Abstract
Background & Objectives
Methotrexate (MTX) is commonly used to manage psoriasis. The drug has erratic absorption characteristics and shows several complications. The present study uses different experimental models to evaluate the solid-lipid nanoparticles of MTX (SLN-MTX) for the anti-psoriatic effect.
Methods
A prepared SLN-MTX formulation was used and its permeability studies were conducted on Wistar rat abdominal skin. The organ-level distribution of the drug in the formulation was tested in mice and the in-vitro anti-psoriatic activity was determined in CL-177; XB-2 keratinocytes cell lines. The efficacy of SLN-MTX formulation was compared with standard MTX and marketed MTX preparations. The results are analyzed statistically using the student’s t-test.
Results
The data suggested that MTX from the formulation was slowly released and completely (80.36%) permeated through the skin. The flux and permeation data were found to be maximum for SLN-MTX compared to marketed and standard preparations. MTX in the formulation was found to be distributed more in the liver (67.5%) and kidney (2.34%). Further, SLN-MTX formulation showed dose-dependent inhibition on the growth of keratinocytes, and the cytotoxic concentration (CTC50) was found to be the least (518 mcg/ml).
Interpretation & Conclusion
The findings suggested that MTX in solid-lipid nanoparticles could be a promising formulation for the management of psoriasis since the drug was slowly released, progressively inhibited the growth of keratinocytes, and distributed mostly in organs meant for elimination. More studies in this direction might establish the precise safety and efficacy of SLN-MTX formulation in psoriasis.
Keywords: Solid-lipid nanoparticles, Methotrexate, Permeability, Distribution, Keratinocytes
1. Introduction
Psoriasis is a type of chronic skin disease that has affected more than 125 million people worldwide. The disease is considered not contagious and often seen in the middle-aged population (Armstrong et al., 2020). The precise cause of psoriasis is not known but is associated with the development of autoantibodies. A genetically programmed inflammation dysfunction driven by multiple immune system components has also been reported (Kamiya et al., 2019). The symptoms of the disease are due to abnormality in the formation of superficial skin layers, mainly in the palms and soles. About 10% of the patients diagnosed with psoriasis have been reported with other complications such as pain, inflammation, and tenderness of joints and tissues around joints (Amin et al., 2020).
Several classes of drugs have been tested and approved for psoriasis. Most of them target the immune cells, such as T-cells, and clusters of differentiation cells and neutralize the circulating cytokines responsible for the inflammatory process (Martins et al., 2020). Methotrexate (MTX) is one of the important constituents of anti-psoriatic therapy. The drug chemically is a 4-amino-4-deoxy-10-methypteroyl-L-glutamic acid (Kim et al., 2017).
MTX is known to exhibit its action by inhibiting the enzyme dihydrofolate reductase. This causes failure in converting folate to tetrahydrofolate, essential for synthesizing nucleic acid components. The action of MTX primarily results in the inhibition of DNA and cell replication and is highly toxic to rapidly multiplying cells such as bone marrow (Wang et al., 2018). MTX is a chemotherapeutic agent that treats different cancers, such as breast, lung, leukemia, and lymphoma. The drug is frequently utilized in auto-immune disorders because it suppresses both B and T-lymphocytes (Uddin et al., 2020). MTX is also popular for its anti-inflammatory action since it reduces lymphocyte proliferation, rheumatoid factor production, and leucocyte endothelial interaction and interferes with the release of several inflammatory cytokines (Alqarni and Zeidler, 2020). The non-significant variation in the clinical parameters associated with metabolic syndrome suggested that MTX could be a promising agent in autoimmune disease patients diagnosed with diabetes, hypertension, obesity and atherosclerosis (Dehpouri et al., 2019).
Systemic administration of MTX is associated with several complications, such as ulcerative stomatitis, leucopenia, hepatotoxicity, renal failure, bone marrow suppression, and vision defects (Pannu, 2019). Several consensuses are being studied for the biological dose reduction and to minimize the complications of MTX. However, these consensuses are debatable as the complications are reported to be observed once MTX achieves the therapeutic concentration in the patients (van Huizen et al., 2022). Topical administration of the drug can achieve a higher concentration at the disease site without exposing it to systemic toxicity. Since MTX is practically insoluble in water, the topical form of the drug has issues in penetration to the skin, where the drug’s action is most essential (Rajitha et al., 2017).
Nanotechnology is one of the advancement in the treatment of psoriasis. This mode of administration was reported to offer targeted drug delivery system. There are different varieties of nanotechnology colloidal carriers popular for treating psoriasis and one among them is the solid-lipid nanoparticles (Damiani et al., 2019). Solid lipid nanoparticles (SLNs), nanostructured lipid carriers, liposomes, niosomes, and transferosomes are some lipid-based nanoparticle techniques used for drug-targeted controlled delivery (Mirchandani and Patravale, 2021). Nanostructured lipid carrier is mostly preferred for solubilized drugs; liposomes have shortened half-life, while niosomes and transferosomes are associated with leakage of entrapped drugs (Kathe et al., 2014). Solid-lipid nanoparticles (SLN) include traditional colloidal carriers such as emulsions, liposomes, polymeric micro-, and nanoparticles (Scioli Montoto et al., 2020). Several applications of SLN have been reported, such as targeted delivery, controlled release, enhanced stability, lower toxicity of carriers, and the possibility of large-scale production, besides being useful for water-insoluble drugs (Rajpoot, 2019). Since methotrexate is not soluble in water, several attempts, such as nanogel formulation using the nanostructured lipid carrier’s technique, have been tried and were reported to be efficacious in attaining therapeutic concentration of MTX at the site of disease (Tripathi et al., 2018).
In another study, the SLN formulation of MTX gel was tested in-vivo and in-vitro for the treatment of psoriasis. This gel form of MTX was reported to have shown improved accumulation in human skin where drug effects are needed the most. However, the study did not identify the MTX accumulation in other body organs that might cause MTX-mediated toxicity (Agrawal et al., 2020). Considering these facts, the present study was undertaken to test a novel SLN formulation of MTX prepared by the oil-in-water micro-emulsion technique. The SLN formulation of MTX was evaluated for establishing the anti-psoriatic activity by determining the skin permeability characteristics, organ level distribution, and keratinocyte inhibitory effect.
2. Methods
2.1. Design of the study
The study was designed to evaluate the solid-lipid nanoparticle formulation of MTX for anti-psoriatic activity. Before designing the study protocol, literature review was conducted and the published articles from the indexed journals were screened for anti-psoriatic activity. Feasibility trials were conducted to perform different parameters related to anti-psoriatic activity in the laboratory, and based on this, the aim and objectives of the study were established.
2.2. Chemicals, reagents, and drugs
The chemicals and reagents used in the study were procured from the authorized supplier of the Institution. All the chemicals were of analytical grade and meant for research purposes. A pure sample of methotrexate (MTX) was obtained from Uni-Med Technologies Limited, India. Dulbecco’s modified eagles’ medium and other chemicals needed for the in-vitro studies were procured from Hi-Media, India. Reagents required for preparing the formulations of MTX were obtained from LOBA Chemie, India.
2.3. Animals
Swiss albino mice of either sex weighing 20–30 gm were used in the study. The animals were in-bred at the central animal house, Al Ameen College of Pharmacy (AACP), Bangalore. The animals were maintained with free access to food (standard pellet diet, Amrut feed, Bangalore) and water at room temperature. Albino rats’ Wistar strain was used for the permeability studies. The skins were obtained from anesthetized rats sacrificed for other experiments and utilized for the permeability studies. All experiments were conducted as per the guidelines and after approval from Institution Animal Ethics Committee (AACP/IAEC/M−47/2006).
2.4. Preparation of SLN-MTX formulation
The formulations of MTX were prepared in the Department of Pharmaceutics, Al-Ameen College of Pharmacy, India. In brief, the procedure includes the first dissolution of solid lipids. The solid lipid was heated above the melting point and gradually added to MTX with continuous stirring until a clear molten lipid was obtained. Further, the micro-emulsion technique was adopted for the preparation of solid-lipid nanoparticles. MTX in solid-lipid form is melted with glyceryl monostearate (1 % w/w), egg lecithin (0.5 % w/w), and co-surfactants (tween 80 – 1 % w/w). Constant stirring was done during the addition. The warm emulsion was then carefully added dropwise into ice-cold water. The ratio between dispersion and micro-emulsion medium was 10:1. The physicochemical characteristics such as particle size, particle shape, entrapment efficiency, stability, and zeta potential of the formulation were determined (Mulla et al., 2009).
2.5. Permeability studies
2.5.1. Preparation of standard MTX solution
The standard solution of methotrexate was prepared by dissolving 10 mg of methotrexate in 10 ml of dimethyl sulfoxide (DMSO) of pH 7.4 and further diluted with phosphate buffer solution (PBS), according to the dosage requirement.
2.5.2. Preparation of skin sample
Wistar rat skin from the abdominal region was collected and washed thoroughly in distilled water. The skin was first dipped in a robust ammonia solution to remove fur, and it was then washed with distilled water until all the ammonia was removed. The skin thus prepared was placed immediately in PBS (pH 7.4) and was used for the permeability study (Liu et al., 2020).
2.5.3. Permeation studies for different formulations of MTX
Permeability studies were carried out using excised rat abdominal skin tied to a modified Franz diffusion cell having a surface area of 2.545 cm2, and 75 ml capacity was employed for permeation studies (Takeuchi et al., 2011). The diffusion cell consists of a donor compartment and a receptor compartment.
A volume of different MTX formulations containing 5 mg of Methotrexate was placed on the skin, which was tied to the mouth of the receptor compartment filled with phosphate buffer (pH 7.4). The cell contents were stirred with a magnetic stirrer at 37° C throughout the experiment. Samples were collected at regular time intervals from the receptor compartment’s side tube and immediately replaced with fresh buffer.
The concentrations of the drug present in the sample solutions were determined spectrophotometrically at 303 nm agains phosphate buffer blank. The data obtained from the above procedures were analyzed to obtain the cumulative release percentage. These data were used to get the drug concentration in the samples by the mathematical expression obtained from the standard graph by interpolation method. The following formula obtained the cumulative percentage release:
2.6. In-vivo studies
2.6.1. Preparation of stock and standard methotrexate solution using HPLC
The mobile phase was prepared by mixing phosphate buffer (pH 6.0) and acetonitrile in the ratio 92: 8. Using this, a stock solution of 1000 µg/ml was prepared by dissolving 10 mg of MTX in 10 ml with mobile phase [(phosphate buffer pH 6.0: Acetonitrile in (92:8 v/v)]. The solution was diluted with mobile phase as per the standard curve's dose (2.5 – 10 µg/ml) (Shi et al., 2017).
2.6.2. HPLC system and chromatographic conditions
The HPLC system consisted of 10 at Shimadzu-Spd10a detector (Shimadzu Corp., Kyoto, Japan) set at a wavelength of 307 nm (λmax). The samples were chromatographed on a reverse phase SS Wakosil II-C18 (250x4.6 mm, 5 µM) column. The mobile phase was prepared by mixing phosphate buffer (pH6.0) and acetonitrile in the ratio 92:8 and was filtered through a 0.45 µm Millipore filter. The flow rate was maintained at 1.4 ml/min, and the run time was set for 3.5 min under the experimental conditions with an injection volume of 100 µl (Feng et al., 2021).
The gradient elution was run as follows: 0.01–1 min, 95% A; 1–2 min, 95–40% A; 2–2.5 min, 40–95% A; 2.5–3.5, 95% A. The total run time was 3.5 min for each sample and the auto-sampler was set for 4 0C. Detection was performed using a mass spectrometer (4500 QQQ). Instrument control and data collection was conducted using Analyst software 1.6 (Zuben et al., 2018).
2.6.3. Drug treatments
Thirty healthy adult mice of either sex weighing 25 to 30 g were ramdomly selected and divided equally into three groups (ten animals in each group). Group 1 - received a single dose of the standard drug (STD-MTX), 20 mg/kg, orally. Group 2 - received a single dose of SLN-MTX equivalent to 20 mg/kg of MTX orally. Group 3 – received a single-dose of marketed MTX [(MKT-MTX), Methox, Generic drug)] equivalent to 20 mg/kg of MTX orally. Animals, after 24 h of drug treatment, were sacrificed under ether anesthesia and vital organs Viz. liver and kidney were isolated.
2.6.4. Calibration of methotrexate in plasma
0.5 ml of blood was collected from the retro-orbital bleeding method just before sacrifice in a glass tube containing sodium citrate (3.3%w/v) solution. All samples were centrifuged at 5000 rpm for 15 min at 4˚C to separate the plasma using a cold centrifuge. After collecting plasma, one volume (eg, 500 µl) of heparinized plasma and two volumes of acetonitrile were thoroughly mixed to form a uniform solution. The deproteinized supernatant mixture was transferred into a glass tube and added four chloroform volumes. The aqueous supernatant obtained by this process was collected and used to estimate the drug concentration (Chaigneau et al., 2021).
The above procedure was followed for the spiked plasma samples with concentrations of 2.5 – 10 µg/mL of MTX to evaluate the drug in the sample. Each sample containing 100 µl was injected, and the effluent was monitored at 307 nm. The AUC or chromatogram values were plotted against concentration to obtain the standard graph of methotrexate in plasma. The procedure was repeated three times, and the average values of the AUC or chromatogram obtained were calculated. The data was then subjected to regression analysis, and regression coefficients were calculated.
2.6.5. Extraction and isolation of methotrexate from the isolated organs
Extraction and isolation of methotrexate from the isolated organs were carried out by adding 1 ml of 10% w/v homogenate of liver and kidney to 0.4 ml of 1 M perchlorate solution followed by centrifugation at 10,000 rpm for 10 min at 4 °C (Giulio et al., 2022). The supernatant (100 µl) was neutralized with 25 µl of 3 M dipotassium hydrogen phosphate. The resultant sample solution was subjected to HPLC, wherein the drug was eluted using a mobile phase consisting of acetonitrile and phosphate buffer (pH 6.0).
2.6.6. Calculation
The AUC or chromatogram obtained for the two organ homogenates was used to determine the drug concentration accumulated in the organs, which was calculated from the regression coefficient for standard methotrexate in plasma (Joerger et al., 2010).
2.7. In-vitro studies
2.7.1. Dulbecco’s modified eagles medium (DMEM)
The DMEM was prepared as per the procedure described in the literature. The medium was filtered in sterile glass bottles and tested for sterility by inoculating 1 ml each in Nutrient broth, Soybean Casein Digest broth, Fluid Thioglycolate and Sabouraud Dextrose broth. Antibiotics (Penicillin 100 units/ml, Streptomycin 100 µg/ml, and Amphotericin-B 5 µg/ml) were added to the sterile medium aseptically and stored at 4 °C until use. Further, newborn calf serum was added into the medium to achieve 10% growth and 2 % maintenance of the cells (Fatimah et al., 2013).
2.7.2. Preparation of methotrexate dilution for in-vitro anti-psoriatic study
Preparation of stock solution of methotrexate formulations was carried out by weighing 10 mg of the drug and diluting with 1 ml of dimethyl sulfoxide (DMSO). The volume was made up to 10 ml with diluent (2% serum with DMEM media). Different concentrations of the MTX formulation, such as 7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 mcg/ml, were used for the study.
2.7.3. Procedure for in-vitro study
The monolayer cell line was CL-177™ (ATCC® Number); designations were XB-2 keratinocytes epithelial. The study was conducted as per the procedure described. The aseptic technique was maintained using laminar flow while experimenting (Adwent et al., 2020). The monolayer cell culture was trypsinized, and the cell count was adjusted to 0.5–1.0 X 105 cells/ml using a medium containing 10% newborn calf serum. To each well of the 96-well microtiter plate, 0.1 ml of the diluted cell suspension (approximately 10,000 cells) was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off and washed once, and 100 µl of different drug concentrations was added to the cells in microtitre plates. The plates were incubated at 37 °C for 3 days in a 5% CO2 atmosphere. After 72 h, 50 µl of 50% trichloro acetic acid was added to the wells gently to form a thin layer over the drug dilutions and to achieve an overall concentration of 10%. The plates were incubated at 4 °C for one hour.
The plates were flicked and washed five times with distilled water to remove traces of medium, drug, and serum and were then air-dried. The air-dried plates were stained with sulphorhodamine B for 30 min. The unbound dye was removed by rapidly washing it with 1% acetic acid four times. The plates were then air-dried. 100 µl of 10 mM tris base was added to the wells to solubilize the dye. The plates were shaken vigorously for 5 min. The absorbance was measured using a microplate reader at a wavelength of 540 nm and calculated by the formula given below:
% Growth inhibition = 100 – (Mean OD of individual test group / Mean OD of the control group) X 100.
The absorbance was measured using an ELISA reader, and the average and the percentage growth inhibition were calculated. The CTC50 was also calculated graphically using the drug concentration in µg/ml against the percentage growth inhibition.
2.8. Statistical analysis
Statistical analysis was performed with SPSS 20.0 software package. Results are expressed as the mean ± standard deviation (X ± SD). The obtained data was analyzed using the Student’s t-test and analysis of variance (ANOVA).
3. Results
3.1. Permeability studies
The cumulative release characteristics of different formulations of MTX indicated a time-dependent release of the drug when the standard MTX (STD-MTX) preparation was tested. At 6 h (50% of tested time duration) interval, a cumulative concentration of 4.082 mcg was released, and at 12 h, the concentration was found to be 4.69 mcg. The data for SLN-MTX indicated a moderate level of MTX release. At 6 h interval, the cumulative amount of drug released was 2.84 mcg, which became 3.993 mcg at 12 h. MKT-MTX exhibited a slower release pattern. At 6 h, the amount released was found to be 1.996 mcg; at 12 h, it was 3.867 mcg/ml (Fig. 1).
Fig. 1.
Cumulative amount release of different formulations of MTX Values are represented as Mean ± SE.
The Fig. 2 represents the cumulative release percentage of MTX from different formulations when tested in rat skin. The STD-MTX preparation at 30 min showed 17.58% of release, which became 34.2% at 1 h and 53.2% at 2 h. At 6 h, about 81% of the drug was released, and at 12 h, the cumulative percentage was 93.8%. SLN-MTX formulation at 30 min showed 7.15% release that rose to 15.3% at 1 h and 37.5% at 2 h. About 50% of the drug was observed to be released between 4 and 6 h, and at 12 h, the percentage was found to be 80.26%. On the other hand, MKT-MTX released 17.3% at 30 min; about 50% of the drug was released after 8 h, and at 12 h, 77.34% of MTX was released from the preparation.
Fig. 2.
Cumulative percentage release of different formulations of MTX.
Values are represented as Mean ± SE.
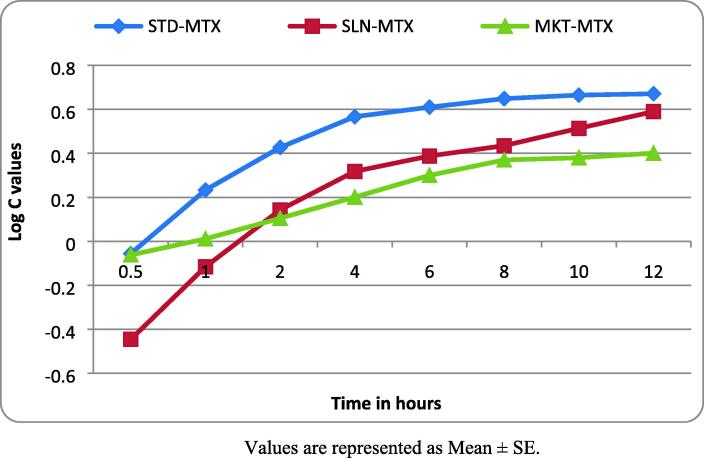
The log C of MTX released from different formulations indicated that STD-MTX at 30 min showed a negative value (- 0.053), and it became 0.233 at 1 h. At 6 h, the log C value was 0.61; at 12 h, it was 0.671. SLN-MTX was found to show a negative value at 30 min (- 0.446) and 1 h (- 0.116). At 6 h, the values rose to 0.388; at 12 h, it was 0.59. Similar to STD-MTX, the log C values of MKT-MTX showed negative (- 0.062) only at 30 min and afterward increased progressively. At 6 h, the log C value for MKT-MTX was found to be 0.3; at 12 h, it was 0.401 (Fig. 3).
Fig. 3.
Log C values of MTX release at different time intervals Values are represented as Mean ± SE.
The permeation parameters recorded for different formulations of MTX are represented in Table 1. The data analysis indicated that SLN-MTX has a significant (P < 0.05) more drug release than STD-MTX. The steady-state flux (Jss) and permeability coefficient (Kp) values were also found to be high for SLN-MTX when compared with STD-MTX and MKT-MTX. The Jss and Kp values recorded for SLN-MTX were 149.48 and 8.01, respectively.
Table 1.
Analysis of permeation parameters recorded for different MTX formulations against time.
| Type of MTX formulation |
Permeation parameters |
|||
|---|---|---|---|---|
| Drug release (µg/cm2) | Jss (µg/cm2/h) | Kp (cm/h) | tL (h) | |
| STD-MTX | 643.12 ± 36.19 | 137.02 ± 10.31 | 6.03 ± 0.32 | 0.34 |
| SLN-MTX | 809.35 ± 41.70* | 149.48 ± 9.73 | 8.01 ± 0.40 | 0.38 |
| MKT-MTX | 679.63 ± 38.40 | 139.23 ± 9.05 | 6.20 ± 0.35 | 0.35 |
Note. STD-MTX – Standard methotrexate, SLN-MTX – Solid lipid nanoparticles of methotrexate and MKT-MTX – Marketed methotrexate preparation. Jss – Steady state flux, Kp – Permeability coefficient, tL – Lag time. Values are represented as Mean ± SD, Statistics. One way ANOVA. * P < 0.05 compared with STD-MTX.
3.2. In-vivo studies
The linearity graph plotted for different concentrations of MTX versus the area of the chromatogram is represented in Fig. 4. The observation from the graph indicated a direct relationship between the concentration and area of the chromatogram, which is represented as a straight line.
Fig. 4.
Linearity graph of MTX in plasma Values are represented as Mean ± SE.
MTX distribution in the liver and kidney when different formulations were tested is represented in Table 2. The data indicated that administration of SLN-MTX formulation resulted in the highest drug concentration in the liver and kidney. The MKT-MTX formulation showed the second-highest distribution of MTX in the liver, but the drug concentration in the kidney was the lowest among the three. Furthermore, the STD-MTX formulation resulted in the second-highest distribution of the drug in the kidney. Still, the drug concentration in the liver was the least among the three MTX preparations.
Table 2.
Drug distribution data of different formulations of MTX in liver and kidney.
| Type of MTX formulation | Organs | Area under curve (mmol/L) | Quantity of drug distribution (mcg/ml) | Percentage of drug distribution |
|---|---|---|---|---|
| STD-MTX | Liver | 460465 ± 233.69 | 157.21 ± 6.48 | 35.73 ± 4.98 |
| Kidney | 263127 ± 197.64 | 9.87 ± 1.95 | 2.24 ± 0.09 | |
| SLN-MTX | Liver | 864055 ± 326.84 | 297.04 ± 8.95 | 67.5 ± 5.99 |
| Kidney | 274662 ± 226.84 | 10.3 ± 2.06 | 2.34 ± 0.08 | |
| MKT-MTX | Liver | 552698 ± 175.64 | 188.96 ± 7.95 | 50.34 ± 4.73 |
| Kidney | 269841 ± 221.26 | 8.84 ± 3.94 | 2.02 ± 0.05 |
Values are represented as Mean ± SE.
3.3. In-vitro studies
Fig. 5 represents the percentage growth inhibition of different MTX formulations against CL-177; XB-2 keratinocytes. A dose-dependent inhibition of keratinocytes’ growth was observed when the different concentration of STD-MTX was tested. The formulation up to 125 mcg/ml showed a mild to moderate level of keratinocyte growth inhibition, but a potent action was observed from 250 mcg/ml. The highest tested dose (1000 mcg/ml) produced 85.3% growth inhibition. In comparison, the SLN-MTX formulation showed a steady inhibition in the growth of keratinocytes when the doses were progressively increased. At 1000 mcg/ml, the formulation was observed to inhibit growth to 66.46%. When tested at different concentrations, MKT-MTX showed a dose-dependent inhibition of keratinocyte growth. The inhibition at 1000 mcg/ml was found to be 88.24% on the growth of keratinocytes.
Fig. 5.
Effect of different MTX formulations on keratinocytes growth Values are represented as Mean ± SE.
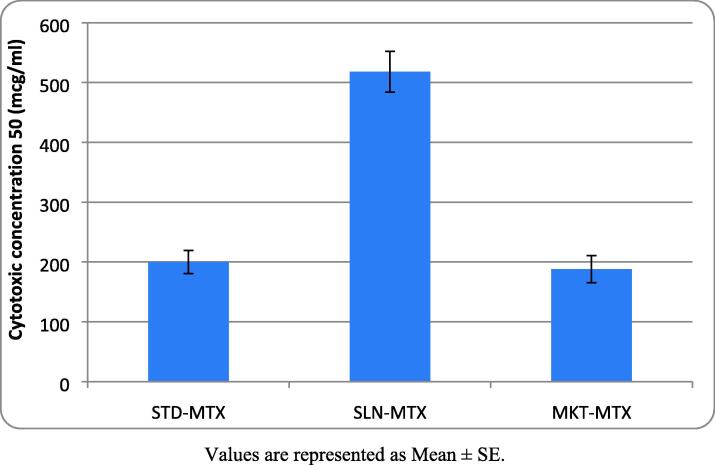
The cytotoxic concentration (CTC50) against CL-177; XB-2 keratinocytes when different MTX formulations were tested indicated that SLN-MTX has the highest CTC50 value (518 mcg/ml), followed by MKT-MTX (188 mcg/ml). The lowest CTC50 value was found with STD-MTX (200 mcg/ml) (Fig. 6 and Fig. 7).
Fig. 6.
CTC50 of different MTX formulations on keratinocytes Values are represented as Mean ± SE.
Fig. 7.
Methotrexate-treated keratinocytes and their percentage growth inhibition.
4. Discussion
The present study evaluated the anti-psoriatic activity of solid-lipid nanoparticles of methotrexate (SLN-MTX) using three models Viz., skin permeability assay, in-vivo organ distribution of drug and in-vitro anti-psoriatic activity on keratinocytes cell lines. The study compared the effect of SLN-MTX with standard MTX (STD-MTX) and marketed MTX (MKT-MTX). The observations from the study indicated that cumulative release (both amount and percentage) of SLN-MTX falls between STD-MTX and MKT-MTX. The STD-MTX was found to be rapidly released and completely (93.8%) permeated the drug through the skin. While MKT-MTX showed slow release, the permeability of MTX through the skin was found to be 77.3% (Fig. 1, Fig. 2). This data when correlated with the previous studies suggested that SLN-MTX formulation might had exhibited a slow and sustained release of the drug, essential for prolong action (Deng et al., 2022).
SLN is a type of controlled drug delivery system. Targeted therapeutic agent delivery to a specific body region is one of the most exciting and challenging fields in medical research (Mirchandani and Patravale, 2021). Nanoparticles present interesting properties such as small size, large surface area, and ability to modify their surface characteristics have several advantages. Primarily, SLN is aqueous colloidal dispersion-emulsion, and its matrix of it is comprised of biodegradable lipids. These formulations have advantages because they are stable, protect labile drugs from degradation, control release, and have better tolerability (Mendoza-Muñoz et al., 2021)
The permeability studies indicated that SLN-MTX formulation released the drug slowly and almost completely permeated (80.3%) (Fig. 1, Fig. 2). MTX’s permeability and release pattern follow first-order kinetics (Fig. 3). Skin permeability studies using rats are considered a vital tool for determining the permeation characteristic of the test compound. It provides basically similar features as observed with the human skin and are useful for testing the efficacy of topical preparations (Shinde et al., 2020). Permeability data analysis suggested that SLN-MTX has the highest drug release values, steady state flux, and permeability coefficient. A significant (P < 0.05) more release of MTX was observed with SLN-MTX compared to STD-MTX (Table 1). These observations indicated that SLN-MTX has slow, steady, and almost complete permeation of MTX. In earlier studies, these properties of a formulation have been attributed to better therapeutic outcomes (Mirchandani et al., 2021, Agrawal et al., 2020).
Another interesting finding of the present study is that SLN-MTX formulation, when administered to experimental mice, showed more distribution of MTX in the liver (67.5%) and kidney (2.34%). STD-MTX distribution in the liver and kidney was 35.73% and 2.24%, respectively. And the distribution of MKT-MTX in the liver was 50.34%, and in the kidney, is 2.02% (Table 2). Evaluating the drug distribution characteristics is important for understanding the pharmacokinetic profile of a compound such as MTX known to induce systemic complications in the patients (Luo et al., 2020). As reported, the metabolism of MTX mainly occurs in the liver through the enzyme called folylpolyglutamate synthetase to methotrexate polyglutamate. The gamma glutamyl hydrolase then hydrolyses the metabolite and re-converts back to methotrexate. Thus, over 80% of the systemically available MTX is excreted unchanged in the urine. A small fraction of MTX is also converted to 7-hydroxy methotrexate (Inoue and Yuasa, 2014). Another metabolite of MTX called 2,4-diamino-N (10)-methyl pteroic acid (DAMPA) is reported to be biologically active. However, this metabolite is excreted more rapidly than MTX and does not possess the cytotoxic property (Schofield et al., 2016). Earlier studies suggested that more availability of drugs in organs of metabolism and excretion is indirectly proportional to their availability in another part of the body. The preparations of drugs having such characteristics could cause lesser toxicity since the bioavailable drug might rapidly get metabolized in the liver and then excreted through the kidney (Li et al., 2021).
The in-vitro anti-psoriatic studies suggested that SLN-MTX formulation showed a dose-dependent inhibition on the growth of XB-2 keratinocytes. The available evidences from the literature suggest that in-vitro activity using keratinocytes establishes the precise role of a drug on the skin disorders such as psoriasis (Gao et al., 2020). Maximum inhibition was observed at 66.46% when 1000 mcg/ml of SLN-MTX was tested. STD-MTX showed slow inhibition at initial doses but beyond 250 mcg/ml, suddenly produced 80% inhibition, and at 1000 mcg/ml showed, 85.3% inhibition of keratinocytes’ growth. On the other hand, MKT-MTX showed progressive dose-dependent inhibition on the keratinocytes growth and, at 1000 mcg/ml, exhibited 88.24% inhibition (Fig. 5). The cytotoxicity concentration (CTC50) suggested that STD-MTX (200 mcg/ml) and MKT-MTX (188 mcg/ml) have similar values (Fig. 6) and are more likely to cause lethality of keratinocytes.
The observations further suggested that SLN-MTX formulation produced slow and steady inhibition on the growth of keratinocytes, and the potential to cause lethality is low (CTC50 = 518 mcg/ml) (Plate-1). The observed characteristics of SLN-MTX might help treat psoriasis, where the growth of normal cells will not be interfered with by administering anti-psoriatic medication (Zhang et al., 2018). These properties could be linked to the particle size, polydispersity index, entrapment efficacy, and zeta potential of MTX formulated as solid-lipid nanoparticles. The SLN-MTX formulation was also stable for one month when stored at 45 °C/75% humid conditions (Shankar et al., 2018). Keratinocytes has been utilized to study the mitochondria targeting activity. A study conducted in the past has suggested that compounds exhibiting such property has the potential to induce apoptosis and can be used to target tumors as well psoriasis (Li et al., 2021). Disruption in the activity of mitochondria can contribute in inflammation, ageing, metabolic and neurodegenerative diseases (Chodari et al., 2021). Besides, it is required to study the role of MTX on proteomics and genomics as well the electrochemical and optical biosensors known to modulate the functioning of several cells and reported to be involved in the pathophysiology of several disease states including auto-immunity (Eftekhari et al., 2019, Ahmadian et al., 2022).
MTX in earlier studies has been used in the treatment of psoriasis. The precise mechanism of action of MTX in psoriasis is not clear. Some of the possibilities reported include inhibiting DNA synthesis by affecting the dihydrofolate reductase essential for forming folic acid. Without folic acid, purines and pyrimidines, vital DNA components, are not synthesized (Alqarni and Zeidler, 2020). MTX appears to affect the S-phase of the cell cycle and inhibits cell growth, and causes cell death (apoptosis). MTX, by inhibiting DNA synthesis, limits epithelial hyperplasia, reinforces the apoptosis of activated T cells and prevents chemotaxis of neutrophils. Besides, MTX is also reported to prevent the synthesis of several pro-inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-1 (El-Esawy et al., 2022).
The research conducted so far could not precisely establish the cause and mechanism for psoriasis. Several environmental factors such as stress, infection, certain medications, alcohol and obesity have been linked as risks (Griffiths 2021). And among the mechanisms, epigenetic related changes like DNA methylation, histone modification and non-coding RNAs are implicated for psoriasis. These epigenetic changes are reported to alter the innate as well the adaptive immune cell differentiation, migration, proliferation and apoptosis, triggering several immune related inflammatory pathways observed in psoriasis (Dopytalska et al., 2021). Available evidences suggested that MTX treatment in rheumatoid arthritis patients influenced DNA methylation at multiple CpG sites, and modulated the level and functioning of CD4+ and memory T cells, previously identified as the possible mechanism for rheumatoid arthritis (Liszewska et al., 2020). Besides, studies have also indicated that MTX augmented the level of those microbiota in the gut known for reducing the inflammatory processes (Olejniczak-Staruch et al., 2021). Further, the role of bioactive metabolite of methotrexate (DAMPA) and the influence of the SLN-MTX formulation on different reported mechanism might present interesting findings on the efficacy of MTX in autoimmune diseases such as psoriasis.
5. Conclusion
The findings from the present study suggested promising effects of t solid-lipid nanoparticles of MTX in the management of psoriasis in the tested models. The formulation exhibited slow, sustained and targeted activity without getting accumulated in the different parts of the Body. MTX in solid-lipid particles could become a new therapeutic option for psoriasis devoid of major systemic complications. More research in this direction might authenticate the findings observed in this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to acknowledge the Researchers Supporting Project number (RSP2023R115), King Saud University, Riyadh, Saudi Arabia, for extending financial support to do this research project.
Funding
The authors are thankful to AlMaarefa University for providing support to do this research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Debarati Maiti, Email: qualityasdaq@gmail.com.
Mohammed Naseeruddin Inamdar, Email: inamdarn@gmail.com.
Mansour Almuqbil, Email: mmetwazi@ksu.edu.sa.
Sarasija Suresh, Email: sarasija_s@hotmail.com.
Syed Mohammed Basheeruddin Asdaq, Email: sasdaq@gmail.com, sasdag@mcst.edu.sa.
Sultan Alshehri, Email: sshehri.c@mcst.edu.sa.
Saad Ali Al Arfaj, Email: arfajs@ngha.med.sa.
Ali Musharraf Alamri, Email: emadfaiqa@gmail.com.
Meshal Meshary Aldohyan, Email: mmd.444@hotmail.com.
Misfir Theeb Alqahtani, Email: misfir_qh@hotmail.com.
Turki Mohammed Alosaimi, Email: researcher1822@gmail.com.
Sami Haran Alenazi, Email: S.am-5@hotmail.com.
Moneer E. Almadani, Email: researcher072022@gmail.comres, earcher072022@gmail.com.
Jameel Ahmed S. Mulla, Email: jameelahmed5@rediffmail.com.
Syed Imam Rabbani, Email: syedrabbani09@yahoo.com.
References
- Adwent I., Grabarek B.O., Kojs-Mrożkiewicz M., Brus R., Staszkiewicz R., Plewka A., Stasiowski M., Lyssek-Boroń A. The influence of Adalimumab and Cyclosporine A on the expression profile of the genes related to TGFβ signaling pathways in keratinocyte cells treated with Lipopolysaccharide A. Mediators Inflamm. 2020 Jul;26(2020):3821279. doi: 10.1155/2020/3821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y.O., Mahajan U.B., Mahajan H.S., Ojha S. Methotrexate-loaded nanostructured lipid carrier gel alleviates imiquimod-induced psoriasis by moderating inflammation: formulation, optimization, characterization, in-vitro and in-vivo studies. Int. J. Nanomed. 2020 Jul;7(15):4763–4778. doi: 10.2147/IJN.S247007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian E., Janas D., Eftekhari A., Zare N. Application of carbon nanotubes in sensing/monitoring of pancreas and liver cancer. Chemosphere. 2022 Sep;302 doi: 10.1016/j.chemosphere.2022.134826. [DOI] [PubMed] [Google Scholar]
- Alqarni A.M., Zeidler M.P. How does methotrexate work? Biochem. Soc. Trans. 2020 Apr 29;48(2):559–567. doi: 10.1042/BST20190803. [DOI] [PubMed] [Google Scholar]
- Amin M, Lee EB, Tsai TF, Wu JJ. Psoriasis and Co-morbidity. Acta Derm Venereol. 2020 Jan 30;100(3):adv00033 [DOI] [PMC free article] [PubMed]
- Armstrong A.W., Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020 May 19;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- Chaigneau T., Pallotta A., Benaddi F.Z., Sancey L., Chakir S., Boudier A., Clarot I. Monitoring of gold biodistribution from nanoparticles using a HPLC-visible method. Separations. 2021;8:215–227. [Google Scholar]
- Chodari L., Dilsiz Aytemir M., Vahedi P., Alipour M., Vahed S.Z., Khatibi S.M.H., Ahmadian E., Ardalan M., Eftekhari A. Targeting mitochondrial biogenesis with polyphenol compounds. Oxid. Med. Cell Longev. 2021 Jul;12(2021):4946711. doi: 10.1155/2021/4946711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani G., Pacifico A., Linder D.M., Pigatto P.D.M., Conic R., Grada A., Bragazzi N.L. Nanodermatology-based solutions for psoriasis: state-of-the art and future prospects. Dermatol. Ther. 2019 Nov;32(6):e13113. doi: 10.1111/dth.13113. [DOI] [PubMed] [Google Scholar]
- Dehpouri T., Rokni G.R., Narenjbon N.A., Goldust M., Yamauchi P.S., Wollina U., et al. Evaluation of the glycemic effect of methotrexate in psoriatic arthritis patients with metabolic syndrome: a pilot study. Dermatol. Rep. 2019 May 9;11(1):7965. doi: 10.4081/dr.2019.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Gu J., Jiang Z., Cao Y., Mao F., Xue Y., Wang J., Dai K., Qin L., Liu K., Wu K., He Q., Cai K. Application of nanotechnology in the early diagnosis and comprehensive treatment of gastrointestinal cancer. J. Nanobiotechnol. 2022 Sep 15;20(1):415. doi: 10.1186/s12951-022-01613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopytalska K., Ciechanowicz P., Wiszniewski K., Szymańska E., Walecka I. The role of epigenetic factors in psoriasis. Int. J. Mol. Sci. 2021 Aug 27;22(17):9294. doi: 10.3390/ijms22179294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A., Ahmadian E., Salatin S., Sharifi S., Dizaj S.M., Khalilov R., Hasanzadeh M. Current analytical approaches in diagnosis of melanoma. TrAC Trends Anal. Chem. 2019;116:122–135. [Google Scholar]
- El-Esawy F.M., Ahmed I.A., El-Fallah A.A., Salem R.M. Methotrexate mechanism of action in plaque psoriasis: something new in the old view. J. Clin. Aesthet. Dermatol. 2022 Aug;15(8):42–46. [PMC free article] [PubMed] [Google Scholar]
- Fatimah S.S., Tan G.C., Chua K., Tan A.E., Nur Azurah A.G., Hayati A.R. Effects of keratinocyte growth factor on skin epithelial differentiation of human amnion epithelial cells. Burns. 2013 Aug;39(5):905–915. doi: 10.1016/j.burns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Feng Z., Gao J., Gao X., Hua L., Nie X., Sun Y., Wang M. A validated HPLC-MS/MS method for quantification of methotrexate and application for therapeutic drug monitoring in children and adults with Non-Hodgkin Lymphoma. Drug Des. Devel. Ther. 2021 Nov;5(15):4575–4583. doi: 10.2147/DDDT.S335122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Chen F., Fang H., Mi J., Qi Q., Yang M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol. Res. 2020 Oct 20;53(1):48. doi: 10.1186/s40659-020-00316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulio L., Padula C., Pescina S., Nicoli S., Santi P. Development and validation of a HPLC-UV based method for the extraction and quantification of methotrexate in the skin. Biomed. Chromatogr. 2022 May;36(5):e5349. doi: 10.1002/bmc.5349. [DOI] [PubMed] [Google Scholar]
- Griffiths C.E.M., Armstrong A.W., Gudjonsson J.E., Barker J.N.W.N. Psoriasis. Lancet. 2021 Apr 3;397(10281):1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- Inoue K., Yuasa H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab. Pharmacokinet. 2014;29(1):12–19. doi: 10.2133/dmpk.dmpk-13-rv-119. [DOI] [PubMed] [Google Scholar]
- Joerger M., Huitema A.D., Krähenbühl S., Schellens J.H., Cerny T., Reni M., Zucca E., Cavalli F., Ferreri A.J. Methotrexate area under the curve is an important outcome predictor in patients with primary CNS lymphoma: a pharmacokinetic-pharmacodynamic analysis from the IELSG no. 20 trial. Br. J. Cancer. 2010 Feb 16;102(4):673–677. doi: 10.1038/sj.bjc.6605559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K., Kishimoto M., Sugai J., Komine M., Ohtsuki M. Risk factors for the development of psoriasis. Int. J. Mol. Sci. 2019 Sep 5;20(18):4347. doi: 10.3390/ijms20184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathe N., Henriksen B., Chauhan H. Physicochemical characterization techniques for solid lipid nanoparticles: principles and limitations. Drug Dev. Ind. Pharm. 2014 Dec;40(12):1565–1575. doi: 10.3109/03639045.2014.909840. [DOI] [PubMed] [Google Scholar]
- Kim W.B., Jerome D., Yeung J. Diagnosis and management of psoriasis. Can. Fam. Phys. 2017 Apr;63(4):278–285. [PMC free article] [PubMed] [Google Scholar]
- Li N., Li X., Cheng P., Yang P., Shi P., Kong L., Liu H. Preparation of Curcumin solid lipid nanoparticles loaded with flower-shaped lactose for lung inhalation and preliminary evaluation of cytotoxicity in vitro. Evid. Based Complement. Alternat. Med. 2021 Oct;28(2021):4828169. doi: 10.1155/2021/4828169. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li C., Liu W., Wang F., Hayashi T., Mizuno K., Hattori S., Fujisaki H., Ikejima T. DNA damage-triggered activation of cGAS-STING pathway induces apoptosis in human keratinocyte HaCaT cells. Mol. Immunol. 2021 Mar;131:180–190. doi: 10.1016/j.molimm.2020.12.037. [DOI] [PubMed] [Google Scholar]
- Liszewska A., Robak E., Bernacka M., Bogaczewicz J., Woźniacka A. Methotrexate use and NAD+/NADH metabolism in psoriatic keratinocytes. Postepy Dermatol. Alergol. 2020 Feb;37(1):19–22. doi: 10.5114/ada.2020.93379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Qu X., Song L., Shang R., Wan X., Fang L. Investigation on the effect of deep eutectic formation on drug-polymer miscibility and skin permeability of rotigotine drug-in-adhesive patch. Int. J. Pharm. 2020 Jan;25(574) doi: 10.1016/j.ijpharm.2019.118852. [DOI] [PubMed] [Google Scholar]
- Luo Y., Gong C., Wei M., Chen Y., Song T., Wu C., Mo L., Zhang J. Evaluation of Mogroside V as a promising carrier in drug delivery: improving the bioavailability and liver distribution of Silybin. AAPS PharmSciTech. 2020 Apr 26;21(4):123. doi: 10.1208/s12249-020-01645-9. [DOI] [PubMed] [Google Scholar]
- Martins A.M., Ascenso A., Ribeiro H.M., Marto J. Current and future therapies for psoriasis with a focus on serotonergic drugs. Mol. Neurobiol. 2020 May;57(5):2391–2419. doi: 10.1007/s12035-020-01889-3. [DOI] [PubMed] [Google Scholar]
- Mendoza-Muñoz N., Urbán-Morlán Z., Leyva-Gómez G., Zambrano-Zaragoza M.L., Piñón-Segundo E., Quintanar-Guerrero D. Solid lipid nanoparticles: an approach to improve oral drug delivery. J. Pharm. Pharm. Sci. 2021;24:509–532. doi: 10.18433/jpps31788. [DOI] [PubMed] [Google Scholar]
- Mirchandani Y, Patravale VB, S B. Solid lipid nanoparticles for hydrophilic drugs. J Control Release. 2021 Jul 10;335:457-464 [DOI] [PubMed]
- Mulla J.A., Suresh S., Khazi I.A. Formulation, characterization and in-vitro evaluation of methotrexate solid-lipid nanoparticles. Res. J. Pharm. Tech. 2009;2:685–689. [Google Scholar]
- Olejniczak-Staruch I., Ciążyńska M., Sobolewska-Sztychny D., Narbutt J., Skibińska M., Lesiak A. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. Int. J. Mol. Sci. 2021 Apr 13;22(8):3998. doi: 10.3390/ijms22083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu A.K. Methotrexate overdose in clinical practice. Curr. Drug Metab. 2019;20(9):714–719. doi: 10.2174/1389200220666190806140844. [DOI] [PubMed] [Google Scholar]
- Rajitha P., Biswas R., Sabitha M., Jayakumar R. Methotrexate in the treatment of psoriasis and rheumatoid arthritis: mechanistic insights, current issues and novel delivery approaches. Curr. Pharm. Des. 2017;23(24):3550–3566. doi: 10.2174/1381612823666170601105439. [DOI] [PubMed] [Google Scholar]
- Rajpoot K. Solid lipid nanoparticles: a promising nanomaterial in drug delivery. Curr. Pharm. Des. 2019;25(37):3943–3959. doi: 10.2174/1381612825666190903155321. [DOI] [PubMed] [Google Scholar]
- Schofield R.C., Ramanathan L.V., Murata K., Fleisher M., Pessin M.S., Carlow D.C. Development of an assay for methotrexate and its metabolites 7-hydroxy methotrexate and DAMPA in serum by LC-MS/MS. Methods Mol. Biol. 2016;1383:213–222. doi: 10.1007/978-1-4939-3252-8_23. [DOI] [PubMed] [Google Scholar]
- Scioli Montoto S., Muraca G., Ruiz M.E. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020 Oct;30(7) doi: 10.3389/fmolb.2020.587997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar R., Joshi M., Pathak K. Lipid nanoparticles: a novel approach for brain targeting. Pharm. Nanotechnol. 2018;6(2):81–93. doi: 10.2174/2211738506666180611100416. [DOI] [PubMed] [Google Scholar]
- Shi H., Hou C., Gu L., Wei Z., Xing H., Zhang M., Wang S., Zhao L., Bi K., Chen X. Influence of pretreatment of piperazine ferulate on pharmacokinetic parameters of methotrexate in methotrexate-induced renal injury model rats by HPLC-MS. Asian J. Pharm. Sci. 2017 Mar;12(2):202–208. doi: 10.1016/j.ajps.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde M., Bali N., Rathod S., Karemore M., Salve P. Effect of binary combinations of solvent systems on permeability profiling of pure agomelatine across rat skin: a comparative study with statistically optimized polymeric nanoparticles. Drug Dev. Ind. Pharm. 2020 May;46(5):826–845. doi: 10.1080/03639045.2020.1757697. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Mano Y., Terasaka S., Sakurai T., Furuya A., Urano H., Sugibayashi K. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp. Anim. 2011;60(4):373–384. doi: 10.1538/expanim.60.373. [DOI] [PubMed] [Google Scholar]
- Tripathi P., Kumar A., Jain P.K., Patel J.R. Carbomer gel bearing methotrexate loaded lipid nanocontainers shows improved topical delivery intended for effective management of psoriasis. Int. J. Biol. Macromol. 2018 Dec;120(Pt A):1322–1334. doi: 10.1016/j.ijbiomac.2018.08.136. [DOI] [PubMed] [Google Scholar]
- Uddin N., Ahmed S., Khan A.M., Mazharol Hoque M., Halim M.A. Halogenated derivatives of methotrexate as human dihydrofolate reductase inhibitors in cancer chemotherapy. J. Biomol. Struct. Dyn. 2020 Feb;38(3):901–917. doi: 10.1080/07391102.2019.1591302. [DOI] [PubMed] [Google Scholar]
- van Huizen A.M., Menting S.P., Gyulai R., Iversen L., van der Kraaij G.E., Middelkamp-Hup M.A., et al. International eDelphi study to reach consensus on the methotrexate dosing regimen in patients with psoriasis. JAMA Dermatol. 2022 May 1;158(5):561–572. doi: 10.1001/jamadermatol.2022.0434. [DOI] [PubMed] [Google Scholar]
- Wang W., Zhou H., Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur. J. Med. Chem. 2018 Oct;5(158):502–516. doi: 10.1016/j.ejmech.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zi Z., Lee E.E., Zhao J., Contreras D.C., South A.P., Abel E.D., Chong B.F., Vandergriff T., Hosler G.A., Scherer P.E., Mettlen M., Rathmell J.C., DeBerardinis R.J., Wang R.C. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat. Med. 2018 May;24(5):617–627. doi: 10.1038/s41591-018-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuben E.S.V., Oliveira A.G., Chorilli M., Scarpa M.V. Development and validation of a rapid reverse-phase HPLC method for the determination of methotrexate from nanostructured liquid crystalline systems. Pharmazie. 2018 Mar 5;73(3):128–132. doi: 10.1691/ph.2018.7140. [DOI] [PubMed] [Google Scholar]