Abstract
Evidence regarding the relationship between age-adjusted Charlson comorbidity index (ACCI) and in-hospital mortality is limited. Therefore, the present study investigated whether there was an independent association between ACCI and in-hospital mortality in critically ill patients with cardiogenic shock (CS) after adjusting for other covariates (age, sex, history of disease, scoring system, in-hospital management, vital signs at presentation, laboratory findings and vasopressors). ACCI, calculated retrospectively after hospitalization between 2008 and 2019, was derived from intensive care unit (ICU) admissions at the Beth Israel Deaconess Medical Center (Boston, MA, USA). Patients with CS were classified into two categories based on predefined ACCI scores (low, <8; high, ≥8). Based on baseline ACCI, the risk of in-hospital mortality in patients with CS was calculated using a multivariate Cox proportional risk model, and the threshold effect was calculated using a two-piece linear regression model. The in-hospital mortality rate was ~1.5 times greater in the ACCI high group compared with that in the ACCI low group [hazard ratio (HR)=1.45; 95% confidence interval (CI), 1.14-1.86]. Additional analysis showed that ACCI had a curvilinear association with in-hospital mortality risk in patients with CS, with a saturation effect predicted at 4.5. When ACCI was >4.5, the risk of in-hospital CS death increased significantly with increasing ACCI (HR=1.122; 95% CI, 1.054-1.194). Overall, ACCI was an independent predictor of in-hospital mortality in ICU patients with CS. A non-linear relationship was revealed between ACCI and in-hospital mortality, where in-hospital mortality increased significantly when ACCI was >4.5.
Keywords: age-adjusted Charlson comorbidity index, cardiogenic shock, mortality, intensive care unit, medical information mart for intensive care-IV
Introduction
Cardiogenic shock (CS) is a low-cardiac-output clinical syndrome caused by severe impairment of myocardial performance and characterized by inadequate tissue perfusion and microcirculation disorders (1). Left ventricular failure caused by acute myocardial infarction (AMI) is the main cause of CS and accounts for 81% of cases of CS (2). Despite significant advances in revascularization techniques, pharmacological therapeutics and mechanical support devices, CS remains associated with a high mortality rate of 27-51% (3).
Mortality risk stratification should be routinely performed in patients with CS. Several predictive tools for critically ill patients are available, including the simplified acute physiology score, and acute physiology and chronic health evaluation score (4,5). However, these predictive tools are poorly calibrated suggesting that they may not be appropriate to apply in patients with CS (6). The CardShock and intra-aortic balloon pump in cardiogenic shock II (IABP-SHOCK II) risk scores have been developed specifically for patients with CS (7-9) and used as predictive models for in-hospital mortality; they also show good discrimination (area under the curve 0.76 and 0.73, respectively) (6). However, the IABP-SHOCK II score is only applicable to patients with acute coronary syndrome (ACS), whereas the CardShock score, although applicable to patients without ACS, does not apply to those with postoperative CS. Moreover, the above two scoring systems require a combination of biochemical examination indexes and coronary angiography, which are time-consuming, thus affecting evaluation of patient's condition (7-9). Therefore, a simple and effective scoring system with relatively wide applicability is required.
The age-adjusted Charlson Comorbidity Index (ACCI) is a weighted system based on the age of an individual and their chronic condition (10). ACCI is commonly used to predict long-term prognosis in patients with cancer (11) and the risk of postoperative complications after orthopedic surgery (12). ACCI can be a predictor of in-hospital death and long-term prognosis in critically ill patients, such as those with sepsis (13) and postoperative cardiac disease (14). However, to the best of our knowledge, the relationship between ACCI and in-hospital mortality in patients with CS has not been investigated thoroughly. ACCI is readily available upon patient admission through history-taking (15). To explore the relationship between ACCI and in-hospital mortality in critically ill patients with CS, high-risk patients should be identified early and provided with extra care. Therefore, the present study investigated whether ACCI was independently associated with mortality in critically ill patients with CS.
Materials and methods
Database
Data for this retrospective, single-center, observational cohort study were obtained from the Medical Information Mart for Intensive Care (MIMIC)-IV database (https://www.physionet.org/content/mimiciv/2.2/). MIMIC-IV provides real-time data on more than 70,000 critically ill patients treated at the Beth Israel Deaconess Medical Center between 2008 and 2019(16).
After completing the collaborative institutional training initiative course and passing the ethics examination (certification no. 48693003), access to the database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center, and access to the database was granted for download and use.
Ethical review and approval was not required for the study in accordance with the local legislation and institutional requirements.
Study cohort
Patients with CS between 2008 and 2019 were eligible for inclusion. The inclusion criteria were as follows: i) International Classification of Diseases (ICD)-9(17): 785.51 and 998.01 and ICD-10(18): R57.0, T81.11, T81.11XA, T81.11XD and T81.11XS); ii) age, ≥18 years; iii) No restrictions on sex.
The exclusion criteria were as follows: i) Age <18 years; ii) intensive care unit (ICU) length of stay <24 h; and iii) incomplete or unobtainable ACCI data. Only the first admission was included in the analysis of patients admitted to the ICU multiple times.
Exposure variable
Upon admission, the comorbidity score was calculated based on the clinical history of the patient. ACCI was generated based on comorbidity score and age of the patients. A total of 19 medical conditions were included in the comorbidity score, each rated from 1 to 6 points to calculate the index score (Table SI). A comorbidity score of ‘1’ was assigned every decade for patients over the age of 40 when calculating ACCI scores (19). The ACCI cut-off points were determined using the mean and median ACCI scores of participants, as well as referring to the cut-off values stated in other studies (14,20).
Covariates
During the first 24 h after admission to the ICU, data on baseline characteristics of the patients were collected, including demographics (age and sex), vital signs [heart rate and mean blood pressure (MBP)], laboratory findings [serum creatinine (Scr), white blood cell (WBC), hemoglobin and platelet counts], comorbidities [hypertension, chronic kidney disease (CKD), stroke, chronic pulmonary disease (COPD), dementia, paraplegia, peptic ulcer disease, diabetes, severe liver disease, malignant cancer and peripheral vascular disease (PVD)], and scores [ACCI and Oxford Acute Severity of Illness Score (OASIS)]. In-hospital management included mechanical ventilation (MV) and extracorporeal membrane oxygenation (ECMO). Etiologies of CS included AMI and acute heart failure (AHF). The use of vasoactive drugs, including dobutamine, norepinephrine and dopamine, was also recorded.
Outcomes
In-hospital mortality was the primary outcome.
Statistical analysis
The characteristics of patients were analyzed based on a predefined ACCI. Continuous variables are reported as means and standard deviations, and categorical variables are presented as percentages. Median values and interquartile ranges were calculated for parameters with a skewed distribution. Pearson's χ2, unpaired Student's t-test and Mann-Whitney U test were used to compare categorical variables, continuous variables with normal distribution and continuous variables with skewed distribution. The Kaplan-Meier and log-rank analyses were used to determine in-hospital survival curves. Multivariate Cox regression analysis was used to estimate the correlation between ACCI and in-hospital mortality. Following the Strengthening the Reporting of Observational Studies in Epidemiology statement (21), four multivariate adjustment models were used: Model I, not adjusted for any variable; Model II, adjusted for age and sex; Model III adjusted for Model II plus hypertension, CKD, stroke, COPD, dementia, paraplegia, peptic ulcer disease, diabetes, severe liver disease, malignant cancer, PVD, AMI and AHF; and Model IV adjusted for Model III plus heart rate, MBP, hemoglobin, platelets, WBC, Scr, OASIS, MV, ECMO, dobutamine, norepinephrine and dopamine. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. A generalized additive model was used to evaluate the nonlinear relationship between ACCI scores and in-hospital mortality in patients with CS (22,23). Based on the Cox curve fitting, a two-piecewise linear regression model was used to determine the threshold (22). The median value for each ACCI group was entered into the model as a continuous variable to test for linear trends (22). Tests were performed for linear trends by entering the median value of each ACCI category as a continuous variable in the models (24). Statistical analyses were conducted using the statistical software packages R version 3.6.3 (https://www.r-project.org/) and Free Statistics software (version 1.6) (FreeClinical Medical Technology Co., Ltd.). P<0.05 was considered to indicate a statistically significant difference.
Sensitivity analysis
Initially, patients who had an ICD-9 code of 78551 (cardiogenic shock) or 99801 (postoperative shock, cardiogenic), an ICD-10 code of R570 (cardiogenic shock) or T8111 (postprocedural cardiogenic shock) or T8111XA (postprocedural cardiogenic shock, initial encounter) or T8111XD (postprocedural cardiogenic shock, subsequent encounter) or T8111XS (postprocedural cardiogenic shock, sequela) were included. Postoperative cardiogenic shock is mostly caused by cardiac surgery and is more severe, which may affect the present conclusions. Additionally, in the current study, patients were included whenever cardiogenic shock was present, but there may be a large heterogeneity between patients with cardiogenic as the primary diagnosis or not, which may affect the conclusions. To test the robustness of the present findings, a sensitivity analysis was conducted wherein patients who presented postoperatively with CS or patients for whom CS was not indicated in the primary diagnosis were excluded.
Results
Participant selection
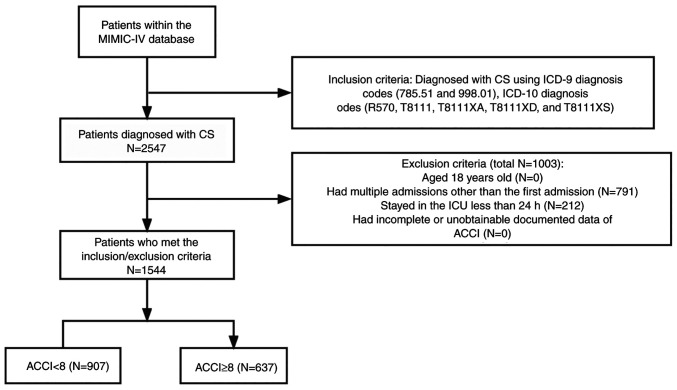
A total of 2,547 patients with CS were identified from 71,532 MIMIC-IV admissions. The flowchart in Fig. 1 illustrates the research process. In the entire CS population drawn from the MIMIC-V database, in-hospital mortality rates were 44.9 and 35.6% for patients with <24 h of hospitalization and those with >24 h of hospitalization, respectively, with a statistically significant difference (P=0.0071; Table SII); The in-hospital mortality rates were 41.2 and 33.3% for patients with repeat ICU admissions and first ICU admission, respectively, with a statistically significant difference (P=0.0002; Table SIII). After excluding patients <18 years of age, ICU length of stay <24 h and those with incomplete information, 1,544 patients were included in the analysis.
Figure 1.
Flowchart of patient selection. CS, cardiogenic shock; MIMIC-IV, medical information mart for intensive care-IV; ICU, intensive care unit; ACCI, age-adjusted Charlson comorbidity index.
Baseline characteristics
Baseline characteristics are presented in Table I. The enrolled patients were classified into two categories: Low ACCI group (ACCI <8) and high ACCI group (ACCI ≥8). The mean ACCI for the entire cohort was 7.0±2.7, while the high ACCI group included 637 patients (41.3%). The average age of all study participants was 70.2±14.5 years, and 920 (59.6%) were men. No significant differences were revealed between the two groups in terms of sex (P=0.34). A higher frequency of individual comorbidities, such as CKD, PVD, dementia, COPD, peptic ulcer, diabetes mellitus, malignant cancer, paraplegia and severe liver disease, as well as CS etiologies, such as AMI and AHF, were found in the high ACCI group. Patients in the high ACCI group had higher age and OASIS score and lower MBP, hemoglobin, WBC, hypertension and epinephrine values. There was no relationship between length of ICU stay and hospital stay between the two groups.
Table I.
Baseline characteristics of the study participants.
| ACCI | All patients | Low ACCI (<8) | High ACCI (≥8) | P-value |
|---|---|---|---|---|
| Number, n | 1544 | 907 | 637 | |
| Sex, n (%) | 0.340 | |||
| Male | 920 (59.6) | 550 (60.6) | 370 (58.1) | |
| Female | 624 (40.4) | 357 (39.4) | 267 (41.9) | |
| Age, years | 70.2±14.5 | 65.8±15.5 | 76.4±9.9 | <0.001 |
| Etiology | ||||
| AMI, n (%) | 562 (36.4) | 307 (33.8) | 255 (40.0) | 0.015 |
| AHF, n (%) | 988 (64.0) | 519 (57.2) | 469 (73.6) | <0.001 |
| History of disease | ||||
| Hypertension, n (%) | 326 (21.1) | 222 (24.5) | 104 (16.3) | <0.001 |
| CKD, n (%) | 197 (12.8) | 37 (4.1) | 160 (25.1) | <0.001 |
| Stroke, n (%) | 76 (4.9) | 37 (4.1) | 39 (6.1) | 0.088 |
| PVD, n (%) | 294 (19.0) | 103 (11.4) | 191 (30.0) | <0.001 |
| Dementia, n (%) | 48 (3.1) | 15 (1.7) | 33 (5.2) | <0.001 |
| COPD, n (%) | 473 (30.6) | 229 (25.2) | 244 (38.3) | <0.001 |
| Peptic ulcer, n (%) | 35 (2.3) | 11 (1.2) | 24 (3.8) | 0.002 |
| Diabetes, n (%) | 207 (13.4) | 25 (2.8) | 182 (28.6) | <0.001 |
| Paraplegia, n (%) | 44 (2.8) | 8 (0.9) | 36 (5.7) | <0.001 |
| Malignant cancer, n (%) | 114 (7.4) | 19 (2.1) | 95 (14.9) | <0.001 |
| Severe liver disease, n (%) | 46 (3.0) | 11 (1.2) | 35 (5.5) | <0.001 |
| Scoring system | ||||
| ACCI | 7.0±2.7 | 5.2±1.6 | 9.5±1.6 | <0.001 |
| OASIS | 37.9±10.1 | 37.2±10.1 | 38.9±10.0 | <0.001 |
| In-hospital management | ||||
| MV, n (%) | 487 (31.5) | 298 (32.9) | 189 (29.7) | 0.204 |
| ECMO, n (%) | 48 (3.1) | 35 (3.9) | 13 (2.0) | 0.060 |
| Vital signs at presentation | ||||
| HR (beats/minute) | 88.3±17.9 | 89.1±18.3 | 87.3±17.2 | 0.062 |
| MBP (mmHg) | 75.1±9.4 | 76.4±9.6 | 73.2±8.9 | <0.001 |
| Laboratory findings | ||||
| Hemoglobin (g/dl) | 12.0±2.3 | 12.5±2.3 | 11.2±2.0 | <0.001 |
| Platelet (K/µl) | 166.0 (115.0, 228.0) | 167.0 (118.0, 230.0) | 165.0 (114.0, 226.2) | 0.637 |
| WBC (109/l) | 14.9 (11.0, 20.0) | 15.8 (11.7, 20.9) | 13.7 (10.5, 19.0) | <0.001 |
| Scr (mg/dl) | 1.6 (1.1, 2.4) | 1.3 (1.0, 2.0) | 2.1 (1.5, 3.1) | <0.001 |
| Vasopressors, n (%) | ||||
| Dobutamine, n (%) | 374 (24.2) | 197 (21.7) | 177 (27.8) | 0.007 |
| Norepinephrine, n (%) | 986 (63.9) | 575 (63.4) | 411 (64.5) | 0.69 |
| Dopamine, n (%) | 351 (22.7) | 202 (22.3) | 149 (23.4) | 0.649 |
| Outcomes | ||||
| In-hospital mortality, n (%) | 495 (32.1) | 230 (25.4) | 265 (41.6) | <0.001 |
| Los hospital (days) | 13.9±12.6 | 13.6±12.7 | 14.3±12.5 | 0.254 |
| Los ICU (days) | 7.3±7.9 | 7.2±8.1 | 7.3±7.7 | 0.803 |
AMI, acute myocardial infarction; AHF, acute heart failure; PVD, peripheral vascular disease; CKD, chronic kidney disease; COPD, chronic pulmonary disease; ACCI, age-adjusted Charlson comorbidity index; OASIS, Oxford Acute Severity of Illness Score; HR, heart rate; WBC, white blood cell; Scr, serum creatinine; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation; MBP, mean arterial blood pressure; Los hospital, length of hospital stay time; Los ICU, length of intensive care unit stay time.
Outcomes
In the index hospitalization, 495 patients (32.1%) died, and those with high ACCI were more likely to die during hospitalization (41.6 vs. 25.4%; P<0.001; Table I).
ACCI score and in-hospital mortality
Univariate logistic regression analysis showed that variables including age, acute heart failure, chronic kidney disease, malignant cancer, Charlson comorbidity index, ACCI score ≥8, Oxford Acute Severity of Illness Score, hemoglobin, white blood cells, serum creatinine, mechanical ventilation, mean blood pressure, and use of norepinephrine and dopamine were associated with in-hospital mortality (Table SIV). Kaplan-Meier analysis showed poorer survival rate in the high ACCI group compared with in the low ACCI group during hospitalization (P<0.001; Fig. 2). As a continuous variable in the unadjusted Cox hazard regression model, ACCI was significantly associated with increased risk of in-hospital mortality (HR=1.1; 95% CI, 1.06-1.13). After further adjustment for all potential covariates, associations were slightly attenuated but remained significant, with an HR of 1.09 (95% CI, 1.03-1.16; Table II). As a categorized variable in the fully adjusted model, participants in the high ACCI group had a 45% increased risk of in-hospital mortality compared with the low ACCI group (Table II).
Figure 2.
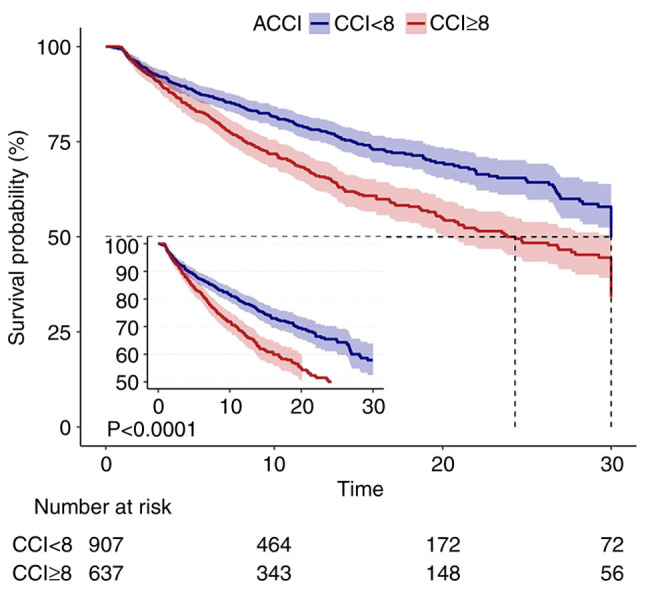
Kaplan-Meier survival curves showing in-hospital mortality rate according to ACCI in patients with CS. ACCI, age-adjusted Charlson comorbidity index; CS, cardiogenic shock.
Table II.
Association between ACCI scores and in-hospital mortality in multiple regression model.
| Model I | Model II | Model III | Model IV | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| ACCI | 1.10 (1.06-1.13) | <0.001 | 1.05 (1.01-1.10) | 0.007 | 1.12 (1.06-1.18) | <0.001 | 1.09 (1.03-1.16) | 0.004 |
| ACCI <8 | 1.00 (Ref) | - | 1.00 (Ref) | - | 1.00 (Ref) | - | 1.00 (Ref) | - |
| ACCI ≥8 | 1.56 (1.31-1.86) | <0.001 | 1.30 (1.08-1.57) | 0.007 | 1.64 (1.29-2.09) | <0.001 | 1.45 (1.14-1.86) | 0.003 |
Model I: Did not adjust for confounders. Model II: Adjusted for age + sex. Model III: Model II + hypertension, chronic kidney disease, stroke, chronic pulmonary disease, dementia, paraplegia, peptic ulcer disease, diabetes, severe liver disease, malignant cancer, peripheral vascular disease, acute myocardial infarction, acute heart failure. Model IV: Model III + HR, mean blood pressure, hemoglobin, platelets, white blood cell, serum creatinine, Oxford Acute Severity of Illness Score, mechanical ventilation, extracorporeal membrane oxygenation, dobutamine, norepinephrine, dopamine. HR, hazard Ratio; CI, Confidence interval; ACCI, age-adjusted Charlson comorbidity.
Non-linear relationship between ACCI and in-hospital mortality
After adjustment for a series of covariates, ACCI and in-hospital mortality exhibited a non-linear dose-response relationship (Fig. 3). Based on a two-piecewise linear regression model, the ACCI threshold was defined at 4.5 (Table III). When the threshold was reached, the in-hospital mortality rate continued to rise (HR=1.122; 95% CI, 1.054-1.194; P<0.001), whereas when ACCI was below the threshold, no significant dose-response relationship was observed (HR=0.717; 95% CI, 0.458-1.123; P=0.1467).
Figure 3.
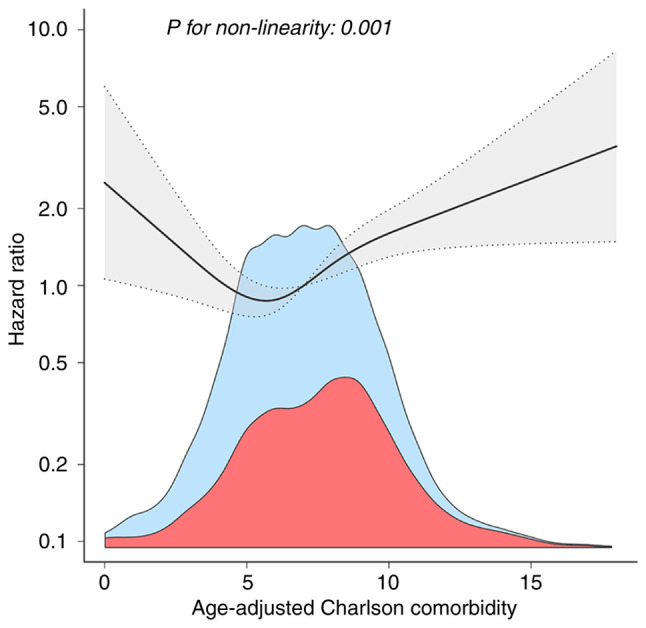
Non-linear dose-response relationship between ACCI and in-hospital mortality. The black line and gray area represent the estimated values and their corresponding 95% confidence intervals, respectively. HRs are adjusted for age, sex, hypertension, chronic kidney disease, stroke, chronic pulmonary disease, dementia, paraplegia, peptic ulcer disease, diabetes, severe liver disease, malignant cancer, peripheral vascular disease, acute myocardial infarction, acute heart failure, heart rate, mean blood pressure, hemoglobin, platelets, white blood cell, serum creatinine, Oxford Acute Severity of Illness Score, mechanical ventilation, extracorporeal membrane oxygenation, dobutamine, norepinephrine and dopamine. ACCI, age-adjusted Charlson comorbidity index; HR, hazard ratio.
Table III.
Threshold effect analysis of age-adjusted Charlson comorbidity on in-hospital mortality.
| Threshold of ACCI | HR | 95% confidence intervals | P-value |
|---|---|---|---|
| <4.5 | 0.717 | 0.458,1.123 | 0.147 |
| ≥4.5 | 1.122 | 1.054,1.194 | <0.001 |
| Log-likelihood ratio test | <0.001 |
HRs were adjusted for age, sex, hypertension, chronic kidney disease, stroke, chronic pulmonary disease, dementia, paraplegia, peptic ulcer disease, diabetes, severe liver disease, malignant cancer, peripheral vascular disease, acute myocardial infarction, acute heart failure, HR, mean blood pressure, hemoglobin, platelets, white blood cell, serum creatinine, Oxford Acute Severity of Illness Score, mechanical ventilation, extracorporeal membrane oxygenation, dobutamine, norepinephrine, dopamine. HR, hazard ratio.
Sensitivity analysis
To further clarify the influence of surgery and diagnosis sequence on the results, 175 patients who received postprocedural CS and 476 patients with CS who showed diagnosis sequence number >5 were excluded from sensitivity analyses. After excluding these patients, the HR of in-hospital mortality generally increased in patients with high ACCI (Table SV, Table SVI, Table SVII and Table VIII).
Discussion
In the current retrospective cohort study, ACCI was independently associated with in-hospital mortality in critically ill patients with CS. Higher ACCI was associated with higher in-hospital mortality rate. This relationship persisted after adjusting for the appropriate variables and confounders. To the best of our knowledge, this is the first report examining the relationship between ACCI and in-hospital mortality in critically ill patients with CS. Non-equidistant trends were revealed in the effect values in different ACCI subgroups, suggesting a possible non-linear relationship between ACCI and in-hospital mortality in these patients. The effect of ACCI on in-hospital mortality rate depended on whether the ACCI score was >4.5 or <4.5; ACCI >4.5 was positively associated with in-hospital mortality, whereas ACCI <4.5 did not demonstrate a statistically significant association with in-hospital mortality, suggesting a threshold effect.
Studies have shown that patients admitted to the ICU with CS have a significantly higher mortality rate within 24 or 48 h of admission (25,26), and repeat ICU admissions also have a higher in-hospital mortality rate compared with first ICU admission (27). Therefore, these aforementioned patients with high heterogeneity were excluded given the stability of the study results. In articles examining in-hospital mortality in a variety of critically ill patients, patients with a length of stay of <24 h were also usually excluded, and for patients admitted to the ICU multiple times, only the first admission data were selected for analysis (28-30). Alvan R. Feinstein defined the Charlson comorbidity index (CCI) as ‘any distinct additional clinical entity that has existed or that may occur during the clinical course of a disease that is under study’ (31). ACCI is based on CCI after adjusting for age. ACCI was used for the first time to predict mortality due to comorbidities in 685 patients with breast cancer over 10 years (32). ACCI has been useful for predicting long-term mortality in patients with tumors (11,33). A study reported that in 4,508 patients with lung cancer, ACCI showed better predictive value for 3-year mortality compared with CCI alone or the Elixhauser index (34). ACCI also predicts long-term mortality in ICU patients, including those with cardiovascular disease and cancer (35-38). It was not specifically designed to predict in-hospital mortality but has often been used for this purpose. ACCI was a predictor of in-hospital and one-year mortality rates in 29,620 patients hospitalized for ACS in Switzerland (39) and in-hospital mortality rate in 529 older patients with cardiac disease (40). Results between these studies were consistent and showed that ACCI was positively correlated with mortality. However, other studies have shown that ACCI was not independently associated with 30-day mortality in patients undergoing transcatheter aortic valve implantation after multivariate adjustment (41), although it may be a useful variable in other models to help patients decide on this procedure.
Evidence suggests that ACCI is a valid and widely used measure for predicting mortality risk. This tool can be used to predict clinical outcomes and to screen for sensitive conditions (15). The present study demonstrated that ACCI is significantly associated with in-hospital mortality and that it may reliably predict in-hospital mortality in patients with CS. There are several possible explanations for this finding. Notably, some comorbidities included in ACCI, such as myocardial infarction and congestive heart failure, are underlying conditions and predisposing factors for the development of CS and affect CS instability (42,43). Multiple mechanisms link comorbidities and CS, which influence each other bidirectionally, including inflammation (44,45), cytokine cascades (44) and microvascular disorders (46).
Several studies have observed the relationship between ACCI and in-hospital mortality in critically ill patients (47,48). A study showed that ACCI is a valid indicator to predict death in ICU patients with cardiac arrest (49); however, to the best of our knowledge, no previous studies have investigated the linear relationship between ACCI and in-hospital mortality in patients with CS. Curve fitting was performed in a study that examined the prognostic relationship between ACCI and minimally invasive mitral valve surgery, wherein the spline curve analysis showed that the odds ratio increased considerably when ACCI >8 in reference to the adverse events of 1-year and 30-day mortality (14). This threshold was not similar to the cut-off values reported in the present study, probably because of the different types of disease and distribution of the sample. A threshold of ~4.5 was determined, and the effects below and above this threshold were completely different. Although in-hospital mortality rate decreased in patients with ACCI <4.5, P-values showed no statistical significance. This may be related to patient age, different comorbid components, and a small sample size with ACCI <4.5. To verify the robustness of the present conclusions, multivariate Cox regression analyses and curve fitting were performed, excluding patients who developed CS after surgery or those who were ranked fifth in cardiogenic diagnosis. The HRs for in-hospital mortality from ACCI as a continuous variable for CS were 1.09 (1.03-1.16) and 1.11 (1.03-1.2), similar to those in the overall population. The cut-off values were 4.5 and 3.9, which remained robust.
The present study had several limitations. First, as a single-center study and owing to the strict inclusion criteria, conclusions may be extrapolated only for patients with CS who were in the ICU for >24 h. Second, some undetected and uncontrolled confounding factors may have affected the conclusions, which is an inherent problem for all observational studies. Third, the current study could not find a plausible explanation for the lack of a correlation between ACCI and in-hospital mortality below the threshold; hence, further investigation of possible causes and mechanisms is required. Forth, the reason for patient mortality; whether this was expected; and if the patients were subjected to standard clinical practice were not provided in the database, so we cannot identify the exact cause of death. This has somewhat influenced the further search for ways to reduce mortality. However, after excluding some special populations from the sensitivity analysis, the present results were consistent with those in the whole population, indicating the robustness of the results.
In conclusion, a non-linear relationship between ACCI and mortality in critically ill patients with CS exists, with a significant increase in in-hospital mortality when the ACCI score exceeds 4.5.
Supplementary Material
Acknowledgements
The authors wish to thank Dr Liu Jie (People's Liberation Army of the China General Hospital, Beijing, China) and Dr Yang Qilin (The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China) for helping with the revision of the manuscript.
Funding Statement
Funding: This study was supported by the Guangxi Zhuang Autonomous Region Suitable Technology Development and Promotion Project of Traditional Chinese Medicine (grant no. GZSY22-80) and the Liuzhou Medical Health and Biomedical Science and Technology Innovation Project (grant no. 2022CAC0216).
Availability of data and materials
The clinical data used to support the findings of this study were acquired from the Monitoring in Intensive Care Database IV version 2.0 (MIMIC-IV v.2.0). Although the database is publicly and freely available, users must complete the National Institutes of Health's web-based course known as Protecting Human Research Participants to apply for permission to access the database. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors have contributed to this work and take public responsibility for appropriate portions of the content and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. DW was responsible for the design of this study. RC contributed to data acquisition and analysis. WW contributed to interpretation of the data. RC and WW confirm the authenticity of all the raw data. YS made substantial contributions to analysis of data. DW and YM made substantial contributions to interpretation of data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The use of the MIMIC-IV database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Ethical review and approval was not required for the study in accordance with the local legislation and institutional requirements. And the data used in the present study were obtained from a public database, which do not contain protected health information, so individual patient consent was not needed. The protocol was performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8(e011991) doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: A scientific statement from the American heart association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Makam RCP, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-long trends (2001-2011) in the incidence and hospital death rates associated with the in-hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–125. doi: 10.1161/CIRCOUTCOMES.115.002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahtouee M, Eghbali SS, Maleki N, Rastgou V, Motamed N. Acute physiology and chronic health evaluation II score for the assessment of mortality prediction in the intensive care unit: A single-centre study from Iran. Nurs Crit Care. 2019;24:375–380. doi: 10.1111/nicc.12401. [DOI] [PubMed] [Google Scholar]
- 5.Alizadeh AM, Hassanian-Moghaddam H, Shadnia S, Zamani N, Mehrpour O. Simplified acute physiology score II/acute physiology and chronic health evaluation II and prediction of the mortality and later development of complications in poisoned patients admitted to intensive care unit. Basic Clin Pharmacol Toxicol. 2014;115:297–300. doi: 10.1111/bcpt.12210. [DOI] [PubMed] [Google Scholar]
- 6.Miller RJH, Southern D, Wilton SB, James MT, Har B, Schnell G, van Diepen S, Grant ADM. Comparative prognostic accuracy of risk prediction models for cardiogenic shock. J Intensive Care Med. 2020;35:1513–1519. doi: 10.1177/0885066619878125. [DOI] [PubMed] [Google Scholar]
- 7.Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 8.Pöss J, Köster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, Lassus J, Harjola VP, Zeymer U, Thiele H, Desch S. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:1913–1920. doi: 10.1016/j.jacc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Sleeper LA, Reynolds HR, White HD, Webb JG, Dzavík V, Hochman JS. A severity scoring system for risk assessment of patients with cardiogenic shock: A report from the SHOCK trial and registry. Am Heart J. 2010;160:443–450. doi: 10.1016/j.ahj.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Lin JX, Huang YQ, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu R, Huang ZN, et al. Association of the age-adjusted Charlson comorbidity index and systemic inflammation with survival in gastric cancer patients after radical gastrectomy. Eur J Surg Oncol. 2019;45:2465–2472. doi: 10.1016/j.ejso.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Chou ACC, Nadkarni N, Ng CEQ, Chong YS, Howe TS, Koh JSB. Charlson comorbidity index predicts 5-year survivorship of surgically treated hip fracture patients. Geriatr Orthop Surg Rehabil. 2018;9(2151459318806442) doi: 10.1177/2151459318806442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stavem K, Hoel H, Skjaker SA, Haagensen R. Charlson comorbidity index derived from chart review or administrative data: Agreement and prediction of mortality in intensive care patients. Clin Epidemiol. 2017;9:311–320. doi: 10.2147/CLEP.S133624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minol JP, Dimitrova V, Petrov G, Langner R, Boeken U, Rellecke P, Aubin H, Kamiya H, Sixt S, Huhn R, et al. The age-adjusted Charlson comorbidity index in minimally invasive mitral valve surgery. Eur J Cardiothorac Surg. 2019;56:1124–1130. doi: 10.1093/ejcts/ezz240. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: A critical review of clinimetric properties. Psychother Psychosom. 2022;91:8–35. doi: 10.1159/000521288. [DOI] [PubMed] [Google Scholar]
- 16.Johnson Alistair, Bulgarelli Lucas, Pollard Tom, Horng Steven, Celi Leo Anthony, Roger Mark. doi: 10.13026/7vcr-e114. MIMIC-IV (version 2.0). Available from: [DOI] [Google Scholar]
- 17. World Health Organization (WHO). International classification of diseases: [9th] ninth revision, basic tabulation list with alphabetic index. WHO, Geneva, 1978. https://apps.who.int/iris/handle/10665/39473. [Google Scholar]
- 18. World Health Organization (WHO). International statistical classification of diseases and related health problems. 10th revision. 5th edition. WHO, Geneva, 2016. https://apps.who.int/iris/handle/10665/246208. [Google Scholar]
- 19.Chang CM, Yin WY, Wei CK, Wu CC, Su YC, Yu CH, Lee CC. Adjusted age-adjusted charlson comorbidity index score as a risk measure of perioperative mortality before cancer surgery. PLoS One. 2016;11(e0148076) doi: 10.1371/journal.pone.0148076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saji M, Katz MR, Ailawadi G, Fowler DE, Ragosta M, Lim DS. Predictive value of age-adjusted charlson co-morbidity index for 1-, 3-, and 5-year mortality in patients requiring transcatheter mitral valve repair. Am J Cardiol. 2017;120:309–314. doi: 10.1016/j.amjcard.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Yu X, Chen J, Li Y, Liu H, Hou C, Zeng Q, Cui Y, Zhao L, Li P, Zhou Z, et al. Threshold effects of moderately excessive fluoride exposure on children's health: A potential association between dental fluorosis and loss of excellent intelligence. Environ Int. 2018;118:116–124. doi: 10.1016/j.envint.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 23.Kong X, Huang X, Zhao M, Xu B, Xu R, Song Y, Yu Y, Yang W, Zhang J, Liu L, et al. Platelet count affects efficacy of folic acid in preventing first stroke. J Am Coll Cardiol. 2018;71:2136–2146. doi: 10.1016/j.jacc.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med. 2017;167:228–235. doi: 10.7326/M16-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barssoum K, Patel HP, Abdelmaseih R, Hassib M, Victor V, Mohamed A, Jazar DA, Mai S, Ibrahim F, Patel B, et al. Characteristics and outcomes of early vs late initiation of mechanical circulatory support in non-acute myocardial infarction related cardiogenic shock: An analysis of the national inpatient sample database. Curr Probl Cardiol. 2023;48(101584) doi: 10.1016/j.cpcardiol.2023.101584. [DOI] [PubMed] [Google Scholar]
- 26.Assali AR, Iakobishvili Z, Zafrir N, Solodky A, Teplitsky I, Rechavia E, Butto N, Shor N, Hasdai D, Fuchs S, et al. Characteristics and clinical outcomes of patients with cardiogenic shock complicating acute myocardial infarction treated by emergent coronary angioplasty. Int J Cardiovasc Intervent. 2005;7:193–198. doi: 10.1080/14628840500445566. [DOI] [PubMed] [Google Scholar]
- 27.Lee SI, Koh Y, Huh JW, Hong SB, Lim CM. Factors and outcomes of intensive care unit readmission in elderly patients. Gerontology. 2022;68:280–288. doi: 10.1159/000516297. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Yuan M, Ma Y, Shao C, Wang Y, Qi M, Ren B, Gao D. The admission (Neutrophil+Monocyte)/lymphocyte ratio is an independent predictor for in-hospital mortality in patients with acute myocardial infarction. Front Cardiovasc Med. 2022;9(870176) doi: 10.3389/fcvm.2022.870176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, Schwartzstein RM, Vieillard-Baron A, Celi LA. Transthoracic echocardiography and mortality in sepsis: Analysis of the MIMIC-III database. Intensive Care Med. 2018;44:884–892. doi: 10.1007/s00134-018-5208-7. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Zhu Z, Zhao C, Guo Y, Chen D, Wei Y, Jin J. Central venous pressure measurement is associated with improved outcomes in septic patients: An analysis of the MIMIC-III database. Crit Care. 2020;24(433) doi: 10.1186/s13054-020-03109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Wu CC, Hsu TW, Chang CM, Yu CH, Lee CC. Age-adjusted Charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine (Baltimore) 2015;94(e431) doi: 10.1097/MD.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang CC, Fong Y, Lin LC, Que J, Ting WC, Chang CL, Wu HM, Ho CH, Wang JJ, Huang CI. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53:235–240. doi: 10.1093/ejcts/ezx215. [DOI] [PubMed] [Google Scholar]
- 35.Hoang TH, Maiskov VV, Merai IA, Kobalava ZD. Development and validation of a model for predicting 18-month mortality in type 2 myocardial infarction. Am J Emerg Med. 2021;48:224–230. doi: 10.1016/j.ajem.2021.04.060. [DOI] [PubMed] [Google Scholar]
- 36.Shebeshi DS, Dolja-Gore X, Byles J. Charlson Comorbidity index as a predictor of repeated hospital admission and mortality among older women diagnosed with cardiovascular disease. Aging Clin Exp Res. 2021;33:2873–2878. doi: 10.1007/s40520-021-01805-2. [DOI] [PubMed] [Google Scholar]
- 37.Hsu YT, He YT, Ting CK, Tsou MY, Tang GJ, Pu C. Administrative and claims data help predict patient mortality in intensive care units by logistic regression: A nationwide database study. Biomed Res Int. 2020;2020(9076739) doi: 10.1155/2020/9076739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadique S, Culp S, Sangani RG, Chapman KD, Khan S, Parker JE, Moss AH. Derivation and validation of a prognostic model to predict 6-month mortality in an intensive care unit population. Ann Am Thorac Soc. 2017;14:1556–1561. doi: 10.1513/AnnalsATS.201702-159OC. [DOI] [PubMed] [Google Scholar]
- 39.Radovanovic D, Seifert B, Urban P, Eberli FR, Rickli H, Bertel O, Puhan MA, Erne P. Validity of Charlson Comorbidity index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart. 2014;100:288–294. doi: 10.1136/heartjnl-2013-304588. [DOI] [PubMed] [Google Scholar]
- 40.Jepma P, Verweij L, Tijssen A, Heymans MW, Flierman I, Latour CHM, Peters RJG, Reimer WJM, Buurman BM, Riet GT. The performance of the dutch safety management system frailty tool to predict the risk of readmission or mortality in older hospitalised cardiac patients. BMC Geriatr. 2021;21(299) doi: 10.1186/s12877-021-02243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George S, Kwok CS, Martin GP, Babu A, Shufflebotham A, Nolan J, Ratib K, Bagur R, Gunning M, Mamas M. The influence of the Charlson Comorbidity index on procedural characteristics, VARC-2 endpoints and 30-day mortality among patients who undergo transcatheter aortic valve implantation. Heart Lung Circ. 2019;28:1827–1834. doi: 10.1016/j.hlc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Abraham J, Blumer V, Burkhoff D, Pahuja M, Sinha SS, Rosner C, Vorovich E, Grafton G, Bagnola A, Hernandez-Montfort JA, Kapur NK. Heart failure-related cardiogenic shock: Pathophysiology, evaluation and management considerations: Review of heart failure-related cardiogenic shock. J Card Fail. 2021;27:1126–1140. doi: 10.1016/j.cardfail.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Dalzell JR. Review of cardiogenic shock after acute myocardial infarction. JAMA. 2022;327(878) doi: 10.1001/jama.2021.25175. [DOI] [PubMed] [Google Scholar]
- 44.Bertini P, Guarracino F. Pathophysiology of cardiogenic shock. Curr Opin Crit Care. 2021;27:409–415. doi: 10.1097/MCC.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 45.Lassus J, Tarvasmäki T, Tolppanen H. Biomarkers in cardiogenic shock. Adv Clin Chem. 2022;109:31–73. doi: 10.1016/bs.acc.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Krychtiuk KA, Vrints C, Wojta J, Huber K, Speidl WS. Basic mechanisms in cardiogenic shock: Part 1-definition and pathophysiology. Eur Heart J Acute Cardiovasc Care. 2022;11:356–365. doi: 10.1093/ehjacc/zuac021. [DOI] [PubMed] [Google Scholar]
- 47.Wireklint SC, Elmqvist C, Fridlund B, Göransson KE. A longitudinal, retrospective registry-based validation study of RETTS©, the Swedish adult ED context version. Scand J Trauma Resusc Emerg Med. 2022;30(27) doi: 10.1186/s13049-022-01014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu KJ, Kearney LG, Ord M, Jones E, Burrell LM, Srivastava PM. Age adjusted Charlson Co-morbidity index is an independent predictor of mortality over long-term follow-up in infective endocarditis. Int J Cardiol. 2013;168:5243–5248. doi: 10.1016/j.ijcard.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N, Lin Q, Jiang H, Zhu H. Age-adjusted Charlson Comorbidity index as effective predictor for in-hospital mortality of patients with cardiac arrest: A retrospective study. BMC Emerg Med. 2023;23(7) doi: 10.1186/s12873-022-00769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data used to support the findings of this study were acquired from the Monitoring in Intensive Care Database IV version 2.0 (MIMIC-IV v.2.0). Although the database is publicly and freely available, users must complete the National Institutes of Health's web-based course known as Protecting Human Research Participants to apply for permission to access the database. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.