Key Points
Question
What is the prevalence and variability of sodium-glucose cotransporter-2 inhibitor (SGLT2i) use among patients with heart failure with reduced ejection fraction (HFrEF) in the US?
Findings
In this cohort study including 49 399 patients hospitalized for HFrEF in the Get With The Guidelines–Heart Failure registry, 1 in 5 patients were discharged with prescriptions for SGLT2i therapy; rates were similarly low in patients with comorbid type 2 diabetes and chronic kidney disease. Hospital-level discharge prescription of SGLT2i varied widely, independent of patient and hospital characteristics.
Meaning
In this study, use of SGLT2i for HFrEF in the US was overall low and highly variable across hospitals.
This cohort study characterizes patterns of sodium-glucose cotransporter-2 inhibitor use among eligible patients hospitalized for heart failure with reduced ejection fraction (HFrEF).
Abstract
Importance
Clinical guidelines for patients with heart failure with reduced ejection fraction (HFrEF) strongly recommend treatment with a sodium-glucose cotransporter-2 inhibitor (SGLT2i) to reduce cardiovascular mortality or HF hospitalization. Nationwide adoption of SGLT2i for HFrEF in the US is unknown.
Objective
To characterize patterns of SGLT2i use among eligible US patients hospitalized for HFrEF.
Design, Setting, and Participants
This retrospective cohort study analyzed 49 399 patients hospitalized for HFrEF across 489 sites in the Get With The Guidelines–Heart Failure (GWTG-HF) registry between July 1, 2021, and June 30, 2022. Patients with an estimated glomerular filtration rate less than 20 mL/min/1.73 m2, type 1 diabetes, and previous intolerance to SGLT2i were excluded.
Main Outcomes and Measures
Patient-level and hospital-level prescription of SGLT2i at hospital discharge.
Results
Of 49 399 included patients, 16 548 (33.5%) were female, and the median (IQR) age was 67 (56-78) years. Overall, 9988 patients (20.2%) were prescribed an SGLT2i. SGLT2i prescription was less likely among patients with chronic kidney disease (CKD; 4550 of 24 437 [18.6%] vs 5438 of 24 962 [21.8%]; P < .001) but more likely among patients with type 2 diabetes (T2D; 5721 of 21 830 [26.2%] vs 4262 of 27 545 [15.5%]; P < .001) and those with both T2D and CKD (2905 of 12 236 [23.7%] vs 7078 vs 37 139 [19.1%]; P < .001). Patients prescribed SGLT2i therapy were more likely to be prescribed background triple therapy with an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor, β-blocker, and mineralocorticoid receptor antagonist (4624 of 9988 [46.3%] vs 10 880 of 39 411 [27.6%]; P < .001), and 4624 of 49 399 total study patients (9.4%) were discharged with prescriptions for quadruple medical therapy including SGLT2i. Among 461 hospitals with 10 or more eligible discharges, 19 hospitals (4.1%) discharged 50% or more of patients with prescriptions for SGLT2i, whereas 344 hospitals (74.6%) discharged less than 25% of patients with prescriptions for SGLT2i (including 29 [6.3%] that discharged zero patients with SGLT2i prescriptions). There was high between-hospital variance in the rate of SGLT2i prescription in unadjusted models (median odds ratio, 2.53; 95% CI, 2.36-2.74) and after adjustment for patient and hospital characteristics (median odds ratio, 2.51; 95% CI, 2.34-2.71).
Conclusions and Relevance
In this study, prescription of SGLT2i at hospital discharge among eligible patients with HFrEF was low, including among patients with comorbid CKD and T2D who have multiple indications for therapy, with substantial variation among US hospitals. Further efforts are needed to overcome implementation barriers and improve use of SGLT2i among patients with HFrEF.
Introduction
Clinical guidelines strongly recommend use of sodium-glucose cotransporter-2 inhibitor (SGLT2i) therapy for the treatment of heart failure (HF) with reduced ejection fraction (HFrEF) to reduce the risk of cardiovascular mortality and worsening HF events.1,2 This class I recommendation is in the context of patients with HFrEF facing substantial risks of adverse clinical outcomes, often in spite of perceived clinical stability by the patient and/or treating clinician.3,4 Recent analyses have suggested that optimal implementation of SGLT2i therapy has the potential to dramatically improve survival and reduce hospitalizations in this vulnerable population.5,6,7
Prior studies of other therapies proven to reduce mortality and morbidity in HFrEF have observed slow and varied adoption within routine clinical practice, resulting in potentially preventable deaths and hospitalizations.8,9,10,11,12 At the same time, SGLT2i possess unique features that may favor improved early implementation relative to prior therapies, including simplicity of use (1 dose, once daily administration), minimal effect on blood pressure, and separate guideline recommendations for common comorbidities, including type 2 diabetes (T2D) and chronic kidney disease (CKD).13,14 Nonetheless, US nationwide utilization of SGLT2i therapy among eligible patients with HFrEF has not been well characterized. As a class I guideline-recommended therapy, understanding potential gaps, temporal changes, variation, and disparities in SGLT2i therapy may be central to future quality-improvement initiatives and efforts to improve outcomes in clinical practice. Herein, we leverage the American Heart Association Get With The Guidelines–Heart Failure (GWTG-HF) registry to assess contemporary nationwide treatment patterns of SGLT2i use among patients hospitalized with HFrEF in the US. Additionally, we assess hospital-level variability in rates of SGLT2i prescription at hospital discharge and characterize patient-level and hospital-level characteristics associated with the use of SGLT2i therapy.
Methods
GWTG-HF
This was a retrospective cohort study evaluating patients hospitalized for HFrEF at participating GWTG-HF sites. The GWTG-HF registry is an ongoing quality-improvement initiative sponsored by the American Heart Association.15,16 Trained personnel at each participating site prospectively collect data for patients hospitalized for HF using standardized case report forms (IQVIA), including demographic and clinical characteristics, medical history, vital signs and laboratory values, discharge therapies, and in-hospital outcomes. Study protocols were approved by institutional review boards at each participating site. Because data are used for quality improvement, patient-level consent was waived under the Common Rule. Data from participating GWTG-HF sites were linked to the American Hospital Association Annual Survey to obtain select characteristics at individual hospitals.
Patient Population
We identified patients 18 years and older hospitalized for HF at participating GWTG-HF sites between July 1, 2021, and June 30, 2022, with a left ventricular ejection fraction (EF) of 40% or lower (eFigure 1 in Supplement 1). Patients missing data for age, sex, disposition, or SGLT2i status at discharge were excluded. Race and ethnicity were self-reported. We excluded patients who died during index hospitalization, were transferred to an acute care facility, discharged to hospice or palliative care, left against medical advice, or had a history of heart transplant, left ventricular assist device, dialysis, or contraindication to SGLT2i. Contraindications to SGLT2i therapy included (1) type 1 diabetes, (2) estimated glomerular filtration rate (eGFR) less than 20 mL/min/1.73 m2, (3) history of ketoacidosis, (4) hypersensitivity to SGLT2i, and (5) prior documented intolerance to SGLT2i.
Statistical Analysis
Baseline characteristics of patients who were and were not prescribed an SGLT2i at the time of hospital discharge were compared using absolute standardized differences, whereby a percentage absolute standardized difference more than 10% indicates nonnegligible imbalance between groups. Because SGLT2is are also indicated for the treatment of T2D and CKD by the US Food and Drug Administration and recommended by corresponding treatment guidelines, we assessed rates of SGLT2i prescription among patients hospitalized with HFrEF with and without comorbid T2D, CKD, and both T2D and CKD. Quarterly rates of SGLT2i prescription over the study period were assessed among the entire study cohort as well as stratified by demographic characteristics (age, sex, race and ethnicity, and insurance) and clinical characteristics (comorbid T2D, CKD [defined as discharge eGFR less than 60 mL/min/1.73 m2 or documented medical history of kidney insufficiency], and both T2D and CKD). The Cochran-Armitage trend test was used to assess for significance of the quarterly temporal trend.
To assess hospital-level variability, participating GWTG-HF hospital sites with 10 or more eligible discharges were grouped according to the proportion of eligible discharges with an SGLT2i prescription (0%, more than 0% to less than 10%, 10% to less than 25%, 25% to less than 50%, and 50% or more). Hospital characteristics were compared between groups, including number of beds, geographic region, rural location teaching status, interventional cardiology catheterization on site, and heart transplant services.
Multivariable-adjusted logistic regression modeling was used to identify patient-level and hospital-level characteristics independently associated with SGLT2i therapy. A logistic random-effects model was fitted with 30 prespecified variables, including demographic characteristics, medical history, vitals and laboratory values, and hospital characteristics (eMethods in Supplement 1). Backward variable selection with a P value threshold of .20 was used to identify factors associated with SGLT2i prescription.
To further quantify interhospital variation in discharge use of SGLT2i therapy among eligible patients, hierarchical logistic regression modeling with hospital-specific random intercepts was then used to assess the interhospital variation in discharge use of SGLT2i therapy. Three models were fitted, including an unadjusted model (model 1) and 2 adjusted models: one adjusted for patient characteristics alone (model 2) and another adjusted for the patient and hospital characteristics identified to be independently associated with SGLT2i prescription by backward variable selection (model 3). For each model, we report the between-hospital variance and the median odds ratio (OR), which quantifies the median of the set of ORs when comparing the odds of SGLT2i prescription in 2 randomly selected patients with identical covariates discharged from different hospitals. P values were 2-sided, and a P value less than .05 was considered statistically significant. All statistical analyses were performed at the Duke Clinical Research Institute using SAS version 9.4 (SAS Institute).
Results
Rates of SGLT2i Prescription
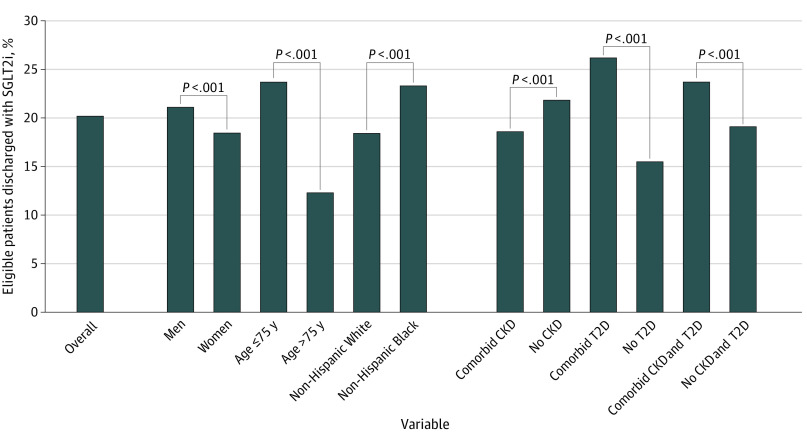
Of 49 399 included patients, 16 548 (33.5%) were female, and the median (IQR) age was 67 (56-78) years. A total of 1125 patients (2.3%) were Asian, 14 793 (29.9%) were non-Hispanic Black, 4311 (8.7%) were Hispanic, 27 274 (55.2%) were non-Hispanic White, and 1896 (3.8%) were another race (including American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and unknown race). Among 49 399 patients hospitalized with HF across 489 participating sites, 9988 (20.2%) were prescribed SGLT2i at hospital discharge (Figure 1). Rates of SGLT2i prescription varied among patient subgroups, including by sex (6944 of 32 851 men [21.1%] vs 3044 of 16 548 women [18.4%]; P < .001), age (8141 of 34 400 [23.7%] 75 years and younger vs 1847 of 14 999 [12.3%] older than 75 years; P < .001), and race (Asian, 191 of 1125 [17.0%]; Black, 3453 of 14 793 [23.3%]; Hispanic, 866 of 4311 [20.1%]; White, 5026 of 27 274 [18.4%]; other race, 452 of 1896 [23.8%]; P < .001). Patients with comorbid CKD were less likely to receive SGLT2i therapy than those without CKD (4550 of 24 437 [18.6%] vs 5438 of 24 962 [21.8%]; P < .001), whereas SGLT2i prescription was more common among patients with T2D compared with those without T2D (5721 of 21 830 [26.2%] vs 4262 of 27 545 [15.5%]; P < .001) and more common among those with both T2D and CKD compared with those without (2905 of 12 236 [23.7%] vs 7078 vs 37 139 [19.1%]; P < .001). Rates of SGLT2i prescription increased quarterly over the study period from 12.0% (1338 of 11 157) in July-September 2021 to 29.0% (3507 of 12 095) in April-June 2022 (P < .001) (eFigure 2 in Supplement 1). Similar trends were seen among demographic and clinical subgroups (eFigures 3 and 4 in Supplement 1).
Figure 1. Discharge Use of Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) Therapy Overall and Among Demographic and Clinical Subgroups.
Rates of discharge prescription of SGLT2i overall and among demographic subgroups (sex, age, race and ethnicity) and clinical subgroups (type 2 diabetes [T2D], chronic kidney disease [CKD], and a combination of T2D and CKD).
Patient Characteristics
Compared with patients who were not prescribed SGLT2i, those prescribed SGLT2i were more likely to be younger (median [IQR] age, 63 [53-73] years vs 68 [57-79] years; standardized difference, 34.9), Black (3453 [34.6%] vs 11 340 [28.8%]; standardized difference, 14.3), and with Medicaid insurance (2530 [30.0%] vs 7825 [23.1%]; standardized difference, 20.4) (Table 1). Patients prescribed SGLT2i were also more likely to have lower EF (median [IQR] of 23% [19-30] vs 27% [20-33]; standardized difference, 24.2), T2D (5721 [57.3%] vs 16 109 [40.9%]; standardized difference, 33.3), and an implantable cardioverter-defibrillator (2963 [29.7%] vs 8276 [21.0%]; standardized difference, 20.0). Among those prescribed SGLT2i, the median (IQR) body mass index (calculated as weight in kilograms divided by height in meters squared; 30.2 [25.7-36.3] vs 28.7 [24.4-34.3]; standardized difference, 18.9) and estimated glomerular filtration rate (63.1 [46.5-82.5] mL/min/1.73 m2 vs 59.0 [41.6-79.3] mL/min/1.73 m2; standardized difference, 15.7) were higher, while serum N-terminal pro–brain natriuretic peptide (5201 [2590-10 246] pg/mL vs 6450 [3164-13 074] pg/mL; standardized difference, 19.7) and systolic blood pressure at hospital admission (131 [115-150] mm Hg vs 135 [118-155] mm Hg; standardized difference, 14.1) were lower. Those prescribed SGLT2i also had a longer median (IQR) length of stay (5 [3-7] days vs 4 [3-6] days; standardized difference, 12.8).
Table 1. Baseline Characteristics of Patients Discharged With and Without Prescription for Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) Therapy.
| Characteristic | No. (%) | Absolute standardized difference, %a | ||
|---|---|---|---|---|
| Overall (N = 49 399) | Prescribed SGLT2i (n = 9988) | No SGLT2i (n = 39 411) | ||
| Age, median (IQR range), y | 67 (56-78) | 63 (53-73) | 68 (57-79) | 34.9 |
| Sex | ||||
| Female | 16 548 (33.5) | 3044 (30.5) | 13 504 (34.3) | 8.1 |
| Male | 32 851 (66.5) | 6944 (69.5) | 25 907 (65.7) | |
| Race and ethnicityb | ||||
| Asian | 1125 (2.3) | 191 (1.9) | 934 (2.4) | 14.3 |
| Non-Hispanic Black | 14 793 (29.9) | 3453 (34.6) | 11 340 (28.8) | |
| Hispanic | 4311 (8.7) | 866 (8.7) | 3445 (8.7) | |
| Non-Hispanic White | 27 274 (55.2) | 5026 (50.3) | 22 248 (56.5) | |
| Other race | 1896 (3.8) | 452 (4.5) | 1444 (3.7) | |
| Insurance status | ||||
| Private, HMO, or other | 11 485 (27.2) | 2481 (29.4) | 9004 (26.6) | 20.4 |
| Medicaid | 10 355 (24.5) | 2530 (30.0) | 7825 (23.1) | |
| Medicare | 17 731 (42.0) | 3019 (35.7) | 14 712 (43.5) | |
| Uninsured | 2694 (6.4) | 416 (4.9) | 2278 (6.7) | |
| Ejection fraction, median (IQR), % | 25 (20-33) | 23 (19-30) | 27 (20-33) | 24.2 |
| Length of stay, median (IQR), d | 4 (3-6) | 5 (3-7) | 4 (3-6) | 12.8 |
| Medical history | ||||
| Diabetes | 21 830 (44.2) | 5721 (57.3) | 16 109 (40.9) | 33.3 |
| Chronic kidney disease | 24 437 (49.5) | 4550 (45.6) | 19 887 (50.5) | 9.8 |
| Coronary artery disease | 23 202 (47.0) | 4685 (46.9) | 18 517 (47.0) | 0.2 |
| Prior myocardial infarction | 11 705 (23.7) | 2416 (24.2) | 9289 (23.6) | 1.5 |
| Peripheral vascular disease | 5023 (10.2) | 925 (9.3) | 4098 (10.4) | 3.8 |
| Stroke/transient ischemic attack | 7814 (15.8) | 1515 (15.2) | 6299 (16.0) | 2.3 |
| Hypertension | 40 608 (82.3) | 8147 (81.6) | 32 461 (82.4) | 2.1 |
| Hyperlipidemia | 28 002 (56.7) | 5804 (58.2) | 22 198 (56.4) | 3.6 |
| Atrial fibrillation/flutter | 17 441 (35.4) | 3237 (32.5) | 14 204 (36.2) | 7.6 |
| COPD or asthma | 15 104 (30.6) | 3091 (31.0) | 12 013 (30.5) | 1.0 |
| CRT | 4693 (9.5) | 1191 (11.9) | 3502 (8.9) | 10.0 |
| ICD | 11 239 (22.8) | 2963 (29.7) | 8276 (21.0) | 20.0 |
| Current smoking | 12 592 (25.6) | 2569 (25.8) | 10 023 (25.5) | 0.8 |
| Vital sign and laboratory values at admission, median (IQR) | ||||
| Systolic blood pressure, mm Hg | 134 (117-154) | 131 (115-150) | 135 (118-155) | 14.1 |
| Heart rate, beats per min | 92 (78-107) | 94 (79-108) | 92 (78-107) | 3.7 |
| BMIc | 29.0 (24.7-34.7) | 30.2 (25.7-36.3) | 28.7 (24.4-34.3) | 18.9 |
| Potassium, mEq/L | 4.1 (3.8-4.5) | 4.1 (3.8-4.5) | 4.1 (3.8-4.5) | 7.0 |
| Serum sodium, mEq/L | 138 (136-141) | 138 (136-140) | 138 (136-141) | 1.3 |
| eGFR, mL/min/1.73 m2d | 59.8 (42.5-79.9) | 63.1 (46.5-82.5) | 59.0 (41.6-79.3) | 15.7 |
| NT-proBNP, pg/mL | 6163 (2999-12 400) | 5201 (2590-10 246) | 6450 (3164-13 074) | 19.7 |
| Vital sign and laboratory values at discharge, median (IQR) | ||||
| Systolic blood pressure, mm Hg | 116 (104-130) | 112 (102-126) | 117 (105-131) | 22.6 |
| Heart rate, beats per min | 79 (70-90) | 80 (70-90) | 79 (69-89) | 6.8 |
| BMIc | 28.4 (24.2-34.1) | 29.7 (25.1-35.5) | 28.2 (24.0-33.7) | 16.3 |
| Potassium, mEq/L | 4.0 (3.7-4.3) | 4.1 (3.8-4.4) | 4.0 (3.7-4.3) | 9.6 |
| Serum sodium, mEq/L | 138 (135-140) | 137 (135-140) | 138 (135-140) | 8.8 |
| eGFR, mL/min/1.73 m2d | 60.8 (43.3-80.7) | 63.3 (47.1-82.3) | 60.1 (42.3-80.3) | 13.4 |
| Medications at discharge | ||||
| ACEi/ARB/ARNI | 33 709 (68.2) | 7719 (77.3) | 25 990 (65.9) | 28.0 |
| ACEi/ARB | 19 626 (39.7) | 2879 (28.8) | 16 747 (42.5) | 41.2 |
| ARNI | 14 083 (28.5) | 4840 (48.5) | 9243 (23.5) | 55.2 |
| β-Blocker | 43 950 (89.0) | 8873 (88.8) | 35 077 (89.0) | 7.2 |
| MRA | 20 276 (41.0) | 5906 (59.1) | 14 370 (36.5) | 49.1 |
| Triple therapy (ACEi/ARB/ARNI plus β-blocker plus MRA) | 15 504 (31.4) | 4624 (46.3) | 10 880 (27.6) | 39.7 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HMO, health maintenance organization; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro–brain natriuretic peptide.
Percentage absolute standardized difference greater than 10% indicates nonnegligible imbalance between groups.
Race and ethnicity were identified by self-report. The other race category included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and unknown race.
Calculated as weight in kilograms divided by height in meters squared.
eGFR was calculated using the 2021 Chronic Kidney Disease–Epidemiology Collaboration equation.
Discharge use of other guideline-recommended therapies for HFrEF was higher among those prescribed SGLT2i, including use of angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), or angiotensin receptor–neprilysin inhibitors (ARNI; 7719 [77.3%] vs 25 990 [65.9%]; standardized difference, 28.0) and mineralocorticoid receptor antagonists (MRA; 5906 [59.1%] vs 14 370 [36.5%]; standardized difference, 49.1). Background simultaneous use of ACEi/ARB/ARNI, β-blocker, and MRA was also higher among those prescribed SGLT2i (4624 [46.3%] vs 10 880 [27.6%]; standardized difference, 39.7). Based on the rate of background triple therapy (ACEi/ARB/ARNI, β-blocker, and MRA) among patients prescribed SGLT2i, 4624 of 49 399 patients (9.4%) in the overall study cohort were prescribed quadruple medical therapy for HFrEF at discharge.
Hospital Characteristics and Hospital-Level Variation in SGLT2i Prescription
Higher rates of SGLT2i prescription were concentrated among select hospitals (Figure 2). Among 461 hospitals with 10 or more eligible discharges, 19 hospitals (4.1%) discharged 50% or more of patients with prescriptions for SGLT2i, whereas 344 (74.6%) discharged less than 25% of eligible patients with prescriptions for SGLT2i therapy (including 29 [6.3%] that discharged zero patients with a SGLT2i prescription). Hospitals with lower rates of SGLT2i use more often had fewer beds and were more likely to be located in the West region, rural, nonteaching, and without interventional cardiac catheterization and heart transplantation services (Table 2). There was high between-hospital variance in the rate of SGLT2i use in unadjusted models (median OR, 2.53; 95% CI, 2.36-2.74; P < .001), which was similar after adjustment for patient characteristics (median OR, 2.61; 95% CI, 2.43-2.83; P < .001) and both patient and hospital characteristics (median OR, 2.51; 95% CI, 2.34-2.71; P < .001) (eTable 1 in Supplement 1).
Figure 2. Hospital-Level Variation in Percentage of Patients Discharged With Prescription for Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i).
Hospital-level variation in discharge prescription of SGLT2i among eligible hospital discharges. Data are from only those hospitals contributing 10 or more eligible discharges (461 of 489 total hospitals [94.2%]). GWTG-HF indicates Get With The Guidelines–Heart Failure.
Table 2. Hospital Characteristics Grouped by Percentage of Patients Discharged With Prescription for Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i)a.
| Characteristic | Overall (N = 461) | Discharged 0% with SGLT2i (n = 29) | Discharged >0% to <10% with SGLT2i (n = 150) | Discharged 10% to <25% with SGLT2i (n = 165) | Discharged 25% to <50% with SGLT2i (n = 98) | Discharged ≥50% with SGLT2i (n = 19) | P valueb |
|---|---|---|---|---|---|---|---|
| Patients discharged with SGLT2i, median (IQR), % | 13.6 (6.3-25.0) | 0 (0-0) | 5.9 (3.4-8.2) | 16.1 (12.4-19.0) | 31.7 (29.1-37.9) | 56.0 (53.1-64.3) | NA |
| No. of beds, median (IQR) | 294 (176-473) | 168 (71-272) | 232 (155-369) | 327 (200-516) | 399 (238-581) | 419 (230-585) | <.001 |
| Geographic region, No. (%) | |||||||
| Northeast | 114 (24.7) | 4 (13.8) | 27 (18.0) | 43 (26.1) | 34 (34.7) | 6 (31.6) | .001 |
| Midwest | 111 (24.1) | 7 (24.1) | 36 (24.0) | 37 (22.4) | 28 (28.6) | 3 (15.8) | |
| South | 134 (29.1) | 4 (13.8) | 43 (28.7) | 56 (33.9) | 25 (25.5) | 6 (31.6) | |
| West | 102 (22.1) | 14 (48.3) | 44 (29.3) | 29 (17.6) | 11 (11.2) | 4 (21.1) | |
| Rural location, No. (%) | 25 (5.4) | 5 (17.2) | 12 (8.1) | 6 (3.6) | 2 (2.0) | 0 | .007 |
| Teaching status, No. (%) | 312 (70.0) | 14 (50.0) | 88 (61.5) | 119 (74.4) | 76 (79.2) | 15 (78.9) | .003 |
| Interventional cardiac catheterization, No./total No. (%) | 215/296 (72.6) | 7/16 (43.8) | 61/97 (62.9) | 83/105 (79.0) | 56/70 (80.0) | 8/8 (100.0) | .001 |
| Heart transplant services, No./total No. (%) | 31/306 (10.1) | 0/18 (0.0) | 1/102 (1.0) | 9/108 (8.3) | 17/69 (24.6) | 4/9 (44.4) | <.001 |
Data reflect hospitals with 10 or more eligible discharges for SGLT2i.
P values are based on test for differences between groups using the Pearson χ2 test or Fisher exact test for categorical variables and the Wilcoxon rank sum tests for continuous variables.
Factors Associated With SGLT2i Prescription
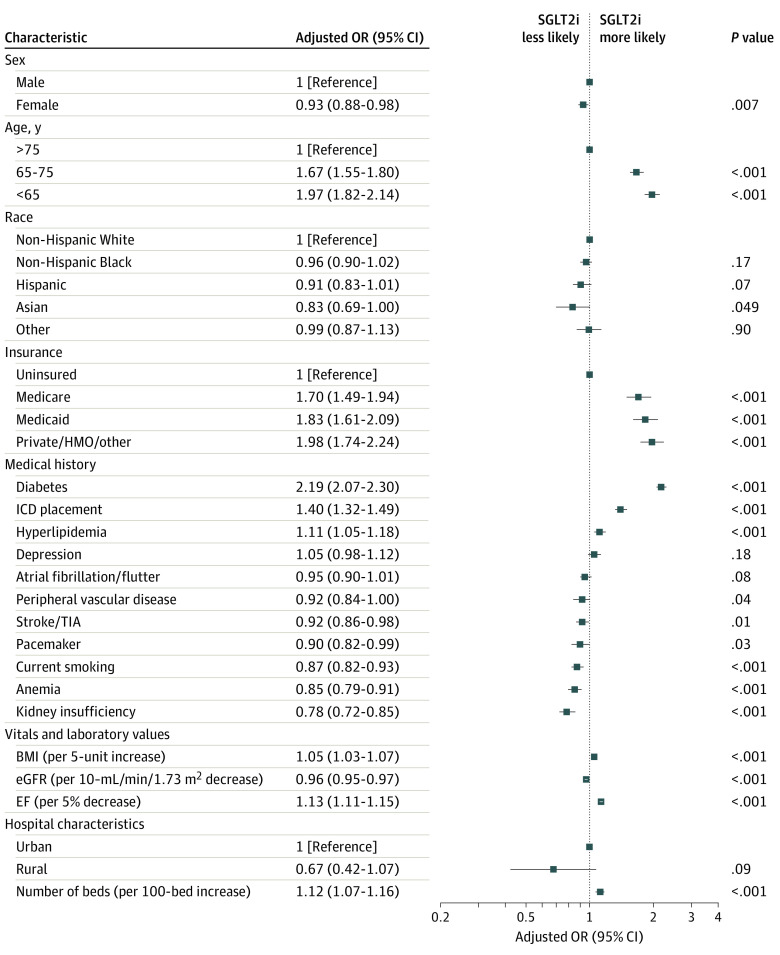
In multivariable analysis, several patient factors were independently associated with SGLT2i prescription, including female sex vs male sex (OR, 0.93; 95% CI, 0.88-0.98), age 65-75 years vs older than 75 years (OR, 1.67; 95% CI, 1.55-1.80), age younger than 65 years vs older than 75 years (OR, 1.98; 95% CI, 1.82-2.14), and Asian race vs White race (OR, 0.83; 95% CI, 0.69-1.00) (Figure 3). Insurance status was also independently associated with SGLT2i use, including Medicare (OR, 1.70; 95% CI, 1.49-1.94), Medicaid (OR, 1.83; 95% CI, 1.61-2.09), and private insurance (OR, 1.98; 95% CI, 1.74-2.24), compared with those who were uninsured. Medical history elements positively associated with SGLT2i prescription were diabetes (OR, 2.19; 95% CI, 2.07-2.30), implantable cardioverter-defibrillator (OR, 1.40; 95% CI, 1.32-1.49), and hyperlipidemia (OR, 1.11; 95% CI, 1.05-1.18), while kidney insufficiency (OR, 0.78; 95% CI, 0.72-0.85), anemia (OR, 0.85; 95% CI, 0.79-0.91), and current smoking (OR, 0.87; 95% CI, 0.82-0.93) had the strongest negative associations with prescription of SGLT2i. Patients with lower EF (per 5% decrease; OR, 1.13; 95% CI, 1.11-1.15) and higher body mass index (per 5-unit increase; OR, 1.05; 95% CI, 1.03-1.07) were more likely to receive SGLT2i. The only hospital characteristic significantly associated with SGLT2i prescription in the multivariable model was number of beds (per 100-bed increase; OR, 1.12; 95% CI, 1.07-1.16).
Figure 3. Patient-Level and Hospital-Level Characteristics Independently Associated With Discharge Use of Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) Therapy.
Forest plot depicting adjusted odds ratios (ORs) for SGLT2i prescription for patient and hospital characteristics included in the multivariable logistic regression model after backward variable selection. A P value threshold of .20 was used for backward variable selection. Kidney insufficiency was defined as chronic serum creatinine elevation greater than 2.0 mg/dL (to convert to micromoles per liter, multiply by 88.4). BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); EF, ejection fraction; eGFR, estimated glomerular filtration rate; HMO; health maintenance organization; ICD, implantable cardioverter-defibrillator; TIA, transient ischemic attack.
Discussion
In this nationwide retrospective cohort study of 49 399 patients hospitalized with HFrEF in the US, we found that only 1 in 5 eligible patients were prescribed an SGLT2i at discharge. SGLT2i use varied by demographic and clinical subgroups, but rates of prescription remained low even among patients with multiple indications for use, including those with comorbid T2D and CKD. While patients prescribed SGLT2i were more likely to be prescribed other evidence-based HFrEF therapies, less than 10% of the overall cohort was discharged with prescriptions for quadruple medical therapy with SGLT2i, ACEi/ARB/ARNI, β-blocker, and MRA. We also found marked hospital-level variation in SGLT2i use, with nearly three-quarters of hospitals discharging less than 25% of eligible patients with an SGLT2i (including some with zero prescriptions) while others discharging well above 50% with prescriptions for SGLT2i therapy. This high between-hospital variance in discharge prescription among eligible patients was observed independent of patient and hospital characteristics. Despite the overall low and variable use of SGLT2i, quarterly rates of prescription more than doubled over the 1-year study period.
The importance of SGLT2i in the clinical management of HFrEF is reflected in recent class I recommendations from professional society guidelines for the reduction of morbidity and mortality in individuals with HFrEF.1,2 To our knowledge, the current study is the first to examine nationwide contemporary use of SGLT2i among patients with HFrEF in the US, inclusive of patients with and without T2D. Prior studies evaluating prescribing practices of evidence-based HFrEF therapies have shown slow adoption among eligible patients in routine clinical practice. For instance, in the year following Food and Drug Administration approval of ARNI for HFrEF in July 2015, an analysis of the GWTG-HF registry found only 2.3% of patients hospitalized with HFrEF were prescribed ARNI at discharge.11 A subsequent analysis from the CHAMP-HF registry examining outpatients with HFrEF from December 2015 to March 2017 found that only 12.8% of patients were prescribed ARNI, with most receiving subtarget doses.9 In contrast, we demonstrate relatively more rapid adoption of SGLT2i with approximately 20% of eligible patients prescribed an SGLT2i at hospital discharge during the overall study period, with nearly 30% discharged with prescriptions for therapy during the last study quarter of April to June 2022. Although more rapid adoption of SGLT2i could relate to the therapy being commercially available for glycemic control among patients with T2D since 2013, first Food and Drug Administration approval for HFrEF did not occur until May 2020 with dapagliflozin. Rather, relative differences in the pace of early adoption of ARNI vs SGLT2i may be potentially explained by differing characteristics between medications. While ARNI requires twice-daily administration with 3 available doses, prescription of SGLT2i is simpler, with only a single dose and once-daily administration. In addition, SGLT2i exhibit minimal effects on blood pressure, facilitating prescription among patients whose HFrEF therapies may be otherwise limited by hypotension.17,18
Although the speed of adoption of SGLT2i may compare favorably with that of ARNI, it remains concerning that the vast majority of patients expected to derive benefit are not receiving this medication. Our findings that women, older adults, and people who lack health insurance were significantly less likely to receive SGLT2i prescriptions echo findings from other studies.9,19,20 While these groups may face different barriers to receiving appropriate care, including bias, greater financial limitations, or less access to high-quality clinicians, efforts are needed to ensure such disparities do not persist.
The current study observed significant hospital-level variability in SGLT2i prescription at discharge. Rates of SGLT2i prescription among eligible patients ranged from 0% to 100%, with 6.3% of hospitals discharging zero eligible patients with an SGLT2i prescription. Despite adjustment for both patient and hospital characteristics (including patient insurance coverage), we observed high between-hospital variance in rates of SGLT2i prescription, suggesting that heterogeneity in clinician and institutional practice patterns were responsible for variability in SGLT2i use rather than patient case mix or intrinsic hospital characteristics. Indeed, after adjustment for patient and hospital factors, a median OR of approximately 2.5 (median OR, 2.51; 95% CI, 2.34-2.71; P < .001) suggests that 2 randomly selected patients with identical covariates have substantially different likelihood of being discharged with a prescription for SGLT2i therapy at 2 different hospitals. By comparison, prior US analyses of ACEi, ARB, β-blocker, and MRA have shown much less practice-level variation, with adjusted median ORs ranging from 1.08 to 1.44.21,22 Reasons underlying such inconsistent prescribing of SGLT2i are likely multifactorial. There may be varying awareness of guideline recommendations and evidence-based therapies in HFrEF, as potentially suggested by patients prescribed SGLT2i in our study also being more likely to receive other evidence-based therapies. There may also be variable levels of clinician comfort with prescribing SGLT2i therapy or underappreciation of the risk of death or readmission associated with HFrEF despite symptomatic improvement or stability.23 Additionally, variations in health system formularies, patient out-of-pocket costs, insurance coverage, and social determinants of heath may be larger contributors to variation in SGLT2i prescription compared with variation in other HF medication with generic formulations.
With less than 1 in 10 patients discharged with prescriptions for quadruple medical therapy, the current data highlight the need for improvements regarding in-hospital initiation of evidence-based therapies for HFrEF. Prior studies have demonstrated that deferring in-hospital initiation to the outpatient setting carries a greater than 75% risk that patients will not start therapy within the subsequent year.24,25 With SGLT2i therapy, deferring in-hospital initiation may significantly increase risk of early postdischarge death and readmission, with clinical trial analyses demonstrating statistically and clinically meaningful reductions in death and worsening HF events within 12 days of SGLT2i intiation.5,26 Similarly, randomized clinical trials have shown in-hospital initiation of SGLT2i to be safe and provide significant early postdischarge benefits on patient symptoms and quality of life.27 Such early benefits on clinical and patient-reported outcomes may be particularly important for patients hospitalized for HFrEF, a population where risks of death and clinical worsening are particularly high.28
Limitations
This study has limitations. First, although prior studies have supported the generalizability of the GWTG-HF registry,29 participation in the registry is voluntary and may select for hospitals with increased interest in quality improvement. Data from the US Centers for Medicare and Medicaid Services Hospital Compare program suggest that the current analysis of 489 US hospitals may comprise approximately 10% to 12% of US hospitals that admit patients with HF.30 In a comparison with 4245 US hospitals in the Centers for Medicare and Medicaid Services Hospital Compare program, GWTG-HF hospitals were more likely to be teaching hospitals and have larger bed size and had modestly better processes of care.30 Thus, the current data may not reflect all patients hospitalized for HFrEF across all US hospitals and may potentially overestimate the quality of care delivered nationally. Second, data regarding medications prescribed prior to admission were limited and did not allow for determination of whether patients discharged with SGLT2i prescriptions had therapy initiated during the index hospitalization or continued from prehospital use. Third, the possibility of residual or unmeasured confounding in adjusted regression models cannot be excluded. Fourth, although cardiovascular outcome trials demonstrating the efficacy and safety of SGLT2i as treatment for HFrEF were published in the years preceding our study period, the American College of Cardiology/American Heart Association/Heart Failure Society of America heart failure guidelines with the class I recommendation for SGLT2i in HFrEF were published online on April 1, 2022, at the start of the last quarter of the study period.1,31,32 Similarly, initial cardiovascular outcome trials with SGLT2i in HFrEF enrolled patients in the outpatient setting, and a randomized trial clearly demonstrating the safety and efficacy of in-hospital initiation of SGLT2i for HF was not published until February 2022.33 Fifth, although this study included patients without obvious medical contraindications or intolerance to SGLT2i therapy, the degree to which patient-level barriers (eg, patient preference) or systems-level barriers (eg, out-of-pocket patient costs, insurance coverage, prior authorizations) contributed to low overall rates of discharge prescription remains unclear. Likewise, this study could not determine the precise level to which suboptimal clinician education (eg, eGFR eligibility criteria and kidney safety) or experience regarding use of SGLT2i use for HFrEF may explain gaps in SGLT2i discharge prescriptions.
Conclusions
In a large, contemporary cohort of patients hospitalized with HFrEF in the US, only 1 in 5 eligible patients were prescribed SGLT2i at discharge, and less than 1 in 10 patients were prescribed quadruple medical therapy at discharge. Use of SGLT2i was low even among patients with multiple indications for therapy, including T2D and CKD. Although rates of SGLT2i use more than doubled over the 1-year study period, there was marked hospital-level variability in prescription of SGLT2i at discharge, even after adjustment for patient and hospital characteristics. These data suggest substantial opportunities to optimize and standardize use of SGLT2i in current HFrEF care.
eMethods.
eFigure 1. CONSORT Diagram for Selection of the Study Cohort From GWTG-HF Registry
eFigure 2. Overall Quarterly Trend in Prescription of SGLT-2 Inhibitors at the Time of Hospital Discharge
eFigure 3. Quarterly Trend in Prescription of SGLT-2 Inhibitors at the Time of Hospital Discharge by Demographic Subgroups
eFigure 4. Quarterly Trend in Prescription of SGLT-2 Inhibitors at the Time of Hospital Discharge by Chronic Kidney Disease and Type 2 Diabetes Status
eTable. Nested Regression Modeling of Hospital-Level Variation in Prescription of SGLT-2 Inhibitors
Data Sharing Statement
References
- 1.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421. doi: 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 2.McDonagh TA, Metra M, Adamo M, et al. ; ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 3.Fiuzat M, Ezekowitz J, Alemayehu W, et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2020;5(7):757-764. doi: 10.1001/jamacardio.2020.0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Fonarow GC. Contextualizing risk among patients with heart failure. JAMA. 2021;326(22):2261-2262. doi: 10.1001/jama.2021.20739 [DOI] [PubMed] [Google Scholar]
- 5.Berg DD, Jhund PS, Docherty KF, et al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(5):499-507. doi: 10.1001/jamacardio.2020.7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121-128. doi: 10.1016/S0140-6736(20)30748-0 [DOI] [PubMed] [Google Scholar]
- 7.Bassi NS, Ziaeian B, Yancy CW, Fonarow GC. Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol. 2020;5(8):948-951. doi: 10.1001/jamacardio.2020.0898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365-2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72(4):351-366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 10.Pierce JB, Li Z, Greiner MA, et al. Adoption of sacubitril/valsartan among patients with heart failure with mildly reduced or preserved ejection fraction: the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2023;16(1):e010176. doi: 10.1161/CIRCHEARTFAILURE.122.010176 [DOI] [PubMed] [Google Scholar]
- 11.Luo N, Fonarow GC, Lippmann SJ, et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from Get With the Guidelines–Heart Failure (GWTG-HF). JACC Heart Fail. 2017;5(4):305-309. doi: 10.1016/j.jchf.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Claggett BL, Greene SJ, et al. Potential implications of expanded US Food and Drug Administration labeling for sacubitril/valsartan in the US. JAMA Cardiol. 2021;6(12):1415-1423. doi: 10.1001/jamacardio.2021.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossing P, Caramori ML, Chan JC, et al. ; Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1-S127. doi: 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 14.ElSayed NA, Aleppo G, Aroda VR, et al. ; American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Care in diabetes—2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi: 10.2337/dc23-S009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Y, LaBresh KA. Overview of the American Heart Association “Get With The Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5(4):179-186. doi: 10.1097/01.hpc.0000243588.00012.79 [DOI] [PubMed] [Google Scholar]
- 16.Smaha LA; American Heart Association . The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148(5)(suppl):S46-S48. doi: 10.1016/j.ahj.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 17.Serenelli M, Böhm M, Inzucchi SE, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J. 2020;41(36):3402-3418. doi: 10.1093/eurheartj/ehaa496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böhm M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Committees and Investigators . Empagliflozin improves cardiovascular and renal outcomes in heart failure irrespective of systolic blood pressure. J Am Coll Cardiol. 2021;78(13):1337-1348. doi: 10.1016/j.jacc.2021.07.049 [DOI] [PubMed] [Google Scholar]
- 19.Greene SJ, Ezekowitz JA, Anstrom KJ, et al. Medical therapy during hospitalization for heart failure with reduced ejection fraction: the VICTORIA registry. J Card Fail. 2022;28(7):1063-1077. doi: 10.1016/j.cardfail.2022.02.011 [DOI] [PubMed] [Google Scholar]
- 20.Breathett KK, Xu H, Sweitzer NK, et al. Is the Affordable Care Act Medicaid expansion associated with receipt of heart failure guideline-directed medical therapy by race and ethnicity? Am Heart J. 2022;244:135-148. doi: 10.1016/j.ahj.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson PN, Chan PS, Spertus JA, et al. Practice-level variation in use of recommended medications among outpatients with heart failure: insights from the NCDR PINNACLE program. Circ Heart Fail. 2013;6(6):1132-1138. doi: 10.1161/CIRCHEARTFAILURE.113.000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dev S, Lacy ME, Masoudi FA, Wu WC. Temporal trends and hospital variation in mineralocorticoid receptor antagonist use in veterans discharged with heart failure. J Am Heart Assoc. 2015;4(12):e002268. doi: 10.1161/JAHA.115.002268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Peterson E, Pagidipati N. Barriers to prescribing glucose-lowering therapies with cardiometabolic benefits. Am Heart J. 2020;224:47-53. doi: 10.1016/j.ahj.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 24.Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure—optimizing therapy with the need for speed. JAMA Cardiol. 2021;6(7):743-744. doi: 10.1001/jamacardio.2021.0496 [DOI] [PubMed] [Google Scholar]
- 25.Rao VN, Murray E, Butler J, et al. In-hospital initiation of sodium-glucose cotransporter-2 inhibitors for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;78(20):2004-2012. doi: 10.1016/j.jacc.2021.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation. 2021;143(4):326-336. doi: 10.1161/CIRCULATIONAHA.120.051783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosiborod MN, Angermann CE, Collins SP, et al. Effects of empagliflozin on symptoms, physical limitations, and quality of life in patients hospitalized for acute heart failure: results from the EMPULSE trial. Circulation. 2022;146(4):279-288. doi: 10.1161/CIRCULATIONAHA.122.059725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi: 10.1001/jama.2018.19232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis LH, Greiner MA, Hammill BG, et al. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and non-OPTIMIZE-HF Medicare patients. Circ Cardiovasc Qual Outcomes. 2009;2(4):377-384. doi: 10.1161/CIRCOUTCOMES.108.822692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidenreich PA, Hernandez AF, Yancy CW, Liang L, Peterson ED, Fonarow GC. Get With The Guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5(1):37-43. doi: 10.1161/CIRCOUTCOMES.110.959122 [DOI] [PubMed] [Google Scholar]
- 31.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 32.Packer M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 33.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568-574. doi: 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. CONSORT Diagram for Selection of the Study Cohort From GWTG-HF Registry
eFigure 2. Overall Quarterly Trend in Prescription of SGLT-2 Inhibitors at the Time of Hospital Discharge
eFigure 3. Quarterly Trend in Prescription of SGLT-2 Inhibitors at the Time of Hospital Discharge by Demographic Subgroups
eFigure 4. Quarterly Trend in Prescription of SGLT-2 Inhibitors at the Time of Hospital Discharge by Chronic Kidney Disease and Type 2 Diabetes Status
eTable. Nested Regression Modeling of Hospital-Level Variation in Prescription of SGLT-2 Inhibitors
Data Sharing Statement