Abstract
Induction of cell-mediated immunity may be essential for an effective AIDS vaccine. Listeria monocytogenes is an attractive bacterial vector to elicit T-cell immunity to human immunodeficiency virus (HIV) because it specifically infects monocytes, key antigen-presenting cells, and because natural infection originates at the mucosa. Immunization with recombinant L. monocytogenes has been shown to protect mice from lymphocytic choriomeningitis virus, influenza virus, and tumor inoculation. L. monocytogenes expressing HIV gag elicits sustained high levels of Gag-specific cytotoxic T lymphocytes (CTLs) in mice. We have examined the ability of Listeria to infect human monocytes and present HIV antigens to CD8 T lymphocytes of HIV-infected donors to induce a secondary T-cell immune response. Using this in vitro vaccination protocol, we show that L. monocytogenes expressing the HIV-1 gag gene efficiently provides a strong stimulus for Gag-specific CTLs in HIV-infected donor peripheral blood mononuclear cells. Listeria expressing Nef also elicits a secondary in vitro anti-Nef CTL response. Since L. monocytogenes is a pathogen, before it can be seriously considered as a human vaccine vector, safety concerns must be addressed. We therefore have produced a highly attenuated strain of L. monocytogenes that requires d-alanine for viability. The recombinant bacteria are attenuated at least 105-fold. We show that when these hyperattenuated bacteria are engineered to express HIV-1 Gag, they are at least as efficient at stimulating Gag-specific human CTLs in vitro as wild-type recombinants. These results suggest that attenuated Listeria is an attractive candidate vaccine vector to induce T-cell immunity to HIV in humans.
Growing evidence suggests that T-cell-mediated immunity plays an essential role in controlling human immunodeficiency virus (HIV) infection. Exposed but uninfected individuals often have specific antiviral T-cell responses, but no antibody response (7, 8, 32). The viremia of primary infection resolves coincident with the development of virus-specific cytotoxic T lymphocytes (CTLs), before the development of specific antibodies (2, 20). Rhesus macaques depleted of CD8 T cells are unable to control simian immunodeficiency virus during primary infection (33). These data suggest that the ability to induce anti-HIV cell-mediated immunity may be an essential feature of an effective AIDS vaccine. This is particularly important, since generating neutralizing antibodies to a broad range of HIV isolates by vaccination has proven difficult.
Eliciting effective T-cell responses with a vaccine is a challenge, both theoretically and practically. Although subunit vaccines can prime antigen-presenting cells for presentation and activation of CD4 T-cell responses, they do not efficiently induce CD8 T-cell responses. Since CD8 T-cell priming requires antigen delivery to the cytosol for processing into the major histocompatibility complex (MHC) class I pathway, the obstacle is to deliver recombinant protein antigens into the cytosol (27). Live intracellular bacterial or attenuated viral vectors do this efficiently, although there are safety concerns associated with these approaches.
Listeria monocytogenes is an attractive intracellular bacterial vector to introduce foreign antigens into the MHC class I pathway for presentation to T cells. Because it is transmitted by oral exposure, it should be especially suitable for generating mucosal immunity. Moreover it preferentially infects monocytes, professional antigen-presenting cells that are particularly good at inducing cellular immune responses. There is also good evidence that L. monocytogenes infects dendritic cells, since it has been shown that it infects a dendritic cell line as well as cytokine-generated monocyte-derived dendritic cells in vitro (16). Moreover, cells with the morphological features of dendritic cells are infected in the spleens of mice intravenously inoculated with L. monocytogenes (10). Despite the fact that L. monocytogenes is pervasive in the environment, the annual incidence of listeriosis is only 1/100,000 (39). Thus, few patients are expected to have preexisting immunity, which can interfere with the generation of a neoantigen response.
After infection, L. monocytogenes escapes from phagolysosome proteolysis by exiting from the phagosome into the host cytosol with the help of its pore-forming protein, listeriolysin (31). In the cytosol, it replicates and can spread directly into neighboring cells via the engulfment of actin-mediated cellular protrusions containing the bacterium (41). L. monocytogenes infection induces a potent CD8 T-cell response which is critical for protection (18). Inoculation with recombinant bacteria that express foreign proteins, particularly if they are secreted into the host cell cytoplasm for processing by the endogenous MHC class I pathway (34), is able to induce strong CD8 T-cell responses and provide protection to mice challenged with tumors, lymphocytic choriomeningitis virus, and influenza virus (17, 28, 30, 35). We previously showed that a modestly attenuated Listeria recombinant was able to elicit a powerful and sustained HIV-specific CTL response in mice (13). More recently, L. monocytogenes has been used as a carrier of plasmid DNA encoding foreign antigens (11).
Since T-cell responses to antigens are MHC restricted and vaccine and immunogenicity studies in one species do not necessarily translate into another, we wished to determine whether L. monocytogenes might also be useful as a vaccine vector to elicit a T-cell response in humans, particularly against HIV-1. We therefore developed a model in vitro “vaccination” protocol to evaluate the ability of this candidate HIV-1 vaccine to elicit a secondary T-cell response from peripheral blood mononuclear cells (PBMCs) of HIV-infected donors. The protocol was used to evaluate the induction of Gag and Nef-specific T cells by using L. monocytogenes strains expressing gag (Lm-gag) or nef (Lm-nef) driven from the hly promoter. This “vaccination” assay may be useful for in vitro evaluation and comparison of other candidate human vaccines early in their development.
Because L. monocytogenes can cause serious disease in even mildly immunocompromised hosts, including pregnant women and neonates, the safe use of L. monocytogenes as a vaccine vector requires significant attenuation. Although the expression of stably transfected foreign genes often results in some attenuation of virulence, more significant attenuation may be required. Strategies for attenuation of L. monocytogenes have included inoculation with killed bacteria (38); mutation of the hly gene, which encodes the bacterial hemolysin, listeriolysin (3, 26); deletion of the actA gene, whose product is required for actin-mediated cell-to-cell transmission (14, 15); and inactivation of the genes required for bacterial d-alanine synthesis (40). However, attenuation often comes at the price of loss of immunogenicity. In this paper, we investigated whether L. monocytogenes attenuated through crippling the biosynthetic enzymes for d-alanine was effective at eliciting HIV-specific CD8 T cells. We therefore introduced the HIV gag gene into this highly attenuated L. monocytogenes strain (Lmdaldat), in which the genes for d-alanine biosynthesis, alanine racemase (dal) and d-amino acid aminotransferase (dat), were inactivated. We found that the resulting strain (Lmdaldat-gag) was as immunogenic in vitro as Lm-gag, the analogous L. monocytogenes strain with intact d-alanine synthesis. Thus, L. monocytogenes would appear to be highly efficient for the induction of immune responses in human T cells, and the hyperattenuated strain of Listeria is as effective as wild-type Listeria in this process.
MATERIALS AND METHODS
Bacterial strains.
L. monocytogenes strain 10403S (13) was the wild-type organism used in these studies. It was grown in brain heart infusion medium (BHI) (Difco Laboratories). The chromosome of strain 10403S was stably modified to synthesize and secrete HIV Gag encoded by the molecular clone HXB and 89.6 (9) by using the shuttle vector pKSV7 (36) and a previously described protocol (4, 13). The resulting plasmid was introduced into wild-type L. monocytogenes by electroporation of penicillin-treated bacteria and allowed to participate in the two-step allelic exchange reaction (29). Bacteria which had undergone chromosomal integration into the sepA region of the L. monocytogenes chromosome followed by excision of extraneous vector sequences were selected by sequential culture in media containing chloramphenicol followed by erythromycin. The expected recombination product was verified by Southern blotting and sequencing, and Gag protein expression was verified by Western blotting. The Gag-expressing mutant bacterium was termed Lm-gag. A similar approach was used to produce a modified wild-type bacterium, Lm-nef, expressing full-length HIV nef from molecular clone pNL432. In BALB/c mice, the 50% lethal dose (LD50) of Lm-gag and Lm-nef is approximately 5 × 107, 3 logs greater than that of wild-type strain 10403S (3 × 104) (13).
A hyperattenuated strain of L. monocytogenes, Lmdaldat, which can only grow in the presence of d-alanine, was also constructed from the wild-type strain as described previously (40). Lmdaldat is a double-deletion mutant of L. monocytogenes in which 82% of the alanine racemase gene (dal) and 31% of the d-amino acid aminotransferase gene (dat) were removed in frame. The LD50 of Lmdaldat in BALB/c mice is >8 × 108, or, when injected in the presence of 20 mg of d-alanine in the 0.2-ml injection volume (Lmdaldat + d-Ala), was approximately 7 × 107 (40) or greater (M. V. Rayevskaya and F. R. Frankel, unpublished). The chromosome of Lmdaldat was stably modified with the pKSV7 shuttle vector and the protocol described above and in reference 13 to express and secrete HIV HXB Gag. In that way, a strain of Lmdaldat-gag (or Lmdd-gag) otherwise exactly analogous to Lm-gag (13) was constructed with the Gag cassette inserted into the bacterial sepA gene. The LD50 of Lmdaldat-gag in BALB/c mice is >8 × 108 (Rayevskaya and Frankel, unpublished).
Preparation of L. monocytogenes for in vitro infection.
A single L. monocytogenes colony was inoculated into 3 ml of BHI and grown overnight at 30°C. The Lmdaldat-gag strain was grown and manipulated in the presence of 100 μg of d-alanine per ml. Bacteria were pelleted by microcentrifuge for 8 min and washed twice with 1 ml of phosphate-buffered saline (PBS). Before inoculation into monocyte cultures, bacteria were treated with complement by incubation for 30 min at 37°C with 10% human pooled AB serum, which had not been heat inactivated, in PBS.
In vitro vaccination protocol.
Human PBMCs were isolated from heparinized asymptomatic HIV-seropositive donor blood by separation on Ficoll-Hypaque density gradients and resuspended in T-cell medium (22). Samples were obtained with informed consent, and the study was approved by the institutional Human Investigation Review Committee. Adherent cells, obtained after incubation of 2 × 106 PBMCs/well in 1 ml in 24-well plates at 37°C for 2 h, were cultured in medium containing 20% pooled human AB serum and 5 ng of recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (R and D Systems, Minneapolis, Minn.). Complement-treated freshly prepared L. monocytogenes cells (at titrated doses) were inoculated 7 days later into replicate wells in medium containing no antibiotics. An inoculate corresponding to a multiplicity of infection of approximately 5 to 10 bacteria/adherent cell was optimal. For experiments using the Lmdaldat-gag strain, the medium contained 100 μg of d-alanine per ml until the addition of penicillin and streptomycin. Control wells were treated with nothing, 2 μg of phytohemagglutinin (PHA-P) per ml (Difco, Detroit, Mich.), or HIV immunodominant peptides (1 μg/ml) recognized by the subject's CD8 T cells (22). After 1 h at 37°C, adherent cells were washed to remove excess bacteria, and gentamicin was added to a final concentration of 50 μg/ml to kill extracellular bacteria. Autologous PBMCs (2 × 106/well) were added 1.5 h after infection, and penicillin (100 U/ml) and streptomycin (100 μg/ml) were added 3 or 16 h after the addition of PBMCs to kill intracellular bacteria. d-Alanine was removed from the cultures infected with Lmdaldat-gag, and colony counts were obtained from hypotonically lysed replicate wells at the time of penicillin-streptomycin addition. Recombinant human interleukin 2 (IL-2) (50 IU/ml; a gift of Chiron Oncology, Emeryville, Calif.) was added the next day. Cultures were fed with medium containing penicillin, streptomycin, and IL-2 biweekly and assayed after 7 to 15 days of culture.
CTL assays.
HIV-specific cytotoxicity of in vitro-stimulated PBMCs was analyzed by 4-h 51Cr release assay against autologous B-lymphoblastoid cell line (B-LCL) targets infected with vaccinia virus recombinant vectors encoding HIV genes as previously described (22). Target cells were infected with vaccinia virus vectors containing lacZ (vSC8), the gag gene of the HXB.2 subclone (vDK1), or nef from the molecular clone pNL432 (vTFnef) (6, 19). Epitope mapping of the CTL response of PHA-stimulated or L. monocytogenes-stimulated cell lines was performed as described previously (21).
Apoptosis assay.
Infected adherent cells were harvested by EDTA treatment 4.5 or 7 h after infection performed as described above. Cells were resuspended in 100 μl of PBS containing 5 μg of propidium iodide per ml and 5 μl of annexin V-fluorescein isothiocyanate (Pharmingen). After staining for 15 min at room temperature, cells were analyzed immediately on a loosely gated monocyte population by using a FACscalibur (Becton Dickinson) fluorescence-activated cell sorter. Gates were set using unstained control samples.
RESULTS
Construction of stable recombinants of L. monocytogenes and an attenuated d-alanine-requiring strain of Listeria that express and secrete HIV Gag and Nef proteins.
We previously produced a recombinant L. monocytogenes strain termed Lm-gag in which gag was inserted into the sepA gene of the bacterial chromosome of wild-type strain 10403S by using the reptsCmr shuttle vector pKSV7 and the method of Camilli (4, 13). The LD50 of the recombinant bacterium in BALB/c mice is about 3 logs greater than that of wild-type bacteria as a result of the physiological burden of expressing a foreign protein. A single inoculation of these bacteria results in a strong CD8 CTL response to Gag, which persists for at least 15 weeks or, after an additional boost, for at least 6 months. An analogous strategy was employed to express a secreted form of the nef gene from the molecular HIV clone pNL432 to produce Lm-nef. Expression of Gag and Nef by the recombinant bacteria was verified by the presence of 55- and 30-kDa immunoreactive bands, respectively, in extracts from the recombinant, but not wild-type, bacteria on immunoblots probed with antiserum (data not shown).
A major obstacle to using L. monocytogenes as a vaccine vector is its virulence in immunocompromised patients, pregnant women, and newborns. A hyperattenuated strain, termed Lmdaldat (or Lmdd), was produced by deleting large portions of the genes encoding the two enzymes responsible for d-alanine biosynthesis, alanine racemase and d-amino acid aminotransferase (40). The LD50 of Lmdaldat is approximately 7 × 107 or greater when inoculated with d-alanine and >8 × 108 when given without d-alanine. Inoculation with Lmdaldat protects BALB/c mice from challenge with the wild-type strain. To determine whether the hyperattenuated strain is efficient at presenting foreign proteins to the class I pathway for CD8 T-cell induction, gag was inserted into the sepA gene of Lmdaldat to produce Lmdaldat-gag. Expression of the p55Gag protein was verified by immunoblotting of cell lysates from monocytes infected with Lmdaldat-gag (data not shown).
Development of an in vitro protocol to measure CD8 T-cell induction from human PBMCs.
L. monocytogenes has proven to be a potent agent for the induction of strong CD8 T-cell responses in mice. Evaluation of the immunogenicity of this candidate human vaccine in nonhuman primates is expensive, and in humans, it would be dangerous and unethical before accumulating extensive preclinical safety and immunogenicity data. We have therefore developed an in vitro vaccination protocol to measure a secondary response to antigen in vitro, using PBMCs from HIV-infected donors. This procedure was used to examine the in vitro immunogenicity of wild-type and hyperattenuated Listeria strains to present HIV proteins for CD8 T-cell activation. To develop the assay, adherent PBMCs from HIV-seropositive donors were treated for 7 days with no cytokines or were activated with 5 ng of GM-CSF per ml with and without 500 U of IL-4 per ml. The combination of GM-CSF and IL-4 induces the differentiation of monocytes into “dendrophages,” cells with the morphology and functional antigen-presenting properties of dendritic cells (5).
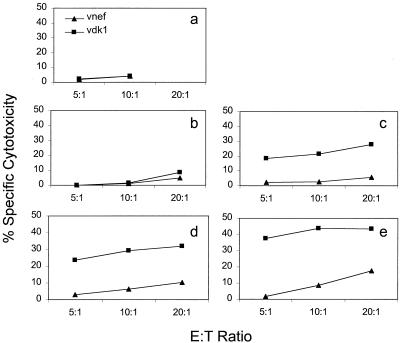
The adherent cells were infected in antibiotic-free medium with approximately 5 to 10 Lm-gag bacteria per adherent cell for 1 h before addition of gentamicin to kill extracellular bacteria. Autologous PBMCs were added 30 min later, and penicillin and streptomycin were added to the medium either 3 or 16 h after addition of PBMCs to kill intracellular bacteria. IL-2 was added to the in vitro-stimulated cultures 16 h after infection. They were then tested 15 days later for Gag-specific cytotoxicity by using autologous B cells infected with recombinant vaccinia viruses expressing gag or lacZ (Fig. 1). Antigen-specific cytotoxicity was found to be maximal 10 to 16 days after in vitro stimulation (data not shown). The cytotoxicity assays were performed at low effector/target (E:T) ratios (1.25 to 5:1), at which it is rare to detect any HIV-specific cytotoxicity above background levels without in vitro stimulation. Unactivated adherent PBMCs did not effectively present Lm-gag to boost the CD8 T-cell response. Adherent cells stimulated with GM-CSF were as efficient at antigen presentation as those activated with both GM-CSF and IL-4. The addition of penicillin and streptomycin at 3 h gave a modestly increased response compared to that of cultures in which intracellular bacteria were allowed to persist for 16 h.
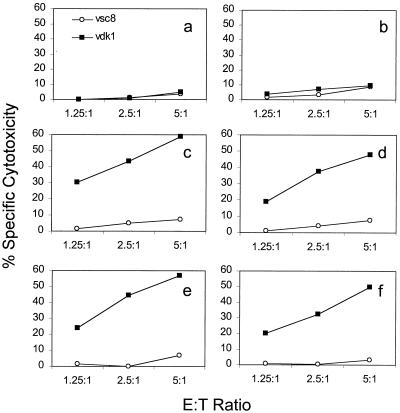
FIG. 1.
Development of an in vitro protocol to measure boosting of a CD8 CTL response to HIV Gag. Adherent PBMCs from HIV-seropositive donors were activated with nothing (b), GM-CSF (a, c, and d), or GM-CSF plus IL-4 (e and f) for 7 days before mock infection (a) or infection with Lm-gag (b to f). Gentamicin was added to all cultures 1 h after infection, and penicillin and streptomycin were added either 3 h after infection (a to c and e) or 16 h after infection (d and f). CTL assays against gag-expressing (vDK1) and lacZ (vsc8) control-expressing autologous targets were performed 15 days later. CD8 T-cell boosting required GM-CSF treatment, but was not enhanced by adding IL-4. Addition of penicillin and streptomycin at the earlier time modestly enhanced the response.
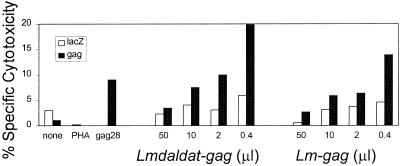
To optimize the in vitro stimulation protocol further, serial fivefold dilutions of Lm-gag cultures were added to replicate wells. The number of intracellular bacteria was counted 1 h after addition of gentamicin by plating dilutions of the supernatant after hypotonic lysis of the adherent cells (Fig. 2). The lowest concentration of bacteria (0.4 μl of bacterial culture or 40 CFU of intracellular bacteria/well) was most efficient at boosting Gag-specific CTLs. In the samples inoculated with the highest concentration (50 μl) of bacteria, there was a significant amount of adherent cell death by cell morphology; in the samples infected with fivefold fewer bacteria, there was some cell death; and at the two lowest concentrations, there was no apparent monocyte cell death. The cell death at higher concentrations or other less visible disruptions of monocyte function at high multiplicities of infection may help explain the dose response we observed. In vitro CTL induction by this low dose of Lm-gag was comparable to the response induced by stimulation with an excess (1 μg/ml) of the immunodominant Gag peptide. This concentration of bacteria was used in subsequent experiments.
FIG. 2.
A low inoculum of bacteria optimizes the CTL response in vitro. Adherent PBMCs from an asymptomatic HIV-seropositive donor were activated by exposure to GM-CSF and infected 1 week later with fivefold dilutions (50, 10, 2, and 0.4 μl) of washed Lm-gag or Lmdaldat-gag cultures. The corresponding numbers of intracellular colonies of Lm-gag per well after gentamicin treatment were 62,600, 49,200, 488, and 40, respectively. Specific cytotoxicity against gag- or lacZ-expressing targets was measured 12 days later at E:T ratios of 1.25 to 5:1. Representative data are shown for the ratio 2.5:1. Control wells were unstimulated or stimulated with PHA or an immunodominant Gag peptide recognized by this donor. The response induced by Lm-gag is comparable to that boosted by an excess of the immunodominant peptide (1 μg/ml).
Effect of infection by Lm-gag or Lmdaldat-gag on activated monocytes.
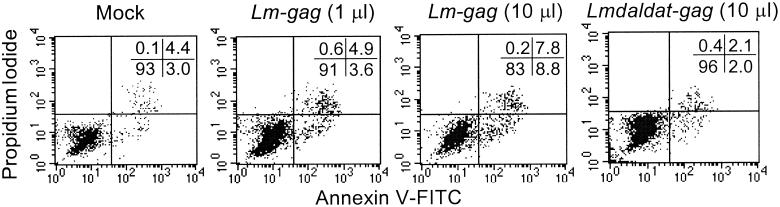
Because Lm-gag induces an unusually strong and durable CTL response in mice (13), we examined by flow cytometry the effect of in vitro infection on the expression of cell surface molecules involved in antigen presentation. There was no discernible difference in either the mean fluorescence intensity or proportion of cells that express HLA-DR, CD40, CD80, CD86, CD11a, or CD11b after infection with either Lm-gag or Lmdaldat-gag (data not shown). Adherent cells from both normal and HIV-infected donors were analyzed at 4.5 and 7 h after infection. Efficient infection was verified by counting bacterial colonies after hypotonic lysis of infected cells. We also looked to see whether high levels of p24 were expressed in the infected cells by staining permeabilized infected cells every day for 3 days after infection with antibodies to p24. The level of p24 expression was not high enough to be detected by flow cytometry, although it was detected by immunoblotting (data not shown). Infection with wild-type L. monocytogenes causes infected cells to die, but whether they die by necrosis or apoptosis is controversial (1, 16). To monitor apoptosis, we looked at whether Lm-gag- or Lmdaldat-gag-infected activated monocytes incorporate propidium iodide (PI) or stain with annexin V 4.5 or 7 h after infection (Fig. 3). In three independent experiments, there was a modest, but reproducible, dose-dependent increase in cells that stain for annexin V or are dually positive for annexin V staining and PI incorporation after Lm-gag infection compared to mock-infected cells. However, infection with Lmdaldat-gag leads to little or no induction of apoptosis.
FIG. 3.
Lm-gag infection induces a small amount of apoptosis, but Lmdaldat-gag does not. Activated monocytes from a stage A HIV-infected donor were analyzed for PI incorporation and annexin V staining 7 h after mock infection or after infection with the indicated amounts of bacteria. Additional experiments with normal donor cells yielded similar results.
In vitro CTL induction by Lm-gag and Lm-nef.
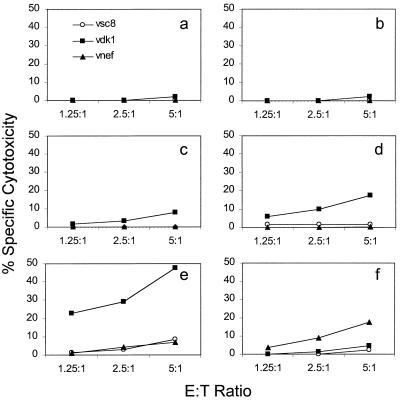
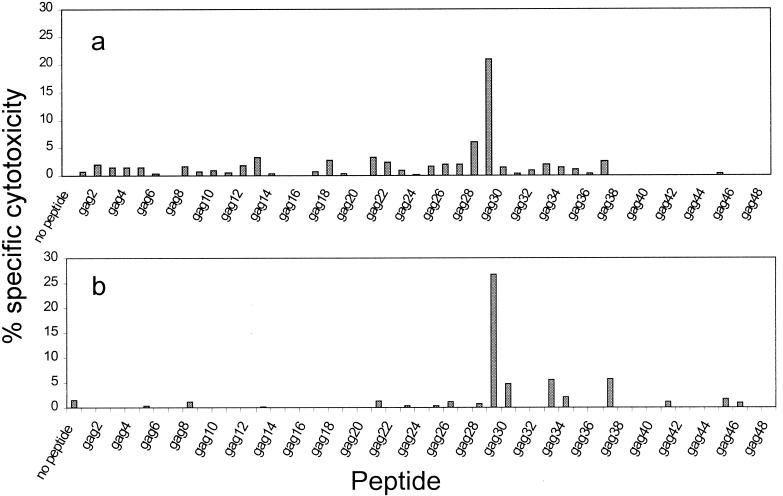
Samples from three asymptomatic HIV-infected donors were used to test the in vitro immunogenicity of Lm-gag and Lm-nef. Inoculation with Lm-nef or Lm-gag enhanced CTL responses in all cases (and only in those cases) in which cell lines from the donor had antigen-specific cytotoxicity that was detectable above background levels at higher E:T ratios. The criterion for antigen-specific cytotoxicity (before in vitro stimulation) was a specific cytotoxicity of at least 5% above the background level at an E:T ratio of 25:1 in at least two independent experiments with PHA-stimulated cell lines as effector cells (22). Results from a representative donor whose CTL response was dominated by the response to the gag29 (VHQAISPRTLNAWVKVVEEK) and nef12 (LWIYHTQGYFPDWQNYTPGPGV) peptides are shown in Fig. 4. The strength of the response was comparable to or greater than that of the response to immunodominant peptides. When the Lm-gag-boosted cell line was analyzed for epitope recognition, recognition of the same immunodominant epitope seen by PHA-stimulated PBMCs dominated the response, and no cryptic epitopes were induced (Fig. 5). Flow cytometry analysis of the responding cell line revealed a predominance of CD8 T cells (data not shown).
FIG. 4.
Lm-gag and Lm-nef boost the HIV-specific CTL response in vitro. Adherent cells from an asymptomatic HIV-infected donor were stimulated in vitro with nothing (a), PHA (b), Gag immunodominant peptide (c), or Nef immunodominant peptide (d) or were infected with Lm-gag (e) or Lm-nef (f). The Listeria recombinants were more efficient at boosting the CTL response than the dominant peptides. The response was measured against target cells infected with vaccinia viruses expressing lacZ (vcs8), gag (vDK1), or nef (vnef).
FIG. 5.
In vitro stimulation with either Lm-gag (a) or Lmdaldat-gag (b) enhances the response to the immunodominant peptide (gag29) that is recognized in vivo. Cell lines were analyzed 15 days after in vitro vaccination by a 4-h 51Cr release assay against autologous B-LCL target cells incubated with 20-mer overlapping peptides encoded by HXB.2 p24Gag gene (21). The experiment was performed at an E:T ratio of 20:1.
Hyperattenuated Lmdaldat-gag is as efficient as Lm-gag at boosting Gag-specific CTLs in vitro.
We used the in vitro stimulation protocol to compare the effectiveness of Lmdaldat-gag and Lm-gag for boosting CD8 T cells. In samples from three donors whose PBMCs were known to respond to p24Gag, the secondary boost to Lmdaldat-gag was at least as strong as the response to Lm-gag. Results for a representative sample are shown in Fig. 6. The epitopes recognized by the boosted CTLs were the same as the immunodominant epitope recognized in vivo (Fig. 5). As was found for Lm-gag, the response to Lmdaldat-gag was optimal at the lowest concentrations of bacteria tested (Fig. 2).
FIG. 6.
Hyperattenuated Lmdaldat-gag is as efficient at in vitro CTL induction as Lm-gag. Specific lysis of Gag-expressing targets is depicted by squares, and that of control targets is depicted by triangles. GM-CSF-activated adherent autologous PBMCs were mock treated (a) or treated with PHA (b), immunodominant Gag peptide (c), Lm-gag (d), or Lmdaldat-gag (e).
DISCUSSION
Since the 1960s, L. monocytogenes has been known to be an effective agent for the induction of cell-mediated immune responses, which could provide protection against subsequent challenge by the organism (24, 25). Subsequent studies showed that protection was provided predominantly by CD8 T cells (18). Recombinants of this organism have since been shown to be able to elicit protection against influenza virus, lymphocytic choriomeningitis virus, and cancer, and we have shown that a strong anti-HIV-1 Gag response occurs in mice following infection with HIV-1 Gag-expressing Listeria (Lm-gag). It is not practical to test the ability of this microorganism, a human pathogen, to induce similar anti-HIV responses in humans. Therefore, we have devised a model in vitro vaccination protocol to explore the ability of Listeria to infect human monocytes and present appropriate antigenic epitopes on the surface of infected cells to T cells, leading to their activation and proliferation. The results of this study demonstrate that Listeria is as effective for antigen presentation in human cells as the peptide epitope recognized by the CTLs derived from the HIV-infected patients used in these studies.
Although L. monocytogenes may be an attractive candidate vaccine vector for humans, it is widely agreed that its use in humans requires attenuation. Previous attempts at attenuation have either been incomplete or have come at the price of reduced immunogenicity. Heat-killed bacteria induce protective immunity, but it is short-lived (38). Genetically modified bacteria with an inactivated hly gene are avirulent, but fail to present antigens for CD8 T-cell activation (3, 26). Although disruption of actA does not interfere with the ability of bacteria to replicate in the cytoplasm and present antigens via the class I pathway, the attenuation may not be sufficient. The LD50 of the actA mutant bacteria is 3 logs higher than that of wild-type bacteria and comparable to that of the Lm-gag and Lm-nef bacteria. However, these bacteria are still virulent and persist in mice in the liver for up to 7 days (14, 15).
In this study, we examined the immunogenicity of a new avirulent strain of Listeria, Lmdaldat, in which d-alanine synthesis is crippled. The strain has been modified to express HIV-1 gag. Inoculation of BALB/c mice with Lmdaldat protected against challenge by wild-type L. monocytogenes and induced anti-Listeria CTLs under conditions in which the mutant phenotype was transiently suppressed by supplying d-alanine in the inoculate or in the drinking water (40). A single intraperitoneal injection of Lmdaldat-gag stimulates a strong CTL response to Gag in BALB/c mice that persists for 6 months. Moreover, oral immunization results in powerful CTL responses to Gag in Peyer's patches and mesenteric lymph nodes that are as potent as those induced by Lm-gag, which itself is modestly attenuated due to the physiological burden of foreign protein secretion (Rayevskaya and Frankel, unpublished). In this study, we found that the hyperattenuated avirulent Lmdaldat-gag was as effective at boosting Gag-specific CTLs in human samples in vitro as the wild-type Lm-gag or an excess of immunodominant peptide or recombinant vaccinia virus (data not shown).
At least in vitro, effective antigen presentation does not require the presence of d-alanine in the medium after the first few hours of infection. This result suggests that it may be desirable to incorporate d-alanine into the vaccine formulation when the hyperattenuated bacteria are used as a vaccine vector, but that it won't be necessary to have it available beyond that. We did not test whether infection in the absence of d-alanine also resulted in effective antigen presentation. This requires further study. In mouse cells, hyperattenuated Lmdaldat-gag cannot escape from the phagolysosome in the absence of exogenous d-alanine, and mice are not protected from wild-type L. monocytogenes infection after inoculation with Lmdaldat if d-alanine is not provided (Rayevskaya and Frankel, unpublished). Although an in vitro assay can give some guidance, the true test of immunogenicity and the d-alanine requirement will be in vivo.
In trying to understand why L. monocytogenes so potently induces T-cell immunity, we looked at the changes in L. monocytogenes-infected GM-CSF-activated adherent cells. There was no significant change in cell surface expression of costimulatory B7 molecules or CD40. Nor were there differences in class II molecule expression or adhesion molecule expression at least up to 7 h after infection. Infection with mildly attenuated Lm-gag induces only small amounts of apoptosis in the infected cells, which may aid antigen presentation by allowing the infected cell to persist for longer periods of time. This may be a further advantage for the hyperattenuated strain, which does not appear to induce any apoptosis in infected cells. The levels of p24 expression induced by infection are modest, since no p24 was detected by flow cytometry of stained permeabilized cells, although protein expression was verified by immunoblotting. CTLs are notoriously sensitive at detecting antigens; one estimate suggests that CTLs may be triggered by only one MHC-peptide complex on the cell surface (37), well below the sensitivity for flow cytometry detection. In fact, the dose response curve (Fig. 2) and improved CTL induction when antibiotics are added early (Fig. 1) suggest that less antigen may be better in the in vitro reaction—and possibly in vivo.
The in vitro vaccination protocol developed here may be a useful inexpensive screening test to evaluate candidate vaccines before performing animal studies. The procedure requires the presence of memory antigen-specific T cells in the sample, which are generally found circulating in the blood of individuals with a prior history of cleared or chronic intracellular infections. This may explain why we did not find any in vitro stimulation of Gag-specific CD4 T cells in samples from two HIV-seropositive subjects studied, who, like most HIV-infected persons, did not have a detectable proliferative response to p24 in their unstimulated PBMCs (data not shown). We were able to demonstrate a weak in vitro CD4 T-cell response to Lm-nef by using PBMCs from an HIV-seronegative donor (data not shown). However, the generation of a detectable response above background levels (stimulation index of 8.5 against vTFnef-infected B cells versus 1.6 against vSC8-infected B cells) required five in vitro stimulations. The ability of recombinant Listeria to stimulate antigen-specific CD4 T cells and cytokine production, as well as antibodies, requires further study. The keys to developing the in vitro “vaccination” assay were the cytokine activation of antigen presentation cells, the titration of infection for optimal antigen presentation, and the low background level in samples that had not been specifically stimulated when low E:T ratios were used. We have been able to use the same procedure (omitting the antibiotic manipulations) to study the effectiveness of a recombinant anthrax toxin vaccine at boosting human CTLs (23). Anthrax toxin constructs, which were able to internalize antigen, boosted a CTL response in vitro, while constructs that failed to translocate protein did not. The in vitro assay also allows a preliminary assessment of epitope presentation in the context of human MHC molecules prior to clinical testing.
Differentiation of monocytes to dendrophages with IL-4 did not enhance antigen presentation in the in vitro assay. This may be because monocytes are natural targets of L. monocytogenes infection or because the assay measures the boosting of a secondary response. If the effector cells had been from naïve individuals, then IL-4 might have been crucial to induce a primary response. A similar in vitro approach has been used to evaluate CD8 CTL induction by replication-defective canarypox virus vectors. In that case, unlike in ours, because poxviruses are promiscuous in the cells they infect, it was not necessary to activate the stimulator cells with cytokines (12). The in vitro vaccination assay, therefore, will need to be customized to optimize the response for each separate vaccine strategy, and for some approaches, stimulation of the antigen-presenting cells with IL-4 or tumor necrosis factor alpha may be desirable.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant AI-42509.
We thank Chiron Oncology for IL-2 and P. Shankar for useful discussions.
REFERENCES
- 1.Barsig J, Kaufmann S H E. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect Immunity. 1997;65:4075–4081. doi: 10.1128/iai.65.10.4075-4081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt L M, Portnoy D A, Unanue E R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 4.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheynier R, Langlade-Demoyen P, Marescot M R, Blanche S, Blondin G, Wain-Hobson S, Griscelli C, Vilmer E, Plata F. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur J Immunol. 1992;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Giorgi J V, Chou C C, et al. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 9.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlan J W. Early pathogenesis of Listeria monocytogenes infection in the mouse spleen. J Med Microbiol. 1996;44:295–302. doi: 10.1099/00222615-44-4-295. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, Kaufmann S H, Hess J, Szalay A A, Goebel W. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari G, Berend C, Ottinger J, Dodge R, Bartlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, Weinhold K J. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 13.Frankel F R, Hegde S, Lieberman J, Paterson Y. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J Immunol. 1995;155:4775–4782. [PubMed] [Google Scholar]
- 14.Goossens P L, Milon G. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int Immunol. 1992;4:1413–1418. doi: 10.1093/intimm/4.12.1413. [DOI] [PubMed] [Google Scholar]
- 15.Goossens P L, Milon G, Cossart P, Saron M F. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int Immunol. 1995;7:797–805. doi: 10.1093/intimm/7.5.797. [DOI] [PubMed] [Google Scholar]
- 16.Guzman C A, Domann E, Rohde M, Bruder D, Darji A, Weiss S, Wehland J, Chakraborty T, Timmis K N. Apoptosis of mouse dendritic cells is triggered by listeriolysis, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomidis G, Portnoy D A, Gerhard W, Paterson Y. Influenza-specific immunity induced by recombinant Listeria monocytogenes vaccines. Vaccine. 1997;15:433–440. doi: 10.1016/s0264-410x(96)00188-0. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 19.Koenig S, Fuerst T R, Wood L V, Woods R M, Suzich J A, Jones G M, de la Cruz V F, Davey R T, Jr, Venkatesan S, Moss B, et al. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990;145:127–135. [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman J, Fabry J A, Fong D M, Parkerson G R., III Recognition of a small number of diverse epitopes dominates the cytotoxic T lymphocytes response to HIV type 1 in an infected individual. AIDS Res Hum Retrovir. 1997;13:383–392. doi: 10.1089/aid.1997.13.383. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman J, Fabry J A, Kuo M C, Earl P, Moss B, Skolnik P R. Cytotoxic T lymphocytes from HIV-1 seropositive individuals recognize immunodominant epitopes in Gp160 and reverse transcriptase. J Immunol. 1992;148:2738–2747. [PubMed] [Google Scholar]
- 23.Lu Y, Friedman R, Kushner N, Doling A, Thomas L, Touzjian N, Starnbach M, Lieberman J. Genetically modified anthrax lethal toxin safely delivers whole HIV protein antigens into the cytosol to induce T cell immunity. Proc Natl Acad Sci USA. 2000;97:8027–8032. doi: 10.1073/pnas.97.14.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 25.Mackaness G B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969;129:973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 27.Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 28.Pan Z K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 29.Park S F, Stewart G S. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 30.Paterson Y, Ikonomidis G. Recombinant Listeria monocytogenes cancer vaccines. Curr Opin Immunol. 1996;8:664–669. doi: 10.1016/s0952-7915(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 31.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 34.Shen H, Miller J F, Fan X, Kolwyck D, Ahmed R, Harty J T. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 35.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith K, Youngman P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie. 1992;74:705–711. doi: 10.1016/0300-9084(92)90143-3. [DOI] [PubMed] [Google Scholar]
- 37.Sykulev Y, Joo M, Vturina I, Tsomides T J, Eisen H N. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 38.Szalay G, Ladel C H, Kaufmann S H. Stimulation of protective CD8+ T lymphocytes by vaccination with nonliving bacteria. Proc Natl Acad Sci USA. 1995;92:12389–12392. doi: 10.1073/pnas.92.26.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tappero J W, Schuchat A, Deaver K A, Mascola L, Wenger J D. Reduction in the incidence of human listeriosis in the United States. Effectiveness of prevention efforts? The Listeriosis Study Group. JAMA. 1995;273:1118–1122. doi: 10.1001/jama.1995.03520380054035. [DOI] [PubMed] [Google Scholar]
- 40.Thompson R J, Bouwer H G A, Portnoy D A, Frankel F R. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires d-alanine for growth. Infect Immun. 1998;66:3552–3561. doi: 10.1128/iai.66.8.3552-3561.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]