Key Points
Question
In patients with chronic obstructive pulmonary disase (COPD), are combination inhalers containing long-acting muscarinic antagonists (LAMAs) and long-acting β-agonists (LABAs) associated with a reduced incidence of exacerbations and pneumonia hospitalizations compared with inhalers containing inhaled corticosteroids (ICSs) and LABAs?
Findings
In this 1:1 propensity score–matched cohort study of 60 432 patients with COPD in a large US commercial insurance claims database, LAMA-LABA therapy was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation and a 20% reduction in the rate of first pneumonia hospitalization compared with ICS-LABA therapy.
Meaning
The results of this study suggest that LAMA-LABA therapy should be preferred to ICS-LABA for patients with COPD.
Abstract
Importance
Clinical guidelines on chronic obstructive pulmonary disease (COPD) recommend inhalers containing long-acting muscarinic antagonists (LAMAs) and long-acting β-agonists (LABAs) over inhalers containing inhaled corticosteroids (ICSs) and LABAs. However, data from randomized clinical trials comparing these combination inhalers (LAMA-LABAs vs ICS-LABAs) have been conflicting and raised concerns of generalizability.
Objective
To assess whether LAMA-LABA therapy is associated with reduced COPD exacerbations and pneumonia hospitalizations compared with ICS-LABA therapy in routine clinical practice.
Design, Setting, and Participants
This was a 1:1 propensity score–matched cohort study using Optum’s Clinformatics Data Mart, a large commercial insurance–claims database. Patients must have had a diagnosis of COPD and filled a new prescription for a combination LAMA-LABA or ICS-LABA inhaler between January 1, 2014, and December 31, 2019. Patients younger than 40 years were excluded, as were those with a prior diagnosis of asthma. The current analysis was performed from February 2021 to March 2023.
Exposures
Combination LAMA-LABA inhalers (aclidinium-formoterol, glycopyrronium-formoterol, glycopyrronium-indacaterol, tiotropium-olodaterol, or umeclidinium-vilanterol) and combination ICS-LABA inhalers (budesonide-formoterol, fluticasone-salmeterol, fluticasone-vilanterol, or mometasone-formoterol).
Main Outcome
The primary effectiveness outcome was first moderate or severe COPD exacerbation, and the primary safety outcome was first pneumonia hospitalization. Propensity score matching was used to control for confounding between the 2 groups. Logistic regression analysis was used to estimate propensity scores. Hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards models stratified on matched pairs.
Results
Among 137 833 patients (mean [SD] age, 70.2 [9.9] years; 69 530 [50.4%] female) (107 004 new ICS-LABA users and 30 829 new LAMA-LABA users), 30 216 matched pairs were identified for the primary analysis. Compared with ICS-LABA use, LAMA-LABA use was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation (HR, 0.92; 95% CI, 0.89-0.96) and a 20% reduction in the rate of first pneumonia hospitalization (HR, 0.80; 95% CI, 0.75-0.86). These findings were robust across a range of prespecified subgroup and sensitivity analyses.
Conclusion
In this cohort study, LAMA-LABA therapy was associated with improved clinical outcomes compared with ICS-LABA therapy, suggesting that LAMA-LABA therapy should be preferred for patients with COPD.
This cohort study uses commercial insurance claims to compare the incidence of first moderate or severe chronic obstructive pulmonary disease (COPD) exacerbation and first pneumonia hospitalization among patients with COPD who started using inhalers with long-acting muscarinic antagonists and long-acting β-agonists (LABAs) vs inhalers containing inhaled corticosteroids and LABAs.
Introduction
Clinical guidelines for chronic obstructive pulmonary disease (COPD) recommend inhalers containing long-acting muscarinic antagonists (LAMAs) and long-acting β-agonists (LABAs) over inhalers containing inhaled corticosteroids (ICSs) and LABAs.1,2 Data from several randomized clinical trials,3,4,5 particularly the Effect of Indacaterol-Glycopyrronium Versus Fluticasone Salmeterol on COPD Exacerbations (FLAME) trial in 2016,6 found combined LAMA-LABA therapy to result in fewer exacerbations, longer time to first moderate or severe exacerbation, and fewer pneumonias when compared with combined ICS-LABA therapy. However, 2 large randomized clinical trials showed that ICS-LABA therapy, while leading to more pneumonias than LAMA-LABA therapy, reduced the rate of COPD exacerbations (eTable 1 in Supplement 1).7,8
Uncertainty about optimal COPD therapy has been further compounded by problems of generalizability in clinical trials for COPD. Populations treated in routine clinical practice tend to be older, include more women, have fewer spirometry measurements (though higher forced expiratory volume in 1 second when spirometry is performed), and have more cardiovascular comorbidities compared with patients enrolled in randomized clinical trials.9,10,11,12,13,14 Run-in periods, during which patients are taken off their usual inhalers and given an alternative treatment or placebo before randomization, have also raised concerns about external validity.15,16
When data from randomized clinical trials are conflicting or lack generalizability, high-quality observational studies can serve as an important adjunct in shaping clinical practice guidelines.17 We performed a cohort study using commercial insurance claims to compare the incidence of first moderate or severe COPD exacerbation and first pneumonia hospitalization among typical patients with COPD in routine care who were started on LAMA-LABA inhalers vs ICS-LABA inhalers.
Methods
Study Cohort
Our study cohort included enrollees in Optum’s deidentified Clinformatics Data Mart, a large US commercial insurance claims database. We included new initiators of combination LABA-LAMA and combination ICS-LABA inhalers from January 1, 2014, to December 31, 2019 (eTable 2 in Supplement 1). The current analysis was performed from February 2021 to March 2023. This study was approved by the Mass General Brigham Institutional Review Board and granted a waiver of informed consent because only deidentified claims data were used. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Patients were required to have 365 days of continuous enrollment in the database prior to cohort entry. Patients entered the cohort when they filled a new (index) prescription for a LAMA-LABA inhaler (containing aclidinium-formoterol, glycopyrronium-formoterol, glycopyrronium-indacaterol, tiotropium-olodaterol, or umeclidinium-vilanterol) or ICS-LABA inhaler (containing budesonide-formoterol, fluticasone-salmeterol, fluticasone-vilanterol, or mometasone-formoterol) with no use of a combined LAMA-LABA inhaler (or separate LAMA and LABA inhalers within 30 days of one another) or of a combined ICS-LABA inhaler (or separate ICS and LABA inhalers within 30 days of one another) during the 365 days before cohort entry. We also excluded patients who received an ICS-LAMA-LABA inhaler during the look-back window, as well as patients who filled an ICS or LAMA prescription on the cohort entry date, since these patients were initiating triple therapy rather than dual therapy. All patients were required to have a diagnosis of COPD, defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 491.xx, 492.xx, or 496, and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes J41.x, J42, J43.x, J44.x. Those with a prior diagnosis of asthma (ICD-9-CM code 493.xx or ICD-10-CM code J45.xx) and those younger than 40 years of age were excluded.
Adjustment for Confounders
We measured potential confounders during the 365 days before cohort entry and included them in a propensity score model. These included age (at cohort entry), sex, year of cohort entry, number of moderate COPD exacerbations, number of severe COPD exacerbations, GOLD (Global Initiative for Obstructive Lung Disease) stage E (which includes stages C and D from prior guidelines and is defined as at least 2 moderate COPD exacerbations and/or 1 severe COPD exacerbation in the prior year), number of short-acting β-agonist (SABA) fills (prescriptions filled), number of short-acting muscarinic antagonist (SAMA) fills, number of SAMA-SABA fills, number of pneumonia hospitalizations, number of antibiotic fills (eTable 3 in Supplement 1), home oxygen or equipment claim, whether spirometry measurements were taken, whether the index prescription was written by a pulmonologist, smoking, pulmonary rehabilitation, baseline use of maintenance medications (including LAMA, LABA, and ICS inhalers, roflumilast, chronic azithromycin [an azithromycin prescription for 30 days or more] and chronic prednisone [a prednisone prescription for 30 days or more]), events within 30 days before cohort entry (including moderate or severe COPD exacerbations, antibiotic fills, and ICS fills), and eosinophil counts. We also adjusted for other comorbidities, including a combined comorbidity score,18 a frailty index score,19 obstructive sleep apnea, hypertension, diabetes, obesity, coronary artery disease, peripheral vascular disease, congestive heart failure, gastroesophageal reflux disease, kidney failure, osteoporosis, dementia or other neurological disease, nonmetastatic malignant cancer, and metastatic solid organ tumor. Propensity scores also included measurements of health care use (emergency department visits, hospitalizations, 90-day readmissions, office visits, prescription drug claims, basic or comprehensive metabolic panels, electrocardiograms, echocardiograms, computed tomographic scans, bronchoscopies, colonoscopies, mammography, bone-mineral density scans, and influenza vaccination), and nonpulmonary medication use (statins, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, thiazide diuretics, loop diuretics, proton pump inhibitors, and histamine 2 receptor blockers). Data on self-reported race were not available for analysis.
Outcomes and Follow-up
The primary effectiveness outcome was the incidence of a first moderate or severe COPD exacerbation. A severe exacerbation was defined as a hospitalization with a primary diagnosis of COPD (using the same codes as above (see Study Cohort), with the exclusion of ICD-9-CM code 491.20, obstructive chronic bronchitis without exacerbation). A moderate COPD exacerbation was defined as a prednisone prescription fill for 5 to 14 days (with no prednisone fills or COPD hospitalizations in the prior 14 days, to avoid counting exacerbations twice). Patients who filled a prednisone prescription and were subsequently admitted to the hospital for a COPD exacerbation within 14 days were considered to have had a severe COPD exacerbation. This distinction between moderate COPD exacerbations requiring oral steroids and severe COPD exacerbations requiring hospitalization has been widely used in clinical guidelines1 and randomized clinical trials.6,7,8 The primary safety outcome was first hospitalization for pneumonia (ICD-9-CM code 480.x-488.xx or ICD-10-CM codes J09.X1, J10.xx-J18.x, A01.03, A02.22, A37.01, A37.11 A37.81, A37.91, A54.84, B01.2, B05.2, B06.81, B77.81, J85.1, or J22). The outcome definitions chosen for COPD exacerbations and pneumonia follow approaches in other studies using electronic health care data20,21,22,23,24,25,26 and have good positive predictive values27,28,29 (eMethods in Supplement 1).
We followed up patients for up to 1 year after cohort entry or until any of the following occurred: discontinuation of treatment (with a 60-day grace period between prescription fills), a switch from exposure to referent or vice versa, the addition of an ICS or LAMA, initiation of triple therapy with an ICS-LAMA-LABA, death, or the end of insurance coverage (eFigure 1 in Supplement 1).
Statistical Analysis
We used 1:1 propensity score matching to control for confounding between the 2 groups using nearest-neighbor matching without replacement and a caliper of 0.01 on the propensity scale. Logistic regression was used to estimate propensity scores. We estimated hazard ratios (HRs) and 95% CIs using Cox proportional hazards models stratified on matched pairs.30,31 For sensitivity analyses that adjusted for basic confounders and deciles of propensity scores, we used a Cox proportional hazards regression model. We estimated rate differences using a modified generalized linear model. We compared rates of total COPD exacerbations and pneumonia hospitalizations via a random effects logistic regression with patient-specific intercepts to account for repeated events for a given patient (CIs were computed using nonparametric bootstrap).
We performed several prespecified secondary, subgroup, and sensitivity analyses. As secondary outcomes, we analyzed first moderate COPD exacerbation separately from first severe COPD exacerbation. We also analyzed the rate of total moderate or severe COPD exacerbations (as composite and separate outcomes) and the rate of total hospitalizations for pneumonia. Subgroup analysis included patients with 1 or more COPD exacerbations at baseline, 1 or more COPD hospitalizations, and GOLD stage E disease. We analyzed patients with measurements of peripheral blood eosinophils in the 6 months prior to cohort entry and categorized them based on their most recent laboratory values: less than or equal to 100/μL, greater than 100/μL but less than or equal to 300/μL, and greater than 300/μL (to convert to ×109/L, multiply by 0.001) (eMethods in Supplement 1). We performed post hoc subgroup analyses based on the individual inhaler prescribed, whether patients had undergone baseline spirometry measurement, and whether patients received their index prescription from a pulmonologist. We also performed several sensitivity analyses that are described in further detail in the eMethods in Supplement 1. All statistical analyses were conducted using the Aetion Evidence Platform, version 4.73 (Aetion Inc), Stata, version 16.1 (StataCorp LLC), and RStudio, version 4.2 (Posit PBC).
Results
Among 137 833 patients included in the cohort (mean [SD] age, 70.2 [9.9] years; 69 530 [50.4%] female), 30 829 started LAMA-LABA therapy and 107 004 started ICS-LABA therapy (Table 1; eFigure 2 in Supplement 1). Women were less likely to receive LAMA-LABA therapy than men (47.3% vs 52.6%) and more likely to receive ICS-LABA therapy (51.3% vs 48.6%). Patients starting on LAMA-LABA therapy had fewer moderate exacerbations (mean [SD], 0.37 [0.78] vs 0.41 [0.81] exacerbations), severe exacerbations (mean [SD], 0.04 [0.23] vs 0.08 [0.32] exacerbations), and pneumonia hospitalizations (mean [SD], 0.09 [0.36] vs 0.12 [0.44] exacerbations) and were less likely to have had baseline GOLD stage E disease (3869 patients [12.5%] vs 17 842 patients [16.7%]). By contrast, LAMA-LABA (n = 30 829) users were more likely than ICS-LABA users (n = 107 004) to have undergone baseline spirometry measurement (15 478 patients [50.2%] vs 32 890 patients [30.7%]) and more likely to have received their index prescription from a pulmonologist (3500 patients [11.4%] vs 4843 patients [4.5%]). The 2 treatment groups were otherwise largely similar in terms of baseline comorbidities, health care use, and medication use. Propensity score matches were found for 30 216 patients (Table 1). The groups were well-balanced after matching (eFigure 3 in Supplement 1).
Table 1. Characteristics of Patients Initiating Combination Maintenance Inhalers Included in the Cohort.
| Characteristic | Total eligible cohort | 1:1 Propensity score–matched cohort | ||||
|---|---|---|---|---|---|---|
| No. (%) | Absolute standardized difference | No. (%) | Absolute standardized difference | |||
| ICS-LABA (n = 107 004) | LAMA-LABA (n = 30 829) | ICS-LABA (n = 30 216) | LAMA-LABA (n = 30 216) | |||
| Demographic characteristics | ||||||
| Age, mean (SD), y | 70.2 (10.0) | 70.4 (9.4) | 0.023 | 70.3 (9.7) | 70.4 (9.5) | 0.005 |
| Sexa | ||||||
| Female | 54 937 (51.3) | 14 593 (47.3) | 0.080 | 14 312 (47.4) | 14 378 (47.6) | 0.005 |
| Male | 52 048 (48.6) | 16 230 (52.6) | 0.080 | 15 900 (52.6) | 15 832 (52.4) | 0.004 |
| Year of cohort entry | ||||||
| 2014 | 14 782 (13.8) | 205 (0.7) | 0.525 | 200 (0.7) | 205 (0.7) | 0.002 |
| 2015 | 14 051 (13.1) | 1608 (5.2) | 0.277 | 1684 (5.6) | 1608 (5.3) | 0.011 |
| 2016 | 16 073 (15.0) | 3390 (11.0) | 0.120 | 3406 (11.3) | 3385 (11.2) | 0.002 |
| 2017 | 19 823 (18.5) | 7168 (23.3) | 0.116 | 7043 (23.3) | 7069 (23.4) | 0.002 |
| 2018 | 20 578 (19.2) | 8753 (28.4) | 0.216 | 8489 (28.1) | 8546 (28.3) | 0.004 |
| 2019 | 21 697 (20.3) | 9705 (31.5) | 0.258 | 9394 (31.1) | 9403 (31.1) | 0.001 |
| Clinical characteristics in the year before cohort entry | ||||||
| Moderate COPD exacerbations, mean (SD) | 0.41 (0.81) | 0.37 (0.78) | 0.062 | 0.37 (0.76) | 0.37 (0.78) | 0.005 |
| Severe COPD exacerbations, mean (SD) | 0.08 (0.32) | 0.04 (0.23) | 0.118 | 0.04 (0.23) | 0.04 (0.23) | 0.001 |
| GOLD stage E diseaseb | 17 842 (16.7) | 3869 (12.5) | 0.117 | 3893 (12.9) | 3822 (12.6) | 0.007 |
| SABA fills, mean (SD) | 1.54 (2.79) | 1.46 (2.69) | 0.030 | 1.47 (2.60) | 1.46 (2.71) | 0.003 |
| SAMA fills, mean (SD) | 0.07 (0.69) | 0.06 (0.57) | 0.025 | 0.06 (0.55) | 0.06 (0.57) | 0.005 |
| SAMA-SABA fills, mean (SD) | 0.48 (1.86) | 0.37 (1.58) | 0.066 | 0.38 (1.56) | 0.37 (1.59) | 0.008 |
| Pneumonia hospitalizations, mean (SD) | 0.12 (0.44) | 0.09 (0.36) | 0.091 | 0.09 (0.35) | 0.09 (0.36) | 0.003 |
| Antibiotic fills, mean (SD) | 1.57 (1.89) | 1.40 (1.80) | 0.092 | 1.42 (1.79) | 1.41 (1.81) | 0.011 |
| Home oxygen or equipment claim | 19 247 (18.0) | 5592 (18.1) | 0.004 | 5482 (18.1) | 5441 (18.0) | 0.004 |
| Underwent spirometry measurement | 32 890 (30.7) | 15 478 (50.2) | 0.405 | 15 080 (49.9) | 14 883 (49.3) | 0.013 |
| Index prescription by pulmonologist | 4843 (4.5) | 3500 (11.4) | 0.255 | 2912 (9.6) | 3019 (10.0) | 0.012 |
| Smoking | 58 838 (55.0) | 20 120 (65.3) | 0.211 | 19 561 (64.7) | 19 582 (64.8) | 0.001 |
| Underwent pulmonary rehabilitation | 495 (0.5) | 222 (0.7) | 0.034 | 221 (0.7) | 208 (0.7) | 0.005 |
| Baseline use of maintenance medication | ||||||
| LAMA | 14 818 (13.8) | 4608 (14.9) | 0.031 | 4575 (15.1) | 4478 (14.8) | 0.009 |
| LABA | 430 (0.4) | 199 (0.6) | 0.034 | 202 (0.7) | 178 (0.6) | 0.010 |
| ICS | 3392 (3.2) | 1095 (3.6) | 0.021 | 1116 (3.7) | 1047 (3.5) | 0.012 |
| Chronic azithromycin | 432 (0.4) | 143 (0.5) | 0.009 | 138 (0.5) | 139 (0.5) | <0.001 |
| Roflumilast | 306 (0.3) | 74 (0.2) | 0.009 | 82 (0.3) | 73 (0.2) | 0.006 |
| Chronic prednisone | 5433 (5.1) | 1393 (4.5) | 0.026 | 1385 (4.6) | 1371 (4.5) | 0.002 |
| Events within 30 d before cohort entry | ||||||
| Moderate or severe COPD exacerbation | 17 412 (16.3) | 2916 (9.5) | 0.205 | 2898 (9.6) | 2906 (9.6) | 0.001 |
| Antibiotic fill | 29 615 (27.7) | 5699 (18.5) | 0.219 | 5682 (18.8) | 5677 (18.8) | <0.001 |
| ICS fill | 939 (0.9) | 378 (1.2) | 0.034 | 366 (1.2) | 350 (1.2) | 0.005 |
| Baseline eosinophil counts | ||||||
| CBC with differential performed | 60 528 (56.6) | 18 481 (59.9) | 0.069 | 18 150 (60.1) | 18 065 (59.8) | 0.006 |
| Eosinophil results available | 25 202 (23.6) | 7965 (25.8) | 0.053 | 7788 (25.8) | 7795 (25.8) | 0.001 |
| Eosinophil categories, cells/μL | ||||||
| ≤100 | 8719 (8.1) | 2712 (8.8) | 0.023 | 2632 (8.7) | 2651 (8.8) | 0.002 |
| >100 to ≤300 | 11 756 (11.0) | 3860 (12.5) | 0.048 | 3731 (12.3) | 3771 (12.5) | 0.004 |
| >300 | 4727 (4.4) | 1392 (4.5) | 0.005 | 1425 (4.7) | 1373 (4.5) | 0.008 |
| Other comorbidities | ||||||
| Combined comorbidity index score, mean (SD)c | 2.54 (2.67) | 2.60 (2.64) | 0.021 | 2.61 (2.63) | 2.59 (2.64) | 0.005 |
| Frailty index score, mean (SD)d | 0.18 (0.06) | 0.18 (0.06) | 0.066 | 0.18 (0.06) | 0.18 (0.06) | 0.005 |
| Obstructive sleep apnea | 14 991 (14.0) | 5364 (17.4) | 0.093 | 5214 (17.3) | 5198 (17.2) | 0.001 |
| Hypertension | 82 633 (77.2) | 24 120 (78.2) | 0.024 | 23 591 (78.1) | 23 619 (78.2) | 0.002 |
| Diabetes | 35 226 (32.9) | 9619 (31.2) | 0.037 | 9490 (31.4) | 9466 (31.3) | 0.002 |
| Obesity | 19 977 (18.7) | 6031 (19.6) | 0.023 | 5986 (19.8) | 5921 (19.6) | 0.005 |
| Coronary artery disease | 35 468 (33.1) | 11 156 (36.2) | 0.064 | 10 806 (35.8) | 10 844 (35.9) | 0.003 |
| Peripheral vascular disease | 29 569 (27.6) | 9062 (29.4) | 0.039 | 8901 (29.5) | 8864 (29.3) | 0.003 |
| Congestive heart failure | 26 496 (24.8) | 6778 (22.0) | 0.066 | 6620 (22.0) | 6650 (22.0) | 0.001 |
| GERD | 29 774 (27.8) | 8900 (28.9) | 0.023 | 8748 (29.0) | 8725 (28.9) | 0.002 |
| Kidney failure | 23 257 (21.7) | 6435 (20.9) | 0.021 | 6353 (21.0) | 6331 (21.0) | 0.002 |
| Osteoporosis | 8510 (8.0) | 2603 (8.4) | 0.018 | 2589 (8.6) | 2546 (8.4) | 0.005 |
| Dementia or other neurological disease | 7842 (7.3) | 2077 (6.7) | 0.023 | 1984 (6.6) | 2049 (6.8) | 0.009 |
| Cancer, nonmetastatic | 14 349 (13.4) | 4796 (15.6) | 0.061 | 4636 (15.3) | 4634 (15.3) | 0.000 |
| Metastatic solid organ tumor | 2839 (2.7) | 973 (3.2) | 0.030 | 943 (3.1) | 934 (3.1) | 0.002 |
| Health care use in baseline period | ||||||
| Emergency department visits, mean (SD) | 2.11 (3.97) | 1.76 (3.15) | 0.098 | 1.78 (3.06) | 1.77 (3.16) | 0.004 |
| Hospitalizations, mean (SD) | 0.64 (1.32) | 0.46 (1.01) | 0.155 | 0.46 (1.00) | 0.46 (1.01) | 0.001 |
| 90-d Readmissions, mean (SD) | 0.13 (0.62) | 0.09 (0.46) | 0.078 | 0.09 (0.48) | 0.09 (0.46) | 0.004 |
| Office visits, mean (SD) | 10.22 (8.14) | 11.18 (8.15) | 0.117 | 11.20 (8.54) | 11.11 (8.09) | 0.012 |
| Prescription drug claims, mean (SD) | 47.99 (40.10) | 45.75 (36.28) | 0.059 | 45.83 (37.18) | 45.81 (36.37) | 0.001 |
| BMP or CMP | 87 680 (81.9) | 26 263 (85.2) | 0.088 | 25 775 (85.3) | 25 703 (85.1) | 0.007 |
| Electrocardiogram | 64 673 (60.4) | 18 666 (60.5) | 0.002 | 18 214 (60.3) | 18 294 (60.5) | 0.005 |
| Echocardiogram | 35 057 (32.8) | 10 334 (33.5) | 0.016 | 10 054 (33.3) | 10 100 (33.4) | 0.003 |
| CT scan | 44 914 (42.0) | 14 071 (45.6) | 0.074 | 13 674 (45.3) | 13 700 (45.3) | 0.002 |
| Bronchoscopy | 2732 (2.6) | 1007 (3.3) | 0.042 | 947 (3.1) | 966 (3.2) | 0.004 |
| Colonoscopy | 736 (0.7) | 156 (0.5) | 0.024 | 167 (0.6) | 155 (0.5) | 0.005 |
| Mammography | 802 (0.7) | 89 (0.3) | 0.064 | 92 (0.3) | 88 (0.3) | 0.002 |
| Bone-mineral density scan | 8338 (7.8) | 2642 (8.6) | 0.028 | 2632 (8.7) | 2591 (8.6) | 0.005 |
| Influenza vaccination | 37 788 (35.3) | 11 120 (36.1) | 0.016 | 10 883 (36.0) | 10 866 (36.0) | 0.001 |
| Nonpulmonary medications | ||||||
| Statins | 60 209 (56.3) | 18 033 (58.5) | 0.045 | 17 585 (58.2) | 17 637 (58.4) | 0.003 |
| β-Blockers | 45 368 (42.4) | 13 455 (43.6) | 0.025 | 13 052 (43.2) | 13 132 (43.5) | 0.006 |
| ACE inhibitors | 35 075 (32.8) | 10 026 (32.5) | 0.005 | 9877 (32.7) | 9844 (32.6) | 0.002 |
| ARBs | 22 807 (21.3) | 7125 (23.1) | 0.043 | 7021 (23.2) | 6969 (23.1) | 0.004 |
| Calcium channel blockers | 31 047 (29.0) | 9016 (29.2) | 0.005 | 8759 (29.0) | 8835 (29.2) | 0.006 |
| Thiazide diuretics | 25 083 (23.4) | 7429 (24.1) | 0.015 | 7299 (24.2) | 7278 (24.1) | 0.002 |
| Loop diuretics | 26 548 (24.8) | 7044 (22.8) | 0.046 | 6953 (23.0) | 6919 (22.9) | 0.003 |
| Proton-pump inhibitors | 37 276 (34.8) | 10 231 (33.2) | 0.035 | 10 145 (33.6) | 10 078 (33.4) | 0.005 |
| H2-receptor blockers | 8466 (7.9) | 2440 (7.9) | <0.001 | 2410 (8.0) | 2390 (7.9) | 0.002 |
Abbreviations: ACE, angiotensin converting; ARB, angiotensin-receptor blocker; BMP, basic metabolic panel; CBC, complete blood cell count; CMP, complete metabolic panel; COPD, chronic obstructive pulmonary disease; CT, computed tomographic; GERD, gastroesophageal reflux disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; H2, histamine 2; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist.
SI conversion factor: To convert microliters to ×109/L, multiply by 0.001.
In the full cohort, data on sex were missing for 25 people (19 in the ICS-LABA group and 6 in the LAMA-LABA group). In the propensity score–matched cohort, data on sex were missing for 10 people (4 in the ICS-LABA group and 6 in the LAMA-LABA group).
Those with GOLD stage E disease had at least 2 moderate exacerbations and/or 1 severe exacerbation. This category, which was first included in the 2023 guidelines,2 includes GOLD stages C and D from prior guidelines.
Range, 0 to 27, with 0 representing no comorbidities included in the index and 27 representing all comorbidities included in the index.
Range, 0 to 1 based on the proportion of 93 different health deficits met, with 0 representing lower frailty and 1 representing higher frailty.
Primary Effectiveness and Safety End Points
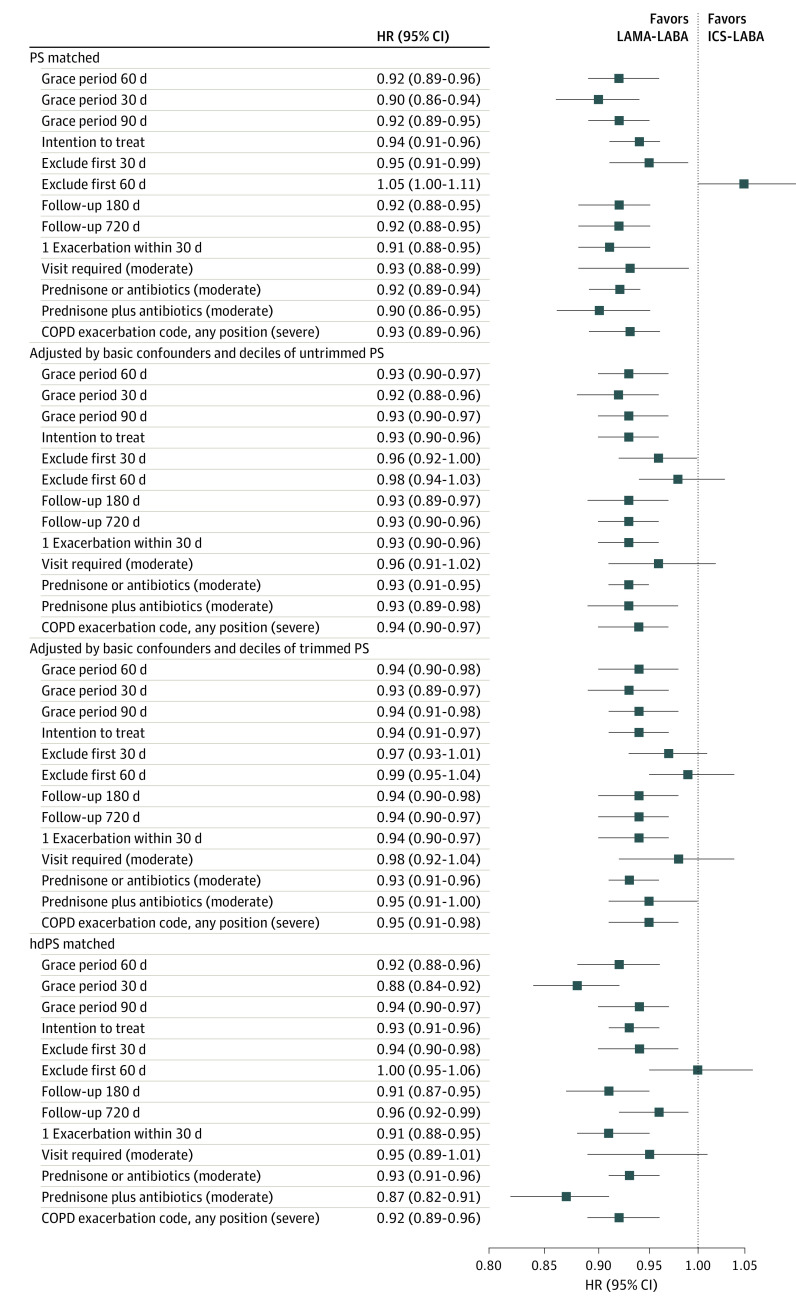
Among patients in the matched cohort, 8151 had a first moderate or severe exacerbation during 23 983 person-years of follow-up, giving a crude incidence of 340.0 events per 1000 person-years. We found an 8% reduction in the rate of first moderate or severe COPD exacerbation among new users of LAMA-LABA therapy compared with new users of ICS-LABA therapy (HR, 0.92; 95% CI, 0.89-0.96) (Table 2). The absolute rate reduction was 43.0 events per 1000 person-years (95% CI, −90.6 to 4.7 events per 1000 person-years).
Table 2. First Chronic Obstructive Pulmonary Disease Exacerbation and Pneumonia Hospitalization in Patients Using LAMA-LABAs vs ICS-LABAs in the Propensity Score–Matched Cohort.
| Event | ICS-LABA–associated events per 1000 PY | LAMA-LABA–associated events per 1000 PY | HR (95% CI) |
|---|---|---|---|
| Moderate or severe COPD exacerbation | 363.6 | 320.6 | 0.92 (0.89-0.96) |
| Moderate COPD exacerbation | 321.5 | 286.6 | 0.93 (0.90-0.97) |
| Severe COPD exacerbation | 47.3 | 39.8 | 0.85 (0.77-0.94) |
| Pneumonia hospitalization | 104.0 | 82.1 | 0.80 (0.75-0.86) |
Abbreviations: COPD, chronic obstructive pulmonary disease; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; PY, person-years.
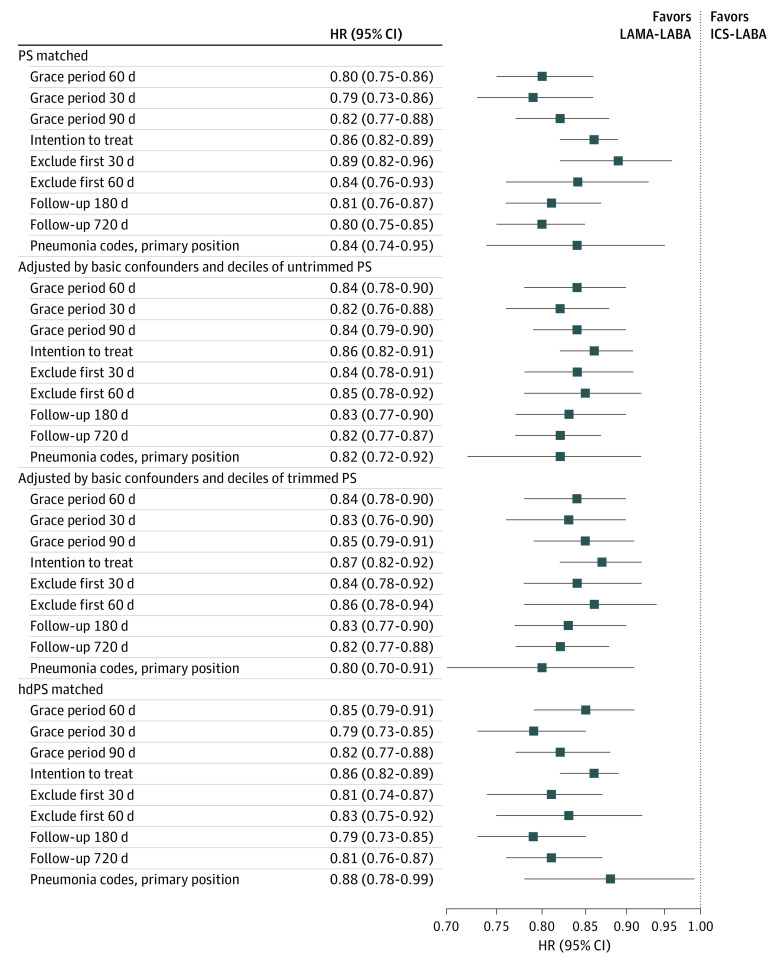
The primary safety end point of first hospitalization for pneumonia occurred 2378 times during 25 891 person-years of follow-up, giving a crude incidence of 91.8 events per 1000 person-years. There was a 20% reduction in the rate of first pneumonia hospitalization among new users of LAMA-LABA therapy compared with new users of ICS-LABA therapy (HR, 0.80; 95% CI, 0.75-0.86). This represented an absolute rate reduction of 21.9 events per 1000 person-years (95% CI, −47.3 to 3.4 events per 1000 person-years).
Mean follow-up time and reasons for censoring in the primary effectiveness and safety analyses are provided in eTables 4 and 5 in Supplement 1. The HRs for the primary end points are provided in Figure 1 and Figure 2. Sensitivity analyses yielded effect sizes similar to those in the primary analysis, including in our intention-to-treat analysis.
Figure 1. Sensitivity Analysis Showing the Incidence of First Moderate or Severe Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Among Patients Treated With a Long-Acting Muscarinic Antagonist Plus a Long-Acting β-Agonist (LAMA-LABA) vs Those Receiving Inhaled-Corticosteroid Plus a LABA (ICS-LABA).
The primary effectiveness analysis included a grace period of 60 days. COPD codes refers to International Classification of Diseases, Ninth Revision, Clinical Modification, and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification codes for COPD. hdPS indicates high-dimensional propensity score; HR, hazard ratio; and PS, propensity score.
Figure 2. Sensitivity Analysis Showing the Incidence of First Pneumonia Hospitalization Among Patients Treated With a Long-Acting Muscarinic Antagonist Plus a Long-Acting β-Agonist (LAMA-LABA) vs Those Receiving an Inhaled Corticosteroid Plus a LABA (ICS-LABA).
The primary effectiveness analysis included a grace period of 60 days. Pneumonia codes refer to International Classification of Diseases, Ninth Revision, Clinical Modification, and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification, codes for pneumonia. hdPS indicates high-dimensional propensity score; HR, hazard ratio; and PS, propensity score.
Total COPD Exacerbations and Pneumonia Hospitalizations
Patients had 10 993 total moderate or severe exacerbations during 26 806 person-years of follow up, yielding a crude incidence of 410.1 events per 1000 person-years. Therapy with LAMA-LABA was associated with a 5% reduction in the overall rate of moderate or severe COPD exacerbations (incidence rate ratio [IRR], 0.95; 95% CI, 0.93-0.98). Patients had a total of 2985 pneumonia hospitalizations during 26 806 person-years of follow-up for a crude incidence of 111.4 events per 1000 person-years. The overall rate of pneumonia hospitalizations among LAMA-LABA users was 17% lower than among ICS-LABA users (IRR, 0.83 95% CI, 0.78-0.88).
Separate Analyses of Composite COPD End Point
When analyzing moderate and severe COPD exacerbations separately, we found that LAMA-LABA therapy was associated with a 7% reduction in the rate of first moderate exacerbation (HR, 0.93; 95% CI, 0.90-0.97) and a 4% reduction in the overall rate of moderate COPD exacerbations (IRR, 0.96; 95% CI, 0.93-1.00) compared with ICS-LABA therapy (eFigure 4 in Supplement 1). In addition, LAMA-LABA therapy was associated with a 15% reduction in the rate of first severe exacerbation (HR, 0.85; 95% CI, 0.77-0.94) and 10% reduction in the overall rate of severe COPD exacerbations (IRR, 0.90; 95% CI, 0.80-1.01) compared with ICS-LABA therapy (eFigure 5 in Supplement 1).
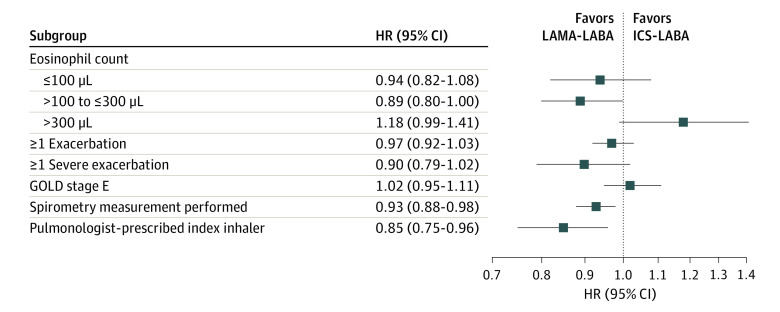
Subgroup Analysis
ICS-LABA therapy was not associated with lower rates of first moderate or severe COPD exacerbation for any subgroup studied, including those with GOLD stage E disease (HR, 1.02; 95% CI, 0.95-1.11) and those with eosinophil counts greater than 300/μL (HR, 1.18; 95% CI, 0.99-1.41) (Figure 3; eFigure 6 in Supplement 1). LAMA-LABA therapy was associated with lower rates of first moderate or severe COPD exacerbation among those who underwent baseline spirometry measurements (HR, 0.93, 95% CI, 0.88-0.98) and those who received their index prescription from a pulmonologist (HR, 0.85; 95% CI, 0.75-0.96).
Figure 3. Subgroup Analysis Showing the Incidence of First Moderate or Severe Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Among Patients Who Were Treated With a Long-Acting Muscarinic Antagonist Plus a Long-Acting β-Agonist (LAMA-LABA) vs Those Receiving an Inhaled Corticosteroid Plus a LABA (ICS-LABA).
GOLD indicates Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio.
Point estimates for all subgroup analyses of pneumonia hospitalizations favored the LAMA-LABA group over the ICS-LABA group, although the CIs were wide for some subgroups, indicating nonsignificance (eFigure 7 in Supplement 1).
LAMA-LABA Therapy vs Individual ICS-LABA Inhalers
When comparing LAMA-LABA therapy with individual ICS-LABAs on the outcomes of first moderate or severe COPD exacerbation (eFigure 8 in Supplement 1) and first pneumonia hospitalization (eFigure 9 in Supplement 1), point estimates favored LAMA-LABA therapy for nearly all individual inhalers, although CIs were wide for some products, indicating nonsignificance. The 2 most commonly used ICS-LABAs in the cohort were fluticasone-salmeterol (Advair Diskus) and budesonide-formoterol (Symbicort). LAMA-LABAs were associated with a lower hazard of first moderate or severe COPD exacerbation (HR, 0.91; 95% CI, 0.86-0.96) and a lower hazard of first pneumonia hospitalization (HR, 0.75; 95% CI, 0.69-0.83) compared with Advair Diskus and a lower hazard of first moderate or severe COPD exacerbation (HR 0.92; 95% CI, 0.88-0.96) and a lower hazard of first pneumonia hospitalization (HR 0.83; 95% CI, 0.77-0.89) compared with Symbicort.
Discussion
In this cohort study, we found a lower incidence of moderate or severe COPD exacerbations and a lower incidence of pneumonia hospitalizations in patients with COPD who were prescribed LAMA-LABA therapy compared with ICS-LABA therapy in routine care. This association persisted after adjustment for a wide variety of clinical and demographic factors.
Our findings are largely consistent with those of the FLAME trial,6 which found a 22% reduction in the rate of first moderate or severe COPD exacerbation in patients receiving LAMA-LABA therapy compared with ICS-LABA therapy and a 17% reduction in the annual rate of moderate or severe exacerbations.6 However, the decrease in exacerbations observed in FLAME6 was greater than in the present study. Our findings differ from those in the Informing the Pathway of COPD Treatment (IMPACT)7 and Efficacy and Safety of Triple Therapy in Obstructive Lung Disease (ETHOS)8 trials, which found no benefit of LAMA-LABA therapy in increasing the time to first moderate or severe COPD exacerbation compared with ICS-LABA therapy and a 13%7 and 15%8 higher annual rate, respectively, of moderate or severe exacerbations among those receiving LAMA-LABAs.
Caution must be applied when comparing our findings with those of FLAME,6 IMPACT,7 and ETHOS,8 since the cohorts under investigation differed so markedly. Fewer than half of patients in our study underwent spirometry measurement in the year before cohort entry and fewer than a third experienced moderate or severe COPD exacerbations, while all patients in FLAME,6 IMPACT,7 and ETHOS8 underwent spirometry measurement and had 1 or more moderate or severe COPD exacerbations in the year before enrollment. Reflecting routine practice, few people starting combination therapy in our study had received any maintenance inhaler therapy in the year before cohort entry compared with the overwhelming majority of patients in FLAME,6 IMPACT,7 and ETHOS.8
When parsing the conflicting findings of FLAME6 on the one hand and IMPACT7 and ETHOS8 on the other, some commentators point to the features associated with high risk among patients enrolled in IMPACT7 and ETHOS8 as a potential explanation for why ICS-LABA therapy may have been effective in these trials. The cohort in our study is certainly closer to the population of patients enrolled in FLAME,6 but the patients in our study appear to have substantially lower baseline risk than in all 3 of the other trials. Because physicians frequently prescribe inhalers to patients who would not meet entry criteria for inclusion in COPD trials, the relative effectiveness of LAMA-LABA therapy compared with ICS-LABA therapy may differ in routine clinical practice. Our study should not be interpreted as a decisive verdict on the conflicting clinical trial data in FLAME,6 IMPACT,7 and ETHOS8 but rather as 1 piece of additional evidence to help shape clinical guidelines.
To our knowledge, this is the largest observational study to date comparing LAMA-LABA with ICS-LABA therapy. Our findings differ from those of earlier cohort studies in important ways. Two such studies23,25 found no difference in COPD exacerbations when comparing LAMA-LABA with ICS-LABA therapy, but these studies were completed before the widespread availability of combined LAMA-LABA inhalers. Most patients in the exposure groups received LAMA and LABA therapy via 2 separate inhalers, while many in the reference group received ICS and LABA therapy via the same inhaler.23,25 This may have biased findings, since patients prescribed multiple inhalers are more likely to be nonadherent to treatment and require urgent care visits, emergency department visits, and hospitalizations compared with patients receiving the same therapy via combination inhalers.32,33,34 A third study,35 which found a benefit for LAMA-LABA therapy via a single inhaler, analyzed only 1 LAMA-LABA (tiotropium-olodaterol) and included patients using other LAMA-LABAs during the washout period while excluding those who used ICS-LABAs, which may have biased findings toward tiotropium-olodaterol. All 3 studies23,25,35 were limited by small sample sizes in the exposure group.
A recent study36 from Taiwan found that patients receiving LAMA-LABA inhalers had better outcomes than those receiving fluticasone-salmeterol but not budesonide-formoterol or beclomethasone-formoterol, leading some to wonder whether clinical guidelines may ignore potential intraclass differences among inhaled corticosteroids.37 Our study found LAMA-LABAs to be associated with better outcomes than ICS-LABAs regardless of whether fluticasone or budesonide was the ICS included—although statistically significant differences in rates of exacerbations were not observed for less commonly used fluticasone products or for mometasone-containing products. More work is needed to understand potential intraclass differences in inhaled therapy, but our findings do not support treating individual ICS-LABAs differently from one another in treatment guidelines.
Limitations
This article has several limitations. First, short follow-up time in our analysis underscores the problems of nonadherence in routine clinical practice and raises concerns about the generalizability of our findings to patients who continue therapy for longer durations. The fact that LAMA-LABA therapy was associated with fewer exacerbations and pneumonia hospitalizations on intention-to-treat analysis may help assuage some of these concerns, but questions remain about the comparative effectiveness and safety of LAMA-LABA and ICS-LABA therapy among patients who are adherent to their medications for long durations.
Second, our analysis relied on prescription fills, leaving open the possibility of exposure misclassification since patients may not actually have been using their inhalers or may have been doing so incorrectly. This may partially explain why the effect sizes in our study were smaller than those in the FLAME trial.6
Third, the patients receiving ICS-LABAs in our unmatched cohort before propensity score matching differed in key ways from those receiving LAMA-LABAs. They tended to have more severe COPD (as measured by GOLD stage) and less access to specialty respiratory care (with lower rates of spirometry measurement and fewer prescriptions by pulmonologists at cohort entry), which may have led to worse outcomes. Although we adjusted for numerous confounders using propensity score matching and performed several sensitivity analyses, we cannot completely exclude the possibility of residual confounding.
Fourth, our analysis did not compare LAMA-LABA or ICS-LABA therapy with triple therapy (ICS-LAMA-LABA). Further studies in clinical settings comparing dual with triple therapy will be important, since the 2023 GOLD guidelines2 reserve a role for ICS-LAMA-LABAs over LAMA-LABAs among those with frequent exacerbations and high eosinophil counts.
Fifth, use of ICS-LABAs has been associated with other safety concerns besides pneumonia hospitalizations, including milder episodes of pneumonia and thrush.38 Our analysis focused on only 1 key safety concern.
Conclusions
In this cohort study including a large population of patients with COPD receiving usual care, those receiving LAMA-LABA therapy had a lower incidence of moderate or severe COPD exacerbations and a lower incidence of pneumonia hospitalizations than those receiving ICS-LABA therapy. These findings provide further support for recommendations in clinical guidelines favoring LAMA-LABA over ICS-LABA therapy.
eMethods
eTable 1. Key Randomized Controlled Trials Comparing LAMA-LABA to ICS-LABA Therapy in COPD
eTable 2. Inhalers in the Exposure and Referent Groups
eTable 3. Identification of Oral Antibiotics Used to Treat COPD Exacerbations
eTable 4. Reasons for Censoring in the Primary Effectiveness Analysis
eTable 5. Reasons for Censoring in the Primary Safety Analysis
eFigure 1. Study Design Comparing New LAMA-LABA Users to ICS-LABA Users
eFigure 2. Cohort Composition
eFigure 3. Propensity Score Distributions Before and After Matching
eFigure 4. Sensitivity Analysis for the Incidence of First Moderate COPD Exacerbation
eFigure 5. Sensitivity Analysis for the Incidence of First Severe COPD Exacerbation
eFigure 6. Sensitivity Analysis for Subgroup Comparison of First Moderate or Severe COPD Exacerbation Based on Eosinophil Level
eFigure 7. Subgroup Analysis for the Incidence of First Pneumonia Hospitalization
eFigure 8. Incidence of First Moderate or Severe COPD Exacerbation by Type of ICS-LABA
eFigure 9. Incidence of First Pneumonia Hospitalization by Type of ICS-LABA
eReferences
Data Sharing Statement
References
- 1.Global Initiative on Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report. 2020. Accessed January 23, 2023. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 2.Global Initiative on Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2023 report. 2022, 2023. Accessed January 23, 2023. https://goldcopd.org/wp-content/uploads/2023/01/GOLD-2023-ver-1.2-7Jan2023_WMV.pdf
- 3.Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008;134(2):255-262. doi: 10.1378/chest.07-2138 [DOI] [PubMed] [Google Scholar]
- 4.Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51-60. doi: 10.1016/S2213-2600(12)70052-8 [DOI] [PubMed] [Google Scholar]
- 5.Zhong N, Wang C, Zhou X, et al. ; LANTERN Investigators . LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Banerji D, Chapman KR, et al. ; FLAME Investigators . Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222-2234. doi: 10.1056/NEJMoa1516385 [DOI] [PubMed] [Google Scholar]
- 7.Lipson DA, Barnhart F, Brealey N, et al. ; IMPACT Investigators . Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 8.Rabe KF, Martinez FJ, Ferguson GT, et al. ; ETHOS Investigators . Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35-48. doi: 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 9.Herland K, Akselsen JP, Skjønsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99(1):11-19. doi: 10.1016/j.rmed.2004.03.026 [DOI] [PubMed] [Google Scholar]
- 10.Travers J, Marsh S, Caldwell B, et al. External validity of randomized controlled trials in COPD. Respir Med. 2007;101(6):1313-1320. doi: 10.1016/j.rmed.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Walker S, Fingleton J, Weatherall M, Beasley R. Limited generalisability of UPLIFT findings to clinical practice. Thorax. 2013;68(11):1066-1067. doi: 10.1136/thoraxjnl-2013-203724 [DOI] [PubMed] [Google Scholar]
- 12.Scichilone N, Basile M, Battaglia S, Bellia V. What proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials? Respiration. 2014;87(1):11-17. doi: 10.1159/000355082 [DOI] [PubMed] [Google Scholar]
- 13.Halpin DM, Kerkhof M, Soriano JB, Mikkelsen H, Price DB. Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy. Respir Res. 2016;17(1):120. doi: 10.1186/s12931-016-0433-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodcock A, Boucot I, Leather DA, et al. Effectiveness versus efficacy trials in COPD: how study design influences outcomes and applicability. Eur Respir J. 2018;51(2):1701531. doi: 10.1183/13993003.01531-2017 [DOI] [PubMed] [Google Scholar]
- 15.Suissa S. Run-in bias in randomised trials: the case of COPD medications. Eur Respir J. 2017;49(6):1700361. doi: 10.1183/13993003.00361-2017 [DOI] [PubMed] [Google Scholar]
- 16.Suissa S, Drazen JM. Making sense of triple inhaled therapy for COPD. N Engl J Med. 2018;378(18):1723-1724. doi: 10.1056/NEJMe1716802 [DOI] [PubMed] [Google Scholar]
- 17.Gershon AS, Lindenauer PK, Wilson KC, et al. Informing healthcare decisions with observational research assessing causal effect: an official American Thoracic Society research statement. Am J Respir Crit Care Med. 2021;203(1):14-23. doi: 10.1164/rccm.202010-3943ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980-987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavakoli H, Chen W, Sin DD, FitzGerald JM, Sadatsafavi M. Predicting severe chronic obstructive pulmonary disease exacerbations. developing a population surveillance approach with administrative data. Ann Am Thorac Soc. 2020;17(9):1069-1076. doi: 10.1513/AnnalsATS.202001-070OC [DOI] [PubMed] [Google Scholar]
- 21.Suissa S, Dell’Aniello S, Ernst P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: Cohort Study in Real-World Clinical Practice. Chest. 2020;157(4):846-855. doi: 10.1016/j.chest.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 22.Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855-862. doi: 10.1016/S2213-2600(18)30368-0 [DOI] [PubMed] [Google Scholar]
- 23.Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158-1165. doi: 10.1016/j.chest.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Stanford RH, Nag A, Mapel DW, et al. Claims-based risk model for first severe COPD exacerbation. Am J Manag Care. 2018;24(2):e45-e53. [PubMed] [Google Scholar]
- 25.Samp JC, Joo MJ, Schumock GT, Calip GS, Pickard AS, Lee TA. Comparative effectiveness of long-acting beta2-agonist combined with a long-acting muscarinic antagonist or inhaled corticosteroid in chronic obstructive pulmonary disease. Pharmacotherapy. 2017;37(4):447-455. doi: 10.1002/phar.1913 [DOI] [PubMed] [Google Scholar]
- 26.Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771-782. doi: 10.2147/CLEP.S117867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi: 10.1371/journal.pone.0151357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87-93. doi: 10.1378/chest.11-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern DM, Davis J, Williams SA, et al. Validation of an administrative claims-based diagnostic code for pneumonia in a US-based commercially insured COPD population. Int J Chron Obstruct Pulmon Dis. 2015;10:1417-1425. doi: 10.2147/COPD.S83135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinozaki T, Mansournia MA. Hazard ratio estimators after terminating observation within matched pairs in sibling and propensity score matched designs. Int J Biostat. 2019;15(1):20170103. doi: 10.1515/ijb-2017-0103 [DOI] [PubMed] [Google Scholar]
- 31.Lin DYWL. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074-1078. doi: 10.1080/01621459.1989.10478874 [DOI] [Google Scholar]
- 32.Zhang S, King D, Rosen VM, Ismaila AS. Impact of single combination inhaler versus multiple inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:417-438. doi: 10.2147/COPD.S234823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu AP, Guérin A, de Leon DP, et al. Clinical and economic outcomes of multiple versus single long-acting inhalers in COPD. Respir Med. 2011;105(12):1861-1871. doi: 10.1016/j.rmed.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Hagedorn C, Kässner F, Banik N, Ntampakas P, Fielder K. Influence of salmeterol/fluticasone via single versus separate inhalers on exacerbations in severe/very severe COPD. Respir Med. 2013;107(4):542-549. doi: 10.1016/j.rmed.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 35.Quint JK, Montonen J, Esposito DB, et al. Effectiveness and safety of COPD maintenance therapy with tiotropium/olodaterol versus LABA/ICS in a US claims database. Adv Ther. 2021;38(5):2249-2270. doi: 10.1007/s12325-021-01646-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang MT, Lai JH, Huang YL, et al. Comparative effectiveness and safety of different types of inhaled long-acting β2-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting β2-agonist plus inhaled corticosteroid fixed-dose combinations in COPD: a propensity score-inverse probability of treatment weighting cohort study. Chest. 2021;160(4):1255-1270. doi: 10.1016/j.chest.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 37.Cazzola M, Matera MG. An obvious paradigm: choosing bronchodilators and inhaled corticosteroids for their pharmacologic characteristics. Chest. 2021;160(4):1157-1159. doi: 10.1016/j.chest.2021.06.025 [DOI] [PubMed] [Google Scholar]
- 38.Dekhuijzen PNR, Batsiou M, Bjermer L, et al. Incidence of oral thrush in patients with COPD prescribed inhaled corticosteroids: effect of drug, dose, and device. Respir Med. 2016;120:54-63. doi: 10.1016/j.rmed.2016.09.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Key Randomized Controlled Trials Comparing LAMA-LABA to ICS-LABA Therapy in COPD
eTable 2. Inhalers in the Exposure and Referent Groups
eTable 3. Identification of Oral Antibiotics Used to Treat COPD Exacerbations
eTable 4. Reasons for Censoring in the Primary Effectiveness Analysis
eTable 5. Reasons for Censoring in the Primary Safety Analysis
eFigure 1. Study Design Comparing New LAMA-LABA Users to ICS-LABA Users
eFigure 2. Cohort Composition
eFigure 3. Propensity Score Distributions Before and After Matching
eFigure 4. Sensitivity Analysis for the Incidence of First Moderate COPD Exacerbation
eFigure 5. Sensitivity Analysis for the Incidence of First Severe COPD Exacerbation
eFigure 6. Sensitivity Analysis for Subgroup Comparison of First Moderate or Severe COPD Exacerbation Based on Eosinophil Level
eFigure 7. Subgroup Analysis for the Incidence of First Pneumonia Hospitalization
eFigure 8. Incidence of First Moderate or Severe COPD Exacerbation by Type of ICS-LABA
eFigure 9. Incidence of First Pneumonia Hospitalization by Type of ICS-LABA
eReferences
Data Sharing Statement