Abstract
The prevalence of heart failure is increasing, causing a tremendous burden on health care systems around the world. Although mortality rate of heart failure has been significantly reduced by several effective agents in the past 3 decades, yet it remains high in observational studies. More recently, several new classes of drugs emerged with significant efficacy in reducing mortality and hospitalization in chronic heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF). To integrate these effective therapies and prioritize them in the management of Asian patients, Taiwan Society of Cardiology has recently appointed a working group to formulate a consensus of pharmacological treatment in patients with chronic heart failure. Based on most updated information, this consensus provides rationales for prioritization, rapid sequencing, and in-hospital initiation of both foundational and additional therapies for patients with chronic heart failure.
Keywords: Asia, Chronic heart failure, Consensus, Foundational therapy, Left ventricular ejection fraction
Abbreviations
ACEI, Angiotensin converting enzyme inhibitor
A-HeFT, African-American Heart Failure Trial
aHR, Adjusted hazard ratio
ARB, Angiotensin receptor blocker
ARNI, Angiotensin receptor-neprilysin inhibitor
ATLAS, Assessment of Treatment with Lisinopril and Survival
CI, Confidence interval
cGMP, Cyclic guanosine monophosphate
COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival
DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure
DELIVER, Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure
DIG, Digitalis Investigation Group
EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction
EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure
EMPULSE, Effect of Empagliflozin in Patients Who Are in Hospital for Acute Heart Failure
HEAAL, Heart failure Endpoint evaluation of Angiotensin II Antagonist Losartan
HfimpEF, Heart failure with improved EF
HFmrEF, Heart failure with mildly reduced ejection fraction
HFnEF, Heart failure with normal ejection fraction
HFpEF, Heart failure with preserved ejection fraction
HFrEF, Heart failure with reduced ejection fraction
HFsrEF, Heart failure with severely reduced ejection fraction
IMPACT-HF, Initiation Management Pre-Discharge: Assessment of Carvedilol Therapy for Heart Failure
KCCQ, Kansas City Cardiomyopathy Questionnaire
LVEF, Left ventricular ejection fraction
MRA, Mineralocorticoid receptor antagonist
NYHA, New York Heart Association
NT-proBNP, N-terminal prohormone of brain natriuretic peptide
PARADIGM-HF, Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure
PARAGON-HF, Prospective Comparison of ARNI With ARB Global Outcomes in HF With Preserved Ejection Fraction
PEP-CHF, Perindopril in Elderly People with Chronic Heart Failure
PIONEER-HF, Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode
RALES, Randomized Aldactone Evaluation Study
SGLT2, Sodium-glucose co-transporter 2
SHIFT, Systolic Heart failure treatment with the If inhibitor ivabradine Trial
SOLOIST-WHF, Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure
SOLVD, Studies of Left Ventricular Dysfunction
STRONG-HF, Safety, Tolerability and Efficacy of Rapid Optimization, Helped by NT-proBNP Testing, of Heart Failure Therapies
TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist
V-HeFT, Vasodilator Heart Failure Trial
VICTORIA, Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction
WHF, Worsening heart failure
1. INTRODUCTION
Heart failure is a clinical syndrome characterized by cardinal symptoms of shortness of breath, ankle swelling, and fatigue, and commonly accompanied by typical signs of elevated jugular venous pressure, lung rales, and peripheral edema.1 The prevalence of heart failure is around 1-2% in adults, but the clinical course of heart failure is grave, characterized by repetitive hospitalization and high cardiovascular mortality.1 Mortality rate of heart failure has been significantly reduced by several effective drugs in the past 3 decades,2-4 but it remains high in observational studies.5 More recently, several new classes of drugs emerged with significant efficacy in reducing mortality and hospitalization in chronic heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF).6-12 How to integrate these effective therapies and prioritize them is unclear in recent heart failure guidelines and consensuses.1,13 Taiwan Society of Cardiology has recently appointed a working group to formulate a consensus of pharmacological treatment in patients with chronic heart failure.
2. BURDEN OF HEART FAILURE IN ASIA AND TAIWAN
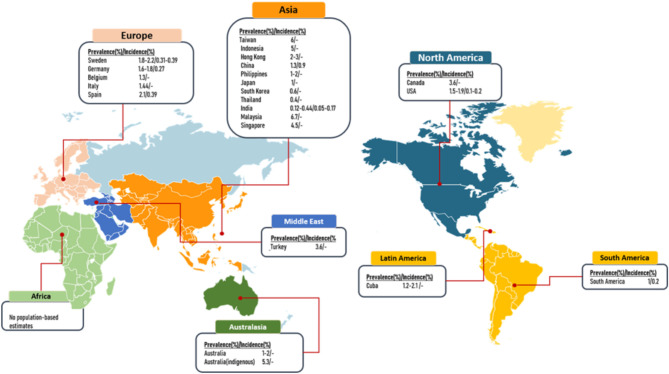
The incidence of heart failure in developed countries has been stabilized and ranges from 1% to 14%.14 Although the global age-adjusted incidence of heart failure is decreasing, the absolute number and heart failure prevalence is increasing15,16 (Figure 1). Heart failure mortality remains high, approximately 50% at 5 years in recent years.17 With significant regional and ethnic heterogeneity, the reported 6-month and 12-month crude mortality rates were 6.9% and 9.6%, respectively, among heart failure patients in Asia.16
Figure 1.
Prevalence and incidence of heart failure in population-based studies around the world.
The incidence of heart failure in Taiwan in 2016 was 2.19 per 1000 person-years with a stepwise increase with age, with an overall temporal trend of slightly decreased incidence from 2001 to 2016 (2.44 to 2.19 per 1,000 person-years, respectively).18 The prevalence increased from 0.63% in 2001 to 1.40% in 2016, with a 2.22-fold increase over the 16 years. The projected prevalence rate was estimated to be 1.99% in 2025, and would step up every 5 years to 2.44%, 2.88%, 3.36%, 3.89%, and 4.45% (803,401 patients estimated) in 2050.18 The lifetime risk of heart failure was 1 in 5 for Taiwanese adults aged ≥ 20 years, being higher for males (1 in 4) compared to females (1 in 5). The incident mortality after newly diagnosed heart failure during follow-up was estimated to be 38.5%, 52.2%, 62.1%, 69.6% and 75.5% at 2-year, 4-year, 6-year, 8-year and 10-year follow-up, respectively. The annual rate of all-cause death was 16.53%, higher than those without heart failure [adjusted hazard ratio (aHR): 1.80, 95% confidence interval (CI): 1.38-2.36, p < 0.01].18
Consensus statements
• Despite a decrease in the incidence rate of heart failure in Taiwan in the recent 2 decades, the prevalence rate is increasing, similar to current Asia status.
• For adult Taiwanese, the lifetime risk of heart failure is 1 in 5 for women and 1 in 4 for men.
• The all-cause death rate of heart failure in Taiwan is very high, around 50% in 4 years and 75% in 10 years.
3. PROPOSED RE-CLASSIFICATION OF HEART FAILURE
Classification of heart failure based on left ventricular ejection fraction (LVEF) is the most practical approach because it relates to prognosis and response to treatment. More importantly, almost all clinical trials enrolled patients accordingly to individual LVEF. Previously, HFrEF and HFpEF were defined as LVEF ≤ 40% and ≥ 50%, respectively, and LVEF 41-49% was defined as heart failure with mildly reduced EF (HFmrEF).1,19 It seems somewhat awkward to classify patients with LVEF < 50% into HFrEF and HFmrEF. Are patients with HFrEF more severe than and responding to therapy differently to patients with HFmrEF? Several analyses suggested that patients with HFmrEF were less severe than HFrEF, but benefit from, though to a lesser degree, mineralocorticoid receptor antagonist (MRA),20,21 beta-blockers,22 angiotensin receptor blocker (ARB),21,23 angiotensin receptor-neprilysin inhibitor (ARNI),21,24 and, most recently, sodium-glucose co-transporter 2 (SGLT2) inhibitor.25 A more straightforward way is to classify patients with LVEF < 50% as HFrEF, and further classify HFrEF into HFmrEF (LVEF 41%-49%) and heart failure with severely reduced EF (HFsrEF) (LVEF ≤ 40%) (Figure 2 and Table 1). It is also reasonable to name those patients with LVEF ≥ 60% as heart failure with normal EF (HFnEF).26,27 There is an argument that male, when compared with female, have a lower cutoff EF (EF ≥ 55% vs. ≥ 60%) for HFnEF.26 We did not propose different cutoff of LVEF for gender, as the efficacy of heart failure treatment was similar between genders in more recent trials,11,12 though in the only ARNI trial we did observe a difference.28 In a subgroup analysis of the DELIVER trial, patients with HFnEF got similar degree of benefits from SGLT2 inhibitor.12,29 Nevertheless, other underlying causes, such as amyloidosis and hypertrophic cardiomyopathy, need to be thoroughly investigated in patients with HFnEF.
Figure 2.
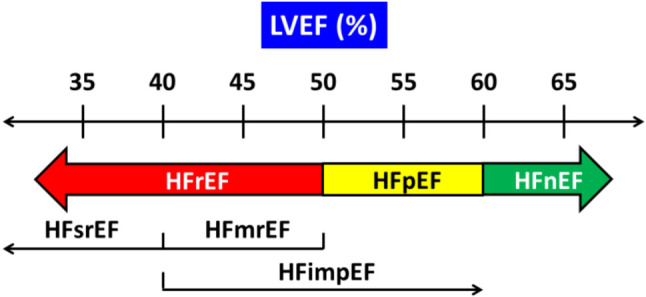
Proposed new classification of heart failure. HFimpEF, HF with improved ejection fraction (previous LVEF < 40% that improved by 10% in LVEF and now > 40%); HFimpEF,heart failure with improved EF; HFmrEF, heart failure with mildly reduced ejection fraction; HFnEF, heart failure with normal ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFsrEF, heart failure with severely reduced ejection fraction; LVEF, left ventricular ejection fraction.
Table 1. Classification of heart failure based on LVEF.
| Type of HF | HFrEF | HFpEF | HFnEF | |
| HFsrEF | HFmrEF | |||
| CRITERIA | ||||
| 1 | Symptoms ± signs | Symptoms ± signs | Symptoms ± signs | Symptoms ± signs |
| 2 | LVEF ≤ 40% | LVEF 41-49% | LVEF 50-59% | LVEF ≥ 60% |
| 3 | – | – | Objective evidence of cardiac structural and functional abnormalities consistent with spontaneous or provokable increased LV filling pressures; and elevated natriuretic peptides* | Objective evidence of cardiac structural and functional abnormalities consistent with spontaneous or provokable increased LV filling pressures; and elevated natriuretic peptides* |
HF, heart failure; HfmrEF, heart failure with mildly reduced ejection fraction; HfnEF, heart failure with normal ejection fraction; HfpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HfsrEF, heart failure with severely reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction.
* B-type natriuretic peptide ≥ 35 pg/mL and/or N-terminal pro-B type natriuretic peptide ≥ 125 pg/mL.
Heart failure patients with an initial LVEF ≤ 40%, but that improved to > 40% or even ≥ 50%, are classified as heart failure with improved EF (HFimpEF). Patients with HFimpEF can be looked at as a subgroup of HFsrEF, who have baseline characteristics similar to HFsrEF but different from those with HFpEF.30 The DELIVER trial is the first to enroll this particular type of patients and confirmed that patients with HFimpEF obtained similar magnitude of benefits compared with that for HFrEF.12
Additionally, objective evidence of cardiac structural and functional abnormalities consistent with spontaneous or provocable increased LV filling pressures, and elevated natriuretic peptides, are required to define HFpEF and HFnEF (Table 1).
Consensus statements
• Based on LVEF, Taiwan Society of Cardiology has proposed a new classification of heart failure.
• Heart failure with LVEF ≤ 40%, 41%-49%, 50%-59%, and ≥ 60% are classified as HFsrEF, HFmrEF, HFpEF, and HFnEF, respectively.
• Patients with HFrEF include those with HFsrEF and HFmrEF.
• Heart failure with an initial LVEF ≤ 40%, but that improves to > 40% or even ≥ 50%, are classified as HFimpEF.
• Objective evidence of cardiac structural and functional abnormalities, combined with elevated natriuretic peptides, is required to define HFpEF and HFnEF.
• For patients with HFnEF, underlying causes, such as amyloidosis and hypertrophic cardiomyopathy, should be thoroughly investigated.
4. CLASSIFICATION OF MEDICATIONS FOR HFrEF
4.1. Diuretics
Given the central role of volume expansion in the pathogenesis of congestion, diuretics are among the cornerstones of treatment of heart failure, though the effects of diuretics, except MRAs, on morbidity and mortality are uncertain. Despite that the recent OPTIMIZE-HF registry with diuretic use compared with no diuretic use after discharge for heart failure demonstrated reductions in all-cause death and hospitalization for heart failure,31 diuretics should not be used in isolation, since they need to be combined with other evidence-based therapy. Loop diuretics are the mainstays of diuretic agents in most patients with heart failure, irrespective of LVEF.
4.2. Foundational therapy and additional therapy
In this consensus, we classify medications for HFrEF into two groups: foundational therapy and additional therapy. We consider a drug to be foundational therapy if it reduces cardiovascular death and/or all-cause death, and the risk of hospitalization for heart failure in large-scale clinical trials.32 This group includes angiotensin converting enzyme inhibitor (ACEI), ARB, beta-blocker, MRA, ARNI, and SGLT2 inhibitor. Additional therapy includes digoxin, hydralazine/isosorbide dinitrate, ivabradine, and vericiguat, because the magnitude of the overall treatment effects has been modest, the strength of evidence (the level of statistical significance) is not robust, or the benefits are limited to specific subgroups.32 In general, foundational therapy should be used first, whereas additional therapy can be added when patients still have symptoms despite of foundational therapy, or in certain conditions when foundational therapy cannot be applied or contraindicated. The characteristics, inclusion criteria, and event reductions of major placebo-controlled HFsrEF trials for both the foundational therapies and additional therapies are shown in Table 2.
Table 2. Characteristics, inclusion criteria, and efficacy of major trials of HFsrEF.
| Characteristics | Inclusion/exclusion criteria | Events | |||||||||||
| Class of drug | Name of trial | Drug name | Number of patient | EF (%) | SBP (mmHg) | HR (bpm) | Cr (mg/dL) | eGFR (mL/min) | K (meq/L) | All-cause death | CV death + HHF | CV death | HHF |
| Foundational therapy | |||||||||||||
| ACEI | CONSENSUS33 | Enalapril | 253 | NR | NR | NR | ≤ 3.7 | NR | NR | ↓ | NR | ↓ | NR |
| SOLVD34 | Enalapril | 2569 | ≤ 35 | NR | NR | ≤ 2 | NR | NR | ↓ | ↓ | ↓ | ↓ | |
| ARB | Val-HeFT39 | Valsartan | 5010 | ≤ 40 | ≥ 90 | NR | ≤ 2.5 | NR | NR | ↔ | ↓ | ↔ | ↓ |
| CHARM-Alternative40 | Candesartan | 2028 | ≤ 40 | NR | NR | < 3 | NR | < 5.5 | ↔ | ↓ | ↓ | ↓ | |
| BB | US Carvedilol49 | Carvedilol | 1094 | ≤ 35 | ≥ 85 | ≥ 68 | NR | NR | NR | ↓ | ↓ | ↓ | ↓ |
| MERIT-HF3 | Metoprolol succinate | 3991 | ≤ 40 | ≥ 100 | ≥ 68 | NR | NR | NR | ↓ | ↓ | ↓ | ↓ | |
| CIBIS-II51 | Bisoprolol | 2647 | ≤ 35 | ≥ 100 | ≥ 60 | NR | NR | NR | ↓ | ↓ | ↓ | ↓ | |
| COPERNICUS50 | Carvedilol | 2289 | ≤ 25 | ≥ 85 | ≥ 68 | ≤ 2.8 | NR | 3.5-5.2 | ↓ | ↓ | ↓ | ↓ | |
| SENIORS52 | Nebivolol | 2128 | ≤ 35 | ≥ 90 | ≥ 60 | NR | NR | NR | ↔ | ↓ | ↔ | ↔ | |
| MRA | RALES4 | Spironolactone | 1663 | ≤ 35 | NR | NR | ≤ 2.5 | NR | ≤ 5 | ↓ | ↓ | ↓ | ↓ |
| EMPHASIS-HF55 | Eplerenone | 2737 | ≤ 35 | NR | NR | NR | ≥ 30 | ≤ 5 | ↓ | ↓ | ↓ | ↓ | |
| ARNI | PARADIGM-HF6 | Sacubitril/valsartan | 8442 | ≤ 40 | ≥ 100 | NR | NR | ≥ 30 | ≤ 5.2 | ↓ | ↓ | ↓ | ↓ |
| SGLT2 i | DAPA-HF7 | Dapagliflozin | 4744 | ≤ 40 | ≥ 95 | NR | NR | ≥ 30 | NR | ↓ | ↓ | ↓ | ↓ |
| EMPEROR-Reduced8 | Empagliflozin | 3730 | ≤ 40 | ≥ 100 | NR | NR | ≥ 20 | NR | ↔ | ↓ | ↔ | ↓ | |
| SOLOIST-WHF9 | Sotagliflozin | 1222 | NL | ≥ 100 | NR | NR | ≥ 30 | NR | ↔ | ↓ | ↔ | ↓ | |
| Additional therapy | |||||||||||||
| Digoxin | DIG72 | Digoxin | 6800 | ≤ 45 | NR | NR | NR | NR | NR | ↔ | NR | ↔ | ↓ |
| Vasodilator | V-HeFT I76 | Hydralazine/isosorbide dinitrate | 642 | < 45 | NR | NR | NR | NR | NR | ↓ | NR | NR | NR |
| A-HeFT78 | Isosorbide/hydralazine | 1050 | ≤ 35 | NR | NR | NR | NR | NR | ↓ | NR | NR | ↓ | |
| Sinus node inhibitor | SHIFT79 | Ivabradine | 6558 | ≤ 35 | ≥ 85 | ≥ 70 | NR | NR | NR | ↔ | ↓ | ↔ | ↓ |
| sGC stimulator | VICTORIA10 | Vericiguat | 5050 | ≤ 45 | ≥ 100 | NR | NR | ≥ 15 | NR | ↔ | ↓ | ↔ | ↓ |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blocker; bpm, beat per minute; Cr, serum creatinine; CV, cardiovascular; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HFsrEF, heart failure with severely reduced ejection fraction; HHF, heart failure hospitalization; HR, heart rate; K, potassium; MRA, mineralocorticoid receptor antagonist; NL, no limit; NR, not reported; SBP, systolic blood pressure; sGC, soluble guanylate cyclase; SGLT2 i, sodium-glucose co-transporter 2 inhibitor.
Asian patients were not included in some remote trials, and only recent trials of ARNI,6 SGLT2 inhibitors,7,8 and vericiguat enrolled Asian patients.10 In general, the efficacy in Asian subgroup did not differ from other races and the main trial.6-8,10
4.3. ACEI/ARB/ARNI
4.3.1. ACEI
ACEIs are the first class of foundational therapy that could reduce all-cause death, cardiovascular death, and hospitalization for heart failure33,34 (Table 2). They should be used in patients with systolic blood pressure ≥ 90 mmHg.1 Patients with severely impaired renal function (serum creatinine level > 3.7 mg/dL) were excluded.33 Based on one important study in Asians, ACEI can be used in patients with an estimated glomerular filtration rate of 20 mL/min.35 The major issue of ACEI in Asian population is higher prevalence of cough compared with Caucasians. The starting doses, target doses, and the mean doses achieved in clinical trials are shown in Table 3.
Table 3. Doses and early effects of foundational therapies in randomized controlled trials.
| Drug name | Trial name | Ref. # | Starting dose | Steps of titration | Target dose | Mean dose achieved in RCTs | Proportion reaching target dose | Mean dose at earliest time of efficacy (% of target dose) | Earliest time of efficacy |
| Enalapril | SOLVD | 34, 36 | 2.5-5 mg BID | 44960 | 10 mg BID | 16.6 mg/day | 49% | NR | 30 days |
| Candesartan | CHARM-low LVEF | 41, 42 | 4-8 mg QD | 44960 | 32 mg QD | 24 mg/day | 60% | 8.5 mg QD (26.6%) | 28 days |
| Carvedilol | COPERNICUS | 50, 54 | 3.125 mg BID | 4 | 25 mg BID | 37 mg/day | 65% | 6.5 mg BID (26%) | 28 days |
| Eplerenone | EMPHASIS-HF | 42, 55 | 25 mg QD | 2 | 50 mg QD | 42 mg/day | 85% | 27.6 mg QD (55.2%) | 28 days |
| Eplerenone | EPHESUS | 58, 59 | 25 mg QD | 2 | 50 mg QD | 42.6 mg/day | NR | 25 mg QD (50%) | 30 days |
| Sacubitril/valsartan | PARADIGM-HF | 6, 48 | 49/51 mg BID | 2 | 97/103 BID | 182 mg/193 mg/day | NR | NR (Most patients maintained at target dose) | 30 days |
| Dapagliflozin | DAPA-HF | 7, 69 | 10 mg QD | 1 | 10 mg QD | 9.8 mg/day | 98.1% | 10 mg QD (100%) | 28 days |
| Empagliflozin | EMPEROR-Reduced | 8, 70 | 10 mg QD | 1 | 10 mg QD | NR | NR | 10 mg QD (100%) | 28 days |
BID, twice daily; NR, not reported; QD, once daily; Ref, reference.
In the Studies of Left Ventricular Dysfunction (SOLVD) trial,34 HRs for all-cause death, heart failure hospitalization, and the combined endpoint of heart failure hospitalization or all-cause death at 14 days after randomization were 0.80 (95% CI: 0.32-2.03), 0.63 (95% CI: 0.35-1.12), and 0.65 (95% CI: 0.39-1.06), respectively. Corresponding HRs at 30 days were 0.82 (95% CI: 0.41-1.67), 0.43 (95% CI: 0.27-0.68), and 0.43 (95% CI: 0.27-0.68), respectively36 (Table 3). The magnitude of these early effects of starting doses of enalapril is similar to its previously reported long-term effects at the target dose.36 These data prompt early initiation of ACEI in HFsrEF. Given that enalapril have been safely initiated in-hospital in the Comparison of Sacubitril-Valsartan versus Enalapril on Effect on N-terminal prohormone of brain natriuretic peptide (NT-proBNP) in Patients Stabilized from an Acute Heart Failure Episode (PIONEER-HF) trial,37 ACEI can be initiated before discharge to provide maximal protection, if patients are in the convalescent phase with hemodynamic stability.
The effect of ACEI extending to patients with HFmrEF was shown in the Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) trial.38 Patients with LVEF 41-49% (i.e. HFmrEF) were enrolled to compare perindopril versus placebo. Perindopril did not reduce the primary endpoint (composite of all-cause death and heart failure hospitalization) (HR: 0.919, 95% CI: 0.700-1.208; p = 0.545), due to lower enrollment and event rates. Moreover, many patients withdrew from perindopril (28%) and placebo (26%) after 1 year and started taking open-label ACEI. Interestingly, there were reductions in hospitalization for heart failure (HR: 0.63, 95% CI: 0.41-0.97; p = 0.033) (Table 4). Func-tional class (p < 0.030) and 6-minute corridor walk distance (p = 0.011) improved in those assigned to perindopril. Though uncertainty remains about the effects of perindopril on long-term morbidity and mortality in this clinical setting (HFmrEF), improved symptoms and exercise capacity and fewer hospitalizations for heart failure were observed for perindopril in the first year.38
Table 4. Evidence of foundational therapies in patients with HFmrEF.
| Drug class/drug name | Trial name | Number of patients | Inclusion LVEF (patient number) | Primary endpoints | Effect on primary endpoint by LVEF, HR (95% CI) (p value) | Pint | Effect on HHF by LVEF, HR (95% CI) (p value) | Pint | ||||||
| ACEI/perindopril | PEP-CHF38 | 850 | < 40% (0) | 41-49% (850) | ≥ 50% (0) | All-cause death + HHF | < 40%, – | 41-49%, HR: 0.69 (0.47-1.01) (p = 0.055) | ≥ 50%, – | – | < 40%, – | 41-49%, HR: 0.63 (0.41-0.97) (p = 0.033) | ≥ 50%, – | – |
| ARB/candesartan | CHARM-Program23 | 7598 | < 40% (4,323) | 41-49% (1,322) | ≥ 50% (1,953) | CV death + HHF | < 40%, HR: 0.82 (0.75-0.91) (p < 0.001) | 41-49%, HR: 0.76 (0.61-0.96) (p = 0.02) | ≥ 50%, HR: 0.95 (0.79-1.14) (p = 0.57) | 0.27 | < 40%, HR: 0.68* (0.58-0.80) (p < 0.001) | 41-49%, HR: 0.48* (0.33-0.70) (p < 0.001) | ≥ 50%, HR: 0.78* (0.59-1.03) (p = 0.08) | 0.60 |
| ARNI | PARADIGM/PARAGON Pooled24 | 13195 | 32.5-42.5% (3,143) | 42.5-52.5% (1,427) | 52.5-62.5% (2,166) | CV death + HHF | 32.5-42.5%, HR: 0.81 (0.69-0.94) (p = 0.005) | 42.5-52.5%, HR: 0.89 (0.73-1.10) (p = 0.28) | 52.5-62.5%, HR: 0.89, (0.74-1.06) (p = 0.05) | 0.05 | 32.5-42.5%, HR: 0.81 (0.69-0.94) (p = 0.005) | 42.5-52.5%, HR: 0.89 (0.73-1.10) (p = 0.28) | 52.5-62.5%, HR: 0.89 (0.74-1.06) (p = 0.05) | 0.15 |
| Beta-blocker | 11 trials/Meta-analysis22 | 14262 | – | 41-49% (5,750) | ≥ 50% (244) | All-cause death or CV death | – | 41-49%, HR: 0.59 (0.34-1.03) (p = 0.066) | ≥ 50%, HR: 1.79 (0.78-4.10) (p = 0.17) | NR | – | 41-49%#, HR: 0.48 (0.24-0.97) (p = 0.04) | ≥ 50%#, HR: 1.77 (0.61-5.14) (p = 0.29) | NR |
| MRA/eplerenone | TOPCAT20 | 1766 | 45 to < 50% (197) | 50 to 55% (289) | 55 to 60% (422) | CV death + HHF | 45 to < 50%, HR: 0.55 (0.33-0.91) | 50 to 55%, HR: 0.83 (0.56-1.25) | 55 to 60%, HR: 0.85 (0.60-1.21) | 0.069 | 45 to < 50%, HR: 0.60 (0.32-1.10) | 50 to 55%, HR: 0.80 (0.65-1.06) | 55 to 60%, HR: 0.70 (047-1.06) | 0.037 |
| SGLT2 inhibitor/empagliflozin | EMPEROR-Preserved11 | 5988 | < 50% (1,983) | 50 to < 60% (2,058) | ≥ 56% (1,947) | CV death + HHF | < 50%, HR: 0.71 (0.57-0.88) | 50 to < 60%, HR: 0.80 (0.64-0.99) | ≥ 56%, HR: 0.87 (0.69-1.10) | NR | NR | NR | NR | NR |
| SGLT2 inhibitor/empagliflozin | EMPEROR-Reduced/EMPEROR-Preserved pooled25 | 9718 | 35-44% (1,272) | 45-54% (2,260) | 55-64% (2,092) | CV death + HHF | 35-44%, HR: 0.82 (0.63-1.05) | 45-54%, HR: 0.74 (0.61-0.91) | 55-64%, HR: 0.78 (0.62-0.97) | NR | 35-44%, HR: 0.72 (0.52-0.98) | 45-54%, HR: 0.66 (0.50-0.86) | 55-64%, HR: 0.70 (0.53-0.92) | 0.32 |
| SGLT2 inhibitor/dapagliflozin | DELIVER12 | 6263 | < 49% (2,116) | 50 to 59% (2,256) | ≥ 60% (1,891) | CV death + WHF | < 49%, HR: 0.87 (0.72-1.04) | 50 to 59%, HR: 0.79 (0.65-0.97) | ≥ 60%, HR: 0.78 (0.62-0.96) | > 0.05 | > 44% and ≤ 51%†, HR: 0.83 (0.64-1.07) | > 51% and ≤ 60%†, HR: 0.66 (0.51-0.84) | ≥ 60%†, HR: 0.88 (0.64-1.22) | > 0.05 |
| SGLT1+2 inhibitor/sotagliflozin | SOLOIST-WHF9 | 1222 | < 50% | ≥ 50% | CV death + total HHF + urgent HF visit | < 50%, HR: 0.72 (0.56-0.94) | ≥ 50%, HR: 0.48 (0.27-0.86) |
* Recurrent heart failure hospitalization; # Cardiovascular death; † Data from Jhund et al.29 Data shown in bold characters are for HFmrEF.
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CI, confidence interval; CV, cardiovascular; HF, heart failure; HfmrEF, heart failure with mildly reduced ejection fraction; HHF, heart failure hospitalization; HR, hazard ratio; int, interaction; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NR, not reported; SGLT2 I, sodium-glucose co-transporter 2 inhibitor; WHF, worsening heart failure (heart failure hospitalization + urgent heart failure visit).
4.3.2. ARB
ARBs reduced cardiovascular death and hospitalization for heart failure, but not all-cause death39,40 (Table 2). They should be used in patients with systolic blood pressure ≥ 90 mmHg.39 It is reasonable to have the similar lower limit of estimated glomerular filtration rate as ACEI (20 mL/min). Patients who are intolerant to ACEI because of cough or angioedema should be started on or changed to an ARB.
Early efficacy of ARB was shown in a pre-defined sub-analysis of patients with low LVEF in the CHARM Program.41,42 Treatment with candesartan led to a significant reduction in the composite of all-cause death and heart failure hospitalization within 28 days of randomization (HR: 0.61, 95% CI: 0.43-0.86) when the daily dose was only 8.5 mg daily, 26.6% of the target dose (Table 3).
The effect of ARB extending to patients with HFmrEF was demonstrated by in an analysis of 7,598 patients in the whole CHARM Program23 (Table 4). Patients with HFmrEF were similar to those with HFsrEF with respect to some characteristics, and intermediate between HFsrEF and HFpEF with respect to others.23 The incidence of primary endpoint (cardiovascular death and heart failure hospitalization) for candesartan vs. placebo were 14.4 versus 17.5%/y in HFsrEF (HR: 0.82, 95% CI: 0.75-0.91; p < 0.0010), 7.4 vs. 9.7%/y in HFmrEF (HR: 0.76, 95% CI: 0.61-0.96; p = 0.02), and 8.6 vs. 9.1 %/y in HFpEF (HR: 0.95, 95% CI: 0.79-1.14; p = 0.57). For recurrent hospitalization due to heart failure, the incidence rate ratios were 0.68 in HFsrEF (95% CI: 0.58-0.80; p < 0.001), 0.48 in HFmrEF (95% CI: 0.33-0.70; p < 0.001), and 0.78 in HFpEF (95% CI: 0.59-1.03; p = 0.08) (Table 4). With EF as a continuous spline variable, candesartan significantly reduced the primary outcome until LVEF well over 50% and recurrent HF hospitalizations until LVEF well over 60%.23 These findings were confirmed by a recent pooled analysis of individual patient-level data from the CHARM-Program.21 An ARB may be of benefit beyond the upper limit of LVEF eligibility used in contemporary HFrEF trials (40%) and may extend to HFmrEF (LVEF 41-49%) and even to the lower part of the LVEF range currently categorized as HFpEF (LVEF ≥ 50%).21
4.3.3. ARNI
An ARNI is comprised of an ARB and a neprilysin inhibitor. In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Mor-bidity in Heart Failure (PARADIGM-HF) trial,6 sacubitril/valsartan, when compared with enalapril, reduced the primary endpoint (composite of cardiovascular death and heart failure hospitalization) by 20% (HR: 0.80, 95% CI: 0.73-0.87; p < 0.001) (Table 2). Cardiovascular death (HR: 0.80, 95% CI: 0.71-0.89, p < 0.001), heart failure hospitalization (HR: 0.79, 95% CI: 0.71-0.89, p < 0.001), and all-cause death (HR: 0.84, 95% CI: 0.76-0.93, p < 0.001) were all reduced. Based on a putative placebo analysis of the PARADIGM-HF trial, sacubitril/ valsartan significantly reduced cardiovascular death by 34% (p < 0.0001) and heart failure hospitalization by 49% (p < 0.0001).43
Additional benefits of sacubitril/valsartan included an improvement in symptoms and quality-of-life,6 a reduction in the incidence of diabetes requiring insulin treatment,44 and a reduction in the decline in renal function,45 as well as a reduction in hyperkalemia.46 We recommend that an ACEI or ARB can be replaced by sacubitril/valsartan in ambulatory patients with HFsrEF, who remain symptomatic despite optimal treatment. Symptomatic hypotension was more common in patients treated with sacubitril/valsartan.6 Sacubitril/valsartan should be used in patients with systolic blood pressure ≥ 100 mmHg.6 It is reasonable to have the lower limit of estimated glomerular filtration rate at 20 mL/min.47
In the PARADIGM-HF trial,6 the reduction in heart failure hospitalization with ARNI was evident within the first 30 days (HR: 0.60, 95% CI: 0.38-0.94),48 suggesting a benefit of early initiation of ARNI (Table 3). This was supported by the PIONEER-HF trial that enrolled in-hospital patients.37 The time-averaged reduction in NT-proBNP concentration was significantly greater in the sacubitril/valsartan group than in the enalapril group (percent change, -46.7% vs. -25.3%; ratio of change with sacubitril/valsartan vs. enalapril, 0.71; 95% CI: 0.63 to 0.81; p < 0.001).37 The greater reduction in the NT-proBNP concentration with sacubitril/valsartan was evident as early as week 1 (ratio of change, 0.76; 95% CI: 0.69 to 0.85). Rehospitalization for heart failure was also reduced (HR: 0.56, 95% CI: 0.37 to 0.84). It should be noted that before randomization patients were required to be hemodynamically stable, defined by maintenance of a systolic blood pressure of at least 100 mmHg for the preceding 6 hours, with no increase in the dose of intravenous diuretics and no use of intravenous vasodilators during the preceding 6 hours and no use of intravenous inotropes during the preceding 24 hours.37
The effect of ARNI in patients with HFmrEF was demonstrated in a recent pooled analysis from PARADIGM-HF and Prospective Comparison of ARNI With ARB Global Outcomes in HF With Preserved Ejection Fraction (PARAGON-HF)-HF trial.24 A total of 13,195 patients were re-classified into six LVEF categories: ≤ 22.5% (n = 1269), > 22.5% to 32.5% (n = 3987), > 32.5% to 42.5% (n = 3143), > 42.5% to 52.5% (n = 1427), > 52.5% to 62.5% (n = 2166), and > 62.5% (n = 1202). The effect of sacubitril/valsartan on the primary endpoints (cardiovascular death and heart failure hospitalization) was modified by LVEF (treatment-by-continuous LVEF interaction p = 0.02), and benefit appeared to be present for individuals with LVEF primarily below the normal range, including patients with HFmrEF, although the treatment benefit for cardiovascular death diminished at a higher ejection fraction21,24 (Table 4).
4.4. Beta-blocker
Carvedilol,49,50 metoprolol succinate,3 and bisoprolol51 consistently reduced all-cause death, cardiovascular death, and hospitalization for heart failure (Table 2). Both sudden death and death due to progressive heart failure were reduced. In the SENIORS trial,52 nebivolol reduced the primary composite endpoints of all-cause death plus hospitalization for heart failure and the secondary composite endpoints of cardiovascular death plus heart failure hospitalization, though all-cause death and cardiovascular death were not reduced (Table 2). Beta-blockers should be used in patients with heart rate ≥ 60/min, and systolic blood pressure ≥ 85 mmHg, but renal function and serum potassium level did not impose limitation for their use (Table 2).
The safety and efficacy of early initiation of beta-blocker was demonstrated in the Initiation Management Pre-Discharge: Assessment of Carvedilol Therapy for Heart Failure (IMPACT-HF) trial.53 Early initiation of carvedilol in stabilized patients hospitalized for HF improved the use of beta-blocker at 60 days without increasing side effects or length of stay. The early effects of beta-blocker in patients with severe heart failure was shown in a subanalysis of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial that enrolled patients with very high risk, defined by 1 or more of the following: the presence of pulmonary rales, ascites, or edema at randomization; 3 or more hospitalizations for heart failure within the last year; hospitalization at the time of screening or randomization; need for intravenous positive inotropic agent or vasodilator drug within 14 days before randomization; or left ventricular ejection fraction of 15%.54 Kaplan-Meier curves suggested that the all-cause death separated as early as 14-21 days among all randomized patients (HR: 0.75, 95% CI: 0.41-1.35), especially in very-high risk group (HR: 0.20, 95% CI: 0.06-0.70).54 It is noteworthy that 21 days of treatment is at a time when patients were generally receiving a dosage of only 6.25 mg of carvedilol twice a day, 26% of the target dose (Table 3).
The efficacy of beta-blocker in patients with HFmrEF was demonstrated in a recent individual patient-level meta-analysis.22 Among 14,262 patients in sinus rhythm, median LVEF was 27%, including 575 patients with LVEF 40-49% and 244 ≥ 50%. Beta-blockers reduced all-cause and cardiovascular death compared to placebo in sinus rhythm, an effect that was consistent across LVEF strata, except for those in the small subgroup with LVEF ≥ 50%. For LVEF 40-49%, death occurred in 7.2% patients randomized to beta-blockers compared to 12.4% with placebo (aHR: 0.59, 95% CI: 0.34-1.03). Cardiovascular death occurred in 4.5% with beta-blockers and 9.2% with placebo (aHR: 0.48, 95% CI: 0.24-0.97) (Table 4). Over a median of 1.0 year following randomization (n = 4,601), LVEF increased with beta-blockers in all groups in sinus rhythm except those with LVEF ≥ 50%. For patients in atrial fibrillation at baseline (n = 3,050), beta-blockers increased LVEF if baseline LVEF was < 50%, but did not improve prognosis. These data support the efficacy of beta-blockers in patients with HFmrEF.22
4.5. MRA
MRAs consistently reduced all-cause death, cardiovascular death, and heart failure hospitalization (Table 2). Both sudden death and death due to progressive heart failure were reduced.4 In patients with a baseline serum potassium level greater than 5 meq/L, MRA is contraindicated or should be used with caution.4,55 In regarding to renal function, patients with advanced chronic kidney disease, defined by estimated glomerular filtration rate < 30 mL/min in the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trial55 or serum creatinine > 2.5 mg/dL (≈ glomerular filtration rate < 20-26 mL/min) in the Randomized Aldactone Evaluation Study (RALES) trial, were excluded.4 Given that MRAs provide huge benefits in the reduction in mortality, it is generally accepted that they can be used cautiously in patients with an estimated glimerular filtration rate ≥ 20 mL/min if the dose can be properly reduced and the risk of hyperkalemia and acute kidney injury be carefully monitored.56
In a subanalysis of the RALES trial, patients with baseline estimated glomerular rate < 60 mL/min exhibited similar relative risk reductions in all-cause death and the combined endpoint of death plus heart failure hospitalization as those with an estimated glomerular rate ≥ 60 mL/min, but with greater absolute risk reduction (10.3% vs. 6.4%).57 Moreover, worsening renal function (defined as a 30% reduction in estimated glomerular filtration rate from baseline to 12 weeks post-randomization) was associated with an increased adjusted risk of death in the placebo group (HR: 1.9, 95% CI: 1.3 to 2.6) but not in those randomized to spironolactone (HR: 1.1, 95% CI: 0.79 to 1.5, p for interaction = 0.009). The risk of hyperkalemia and renal failure was higher in the spironolactone arm, but the substantial net clinical benefit remained.57
The early benefits of MRA were described in both the EMPHASIS-HF trial55 and the EPHESUS trial.58 In the EMPHASIS-HF trial, eplerenone reduced the primary endpoint (composite of cardiovascular death and heart failure hospitalization) within 28 days (HR: 0.51, 95% CI: 0.30-0.87) when the daily dose was only 27.6 mg, 55.2% of the target dose42,55 (Table 3). In a subanalysis of EPHESUS,58 eplerenone reduced the risk of all-cause death by 31% (HR: 0.69, 95% CI: 0.54-0.89, p = 0.004) and cardiovascular death by 32% (HR: 0.68, 95% CI: 0.53-0.88, p = 0.003) at 30 days after randomization, when patients were treated with eplerenone 25 mg/day, 50% of the target dose59 (Table 3). The benefits of predischarge administration of MRA was confirmed by another study from Asia.60
The effect of MRA in patients with HFmrEF was demonstrated in a recent sub-analysis of patients from America in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial.20 Patients were categorized into four groups according to their LVEF: < 50%, 50-55%, 55-60%, and ≥ 60%. The efficacy (HRs and 95% CI) for the primary endpoint (cardiovascular death, aborted cardiac arrest, and heart failure hospitalization) were 0.55 (95% CI: 0.33-0.91), 0.83 (0.56-1.25), 0.85 (0.60-1.21, and 0.89 (0.69-1.15) (p for interaction 0.069); for cardiovascular death (0.46, [0.23-0.94]; 0.76 [0.40-1.45]; 0.97 [0.57-1.64], and 0.73 [0.49-1.10] (p for interaction 0.93); for heart failure hospitalization (0.60 [0.32-1.10]; 0.80 [0.51-1.25]; 0.70 [0.47-1.06]; and 0.85 [0.71-1.26] (p for interaction 0.037); for all-cause death (0.58 [0.34-0.99]; 0.92 [0.56-1.50]; 1.12 [0.75-1.66], and 0.75 [0.55-1.03] (p for interaction 0.54) (Table 4). Therefore, MRA was effective in patients with HFmrEF. These findings were supported by a recent meta-analysis of individual patient-level data from the three MRA trials:21 RALES,4 EMPHASIS-HF,55 and the TOPCAT trials.61 MRA are benecifial not only in HFsrEF patients (≤ 40%), but in HFmrEF patients (LVEF 41-49%) and even to patients with lower part of LVEF range currently categorized as HFpEF (LVEF 50-59%).21
4.6. SGLT2 inhibitor
SGLT2 inhibitors consistently reduced the composite endpoints of cardiovascular death plus heart failure hospitalization (Table 2). Sotagliflozin inhibits both SGLT1 and SGLT2, but the Effect of Sotagliflozin on Cardiovascular Eventsin Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial only enrolled diabetic patients.9 Interestingly, only dapagliflozin reduced all-cause death and cardiovascular death.7 Dapagliflozin also reduced the risk of any serious ventricular arrhythmia, cardiac arrest, or sudden death.62 SGLT2 inhibitors can be safely used in patients with systolic blood pressure ≥ 95 mmHg and an estimated glomerular filtration rate ≥ 20 mL/min. For patients with baseline systolic blood pressure < 110 mmHg, dapagliflozin increased systolic blood pressure with time.63 SGLT2 inhibitors increase hematocrit,64 decrease MRA-induced severe hyperkalemia,65 and prevent new-onset diabetes.66 Asian patients seem to benefit more from the use SGLT2 inhibitors, compared with Caucasian patients.67 In a recent Asian-subanalysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial,68 dapagliflozin reduced the risk of the primary endpoint (cardiovascular death plus heart failure hospitalization) to the same extent in patients from Asia (HR: 0.65, 95% CI: 0.49 to 0.87) as elsewhere (HR: 0.77; 95% CI: 0.66 to 0.89; p for interaction = 0.32). The absolute risk reduction, however, was numerically greater in Asians (5.8%/ year vs. 3.5%/year) that translated to number-need-to-treat of 18 vs. 29. East-Asian patients (China, Japan, and Taiwan) when compared with South-East Asians and South Asians, seem to benefit the most (HRs: 0.61, 95% CI: 0.43-0.86; 0.69, 95% CI: 0.26-1.85; 0.87, 95% CI: 0.45-1.72, respectively; p for interaction 0.72) with an absolute risk reduction of 8.1%/year and a number-need-to-treat of only 13.68
The efficacy of SGLT2 inhibitors appeared within 30 days of initiation69,70 (Table 3). The safety and efficacy of initiation of SGLT2 before discharge has been shown in the Effect of Empagliflozin in Patients Who Are in Hospital for Acute Heart Failure (EMPULSE) trial.71 Patients were randomized in the hospital when they were clinically stable (median time from hospital admission to randomization, 3 days) and were treated for up to 90 days. The primary outcome of the trial was clinical benefit, defined as a hierarchical composite of death from any cause, number of heart failure events and time to first heart failure event, or a 5 point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) Total Symptom Score at 90 days, as assessed using a win ratio.71 When compared with placebo, more patients treated with empagliflozin had clinical benefit (stratified win ratio: 1.36; 95% CI: 1.09-1.68; p = 0.0054), meeting the primary endpoint. Clinical benefit was observed for both acute de novo and decompensated chronic heart failure and was observed regardless of ejection fraction or the presence or absence of diabetes. These findings indicate that initiation of SGLT2 inhibitor in patients hospitalized for acute heart failure was well tolerated and resulted in significant clinical benefit within 90 days after starting treatment.71
The information regarding SGLT2 inhibitor in patients with HFmrEF was provided by the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) trial and the Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER) trial.11,12 SOLOIST-WHF trial also contained patients with HFmrEF.9 In the EMPEROR-Preserved trial, patients with LVEF > 40%, including HFmrEF, HFpEF, and HFnEF, were enrolled.11 The primary endpoint (cardiovascular death and heart failure hospitalization) was reduced by empagliflozin (HR: 0.79, 95% CI: 0.69-0.90, p < 0.001), mainly driven by a significant reduction in heart failure hospitalization (0.71, 95% CI: 0.60-0.83).11 Cardiovascular death was not reduced (HR: 0.91, 95% CI: 0.76-1.09). The HRs and 95% CI for patients with HFmrEF, HFpEF, and HFnEF were 0.71 (0.57-0.88), 0.80 (0.64-0.99), and 0.87 (0.69-1.10), supporting the effectiveness of empagliflozin in patients with HFmrEF. In a pooled analysis of both the EMPEROR-Reduced trial and EMPEROR-Preserved trials, patients were grouped based on LVEF: < 25%, 25-34%, 35-44%, 45-54%, 55-64%, and ≥ 65%.25 The HRs and 95% CI were 0.73 (0.55-0.96), 0.63 (0.50-0.78), 0.72 (0.52-0.98), 0.66 (0.50-0.86), 0.70 (0.53-0.92), 1.05 (0.70-1.58), respectively. These data re-confirm that empagliflozin was effective in patients with HFmrEF and HFpEF, but the efficacy attenuated in patients with HFnEF.
In the DELIVER trial, patients with LVEF > 40%, including HFmrEF, HFpEF, and HFnEF, were enrolled.12 The primary endpoint was worsening heart failure (heart failure hospitalization and urgent heart failure visit) and cardiovascular death. Dapagliflozin reduced the primary endpoint by 18% (HR: 0.82, 95% CI: 0.73-0.92, p < 0.001), mainly driven by a significant reduction in worsening heart failure (0.79, 95% CI: 0.69-0.91).12 Cardiovascular death was not significantly reduced (HR: 0.88, 95% CI: 0.74-1.05). The HRs and 95% CI for patients with HFmrEF, HFpEF, and HFnEF were 0.87 (0.72-1.04), 0.79 (0.65-0.97), and 0.78 (0.62-0.96) (p for interaction > 0.05), supporting the effectiveness of dapagliflozin in patients with HFmrEF, HFpEF, and HFnEF (Table 4). Of note, in the pre-defined pooled analysis of DAPA-HF and the DELIVER trials, the efficacy of dapagliflozin was demonstrated across the full-spectrum of LVEF, without attenuation in patients with HFnEF.29
In the SOLOIST-WHF trial, 79% patients have LVEF < 50% that include patients with HFmrEF and HFsrEF.9 The primary end point was cardiovascular death and total number of heart failure hospitalizations and urgent visits. Though the trial ended early because of loss of funding from the sponsor, sotagliflozin reduced primary endpoint (HR: 0.67, 95% CI: 0.52 to 0.85; p < 0.001), mainly driven by reduction in total number of heart failure hospitalizations and urgent visits (HR: 0.64, 95% CI: 0.49-0.83, p < 0.001). Cardiovascular death was not significantly reduced (HR: 0.84, 95% CI: 0.58-1.22). Patients with LVEF < 50% and ≥ 50% obtained benefits in the reduction of primary endpoint (HR: 0.72, 95% CI: 0.56-0.94; HR: 0.48, 95% CI: 0.27-0.86; respectively) (Table 4). These data suggested that sotagliflozin was effective in patient with HFmrEF and HFpEF, though the trial only enrolled patients with type 2 diabetes.
4.7. Digoxin
In the main trial of Digitalis Investigation Group (DIG) that enrolled patients with sinus rhythm and an LVEF ≤ 45%,72 digoxin did not reduce all-cause death, nor cardiovascular death, but the combined endpoints of all-cause death plus heart failure hospitalization (HR: 0.85, 95% CI: 0.79-0.91, p < 0.001) and heart failure hospitalization alone (HR: 0.72, 95% CI: 0.66-0.79, p < 0.001) were decreased (Table 2). The details of inclusion and exclusion were not reported, but it is generally accept that digoxin should not be used in patients with end-stage renal disease73 and heart rate less than 60/min.
In the ancillary DIG trial that enrolled patients with sinus rhythm and an LVEF > 45%,74 digoxin did not significantly reduced the primary endpoint (death and hospitalization due to heart failure) (HR: 0.82, 95% CI: 0.63-1.07; p = 0.136). In a retrospective analysis of the DIG trial, the effects of digoxin were examined in three groups according to LVEF: < 40% (HFsrEF), 40-49% (HFmrEF), and ≥ 50% (HFpEF).75 Digoxin reduced primary endpoint in patients with HFsrEF (HR: 0.74, 95% CI: 0.68-0.81), but not in patients with HFmrEF (HR: 0.83, 95% CI: 0.66-1.05) and HFpEF (HR: 0.88, 95% CI: 0.65-1.19).75
4.8. Vasodilator
In the Vasodilator Heart Failure Trial (V-HeFT) I, patients with LVEF < 45% were enrolled.76 Vasodilator therapy with hydralazine/isosorbide dinitrate reduced all-cause death by 34% (p < 0.028) (Table 2). The effect on cardiovascular death and heart failure hospitalization was not reported. The detailed inclusion criteria regarding blood pressure and renal function were not mentioned either. It is generally agreed that vasodilator therapy should be used in patients with systolic blood pressure ≥ 100 mmHg. In the V-HeFT II trial, patients with LVEF < 45% were randomized to enelaptril or hydralazine/isosorbide dinitrate.77 Enalapril was more effective than hydralazine/isosorbide dinitrate in reducing all-cause death by 28% (p = 0.016). Interestingly, in the African-American Heart Failure Trial (A-HeFT), hydralazine/isosorbide dinitrate significant reduced all-cause death by 43% (p = 0.01) and heart failure hospitalization by 33% (p = 0.001).78 The data for vasodilator in patients with HFmrEF was lacking. Vasodilator therapy with hydralazine/isosorbide dinitrate might be considered in patients with HFsrEF who are intolerant to ACEI or ARB.
4.9. Ivabradine
The Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) trial demonstrated the efficacy of ivabradine, a sinoatrial node modulator that selectively inhibits If current, in reducing the composite endpoint of cardiovascular death and heart failure hospitalization (HR: 0.82, 95% CI: 0.75-0.90, p < 0.0001) in patients with LVEF ≤ 35% and sinus rhythm79 (Table 2). The effects were mainly driven by a reduction in heart failure hospitalization (HR: 0.74, 95% CI: 0.66-0.83; p < 0·0001), whereas the cardiovascular death was not significantly reduced (HR: 0.91, 95% CI: 0.80-1.03). Ivabradine should be used in patients with systolic blood pressure ≥ 85 mmHg and sinus rhythm with a baseline heart rate ≥ 70/min despite the use of beta-blocker at maximally tolerated doses. The effect of ivabradine in patients with HFmrEF is unknown.
4.10. Vericiguat
Vericiguat stimulates soluble guanyl cyclase and increases cyclic guanosine monophosphate (cGMP) production. In the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial,10 vericiguat reduced primary composite endpoint (cardiovascular death or heart failure hospitalization) (HR: 0.90, 95% CI: 0.82-0.98), mainly driven by a reduction in heart failure hospitalization (HR: 0.90, 95% CI: 0.81-1.00). Cardiovascular death was not reduced (HR: 0.93, 95% CI: 0.81-1.06) (Table 2). Intesteringly, the VICTORIA trial in the first large-scale trial that enrolled patients with worsening heart failure (WHF) who had recently been hospitalized within 6 months or had received intravenous diuretic therapy within 3 months. According to its inclusion/exclusion criteria, vericiguat can be used in patients with an estimated glomerular filtration rate ≥ 15 mL/min, but not in patients with systolic blood pressure < 100 mmHg, though the blood pressure change throughout the trial was minimal.10
In a pre-defined subgroup analysis of the VICTORIA trial, vericiguat was effective in patients with LVEF < 40% (HR: 0.88, 95% CI: 0.80-0.97),10 but not in patients with LVEF ≥ 40% (HR: 1.05, 95% CI: 0.81-1.36). Therefore, vericiguat can be used in patients with HFsrEF, but its effect in HFmrEF is not clear.
Consensus statements
• Loop diuretics are the mainstays of diuretic agents in most patients with heart failure, irrespective of LVEF.
• Foundational therapies include ACEI, ARB, beta-blocker, MRA, ARNI, and SGLT2 inhibitor. They reduce cardiovascular death and/or all-cause death, and the risk of hospitalization for heart failure in large-scale clinical trials.
• Additional therapies include digoxin, hydralazine/isosorbide dinitrate, ivabradine, and vericiguat, because the magnitude of the overall treatment effects has been modest, the strength of evidence is not robust, or the benefits are limited to specific subgroups.
• Additional therapy can be added when patients still have symptoms despite of foundational therapy, or in certain conditions when foundational therapy cannot be applied or contraindicated.
• Asian patients were not included in some remote trials, and only recent trials of ARNI, SGLT2 inhibitors, and vericiguat enrolled Asian patients. In general, the efficacy in Asian subgroup did not differ from other races and the main trial.
• Before prescribing heart failure medications, the following parameters should be examined: systolic blood pressure, heart rate, renal function, and serum potassium level.
5. DOSE OF FOUNDATIONAL THERAPY
Clinical practice guidelines recommened using the same target doses defined by randomzied controlled trials.1,19 Previously, the foundational therapies were started at a low dose and the dose was then increased in steps over several weeks or months to a target dose if each dose increment was tolerated. However, the mean doses that were achieved in trials were lower than the target doses defined by individual trials (Table 3). The achieved doses were approximately 50-70% of the target doses for ACEI/ARB, and 60-70% for beta-blockers. Given that all the foundational therapies at the achieved doses were proven to be effective in reducing mortality and heart failure hospialization, "maximally tolerated dose" seems a better term than "target dose" when we define the adequacy of doses.
One misconception is that only target doses or maximally tolerated doses can provide efficacy. This is probably not true. As shown in Table 3, many foundational therapies were effective at the doses that were far lower than the target doses. More importantly, the efficacy appeared very early, generally within the first 30 days of initiation (Table 3). For instance, in the CHARM-Low LVEF analysis, candesartan reduced the composite of all-cause death or heart failure hospitalizaiton within 28 days of randomization (HR: 0.61, 95% CI: 0.43-0.86) at a dose of 8.5 mg/day, 26.6% of the target dose.41,42 Similarly, in the EMPHASIS-HF trial, eplerenone reduced all-cause death and heart failure hospitalization within 28 days of randomization (HR: 0.51, 95% CI: 0.30-0.87) at a dose of 27.6 mg/day, 55.2% of the target dose.42,55 In the PARADIGM-HF trial,6 those receiving 50% to < 100% and < 50% of target dose of sacubitril/valsartan obtained similar efficacy (HR: 0.79, 95% CI: 0.67-0.92; HR: 0.79, 95% CI: 0.58-1.07; respectively) compared with those receiving 100% target dose (HR: 0.79, 95% CI: 0.71-0.88).80 There are two randomzied trials comparing low dose and high dose therapy in HFrEF, the Assessment of Treatment with Lisinopril and Survival (ATLAS) and the Heart failure Endpoint evaluation of Angiotensin II Antagonist Losartan (HEAAL) trials.81,82 The high dose regimen reduced heart failure hospitalization, but did not further reduce mortality, when compared with the low dose regimen.
Consensus statements
• The mean doses that were achieved in trials were lower than the target doses defined by individual trials.
• Because all the foundational therapies at the achieved doses were effective in reducing mortality and heart failure hospialization, "maximally tolerated dose" is a better term than "target dose" when we define the adequacy of doses.
• The efficacy of foundational therapy appears very early, generally within the first 30 days of initiation.
6. EFFECTS OF FOUNDATIONAL THERAPY ACCORDING TO EVIDENCE-BASED BACKGROUND THERAPIES
When considering combination therapy for HFsrEF, it is important to ensure that the beneficial effect of a new drug are truly additive to the those obtained from other foundational therapies, across different doses and different combinations. Taken SGLT2 inhibitors as examples, consistent benefits have been shown on the primary endpoint of cardiovascular death and heart failure hospitalization irrespective of background use of other foundational therapies at less than 50% or 50% or more of target dose, and in various clinical relevant dual and triple combinations7,8,83,84 (Table 5). Similar findings have been reported for ARNI in the PARADIGM-HF trial.85 These data provide a rationale for early combination of foundational therapies, well before the maximally tolerated dose being achieved.
Table 5. Effect of SGLT2 inhibitor on primary endpoints by background therapy.
| Trial name | DAPA-HF7,83 | EMPEROR-reduced8,84 | ||
| Drug name | Dapagliflozin | Empagliflozin | ||
| HR (95% CI) | p for interaction | HR (95% CI) | p for interaction | |
| Overall effect | 0.74 (0.65-0.85) | 0.75 (0.65-0.86) | ||
| ARNI | 1.00 | NR | ||
| Yes | 0.75 (0.50-1.13) | 0.64 (0.45-0.89) | ||
| No | 0.74 (0.65-0.86) | 0.77 (0.66-0.90) | ||
| ACEI/ARB target dose | 0.21 | 0.18 | ||
| < 50% | 0.78 (0.65-0.94) | 0.85 (0.69-1.06) | ||
| ≥ 50% | 0.64 (0.50-0.82) | 0.67 (0.52-0.88) | ||
| Beta-blocker target dose | 0.76 | 0.15 | ||
| < 50% | 0.71 (0.59-0.86) | 0.66 (0.54-0.80) | ||
| ≥ 50% | 0.74 (0.60-0.90) | 0.81 (0.66-1.00) | ||
| MRA target dose | 0.82 | 0.96 | ||
| < 50% | 0.71 (0.45-1.12) | 0.77 (0.22-2.63) | ||
| ≥ 50% | 0.74 (0.63-0.88) | 0.75 (0.63-0.88) | ||
| ACEI/ARB ≥ 50% target dose + beta-blocker ≥ 50% target dose | 0.40 | 0.96 | ||
| Yes | 0.66 (0.48-0.91) | 0.74 (0.54-1.03) | ||
| No | 0.77 (0.66-0.89) | 0.75 (0.64-0.87) | ||
| ACEI, ARB, or ARNI + beta-blocker (all at any dose) | NR | 0.64 | ||
| Yes | NR | 0.68 (0.60-0.77) | ||
| No | NR | 0.73 (0.56-0.94) | ||
| ARNI + beta-blocker + MRA (all at any dose) | 0.86 | 0.15 | ||
| Yes | 0.70 (0.41-1.19) | 0.55 (0.35-0.86) | ||
| No | 0.74 (0.65-0.85) | 0.77 (0.67-0.89) | ||
| ACEI/ARB + beta-blocker + MRA (all ≥ 50% target dose) | 0.65 | 0.71 | ||
| Yes | 0.70 (0.48-1.01) | 0.80 (0.55-1.17) | ||
| No | 0.75 (0.65-0.87) | 0.74 (0.64-0.86) | ||
| ACEI, ARB, or ARNI + beta-blocker + MRA (all at any dose) | NR | 0.73 | ||
| Yes | NR | 0.68 (0.58-0.79) | ||
| No | NR | 0.70 (0.59-0.84) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CI, confidence interval; HF, heart failure; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist; NR, not reported; SGLT2i, sodium-glucose co-transporter 2 inhibitor.
Consensus statement
• Early combination of foundational therapies can be started before the maximally tolerated dose being achieved.
7. RAPID SEQUENCING STRATEGY OF FOUNDATIONAL THERAPIES
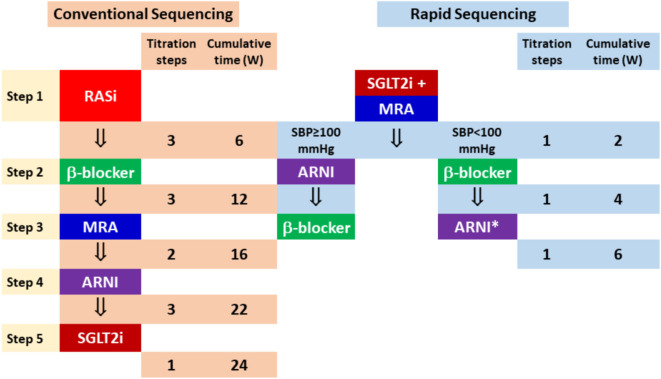
Previously, guidelines suggested initiating foundational therapies in patients with HFsrEF in a conventional sequence that follows the chronological order in which trials were conducted, typically requiring ≥ 6 months. We propose "rapid sequencing" strategy to obtain maximal benefits in shortest duration for the management of HFsrEF. This strategy was based on 5 principles: First, ARNI is a replacement for ACEI/ARB.6 Second, patients should be started on all four foundational therapies within 2-4 weeks because drugs act rapidly to reduce mortality and heart failure hospitalization (Table 3). Third, low starting doses of foundational therapy have substantial therapeutic benefits (Table 3), and achievement of low doses of all four classes of drugs should take precedence over up-titration to target doses.32 Fourth, the efficacy of each foundational therapy is independent of type and doses of other foundational therapies (Table 5). Fifth, the agents that started earlier could enhance the safety of other agents that were started simultaneously or later in the sequence. For instance, early initiation of SGLT2 inhibitor decreased the risk of MRA-induced hyperkalemia,65 increased blood pressure favoring the subsequent use of ARNI,63 and reduced fluid overload that facilitate the addition of a beta-blocker.54
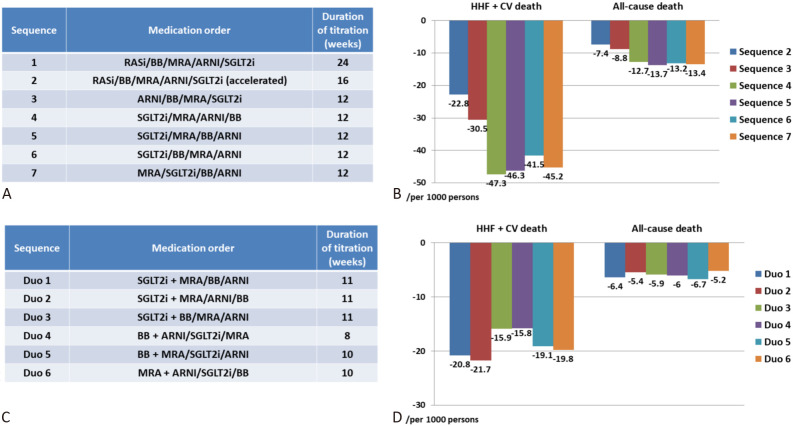
A recent modelling study using data from six pivotal trials in HFrEF provided us the rationale for the strategy to accelerate foundational therapies.86 The investigators compared (i) more rapid up-titration of therapies used in the conventional order (based on the chronology of the trials), and (ii) accelerated up-titration and using treatments in different orders than the conventional ones. The best sequence for reducing the composite of heart failure hospitalization and cardiovascular death was the accelerated sequence of SGLT2 inhibitor/MRA/ARNI/beta-blocker within 12 weeks that could reduced 47 events per 1000/year than the conventional sequence of ACEI (ARB)/beta-blocker/ARNI/SGLT2 inhibitor in 24 weeks (Figure 3A and 3B).86 The investigators further studied the possibility of starting two drugs simultaneously, followed by the remaining two drugs (Figure 3C and 3D). Compared with sequence 3 (ARNI/BB/MRA/SGLT2 inhibitor in 12 weeks) in Figure 3A, the greatest incremental reduction in the composite of heart failure hospitalization and cardiovascular death was with the sequence starting with the combination of SGLT2 inhibitor plus MRA, followed by an ARNI and then beta-blocker (the sequence of SGLT2 inhibitor plus MRA as the same initial, followed by a beta-blocker, and then an ARNI, was almost as effective) (Figure 3C and 3D).86 More recently, the investigators of the Safety, Tolerability and Efficacy of Rapid Optimization, Helped by NT-proBNP Testing, of Heart Failure Therapies (STRONG-HF) trial adopted a more aggressive strategy in patients with heart failure irrespective of LVEF.87 Patients allocated to the high-intensity care group received 3 foundational therapy (beta-blocker, ACEI/ARB/ARNI, MRA) up to at least half the optimal doses before discharge. These medications were up-titrated to 100% of the recommended doses within 2 weeks of discharge. The primary endpoint (180-day heart failure readmission or all-cause death) was reduced by 34% (HR: 0.66, 95% CI: 0.50-0.86, p = 0.0021) in the high-intensity group compared to the usual care group.87 We recommend rapid sequencing, instead of conventional sequencing strategy, for patients with HFsrEF (Figure 4).
Figure 3.
Comparison of different rapid sequencing strategies of foundational therapies. (A) Seven different sequencing strategies. (B) The best sequence for reducing the composite of heart failure hospitalization and cardiovascular death was the accelerated sequence of SGLT2i/MRA/ARNI/beta-blocker within 12 weeks. (C) Six different sequencing strategies starting with combination therapy. (D) The best sequencing for reducing the composite endpoints was the sequence starting with the combination of SGLT2i plus MRA, followed by an ARNI and then beta-blocker. * ARNI can be prescribed when systolic blood pressure ≥ 100 mmHg. ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blocker; CV, cardiovascular; HHF, heart failure hospitalization; MRA, mineralcorticoidreceptor antagonist; RASi, renin-angiotensin system inhibitor; SGLT2i, sodium-glucose co-transporter 2 inhibitor.
Figure 4.
Conventional sequencing and rapid sequencing strategies for HFsrEF. With the rapid sequencing strategy, all 4 classes of foundational therapies can be provided in 4 weeks. SBP, systolic blood pressure; W, week; other abbreviations as in Figure 3.
Consensus statements
• We propose "rapid sequencing" strategy, instead of conventional sequencing strategy, to obtain maximal benefits in shortest duration for the management of HFsrEF.
• Patients should be started on all four foundational therapies within 2-4 weeks.
• ACEI/ARB should be replaced by ARNI to achieve better efficacy.
• We suggest starting with combination of SGLT2 inhibitor and MRA, followed by an ARNI and then beta-blocker (or beta-blocker first, followed by ARNI depending on systolic blood pressure), to achieve maximal benefits.
• The most recent data suggested that all the foundational therapies with half doses could be applied before discharge and were increased to full doses within 2 weeks after discharge.
8. IN-HOSPITAL INITIATION
A quarter of patients hospitalized for worsening HFrEF are either rehospitalized or dead within 30 days of discharge.88 On the other hand, hospitalization for heart failure provides a key opportunity to improve utilization of foundational therapy. Mounting evidence supports hospitalized and ambulatory patients with HFrEF as a common pathophysiology on a continuum,89 and deferring in-hospital initiation of foundational therapies exposes patients to excess risk of early post-discharge deterioration and death,90 and the possibility of never having the medication prescribed.91 Table 6 shows recent trials evaluating in-hospital initiation of foundational therapies for patients with HFrEF. In general, in-hospital initiation of foundational therapies was associated with better primary outcome and/or secondary outcome without an increase in adverse events. The data supporting SGLT2 inhibitors for in-hospital initiation are most robust. The safety data in the hospitalized patients with COVID-19 in the recent DARE-19 (Dapagliflozin in patients with cardiometabolic risk factors hospitalized with COVID-19) trial supported the safety of in-hospital initiation of SGLT2 inhibitor, though patient populations were different.92 Intiation of foundational therapies requires relative stability in hospitalized patients. Supplemental Table 1 shows the timing of in-hospital initiation and definitions of hemodynamically stable conditions in different trials. We propose that patients should fulfill all the following four conditions to be qualified for in-hospital initiation of SGLT2 inhibitor or ARNI: 1) systolic blood pressure ≥ 90 mmHg, 2) intravenous diuretic being stopped or unchanged in the recent 6 hours, 3) intravenous vasodilator being discontinued ≥ 6 hours, and 4) intravenous inotrope being stopped for more than 24 hours.
Table 6. Recent trials evaluating in-hospital initiation of foundational therapies in HFrEF.
| Drug class | Trial name | Number of patients | Design | Primary endpoint | Results (95% CI) | Secondary or tertiary or exploratory endpoints | Results (95% CI) |
| β-blocker | IMPACT-HF53 | 363 | Pre-discharge vs. post-discharge | Persistence of medication at 60 days | 91.2% vs. 73.4%, p < 0.0001 | Composite of death, HHF, and urgent HF visit | 45.4% vs. 46.1% (-0.09-0.11) |
| ARNI | PIONEER-HF37 | 884 | ARNI vs. enalapril | Change of NT-proBNP at 4, 8 weeks | -46.7% vs. 25.3% | Composite of death, HHF, LVAD, list on heart transplantation | 9.3% vs. 16.8% |
| HR: 0.71 (0.63-0.81) | HR: 0.54 (0.37-0.79) | ||||||
| SGLT2i | EMPA-RESPONSE-AHF108 | 80 | Empagliflozin vs. placebo | 1. Change in VAS score | 1. VAS: 1264 vs. 1650 | In-hospital worsening HF, HHF and death at 60 days | 10% vs. 33% (p = 0.014) |
| 2. Diuretic response | 2. -0.35 vs. -0.12 kg | ||||||
| 3. Change in NT-proBNP | 3. -46% vs. -42% | ||||||
| 4. Length of stay | 4. 8 vs. 8 days (All p > 0.05) | ||||||
| SOLOIST-WHF9 | 596 (1,222*) | Sotagliflozin vs. placebo | Total HHF and urgent HF visit, and CV death | 52.1% vs. 76.6 %/y | Total HHF and urgent HF visit | 40.4% vs. 63.9% | |
| HR: 0.71 (0.51-0.99) | HR: 0.64 (0.49-0.83) | ||||||
| EMPULSE71 | 530 | Empagliflozin vs. placebo | Win ratio of the composite (death, HHF, time to HF event, KCCQ-TSS ≥ 5 points) at day 90 | 53.9% vs. 39.7% | KCCQ-TSS change from baseline to day 90 | 36.19 vs. 31.73 | |
| Win ratio 1.36 (1.09-1.68) p = 0.0054 | Difference = 4.45 (0.32-8.59) | ||||||
| GDMT | STRONG-HF87 | 1078 | High-intensity care vs. usual care | 180-day HF readmission and all-cause death | 15.2% vs. 23.3% | 1. All-cause death | 1. HR: 0.84 (0.56-1.26) |
| HR: 0.66 (0.50-0.86) p = 0.0021 | 2. 90-day HF readmission and all-cause death | 2. 0.73 (0.53-1.02) |
* Total patient number in the SOLOIST-WHF trial.
ARNI, angiotensin receptor neprilysin inhibitor; CI, confidence interval; CV, cardiovascular; GDMT, guideline-directed medical therapy; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HHF, heart failure hospitalization; HR, hazard ratio; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire-Total Symptom Score; LVAD, left ventricular assisted device; NT-proBNP, N-terminal pro B type natriuretic peptide; SGLT2i, sodium-glucose co-transporter 2 inhibitor; VAS, Visual Analogue Scale.
Consensus statements
• Deferring in-hospital initiation of foundational therapies exposes patients to excess risk of early post-discharge deterioration and death, and the possibility of never having the medication prescribed.
• In-hospital initiation of foundational therapies was associated with better primary outcome and/or secondary outcome without an increase in adverse events.
• Intiation of foundational therapies requires relative stability in hospitalized patients.
• Clinical stability for in-hospital initiation of SGLT2 inhibitor or ARNI includes all the followings: 1) systolic blood pressure ≥ 90 mmHg, 2) intravenous diuretic being stopped or unchanged in the recent 6 hours, 3) intravenous vasodilator being discontinued ≥ 6 hours, and 4) intravenous inotrope being stopped for more than 24 hours.
9. TREATMENT ALGORITHM FOR HFrEF
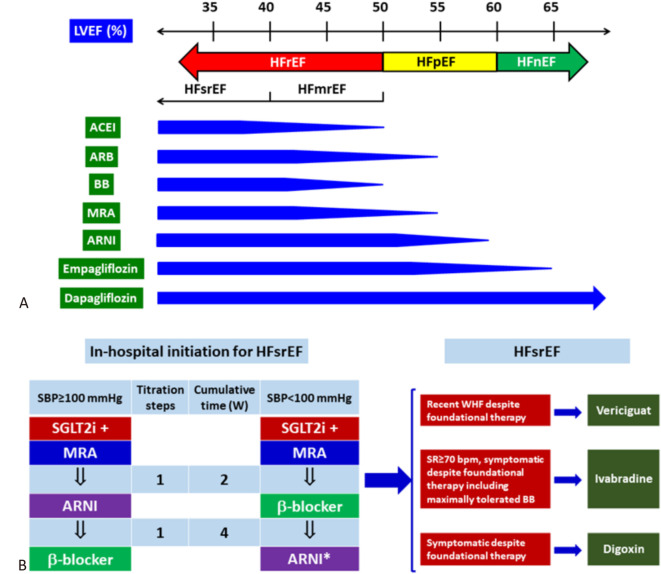
Efficacy of foundational therapies across the full spectrum of LVEF was shown in the Figure 5. Pharmacological treatment of HFsrEF starts with in-hospital initiation with 2-drug combination followed by the third drug in 2 weeks. The fourth drug is applied after another 2 weeks and the four foundational therapies can therefore be put together in 4 weeks. For patients with HFsrEF, vericiguat, ivabradine, and digoxin can be added upon the foundational therapies under different conditions, or when foundational therapies are contraindicated (Figure 5).
Figure 5.
Treatment algorithm for HFrEF. (A) Efficacy of foundational therapy in full spectrum of LVEF. The tapering of the horizontal blue bar suggests decreasing in efficacy in reducing heart failure endpoint. (B) Strategy for in-hospital initiation of foundational therapies for HFsrEF. (C) Role of additional therapy for HFsrEF. Abbreviations as in Figure 2 and 3.
10. TREATMENT OF HFpEF
All foundational therapies for HFrEF, except ACEI38 and beta-blocker,22 have some evidence to support their efficacy in reducing heart failure endpoint in patients with HFpEF (Table 3, Figure 5). The efficacy of MRA remained persistent, though with less effect, in the lower range of LVEF of HFpEF (up to 55%).20 ARNI decreased heart failure endpoints in the range of HFpEF until LVEF around 57%.24,93 Data for SGLT2 inhibitors are the most robust with their efficacy in the spectrum of LVEF from 25% to 65%,25 even to LVEF of 70%.29 A recent individual patient-level meta-analysis also demonstrated efficacy of cardesartan, MRA, and ARNI extending to the lower part of the LVEF range of HFpEF.21
In the recent DELIVER trial, starting dapagliflozin during or shortly after heart failure hospitalization in patients with HFmrEF or HFpEF appears safe and effective.94 Time to first statistical significance for the primary end point was 13 days after randomization (HR: 0.45; 95% CI: 0.20-0.99; p = 0.046).95 These data suggested that foundational therapies should be initiated very early, best before discharged for patients with HFpEF, similar to what we have observed for patients with HFrEF.
The benefit of combination therapy for HFpEF was recently reported, based on individual patient-level analysis from MRA, ARNI, and SGLT2 inhibitor.96 Switching to ARNI from ACEI/ARB, adding an MRA, and empagliflozin reduced cardiovascular death and heart failure hospitalization in the subgroups with LVEF 45% to 54% (HR: 0.49, 95% CI: 0.32-0.74) and LVEF 55% to 64% (HR: 0.54, 95% CI: 0.37-0.80) but not in those with LVEF ≥ 65% (HR: 1.17, 95% CI: 0.65-2.10).96
In-hospital initiation of combination therapy for HFpEF was recently reported in the STRONG-HF trial that contained 15% patients with HFpEF.87 The efficacy in the high-intensity care group was consistent in patients with LVEF ≥ 50% vs. those < 50%. Therefore, we recommend rapid sequencing, instead of conventional sequencing strategy, for HFpEF. Figure 6 shows the treatment algorithm for HFpEF.
Figure 6.
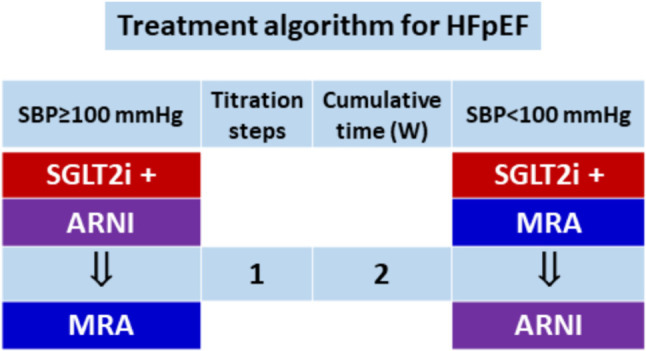
Treatment algorithm for HFpEF. We recommend in-hospital initiation with combination therapy. Abbreviations similar to those in Figure 3 and Figure 4.
Consensus statements
• All foundational therapies for HFrEF, except ACEI and beta-blocker, have evidence in reducing heart failure endpoint in patients with HFpEF.
• SGLT2 inhibitors have the strongest evidence in patients with HFpEF, followed by ARNI, whereas MRA and ARB are only effective in the lower end of LVEF in patients with HFpEF.
• We recommend rapid sequencing strategy for patients with HFpEF, starting with SGLT2 inhibitor and combining it with ARNI or MRA depending on systolic blood pressure.
11. TREATMENT OF HFnEF
The only class of drug that is effective in patients with HFnEF is SGLT2 inhibitors. Empagliflozin’s effect can extend to LVEF of 65%,25 whereas dapagliflozin can extend its efficacy to LVEF of 70% or above.29
Consensus statements
• The only class of drug that is effective in patients with HFnEF is SGLT2 inhibitor.
• In contrast to empagliflozin, the efficacy of dapagliflozin did not attenuate in the range of high LVEF up to 70% or above.
12. WORSENING HEART FAILURE
WHF is traditionally defined by progressive signs and symptoms of heart failure in patients with chronic heart failure, presented with either an unplanned hospitalization or an urgent visit resulting in inravenous diuretic management in the emergency or outpatient setting, despite previously stable therapy97 (Figure 7). After each episode of WHF, the cardiac function does not completely recover to the level before WHF, and the interval between each episode of WHF becomes shorter, resulting in more frequent heart failure hospitalization or urgent heart failure visit97,98 (Figure 7). WHF should be differentiated from the following three conditions: 1) Poor adherence to foundational theapy; 2) Acute heart failure due to other secondary causes; 3) De novo heart failure.97
Figure 7.
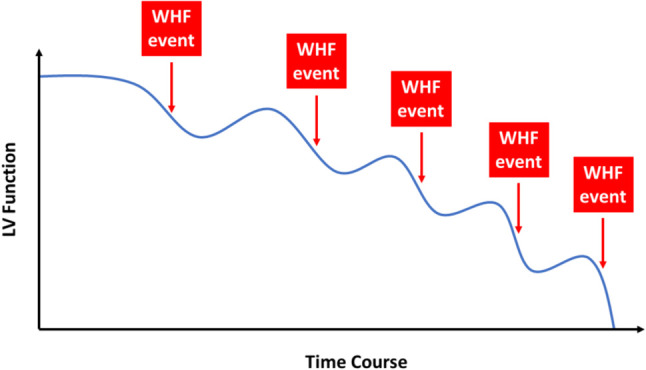
Clinical course of worsening heart failure. After each episode of worsening heart failure (WHF), the left ventricular function does not completely recover to the level before WHF, and the interval between each episode of WHF becomes shorter, resulting in more frequent heart failure hospitalization or urgent heart failure visit. LV, left ventricle.
WHF is not uncommon in patients with HFrEF, affecting 1/6 in the real world setting and 1/8 in clinical trial in a follow-up period of 18 months.99,100 The prognosis of patients with WHF is poor. In a recent registry, patients with WHF had an increased risk of both death (22.5% in 2 years) and heart failure readmission (56% in 30 days).99 The number of heart failure hospitalization is also a strong predictor of mortality in patients with heart failure.101 Based on a health care database, median survival after the first, second, third, and fourth hospitalization was 2.4, 1.4, 1.0, and 0.6 years.101 On the other hand, WHF provides a great opportunity to modify or escalate diuretics and foundational therapy to reduce further deterioration of cardiac function. Interesingly, only a few trials put WHF as a component of the primary endpoint (Supplemental Table 2).
WHF is increasing in numbers in the recent decade. Based on the electronic health recorda from a large, integrated health care system in the US, the annual incidence (events per 100 person-years) of WHF increased from 25.2 in 2010 to 33.0 in 2019, primarily caused by increases in outpatient encounters (from 7 to 10) and ED visits/observation stays (from 4 to 7).102 Altogether, 50.0% of WHF was due to heart failure hospitalization, 22.5% from emergency department visit, and 27.6% from outpatient encounter. In a sub-analysis from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy Post Approval Registry (MADIT-CRT) trial, patients with WHF who received intravenous diuretic therapy during urgent outpatient clinic visits had similar all-cause death to patients who were hospitalized (15.9 vs. 18.5 per 100 patient-years).103 Similar results were observed in the PARADIGM-HF trial.104 Moreover, in the sub-analyses of PARADIGM-HF and DAPA-HF trials patients with WHF who received only outpatient drug intensification not limiting to intravenous diuretic also experienced a significant increase in all-cause death compared to those without WHF (PARADIGM-HF: aHR: 5.2, 95% CI: 4.2-6.3; DAPA-HF: aHR: 3.14, 95% CI: 2.40-4.11).100,104 Therefore, the definition of WHF might need to be modifed to include patients who receive intensification of any foundational therapy, not limiting to intravenous diuretic, in outpatient clinic. These patients should be closely monitored and foundational therapy and diuretics should be escalated.
WHF is generally preceded by gradual progressive "subclinical worsening", and a subclinical high-risk state that follows an apparent clinical recovery and discharge97 (Figure 7). Early detection of the subclinical worsening might be useful to reduce the future risk of WHF. On the other hand, this silent worsening can be unrecognized as patients might limit their daily activity and mask heart failure symptoms. More recently, severel studies have suggested that patient-reported health status such as KCCQ score might have more prognostic value than physician-assessed health status changes in New York Heart Association (NYHA) class.105,106 Regular check of KCCQ score may be useful to detect the subclinical worsening. Moreover, biomarker levels could disclose ongoing cardiac structural and funcitonal deterioration, such as NT-proBNP levels that elevated weeks to months before clinical worsening.107
Consensus statements
• WHF is defined by progressive signs and symptoms of heart failure in patients with chronic heart failure, presented with either an unplanned hospitalization, or an urgent visit requiring intravenous diuretic management in the emergency, or outpatient visit with modification of therapy, not limiting to diuretic, despite previously stable therapy.
• WHF should be differentiated from the following three conditions: 1) Poor adherence to foundational theapy; 2) Acute heart failure due to other secondary causes; 3) De novo heart failure.
• After each episode of WHF, the cardiac function does not completely recover to the level before WHF, and the interval between each episode of WHF becomes shorter, resulting in more frequent heart failure hospitalization or urgent heart failure visit.
• WHF is not uncommon in patients with HFrEF, affecting 1/8 to 1/6 patients in 18 months.
• Patients with WHF have a poor prognosis, with a 2-year mortality rate of more than 20% and a readmission rate above 50% in 30 days.
• WHF, however, also provides a great opportunity for health care providers to modify or escalate diuretics and foundational therapy to reduce further deterioration of cardiac function.
• WHF is generally preceded by gradual progressive "subclinical worsening". Early detection of the subclinical worsening with subsequent initiation or modification of medications is important.
• Patient-reported health status such as KCCQ score is more important than physician-assessed health status in NYHA class for the early detection of WHF.
• Biomarker levels, such as NT-proBNP, elevate weeks to months before clinical events, and may be useful for the early detection of WHF.
13. FUTURE PERSPECTIVES
Though foundational therapies significantly improve outcomes in patients with heart failure, the residual risk of cardiovascular death and heart failure hospitalization is still high. Several neuromormonal modulators and novel drugs with new mechanisms are now being tested in clinical trials. Table 7 shows ongoing phase 2 and 3 drug trials for patients with HFrEF and HFpEF.
Table 7. Ongoing drug trials in chronic heart failure/acute myocardial infarction.
| Class of drug | Trial name (NCT#) | Drug name | Phase | Patient number | Comparison | Primary endpoint | Estimated completion date |
| SGLT2 i | A Study to Test Whether Empagliflozin Can Lower the Risk of Heart Failure and Death in People Who Had a Heart Attack (EMPACT-MI) (NCT04509674). | Empagliflozin | 3 | 6500 | vs. placebo | All-cause death + HHF | March 31, 2023 |
| Dapagliflozin Effects on Cardiovascular Events in Patients With an Acute Heart Attack (DAPA-MI) (NCT04564742). | Dapagliflozin | 3 | 6400 | vs. placebo | CV death + HHH | September 22, 2023 | |
| Effect of Ertugliflozin on Cardiac Function in Diabetes (ERTU-GLS) (NCT03717194) | Ertugliflozin | 3 | 120 | vs. placebo | Change of global longitudinal strain after 24 weeks’ treatment | December 31, 2022 | |
| Ertugliflozin in Chronic Heart Failure (NCT04438213). | Ertugliflozin | 2 | 90 | Ertugliflozin, metolazone, or a placebo | Change in urine sodium concentration and total body water from baseline to day 7 to 6 weeks | July 2023 | |
| GLP1 RA | Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity (STEP-HFpEF) (NCT04788511). | Semaglutide | 3 | 516 | vs. placebo | Changes in KCCQ clinical summary score and body weight | April 25, 2023 |
| Research Study to Look at How Well Semaglutide Works in People Living With Heart Failure, Obesity and Type 2 Diabetes (STEP HFpEF DM) (NCT04916470). | Semaglutide | 3 | 610 | vs. placebo | Changes in KCCQ clinical summary score and body weight | August 8, 2023 | |
| GLP1 RA/GIP | A Study of Tirzepatide (LY3298176) in Participants With Heart Failure With Preserved Ejection Fraction and Obesity (SUMMIT) (NCT04847557). | Tirzepatide | 3 | 700 | vs. placebo | Composite of all-cause death, heart failure events, 6MWD and KCCQ Clinical Summary Score | November 22, 2023 |
| ARNI | Changes in NT-proBNP, Safety, and Tolerability in HFpEF Patients With a WHF Event (HFpEF Decompensation) Who Have Been Stabilized and Initiated at the Time of or Within 30 Days Post-decompensation (PARAGLIDE-HF) (NCT03988634). | Sacubitril/valsartan | 3 | 450 | Sacubitril/valsartan vs. valsartan | Proportional change in NT-proBNP from baseline to the average of weeks 4 and 8 | October 31, 2022 |
| MRA | Study to Evaluate the Efficacy and Safety of Finerenone on Morbidity & Mortality in Participants With Heart Failure and Left Ventricular Ejection Fraction Greater or Equal to 40% (FINEARTS-HF) (NCT04435626). | Finerenone | 3 | 5500 | vs. placebo | CV death + HHF | May 2, 2024 |
| Efficacy, Safety and Tolerability of AZD9977 and Dapagliflozin in Participants With Heart Failure and Chronic Kidney Disease (MIRACLE) (NCT04595370). | AZD9977 | 2 | 500 | AZD9977 + dapagliflozin vs. dapagliflozin | Percent change from baseline in UACR at 12 weeks | June 26, 2023 | |
| sGC activator | Vericiguat in Participants With Chronic Heart Failure With Reduced Ejection Fraction (HFrEF) (VICTOR) (NCT05093933). | Vericiguat | 3 | 6000 | vs. placebo | CV death + HHF | June 15, 2025 |
| Myeloperoxidase (MPO) inhibitor | Study to Evaluate the Efficacy and Safety of AZD4831 in Participants With Heart Failure With Left Ventricular Ejection Fraction > 40% (ENDEAVOR) (NCT04986202). | AZD4831 | 2b and 3 | 1485 | vs. placebo | 1. KCCQ-Total Symptom Score change from baseline at 16 and 24 weeks. 2. 6MWD change from baseline at 16 and 24 weeks | November 13, 2024 |
| Others | A Study of Mavacamten in Participants With HFpEF and Elevation of NT-proBNP With or Without Elevation of cTnT (EMBARK-HFpEF) (NCT04766892). | Mavacamten | 2 | 35 | Single arm | NT-proBNP and cTnT levels at 26 weeks | April 30, 2023 |
| A Study of Ponsegromab in People With Heart Failure (GARDEN TIMI 74) (NCT05492500). | Ponsegromab (growth differentiation factor 15) | 2 | 416 | vs. placebo | Change from baseline in KCCQ 23 Clinical Summary Score | September 13, 2024 | |
| Effectiveness of CRD-740 in Heart Failure (CARDINAL-HF) (NCT05409183). | CRD-740 (a PDE9 inhibitor) | 2 | 660 | vs. placebo | Part A: The change in plasma cGMP at Week 4. Part B: The change in NT-proBNP at Week 12 | January 2024 | |
| REGN5381 in Adult Heart Failure Patients With Elevated Pulmonary Capillary Wedge Pressure and Reduced Left Ventricular Ejection Fraction. | REGN5381 (Natriuretic peptide receptor 1 agonist) | 2 | 80 | vs. placebo | Incidence and severity of treatment-emergent adverse events (TEAEs) | November 22, 2023 | |
| Study to Assess Efficacy and Safety of CDR132L in Patients With Reduced Left Ventricular Ejection Fraction After Myocardial Infarction (HF-REVERT) (NCT05350969). | CDR132L (Cardiac miR-132 inhibitor) | 2 | 280 | vs. placebo | Percent change in Left Ventricular End-Systolic Volume (LVESVI) at Month 6 | November 30, 2024 | |
| Exploratory Ph 2A, Double-Blind, Placebo-Controlled Dose Escalation Study of Safety, Tolerability, PD, & PK of HU6 for Subjects With Obese HFpEF (NCT05284617). | HU6 (Controlled Metabolic Accelerator) | 2 | 62 | vs. placebo | Calculating the PK parameter Cmax | October 30, 2023 | |
| A Study to Assess the Hemodynamic Effects, Safety, Tolerability, and Pharmacokinetics of Intravenous APD418 in Adult Participants With Heart Failure With Reduced Ejection Fraction. | APD418 (beta3-Adrenergic Receptor Antagonist) | 2 | 80 | vs. placebo | Change from baseline in cardiac index measured by right heart catheterization | March 2023 | |
| A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx in Participants With Chronic Heart Failure With Reduced Ejection Fraction (ASTRAAS-HF). | IONIS-AGT-LRx (ASO directed to hepatocyte-derived angiotensinogen) | 2 | 72 | vs. placebo | Percent change in plasma AGT concentration from baseline to study day 85 | January 2023 | |
| OPtimizing Aldosterone Receptor Antagonist Therapy by Sodium Zirconium Cyclosilicate in Heart Failure (OPRA-HF) (NCT04789239). | Sodium zirconium cyclosilicate | 2 | 230 | vs. placebo | Optimization of MRA usage by Sodium Zirconium Cyclosilicate in HFrEF | June 30, 2024 | |
| Modulation of SERCA2a of Intra-myocytic Calcium Trafficking in Heart Failure With Reduced Ejection Fraction (MUSIC-HFrEF1) (NCT04703842). | SRD-001 | 2 | 56 | vs. placebo | Change of NYHA, KCCQ, 6MWT, LV function, HF-related events at month 6 and month 12 | December 2028 | |
| A Phase IIa Clinical Trial on TSG-01 in the Treatment of Chronic Heart Failure in Patients With Coronary Heart Disease. | TSG-01 | 2 | 90 | vs. placebo | NYHA and 6MWT at week 12 | May 7, 2022 | |
| Evaluate the Effect of Injectable Neucardin on the Cardiac Function of Subjects With Chronic Systolic Heart Failure (NCT04468529). | Neucardin | 3 | 140 | vs. placebo | Left Ventricular End-Systolic Volume Index (LVESVI) change from baseline on day 30 | May 20, 2022 | |
| Safety and Efficacy of AT-001 in Patients With Diabetic Cardiomyopathy (NCT04083339). | AT-001 (Aldose Reductase Inhibitor) | 3 | 675 | vs. placebo | Peak VO2 during cardio-pulmonary exercise test at month 15 | December 2025 | |
| Study to Evaluate Effects of INL1 in Patients With Heart Failure and Reduced Ejection Fraction (TRACER-HF) (NCT03875183). | INL1 | 2 | 200 | vs. placebo | Change in NT-proBNP at week 12 | January 2023 | |
| Survival Study of the Recombinant Human Neuregulin-1β in Subjects With Chronic Heart Failure (NCT03388593). | RhNRG-1 (recombinant human neuregulin-1) | 3 | 1600 | vs. placebo | All-cause death | February 2023 | |
| Randomized Placebo-controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency (HEART-FID) (NCT03037931). | Ferric carboxymaltose | 3 | 3068 | vs. placebo | 1. All-cause death. 2. Heart failure hospitalization. 3. Change in 6MWT distance | June 2023 |
AGT, angiotensinogen; ARNI, angiotensin receptor neprilysin inhibitor; Cmax, maximum serum concentration; cTnT, cardiac troponin T; CV, cardiovascular; GIP, gastric inhibitory polypeptide; GLP1 RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HHF, heart failure hospitalization; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricle; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro B type natriuretic peptide; NYHA, New York Heart Association; sGC, soluble guanylate cyclase; SGLT2, sodium-glucose co-transporter 2 inhibitor; UACR, urine albumin-creatinine ratio; VO2, oxygen consumption; 6MWD, 6-minute working distance.
DECLARATION OF CONFLICT OF INTEREST
Dr. C.-E. Chiang has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Menarini, MSD, Novartis, Pfizer, and Sanofi. Dr. C.-L. Hung has received honorarium from Astrazeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Pfizer, and Sanofi. Dr. Y.-W. Wu has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, MSD, Novartis, Pfizer, Sanofi, and Takeda. Dr. T.-H. Lin has received honorarium from Astrazeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, MSD, Novartis, Pfizer, Sanofi, Bayer, Viatris, Menariri, Tanabe, and Takeda. Dr. K.-C. Ueng has received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Merck Sharp and Dohme, Novartis, Pfizer, and Sanofi. Dr. S.-H. Sung has received honorarium from Astrazeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, Sanofi, Abbott, and Edward. Dr. C.-K. Wu has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, Sanofi, and Abbott. Dr. T.-H. Chao has received honorarium from Bayer, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Daiichi-Sankyo, Tanabe Taiwan, and Novartis. Dr. Y.-H. Lin has received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Novartis, Pfizer, and Sanofi. Dr. M. YC Chen has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer. Dr. P.-L. Lin has received honorarium from AstraZeneca, Abbott, Boehringer Ingelheim, Bayer, Daiichi-Sankyo, Eli Lilly, Novartis, Novo Nordisk, Pfizer, and Sanofi. Dr. T.-F. Chao has received speaker honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, and Sanofi. Dr. H.-M. Cheng has received honorarium from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, SERVIER, Sanofi, Takeda, and Eli Lilly. Dr. M.-E. Liu has received honorarium from Abbott, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Medtronic, MSD, Novartis, Pfizer, and Sanofi. Dr. H.-I. Yeh has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, MSD, Novartis, Novo Nordisk, Pfizer, and Sanofi. Dr. Y.-H. Li has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, and Sanofi. Dr. I.-C. Hsieh has received honorarium from AstraZeneca, Boehringer Ingelheim, Novartis, Bayer, MSD, Sanofi, Daiichi Sankyo, Pfizer, and Eli Lilly. Dr. C.-C. Wang has received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer. Dr. C.-H. Chen reports honoraria from Novartis and Daiichi Sankyo. Dr. P.-H. Chu has received honorarium from AstraZeneca. Dr. S.-J. Yeh has received honorarium from Tanabe. Dr. W.-J. Chen has received honorarium from Astrazeneca, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Novartis, Pfizer, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SUPPLEMENTARY MATERIALS
Supplemental Table 1. Timing of in-hospital initiation and definitions of hemodynamically stable conditions.
| Timing | Hemodynamic stability | |||||
| SBP | O2 supply | IV diuretic | IV vasodilator | IV inotrope | ||
| PIONEERS1 | ≥ 24 hours to 10 days after hospitalization | ≥ 100 mmHg | No mention | No increase in dose ≥ 6 hours | No use ≥ 6 hours | No use ≥ 24 hours |
| SOLOIST-WHFS2 | Before discharge to ≤ 3 days after discharge | ≥ 100 mmHg | No O2 supply or mechanical ventilation ≥ 24 hours | No iv diuretic | No iv vasodilator (except nitrates) ≥ 24 hours | No use ≥ 24 hours |
| EMPULSES3 | ≥ 24 hours to < 5 days after hospitalization | ≥ 100 mmHg | No mention | No increase in dose ≥ 6 hours | No use ≥ 6 hours | No use ≥ 24 hours |
| STRONG-HFS4 | Hospital admission within the 72 hours prior to screening | ≥ 100 mmHg | No mention | No mention | No mention | No mention |
IV, intravenous; SBP, systolic blood pressure.
Supplemental Table 2. Primary endpoint in recent heart failure trials.
| Trials | Primary endpoint |
| PARADIGM-HFS5 | Cardiovascular death + hospitalization for heart failure |
| PARAGON-HFS6 | Cardiovascular death + total hospitalizations for heart failure |
| DAPA-HFS7 | Cardiovascular death + worsening heart failure |
| DELIVERS8 | Cardiovascular death + worsening heart failure |
| EMPEROR-ReducedS9 | Cardiovascular death + hospitalization for heart failure |
| EMPEROR-PreservedS10 | Cardiovascular death + hospitalization for heart failure |
| SOLOIST-WHFS2 | Cardiovascular death + total hospitalizations for heart failure + worsening heart failure |
| VICTORIAS11 | Cardiovascular death + hospitalization for heart failure |
| GALACTIC-HFS12 | Cardiovascular death + worsening heart failure |
REFERENCES
- S1.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- S2.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- S3.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400:1938–1952. doi: 10.1016/S0140-6736(22)02076-1. [DOI] [PubMed] [Google Scholar]
- S5.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- S6.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- S7.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- S8.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- S9.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- S10.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- S11.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- S12.Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–116. doi: 10.1056/NEJMoa2025797. [DOI] [PubMed] [Google Scholar]
FUNDING
This work was supported, in part, by grants from the Ministry of Health and Welfare (MOHW112-TDU-B-211-144001), and intramural grants from the Taipei Veterans General Hospital (V111C-194).
REFERENCES
- 1.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 3.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 5.Tsao CW, Lyass A, Enserro D, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 13.Maddox TM, Januzzi JL, Jr., Allen LA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11:404–415. doi: 10.1007/s11897-014-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–580. doi: 10.1016/S0140-6736(17)32520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam CS, Teng TK, Tay WT, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37:3141–3153. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 17.Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128:1421–1434. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 18.Hung CL, Chao TF, Tsai CT, et al. Prevalence, incidence, lifetime risks, and outcomes of heart failure in Asia: a nationwide report. JACC: Heart Failure. 2022;(in press) doi: 10.1016/j.jchf.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–462. doi: 10.1093/eurheartj/ehv464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewan P, Jackson A, Lam CSP, et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail. 2020;22:898–901. doi: 10.1002/ejhf.1776. [DOI] [PubMed] [Google Scholar]
- 22.Cleland JGF, Bunting KV, Flather MD, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26–35. doi: 10.1093/eurheartj/ehx564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund LH, Claggett B, Liu J, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20:1230–1239. doi: 10.1002/ejhf.1149. [DOI] [PubMed] [Google Scholar]
- 24.Solomon SD, Vaduganathan M, B LC, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352–361. doi: 10.1161/CIRCULATIONAHA.119.044586. [DOI] [PubMed] [Google Scholar]
- 25.Butler J, Packer M, Filippatos G, et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam CSP, Solomon SD. Classification of heart failure according to ejection fraction: JACC review topic of the week. J Am Coll Cardiol. 2021;77:3217–3225. doi: 10.1016/j.jacc.2021.04.070. [DOI] [PubMed] [Google Scholar]
- 27.Kondo T, McMurray JJV. Re-emergence of heart failure with a normal ejection fraction? Eur Heart J. 2022;43:427–429. doi: 10.1093/eurheartj/ehab828. [DOI] [PubMed] [Google Scholar]
- 28.McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation. 2020;141:338–351. doi: 10.1161/CIRCULATIONAHA.119.044491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jhund PS, Kondo T, Butt JH, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–1964. doi: 10.1038/s41591-022-01971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon SD, Vaduganathan M, Claggett BL, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction:DELIVER trial. JACC Heart Fail. 2022;10:184–197. doi: 10.1016/j.jchf.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Faselis C, Arundel C, Patel S, et al. Loop diuretic prescription and 30-day outcomes in older patients with heart failure. J Am Coll Cardiol. 2020;76:669–679. doi: 10.1016/j.jacc.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Packer M, McMurray JJV. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23:882–894. doi: 10.1002/ejhf.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 35.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 36.Lam PH, Packer M, Fonarow GC, et al. Early effects of starting doses of enalapril in patients with chronic heart failure in the SOLVD treatment trial. Am J Med. 2020;133:e25–e31. doi: 10.1016/j.amjmed.2019.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 38.Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 39.Cohn JN, Tognoni G the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 40.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 41.Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–2626. doi: 10.1161/01.CIR.0000146819.43235.A9. [DOI] [PubMed] [Google Scholar]
- 42.Kondo T, Jhund PS, McMurray JJV. Drug therapy for heart failure with reduced ejection fraction: what is the ‘right’ dose? Eur J Heart Fail. 2022;24:421–430. doi: 10.1002/ejhf.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurray J, Packer M, Desai A, et al. A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J. 2015;36:434–439. doi: 10.1093/eurheartj/ehu455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Desai AS, Vardeny O, Claggett B, et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2017;2:79–85. doi: 10.1001/jamacardio.2016.4733. [DOI] [PubMed] [Google Scholar]
- 47.Haynes R, Judge PK, Staplin N, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138:1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818. [DOI] [PubMed] [Google Scholar]
- 48.Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
- 49.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 50.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 51.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 52.Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 53.Gattis WA, O'Connor CM, Gallup DS, et al. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–1541. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 54.Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:712–718. doi: 10.1001/jama.289.6.712. [DOI] [PubMed] [Google Scholar]
- 55.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 56.Agrawal S, Agrawal N, Garg J, et al. Heart failure and chronic kidney disease: should we use spironolactone? Am J Med Sci. 2015;350:147–151. doi: 10.1097/MAJ.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 57.Vardeny O, Wu DH, Desai A, et al. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol. 2012;60:2082–2089. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 58.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 59.Pitt B, White H, Nicolau J, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–431. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 60.Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, et al. Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. Am Heart J. 2010;160:1156–1162. doi: 10.1016/j.ahj.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 62.Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42:3727–3738. doi: 10.1093/eurheartj/ehab560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serenelli M, Böhm M, Inzucchi SE, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J. 2020;41:3402–3418. doi: 10.1093/eurheartj/ehaa496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Docherty KF, Curtain JP, Anand IS, et al. Effect of dapagliflozin on anaemia in DAPA-HF. Eur J Heart Fail. 2021;23:617–628. doi: 10.1002/ejhf.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen L, Kristensen SL, Bengtsson O, et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. JACC Heart Fail. 2021;9:254–264. doi: 10.1016/j.jchf.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Inzucchi SE, Docherty KF, Køber L, et al. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA-HF. Diabetes Care. 2021;44:586–594. doi: 10.2337/dc20-1675. [DOI] [PubMed] [Google Scholar]
- 67.Lee MMY, Ghouri N, McGuire DK, et al. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus white patients with and without type 2 diabetes. Diabetes Care. 2021;44:1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 68.Docherty K, Anand IS, Chiang CE, et al. Effects of dapagliflozin in Asian patients with heart failure and reduced ejection fraction in DAPA-HF. JACC Asia. 2022;2:139–153. doi: 10.1016/j.jacasi.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berg DD, Jhund PS, Docherty KF, et al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:499–507. doi: 10.1001/jamacardio.2020.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation. 2021;143:326–336. doi: 10.1161/CIRCULATIONAHA.120.051783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 73.Chan KE, Lazarus JM, Hakim RM. Digoxin associates with mortality in ESRD. J Am Soc Nephrol. 2010;21:1550–1559. doi: 10.1681/ASN.2009101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdul-Rahim AH, Shen L, Rush CJ, et al. Effect of digoxin in patients with heart failure and mid-range (borderline) left ventricular ejection fraction. Eur J Heart Fail. 2018;20:1139–1145. doi: 10.1002/ejhf.1160. [DOI] [PubMed] [Google Scholar]
- 76.Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 77.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 78.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 79.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 80.Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312–2318. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 82.Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 83.Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020;41:2379–2392. doi: 10.1093/eurheartj/ehaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verma S, Dhingra NK, Butler J, et al. Empagliflozin in the treatment of heart failure with reduced ejection fraction in addition to background therapies and therapeutic combinations (EMPEROR-Reduced): a post-hoc analysis of a randomised, double-blind trial. Lancet Diabetes Endocrinol. 2022;10:35–45. doi: 10.1016/S2213-8587(21)00292-8. [DOI] [PubMed] [Google Scholar]
- 85.Okumura N, Jhund PS, Gong J, et al. Effects of sacubitril/valsartan in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003212. [DOI] [PubMed] [Google Scholar]
- 86.Shen L, Jhund PS, Docherty KF, et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur Heart J. 2022;43:2573–2587. doi: 10.1093/eurheartj/ehac210. [DOI] [PubMed] [Google Scholar]
- 87.Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400:1938–1952. doi: 10.1016/S0140-6736(22)02076-1. [DOI] [PubMed] [Google Scholar]
- 88.Greene SJ, Triana TS, Ionescu-Ittu R, et al. Patients hospitalized for de novo versus worsening chronic heart failure in the United States. J Am Coll Cardiol. 2021;77:1023–1025. doi: 10.1016/j.jacc.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 89.Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018;3:252–259. doi: 10.1001/jamacardio.2017.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rao VN, Murray E, Butler J, et al. In-hospital initiation of sodium-glucose cotransporter-2 inhibitors for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;78:2004–2012. doi: 10.1016/j.jacc.2021.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure-optimizing therapy with the need for speed. JAMA Cardiol. 2021;6:743–744. doi: 10.1001/jamacardio.2021.0496. [DOI] [PubMed] [Google Scholar]
- 92.Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:586–594. doi: 10.1016/S2213-8587(21)00180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 94.Cunningham JW, Vaduganathan M, Claggett BL, et al. Dapagliflozin in patients recently hospitalized with heart failure and mildly reduced or preserved ejection fraction. J Am Coll Cardiol. 2022;80:1302–1310. doi: 10.1016/j.jacc.2022.07.021. [DOI] [PubMed] [Google Scholar]
- 95.Vaduganathan M, Claggett BL, Jhund P, et al. Time to clinical benefit of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified secondary analysis of the DELIVER randomized clinical trial. JAMA Cardiol. 2022;7:1259–1263. doi: 10.1001/jamacardio.2022.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaduganathan M, Claggett BL, Inciardi RM, et al. Estimating the benefits of combination medical therapy in heart failure with mildly reduced and preserved ejection fraction. Circulation. 2022;145:1741–1743. doi: 10.1161/CIRCULATIONAHA.121.058929. [DOI] [PubMed] [Google Scholar]
- 97.Greene SJ, Bauersachs J, Brugts JJ, et al. Worsening heart failure: nomenclature, epidemiology, and future directions: JACC review topic of the week. J Am Coll Cardiol. 2023;81:413–424. doi: 10.1016/j.jacc.2022.11.023. [DOI] [PubMed] [Google Scholar]
- 98.Vaduganathan M, Claggett BL, McMurray JJV, et al. Health status trajectories before and after hospitalization for heart failure. Circulation. 2022;145:1872–1874. doi: 10.1161/CIRCULATIONAHA.122.059282. [DOI] [PubMed] [Google Scholar]
- 99.Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:935–944. doi: 10.1016/j.jacc.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 100.Docherty KF, Jhund PS, Anand I, et al. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA-HF. Circulation. 2020;142:1623–1632. doi: 10.1161/CIRCULATIONAHA.120.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 102.Ambrosy AP, Parikh RV, Sung SH, et al. Analysis of worsening heart failure events in an integrated health care system. J Am Coll Cardiol. 2022;80:111–122. doi: 10.1016/j.jacc.2022.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skali H, Dwyer EM, Goldstein R, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur J Heart Fail. 2014;16:560–565. doi: 10.1002/ejhf.71. [DOI] [PubMed] [Google Scholar]
- 104.Okumura N, Jhund PS, Gong J, et al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation. 2016;133:2254–2262. doi: 10.1161/CIRCULATIONAHA.115.020729. [DOI] [PubMed] [Google Scholar]
- 105.Greene SJ, Butler J, Spertus JA, et al. Comparison of New York Heart Association Class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:522–531. doi: 10.1001/jamacardio.2021.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teramoto K, Tay WT, Tromp J, et al. Patient-reported versus physician-assessed health status in heart failure with reduced and preserved ejection fraction from ASIAN-HF registry. Circ Cardiovasc Qual Outcomes. 2023;16:e009134. doi: 10.1161/CIRCOUTCOMES.122.009134. [DOI] [PubMed] [Google Scholar]
- 107.van Vark LC, Lesman-Leegte I, Baart SJ, et al. Prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol. 2017;70:2378–2388. doi: 10.1016/j.jacc.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 108.Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22:713–722. doi: 10.1002/ejhf.1713. [DOI] [PubMed] [Google Scholar]