This cohort study estimates age-dependent risks for cardiovascular events with the amyloidogenic pV142I variant.
Key Points
Question
What are the age-dependent relative and absolute risks for cardiovascular events with the amyloidogenic pV142I variant among Black individuals in the US?
Findings
In this cohort study of 3856 Black participants (including 124 carriers of the amyloidogenic pV142I variant), estimates show increasing risk over time for atrial fibrillation, heart failure hospitalization, mortality, and a composite of heart failure hospitalization or mortality between ages 53 and 80 years. Absolute risk for the composite outcome was particularly heightened in late life.
Meaning
Despite a relatively benign course during earlier years, Black individuals who carry pV142I surviving into later life are at particularly elevated risk for cardiovascular events.
Abstract
Importance
Hereditary transthyretin cardiac amyloidosis is an increasingly recognized cause of heart failure (HF) with distinct treatment. The amyloidogenic pV142I (V122I) variant is present in 3% to 4% of Black individuals in the US and increases the risk for atrial fibrillation (AF), HF, and mortality. Since hereditary transthyretin cardiac amyloidosis demonstrates age-dependent anatomic penetrance, evaluation later in life may identify survivors at particularly high risk.
Objective
To estimate age-dependent risks for cardiovascular events with the variant.
Design, Settings, and Participants
This cohort study analyzed Black participants from the Atherosclerosis Risk in Communities (ARIC) study attending visit 1 (1987-1989) (followed up until 2019; median follow-up, 27.6 years). Data analyses were completed from June 2022 to April 2023.
Exposure
pV142I carrier status.
Main outcomes
The association between the variant and AF, HF hospitalization, mortality, and a composite of HF hospitalization or mortality was modeled by generating 10-year absolute risk differences for each year between ages 53 (the median age at visit 1) and 80 years, adjusting for the first 5 principal components of ancestry and sex. As an example, 5- and 10-year risk differences were specifically estimated for the composite outcome among participants surviving to age 80 years.
Results
Among 3856 Black participants (including 124 carriers) at visit 1, 2403 (62%) were women, 2140 (56%) had hypertension, and 740 (20%) had diabetes, with no differences between groups. The 10-year absolute risk difference between ages 53 and 80 years increased over time for each outcome. Statistical significance for increased 10-year risk difference emerged near ages 65 years for AF, 70 years for HF hospitalization, and 75 years for mortality. Among participants surviving to age 80 years, carriers had a 20% (95% CI, 2%-37%) and 24% (95% CI, 1%-47%) absolute increased risk for HF hospitalization or death at 5 and 10 years, respectively. Thus, at age 80 years, only 4 carriers would need to be identified to attribute 1 HF hospitalization or death over the following decade to the variant.
Conclusions and Relevance
In this study, age-specific risks were provided for relevant outcomes with the pV142I variant. Despite a relatively benign course during earlier years, Black individuals who carry the pV142I variant surviving into later life may be particularly vulnerable. These data may inform timing for screening, risk counseling to patients, and potential strategies for early targeted therapy.
Introduction
Hereditary transthyretin cardiac amyloidosis is an important and increasingly recognized cause of heart failure (HF) with distinct treatment options.1 The amyloidogenic pV142I (V122I) variant is present in 3% to 4% of Black individuals in the US, increases the risk for atrial fibrillation (AF), HF, and mortality2,3,4,5 and has recently been classified as reportable by the American College of Medical Genetics.6 Since hereditary transthyretin cardiac amyloidosis demonstrates age-dependent anatomic penetrance,7 evaluation later in life may identify survivors at particularly high risk for disease, in whom absolute increases in risk may be magnified. Indeed, only subtle cardiovascular differences are observed during middle age.8 While most studies have qualitatively or visually demonstrated this age-dependent risk for HF and mortality, more precise estimates across mid to late life are lacking. Defining age-dependent relative and absolute risks with the variant is critical to informing discussions regarding genetic screening, providing risk counseling to patients, and elucidating potential timing of early treatments to delay disease progression.
Methods
We analyzed participants from the Atherosclerosis Risk in Communities (ARIC) study, a prospective study in 4 US communities comprised of 15 792 individuals, aged 45 to 64 years recruited between 1987 and 1989 (visit 1).9 We included 3856 participants who self-reported as Black attending visit 1, including 124 pV142I carriers (3.2%) (eMethods in Supplement 1). Compared with our earlier report,3 we now include greater follow-up, number of events, and additional outcomes with complete event reporting through December 31, 2019. The institutional review board at each participating site approved the study protocol, and informed consent was obtained in writing at each examination.
Visit characteristics were summarized using descriptive statistics for continuous variables and counts and percentages for categorical variables, stratified by carrier analysis. t Test or χ2/Fisher exact tests were performed, as appropriate.
Over a sequence of 10-year time windows (eg, age 53-63 years), we estimated 10-year cumulative risks of clinical outcomes, by carrier status, using Kaplan-Meier methods, with and without covariate adjustment for the first 5 principal components of ancestry and sex.5 The associated 10-year–adjusted risk difference was then derived from these estimates with corresponding 95% CIs derived from unadjusted Kaplan-Meier estimates. This process was repeated for starting years between ages 53 (the median age at visit 1) and 80 years. No adjustments were made for multiple testing. Sensitivity analyses based on relative, rather than absolute, risks were estimated using 10-year adjusted hazard ratios. To better visualize these resulting estimates, we also applied locally weighted scatterplot smoothing, similar to modeling previously used.10,11 Time-to-first event outcomes included AF, HF, death, and a composite of HF or death. Since participant characteristics were balanced at baseline (Table), sensitivity analyses additionally adjusted for potentially confounding covariates (systolic blood pressure, body mass index [calculated as weight in kilograms divided by height in meters squared], hypertension, diabetes, coronary heart disease, and estimated glomerular filtration rate), specified a priori. Analyses were performed using Stata version 17 (StataCorp), and a 2-sided P value less than .05 was considered significant. Analysis took place between June 2022 to April 2023.
Table. Clinical Characteristics of the Atherosclerosis Risk in Communities Study Sample by pV142I Carrier Status at Visit 1 (1996-1998) and Visit 6 (2016-2017).
| Clinical characteristic | Visit 1 | Visit 6 | ||||
|---|---|---|---|---|---|---|
| pV142I noncarrier (n = 3732) | pV142I carrier (n = 124) | P value | pV142I noncarrier (n = 885) | pV142I carrier (n = 38) | P value | |
| Age, y, mean (SD) | 54.0 (5.8) | 53.5 (5.5) | .37 | 79.1 (4.8) | 78.1 (4.3) | .20 |
| Male, No. (%) | 1408 (37.7) | 45 (36.3) | .75 | 271 (30.6) | 9 (23.7) | .36 |
| Female, No. (%) | 2324 (62.3) | 79 (63.7) | 614 (69.4) | 29 (76.3) | ||
| Physical examination, mean (SD) | ||||||
| Systolic blood pressure, mm Hg | 129 (21) | 131 (20) | .14 | 137 (20) | 136 (22) | .63 |
| Diastolic blood pressure, mm Hg | 80 (12) | 82 (13) | .09 | 67 (10) | 64 (9) | .09 |
| Heart rate, beats/min | 67 (11) | 68 (11) | .24 | 64 (11) | 65 (11) | .60 |
| Body mass indexa | 29.7 (6.2) | 29.1 (6.0) | .35 | 30.1 (6.1) | 27.9 (6.4) | .04 |
| Comorbidities, No. (%) | ||||||
| Hypertension | 2068 (55.7) | 72 (58.5) | .53 | 792 (91.1) | 34 (89.5) | .72 |
| Diabetes | 715 (19.7) | 25 (20.7) | .79 | 395 (49.1) | 16 (43.2) | .49 |
| Coronary heart disease | 143 (3.9) | 5 (4.1) | .92 | 92 (10.5) | 5 (13.2) | .61 |
| Heart failure | 245 (6.7) | 10 (8.2) | .51 | 83 (9.4) | 9 (23.7) | .004 |
| Stroke | 107 (2.9) | 5 (4.1) | .44 | 67 (7.6) | 3 (7.9) | .95 |
| Laboratory testing, mean (SD) | ||||||
| Estimated glomerular filtration rate, mL/min/1.78 m2 | 103 (27) | 103 (24) | .85 | 62 (21) | 64 (18) | .72 |
| Low-density lipoprotein, mg/dL | 138 (43) | 131 (43) | .11 | 108 (33) | 101 (28) | .30 |
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Results
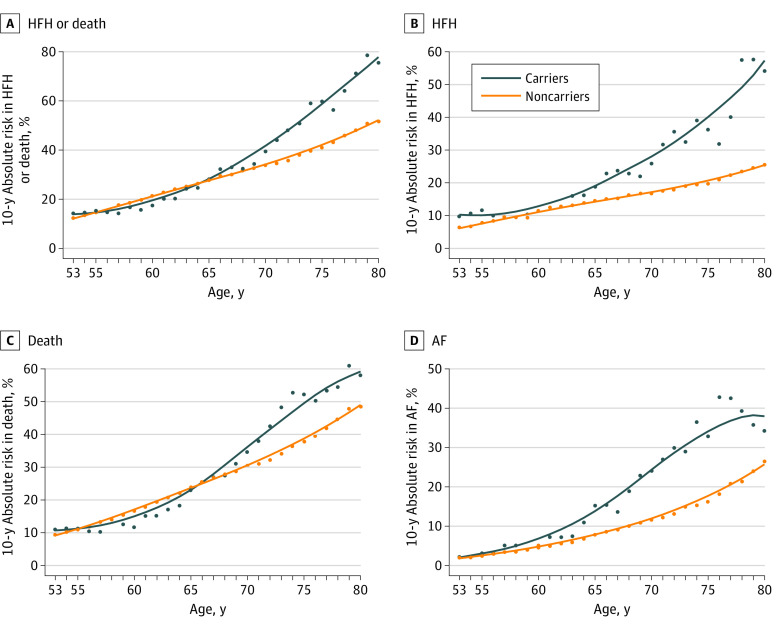
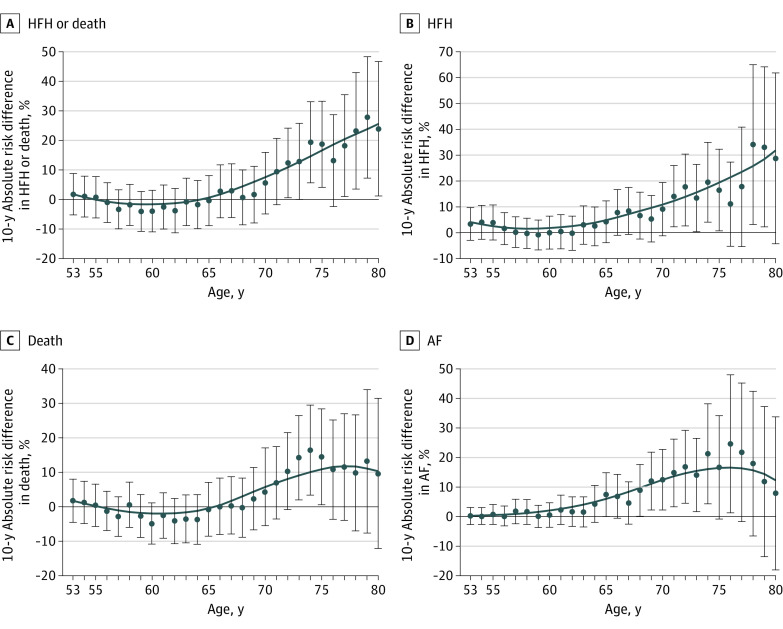
Of 3856 Black individuals, the median (IQR) age at visit 1 was 53 (49-59) years, 2403 (62%) were women, 2140 (56%) had hypertension, and 740 (20%) had diabetes, with no differences between groups (Table).3 Characteristics from participants presenting in late life (visit 6; median [IQR] age, 78 [75-82] years) are also summarized in the Table. Characteristics were overall balanced late in life, aside from a lower body mass index among carriers (mean [SD], 27.9 [6.4] vs 30.1 [6.1]; P = .041) and anticipated higher HF prevalence (9 [23.7%] vs 83 [9.4%]; P = .004). Over a median (IQR) follow-up of 27.6 (17.1-31.0) years, there were 2140 deaths, 1019 incident HF hospitalizations, and 557 cases of incident AF. Figure 1 demonstrates 10-year absolute risks between ages 53 and 80 years by carrier status, while Figure 2 shows increasing absolute risk difference with advancing age. Statistical significance for increased 10-year risk difference emerged near age 65 years for AF, age 70 years for HF, and age 75 years for mortality. Adjusting for potentially confounding variates yielded similar results. Sensitivity analyses showing 10-year hazard ratios were concordant (eFigure 1 in Supplement 1).
Figure 1. Absolute Risks Over 10-Year Windows for Adverse Cardiovascular Events by Age.
Ten-year absolute risks are estimated at each age between 53 and 80 years for heart failure hospitalization (HFH) or death, HFH, death, and atrial fibrillation (AF).
Figure 2. Absolute Risk Differences Over 10-Year Windows for Adverse Cardiovascular Events by Age.
Ten-year absolute risk differences between carriers and noncarriers with 95% CIs are estimated at each age between 53 and 80 years for heart failure hospitalization (HFH), death, and atrial fibrillation (AF). For example, a 75-year-old carrier would have an approximately 20% higher 10-year absolute risk of being hospitalized for HF or dying compared with a noncarrier.
Among participants surviving to age 80 years, carriers were at significantly increased risk for HF hospitalization or death over the next decade of life (hazard ratio, 1.97; 95% CI, 1.24-3.13; P = .004), and this risk was magnified compared with earlier decades (eFigure 2 in Supplement 1), with similar findings noted for other outcomes (eFigures 3, 4, and 5 in Supplement 1). Further, carriers had a 20% (95% CI, 2%-37%) and 24% (95% CI, 1%-47%) absolute increased risk for HF hospitalization or death at 5 and 10 years (P = .03 and .04, respectively) (eTable and eFigure 6 in Supplement 1). Thus, at age 80 years, only 4 carriers would need to be identified to attribute 1 HF hospitalization or death over the following decade to the variant.
Discussion
These data provide age-specific risks for AF, HF, and mortality among pV142I carriers in a large community-based cohort. Despite a relatively benign course during earlier years, Black individuals who carry the pV142I variant surviving into later life may be particularly vulnerable compared with noncarriers, including an increased risk for mortality. These findings are increasingly relevant with the development of targeted therapies for hereditary transthyretin cardiac amyloidosis (including stabilizers such as tafamidis, which is currently approved for clinical use, as well as therapies that are being tested including oligonucleotide inhibitors and gene editing).1,12,13
Signals for increasing 10-year risks for AF, HF, and mortality emerged around ages 65, 70, and 75 years, respectively, highlighting that AF may be a more sensitive marker of variant risk than HF. While a 2022 study highlighted low amyloid penetrance among those with AF,14 this cohort was limited by predominantly White race, in whom the prevalence of this variant is very low3 and with differing risk factors for AF development.15 Screening, counseling, and early intervention efforts likely need to occur several years before risks are heightened, considering greater treatment efficacy of novel therapeutics when started earlier in the disease process.1 We also provide quantitative estimates for 5- and 10-year risk in late life that may be used in discussions between patients and clinicians and demonstrate the large absolute risks in this timeframe attributable to the variant with correspondingly low numbers needed to identify carriers. Of note, ARIC also estimates prevalence of HF that incorporates outpatient diagnoses, and in this study, the prevalence of HF at visit 6 (near 80 years of age) with the variant was nearly 1 in 4, which was significantly higher than noncarriers.
Limitations
Limitations of this analysis include potential survivorship bias for analyses focusing on later life. However, the latter would likely underestimate the association with adverse events since the variant increases the risk for mortality. We further demonstrated that characteristics were generally well balanced at visit 6 in late life, which argues against significant survivor bias. In addition, ARIC did not incorporate comprehensive, advanced phenotyping modalities used in cardiac amyloidosis beyond transthoracic echocardiography, the results of which have been previously reported,3 although study of 99mtechnetium pyrophosphate imaging is underway in this cohort. Moreover, given the sample size, sex-stratified analyses were not performed, although we and others have previously reported no significant interactions by sex with the variant.2,3 Finally, statistical significance is influenced by sample size, and therefore, our data may overestimate ages where enhanced risk for cardiovascular outcomes is delineated.
Conclusions
In conclusion, we provide age-specific relative and absolute risks for several relevant cardiovascular outcomes with the pV142I variant. These data may inform timing for screening, risk counseling to patients, and potential strategies for early targeted therapy.
eMethods.
eTable. Percent of Participants Experiencing an Adverse Event at 5 and 10 Years by Carrier Status, Landmarked at 80 Years of Age
eFigure 1. pV142I Hazard Ratio for Adverse Cardiovascular Events by Age
eFigure 2. pV142I Hazard Ratio for Heart Failure Hospitalization or Death by Decade
eFigure 3. pV142I Hazard Ratio for Death by Decade
eFigure 4. pV142I Hazard Ratio for HF Hospitalization by Decade
eFigure 5. pV142I Hazard Ratio for Atrial Fibrillation by Decade
eFigure 6. Cumulative Heart Failure Hospitalization or Death Among Participants at least 80 Years Old
eReferences.
Data sharing statement
References
- 1.Maurer MS, Schwartz JH, Gundapaneni B, et al. ; ATTR-ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 2.Parcha V, Malla G, Irvin MR, et al. Association of transthyretin Val122Ile variant with incident heart failure among Black individuals. JAMA. 2022;327(14):1368-1378. doi: 10.1001/jama.2022.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly Black Americans. N Engl J Med. 2015;372(1):21-29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selvaraj S, Claggett B, Minamisawa M, et al. Atrial fibrillation and ischemic stroke with the amyloidogenic V122I transthyretin variant among Black Americans. J Am Coll Cardiol. 2021;78(1):89-91. doi: 10.1016/j.jacc.2021.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damrauer SM, Chaudhary K, Cho JH, et al. Association of the V122I hereditary transthyretin amyloidosis genetic variant with heart failure among individuals of African or Hispanic/Latino ancestry. JAMA. 2019;322(22):2191-2202. doi: 10.1001/jama.2019.17935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DT, Lee K, Abul-Husn NS, et al. ; ACMG Secondary Findings Working Group . ACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2022;24(7):1407-1414. doi: 10.1016/j.gim.2022.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466-473. doi: 10.1056/NEJM199702133360703 [DOI] [PubMed] [Google Scholar]
- 8.Sinha A, Zheng Y, Nannini D, et al. Association of the V122I transthyretin amyloidosis genetic variant with cardiac structure and function in middle-aged Black Adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol. 2020;6(6):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 10.Claggett B, Packer M, McMurray JJ, et al. ; PARADIGM-HF Investigators . Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289-2290. doi: 10.1056/NEJMc1509753 [DOI] [PubMed] [Google Scholar]
- 11.Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121-128. doi: 10.1016/S0140-6736(20)30748-0 [DOI] [PubMed] [Google Scholar]
- 12.Gillmore JD, Gane E, Taubel J, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493-502. doi: 10.1056/NEJMoa2107454 [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Adams D, Kristen A, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139(4):431-443. doi: 10.1161/CIRCULATIONAHA.118.035831 [DOI] [PubMed] [Google Scholar]
- 14.Prasad P, Howell S, Sanghai S, et al. Targeted screening for transthyretin amyloid cardiomyopathy in patients with atrial fibrillation. Circulation. 2022;146(22):1730-1732. doi: 10.1161/CIRCULATIONAHA.122.060596 [DOI] [PubMed] [Google Scholar]
- 15.Mou L, Norby FL, Chen LY, et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11(7):e006350. doi: 10.1161/CIRCEP.118.006350 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable. Percent of Participants Experiencing an Adverse Event at 5 and 10 Years by Carrier Status, Landmarked at 80 Years of Age
eFigure 1. pV142I Hazard Ratio for Adverse Cardiovascular Events by Age
eFigure 2. pV142I Hazard Ratio for Heart Failure Hospitalization or Death by Decade
eFigure 3. pV142I Hazard Ratio for Death by Decade
eFigure 4. pV142I Hazard Ratio for HF Hospitalization by Decade
eFigure 5. pV142I Hazard Ratio for Atrial Fibrillation by Decade
eFigure 6. Cumulative Heart Failure Hospitalization or Death Among Participants at least 80 Years Old
eReferences.
Data sharing statement