Abstract
Background.
The objective of this study was to determine the impact of caudate resection on margin status and outcomes during resection of extrahepatic hilar cholangiocarcinoma.
Methods.
A database of 1,092 patients treated for biliary malignancies at institutions of the Extrahepatic Biliary Malignancy Consortium was queried for individuals undergoing curative-intent resection for extrahepatic hilar cholangiocarcinoma. Patients who did versus did not undergo concomitant caudate resection were compared with regard to demographic, baseline, and tumor characteristics as well as perioperative outcomes.
Results.
A total of 241 patients underwent resection for a hilar cholangiocarcinoma, of whom 85 underwent caudate resection. Patients undergoing caudate resection were less likely to have a final positive margin (P = .01). Kaplan-Meier curve of overall survival for patients undergoing caudate resection indicated no improvement over patients not undergoing caudate resection (P = .16). On multivariable analysis, caudate resection was not associated with improved overall survival or recurrence-free survival, although lymph node positivity was associated with worse overall survival and recurrence-free survival, and adjuvant chemoradiotherapy was associated with improved overall survival and recurrence-free survival.
Conclusion.
Caudate resection is associated with a greater likelihood of margin-negative resection in patients with extrahepatic hilar cholangiocarcinoma. Precise preoperative imaging is critical to assess the extent of biliary involvement, so that all degrees of hepatic resections are possible at the time of the initial operation.
Hilar cholangiocarcinoma represents a relatively rare entity with an incidence of approximately 3,000 cases per year in the United States.1 Patients with hilar cholangiocarcinoma often present with locally advanced or metastatic disease, resulting in a high mortality rate. Among patients with resectable disease, treatment typically consists primarily of resection with or without pre- and/or postoperative radiochemotherapy.2–4 Traditionally, resection has involved anatomic liver resection based on the location of the tumor together with en bloc caudate lobe resection.3,5 Proponents of caudate resection have argued that caudate resection maximizes the likelihood of achieving negative margins, which has been independently associated with improved recurrence-free survival (RF) and overall survival (OS).3,6–8
Although the rates of curative hepatectomy for malignancy have increased, caudate resection remains a relatively uncommon operation.9 Inclusion of a caudate resection with a major hepatectomy can prove challenging, particularly in the setting of left-sided liver resections, and also increase operative morbidity.9 Given these factors, the routine inclusion of caudate resection in treatment for hilar cholangiocarcinoma has been challenged.2
To date, there have been no large studies that have investigated specifically the association between caudate lobe resection for hilar cholangiocarcinoma of all types and operative margins or outcomes. Therefore, the objective of the present study was to determine whether caudate resection was associated with improved ability to achieve a margin-negative resection, as well as assess whether caudate resection resulted in improved RFS and OS.
Methods
Patient and variable selection
A multi-institutional database of 1,092 patients treated for biliary malignancies at institutions of the Extrahepatic Biliary Malignancy Consortium (USEBMC) (Emory University; New York University; Johns Hopkins University; Ohio State University; Stanford University; University of Louisville; University of Wisconsin; Vanderbilt University; Wake Forest University; Washington University in St. Louis) was queried for patients undergoing successful resections with curative intent for extrahepatic hilar cholangiocarcinoma between January 1, 2000 and May 30, 2015. Patient contributions by each institution are listed in Supplemental Table S1a with the number of cases per year listed in Supplemental Table S1b. The study was approved by the institutional review boards from each participating institution. Patients were categorized according to whether or not they underwent caudate resection as part of operative intervention. Caudate resection included patients undergoing formal resection of the caudate lobe as well as patients undergoing paracaval caudate resection. The decision to perform caudate resection was at the discretion of each institution and each operating surgeon. There was no defined algorithm to dictate caudate resection. Groups were compared along standard demographic and clinicopathologic variables as well as perioperative and outcomes parameters.
Across institutions, resection margin was defined using the final pathology report. Negative margins were defined as the lack of both macroscopic and microscopic disease at the final resection margin. Anatomic locations of all final positive margins were also identified. Severity of postoperative complication were classified using the Clavien-Dindo scale and were followed and recorded for 90 days postresection. Sequential follow up included triphasic computed tomography (CT) or dynamic magnetic resonance imaging (MRI) with CA 19–9 and/or positron emission tomography when indicated every 3 months for the first year and every 6 months thereafter. Progression-free survival (PFS) was defined as the time to development of local recurrence, distant recurrence, or both as diagnosed on postoperative imaging. OS was defined as time to death or last follow-up from time of resection.
Statistical analysis
Patients undergoing resection with and without inclusion of the caudate lobe were compared across the previously stated variables. Primary outcomes of interest were the presence and location of positive final margins, RFS, disease-specific survival, and OS. Differences between the 2 groups were evaluated using 2-tailed t tests. Kaplan-Meier curves were constructed using log-rank analysis after controlling for patient demographic and tumor characteristics to assess the effect of caudate resection on PFS, OS, and disease-specific survival. Patients lost to follow-up were censored at the time of last follow-up. These patients were coded as dead or alive and with or without evidence of disease progression based on the information available at last follow-up. Patients were not excluded from survival analysis if they were missing information regarding disease status at last follow-up or whether or not they developed recurrence. To evaluate the effect of caudate resection on survival, multivariable analysis using Cox proportional hazard models was performed to assess for effects of tumor and treatment-related factors, including caudate resection, on RFS and OS. Statistics were calculated using JMP software (SAS Institute, Inc, Cary, NC).
Results
Demographic and tumor characteristics
Of the 1,092 patients in the EBMC database, the 256 patients who underwent resection with curative intent for hilar cholangiocarcinoma were identified. Of the 256, 90 underwent resection including caudate resection and 166 underwent resection without caudate resection. Average age was 64 and 66 years, respectively, and average body mass index in both groups was approximately 26. Groups did not differ significantly with respect to baseline laboratory values or incidence of ascites or jaundice. There was also no significant difference in the proportion of patients who had undergone preoperative endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiogram, or portal vein embolization (Table 1). Regarding the type of operation performed in patients, patients undergoing caudate resection were less likely to undergo simple bile duct resection (0% vs 33%, P < .001) and more likely to undergo a left-sided liver resection than those not undergoing caudate resection (60% vs 26%; P < .001). There was no difference in the proportion of patients in each group undergoing right-sided liver resection. They were also more likely to undergo vascular resection (24% vs 8%, P < .001), particularly portal vein resection (16% vs 4%, P = .003), and distant nodal sampling (27% vs 13%, P = .006). The groups did not differ with respect to total number of lymph nodes retrieved.
Table 1.
Baseline patient, operative, and tumor characteristic*.
| Caudate resection (n = 90) | No caudate resection (n=166) | P value | |
|---|---|---|---|
|
| |||
| Male sex | 47 (52%) | 104(63%) | .11 |
| Age | 64 ± 11 | 66 ± 11 | .27 |
| BMI | 25.8 ± 5.4 | 26.5 ± 5.7 | .42 |
| Preoperative total bilirubin (mg/dL) | 3.0 ± 3.1 | 3.8 ± 5.5 | .23 |
| Albumin (g/dL) | 3.4 ± 0.7 | 3.3 ± 0.7 | .44 |
| Ascites | 3 (3%) | 6 (4%) | 1.00 |
| Jaundice | 74 (82%) | 130(79%) | .52 |
| ERCP | 71 (79%) | 122(73%) | .37 |
| PTC | 38 (42%) | 80 (48%) | .43 |
| Portal vein embolization | 6 (7%) | 10(6%) | .79 |
| Type of resection | |||
| Bile duct resection | 0(0%) | 55 (33%) | <.001 |
| Cholecystectomy only | 0(0%) | 1(1%) | 1.00 |
| Radical cholecystectomy + portal lymph node dissection | 0(0%) | 4(2%) | .30 |
| Right hepatectomy | 7 (8%) | 23(14%) | .16 |
| Left hepatectomy | 28 (31%) | 32(19%) | .04 |
| Extended right hepatectomy | 15(l7%) | 24(14%) | .72 |
| Extended left hepatectomy | 13(14%) | 6 (4%) | .003 |
| Right trisectionectomy | 13(14%) | 12(7%) | .08 |
| Left trisectionectomy | 13(14%) | 5 (3%) | .001 |
| Pancreaticoduodenectomy | 0(0%) | 3(2%) | .55 |
| Other | 1(1%) | 1(1%) | 1.00 |
| Right hepatectomy, extended right hepatectomy, right trisectionectomy | 35 (39%) | 59 (36%) | .68 |
| Left hepatectomy, extended left hepatectomy, left trisectionectomy | 54 (60%) | 43(26%) | <.001 |
| Vascular resection | 22 (24%) | 14(8%) | <.001 |
| Portal vein resection | 14(16%) | 7 (4%) | .003 |
| Hepatic artery resection | 7 (8%) | 7 (4%) | .34 |
| Portal vein and hepatic artery resection | 1(1%) | 0 (0%) | .36 |
| Distant nodal sampling | 24 (27%) | 21(13%) | .006 |
| Total nodes retrieved | 4.8 ± 4.6 | 4.7 ± 4.7 | .83 |
| Tumor size (mm) | 32.3 ± 18.6 | 28.3 ± 17.9 | .10 |
| Grade | |||
| 1 | 14(16%) | 30(18%) | .73 |
| 2 | 52 (58%) | 85 (51%) | .36 |
| 3 | 16(18%) | 39 (23%) | .34 |
| 4 | 0(0%) | 1(1%) | 1.00 |
| Not recorded | 8 (9%) | 11 (7%) | .62 |
| AJCCT classification | |||
| 1 | 11 (12%) | 16(10%) | .53 |
| 2a | 19(21%) | 50 (30%) | .14 |
| 2b | 30 (33%) | 38 (23%) | .08 |
| 3 | 13(14%) | 23(14%) | 1.00 |
| 4 | 3 (3%) | 5 (3%) | 1.00 |
| Not recorded | 14(16%) | 34(20%) | .40 |
| Bismuth classification | |||
| 1 | 4 (4%) | 24(14%) | .02 |
| 2 | 12(13%) | 24(14%) | .85 |
| 3a | 17(19%) | 46 (28%) | .13 |
| 3b | 28 (31%) | 18(11%) | <.001 |
| 4 | 22 (24%) | 36 (22%) | .64 |
| Not recorded | 7 (8%) | 18(11%) | .51 |
| Lymph nodes positive | 33 (37%) | 60 (36%) | 1.00 |
| Regional nodes positive | 33 (37%) | 60 (36%) | 1.00 |
| Distant nodes positive | 4 (4%) | 4(2%) | .46 |
| LVI | 26 (29%) | 57 (34%) | .40 |
| PNI | 55 (61%) | 118(71%) | .12 |
| Neoadjuvant external beam radiation | 5 (6%) | 1(1%) | .02 |
| Neoadjuvant chemotherapy | 8 (9%) | 2(1%) | .004 |
Categorical variables expressed as n (%). Continuous variables expressed as mean ± standard deviation.
BMI, body mass index; LVI, lymphovascular invasion; PNI, perineural invasion; PTC, percutaneous transhepatic cholangiogram.
Regarding pathologic characteristics, the majority of patients in both groups had grade II tumors and were American Joint Committee on Cancer (AJCC) T classification 2a or 2b with an average tumor diameter of approximately 30 mm. With regard to the Bismuth classification, patients undergoing caudate resection were less likely to have type I tumors and more likely to have type 3b tumors than patients who did not undergo caudate resection (4% vs 14%; P = .02 and 31% vs 11%; P < .001, respectively). Groups did not differ in the number of patients whose tumors had lymphovascular invasion, perineural invasion, or lymph node positivity. Although few patients received preoperative chemotherapy or external beam radiation therapy, patients undergoing caudate resection were more likely to have received both preoperative chemotherapy and external beam radiation therapy than those not undergoing caudate resection (9% vs 1%; P < .001 and 6% vs 1%; P = .02, respectively). There was no significant difference in proportion of patients in each group receiving postoperative chemotherapy or external beam radiation therapy (Table 1).
Caudate resection impact on margin status
With respect to the final operative margin, there was no difference in the distance of the closest margin to the tumor (2.0 mm vs 2.8 mm, P = .72) or the distribution of location of the closest margin, with the most common location being the proximal bile duct followed by the liver parenchyma (Table 2). Patients undergoing caudate resection, however, were less likely to have a final positive margin (24% vs 40%, P = .01), though the distribution of anatomic location of margin positivity did not differ between groups. Subgroup analysis based on AJCC T classification and anatomic site of resection (right side versus left side of the liver) indicated a difference in margin positivity for T2b tumors only (20% vs 45%, P = .04) (Supplemental Tables S2 and S3).
Table 2.
Caudate resection’s impact on margin status*.
| Caudate resection (n = 90) | No caudate resection (n = 166) | P value | |
|---|---|---|---|
|
| |||
| Closest margin (mm) | 2.0 ± 3.6 | 2.8 ± 15.6 | .72 |
| Proximal bile duct | 32 (36%) | 42 (25%) | .11 |
| Distal bile duct | 7 (8%) | 17(10%) | .65 |
| Pancreatic retroperitoneum | 0(0%) | 2(1%) | .54 |
| Liver parenchyma | 19 (21%) | 25(15%) | .23 |
| Cystic duct | 0(0%) | 1(1%) | 1.00 |
| Not recorded | 32 (36%) | 79 (48%) | .07 |
| Final margin positive | 22 (24%) | 67 (40%) | .01 |
| Proximal bile duct | 8 (36%) | 16(24%) | .28 |
| Distal bile duct | 3(14%) | 8(12%) | 1.00 |
| Pancreatic retroperitoneum | 0(0%) | 2 (3%) | 1.00 |
| Liver parenchyma | 4 (18%) | 10(15%) | .74 |
| Multiple | 7 (32%) | 24 (36%) | .80 |
| Not recorded | 0(0%) | 7(10%) | .19 |
Categorical variables expressed as n (%).
Impact of caudate resection on perioperative outcomes
Patients undergoing resection including the caudate lobe and those undergoing caudate-sparing resection had no difference in the incidence of complications (59% vs 66%, P = .34). There was no difference in rates of bile leak, new postoperative ascites, postoperative liver failure, duration of stay, rates of readmission, or 30- or 90-day mortality (Table 3).
Table 3.
Caudate resection’s impact on perioperative outcome*.
| Caudate resection (n = 90) | No caudate resection (n = 166) | P value | |
|---|---|---|---|
|
| |||
| Complications (Y/N) | 53 (59%) | 109 (66%) | .34 |
| Bile leak | 11 (12%) | 22(13%) | 1.00 |
| New postoperative ascites | 7 (8%) | 17(10%) | .65 |
| Postoperative liver failure | 6 (7%) | 6 (4%) | .35 |
| Duration of stay (days) | 14.9 ± 13.9 | 14.2 ± 12.4 | .69 |
| Readmission | 17(19%) | 42 (25%) | .28 |
| Time to readmission (days) | 12.8 ± 9.0 | 23.0 ± 23.0 | .09 |
| 30-day mortality | 5 (5.6%) | 10(6.0%) | .88 |
| 90-day mortality | 9(10%) | 23 (14%) | .43 |
Categorical variables expressed as n (%). Continuous variables expressed as mean ± standard deviation.
Impact of caudate resection on RFS and OS
Analysis of Kaplan-Meier curves comparing OS, RFS, and disease-specific survival between patients undergoing caudate resection and those not undergoing caudate resection, no differences were noted between the 2 groups (P = .16, P = .22, P = .10, respectively) (Figure, A–C). Caudate resection was not associated with a significant improvement in OS (mean 37.4 vs 32.2 months), RFS (mean 53.2 vs 50.7 months) or DSS (mean 62.2 vs 58.7 months) (Table 2). Patterns of recurrence (local, distant, or both) and distribution of sites of distant recurrence also did not differ between the two groups (Table 4).
Figure.
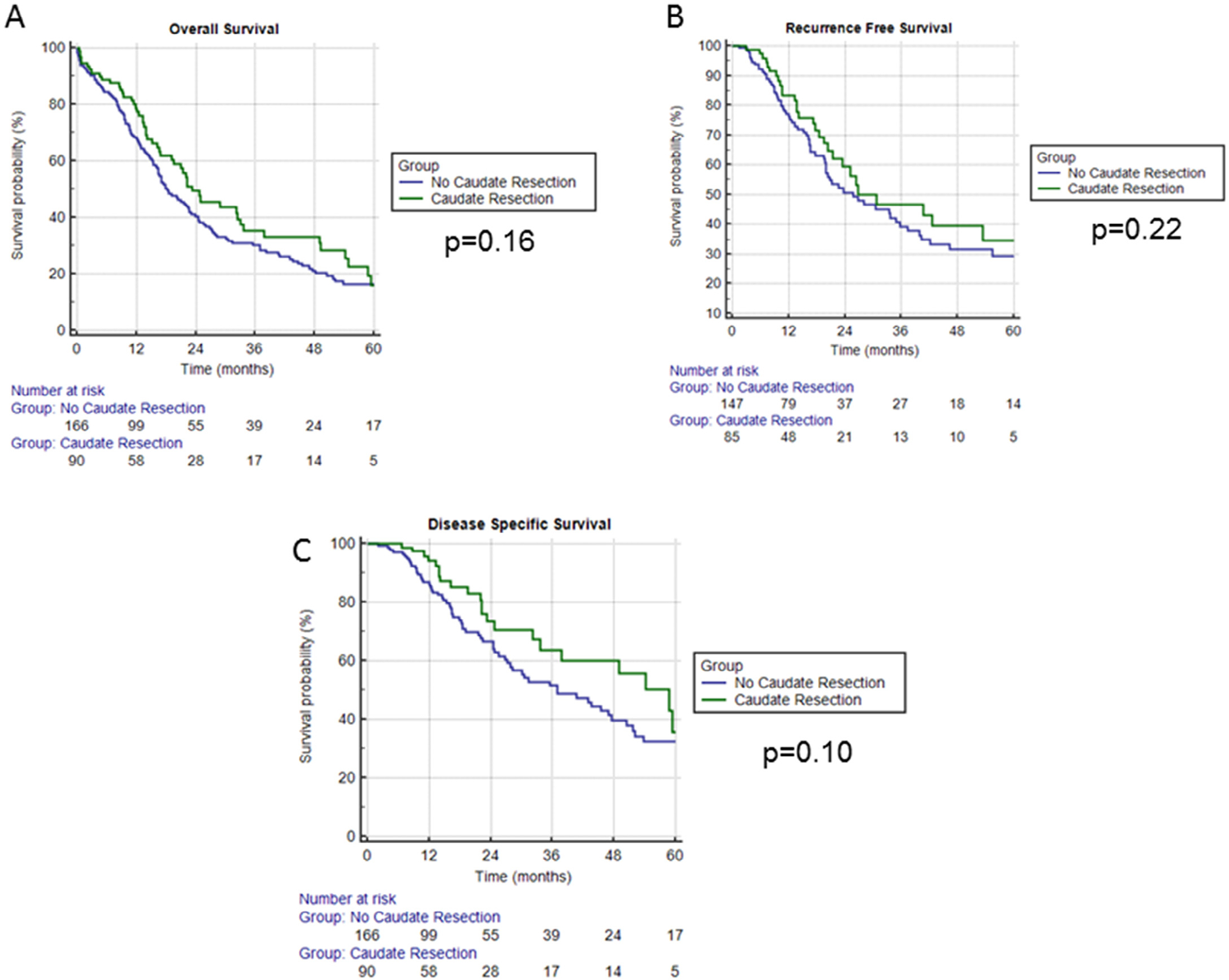
Kaplan-Meier survival curves for patients with hilar cholangiocarcinoma undergoing caudate versus no caudate resection. Differences in overall survival are shown in A, whereas B and C show differences in recurrence-free and disease-specific survival, respectively. In all cases, green lines represent patients undergoing caudate resection, and blue lines represent patients not undergoing caudate resection.
Table 4.
Caudate resection’s impact on OS and RFS (univariate)*.
| Caudate resection (n = 90) | No caudate resection (n = 166) | P value | |
|---|---|---|---|
|
| |||
| OS (months) | 37.4 (28.4–46.3) | 32.2 (26.0–38.3) | .16 |
| RFS (months) | 53.2 (29.2–67.2) | 50.7 (38.4–63.0) | .22 |
| Disease specific survival (months) | 62.2 (47.3–77.1) | 58.7 (46.5–70.9) | .10 |
| Recurrence | 29(32%) | 70(42%) | .14 |
| Local recurrence | 8(28%) | 20(29%) | 1.00 |
| Distant recurrence | 13(45%) | 32 (46%) | 1.00 |
| Both | 8 (28%) | 14(20%) | .43 |
| Not recorded | 0 (0%) | 4 (6%) | .32 |
| Site distant recurrence | |||
| Liver | 11 (52%) | 20 (43%) | .60 |
| Lung | 2(10%) | 2 (4%) | .58 |
| Peritoneum | 3(14%) | 12(26%) | .36 |
| Liver + lung | 2(10%) | 1(2%) | .23 |
| Liver + peritoneum | 2(10%) | 5(11%) | 1.00 |
| Liver + lung + peritoneum | 1 (5%) | 1(2%) | .53 |
| Other | 0(0%) | 5(11%) | .17 |
Categorical variables expressed as n (%). Continuous variables expressed as mean (95% confidence interval).
On multivariable Cox regression analysis, caudate resection was again not associated with an improvement in OS (hazard ratio [HR] = 0.83, 95% confidence interval [CI] = 0.46–1.52 P = .56) (Table 5) or RFS (HR = 0.58, 95% CI = 0.27–1.26; P = .17) (Table 6). Meanwhile, when accounting for caudate resection and various other tumor, operative, and treatment-related variables, lymph node positivity was associated with worse OS (HR = 2.98, 95% CI = 1.68–5.29; P < .001) and RFS (HR = 4.96, 95% CI = 2.28–10.80; P < .001), and adjuvant chemotherapy and/or radiation was associated with improved OS (HR = 0.30, 95% CI = 0.0.17–0.54; P < .001) and RFS (HR = 0.32, 95% CI = 0.15–0.72; P = .01). Additionally, lymphovascular invasion (HR = 1.76, 95% CI = 1.06–2.92; P = .03) and neoadjuvant chemoradiotherapy (HR = 4.89, 95% CI = 1.07–22.32; P = .04) were associated with worse OS. More advanced AJCC T stage (HR = 1.62, 95% CI = 1.13–2.32; P = .01) and perineural invasion (HR = 3.12, 95% CI = 1.24–7.84; P = .02) were associated with worse RFS. No other variables were associated with improved OS or RFS.
Table 5.
Multivariable analysis of tumor, operative, and treatment variables’ impact on OS.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
|
| |||
| Caudate resection | 0.83 | 0.46–1.52 | .56 |
| Left liver resection | 1.03 | 0.54–1.94 | .94 |
| Right liver resection | 1.00 | 0.55–1.82 | 1.00 |
| AJCCT classification | 1.10 | 0.85–1.42 | .49 |
| Bismuth classification | 1.00 | 0.84–1.20 | .98 |
| Lymphovascular invasion | 1.76 | 1.06–2.92 | .03 |
| Perineural invasion | 1.53 | 0.83–2.85 | .18 |
| Lymph node positivity | 2.98 | 1.68–5.29 | <.001 |
| Neoadjuvant chemotherapy/radiation | 4.89 | 1.07–22.32 | .04 |
| Adjuvant chemotherapy/radiation | 0.30 | 0.17–0.54 | <.001 |
Table 6.
Multivariable analysis of tumor, operative, and treatment variables’ impact on RFS.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
|
| |||
| Caudate resection | 0.58 | 0.27–1.26 | .17 |
| Left liver resection | 1.87 | 0.82–4.26 | .14 |
| Right liver resection | 0.53 | 0.22–1.26 | .15 |
| AJCC T classification | 1.62 | 1.13–2.32 | .01 |
| Bismuth classification | 1.04 | 0.82–1.33 | .74 |
| Lymphovascular invasion | 1.43 | 0.76–2.68 | .27 |
| Perineural invasion | 3.12 | 1.24–7.84 | .02 |
| Lymph node positivity | 4.96 | 2.28–10.80 | <.001 |
| Neoadjuvant chemotherapy/radiation | 3.81 | 0.44–33.28 | .23 |
| Adjuvant chemotherapy/radiation | 0.32 | 0.15–0.72 | .01 |
Discussion
This study demonstrated that caudate resection for hilar cholangiocarcinoma is associated with improved margin clearance, but, as has been shown with other hepatopancreaticobiliary malignancies, more aggressive resection does not improve PFS or OS. Thus, these findings confirm that caudate lobe resection for hilar cholangiocarcinoma does not have to be performed in all cases but should be performed to ensure a negative margin is achieved in certain patients. Ultimately, the decision to perform a hepatectomy with caudate resection should be made preoperatively with the use of high-quality, preoperative imaging, including triphasic CT, dynamic MRI, magnetic resonance cholangiopancreatography, and ERCP with direct visualization cholangioscopy.
Though controversial as little as 20 years ago, caudate resection has become largely standard practice for patients with hilar cholangiocarcinoma. Proponents of caudate resection have long argued that, because survival in patients with hilar cholangiocarcinoma is strongly associated with local control, including the caudate lobe in resection increases rates of margin-negative resection and maximizes local control. Improved local control should, in turn, lead to improved OS. The shift toward routine caudate resection began with several Japanese groups reporting improved rates of margin-negative resection, decreased rates of local recurrence, and improved OS.10–14 After groups in the United States and Europe found similar patterns on retrospective analysis with no concomitant increase in complication rates or morbidity, including segment I in the hepatic resection for hilar cholangiocarcinoma gained widespread acceptance as standard surgical practice7,15; however, all these studies have been retrospective analyses of patients undergoing partial hepatectomy with or without caudate resection. This latter distinction bears noting given the importance of partial hepatectomy to obtain clear margins.16
Despite these arguments, the data presented in this study indicate that, despite conferring an improved rate of margin-negative resection, caudate resection does not, in fact, lead to improved RFS or OS in patients with hilar cholangiocarcinoma. We would have expected margins to have only limited influence on OS, because most patients die as a result of distant metastatic disease. This assumption was evidenced by the pattern of recurrence noted in this study; indeed, most patients, in both the entire population and in subgroups, who experienced recurrence developed distant recurrences with or without concomitant local recurrence. Although direct comparison between this study and previous studies proves difficult because of the lack of standardization in surgeon experience with hepatic resections (particularly for hilar cholangiocarcinoma) and extent of lymphadenectomy as well as differences in annual volume among contributing centers, we expected a consistent relationship between margin negativity and improved PFS. Not only was this not found, the only subset of patients in whom an improved rate of margin negative resection was associated with improved RFS was patients not undergoing caudate resection. The inability to detect such a survival benefit conferred by caudate resection may simply be a function of the retrospective nature of this analysis and the highly selective nature of this series. Indeed, the large number of patients not undergoing caudate resection speaks to the number of patients in whom experienced hepatopancreatobiliary surgeons deemed a caudate resection to be unlikely to confer benefit to the patient. Alternatively, this observation begs the question of whether the decision regarding performing a caudate resection may be reflective of a preoperative concern for more advanced or more aggressive disease. Perhaps patients not undergoing caudate resection were thought to have less aggressive disease in which the benefit of a more radical resection would not outweigh the increased perioperative risks. The less aggressive disease was then treated optimally with a margin-negative resection. This approach in conjunction with the tumor’s biology resulted in improved PFS. Furthermore, although no differences in disease stage were noted between the two groups at the time of resection, information regarding the disease stage at presentation was not available for evaluation. In light of the fact that more patients undergoing caudate resection received neoadjuvant chemotherapy and/or radiation, these patients may have had more advanced disease at baseline, which may have influenced their postoperative survival.
Nonetheless, our results indicate improved OS and RFS in patients who receive adjuvant therapy and worse OS and RFS in patients with positive lymph nodes. Although this study lacks the power to draw definitive conclusions with respect to the true impact of adjuvant therapy on oncologic outcomes in patients with hilar cholangiocarcinoma, it suggests that, although margin-negative resection (however that is best achieved, with or without neoadjuvant therapy) provides optimal local control, more aggressive and disseminated disease provide the greatest determinant of prognosis. Indeed, when we compare survival in patients with margin-negative resection to that in patients with positive margins and adjuvant chemotherapy, margin-negative resection is associated with improved RFS (P = .04) and a trend toward improved disease-specific survival (P = .055) but no improvement in OS (P = .53) (Supplemental Fig S1). As has been found with borderline resectable pancreatic cancer, the use of neoadjuvant therapy may have a growing impact on the ability to obtain margin-negative resection given the recent improved response rates of more modern chemotherapy. Additionally, as discussed previously, some of these trends may not have reached significance given the limited number of patients in subgroups within the dataset (eg, various AJCC T classifications) and variation in follow-up protocols, because accurate assessment of disease-specific survival depends on standardization of said protocols. Finally, as this study underscores, the difficulty in operatively treating patients with hilar cholangiocarcinoma lies in the optimal selection of patients for caudate resection. No clinicopathologic characteristics reliably predict reliably which patients will have the aforementioned good oncologic outcomes after resection.
This discussion points to the difficulties inherent in identifying patients with hilar cholangiocarcinoma preoperatively who may benefit most from caudate resection. As mentioned previously, direct cholangioscopy has emerged as a means for direct visualization of the biliary tree and may enable more accurate determination of the extent of biliary involvement in patients with hilar cholangiocarcinoma. As this technology becomes more prevalent and more endoscopists become adept with its use, large-scale trials can be conducted to determine whether it improves accurate assessment of the extent and location of involvement of the biliary tree in this patient population. A more accurate tool or set of criteria for the preoperative biliary assessment still remains the single greatest need in these patients and could help identify patients who would benefit most from a caudate resection in addition to a partial hepatectomy. More specifically, accurately identifying patients in whom a caudate resection would be necessary or highly beneficial in obtaining a margin-negative resection based on tumor location within the biliary tree would allow for selection of ideal candidates for caudate resection. This evaluation could allow for preservation of more liver parenchyma in certain patients in whom a caudate resection might not be necessary to achieve a margin-negative resection.
The conclusions of our study should be viewed with respect to several limitations. The data used for analysis, though collected prospectively, were analyzed retrospectively. Additionally, the multi-institutional nature of the database prevents uniformity in operative technique as well as pre- and postoperative therapy. Selection bias must be considered in institutional decisions regarding which patients would benefit from caudate resection, though there was no difference noted in comorbidities or overall performance status between the 2 groups. Additionally, the extent of lymphadenectomy as well as aggressiveness with regard to vascular resection/reconstruction was left up to the discretion of each institution and each surgeon. Finally, the variability of disease course from patient to patient in the context of a relatively small sample size may have prevented some identified trends from reaching statistical significance, particularly PFS.
All these limitations point to the need for a prospective, randomized trial that standardizes the method of assessing biliary involvement along with neoadjuvant and adjuvant chemotherapy administration to more clearly delineate the benefit of caudate resection in patients undergoing resection for hilar cholangiocarcinoma. Such a trial would include standardized diagnostic and preoperative imaging (specifically a standardized modality and system for assessing biliary involvement), operative, pathology assessment, and both neoadjuvant and adjuvant therapy protocols. Specifically, such a trial would also include criteria governing the use of pre- and postoperative chemotherapy and/or radiation and the extent of lymphadenectomy. Using tumor recurrence as the primary endpoint, based on the identified recurrence rates in this study of 42% in patients not undergoing caudate resection compared with 32% in those who did (an apparent 10% decrease in recurrence), setting α = .05 and β = .80, a total of 730 patients (365 in each arm) would be required to appropriately power a prospective trial with 1:1 randomization evaluating the effect of caudate resection on recurrence in patients undergoing resection for hilar cholangiocarcinoma.
Conclusions
Caudate resection is associated with a greater likelihood of margin-negative resection in patients with extrahepatic hilar cholangiocarcinoma but does not increase OS or DF. Caudate lobe resection may improve disease-specific survival, particularly in patients who do not receive adjuvant chemotherapy. Future randomized controlled trials are required to further assess these questions. Including the caudate lobe in resection for hilar cholangiocarcinoma should be made on a case-by-case basis rather than be performed routinely.
Supplementary Material
Footnotes
None of the authors have any conflicts to disclose.
Supplementary data
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.surg.2017.10.028.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.D’Angelica MI, Jarnagin WR, Blumgart LH. Resectable hilar cholangiocarcinoma: surgical treatment and long-term outcome. Surg Today 2004;34:885–90. [DOI] [PubMed] [Google Scholar]
- 3.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poruk KE, Pawlik TM, Weiss MJ. Perioperative management of hilar cholangiocarcinoma. J Gastrointest Surg 2015;19:1889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos E Principles of surgical resection in hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng QB, Yi B, Wang JH, et al. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol 2012;38:1197–203. [DOI] [PubMed] [Google Scholar]
- 7.Dinant S, Gerhards MF, Busch OR, Obertop H, Gouma DJ, Van Gulik TM. The importance of complete excision of the caudate lobe in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kow AW, Wook CD, Song SC, et al. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg 2012;36:1112–21. [DOI] [PubMed] [Google Scholar]
- 9.Philips P, Farmer RW, Scoggins CR, McMasters KM, Martin RC 2nd. Caudate lobe resections: a single-center experience and evaluation of factors predictive of outcomes. World J Surg Oncol 2013;11:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 2000;7:155–62. [DOI] [PubMed] [Google Scholar]
- 11.Nimura Y, Kamiya J, Nagino M, et al. Aggressive surgical treatment of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg 1998;5:52–61. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M, Ito H, Nakagawa K, et al. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg 1999;189:575–83. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery 1998;123:131–6. [PubMed] [Google Scholar]
- 14.Miyazaki M, Ito H, Nakagawa K, et al. Segments I and IV resection as a new approach for hepatic hilar cholangiocarcinoma. Am J Surg 1998;175:229–31. [DOI] [PubMed] [Google Scholar]
- 15.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg 1996;224:628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998;228:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


