Key Points
Question
Do glucose-lowering medications have different effects on kidney outcomes?
Findings
In this randomized clinical trial including 5047 patients with type 2 diabetes, those receiving metformin treatment and predominantly without kidney disease at baseline were randomly assigned to treatment with a sulfonylurea, a dipeptidyl peptidase 4 inhibitor, a glucagonlike peptide 1 receptor agonist, or basal insulin; all groups had good glycemic and blood pressure management. There were no significant differences in decreased estimated glomerular filtration rate, progression of albuminuria, dialysis, kidney transplant, or death during 5 years of follow-up.
Meaning
In patients with type 2 diabetes treated with metformin, kidney outcomes do not appear to differ by treatment with 1 of 4 second glucose-lowering medication classes evaluated in this randomized clinical trial.
Abstract
Importance
Type 2 diabetes (T2D) is the leading cause of kidney disease in the US. It is not known whether glucose-lowering medications differentially affect kidney function.
Objective
To evaluate kidney outcomes in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) trial comparing 4 classes of glucose-lowering medications added to metformin for glycemic management in individuals with T2D.
Design, Setting, and Participants
A randomized clinical trial was conducted at 36 sites across the US. Participants included adults with T2D for less than 10 years, a hemoglobin A1c level between 6.8% and 8.5%, and estimated glomerular filtration rate (eGFR) greater than or equal to 60 mL/min/1.73 m2 who were receiving metformin treatment. A total of 5047 participants were enrolled between July 8, 2013, and August 11, 2017, and followed up for a mean of 5.0 years (range, 0-7.6 years). Data were analyzed from February 21, 2022, to March 27, 2023.
Interventions
Addition of insulin glargine, glimepiride, liraglutide, or sitagliptin to metformin, with the medication combination continued until the HbA1c was greater than 7.5%; thereafter, insulin was added to maintain glycemic control.
Main Outcomes and Measures
Chronic eGFR slope (change in eGFR between year 1 and trial end) and a composite kidney disease progression outcome (albuminuria, dialysis, transplant, or death due to kidney disease). Secondary outcomes included incident eGFR less than 60 mL/min/1.73 m2, 40% decrease in eGFR to less than 60 mL/min/1.73 m2, doubling of urine albumin-to-creatinine ratio (UACR) to 30 mg/g or greater, and progression of Kidney Disease Improving Global Outcomes stage. Analyses were intention-to-treat.
Results
Of the 5047 participants, 3210 (63.6%) were men. Baseline characteristics were mean (SD) age 57.2 (10.0) years; HbA1c 7.5% (0.5%); diabetes duration, 4.2 (2.7) years; body mass index, 34.3 (6.8); blood pressure 128.3/77.3 (14.7/9.9) mm Hg; eGFR 94.9 (16.8) mL/min/1.73 m2; and median UACR, 6.4 (IQR 3.1-16.9) mg/g; 2933 (58.1%) were treated with renin-angiotensin-aldosterone inhibitors. Mean chronic eGFR slope was −2.03 (95% CI, −2.20 to −1.86) mL/min/1.73 m2 per year for patients receiving sitagliptin; glimepiride, −1.92 (95% CI, −2.08 to −1.75) mL/min/1.73 m2 per year; liraglutide, −2.08 (95% CI, −2.26 to −1.90) mL/min/1.73 m2 per year; and insulin glargine, −2.02 (95% CI, −2.19 to −1.84) mL/min/1.73 m2 per year (P = .61). Mean composite kidney disease progression occurred in 135 (10.6%) patients receiving sitagliptin; glimepiride, 155 (12.4%); liraglutide, 152 (12.0%); and insulin glargine, 150 (11.9%) (P = .56). Most of the composite outcome was attributable to albuminuria progression (98.4%). There were no significant differences by treatment assignment in secondary outcomes. There were no adverse kidney events attributable to medication assignment.
Conclusions and Relevance
In this randomized clinical trial, among people with T2D and predominantly free of kidney disease at baseline, no significant differences in kidney outcomes were observed during 5 years of follow-up when a dipeptidyl peptidase 4 inhibitor, sulfonylurea, glucagonlike peptide 1 receptor agonist, or basal insulin was added to metformin for glycemic control.
Trial Registration
ClinicalTrials.gov Identifier: NCT01794143
This randomized clinical trial evaluates kidney function with the use of medications added to metformin treatment in patients with type 2 diabetes.
Introduction
Diabetes is the leading cause of chronic kidney disease and kidney failure in the US and most of the world. Achieving and maintaining glycemic control is critical for preventing or delaying the development of diabetic kidney disease (DKD).1 Clinical trials in patients with type 1 diabetes and type 2 diabetes (T2D) have consistently demonstrated that intensive glycemic control reduces the risk of developing albuminuria,2,3,4 and long-term follow-up of some cohorts suggests that intensive glycemic control also prevents a decrease in estimated glomerular filtration rate (eGFR) and kidney failure.5,6
More recently, in T2D, several classes of glucose-lowering drugs have been demonstrated to have kidney benefits that appear independent of glycemic effects, an effect mostly seen in studies of people with DKD, atherosclerotic cardiovascular disease, or high atherosclerotic cardiovascular disease risk. Dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagonlike peptide-1 (GLP-1) receptor agonists, and sodium-glucose cotransporter-2 (SGLT2) inhibitors have all been shown to reduce albuminuria in DKD, compared with placebo or other glucose-lowering drugs.7,8,9,10,11,12 In addition, SGLT2 inhibitors have been clearly demonstrated to slow the decrease in eGFR over time,10,11,13 and GLP-1 receptor agonists have shown potential benefits with regard to eGFR loss in short-term studies and secondary analyses of cardiovascular outcome trials.9,12 Whether people at an earlier stage of diabetes, largely free of DKD, derive differential benefit from treatment with non-SGLT2 inhibitor glucose-lowering medication classes is unknown.
The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) Study aimed to compare glycemic and other outcomes among 4 commonly used classes of glucose-lowering medications added to metformin. The medications, selected to represent the most popular classes available at the time of study design and launch in 2013, were the DPP-4 inhibitor sitagliptin, the sulfonylurea glimepiride, the GLP-1 receptor agonist liraglutide, and the basal insulin glargine.14,15 As noted, DPP-4 inhibitors and GLP-1 receptor agonists have shown modest kidney benefits, chiefly albuminuria lowering, in placebo-controlled trials. In contrast to recent cardiovascular outcome trials, GRADE enrolled a diverse US cohort with shorter duration of diabetes, predominantly without cardiovascular and kidney complications at baseline, and maintained overall good glycemic management across all 4 treatment groups. GRADE reported modest differences in time to glycemic progression16 and no significant differences in overall microvascular outcomes in GRADE.17 In this report, we evaluate the effects of the GRADE interventions on detailed kidney outcomes.
Methods
Main Outcomes and Measures
GRADE and its major outcomes have been previously described.14,15,16,17 The GRADE protocol is given in Supplement 1, and the inclusion and exclusion criteria are presented in the eMethods 1 in Supplement 2. The trial was approved by the institutional review boards of the George Washington University and all clinical centers. All participants provided written informed consent and received financial compensation. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Trial Design
GRADE was a parallel-group comparative effectiveness clinical trial conducted in 36 clinical centers across the US. Randomization was performed via a central web-based system and stratified by site. Treatment assignment was unmasked to the participants and clinic staff; laboratories, reading centers, and event adjudicators were masked to treatment assignment.
Participants
Participants had T2D diagnosed at age 30 years or older (≥20 years in self-reported American Indian or Alaska Native individuals), diabetes duration less than 10 years, treated with metformin 1000 to 2000 mg/d, with hemoglobin A1c (HbA1c) 6.8% to 8.5% (to convert to proportion of total hemoglobin, multiply by 0.01) at randomization. Potential participants with a history of a major cardiovascular event within the previous year and New York Heart Association Class II to IV heart failure were excluded. At the outset of the trial, men with a creatinine level greater than 1.5 mg/dL (to convert to micromoles per liter, multiply by 88.4) and women with a creatinine level greater than 1.4 mg/dL were excluded; this threshold was subsequently replaced by estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2 to be consistent with changes in practice and metformin prescribing (protocol version 1.6, August 2016). Participants were recruited between July 8, 2013, and August 11, 2017, and followed up for a mean duration of 5.0 (range, 0-7.6) years through April 30, 2021, with 85.3% completing at least 4 years of follow-up.16,17
Interventions
Participants were randomly assigned to start sitagliptin, glimepiride, liraglutide, or insulin glargine, 100 U/mL, in addition to metformin. The medication combination was continued until the HbA1c was greater than 7.5% and confirmed; thereafter, basal and prandial insulin, if needed, were added to maintain glycemic control. All medications were dosed according to their labeling guidelines. Starting in 2018, updated consensus recommendations on the choice of glucose-lowering medications (including GLP-1 receptor agonists and SGLT2 inhibitors) in the setting of prevalent cardiovascular and, later, kidney disease were issued by the American Diabetes Association/European Association for the Study of Diabetes.18,19 These recommendations were shared with participants eligible for these interventions and their health care professionals. Usual care clinicians managed all medication therapy other than the protocol-assigned glucose-lowering medications, including SGLT2 inhibitors or nonstudy GLP-1 receptor agonists.
Outcomes and Assessments
Participants had in-person study visits every 3 months. Weight and seated standardized blood pressure (mean of 3 measurements using a calibrated sphygmomanometer) were measured per study protocol. Kidney function and damage were assessed by assays of creatinine and albumin.20 Serum creatinine was determined annually, and a spot urinary albumin-to-creatinine ratio (UACR) was determined every 6 months. Creatinine levels were measured in serum and urine by an enzymatic method (creatinase) (Roche cobas 6000 system; Roche Diagnostics) with trueness verified using National Institute of Standards and Technology standard materials.21 The eGFR was calculated from the measured creatinine level using the Chronic Kidney Disease Epidemiology Collaboration equation, including self-reported race and ethnicity as a variable to maintain consistency with previously reported results.14,22 Urine albumin levels were measured using an immunoturbidimetric method (Roche) standardized against a reference preparation. Moderately increased albuminuria was defined as greater than or equal to 30 to less than 300 mg/g of creatinine, confirmed at a subsequent visit, and severely increased albuminuria as greater than or equal to 300 mg/g of creatinine. We also report the prevalence and treatment of hypertension over follow-up and off-protocol use of SGLT2 inhibitors and GLP-1 receptor agonists. Cause of death was adjudicated by an outcomes committee blinded to treatment assignment.
The statistical analysis plan (Supplement 1) for this report was prepared after the primary outcomes of GRADE, including the null result for the primary microvascular outcomes, were known. Therefore, the statistical analysis plan for this report prespecified outcomes that were designed to maximize sensitivity to detect a difference among treatment groups (or convincingly demonstrate a null result) in a cohort with high eGFRs and low rates of albuminuria (and therefore low risk for advanced kidney disease outcomes). We specified the 2 coprimary outcomes. The first of these was the slope of (change in) eGFR between year 1 and the end of follow-up, termed chronic eGFR slope, a valid surrogate for kidney disease progression in low-risk populations.23 Since creatinine levels were measured annually, the year 1 measurement was selected as the starting point to assess slope to exclude the effect of short-term, hemodynamic effects of study medications or resolution of hyperglycemia at study entry with consequent reduction of hyperfiltration on eGFR. The second outcome was a composite kidney disease progression outcome, defined as an increase over time in albuminuria stage according to the Kidney Disease Improving Global Outcomes (KDIGO) categories (eg, for participants with UACR<30 mg/g [KDIGO A1] at baseline, confirmed progression to UACR≥30 mg/g [KDIGO A2], and for those with UACR 30-299 mg/g, progression to UACR≥300 mg/g [KDIGO A3]), kidney transplant, dialysis, or death from kidney disease. This outcome was designed primarily to reflect albuminuria progression as an early marker of kidney disease progression, with kidney-associated severe outcomes included to capture competing risks that would prevent assessment of albuminuria.
Secondary outcomes were also selected to maximize sensitivity: change in eGFR from baseline to year 1 (1-year eGFR slope) and from baseline to end of study (total eGFR slope), time to progression of eGFR to less than 60 mL/min/1.73 m2 among those with baseline eGFR above that threshold, time to a 40% decrease in eGFR to less than 60 mL/min/1.73 m2, time to doubling of the UACR to a level greater than or equal to creatinine 30 mg/g, and time to KDIGO category increase from baseline (ie, moving to a higher eGFR [G1 through G5] or albuminuria category [A1 through A3]).24
Statistical Analysis
Data analysis was performed from February 21, 2022, to March 27, 2023. Continuous variables were modeled over the entire duration of follow-up. Categorical variables and cumulative incidence rates are reported at 4 years of follow-up, when 85.3% of the participants remained in the study. The statistical analysis plan prespecified evaluation of the following subgroups: age (<45, 45-59, ≥60 years), sex (male, female), self-reported race (Black, White, other) and ethnicity (Hispanic, non-Hispanic), HbA1c level (tertiles), body mass index (BMI) (tertiles), duration of diabetes (tertiles), baseline hypertension (defined as measured blood pressure ≥130/80 mm Hg or treatment with blood-pressure-lowering medication, including renin-angiotensin-aldosterone system [RAAS] inhibitors), and eGFR less than 60 mL/min/1.73 m2 vs 60 mL/min/1.73 m2 or higher. GRADE recruited a diverse cohort representative of people affected by T2D. Since kidney outcomes differ by race and ethnicity, we evaluated whether there were differences in outcomes by race and ethnicity in GRADE.
Analyses were intention-to-treat and were restricted to participants with at least 1 postbaseline visit (Figure 1). The eGFR slope models use generalized estimating equation models with annual eGFR starting from year 1 as the response and the following covariates: baseline eGFR, time from baseline in whole years, GRADE treatment group, and time-by-treatment interaction.25,26 The test of the interaction term in this model tests for heterogeneity of slopes. We plotted eGFR over time showing means (SDs). Sensitivity analyses refit the generalized estimating equation model starting from baseline instead of year 1 and removing the adjustment for baseline eGFR.
Figure 1. Consolidated Standards of Reporting Trials Diagram.
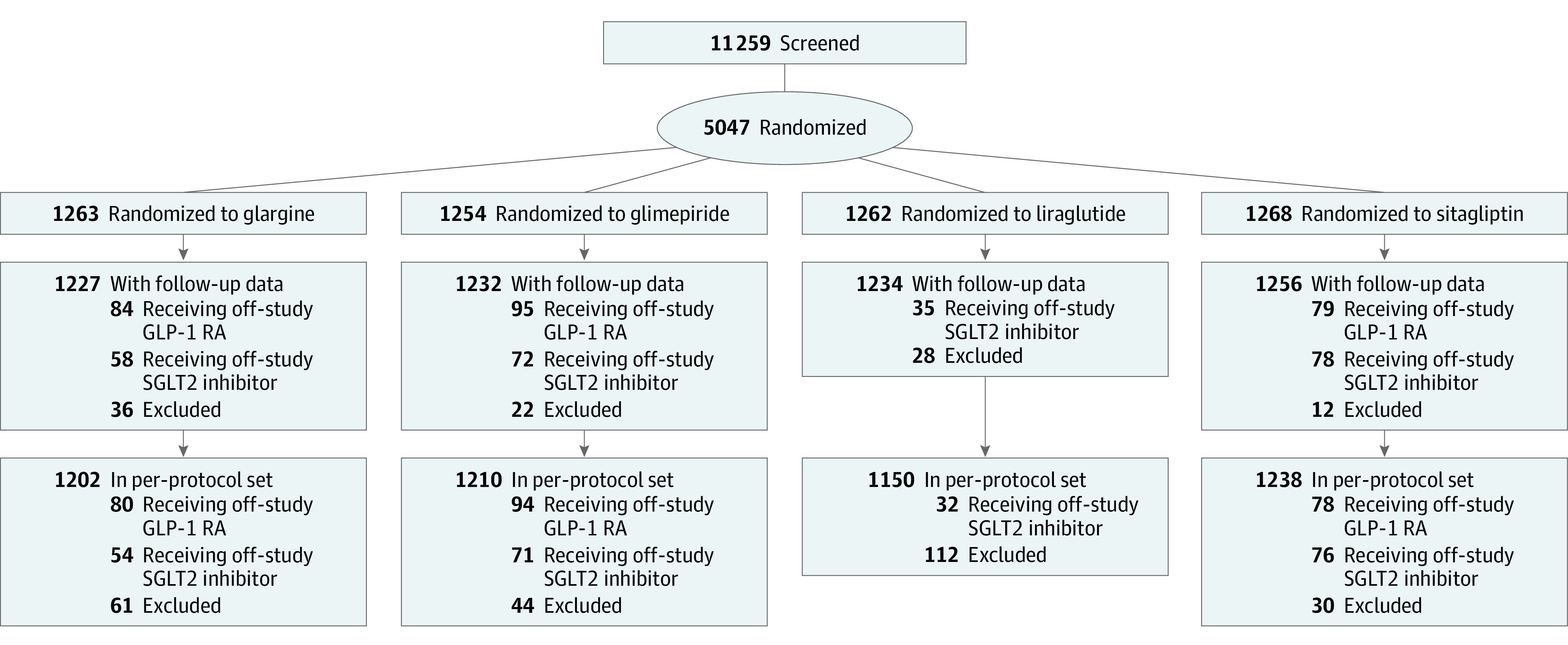
Assignment to the 4 treatment groups, numbers of drop-in/drop-out, and numbers included in intention-to-treat (ITT) and per-protocol analyses. For the ITT analyses, 98 participants in the glargine (n = 36), glimepiride (n = 22), liraglutide (n = 28), and sitagliptin (n = 12) groups were removed from the randomized group (n = 5047) due to not having at least 1 follow-up estimated glomerular filtration rate or 1 follow-up urinary albumin-to-creatinine ratio measure obtained after the baseline visit. For the per-protocol analyses, 247 participants in the glargine (n = 61), glimepiride (n = 44), liraglutide (n = 112), and sitagliptin (n = 30) groups were removed from the randomized group (n = 5047) if they did not attend any follow-up visits or did not take at least 1 dose of their randomized medication. GLP-1 RA indicates glucagonlike peptide-1 receptor agonist; SGLT2, sodium-glucose cotransporter-2.
Evaluation of kidney disease progression over the duration of follow-up is depicted using Kaplan-Meier plots by treatment group and using the log-rank test to assess treatment differences with treatment group as the only covariate. For each secondary outcome, we tested for treatment heterogeneity using similar models and multiple comparison adjustment. Tests of treatment heterogeneity for the 2 primary outcomes across the prespecified subgroups were carried out by adding treatment by subgroup interaction terms to the models described above. The P values from the interaction terms for these models were adjusted for false discovery using the Benjamini-Hochberg procedure (details in eMethods 2 in Supplement 2). For the specified coprimary outcome of composite kidney disease progression, the number of events that occurred in the study allowed a detection of hazard ratios (HRs) of 1.30 assuming 70% power, 1.33 assuming 80% power, and 1.38 assuming 90% power between any 2 treatment groups (for a total of 6 pairwise treatment group comparisons).27
We conducted 2 sets of sensitivity analyses. Per-protocol sensitivity analyses were censored at the last visit before deviation from the assigned treatment regimen. The second set of sensitivity analyses explored the effect of SGLT2 inhibitors and GLP-1 receptor agonists not in the protocol (ie, prescribed by the participants’ personal clinicians) on the primary outcomes. Specific methods are described in eMethods 2 in Supplement 2).
All analyses were conducted with R, version 4.0.3 (R Foundation for Statistical Computing, 2022). All tests were unpaired and 2-sided, with statistical significance set at P < .05.
Results
Baseline Characteristics
Baseline characteristics of the 5047 participants (3210 men [63.6%], 1837 women [36.4%]) are presented in Table 1. Mean (SD) age was 57.2 (10.0) years; BMI, 34.3 (6.8) (calculated as weight in kilograms divided by height in meters squared); and blood pressure, 128.3/77.3 (14.7/9.9) mm Hg. A total of 2933 (58.1%) patients were treated with RAAS inhibitors at baseline. Mean (SD) baseline eGFR was 94.9 (16.8) mL/min/1.73 m2, and 125 (2.5%) participants had an eGFR less than 60 mL/min/1.73 m2. The UACR was moderately elevated in 716 (14.2%) individuals and severely elevated in 84 (1.7%); median UACR was 6.4 (IQR, 3.1-16.9). The mean (SD) duration of the diagnosis of diabetes was 4.2 (2.7) years.
Table 1. Baseline Characteristics of Participants in the GRADE Trial.
| Characteristic | All (N=5047) | Glargine (n=1263) | Glimepiride (n=1254) | Liraglutide (n=1262) | Sitagliptin (n=1268) |
|---|---|---|---|---|---|
| Age at baseline visit, mean (SD), y | 57.2 (10.0) | 57.0 (9.9) | 57.1 (10.1) | 57.4 (9.9) | 57.2 (10.1) |
| Age group, y | |||||
| <45 | 619 (12.3) | 152 (12.0) | 165 (13.2) | 153 (12.1) | 149 (11.8) |
| 45-59 | 2325 (46.1) | 606 (48.0) | 552 (44.0) | 563 (44.6) | 604 (47.6) |
| ≥60 | 2103 (41.7) | 505 (40.0) | 537 (42.8) | 546 (43.3) | 515 (40.6) |
| Sex, No. (%) | |||||
| Men | 3210 (63.6) | 811 (64.2) | 778 (62.0) | 823 (65.2) | 798 (62.9) |
| Women | 1837 (36.4) | 452 (35.8) | 476 (38.0) | 439 (34.8) | 470 (37.1) |
| Race, No. (%)a | |||||
| American Indian or Alaska Native | 137 (2.7) | 33 (2.6) | 30 (2.4) | 40 (3.2) | 34 (2.7) |
| Asian/Hawaiian or Pacific Islander | 210 (4.2) | 39 (3.1) | 54 (4.3) | 55 (4.4) | 62 (4.9) |
| Black or African American | 1000 (19.8) | 253 (20.0) | 271 (21.6) | 251 (19.9) | 225 (17.7) |
| White | 3314 (65.7) | 837 (66.3) | 808 (64.4) | 815 (64.6) | 854 (67.4) |
| Other or unknown | 386 (7.6) | 101 (8.0) | 91 (7.3) | 101 (8.0) | 93 (7.3) |
| Ethnicity, No. (%)a | |||||
| Hispanic | 929 (18.6) | 220 (17.6) | 234 (18.9) | 234 (18.6) | 241 (19.2) |
| Non-Hispanic | 4077 (81.4) | 1032 (82.4) | 1006 (81.1) | 1022 (81.4) | 1017 (80.8) |
| Education completed, No. (%) | |||||
| <High school | 364 (7.2) | 92 (7.3) | 99 (7.9) | 84 (6.7) | 89 (7.0) |
| College degree or above | 2180 (43.2) | 526 (41.6) | 546 (43.5) | 533 (42.2) | 575 (45.4%) |
| High school graduate | 1039 (20.6) | 248 (19.6) | 255 (20.3) | 267 (21.2) | 269 (21.2%) |
| Some college | 1463 (29.0) | 397 (31.4) | 354 (28.2) | 378 (30.0) | 334 (26.4%) |
| Blood pressure, mean (SD), mm Hg | |||||
| Systolic | 128.3 (14.7) | 128.6 (14.8) | 128.2 (14.3) | 128.3 (15.0) | 128.3 (14.9) |
| Diastolic | 77.3 (9.9) | 77.5 (10.0) | 77.0 (9.6) | 77.4 (10.0) | 77.3 (9.8) |
| Treated with ACEi/ARB | 2933 (58.1) | 724 (57.3) | 742 (59.2) | 738 (58.5) | 729 (57.5) |
| History of hypertension | 3339 (66.2) | 845 (66.9) | 834 (66.5) | 843 (66.8) | 817 (64.4) |
| Baseline ASCVD (MI plus stroke) | 328 (6.5) | 80 (6.3) | 78 (6.2) | 78 (6.2) | 92 (7.3) |
| Diabetes | |||||
| BMI | 34.3 (6.8) | 34.4 (6.8) | 34.3 (6.9) | 34.3 (6.7) | 34.1 (6.8) |
| Duration of diabetes, y | 4.2 (2.7) | 4.2 (2.7) | 4.3 (2.8) | 4.2 (2.7) | 4.2 (2.7) |
| HbA1c, mean (SD), % | 7.5 (0.5) | 7.5 (0.5) | 7.5 (0.5) | 7.5 (0.5) | 7.5 (0.5) |
| Kidney parameters | |||||
| eGFR, mean (SD), mL/min/1.73 m2 | 94.9 (16.8) | 94.6 (16.7) | 95.2 (16.9) | 94.2 (17.3) | 95.4 (16.3) |
| eGFR <60 mL/min/1.73 m2, No. (%) | 125 (2.5) | 35 (2.8) | 28 (2.2) | 34 (2.7) | 28 (2.2) |
| UACR median (IQR), mg/g | 6.4 (3.1-16.9) | 6.7 (3.1-17.5) | 6.1 (2.9-16.7) | 6.7 (3.2-18.5) | 6.1 (3.1-15.1) |
| Moderately elevated albuminuria, No. (%) | 716 (14.2) | 174 (13.8) | 174 (13.9) | 191 (15.2) | 177 (14.0) |
| Severely elevated albuminuria, No. (%) | 84 (1.7) | 17 (1.3) | 27 (2.2) | 22 (1.7) | 18 (1.4) |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; MI, myocardial infarction; UACR, albumin-to-creatinine ratio.
SI conversion factor: to convert HbA1c to proportion of total hemoglobin, multiply by 0.01.
Race and ethnicity were determined by participant self-report.
Follow-up
Ninety-eight patients were excluded from the analysis due to missing data (eTable 1 in Supplement 2). Over the course of the trial, participants had mean (SD) HbA1c of 7.2% (1.2%) and blood pressure of 128/76 (16/10) mm Hg, with 2510 (64.4%) treated with RAAS inhibitors and 3201 (82.2%) treated with any blood-pressure-lowering medication at year 4 (eTable 2 in Supplement 2) with minor differences across some treatment groups. Mean (SD) eGFR was measured 5.4 (1.5) times per participant and UACR was measured 9.8 (3.0) times per participant. Rates of permanent discontinuation of assigned study medication were 14% for glargine, 23% for glimepiride, 23% for liraglutide, and 19% for sitagliptin.16
Kidney Outcomes
The mean eGFR decreased during the trial (Figure 2A), with a chronic slope from year 1 to trial end of −2.01 (95% CI, −2.10 to −1.92) mL/min/1.73 m2 per year among all study participants. Mean chronic eGFR slope was −2.03 (95% CI, −2.20 to −1.86) mL/min/1.73 m2 per year for participants receiving sitagliptin; glimepiride, −1.92 (95% CI, −2.08 to −1.75) mL/min/1.73 m2 per year; liraglutide, −2.08 (95% CI, −2.26 to −1.90) mL/min/1.73 m2 per year; and insulin glargine, −2.02 (95% CI, −2.19 to −1.84) mL/min/1.73 m2 per year (P = .61). There were no significant differences in slope among the treatment groups. Similarly, there were no significant differences in mean total slope starting from baseline, change in eGFR during the first year of the study, confirmed progression to eGFR less than 60 mL/min/1.73 m2, or confirmed 40% decrease in eGFR (Table 2, Table 3 and Figure 2A,C,E, and F).
Figure 2. Kidney Parameters Over Time in the GRADE Trial and Secondary Kidney Outcomes.
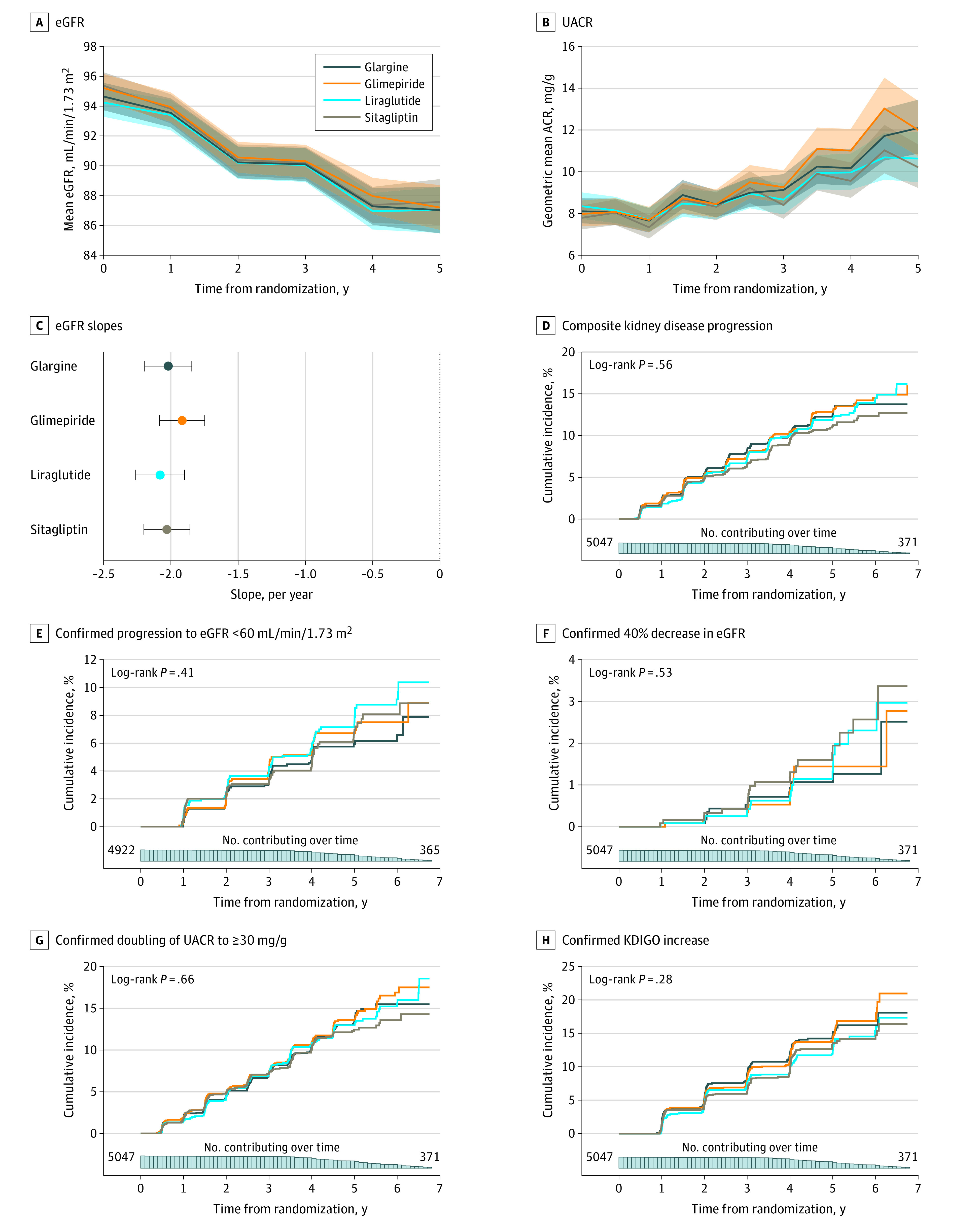
A, Estimated glomerular filtration rate (eGFR) over time by treatment group. Shaded areas indicate 95% CI. B, Urinary albumin-to-creatinine ratio (UACR) over time by treatment group. Shaded areas indicate pointwise 95% CI. C, Slope of eGFR by treatment group from year 1. Error bars indicate 95% CI. D, Composite kidney disease progression (Kaplan-Meier cumulative incidence curve). E, Confirmed progression to eGFR less than 60 mL/min/1.73 m2. F, Confirmed 40% decline in eGFR relative to baseline. G, Confirmed doubling of UACR to a level greater than or equal to 30 mg/g. H, Confirmed Kidney Disease Improving Global Outcomes (KDIGO) category increase.
Table 2. Changes in eGFR Slopea.
| Change | Change (95% CI), mL/min/1.73 m2 per year | |||
|---|---|---|---|---|
| From baseline to 1 y, mean | From 1 y to last observation (chronic slope) | From baseline to trial end, mean | ||
| Mean | Difference in rate of change | |||
| Overall | −1.04 (−1.31 to −0.78) | −2.01 (−2.10 to −1.92) | NA | −1.82 (−1.90 to −1.74) |
| By treatment assignment | ||||
| Glargine | −0.87 (−1.39 to −0.34) | −2.02 (−2.19 to −1.84) | 0 [Reference] | −1.84 (−2.00 to −1.68) |
| Glimepiride | −1.13 (−1.66 to −0.60) | −1.92 (−2.08 to −1.75) | −0.10 (−0.35 to 0.14) | −1.77 (−1.94 to −1.61) |
| Liraglutide | −0.84 (−1.37 to −0.32) | −2.08 (−2.26 to −1.90) | 0.06 (−0.19 to 0.31) | −1.77 (−1.94 to −1.61) |
| Sitagliptin | −1.34 (−1.86 to −0.82) | −2.03 (−2.20 to −1.86) | 0.01 (−0.23 to 0.25) | −1.89 (−2.05 to −1.73) |
Abbreviations: eGFR, estimated glomerular filtration rate; NA, not applicable.
Changes are coprimary outcome. P = .61 for heterogeneity.
Table 3. Kidney Outcomes.
| Treatment assignment | No. (%) | Cumulative incidence at year 4, % (95% CI) | HR (95% CI) | P value for heterogeneity |
|---|---|---|---|---|
| Kidney disease progressiona | ||||
| All treatment groups | 592 (11.7) | 10.02 (9.08-10.96) | NA | .56 |
| Glargine | 150 (11.9) | 10.31 (8.39-12.23) | 1 [Reference] | |
| Glimepiride | 155 (12.4) | 10.39 (8.47-12.32) | 1.04 (0.83-1.30) | |
| Liraglutide | 152 (12.0) | 10.11 (8.21-12.01) | 1.00 (0.80-1.26) | |
| Sitagliptin | 135 (10.6) | 9.27 (7.46-11.08) | 0.88 (0.70-1.12) | |
| Incident eGFR<60 mL/min/1.73 m2 | ||||
| All treatment groups | 293 (5.8) | 5.01 (4.33-5.69) | NA | .63 |
| Glargine | 63 (5.0) | 4.70 (3.37-6.03) | 1 [Reference] | |
| Glimepiride | 74 (5.9) | 5.37 (3.96-6.77) | 1.16 (0.83-1.62) | |
| Liraglutide | 84 (6.7) | 5.62 (4.17-7.06) | 1.32 (0.95-1.83) | |
| Sitagliptin | 72 (5.7) | 4.36 (3.10-5.61) | 1.11 (0.79-1.56) | |
| 40% Decrease in eGFR to <60 mL/min/1.73 m2 | ||||
| All treatment groups | 65 (1.3) | 0.88 (0.59-1.16) | NA | .63 |
| Glargine | 13 (1.0) | 0.93 (0.35-1.52) | 1 [Reference] | |
| Glimepiride | 14 (1.1) | 0.76 (0.23-1.29) | 1.16 (0.54-2.51) | |
| Liraglutide | 17 (1.3) | 0.62 (0.16-1.09) | 1.40 (0.67-2.93) | |
| Sitagliptin | 21 (1.7) | 1.18 (0.53-1.83) | 1.71 (0.84-3.47) | |
| UACR doubling to >30 mg/g | ||||
| All treatment groups | 603 (11.9) | 10.59 (9.59-11.59) | NA | .66 |
| Glargine | 149 (11.8) | 10.57 (8.55-12.58) | 1 [Reference] | |
| Glimepiride | 159 (12.7) | 11.00 (8.96-13.04) | 1.06 (0.85-1.32) | |
| Liraglutide | 155 (12.3) | 10.69 (8.69-12.68) | 1.02 (0.81-1.27) | |
| Sitagliptin | 140 (11.0) | 10.10 (8.16-12.04) | 0.92 (0.73-1.15) | |
| KDIGO stage progression | ||||
| All treatment groups | 594 (11.8) | 10.33 (9.33-11.33) | NA | .63 |
| Glargine | 157 (12.4) | 11.65 (9.49-13.80) | 1 [Reference] | |
| Glimepiride | 160 (12.8) | 11.17 (9.08-13.27) | 1.00 (0.80-1.25) | |
| Liraglutide | 138 (10.9) | 9.17 (7.31-11.03) | 0.86 (0.68-1.08) | |
| Sitagliptin | 139 (11.0) | 9.36 (7.46-11.25) | 0.85 (0.68-1.07) | |
Abbreviations: eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes; UACR, urine albumin-to-creatinine ratio.
Coprimary outcome. Kidney disease progression is progression of albuminuria stage or dialysis, transplant, or kidney death. Other outcomes are prespecified secondary outcomes.
The composite kidney disease progression coprimary outcome occurred among 592 participants (Figure 2D). Most of these events (489 [82.6%]) consisted of progression from normal to moderately elevated albuminuria; 93 patients (15.8%) developed severely elevated albuminuria; and 10 had dialysis, kidney transplant, or death from kidney disease (eFigure 1 and eFigure 2 in Supplement 2). By year 4, the Kaplan-Meier–estimated cumulative incidence of the coprimary outcome of progression of kidney disease was 10.3% in the glargine group, 10.4% in the glimepiride group, 10.1% in the liraglutide group, and 9.3% in the sitagliptin group. There were no significant differences across treatment groups for the duration of study follow-up (log-rank P = .56) (Table 3, Figure 2D). The 5-year cumulative incidence of the coprimary composite kidney disease progression was 11.7% overall at 5 years. Secondary outcomes (Table 3 and Figure 2E-H) did not differ significantly across treatment groups. There was no significant heterogeneity across subgroups, including age, sex, race and ethnicity, HbA1c tertiles, BMI tertiles, duration of diabetes tertiles, presence of baseline hypertension, treatment for hypertension or with RAAS inhibitors at baseline, and eGFR less than 60 mL/min/1.73 m2 in any of the primary or secondary outcomes (eTable 3 in Supplement 2).
Results of exploratory per-protocol analysis were largely consistent with the main findings (eResults and eTables 4 and 5 in Supplement 2); there were lower HRs for kidney disease progression in the liraglutide and sitagliptin groups than in the intention-to-treat analyses with no significant difference across treatment groups (P = .07 for treatment group differences). Use of SGLT2 inhibitors and off-protocol GLP-1 receptor agonists differed across treatment groups, with only 5.5% of the liraglutide group adding an SGLT2 inhibitor or using a different GLP-1 receptor agonist. In the non-liraglutide treatment groups, between 10.5% and 12.0% used either of these medication classes during the trial, with 4.8% of glargine, 5.8% of glimepiride, and 6.2% of sitagliptin-assigned participants adding an SGLT2 inhibitor (eTable 6 in Supplement 2). Sensitivity analyses showed that there were no statistically significant differences in coprimary outcomes in participants with use of SGLT2 inhibitors or off-protocol GLP-1 receptor agonists (eTable 7 in Supplement 2).
Discussion
In people with T2D treated with metformin and predominantly normal clinical kidney parameters at baseline, there were no significant differences in eGFR slope or albuminuria progression by treatment with 4 common classes of glucose-lowering medication during 5 years of follow-up. We evaluated many participants and a range of complementary early sensitive kidney outcomes, but we did not observe any significant differences by treatment assignment in intention-to-treat analyses. These results suggest that neither DPP-4 inhibitor, GLP-1 receptor agonist, sulfonylurea, nor basal insulin has a substantial comparative advantage when added to metformin for preventing the development or progression of DKD in T2D in the first decade after diagnosis.
The 5-year cumulative incidence of the coprimary composite kidney disease progression outcome, which largely represented albuminuria progression, was 11.7% at 5 years. Given its known morbidity, this represents a clinically meaningful incidence of DKD. However, this percentage is lower than the 5-year incidence observed in the UKPDS cohort of patients with newly diagnosed diabetes, in which 17.3% developed moderately increased albuminuria and 3.1% of participants developed severely elevated albuminuria over the same time frame.28 This may reflect better overall glycemic and blood pressure management and more prevalent use of RAAS inhibitors in GRADE.
The eGFR loss in GRADE was significant, with a mean chronic eGFR slope of −2.01 (95% CI, −2.10 to −1.92) mL/min/1.73 m2 from a baseline of 94.9 mL/min 1.73m2. The observed rate of eGFR decrease is more rapid than the median decrease of approximately −1.0 mL/min/1.73 m2 observed in a contemporary, nondiabetic cohort with similar age and eGFR.29 The observed rate of eGFR decrease in GRADE is slower than in the placebo arm of the DECLARE-TIMI 58 study (−2.5 mL/min/1.73 m2 per year from baseline eGFR 85 mL/min/1.73 m2),13,30 but greater than that observed in the placebo arm of the EMPA-REG Outcome Study (−1.46 mL/min/1.73 m2 per year from baseline eGFR 74 mL/min/1.73 m2).31
This rate of eGFR loss and albuminuria progression afforded an opportunity to detect significant differences in outcomes according to treatment assignment, but none were observed. In addition, prespecified subgroup analyses evaluating heterogeneity by age, sex, race and ethnicity, HbA1c level, BMI, duration of diabetes, hypertension, and eGFR were null. Per-protocol sensitivity analyses showed more favorable HRs for sitagliptin and liraglutide with regard to albuminuria-related outcomes. These differences were not statistically significant and should be interpreted with caution, but it is not possible to rule out that benefits might emerge with additional follow-up time. Kidney benefits may take longer to become apparent earlier in the course of T2D, as has been observed in type 1 diabetes, where differences in kidney parameters emerge after 10 years of intensive glycemic management.6,32
Our null results may appear to contrast with findings from randomized clinical cardiovascular outcome trials involving incretin-based therapies, some of which showed improved kidney outcomes.11,12,13,14,15 For example, in clinical trials, the GLP-1 receptor agonists liraglutide (LEADER trial8) and dulaglutide (REWIND trial33) were associated with a reduced risk of a composite kidney outcome, largely due to reduced progression to severely elevated albuminuria and slower eGFR loss in post hoc analyses. A meta-analysis of GLP-1 receptor agonist cardiovascular outcome trials reported an HR of 0.89 (95% CI, 0.82-0.94) for a composite kidney outcome of development of severely elevated albuminuria, doubling of serum creatinine level, 40% or more decrease in eGFR, kidney replacement therapy, or death due to kidney disease.34 Most of the GLP-1 receptor agonist trials showing kidney benefit included participants with substantially higher baseline prevalence of DKD than that in GRADE with beneficial effects of semaglutide demonstrated to be greater in patients with DKD at baseline.35 These observations raise the possibility that GLP-1 receptor agonists are more effective at slowing the progression of established DKD than at preventing the development of DKD. Kidney outcome trials with GLP-1 receptor agonists in established DKD are ongoing.36,37 Meta-analyses of DPP-4 inhibitor trials, which similarly included cardiovascular trials with higher risk populations, have also noted a reduction in albuminuria; this has been attributed to an anti-inflammatory and antifibrotic mechanisms.38,39,40
Limitations
These findings must be interpreted in the context of several limitations. Although GRADE followed up participants with T2D longer than any noncardiovascular outcome study in the current era, 5 years of follow-up remains brief for a low-risk population given that complications of diabetes, including DKD, develop over decades. Consequently, the number of events for the most serious and severe kidney outcomes, such as 40% decrease in eGFR, was low. Slightly greater weight loss in the liraglutide and sitagliptin groups leading to overestimation of UACR due to decreased creatinine excretion rate and lower use of RAAS inhibitors in the liraglutide group could have biased albuminuria results toward the null. A limitation of GRADE overall is the lack of an SGLT2 inhibitor arm owing to the fact that SGLT2 inhibitors were not approved at the time that GRADE was designed and launched; SGLT2 inhibitor treatment reduces eGFR slope decrease and albuminuria progression compared with placebo in lower risk subgroups similar to the GRADE cohort.13 We examined whether the addition of SGLT2 inhibitors influenced outcomes and did not find any effect; however, only 4.9% of participants used SGLT2 inhibitors, with most starting them relatively late in the trial, limiting power to evaluate this outcome. Although there were no observable differences in kidney outcomes across the 4 treatment groups, results from GRADE showing differential effects on total cardiovascular events, weight, and other outcomes may influence the selection of one medication over another.
Conclusions
As a randomized clinical trial of next-step therapy after metformin, GRADE enrolled a low-risk cohort of participants with T2D, largely free of cardiac and kidney disease at baseline. The results of GRADE suggest that, in people with T2D predominantly without kidney complications at baseline, a DPP-4 inhibitor, sulfonylurea, GLP-1 receptor agonist, or basal insulin added to metformin are equivalent with respect to the development or progression of DKD over 5 years.
Trial Protocol and Statistical Analysis Plan
eMethods 1. Inclusion and Exclusion Criteria
eMethods 2. Supplementary Methods
eTable 1. Characteristics of Included and Excluded Participants
eTable 2. Clinical Characteristics Related to Kidney Outcomes at Years 1 and 4
eFigure 1. Distribution of Composite Kidney Outcome Components
eFigure 2. Distribution of KDIGO Category Progression
eTable 3. Heterogeneity Across Subgroups
eResults. Supplementary Results of Per-Protocol and Off-Protocol Use of SGLT2 Inhibitor and GLP-1 Receptor Agonist Analyses
eTable 4. eGFR Slope, Per-Protocol Analyses
eTable 5. Kidney Outcomes, Per-Protocol Analyses
eTable 6. Participants With SGLT2 Inhibitor (SGLT-2i) and Non-Protocol GLP-1 Receptor Agonist (GLP-1 RA) Use (Drop-in)
eTable 7. Effect of SGLT2 Inhibitors or Off-Protocol GLP-1 Receptor Agonists
Nonauthor Collaborators. The GRADE Study Research Group
Data Sharing Statement
References
- 1.American Diabetes Association Professional Practice Committee . 11: Chronic kidney disease and risk management; standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S175-S184. doi: 10.2337/dc22-S011 [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172(10):761-769. doi: 10.1001/archinternmed.2011.2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoungas S, Arima H, Gerstein HC, et al. ; Collaborators on Trials of Lowering Glucose (CONTROL) group . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431-437. doi: 10.1016/S2213-8587(17)30104-3 [DOI] [PubMed] [Google Scholar]
- 4.DCCT/EDIC research group . Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol. 2014;2(10):793-800. doi: 10.1016/S2213-8587(14)70155-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong MG, Perkovic V, Chalmers J, et al. ; ADVANCE-ON Collaborative Group . Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694-700. doi: 10.2337/dc15-2322 [DOI] [PubMed] [Google Scholar]
- 6.de Boer IH, Sun W, Cleary PA, et al. ; DCCT/EDIC Research Group . Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366-2376. doi: 10.1056/NEJMoa1111732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenstock J, Perkovic V, Johansen OE, et al. ; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79. doi: 10.1001/jama.2018.18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann JFE, Ørsted DD, Brown-Frandsen K, et al. ; LEADER Steering Committee and Investigators . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839-848. doi: 10.1056/NEJMoa1616011 [DOI] [PubMed] [Google Scholar]
- 9.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605-617. doi: 10.1016/S2213-8587(18)30104-9 [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 11.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. ; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 12.de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839-848. doi: 10.1016/j.kint.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 13.Mosenzon O, Raz I, Wiviott SD, et al. Dapagliflozin and prevention of kidney disease among patients with type 2 diabetes: post hoc analyses from the DECLARE-TIMI 58 trial. Diabetes Care. 2022;45(10):2350-2359. doi: 10.2337/dc22-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wexler DJ, Krause-Steinrauf H, Crandall JP, et al. ; GRADE Research Group . Baseline Characteristics of Randomized Participants in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2019;42(11):2098-2107. doi: 10.2337/dc19-0901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM, Buse JB, Kahn SE, et al. ; GRADE Study Research Group . Rationale and design of the Glycemia Reduction Approaches in Diabetes: a Comparative Effectiveness Study (GRADE). Diabetes Care. 2013;36(8):2254-2261. doi: 10.2337/dc13-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Lachin JM, Balasubramanyam A, et al. ; GRADE Study Research Group . Glycemia reduction in type 2 diabetes—glycemic outcomes. N Engl J Med. 2022;387(12):1063-1074. doi: 10.1056/NEJMoa2200433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan DM, Lachin JM, Bebu I, et al. ; GRADE Study Research Group . Glycemia reduction in type 2 diabetes—microvascular and cardiovascular outcomes. N Engl J Med. 2022;387(12):1075-1088. doi: 10.1056/NEJMoa2200436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to management of hyperglycemia in type 2 diabetes, 2018; a Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487-493. doi: 10.2337/dci19-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Grams ME, Inker LA. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med. 2022;386(22):2120-2128. doi: 10.1056/NEJMra2201153 [DOI] [PubMed] [Google Scholar]
- 21.Miller WG, Myers GL, Ashwood ER, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129(3):297-304. doi: 10.5858/2005-129-297-CMSOTA [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in Collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84-104. doi: 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17-28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 25.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; 2002. [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121-130. doi: 10.2307/2531248 [DOI] [PubMed] [Google Scholar]
- 27.Schoenfeld D. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika. 1981;68(1):316-319. doi: 10.1093/biomet/68.1.316 [DOI] [Google Scholar]
- 28.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP . Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225-232. doi: 10.1046/j.1523-1755.2003.00712.x [DOI] [PubMed] [Google Scholar]
- 29.Waas T, Schulz A, Lotz J, et al. Distribution of estimated glomerular filtration rate and determinants of its age dependent loss in a German population-based study. Sci Rep. 2021;11(1):10165. doi: 10.1038/s41598-021-89442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606-617. doi: 10.1016/S2213-8587(19)30180-9 [DOI] [PubMed] [Google Scholar]
- 31.Wanner C, Heerspink HJL, Zinman B, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME Trial. J Am Soc Nephrol. 2018;29(11):2755-2769. doi: 10.1681/ASN.2018010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339(2):69-75. doi: 10.1056/NEJM199807093390202 [DOI] [PubMed] [Google Scholar]
- 33.Gerstein HC, Colhoun HM, Dagenais GR, et al. ; REWIND Investigators . Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131-138. doi: 10.1016/S0140-6736(19)31150-X [DOI] [PubMed] [Google Scholar]
- 34.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653-662. doi: 10.1016/S2213-8587(21)00203-5 [DOI] [PubMed] [Google Scholar]
- 35.Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145(8):575-585. doi: 10.1161/CIRCULATIONAHA.121.055459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkovic V, Baeres F, Bakris G, et al. FC 123: baseline characteristics of the flow trial population: kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. Published online May 3, 2022 doi: 10.1093/ndt/gfac126.002 [DOI] [Google Scholar]
- 37.Rossing P, Baeres FMM, Bakris G, et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. 2023;gfad009. doi: 10.1093/ndt/gfad009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae JH, Kim S, Park EG, Kim SG, Hahn S, Kim NH. Effects of dipeptidyl peptidase-4 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta-analysis. Endocrinol Metab (Seoul). 2019;34(1):80-92. doi: 10.3803/EnM.2019.34.1.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daza-Arnedo R, Rico-Fontalvo JE, Pájaro-Galvis N, et al. Dipeptidyl peptidase-4 inhibitors and diabetic kidney disease: a narrative review. Kidney Med. 2021;3(6):1065-1073. doi: 10.1016/j.xkme.2021.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkovic V, Toto R, Cooper ME, et al. ; CARMELINA investigators . Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the CARMELINA randomized trial. Diabetes Care. 2020;43(8):1803-1812. doi: 10.2337/dc20-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods 1. Inclusion and Exclusion Criteria
eMethods 2. Supplementary Methods
eTable 1. Characteristics of Included and Excluded Participants
eTable 2. Clinical Characteristics Related to Kidney Outcomes at Years 1 and 4
eFigure 1. Distribution of Composite Kidney Outcome Components
eFigure 2. Distribution of KDIGO Category Progression
eTable 3. Heterogeneity Across Subgroups
eResults. Supplementary Results of Per-Protocol and Off-Protocol Use of SGLT2 Inhibitor and GLP-1 Receptor Agonist Analyses
eTable 4. eGFR Slope, Per-Protocol Analyses
eTable 5. Kidney Outcomes, Per-Protocol Analyses
eTable 6. Participants With SGLT2 Inhibitor (SGLT-2i) and Non-Protocol GLP-1 Receptor Agonist (GLP-1 RA) Use (Drop-in)
eTable 7. Effect of SGLT2 Inhibitors or Off-Protocol GLP-1 Receptor Agonists
Nonauthor Collaborators. The GRADE Study Research Group
Data Sharing Statement


