Key Points
Question
Is dual thrombolytic treatment with small bolus alteplase and mutant prourokinase safer than treatment with alteplase alone in patients with ischemic stroke?
Findings
In this randomized, phase 2 clinical trial that included 238 participants with ischemic stroke, an intracranial hemorrhage occurred in 13.2% of patients in the intervention group and 13.7% of patients in the control group. Mutant prourokinase led to a nonsignificant shift toward better modified Rankin Scale scores at 30 days.
Meaning
In patients with minor ischemic stroke who were not eligible for endovascular therapy, thrombolytic treatment with small bolus alteplase and mutant prourokinase could not be proven safer than treatment with alteplase alone.
This randomized clinical trial of individuals with ischemic stroke assesses the safety and efficacy of treatment with a dual plasminogen activator vs usual treatment with intravenous alteplase.
Abstract
Importance
Dual thrombolytic treatment with small bolus alteplase and mutant prourokinase has the potential to be a safer and more efficacious treatment for ischemic stroke than alteplase alone because mutant prourokinase is designed to act only on degraded fibrin without affecting circulating fibrinogen.
Objective
To assess the safety and efficacy of this dual thrombolytic treatment compared with alteplase.
Design, Setting, and Participants
This controlled, open-label randomized clinical trial with a blinded end point was conducted from August 10, 2019, to March 26, 2022, with a total follow-up of 30 days. Adult patients with ischemic stroke from 4 stroke centers in the Netherlands were enrolled.
Interventions
Patients were randomized (1:1) to receive a bolus of 5 mg of intravenous alteplase and 40 mg of an intravenous infusion of mutant prourokinase (intervention) or usual care with 0.9 mg/kg of intravenous alteplase (control).
Main Outcomes and Measures
The primary outcome was any intracranial hemorrhage (ICH) on neuroimaging at 24 hours. Secondary outcomes included functional outcome at 30 days, symptomatic ICH, and fibrinogen levels within 24 hours. Analyses were by intention to treat. Treatment effects were adjusted for baseline prognostic factors.
Results
A total of 268 patients were randomized, and 238 (median [IQR] age, 69 [59-77] years; 147 [61.8%] male) provided deferred consent and were included in the intention-to-treat population (121 in the intervention group and 117 in the control group). The median baseline score on the National Institutes of Health Stroke Scale was 3 (IQR, 2-5). Any ICH occurred in 16 of 121 patients (13.2%) in the intervention group and 16 of 117 patients (13.7%) in the control group (adjusted odds ratio, 0.98; 95% CI, 0.46-2.12). Mutant prourokinase led to a nonsignificant shift toward better modified Rankin Scale scores (adjusted common odds ratio, 1.16; 95% CI, 0.74-1.84). Symptomatic ICH occurred in none of the patients in the intervention group and 3 of 117 patients (2.6%) in the control group. Plasma fibrinogen levels at 1 hour remained constant in the intervention group but decreased in the control group (β = 65 mg/dL; 95% CI, 26-105 mg/dL).
Conclusions and Relevance
In this trial, dual thrombolytic treatment with small bolus alteplase and mutant prourokinase was found to be safe and did not result in fibrinogen depletion. Further evaluation of thrombolytic treatment with mutant prourokinase in larger trials to improve outcomes in patients with larger ischemic strokes is needed. Overall, in patients with minor ischemic stroke who met indications for treatment with intravenous thrombolytics but were not eligible for treatment with endovascular therapy, dual thrombolytic therapy with intravenous mutant prourokinase was not superior to treatment with intravenous alteplase alone.
Trial Registration
ClinicalTrials.gov Identifier: NCT04256473
Introduction
Thrombolytic treatment with recombinant tissue plasminogen activator alteplase in patients with ischemic stroke leads to improved reperfusion and increases the likelihood of good clinical outcome.1 Apart from its limited efficacy, it carries a risk of symptomatic intracranial hemorrhage (ICH) of 6% to 7%.2
Treatment with endovascular therapy (EVT) is effective in patients with ischemic stroke due to a large vessel occlusion in the anterior circulation,3 which is present in approximately 10% to 15% of patients with ischemic stroke presenting to the emergency department.4 For patients with ischemic stroke without a large vessel occlusion, thrombolytic treatment is the only available reperfusion therapy.5 However, there is a need for a better and safer thrombolytic treatment that would expand the number of patients who can be treated safely and successfully.
Tenecteplase might be a promising alternative because it is a genetically modified variant of alteplase engineered to improve efficacy through greater fibrin specificity and longer plasma half-life. Noninferiority has been demonstrated, but not superiority.6,7 In addition, the rates of ICH in patients treated with tenecteplase and alteplase are similar.6,7
Preclinical and clinical studies8,9,10 have indicated that dual thrombolytic therapy with a small bolus of alteplase followed by a mutant prourokinase has a noteworthy potential to be safer and more efficacious than standard-dose alteplase alone (0.9 mg/kg). Intra-arterial treatment with prourokinase in patients with ischemic stroke resulted in better reperfusion and more patients with a favorable outcome than controls despite an increased rate of intracerebral hemorrhage.11,12 Mutant prourokinase is a single-point mutation of prourokinase, which makes it less susceptible to nonspecific activation into its enzymatic, 2-chain form, urokinase.10 Mutant prourokinase by itself does not lyse hemostatic fibrin, only partially degraded fibrin.9,13 Mutant prourokinase is designed to specifically continue intravascular clot lysis and spare hemostatic fibrin after alteplase is cleared from the systemic circulation. Administration of mutant prourokinase at therapeutic dosages in healthy volunteers did not result in fibrinogen depletion. A fibrinogen depletion below 200 mg/dL (to convert to grams per liter, multiply by 0.01) is associated with bleeding after thrombolysis for ischemic stroke.14,15,16 The current study—Dual Thrombolytic Therapy With Mutant Pro-urokinase and Small Bolus Alteplase for Ischemic Stroke (DUMAS)—aimed to assess the safety and efficacy of treatment with a dual plasminogen activator, which consists of a small bolus of intravenous alteplase followed by intravenous infusion of mutant prourokinase, against usual treatment with intravenous alteplase in patients presenting with ischemic stroke.
Methods
Study Design and Participants
We conducted an open-label, multicenter, randomized, controlled, phase 2 clinical trial with blinded end point assessment and an adaptive design for dose optimization in 1 primary stroke center and 3 comprehensive stroke centers in the Netherlands. Patients were eligible if they were 18 years or older, had a clinical diagnosis of ischemic stroke with a deficit on the National Institutes of Health Stroke Scale (NIHSS) of at least 1 point, and met the criteria for standard treatment with intravenous alteplase according to national guidelines.17 Patients eligible for EVT (ie, patients with a proximal large artery occlusion on computed tomography [CT] angiography or magnetic resonance angiography); with prestroke disability (ie, modified Rankin Scale [mRS] scores >2), which interferes with assessment of functional outcome at 30 days; with known pregnancy; or with a contraindication for magnetic resonance imaging (MRI) were excluded. The study protocol was approved by the central medical ethics committee at Erasmus MC University Medical Center.18 We used a deferred informed consent procedure because this study evaluated an immediate intervention in an emergency situation concerning a life-threatening disorder, in accordance with national legislation.19 Patients or their legal representatives provided written deferred informed consent. If no deferred informed consent was given, only the following characteristics were collected for a safety registry: study number, treatment allocation, in-hospital symptomatic ICH, and in-hospital death. If a patient died before deferred consent could be obtained, all data were used. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol can be found in Supplement 1.
Randomization, Masking, and Procedures
Patients were randomized 1:1 to standard treatment with alteplase alone or dual treatment with bolus alteplase and mutant prourokinase by treating physicians or local investigators from August 10, 2019, to March 26, 2022, with a total follow-up of 30 days. The randomization procedure was web based using permuted blocks and was stratified for center. The allocation sequence was generated by the independent trial statistician and was unknown to all investigators.
Clinical outcomes, such as NIHSS scores, were collected by trained research personnel unaware of treatment allocation. Neuroimaging was assessed by an imaging core laboratory blinded to study treatment allocation. Members of the imaging core laboratory were unaware of clinical data, including treatment allocation, but were informed about baseline clinical symptoms (ie, side of the hemiparesis, presence of aphasia, or other symptoms for the patients without hemiparesis or aphasia). Follow-up neuroimaging on which the primary outcome was assessed was evaluated by 2 independent members (L.E.M.S. and A.v.d.L.) of the imaging core laboratory. If the 2 imaging core laboratory assessments did not match, disagreements were resolved by consensus.
The intervention group received a 5-mg bolus of intravenous alteplase followed by a continuous intravenous infusion of mutant prourokinase, 40 mg, in 60 minutes (initial dose) independent of patient weight based on previous research with prourokinase.20 Because the optimal dose of intravenous mutant prourokinase for patients with ischemic stroke was still unknown, sequential interim analyses were performed, allowing adaptation of the intravenous mutant prourokinase dose. Depending on the result of interim analyses, the mutant prourokinase dosage could be revised to a dose 25% higher than the initial dose (ie, 50 mg in 60 minutes) or to a dose 25% lower than the initial dose (ie, 30 mg in 60 minutes). A detailed description of this adaptive design can be found in the study protocol in Supplement 1.18 The control group received standard treatment with intravenous alteplase alone in a dose of 0.9 mg/kg (10% bolus plus 90% infusion in 60 minutes; maximum dose, 90 mg).
All patients underwent neurological assessments by certified assessors at baseline, at 24 hours, and at 5 to 7 days (or hospital discharge if earlier). Patients underwent noncontrast CT, CT angiography and CT perfusion, or MRI and magnetic resonance angiography of the brain at baseline. For follow-up imaging, patients underwent MRI of the brain at 24 hours (range, 12-48 hours). The MRI should include the following sequences: (1) susceptibility-weighted imaging, (2) diffusion-weighted imaging (DWI) or apparent diffusion coefficient, (3) dynamic susceptibility contrast MRI, and (4) T2-weighted imaging or fluid attenuation inversion recovery. In case of any contraindication for MRI after randomization (eg, because the contraindication was not known at the time of inclusion or the patient had a new contraindication due to an intervention during hospital admission), a follow-up noncontrast CT and CT perfusion at 24 hours were performed instead. Blood samples were taken at baseline and at 1 hour, 3 hours, and 24 hours after treatment for patients in 2 participating centers. All patients were followed up until the final assessment at 30 days. Standardized telephone interviews to assess the mRS score at 30 days were conducted from a central location by experienced research nurses unaware of treatment allocation.21,22 The assessors instructed patients or relatives before starting the interview to not mention anything about the acute stroke treatment or the admission in the hospital.
Outcomes
The primary outcome was any postintervention ICH confirmed on susceptibility-weighted imaging or noncontrast CT according to the Heidelberg Bleeding Classification at 24 hours (range, 12-48 hours) after study drug administration.23 Secondary clinical outcomes were the NIHSS score at 24 hours, the NIHSS score at 5 to 7 days (or at discharge if earlier), and the mRS score at 30 days.24,25 Secondary imaging outcomes were posttreatment abnormal perfusion volume (defined as tissue with a time to maximum drug concentration [Tmax] greater than 6 seconds) on dynamic susceptibility contrast or CT perfusion Tmax maps and infarct volume on DWI or noncontrast CT at 24 hours after treatment. Secondary blood biomarker outcomes were fibrinogen at 1 hour, 3 hours, and 24 hours. Safety outcomes were symptomatic ICH according to the Heidelberg Bleeding Classification, death from any cause within 30 days, and major extracranial hemorrhage according to the International Society of Thrombosis and Haemostasis criteria within 24 hours of thrombolytic treatment.23,26
Statistical Analysis
We followed the study protocol and the statistical analysis plan, which were published before the database was locked.18 For the sample size calculation, we assumed that the primary outcome, any ICH, would occur with a probability of 20% with standard thrombolytic treatment and a probability of 7% in the patients treated with dual thrombolytic therapy.27 A sample of 200 patients would provide us with a power of at least 77% to detect a statistically significant effect on the primary outcome. This estimate did not take into account the increasing effect on power of the use of multivariable adjustment for differences in baseline characteristics in the primary analysis. To ensure sufficient power, an additional patient was randomized and included for each patient who did not give consent for participation in the study, who for any reason did not receive the full dose of thrombolytics as assigned, or who had a final diagnosis other than ischemic stroke (ie, stroke mimic). The steering committee discussed all patients about whom doubt existed concerning the discharge diagnosis of ischemic stroke or not while being blinded to treatment allocation. Every local principal investigator could propose cases for discussion. We estimated that up to 20% of the included patients would not have a final diagnosis of ischemic stroke.28
The effect of the study treatment on the primary outcome was assessed with multivariable logistic regression with study treatment as a binary independent variable (mutant prourokinase vs control). We reported adjusted and unadjusted effect estimates with corresponding 95% CIs. The effect estimate was adjusted for important prognostic factors at baseline, which included age, time from onset of symptoms to randomization, and stroke severity (NIHSS score). An odds ratio (OR) of more than 1 indicates an increased risk of any ICH of the intervention, and an OR of less than 1 indicates a decreased risk of any ICH.
The effects of the study treatment on the secondary outcomes were assessed with multivariable linear, logistic, or ordinal regression models. The effect parameter was a β, OR, or common OR. These effects were adjusted with variables that are associated with the specific outcome measure.
We performed and reported 4 analyses. The first (primary) analysis was a simple modified intention-to-treat (mITT) analysis to assess overall safety and efficacy. This analysis was an mITT analysis because we excluded patients who did not give consent to participate in the study. The second analysis was a targeted mITT analysis that excluded patients with a final diagnosis other than ischemic stroke to assess safety and efficacy in the target population. The third analysis was a targeted modified receiving-treatment analysis to assess the safety and efficacy in patients who actually received treatment, excluding patients with a final diagnosis other than ischemic stroke. The fourth analysis was a per-protocol analysis.
Additionally, we performed prespecified subgroup analyses on categorized baseline variables, including age, sex, systolic blood pressure, Alberta stroke program early CT score, time from onset to study treatment, NIHSS score, extracranial carotid or vertebral arterial occlusion, prestudy antiplatelet treatment, DWI lesion (yes/no), and lacunar syndrome (yes/no). Subgroup analyses were performed by testing for interaction of the subgroup indicator with treatment.
An independent trial statistician (D.N.) performed the interim analyses. Interim analyses were planned after inclusion of 20, 30, 40, and 50 patients with a discharge diagnosis of ischemic stroke and after that in increments of 50 after start of the trial and after any dose change, until the trial was completed. Additional analyses to adapt the dosing in the study were planned after inclusion of 60 patients with a discharge diagnosis of ischemic stroke and every 20 patients thereafter.
For regression analyses, we assigned the worst score for all unassessed clinical outcome measures for patients who died within the study period. All other missing values, except the primary outcome, were imputed using multiple imputation (n = 5). Statistical analyses were performed using R software, version 4.0.5 (R Foundation for Statistical Computing).
Results
A total of 268 patients were randomized, of whom 15 (5.6%) did not provide deferred consent and 15 (5.6%) declined follow-up imaging (Figure). Therefore, a total of 238 patients (median [IQR] age, 69 [59-77] years; 147 [61.8%] male and 91 [38.2%] female) were included in the mITT population; 121 (50.8%) were randomized to dual thrombolytic treatment and 117 (49.2%) to standard treatment with alteplase alone.
Figure. CONSORT Flow Diagram of Patient Process Throughout the Trial.
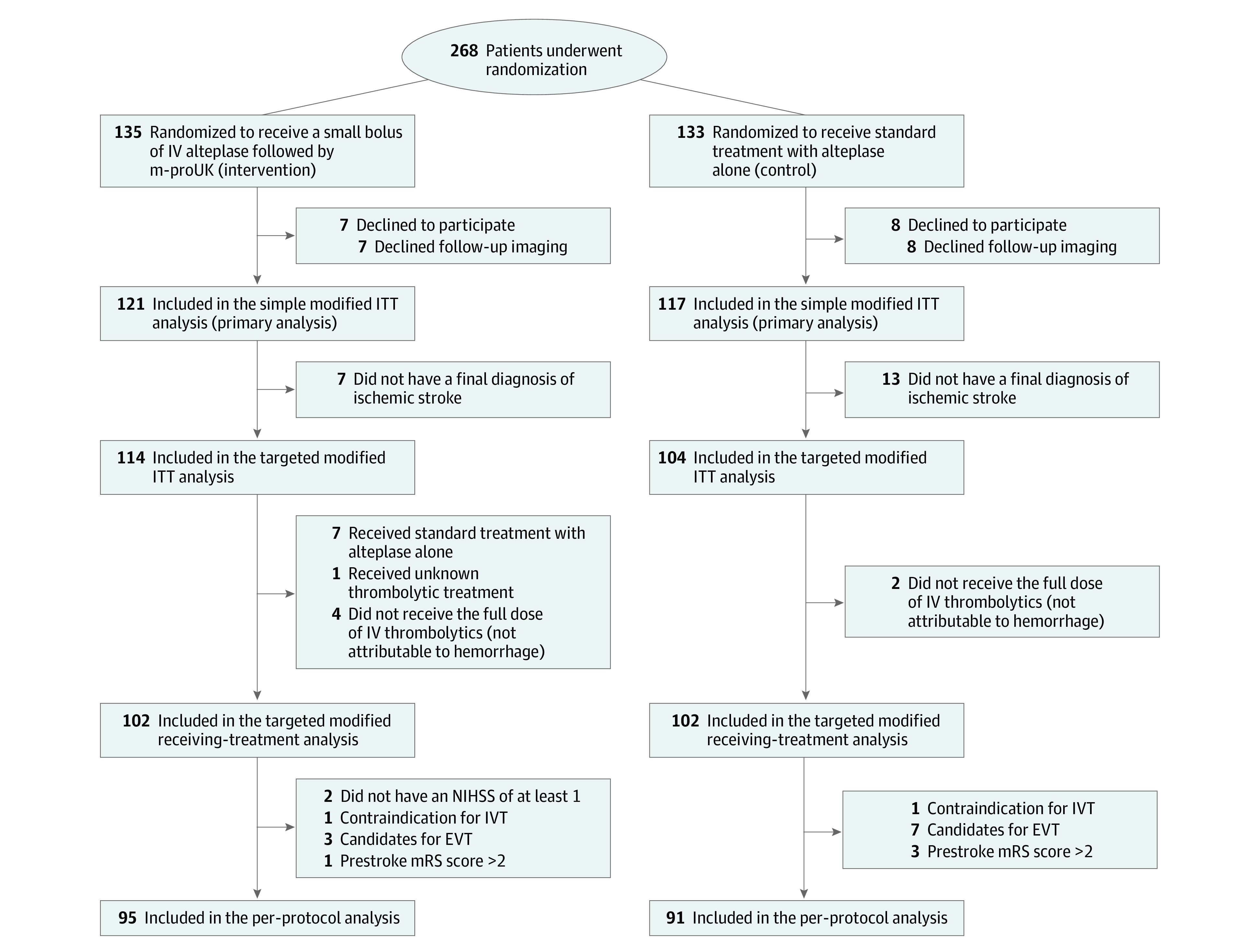
EVT indicates endovascular therapy; ITT, intention to treat; IV, intravenous; IVT, intravenous thrombolytics; m-proUK, mutant prourokinase; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
The median (IQR) time from symptom onset or last seen well to randomization was 114 (80-156) minutes, and the median (IQR) NIHSS score at baseline was 3 (2-5). Baseline demographic and clinical characteristics of patients were similar between the intervention and control group in the mITT population29 (Table 1).
Table 1. Baseline Characteristics and Treatment Process Measures of the Modified Intention-to-Treat Populationa.
| Characteristic | Intervention (n = 121) | Control (n = 117) |
|---|---|---|
| Age, median (IQR), y | 68 (59-74) | 69 (59-78) |
| Sex | ||
| Male | 69 (57.0) | 78 (66.7) |
| Female | 52 (43.0) | 39 (33.3) |
| History of atrial fibrillation | 5 (4.1) | 4 (3.4) |
| History of hypertension | 57 (47.1) | 61 (52.1) |
| History of diabetes | 20 (16.5) | 25 (21.4) |
| Previous ischemic stroke | 25 (20.7) | 27 (23.1) |
| Antiplatelet use | 44 (36.4) | 49 (41.9) |
| Prestroke modified Rankin Scale score | ||
| 0 | 92 (76.0) | 85 (72.6) |
| 1 | 20 (16.5) | 19 (16.2) |
| 2 | 7 (5.8) | 10 (8.5) |
| >2 | 2 (1.7) | 3 (2.6) |
| Systolic blood pressure, median (IQR), mm Hgb | 162 (145-184) | 161 (145-179) |
| NIHSS score, median (IQR) | 3 (2-5) | 3 (2-5) |
| Suspected location of stroke | ||
| Left hemisphere | 57 (47.1) | 50 (42.7) |
| Right hemisphere | 34 (28.1) | 29 (24.8) |
| Posterior circulation | 30 (24.8) | 38 (32.5) |
| Lacunar syndromec | 15 (12.4) | 13 (11.1) |
| Blood glucose, median (IQR), mmol/Ld | 6.8 (5.9-8.1) | 6.6 (5.8-8.2) |
| Platelet count, median (IQR), ×103/μLd | 243 (205-278) | 234 (203-292) |
| Fibrinogen, median (IQR), mg/dLe | 290 (250-350) | 300 (240-350) |
| Ischemia on baseline NCCTf | ||
| None | 109 (90.1) | 103 (88.8) |
| Right anterior circulation | 6 (5.0) | 3 (2.6) |
| Right posterior circulation (PCA territory) | 0 | 0 |
| Left anterior circulation | 3 (2.5) | 4 (3.4) |
| Left posterior circulation (PCA territory) | 1 (0.8) | 0 |
| Infratentorial | 2 (2) | 6 (5.2) |
| Perfusion deficit volume, median (IQR), mLg | 0 (0-4.0) | 0 (0-0.4) |
| Intracranial occlusion on CT angiographyh | ||
| None | 92 (77.3) | 94 (81.7) |
| ICA | 1 (0.8) | 1 (0.9) |
| M1 | 2 (1.7) | 4 (3.5) |
| M2 | 8 (6.7) | 4 (3.5) |
| BA | 1 (0.8) | 3 (2.6) |
| P1 | 1 (0.8) | 1 (0.9) |
| Other | 14 (11.8) | 8 (7.0) |
| Endovascular therapy | 5 (4.1) | 9 (7.7) |
| Time from stroke onset to door, median (IQR), minb | 82 (51-126) | 80 (50-119) |
| Time from stroke onset to randomization, median (IQR), minf | 115 (79-156) | 109 (84-146) |
| Time from stroke onset to bolus alteplase, median (IQR), mini | 118 (83-170) | 121 (89-171) |
| Final diagnosis | ||
| Ischemic stroke | 114 (94.2) | 104 (88.9) |
| No ischemic stroke | 7 (5.8) | 13 (11.1) |
Abbreviations: BA, basilar artery; CT, computed tomography; ICA, intracranial internal carotid artery; M1, middle cerebral artery M1 segment; M2, middle cerebral artery M2 segment; NCCT, noncontrast computed tomography; NIHSS, National Institutes of Health Stroke Scale; P1, posterior cerebral artery P1 segment; PCA, posterior cerebral artery.
SI conversion factors: To convert platelets to ×109/L, multiply by 1; fibrinogen to grams per liter, multiply by 0.01.
Data are presented as number (percentage) of patients unless otherwise indicated.
Data were missing in 1 patient in the intervention group and 1 patient in the control group.
Lacunar syndrome was a clinical diagnosis.29
Data were missing in 1 patient in the intervention group.
Only available for 44 patients in the intervention group and 18 patients in the control group.
Data were missing in 1 patient in the control group.
A perfusion deficit was defined as the presence of brain areas with a time to maximum drug concentration greater than 6 seconds on baseline computed tomography perfusion. The number of patients with no perfusion deficit on baseline computed tomography perfusion was 62 in the intervention group and 69 in the control group. Data were missing for 22 patients in the intervention group and 20 patients in the control group.
Data were missing for 2 patients in the intervention group and 2 patients in the control group.
Data were missing for 6 patients in the intervention group and 3 patients in the control group.
Of the 238 included patients, 222 (93%) had MRI as follow-up imaging. The primary outcome (any ICH) occurred in 16 of 121 patients (13.2%) randomized to the intervention group and 16 of 117 patients (13.7%) randomized to the control group (unadjusted OR, 0.96; 95% CI, 0.45-2.03; adjusted OR, 0.98; 95% CI, 0.46-2.12) (Table 2). Results were similar in the targeted mITT, targeted modified receiving-treatment, and per-protocol analyses. A detailed overview of the ICH subtypes according to the Heidelberg Bleeding Classification is given in eTable 1 in Supplement 2.
Table 2. Primary Outcome (Any ICH) and Treatment Effects in the Primary and Secondary Analyses.
| Analysis | No./total No. of patients (%) | OR (95% CI) | ||
|---|---|---|---|---|
| Intervention | Control | Unadjusted | Adjusteda | |
| Primary analysis | ||||
| Simple modified intention to treat | 16/121 (13.2) | 16/117 (13.7) | 0.96 (0.45-2.03) | 0.98 (0.46-2.12) |
| Secondary analyses | ||||
| Targeted modified intention to treat | 16/114 (14.0) | 16/104 (15.4) | 0.90 (0.42-1.91) | 0.94 (0.43-2.04) |
| Targeted modified receiving-treatment | 12/102 (11.8) | 15/102 (14.7) | 0.77 (0.34-1.75) | 0.82 (0.35-1.91) |
| Per protocol | 12/95 (12.6) | 11/91 (12.1) | 1.04 (0.43-2.50) | 1.26 (0.50-3.18) |
Abbreviations: ICH, intracranial hemorrhage; OR, odds ratio.
Treatment effects were adjusted for age, time from symptom onset or last seen well to randomization, and baseline National Institutes of Health Stroke Scale score.
Occurrence of secondary clinical and imaging outcomes did not differ between the intervention and control groups in the mITT population (Table 3). The intervention group had higher fibrinogen levels than the control group at 1 hour (adjusted β = 65 mg/dL; 95% CI, 26-105 mg/dL), at 3 hours (adjusted β = 47 mg/dL; 95% CI, 2-93 mg/dL), and at 24 hours (adjusted β = 51 mg/dL; 95% CI, 10-92 mg/dL) (Table 3; eFigure in Supplement 2). There were no significant differences in safety outcomes between the intervention and control groups (Table 3). Symptomatic ICH occurred in no patients in the intervention group and 3 of 117 patients (2.6%) in the control group. In the intervention group, 2 of the 121 patients (1.7%) patients died, and 4 of 117 patients (3.4%) in the control group died. Sensitivity analyses in the safety registry showed similar results (eTable 2 in Supplement 2).
Table 3. Secondary Outcomes and Treatment Effects in the Simple Modified Intention-to-Treat Population.
| Outcome | Intervention (n = 121) | Control (n = 117) | Unadjusted effect estimate (95% CI) | Adjusted effect estimate (95% CI)a |
|---|---|---|---|---|
| Clinical | ||||
| NIHSS score | ||||
| At 24 h, mean (IQR) | 1 (0-2) | 1 (0-2) | β = −0.20 (−1.10 to 0.71)b | β = −0.19 (−1.10 to 0.72) |
| Improvement at 24 h, No. (%)c | 77 (63.6) | 83 (70.9) | OR = 0.72 (0.42 to 1.25)b | OR = 0.68 (0.39 to 1.20) |
| At 5-7 d or discharge, mean (IQR) | 0 (0-2) | 0 (0-2) | β = −0.76 (−1.79 to 0.27)b | β = −0.76 (−1.79 to 0.27) |
| Ordinal mRS score at 30 d, mean (IQR) | 2 (1-3) | 2 (1-3) | OR = 1.09 (0.69 to 1.72)c | OR = 1.16 (0.74 to 1.84)c |
| mRS score at 30 d, No. (%) | ||||
| 0 vs 1-6 | 21 (17.4) | 18 (15.4) | OR = 1.16 (0.58 to 2.31) | OR = 1.21 (0.60 to 2.45) |
| 0-1 vs 2-6 | 49 (40.5) | 45 (38.5) | OR = 1.09 (0.65 to 1.84) | OR = 1.18 (0.68 to 2.06) |
| 0-2 vs 3-6 | 89 (73.6) | 87 (74.4) | OR = 0.96 (0.54 to 1.72) | OR = 0.94 (0.52 to 1.70) |
| 0-3 vs 4-6 | 110 (90.9) | 101 (86.3) | OR = 1.58 (0.70 to 3.59) | OR = 1.61 (0.70 to 3.70) |
| 0-4 vs 5-6 | 118 (97.5) | 112 (95.7) | OR = 1.76 (0.41 to 7.58) | OR = 2.04 (0.44 to 9.39) |
| Neuroimaging | ||||
| Infarct volume at 24 h, mean (range), mLd | 5.1 (0-113) | 7.0 (0-263) | β = −1.81 (−7.11 to 3.50) | β = −2.13 (−7.33 to 3.07) |
| Perfusion deficit volume at 24 h, mean (range), mLe | 2.0 (0-62) | 1.6 (0-39) | β = −0.85 (−2.70 to 0.99)f | β = −0.80 (−2.62 to 1.01) |
| Blood biomarker | ||||
| Fibrinogen, mean (IQR), mg/dLg | ||||
| At 1 hh | 3.0 (2.5-3.5) | 1.8 (1.6-3.5) | β = 0.69 (0.29 to 1.10) | β = 0.65 (0.26 to 1.05) |
| At 3 hi | 2.9 (2.4-3.4) | 2.2 (1.7-2.6) | β = 0.52 (0.10 to 0.65) | β = 0.47 (0.02 to 0.93) |
| At 24 hj | 3.0 (2.5-3.6) | 2.5 (2.0-2.7) | β = 0.54 (0.14 to 0.95) | β = 0.51 (0.10 to 0.92) |
| Safety, No. (%) | ||||
| Symptomatic intracranial hemorrhage | 0 | 3 (2.6) | NT | NT |
| Death from any cause at 30 d | 2 (1.7) | 4 (3.4) | OR = 0.47 (0.08 to 2.67) | OR = 0.37 (0.06 to 2.38) |
| Major extracranial hemorrhage according to the ISTH criteria within 24 h of study drug administration | 1 (0.8) | 1 (0.9) | OR = 0.97 (0.06 to 15.9) | OR = 0.83 (0.05 to 14.9) |
Abbreviations: ISTH, International Society of Thrombosis and Haemostasis; mRS, modified Rankin Scale; OR, odds ratio; NIHSS, National Institutes of Health Stroke Scale; NT, not tested.
SI conversion factors: To convert platelets to ×109/L, multiply by 1.
Adjusted for age, time from symptom onset or last seen well to randomization, and baseline NIHSS score.
Adjusted for baseline NIHSS score.
Improvement of at least 4 points or NIHSS 0 or 1 at 24 hours.
Missing for 2 patients in the control group.
Missing for 45 patients in the intervention group and 48 patients in the control group.
Adjusted for baseline perfusion deficit volume.
Blood samples were only drawn in 2 centers. For the regression models, only patients randomized in those 2 centers were included (n = 128). Those regression models were adjusted for baseline fibrinogen.
Available for 42 patients in the intervention group and 31 patients in the control group.
Available for 39 patients in the intervention group and 44 patients in the control group.
Available for 35 patients in the intervention group and 44 patients in the control group.
Up to day 30, a total of 38 serious adverse events were noted in 19 of 121 patients (15.7%) in the intervention group and in 19 of 117 patients (16.2%) patients in the control group (Table 4). No clinically relevant differences in treatment effects were observed in prespecified subgroup analysis (eTable 3 in Supplement 2).
Table 4. Serious Adverse Events in the Modified Intention-to-Treat Population.
| Adverse event | No. (%) of patients | |
|---|---|---|
| Intervention (n = 121) | Control (n = 117) | |
| Patients with at least 1 serious adverse event at 30 d | 19 (15.7) | 19 (16.2) |
| Intracranial hemorrhage | 0 | 3 (2.6) |
| Extracranial hemorrhage | 1 (0.8) | 1 (0.9) |
| Stroke progression | 2 (1.7) | 2 (1.7) |
| New ischemic stroke | 2 (1.7) | 2 (1.7) |
| Allergic reaction | 0 | 0 |
| Pneumonia | 1 (0.8) | 3 (2.6) |
| Other infection | 1 (0.8) | 2 (1.7) |
| Other serious adverse event | 12 (9.9) | 9 (7.7) |
Discussion
In patients with ischemic stroke who met the indications for treatment with intravenous thrombolytics and who were not eligible for treatment with EVT, dual thrombolytic therapy with small bolus intravenous alteplase followed by mutant prourokinase was not superior to treatment with intravenous alteplase alone. Although we found no significant differences in clinical and safety outcomes by treatment, patients in the intervention group had significantly higher fibrinogen levels at 1 hour, 3 hours, and 24 hours after treatment compared with patients in the control group. Previous studies14,15 have shown a strong association between fibrinogen depletion (<200 mg/dL) and occurrence of symptomatic ICH. This finding is also suggested in our data, which showed 3 symptomatic ICHs in the control group vs none in the intervention group, although this association cannot be thoroughly assessed because of the small number of patients with symptomatic ICH in our study population. Therefore, further evaluation of thrombolytic treatment with mutant prourokinase in larger trials with patients with greater symptom severity is needed, especially because these patients are more at risk of symptomatic ICH.
In the Alteplase-Tenecteplase Trial Evaluation for Stroke Thrombolysis (ATTEST), alteplase caused significant disruption of the fibrinolytic system, whereas tenecteplase did not, consistent with the trend toward lower intracerebral hemorrhage incidence with tenecteplase.30 Importantly, the first measurements were taken at 6 hours after treatment, and earlier measurements are not available. Our results indicate that the largest decrease in fibrinogen level occurred much earlier at 1 hour after treatment. This finding makes it difficult to compare the effect of tenecteplase on fibrinogen depletion with the results of our study.
Patients included in our trial had minor infarcts (median [IQR] baseline NIHSS score, 3 [2-5]). The European Stroke Organisation guidelines recommend treatment of patients with minor stroke (NIHSS scores of 0-4) if deficits are disabling, which is defined as a deficit that, if unchanged, would prevent the patient from performing basic activities of daily living or returning to work.31 The American Heart Association/American Stroke Association guideline recommends treatment in patients with disabling mild stroke and deficit. The PRISMS trial (Study of the Efficacy and Safety of Alteplase in Participants With Mild Stroke) of alteplase vs placebo in the treatment of mild, nondisabling stroke was stopped early with neutral results.32 On the other hand, a meta-analysis of individual patient data from 9 randomized clinical trials found that alteplase significantly improved the overall odds of a good outcome irrespective of stroke severity.33 Dutch neurologists are aware of this discussion and adhere to the Dutch guidelines, which are in line with the European Stroke Organisation guidelines. Large registries of patients treated with intravenous thrombolytics mostly included patients with higher NIHSS scores compared with our study.27,34,35
The inclusion of minor infarcts could explain the lower occurrence of any ICH than anticipated in the control group (13.7% instead of 20.0%) because baseline NIHSS score is associated with ICH.36 Moreover, not reaching the anticipated reduction in ICH could be explained by the lack of association between any ICH and thrombolytic treatment as we assumed based on earlier studies.32,37 Recent trials evaluating the efficacy of EVT alone vs intravenous thrombolytics and EVT showed almost similar rates of any ICH in both groups.38,39
We used an adaptive design for dose optimization, but no dose changes were advised by the data safety and monitoring board during the trial. Whether the dose used in DUMAS is the most optimal might depend on the number of tissue plasminogen activator binding sites on fibrin, which depends on the size and the composition of the thrombus. The inclusion of patients with small infarcts and the lack of dose adaptation suggest that the dose is correct for patients included in our trial.
Limitations
This study has some limitations. We excluded patients who were eligible for EVT (ie, patients with a proximal intracranial large artery occlusion on CT angiography or magnetic resonance angiography) because we reasoned that subsequent EVT might disturb the assessment of the intervention effect. However, although we allowed inclusion of all other types of ischemic stroke, including lacunar infarcts, cortical infarcts, and posterior circulation strokes (ie, different stroke etiology), the site of occlusion is known to interfere with the effect of thrombolytic treatment.5 Our results may be generalizable to all patients with an indication for treatment with intravenous thrombolytics, but this needs further evaluation for patients with a large vessel occlusion. A limitation of the use of deferred consent is that selective withdrawal from the trial by patients with a poor outcome may have introduced selection bias. However, because the number of patients with a symptomatic ICH was similar in the mITT population and in the safety registry, a bias associated with symptomatic ICHs can be excluded.
Conclusion
This randomized clinical trial found that dual thrombolytic treatment with small bolus alteplase and mutant prourokinase was safe and did not result in fibrinogen depletion. Further evaluation of thrombolytic treatment with mutant prourokinase in larger trials to improve outcomes in patients with larger ischemic strokes is needed. Overall, in patients with minor ischemic stroke who met the indications for treatment with intravenous thrombolytics but who are not eligible for treatment with EVT, we could not prove superiority of dual thrombolytic therapy with intravenous mutant prourokinase over treatment with intravenous alteplase alone.
Trial Protocol and Statistical Analysis Plan
eTable 1. Any ICH Subtypes According to the Heidelberg Bleeding Classification in the Modified Intention-to-Treat Population
eTable 2. Safety Outcomes With Treatment Effects in the Safety Registry
eTable 3. Treatment Effect Estimates on Any Post-Intervention Intracranial Hemorrhage in Pre-Specified Subgroups
eFigure. Fibrinogen Levels From Baseline to 24-Hour Post Thrombolytic Treatment in the Modified Intention-to-Treat Population
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;2014(7):CD000213. doi: 10.1002/14651858.CD000213.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4.Duvekot MHC, Venema E, Rozeman AD, et al. ; PRESTO Investigators . Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol. 2021;20(3):213-221. doi: 10.1016/S1474-4422(20)30439-7 [DOI] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32(1):78-86. doi: 10.1002/ana.410320113 [DOI] [PubMed] [Google Scholar]
- 6.Menon BK, Buck BH, Singh N, et al. ; AcT Trial Investigators . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. doi: 10.1016/S0140-6736(22)01054-6 [DOI] [PubMed] [Google Scholar]
- 7.Campbell BCV, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Investigators . Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 8.Gurewich V, Pannell R, Simmons-Byrd A, Sarmientos P, Liu JN, Badylak SF. Thrombolysis vs. bleeding from hemostatic sites by a prourokinase mutant compared with tissue plasminogen activator. J Thromb Haemost. 2006;4(7):1559-1565. doi: 10.1111/j.1538-7836.2006.01993.x [DOI] [PubMed] [Google Scholar]
- 9.Liu JN, Liu JX, Liu BF, et al. Prourokinase mutant that induces highly effective clot lysis without interfering with hemostasis. Circ Res. 2002;90(7):757-763. doi: 10.1161/01.RES.0000014825.71092.BD [DOI] [PubMed] [Google Scholar]
- 10.Pannell R, Li S, Gurewich V. Highly effective fibrinolysis by a sequential synergistic combination of mini-dose tPA plus low-dose mutant proUK. PLoS One. 2015;10(3):e0122018. doi: 10.1371/journal.pone.0122018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke. 1998;29(1):4-11. doi: 10.1161/01.STR.29.1.4 [DOI] [PubMed] [Google Scholar]
- 12.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. JAMA. 1999;282(21):2003-2011. doi: 10.1001/jama.282.21.2003 [DOI] [PubMed] [Google Scholar]
- 13.Liu JN, Gurewich V. Fragment E-2 from fibrin substantially enhances pro-urokinase-induced Glu-plasminogen activation: a kinetic study using the plasmin-resistant mutant pro-urokinase Ala-158-rpro-UK. Biochemistry. 1992;31(27):6311-6317. doi: 10.1021/bi00142a021 [DOI] [PubMed] [Google Scholar]
- 14.Romoli M, Giannandrea D, Zini A. Fibrinogen depletion and intracerebral hemorrhage after thrombolysis for ischemic stroke: a meta-analysis. Neurol Sci. 2022;43(2):1127-1134. doi: 10.1007/s10072-021-05441-6 [DOI] [PubMed] [Google Scholar]
- 15.Romoli M, Vandelli L, Bigliardi G, et al. Fibrinogen depletion coagulopathy predicts major bleeding after thrombolysis for ischemic stroke: a multicenter study. Stroke. 2022;53(12):3671-3678. doi: 10.1161/STROKEAHA.122.039652 [DOI] [PubMed] [Google Scholar]
- 16.Yan S, Zhang X, Zhang R, Xu J, Lou M. Early fibrinogen depletion and symptomatic intracranial hemorrhage after reperfusion therapy. Stroke. 2019;50(10):2716-2721. doi: 10.1161/STROKEAHA.119.025711 [DOI] [PubMed] [Google Scholar]
- 17.Nederlandse Vereniging voor Neurologie . Herseninfarct en hersenbloeding. Accessed April 8, 2023. https://www.neurologie.nl/publiek/beroepsinformatie/richtlijnen/nvn-richtlijnen
- 18.van der Ende NAM, Roozenbeek B, Smagge LEM, et al. ; DUMAS Investigators . Dual thrombolytic therapy with mutant pro-urokinase and small bolus alteplase for ischemic stroke (DUMAS): study protocol for a multicenter randomized controlled phase II trial. Trials. 2022;23(1):641. doi: 10.1186/s13063-022-06596-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kompanje EJO, van Dijck JTJM, Chalos V, et al. Informed consent procedures for emergency interventional research in patients with traumatic brain injury and ischaemic stroke. Lancet Neurol. 2020;19(12):1033-1042. doi: 10.1016/S1474-4422(20)30276-3 [DOI] [PubMed] [Google Scholar]
- 20.Zarich SW, Kowalchuk GJ, Weaver WD, et al. Sequential combination thrombolytic therapy for acute myocardial infarction: results of the Pro-Urokinase and t-PA Enhancement of Thrombolysis (PATENT) Trial. J Am Coll Cardiol. 1995;26(2):374-379. doi: 10.1016/0735-1097(95)80009-6 [DOI] [PubMed] [Google Scholar]
- 21.Bruno A, Shah N, Lin C, et al. Improving modified Rankin Scale assessment with a simplified questionnaire. Stroke. 2010;41(5):1048-1050. doi: 10.1161/STROKEAHA.109.571562 [DOI] [PubMed] [Google Scholar]
- 22.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243-2246. doi: 10.1161/01.STR.0000027437.22450.BD [DOI] [PubMed] [Google Scholar]
- 23.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 24.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 25.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. doi: 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 26.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation . Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126. doi: 10.1111/jth.13140 [DOI] [PubMed] [Google Scholar]
- 27.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST Investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 28.Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781-788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 29.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521-1526. doi: 10.1016/0140-6736(91)93206-O [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi: 10.1161/STROKEAHA.115.011290 [DOI] [PubMed] [Google Scholar]
- 31.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I-LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatri P, Kleindorfer DO, Devlin T, et al. ; PRISMS Investigators . Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018;320(2):156-166. doi: 10.1001/jama.2018.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rha JH, Shrivastava VP, Wang Y, et al. ; SITS Investigators . Thrombolysis for acute ischaemic stroke with alteplase in an Asian population: results of the multicenter, multinational Safe Implementation of Thrombolysis in Stroke-Non-European Union World (SITS-NEW). Int J Stroke. 2014;9(suppl A100):93-101. doi: 10.1111/j.1747-4949.2012.00895.x [DOI] [PubMed] [Google Scholar]
- 35.Ahmed N, Kellert L, Lees KR, Mikulik R, Tatlisumak T, Toni D; SITS Investigators . Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): an observational study. JAMA Neurol. 2013;70(7):837-844. doi: 10.1001/jamaneurol.2013.406 [DOI] [PubMed] [Google Scholar]
- 36.Whiteley WN, Emberson J, Lees KR, et al. ; Stroke Thrombolysis Trialists’ Collaboration . Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016;15(9):925-933. doi: 10.1016/S1474-4422(16)30076-X [DOI] [PubMed] [Google Scholar]
- 37.Hacke W, Kaste M, Fieschi C, et al. ; Second European-Australasian Acute Stroke Study Investigators . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998;352(9136):1245-1251. doi: 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 38.LeCouffe NE, Kappelhof M, Treurniet KM, et al. ; MR CLEAN–NO IV Investigators . A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833-1844. doi: 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 39.Yang P, Zhang Y, Zhang L, et al. ; DIRECT-MT Investigators . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Any ICH Subtypes According to the Heidelberg Bleeding Classification in the Modified Intention-to-Treat Population
eTable 2. Safety Outcomes With Treatment Effects in the Safety Registry
eTable 3. Treatment Effect Estimates on Any Post-Intervention Intracranial Hemorrhage in Pre-Specified Subgroups
eFigure. Fibrinogen Levels From Baseline to 24-Hour Post Thrombolytic Treatment in the Modified Intention-to-Treat Population
Nonauthor Collaborators
Data Sharing Statement


