This cohort study analyzes data from 3 commercial claims databases to conduct near–real-time monitoring of health outcomes following BNT162b2 COVID-19 vaccination in the US pediatric population aged 5 to 17 years.
Key Points
Question
Does active monitoring detect potentially elevated risk of health outcomes after BNT162b2 COVID-19 vaccination in the US pediatric population aged 5 to 17 years?
Findings
In this cohort study of more than 3 million children (aged 5-17 years) who received BNT162b2 COVID-19 vaccination through mid-2022 using data from 3 US commercial claims databases, only myocarditis or pericarditis met the statistical threshold for a signal after BNT162b2 COVID-19 vaccination via near–real-time monitoring.
Meaning
Results from near–real-time monitoring of health outcomes after BNT162b2 COVID-19 vaccination are consistent with current evidence and provide additional evidence of vaccine safety in the pediatric population.
Abstract
Importance
Active monitoring of health outcomes after COVID-19 vaccination offers early detection of rare outcomes that may not be identified in prelicensure trials.
Objective
To conduct near–real-time monitoring of health outcomes following BNT162b2 COVID-19 vaccination in the US pediatric population aged 5 to 17 years.
Design, Setting, and Participants
This population-based study was conducted under a public health surveillance mandate from the US Food and Drug Administration. Participants aged 5 to 17 years were included if they received BNT162b2 COVID-19 vaccination through mid 2022 and had continuous enrollment in a medical health insurance plan from the start of an outcome-specific clean window until the COVID-19 vaccination. Surveillance of 20 prespecified health outcomes was conducted in near real time within a cohort of vaccinated individuals from the earliest Emergency Use Authorization date for the BNT162b2 vaccination (December 11, 2020) and was expanded as more pediatric age groups received authorization through May and June 2022. All 20 health outcomes were monitored descriptively, 13 of which additionally underwent sequential testing. For these 13 health outcomes, the increased risk of each outcome after vaccination was compared with a historical baseline with adjustments for repeated looks at the data as well as a claims processing delay. A sequential testing approach was used, which declared a safety signal when the log likelihood ratio comparing the observed rate ratio against the null hypothesis exceeded a critical value.
Exposure
Exposure was defined as receipt of a BNT162b2 COVID-19 vaccine dose. The primary analysis assessed primary series doses together (dose 1 + dose 2), and dose-specific secondary analyses were conducted. Follow-up time was censored for death, disenrollment, end of the outcome-specific risk window, end of the study period, or a receipt of a subsequent vaccine dose.
Main Outcomes
Twenty prespecified health outcomes: 13 were assessed using sequential testing and 7 were monitored descriptively because of a lack of historical comparator data.
Results
This study included 3 017 352 enrollees aged 5 to 17 years. Of the enrollees across all 3 databases, 1 510 817 (50.1%) were males, 1 506 499 (49.9%) were females, and 2 867 436 (95.0%) lived in an urban area. In the primary sequential analyses, a safety signal was observed only for myocarditis or pericarditis after primary series vaccination with BNT162b2 in the age group 12 to 17 years across all 3 databases. No safety signals were observed for the 12 other outcomes assessed using sequential testing.
Conclusions and Relevance
Among 20 health outcomes that were monitored in near real time, a safety signal was identified for only myocarditis or pericarditis. Consistent with other published reports, these results provide additional evidence that COVID-19 vaccines are safe in children.
Introduction
Three vaccines are currently available in the United States to prevent COVID-19 in children, including COVID-19 vaccines from Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) for ages 6 months to 17 years and Novavax (NVX-CoV2373) for ages 12 to 17 years.1,2,3 We present safety results from near–real-time monitoring using commercial claims databases with data for 3 017 352 children aged 5 to 17 years following administration of the COVID-19 BNT162b2 vaccine, which was the first approved vaccine for the pediatric population. This study was conducted under the US Food and Drug Administration (FDA) Biologics Effectiveness and Safety (BEST) Initiative using rapid cycle analysis, or a near–real-time monitoring framework, which enables early detection of potential safety signals. This surveillance effort in near real time is part of the US government’s larger ongoing postauthorization monitoring and collection of real-world evidence to ensure the safety of COVID-19 vaccines in children.
Methods
This surveillance activity was conducted as part of the US FDA public health surveillance mandate. This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. It was exempt from institutional review board approval because these databases contain deidentified data and did not require informed consent procedures. FDA surveillance activities under the Sentinel Initiative and BEST Initiative, which is a component of the Sentinel Initiative, are exempt from institutional review board review and approval.4
Data Sources
This study used administrative claims data from Optum, HealthCore (HealthCore Integrated Research Database), and CVS Health (Aetna Enterprise Data Warehouse) that contain longitudinal medical and pharmacy claims data. At the time of analysis, claims data from Optum and CVS Health were additionally supplemented with vaccination data from participating local and state immunization information systems (eTable 1 in Supplement 1).5 For BNT162b2 vaccines, claims data alone captured 19.3% of the total enrolled population, but IIS-claims linkage increased this percentage to 29.0%.
Study Population
We included health plan members aged 5 to 17 years who received BNT162b2 vaccination from the earliest date of its Emergency Use Authorization by age group through June 25, 2022 (Optum), May 6, 2022 (HealthCore), and May 31, 2022 (CVS Health). The study population was divided into 3 age groups according to the vaccine authorization schedule (5-11, 12-15, and 16-17 years). We required continuous enrollment in a medical health insurance plan from the start of a clean window specific to each health outcome to the COVID-19 vaccination so that only a new incident diagnosis of an outcome during the postvaccination risk window would contribute to the rapid cycle analysis (eTable 3 in Supplement 1). (A clean window is the term for the interval used to define incident outcomes where an individual enters the study cohort only if the outcome of interest did not occur during that interval.)
Exposure and Follow-up
Exposure was defined as the receipt of BNT162b2 COVID-19 vaccine (eTable 2 in Supplement 1). The dose number was assigned chronologically. Follow-up began on the date of eligible vaccination and was censored at subsequent vaccination, death, disenrollment, end of the outcome-specific risk window, or end of the study period. The primary analysis (dose 1 + dose 2) included all follow-up time accrued after dose 1 and after dose 2 combined. Secondary analyses included stratification by dose 1, dose 2, and dose 3 or booster, including follow-up time accrued after the individual dose up until a censoring criterion was met.
Health Outcomes
Using claims-based algorithms, we monitored 20 health outcomes selected through clinical consultation and literature review.6,7 These outcomes included myocarditis, pericarditis, or co-occurring myocarditis and pericarditis (hereafter referred to as myocarditis or pericarditis); encephalitis or encephalomyelitis; anaphylaxis; common thromboses with thrombocytopenia; seizures or convulsions; Bell palsy; deep vein thrombosis; pulmonary embolism; disseminated intravascular coagulation; immune thrombocytopenia; narcolepsy; appendicitis; nonhemorrhagic stroke; Guillain-Barré syndrome; multisystem inflammatory syndrome in children; transverse myelitis; cerebral and abdominal (unusual site) thrombosis with thrombocytopenia; Kawasaki disease; hemorrhagic stroke; and acute myocardial infarction. Among these, 13 were assessed using sequential testing and 7 were monitored descriptively because of a lack of historical comparator data.6 For each outcome for which sequential analysis was conducted, we excluded enrollees who had experienced the outcome during an outcome-specific clean window to ensure that it was not a preexisting condition before vaccination. The myocarditis or pericarditis outcome was assessed with varying outcome-specific risk windows and care settings based on evidence from prior surveillance efforts and clinician input (eTable 3 in Supplement 1).
Sequential Testing
We conducted monthly sequential testing using the Poisson maximized sequential probability ratio test8 and generated incidence rate ratios (RRs) of observed outcome rates compared with database-specific historical (expected) rates. This screening method can detect a relatively large increased risk early in the surveillance period. The method does not establish a causal association and is not intended to quantify its magnitude nor its precision, so it cannot generate confidence intervals around the incidence RRs.
Historical rates before COVID-19 vaccination were adjusted for claims processing delay and standardized by age and sex where case counts permitted.9 One-tailed tests were used with a null hypothesis that the observed rate was no greater than the historical comparator rate beyond a prespecified test margin with an α of 1%, specified for each outcome-dose–age group being sequentially tested. A stringent α level was selected to increase the specificity of signals detected from testing multiple outcomes across different analyses. The log likelihood ratio was calculated comparing the likelihoods of the observed rate ratio and the null hypothesis. At each test, if the log likelihood ratio exceeded a prespecified critical value, the null hypothesis was rejected and a safety signal was declared. A statistical signal occurred if the log likelihood ratio exceeded a critical value. Surveillance continued until a signal was detected or the prespecified maximum surveillance length was reached.6
Medical Record Review
Medical record review was conducted for the myocarditis or pericarditis outcome to assess the validity of cases identified by claims-based algorithms. Clinical adjudicators used the Brighton collaboration definition to classify cases, and records meeting the confirmed or probable Brighton classifications were considered a true case.10
Results
Descriptive Monitoring
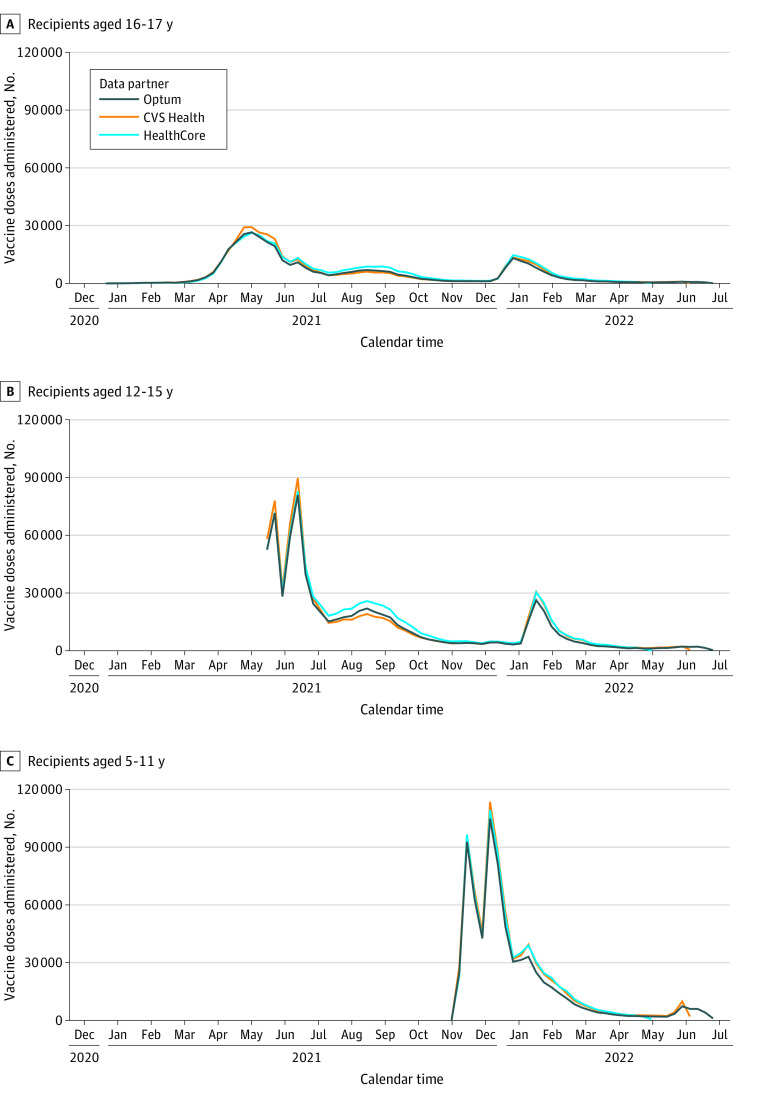
Across the 3 databases, a total of 5 901 825 doses of the BNT162b2 COVID-19 vaccine were administered to 3 017 352 children aged 5 to 17 years enrolled in these commercial health plans; 1 999 550 doses were observed in 1 000 895 enrollees in CVS Health, 2 033 212 doses in 1 078 712 enrollees in HealthCore, and 1 869 063 doses in 937 745 enrollees in Optum (Figure). Demographic characteristics of the vaccinated populations were largely similar across databases (Table 1). Of enrollees across all 3 databases, 1 510 817 (50.1%) were males, 1 506 499 (49.9%) were females, and 2 867 436 (95.0%) lived in an urban area. We observed a low incidence of the 7 outcomes monitored descriptively in all 3 databases (<25 events of each outcome in each database) (eTable 5 in Supplement 1).
Figure. Vaccine Doses Administered Among Recipients Aged 5 to 17 Years With Data From the CVS Health, HealthCore, and Optum Databases.
The study start date was the earliest Emergency Use Authorization date for the BNT162b2 vaccination for each age group: for ages 5 to 11 years, October 29, 2021; ages 12 to 15 years, May 10, 2021; and ages 16 to 17 years, December 11, 2020. For the data cut dates, CVS Health data were available through May 31, 2022; HealthCore data through May 6, 2022; and Optum data through June 25, 2022.
Table 1. Characteristics of Health Plan Members Aged 5 to 17 Years Receiving the BNT162b2 COVID-19 Vaccine From the CVS Health, HealthCore, and Optum Databasesa.
| Characteristic | No. (%) | ||
|---|---|---|---|
| CVS Health | HealthCore | Optum | |
| Total No. of recipients | 1 000 895 (100) | 1 078 712 (100) | 937 745 (100) |
| Age at first dose, y | |||
| 5-11 | 412 159 (41.2) | 416 527 (38.6) | 377 632 (40.3) |
| 12-15 | 389 450 (38.9) | 435 704 (40.4) | 370 219 (39.5) |
| 16-17 | 199 286 (19.9) | 226 481 (21.0) | 189 894 (20.3) |
| Sex | |||
| Female | 499 397 (49.9) | 538 766 (49.9) | 468 336 (49.9) |
| Male | 501 462 (50.1) | 539 946 (50.1) | 469 409 (50.1) |
| Location | |||
| Rural | 24 735 (2.5) | 87 477 (8.1) | 33 158 (3.5) |
| Urban | 973 377 (97.3) | 991 108 (91.9) | 902 951 (96.3) |
| HHS region | |||
| 1: CT, ME, NH, RI, VT | 48 112 (4.8) | 105 064 (9.7) | 45 758 (4.9) |
| 2: NJ, NY, PR, VI | 202 929 (20.3) | 85 839 (8.0) | 86 668 (9.2) |
| 3: DE, DC, MD, PA, VA, WV | 170 769 (17.1) | 114 941 (10.7) | 78 746 (8.4) |
| 4: AL, FL, GA, KY, MS, NC, SC, TN | 133 929 (13.4) | 199 701 (18.5) | 136 965 (14.6) |
| 5: IL, IN, MI, MN, OH, WI | 120 483 (12.0) | 203 959 (18.9) | 177 327 (18.9) |
| 6: AR, LA, NM, OK, TX | 76 940 (7.7) | 64 383 (6.0) | 115 398 (12.3) |
| 7: IA, KS, MO, NE | 25 288 (2.5) | 43 794 (4.1) | 59 449 (6.3) |
| 8: CO, MT, ND, SD, UT, WY | 22 981 (2.3) | 37 834 (3.5) | 55 800 (6.0) |
| 9: AZ, CA, HI, NV, AS, MP, FM, GU, MH, PW | 168 647 (16.8) | 203 081 (18.8) | 149 741 (16.0) |
| 10: AK, ID, OR, WA | 28 038 (2.8) | 20 012 (1.9) | 31 763 (3.4) |
Abbreviation: HHS, Department of Health and Human Services.
Percentages may not sum to 100% because of missing data or rounding. Data are missing for the following: sex (CVS Health only), urban or rural location (all databases), and HHS region (all databases).
Sequential Testing
Of the 13 outcomes sequentially tested, only myocarditis or pericarditis met the threshold for a statistical signal in any of the 3 databases. In the primary series analyses, a signal was detected in all databases for the age groups 12 to 15 years and 16 to 17 years after BNT162b2 COVID-19 vaccination for all definitions of the outcome myocarditis or pericarditis (Table 2). In the dose-specific analyses, a signal was detected after dose 2 in all definitions of the outcome myocarditis or pericarditis in the age groups 12 to 15 years and 16 to 17 years from all data partners. After dose 3, signals were detected for some of the definitions of myocarditis or pericarditis in the age group 16 to 17 years in the CVS Health database and those aged 12 to 15 years in the HealthCore database (eTable 4 in Supplement 1). No signals were observed in the age group 5 to 11 years.
Table 2. Sequential Testing Results by Health Outcome Following BNT162b2 Primary Series Doses for Health Plan Members Aged 5 to 17 Years in CVS Health, HealthCore, and Optum.
| Age group, y | CVS Health | HealthCore | Optum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of doses | Outcomes, No. | Person-time, d | RR | No. of doses | Outcomes, No. | Person-time, d | RR | No. of doses | Outcomes, No. | Person-time, d | RR | |
| Anaphylaxis | ||||||||||||
| 5-11 | 739 974 | <11 | 1 478 203 | 3.50 | 727 202 | <11 | 1 449 597 | 5.39 | 680 186 | 0 | 1 348 722 | 0.00 |
| 12-15 | 689 683 | <11 | 1 378 721 | 3.95 | 772 261 | <11 | 1 542 848 | 0.87 | 669 816 | <11 | 1 337 763 | 5.50 |
| 16-17 | 349 440 | <11 | 698 487 | 4.78 | 393 066 | <11 | 785 267 | 1.48 | 337 391 | <11 | 673 972 | 3.95 |
| Appendicitis | ||||||||||||
| 5-11 | 602 964 | 65 | 19 346 774 | 1.13 | 604 588 | 45 | 19 727 071 | 0.74 | 572 135 | 48 | 18 187 457 | 0.70 |
| 12-15 | 572 563 | 90 | 18 568 541 | 1.10 | 652 741 | 88 | 21 526 254 | 0.87 | 565 049 | 87 | 18 251 418 | 0.94 |
| 16-17 | 291 301 | 44 | 9 505 378 | 0.99 | 334 608 | 55 | 11 200 565 | 1.01 | 285 726 | 47 | 9 336 794 | 0.89 |
| Bell palsy | ||||||||||||
| 5-11 | 675 779 | 13 | 21 785 514 | 1.17 | 673 161 | 12 | 22 150 403 | 1.01 | 635 935 | <11 | 20 175 890 | 0.53 |
| 12-15 | 623 855 | 14 | 20 275 128 | 1.08 | 709 401 | 22 | 23 491 552 | 1.18 | 615 692 | 11 | 19 893 989 | 0.57 |
| 16-17 | 314 933 | <11 | 10 298 281 | 0.74 | 360 346 | <11 | 12 108 973 | 0.78 | 308 945 | <11 | 10 096 665 | 0.84 |
| Common thromboses with thrombocytopenia | ||||||||||||
| 5-11 | 603 631 | 0 | 15 063 681 | 0.00 | 605 189 | 0 | 15 253 024 | 0.00 | 572 768 | 0 | 14 156 025 | 0.00 |
| 12-15 | 573 509 | 0 | 14 326 988 | 0.00 | 653 821 | <11 | 16 493 102 | 1.51 | 566 008 | 0 | 14 069 963 | 0.00 |
| 16-17 | 291 770 | <11 | 7 309 572 | 3.28 | 335 214 | 0 | 8 514 719 | 0.00 | 286 191 | 0 | 7 151 741 | 0.00 |
| Deep vein thrombosis | ||||||||||||
| 5-11 | 603 606 | 0 | 15 062 839 | 0.00 | 605 162 | 0 | 15 252 307 | 0.00 | 572 759 | 0 | 14 155 801 | 0.00 |
| 12-15 | 573 474 | <11 | 14 325 938 | 0.24 | 653 791 | <11 | 16 492 356 | 1.19 | 565 957 | 0 | 14 068 697 | 0.00 |
| 16-17 | 291 691 | <11 | 7 307 399 | 0.30 | 335 152 | <11 | 8 513 141 | 1.14 | 286 133 | <11 | 7 150 307 | 0.34 |
| Disseminated intravascular coagulation | ||||||||||||
| 5-11 | 603 622 | 0 | 14 973 673 | 0.00 | 605 194 | <11 | 15 099 403 | 4.09 | 572 773 | 0 | 14 102 730 | 0.00 |
| 12-15 | 573 509 | 0 | 14 304 512 | 0.00 | 653 820 | 0 | 16 440 576 | 0.00 | 566 010 | 0 | 14 053 504 | 0.00 |
| 16-17 | 291 775 | 0 | 7 298 174 | 0.00 | 335 215 | 0 | 8 488 263 | 0.00 | 286 198 | 0 | 7 144 258 | 0.00 |
| Encephalitis or encephalomyelitis | ||||||||||||
| 5-11 | 675 862 | <11 | 21 498 513 | 0.83 | 673 262 | 0 | 21 766 827 | 0.00 | 636 003 | 0 | 20 177 981 | 0.00 |
| 12-15 | 623 934 | <11 | 20 201 159 | 1.65 | 709 494 | <11 | 23 353 158 | 1.14 | 615 778 | <11 | 19 896 658 | 0.62 |
| 16-17 | 315 002 | <11 | 10 263 177 | 1.29 | 360 397 | 0 | 12 041 649 | 0.00 | 308 985 | 0 | 10 097 982 | 0.00 |
| Immune thrombocytopenia | ||||||||||||
| 5-11 | 603 512 | <11 | 19 489 519 | 2.09 | 605 104 | <11 | 19 960 730 | 1.91 | 572 717 | <11 | 18 205 909 | 1.49 |
| 12-15 | 573 435 | <11 | 18 629 280 | 1.08 | 653 693 | 12 | 21 635 214 | 2.53 | 565 907 | <11 | 18 279 130 | 0.85 |
| 16-17 | 291 710 | <11 | 9 535 013 | 1.00 | 335 123 | <11 | 11 256 120 | 1.78 | 286 147 | <11 | 9 350 455 | 0.60 |
| Myocarditis or pericarditis | ||||||||||||
| 1- to 21-d Risk window, all settings | ||||||||||||
| 5-11 | 603 585 | <11 | 12 485 896 | 3.44 | 605 143 | <11 | 12 561 157 | 3.03 | 572 742 | <11 | 11 698 334 | 4.35 |
| 12-15 | 573 445 | 31 | 11 941 308 | 10.62b | 653 742 | 23 | 13 640 542 | 9.06b | 565 967 | 35 | 11 700 690 | 10.19b |
| 16-17 | 291 721 | 28 | 6 071 623 | 12.65b | 335 160 | 23 | 6 990 316 | 7.44b | 286 151 | 13 | 5 912 687 | 3.47b |
| 1- to 7-d Risk window, all settings | ||||||||||||
| 5-11 | 603 585 | <11 | 4 201 903 | 5.10 | 605 143 | <11 | 4 218 557 | 8.94 | 572 742 | <11 | 3 965 884 | 10.26 |
| 12-15 | 573 445 | 22 | 4 005 827 | 22.44b | 653 742 | 14 | 4 569 263 | 16.43b | 565 967 | 26 | 3 943 897 | 22.44b |
| 16-17 | 291 721 | 22 | 2 037 440 | 29.60b | 335 160 | 19 | 2 341 915 | 18.31b | 286 151 | 11 | 1 993 951 | 8.72b |
| 1- to 21-d Risk window, IP and OP-ED | ||||||||||||
| 5-11 | 603 627 | 0 | 12 415 336 | 0.00 | 605 187 | <11 | 12 519 919 | 16.62 | 572 773 | <11 | 11 698 984 | 3.49 |
| 12-15 | 573 501 | 19 | 11 924 350 | 22.98b | 653 800 | 12 | 13 627 693 | 16.86b | 566 007 | 21 | 11 701 527 | 18.64b |
| 16-17 | 291 767 | 22 | 6 063 130 | 20.76b | 335 210 | 15 | 6 983 839 | 9.07b | 286 174 | <11 | 5 913 153 | 4.98b |
| 1- to 7-d Risk window, IP and OP-ED | ||||||||||||
| 5-11 | 603 627 | 0 | 4 176 754 | 0.00 | 605 187 | <11 | 4 207 157 | 48.87 | 572 773 | <11 | 3 966 101 | 10.29 |
| 12-15 | 573 501 | 19 | 3 999 983 | 68.36b | 653 800 | <11 | 4 565 641 | 41.78b | 566 007 | 19 | 3 944 177 | 50.01b |
| 16-17 | 291 767 | 18 | 2 034 590 | 50.55b | 335 210 | 13 | 2 340 241 | 23.39b | 286 174 | <11 | 1 994 109 | 14.77b |
| Narcolepsy | ||||||||||||
| 5-11 | 603 613 | <11 | 19 492 848 | 3.00 | 605 180 | <11 | 19 963 122 | 0.47 | 572 762 | 0 | 18 207 458 | 0.00 |
| 12-15 | 573 450 | <11 | 18 629 851 | 0.50 | 653 703 | <11 | 21 635 586 | 0.73 | 565 952 | <11 | 18 280 657 | 0.76 |
| 16-17 | 291 655 | <11 | 9 533 109 | 0.82 | 335 066 | <11 | 11 254 465 | 0.76 | 286 088 | <11 | 9 348 810 | 0.60 |
| Nonhemorrhagic stroke | ||||||||||||
| 5-11 | 603 620 | 0 | 14 973 614 | 0.00 | 605 190 | <11 | 15 182 777 | 2.44 | 572 772 | 0 | 14 156 125 | 0.00 |
| 12-15 | 573 510 | 0 | 14 304 561 | 0.00 | 653 814 | 0 | 16 468 867 | 0.00 | 566 004 | 0 | 14 069 866 | 0.00 |
| 16-17 | 291 775 | <11 | 7 298 163 | 1.65 | 335 217 | 0 | 8 502 438 | 0.00 | 286 197 | <11 | 7 151 916 | 0.99 |
| Pulmonary embolism | ||||||||||||
| 5-11 | 603 628 | 0 | 15 109 619 | 0.00 | 605 187 | 0 | 15 312 029 | 0.00 | 572 776 | 0 | 14 156 230 | 0.00 |
| 12-15 | 573 504 | <11 | 14 338 531 | 1.06 | 653 808 | 0 | 16 512 834 | 0.00 | 566 002 | <11 | 14 069 828 | 0.56 |
| 16-17 | 291 724 | <11 | 7 314 350 | 1.58 | 335 190 | <11 | 8 524 694 | 1.79 | 286 159 | 0 | 7 150 951 | 0.00 |
| Seizures or convulsions | ||||||||||||
| 5-11 | 732 503 | 15 | 5 804 883 | 1.81 | 721 478 | 24 | 5 748 418 | 1.79 | 676 710 | 12 | 5 356 934 | 1.33 |
| 12-15 | 685 589 | 17 | 5 467 492 | 1.60 | 768 292 | 20 | 6 136 727 | 1.11 | 666 728 | 13 | 5 310 571 | 1.16 |
| 16-17 | 347 431 | 11 | 2 770 390 | 1.63 | 391 217 | 17 | 3 123 757 | 1.27 | 335 928 | <11 | 2 675 382 | 0.91 |
Abbreviations: RR, rate ratio; IP, inpatient; OP-ED, outpatient emergency department.
The primary analysis (dose 1 + dose 2) included all follow-up time accrued after dose 1 and dose 2 combined. CVS Health data were available through May 31, 2022; HealthCore data through May 6, 2022; and Optum data through June 25, 2022. Cell sizes of 1-10 were masked for confidentiality.
Indicates a safety signal was observed for the health outcome in the data.
Medical Record Review
Of the 153 cases of myocarditis or pericarditis among children aged 12 to 17 years, medical record review was conducted for a sample of 37 cases whose records were obtainable. Twenty-seven of these cases (73.0%) were confirmed as true cases of myocarditis or pericarditis, of which 25 patients were male, and 19 were hospitalized with a mean length of hospital stay of 2.8 days (median, 2 days). The mean time from vaccination to presentation for care for myocarditis or pericarditis was 6.8 days (median, 3 days).
Discussion
Our near–real-time monitoring results for 20 prespecified health outcomes in the pediatric population provide reassuring real-world evidence of the safety of the BNT162b2 COVID-19 vaccine in children and adolescents. The signal detected for myocarditis or pericarditis is consistent with that reported in peer-reviewed publications demonstrating an elevated risk of myocarditis or pericarditis following mRNA vaccines, especially among younger males aged 12 to 29 years.11,12,13 It should be noted that either myocarditis or pericarditis is a rare event, with an average incidence of 39.4 cases per million doses administered in children aged 5 to 17 years within 7 days after BNT162b2 COVID-19 vaccination.14,15 We did not detect a signal for myocarditis or pericarditis in younger children (aged 5-11 years), which is consistent with reports from other surveillance systems.16,17
Strengths and Limitations
This study has several strengths. First, the study included a large, geographically diverse population from 3 commercial health insurance databases in the United States. Because of the availability of more information from claims supplemented with IIS data and a short data lag from health encounters, we were able to monitor BNT162b2 COVID-19 vaccine safety in near real time. Additionally, identified cases of myocarditis or pericarditis were validated through medical record review.
The study also has some limitations. First, this study only covers data from a commercially insured pediatric population and may not be nationally representative. We used a rapid monitoring method designed for early detection of a potentially increased risk of health outcomes with limited confounding adjustment. Therefore, results of this study do not establish a causal relationship between the vaccine and health outcomes; the signals should be further evaluated. Furthermore, the study may have limited power to detect a small increase in risk of outcomes in certain subgroups with more recent authorizations such as booster doses in younger children. Analyses were not stratified by sex, which is an important demographic characteristic for certain outcomes.
Another limitation of the study is that we could not conduct record review for all outcomes included in the study because of resource, time, and legal constraints. For the myocarditis or pericarditis signals detected across the 3 databases, we reviewed medical records of a subset of identified cases because a limited number of records were obtainable. Similarly, although clinical values may have reduced outcome misclassification for some outcomes, we were unable to obtain clinical values from administrative claims without medical record review.
Conclusion
This cohort study monitored 20 health outcomes in near real time and identified a safety signal only for myocarditis or pericarditis, which was consistent with other published reports. These results provide additional evidence for the safety of the COVID-19 vaccines in the pediatric population. FDA continues to monitor vaccine safety and has expanded the framework to include additional age groups and vaccine brands with updated authorizations. The FDA BEST Initiative plays a major role in the larger US federal government vaccine safety monitoring efforts and further supports decision-making concerning the safety of COVID-19 vaccines by health care professionals and the public.
eTable 1. Database Descriptions
eTable 2. Codes for COVID-19 Vaccine Administrations, Utilized in Claims and IIS data
eTable 3. Outcomes, Age Groups, Settings, Clean Windows, Risk Windows, and Analysis Type for the Pediatric Population (Ages 5-17)
eTable 4. Sequential Testing Results in Health Plan Members Aged 5-17 years by Outcome Following BNT162b2 All Doses (Primary Series, Dose 1, Dose 2, and Third/Booster Dose) in CVS Health, HealthCore, and Optum Databases
eTable 5. Descriptive Outcome Counts, Overall and by Data Partners
eReferences
Data sharing statement
References
- 1.US Food and Drug Administration . COVID-19 vaccines. Accessed April 17, 2023. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines#authorized-vaccines
- 2.Centers for Disease Control and Prevention . CDC recommends COVID-19 vaccines for young children. Published June 18, 2022. https://www.cdc.gov/media/releases/2022/s0618-children-vaccine.html
- 3.US Food and Drug Administration . Novavax letter of authorization. Published October 19, 2022. https://www.fda.gov/media/159902/download
- 4.Menikoff J. Office of Human Research Protections (45 CRF part 46). Published January 19, 2010. https://www.hhs.gov/ohrp/index.html
- 5.Centers for Disease Control and Prevention . Immunization information systems (IIS). Published 2019. https://www.cdc.gov/vaccines/programs/iis/index.html
- 6.Center for Biologics Evaluation and Research . BEST Initiative. COVID-19 vaccine safety surveillance: protocol addendum for near real-time surveillance of COVID-19 vaccines in the pediatric population. Published December 12, 2022. https://bestinitiative.org/wp-content/uploads/2022/12/C19-Active-Monitoring-Protocol-Addendum-2022.pdf
- 7.Center for Biologics Evaluation and Research, BEST Initiative . COVID-19 vaccine safety protocol supplemental. Published 2021. https://bestinitiative.org/vaccines-and-allergenics
- 8.Kulldorff M, Davis RL, Kolczak M, Lewis E, Lieu T, Platt R. A maximized sequential probability ratio test for drug and vaccine safety surveillance. Sequential Anal. 2011;30(1):58-78. doi: 10.1080/07474946.2011.539924 [DOI] [Google Scholar]
- 9.Center for Biologics Evaluation and Research, BEST Initiative . Background rates of adverse events of special interest for COVID-19 vaccine safety monitoring. Published January 12, 2021. https://www.bestinitiative.org/wp-content/uploads/2022/01/C19-Vax-Safety-AESI-Bkgd-Rate-Protocol-FINAL-2020.pdf
- 10.Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi: 10.1016/j.vaccine.2021.11.074 [DOI] [PubMed] [Google Scholar]
- 11.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi: 10.1056/NEJMoa2110737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi: 10.15585/mmwr.mm7027e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nygaard U, Holm M, Bohnstedt C, et al. Population-based incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. Pediatr Infect Dis J. 2022;41(1):e25-e28. doi: 10.1097/INF.0000000000003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . Clinical considerations: myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults. Published 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html
- 15.Shimabukuro TT. Update on myocarditis following mRNA COVID-19 vaccination. Presentation at: Advisory Committee on Immunization Practices; June 23, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-covid-shimabukuro-508.pdf
- 16.Hause AM, Baggs J, Marquez P, et al. COVID-19 vaccine safety in children aged 5–11 years—United States, November 3–December 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1755-1760. doi: 10.15585/mmwr.mm705152a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodworth KR, Moulia D, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years—United States, November 2021. MMWR Morb Mortal Wkly Rep. 2021;70(45):1579-1583. doi: 10.15585/mmwr.mm7045e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Database Descriptions
eTable 2. Codes for COVID-19 Vaccine Administrations, Utilized in Claims and IIS data
eTable 3. Outcomes, Age Groups, Settings, Clean Windows, Risk Windows, and Analysis Type for the Pediatric Population (Ages 5-17)
eTable 4. Sequential Testing Results in Health Plan Members Aged 5-17 years by Outcome Following BNT162b2 All Doses (Primary Series, Dose 1, Dose 2, and Third/Booster Dose) in CVS Health, HealthCore, and Optum Databases
eTable 5. Descriptive Outcome Counts, Overall and by Data Partners
eReferences
Data sharing statement