Google Scholar]
- COSEWIC (2016) COSEWIC assessment and status report on the Caribou Rangifer tarandus, barren-ground population, in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. xiii + 123. https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/cosewic-assessments-status-reports/caribou-barren-ground-population-2016.html. [accessed August 6, 2022]. [Google Scholar]
- COSEWIC (2017) COSEWIC assessment and status report on the caribou, Dolphin and Union population, Rangifer tarandus, in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. xii + 51. https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/cosewic-assessments-status-reports/caribou-dolphin-union-2017.html. [accessed August 6, 2022]. [Google Scholar]
- Côté S, Festa-Bianchet M, Dussault C, Tremblay J-P, Brodeur V, Simard M, Taillon J, Hins C, LeCorre M, Sharma S (2010) Caribou herd dynamics: impacts of climate change on traditional and sport harvesting. In Allard M, Lemay M, eds, Integrated Regional Impact Study for the Eastern Subarctic. Arcticnet, Quebec City, Canada, pp. 249–269 [Google Scholar]
- Cuyler C, Oritsland N (2002) Do seasonal changes in Svalbard reindeer fur have relevance for heat transfer? Rangifer 22: 133. 10.7557/2.22.2.1532. [DOI] [Google Scholar]
- Di Francesco J, Kwong GPS, Deardon R, Checkley SL, Mastromonaco GF, Mavrot F, Leclerc L-M, Kutz S (2022) Qiviut cortisol is associated with metrics of health and other intrinsic and extrinsic factors in wild muskoxen (Ovibos moschatus). Conserv Physiol 10: coab103. 10.1093/conphys/coab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco J, Mastromonaco GF, Checkley SL, Blake J, Rowell JE, Kutz S (2021) Qiviut cortisol reflects hypothalamic–pituitary–adrenal axis activity in muskoxen (Ovibos moschatus). Gen Comp Endocr 306: 113737. 10.1016/j.ygcen.2021.113737. [DOI] [PubMed] [Google Scholar]
- Di Francesco J, Navarro-Gonzalez N, Wynne-Edwards K, Peacock S, Leclerc L-M, Tomaselli M, Davison T, Carlsson A, Kutz S (2017) Qiviut cortisol in muskoxen as a potential tool for informing conservation strategies. Conserv Physiol 5: cox052. 10.1093/conphys/cox052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulude-de Broin F, Côté SD, Whiteside DP, Mastromonaco GF (2019) Faecal metabolites and hair cortisol as biological markers of HPA-axis activity in the Rocky Mountain goat. Gen Comp Endocr 280: 147–157. 10.1016/j.ygcen.2019.04.022. [DOI] [PubMed] [Google Scholar]
- Ewacha MVA, Roth JD, Anderson WG, Brannen DC, Dupont DLJ (2017) Disturbance and chronic levels of cortisol in boreal woodland caribou. J Wildl Manage 81: 1266–1275. 10.1002/jwmg.21288. [DOI] [Google Scholar]
- Fauchald P, Park T, Tømmervik H, Myneni R, Hausner Vera H (2017) Arctic greening from warming promotes declines in caribou populations. Sci Adv 3: e1601365. 10.1126/sciadv.1601365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti LA, Schwartz CC, Rye RO, Haroldson MA, Gunther KA, Phillips DL, Robbins CT (2003) Use of sulfur and nitrogen stable isotopes to determine the importance of whitebark pine nuts to Yellowstone grizzly bears. Can J Zool 81: 763–770. 10.1139/z03-054. [DOI] [Google Scholar]
- Festa-Bianchet M, Ray JC, Boutin S, Côté SD, Gunn A (2011) Conservation of caribou (Rangifer tarandus) in Canada: an uncertain future. Can J Zool 89: 419–434. 10.1139/z11-025. [DOI] [Google Scholar]
- Flood PF, Stalker MJ, Rowell JE (1989) The hair follicle density and seasonal shedding cycle of the muskox (Ovibos moschatus). Can J Zool 67: 1143–1147. 10.1139/z89-164. [DOI] [Google Scholar]
- Gormally BMG, Romero LM, Angelier F (2020) What are you actually measuring? A review of techniques that integrate the stress response on distinct timescales. Funct Ecol 34: 2030–2044. 10.1111/1365-2435.13648. [DOI] [Google Scholar]
- Government of the Northwest Territories (GNWT) (2018) Management Plan for the Dolphin and Union Caribou (Rangifer tarandus groenlandicus x pearyi) in the Northwest Territories and Nunavut. Environment and Natural Resources, Yellowknife, NWT, p. 107 [Google Scholar]
- Gunn A, Russell D, Greig L (2014) Insights into integrating cumulative effects and collaborative co-management for migratory tundra caribou herds in the Northwest Territories, Canada. Ecol Soc 19: 4. 10.5751/ES-06856-190404. [DOI] [Google Scholar]
- Heimbürge S, Kanitz E, Otten W (2019) The use of hair cortisol for the assessment of stress in animals. Gen Comp Endocrinol 270: 10–17. 10.1016/j.ygcen.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Heimbürge S, Kanitz E, Tuchscherer A, Otten W (2020) Within a hair’s breadth – factors influencing hair cortisol levels in pigs and cattle. Gen Comp Endocr 288: 113359. 10.1016/j.ygcen.2019.113359. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R (2005) Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J 19: 1332–1334. 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- James G, Witten D, Hastie T, Tibshirani R (eds) (2013) An Introduction to Statistical Learning with Applications in R, Ed1st ed. 2013. Springer New York, New York, NY [Google Scholar]
- Jaspers VLB, Dietz R, Sonne C, Letcher RJ, Eens M, Neels H, Born EW, Covaci A (2010) A screening of persistent organohalogenated contaminants in hair of East Greenland polar bears. Sci Tot Environ 408: 5613–5618. 10.1016/j.scitotenv.2010.07.059. [DOI] [PubMed] [Google Scholar]
- Johnson D, Harms NJ, Larter NC, Elkin BT, Tabel H, Wei G (2010) Serum, serology, and parasitology of boreal caribou (Rangifer tarandus caribou) in the Northwest Territories, Canada. J Wildl Dis 46: 1096–1107. 10.7589/0090-3558-46.4.1096. [DOI] [PubMed] [Google Scholar]
- Jutha N, Jardine C, Schwantje H, Mosbacher J, Kinniburgh D, Kutz S (2022) Evaluating the use of hair as a non-invasive indicator of trace mineral status in woodland caribou (Rangifer tarandus caribou). PLoS One 17: e0269441. 10.1371/journal.pone.0269441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keckeis K, Lepschy M, Schöpper H, Moser L, Troxler J, Palme R (2012) Hair cortisol: a parameter of chronic stress? Insights from a radiometabolism study in guinea pigs. J Comp Physiol B 182: 985–996. 10.1007/s00360-012-0674-7. [DOI] [PubMed] [Google Scholar]
- Koren L, Whiteside D, Fahlman S, Ruckstuhl K, Kutz S, Checkley S, Dumond M, Wynne-Edwards K (2012) Cortisol and corticosterone independence in cortisol-dominant wildlife. Gen Comp Endocr 177: 113–119. 10.1016/j.ygcen.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Kummrow MS, Gilman C, Mackie P, Smith DA, Mastromonaco GF (2011) Non-invasive analysis of fecal reproductive hormone metabolites in female veiled chameleons (Chamaeleo calyptratus) by enzyme immunoassay. Zoo Biol 30: 95–115. 10.1002/zoo.20318. [DOI] [PubMed] [Google Scholar]
- Kutz S, Ducrocq J, Cuyler C, Elkin B, Gunn A, Kolpashikov L, Russell D, White RG (2013) Standardized monitoring of Rangifer health during International Polar Year. Rangifer 33: 91–114. 10.7557/2.33.2.2532. [DOI] [Google Scholar]
- Laaksonen S, Paulsen P (2015) Hunting Hygiene. Wageningen Academic Pub, Wegeningen, Netherlands, p. 304 [Google Scholar]
- Lavergne SG, Peers MJL, Mastromonaco G, Majchrzak YN, Nair A, Boutin S, Boonstra R (2020) Hair cortisol as a reliable indicator of stress physiology in the snowshoe hare: influence of body region, sex, season, and predator–prey population dynamics. Gen Comp Endo 294: 113471. 10.1016/j.ygcen.2020.113471. [DOI] [PubMed] [Google Scholar]
- Lyver POB (2005) Monitoring barren-ground caribou body condition with Denésǫłıné traditional knowledge. Arctic 58: 44–54. [Google Scholar]
- Macbeth B (2013) An Evaluation of Hair Cortisol Concentration as a Potential Biomarker of Long-Term Stress in Free-Ranging Grizzly Bears (Ursus arctos), Polar Bears (Ursus maritimus), and Caribou (Rangifer tarandus sp.). PhD Thesis,. Veterinary Biological Sciences, University of Saskatchewan, Saskatoon, Canada, p. 262 [Google Scholar]
- Macbeth BJ, Cattet MRL, Obbard ME, Middel K, Janz DM (2012) Evaluation of hair cortisol concentration as a biomarker of long-term stress in free-ranging polar bears. Wildl Soc B 36: 747–758. 10.1002/wsb.219. [DOI] [Google Scholar]
- Macbeth BJ, Cattet MRL, Stenhouse GB, Gibeau ML, Janz DM (2010) Hair cortisol concentration as a non-invasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): considerations with implications for other wildlife. Can J Zool 88: 935–949. 10.1139/Z10-057. [DOI] [Google Scholar]
- Madslien K, Stubsjøen SM, Viljugrein H, Ytrehus B, Solberg EJ, Kapronczai L, Mysterud A, Godfroid J, Janz DM, Cattet M (2020) Hair cortisol concentration and body mass in moose (Alces alces) infested with deer keds (Lipoptena cervi). J Wildl Dis 56: 687–692. 10.7589/2019-07-185. [DOI] [PubMed] [Google Scholar]
- Majchrzak YN, Mastromonaco GF, Korver W, Burness G (2015) Use of salivary cortisol to evaluate the influence of rides in dromedary camels. Gene Comp Endocr 211: 123–130. 10.1016/j.ygcen.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Mastromonaco GF, Gunn K, McCurdy-Adams H, Edwards DB, Schulte-Hostedde AI (2014) Validation and use of hair cortisol as a measure of chronic stress in eastern chipmunks (Tamias striatus). Cons Physiol 2: cou055. 10.1093/conphys/cou055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard SP (2013) EnvStats: An R Package for Environmental Statistics. Springer, New York [Google Scholar]
- Miller FL (1972) Eruption and attrition of mandibular teeth in barren-ground caribou. J Wildl Manage 36: 606–612. 10.2307/3799093. [DOI] [Google Scholar]
- Nixon AJ, Gurnseyb MP, Betteridgec K, Mitchellc RJ, Welchc RAS (1991) Seasonal hair follicle activity and fibre growth in some New Zealand cashmere-bearing goats (Caprus hircus). J Zool 224: 589–598. 10.1111/j.1469-7998.1991.tb03787.x. [DOI] [Google Scholar]
- Nowak K, Berger J, Panikowski A, Reid DG, Jacob AL, Newman G, Young NE, Beckmann JP, Richards SA (2020) Using community photography to investigate phenology: a case study of coat molt in the mountain goat (Oreamnos americanus) with missing data. Ecol Evol 10: 13488–13499. 10.1002/ece3.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omsjoe EH, Stien A, Irvine J, Albon SD, Dahl E, Thoresen SI, Rustad E, Ropstad E (2009) Evaluating capture stress and its effects on reproductive success in Svalbard reindeer. Can J Zool 87: 73–85. 10.1139/Z08-139. [DOI] [Google Scholar]
- Otten W, Heimbürge S, Kanitz E, Tuchscherer A (2020) It’s getting hairy – external contamination may affect the validity of hair cortisol as an indicator of stress in pigs and cattle. Gen Comp Endocr 295: 113531. 10.1016/j.ygcen.2020.113531. [DOI] [PubMed] [Google Scholar]
- Paterson S, Lello J (2003) Mixed models: getting the best use of parasitological data. Trends Parasitol 19: 370–375. 10.1016/S1471-4922(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Pereira P, Fandos Esteruelas N, Nakamura M, Rio-Maior H, Krofel M, Di Blasio A, Zoppi S, Robetto S, Llaneza L, García Eet al. (2022) Hair cortisol concentration reflects the life cycle and management of grey wolves across four European populations. Sci Rep 12: 5697–5697. 10.1038/s41598-022-09711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polfus JL, Manseau M, Simmons D, Neyelle M, Bayha W, Andrew F, Andrew L, Klütsch CFC, Rice K, Wilson P (2016) Łeghágots’enetę (learning together). Ecol Soc 21: 18. 10.5751/ES-08284-210218. [DOI] [Google Scholar]
- Potratz EJ, Brown JS, Gallo T, Anchor C, Santymire RM (2019) Effects of demography and urbanization on stress and body condition in urban white-tailed deer. Urban Ecosyst 22: 807–816. 10.1007/s11252-019-00856-8. [DOI] [Google Scholar]
- Prandi A, Peric T, Corazzin M, Comin A, Colitti M (2018) A first survey on hair cortisol of an alpine ibex (Capra ibex ibex) population. Anim Sci Pap Rep 36: 57–74. [Google Scholar]
- R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Russell D, Whitfield P, Cai J, Gunn A, White R, Poole K (2013) CARMA’s MERRA-based caribou range climate database. Rangifer 33: 145–152. 10.7557/2.33.2.2535. [DOI] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S (2012) Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psycho Neuro Endocrino 37: 589–601. 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum SHM (2014) The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Ther Drug Monit 36: 30–34. 10.1097/FTD.0b013e31829daa0a. [DOI] [PubMed] [Google Scholar]
- Samuel WM, Welch DA, Drew ML (1986) Shedding of the juvenile and winter hair coats of moose (Alces Alces) with emphasis on the influence of the winter tick (Dermacentor albipictus). Alces 22: 345–360. [Google Scholar]
- Schell CJ, Young JK, Lonsdorf EV, Mateo JM, Santymire RM (2017) Investigation of techniques to measure cortisol and testosterone concentrations in coyote hair. Zoo Biol 36: 220–225. 10.1002/zoo.21359. [DOI] [PubMed] [Google Scholar]
- Sergiel A, Cattet M, Kapronczai L, Janz DM, Selva N, Bartoń KA, Swenson JE, Zedrosser A (2020) Do follicles matter? Testing the effect of follicles on hair cortisol levels. Conserv Physiol 8: coaa003. 10.1093/conphys/coaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg EJ, Jordhøy P, Strand O, Aanes R, Loison A, Sæther BE, Linnell JDC (2001) Effects of density-dependence and climate on the dynamics of a Svalbard reindeer population. Ecography 24: 441–451. 10.1034/j.1600-0587.2001.d01-200.x. [DOI] [Google Scholar]
- Taillon J, Festa-Bianchet M, Côté SD (2012) Shifting targets in the tundra: protection of migratory caribou calving grounds must account for spatial changes over time. Biol Conserv 147: 163–173. 10.1016/j.biocon.2011.12.027. [DOI] [Google Scholar]
- Tews J, Ferguson MAD, Fahrig L (2007) Modeling density dependence and climatic disturbances in caribou: a case study from the Bathurst Island complex, Canadian High Arctic. J Zool 272: 209–217. 10.1111/j.1469-7998.2006.00257.x. [DOI] [Google Scholar]
- Toupin B, Huot J, Manseau M (1996) Effect of insect harassment on the behaviour of the Rivière George Caribou. Arctic 49: 375–382. 10.14430/arctic1213. [DOI] [Google Scholar]
- Trevisan C, Montillo M, Prandi A, Mkupasi EM, Ngowi HA, Johansen MV (2017) Hair cortisol and dehydroepiandrosterone concentrations in naturally Taenia solium infected pigs in Tanzania. Gen Comp Endocr 246: 23–28. 10.1016/j.ygcen.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikøren T, Kristoffersen AB, Lierhagen S, Handeland K (2011) A comparative study of hepatic element levels in wild moose, roe deer, and reindeer from Norway. J Wildl Dis 47: 661–672. 10.7589/0090-3558-47.3.661. [DOI] [PubMed] [Google Scholar]
- Vors LS, Boyce MS (2009) Global declines of caribou and reindeer: caribou reindeer decline. Glob Chang Biol 15: 2626–2633. 10.1111/j.1365-2486.2009.01974.x. [DOI] [Google Scholar]
- Weladji RB, Holand Ø, Almøy T (2003) Use of climatic data to assess the effect of insect harassment on the autumn weight of reindeer (Rangifer tarandus) calves. J Zool 260: 79–85. 10.1017/S0952836903003510. [DOI] [Google Scholar]
- Witter L, Johnson C, Croft B (2014) Weather-based indices of parasitic fly activity and abundance for the Bathurst caribou post-calving and summer range: users guide. GNWT Manuscript Report No. 246 Government of the Northwest Territories, Department of Environment and Natural Resources, p. 36. https://www.ecc.gov.nt.ca/sites/ecc/files/wildlife_manuscript_report_246.pdf. [accessed May 5, 2021].
- Witter LA, Johnson CJ, Croft B, Gunn A, Poirier LM (2012) Gauging climate change effects at local scales: weather-based indices to monitor insect harassment in caribou. Ecol Appl 22: 1838–1851. 10.1890/11-0569.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.